- 1Unit of Addiction Medicine, Department of Internal Medicine, Integrated University Hospital of Verona, Policlinico “G.B. Rossi”, Verona, Italy
- 2Department of Neurosciences, University of Verona, Verona, Italy
- 3Department of Diagnostics and Public Health, University of Verona, Verona, Italy
- 4Padova Neuroscience Center, University of Padua, Padua, Italy
- 5Department of General Psychology, University of Padua, Padua, Italy
- 6Diagnostics and Public Health-Unit of Epidemiology and Medical Statistics, University of Verona, Verona, Italy
Benzodiazepine (BDZ) abuse, especially concerning high doses of BDZs, is an impairing substance use disorder (SUD) that is often difficult to treat. Craving and cue reactivity (CR) are two important phenomena that have a prominent role in maintaining addiction and triggering relapses in BDZ abuse; nevertheless, they have rarely been addressed in scientific literature. The present study aims to fill these gaps by implementing a highly innovative virtual reality (VR) design to assess the impact of substance-related environmental cues on BDZ craving, as well as their influence on patients’ affective states. Therefore, on one hand, this research will contribute to the assessment of VR feasibility in the study of these phenomena, and, on the other, it will help disentangle the role that CR and craving have on mood and attention, which are equally important factors to consider when treating SUDs. We will recruit a healthy control group and a patient group comprising people seeking treatment for BDZ detoxification. The experimental design will consist of the presentation of three VR scenarios, one neutral, one BDZ-related but without BDZ cues, and another with BDZ cues. The craving will be measured through a virtual analog scale (VAS); the Profile of Mood States (POMS) and Alcohol Attention Scale (AAS) questionnaires in a modified version will also be administered. We will additionally control for VR-induced feelings of sickness by administering the Simulator Sickness Questionnaire (SSQ), and the Presence Questionnaire (PQ) will be used to investigate participants’ sense of presence in virtual environments. We expect patients to exhibit higher levels of craving, and that the craving will be higher after exposure to a cue-related virtual environment as compared to a neutral scenario.
Introduction
Substance use disorders (SUDs) feature craving as one of their most prominent mechanisms and diagnostic criteria (1). Indeed, craving is involved in the long-term maintenance of abstinence in SUDs, as well as having an important impact on the development of the disorder itself and on the course of the treatment (2–4). Craving is defined as an abrupt urge to consume the target substance (5–7), which often escalates into compulsively seeking the substance and other behaviors related to substance use (8, 9).
Benzodiazepines (BDZs) are positive allosteric modulators of the GABA-A (Gamma-Aminobutyric Acid Type A) receptor (10) which are widely prescribed to treat insomnia and anxiety. Despite their widespread use, studies have shown that BDZs should only be employed in specific clinical situations and preferably for short-term use (11–13). Adverse effects and dependence are associated with their long-term use and should be implemented with extreme caution. Clinicians should also consider short or intermittent treatments, which could have important benefits for patients (14).
About 6–76% of total BDZ users are long-term users. Of these, 15–44% present moderate-to-severe withdrawal symptoms, and 3–4% exhibit dependence (15).
High-dose (HD) BDZ dependence is considered a specific SUD (16), and it consistently reduces the quality of life (17, 18). A cross-sectional survey in France, Germany, Italy, and the UK showed that an estimated 0.14% of the general population took higher-than-recommended doses of anxiolytic medications, while 0.06% reportedly abused hypnotics (19). These data are consistent with those reported by a study conducted in Switzerland, which revealed an incidence rate of 0.16% concerning high-dose BDZ use (20) and points toward HD BDZ abusers being around 1.5 million in Europe and 600,000 in the United States.
Long-term BDZ use is particularly problematic because it has been found to be associated with anomalies in cognitive functions such as attention, memory, and learning. It also exposes patients to a higher risk of delirium, cognitive decline, and accidents (21–30).
To alleviate BDZ withdrawal symptoms, which are particularly impairing for patients, gradual tapering of the dosage or substituting the target BDZ with an equivalent dose of another long-acting benzodiazepine and then tapering are the preferred courses of treatment (31, 32).
Furthermore, BDZs are reportedly secondary drugs of abuse for most individuals, with much fewer patients reporting BDZs as primary drugs of abuse. BDZ abuse is mainly associated with the concurrent abuse of opioids (54.2%) and alcohol (24.7%). Jones et al. (33), in their recent review, reported that about one in five people who abuse alcohol are also benzodiazepine abusers.
Cue reactivity (CR) is a hypersensitivity to motivational stimuli and situations (34). It is considered an adaptive response to salient information (cues) that are present in the environment and it can be evaluated by relying on psychological measures (changes in mood and craving ratings), physiological measures (skin conductance and heart rate), and behavioral measures (gestures/actions) (35). CR is particularly relevant in SUDs, in which it increases craving and facilitates relapses: subjects with a history of substance abuse are particularly sensitive to stimuli and situations which have been previously associated with pleasurable substance effects (36). In this respect, CR is an evolutionary response that may be both a risk factor, when cues are present, and a protective one, when cues are absent: for instance, households with no smoking-related cues have been demonstrated to reduce relapses in smokers (37). Likewise, an external environment may present both protective and precipitating elements. In this perspective, studying the characteristics of various contexts and their function as either risk or protective factors is central in treating and preventing abuse-related behaviors by designing motivationally healthy environments (38). Even though the effects of spatial features on affective states and perception have been extensively studied (39), the role of domestic and urban settings in inducing motivated behaviors is still a largely unexplored topic.
Concerning potential research methods, virtual reality (VR) seems a promising technology to implement in CR paradigms (2, 40, 41). VR consists in the simulation of real-life contexts and environments, which are presented in 3D and are multisensory, comprising auditory, olfactory, visual, and/or tactile inputs (42). Such an approach, being more similar to reality, enhances participants’ sense of presence, that is a state of mind in which virtual environments are perceived as similar to real-world ones, and may therefore be more valid than traditional CR paradigms (e.g., 2D screens, photos, etc.) (43–45).
Higher efficacy may be achieved using technical VR features such as immersion within the VR environment and allowing subjects to actively interact with the system through real-time feedback (46). Other important aspects are the inclusion of substance-related stimuli and the presentation of highly realistic environments (47–49).
To the best of our knowledge, there is currently no literature regarding CR and VR in BDZ abuse. Some studies have addressed CR and alcohol abuse (47, 50, 51) and have highlighted the influence that environmental settings have on craving in alcoholics. This work has been inspired by the study by Ryan et al. (50), especially given the scientific rigor they adopted in their research.
Objectives of the study
General objective
The general objective of the study is the implementation of a VR protocol to identify the causal relationship between environmental features of a specific setting and craving responses in BDZ abusers.
Specific objectives
The primary objective of the study is to identify the causal relationship between exposure to environmental cues related to BDZ use and the degree of BDZ craving in abusers.
Secondary objectives
1. Correlation between the degree of BDZ craving in the various scenarios and measures of mood, affect, attention, sense of presence, and VR malaise in subjects who abuse BDZs.
2. Evaluation of the effectiveness that the three different VR environments have in discriminating between BDZ abusers and control subjects by comparing BDZ craving degree and measures of mood, affect, attention, sense of presence, and VR malaise in the control group vs. those in the experimental group.
Materials and methods
Study design
This research will be an experimental study aiming to measure the degree of BDZ craving induced by VR exposure to environments associated with BDZ use (cues) after immersion in a VR scenario of a bedroom only, and then a bedroom in which BDZ bottles will be present. Every subject will be sequentially exposed to both environments to avoid carry-over effects.
There will be two cohorts of participants. The first group will be the control group and it will comprise subjects that do not suffer from SUD. Participants will be recruited from University students, collaborators, and staff of the University or Hospital. The second group will be the experimental one and will be recruited among BDZ-abusing patients seeking treatment at the Department of Addiction Medicine (Department of Internal Medicine, Integrated University Hospital of Verona) due to their inability to autonomously quit using BDZs. All subjects will be informed regarding the procedures and risks associated with the protocol and experimental design and will be asked to sign an informed consent form before participating in the experiment. Before the experimental session starts, we will collect demographic data and administer a series of questionnaires.
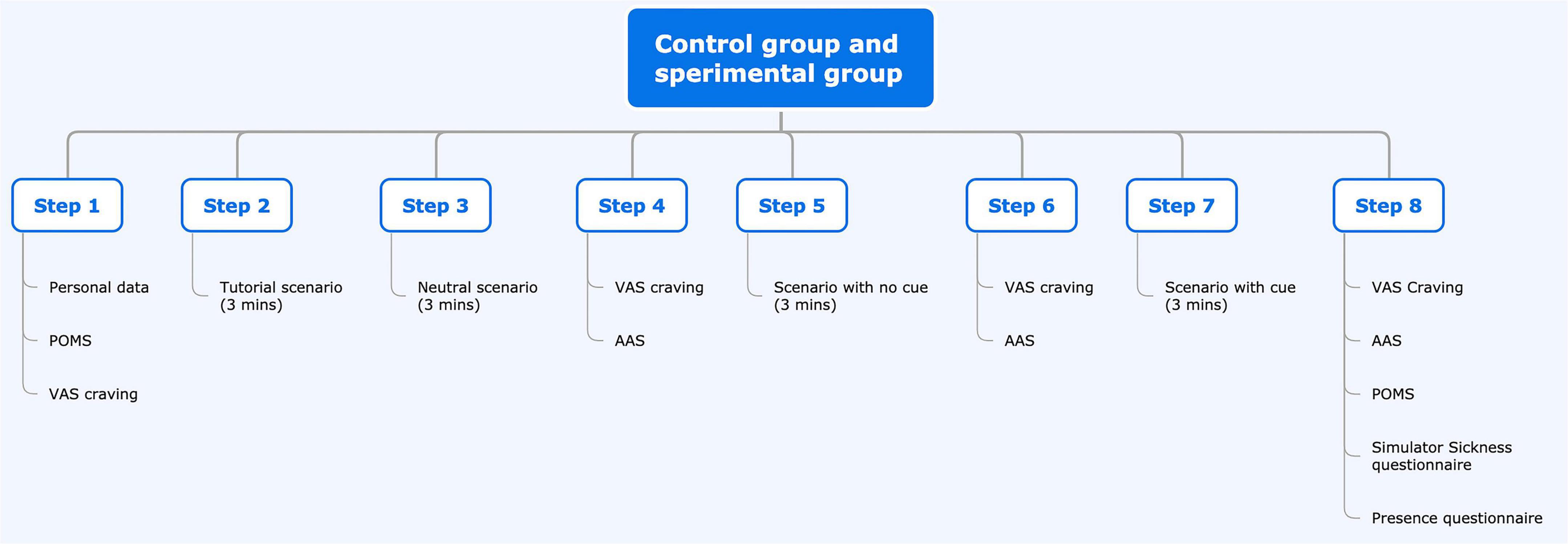
The study will consist of a single session lasting about 45 min. Participants will fill out the Profile of Mood States (POMS) questionnaire before and after the session as a pre-VR baseline measure concerning their mood and affective state. After a 3-min VR baseline, we will administer three scenarios, each lasting 3 min. After the baseline and each of the scenarios, subjects will be required to fill out the VAS to report cravings and a modified version of the Alcohol Attention Scale (AAS) questionnaire. At the end of the experimental session, in addition to the POMS, subjects will also be asked to fill in the Presence Questionnaire (PQ) to assess their sense of presence and the Simulator Sickness Questionnaire (SSQ) to assess the presence of possible adverse effects due to VR exposure.
Materials
Virtual reality instrumentation
HTC-VIVE, which is a VR helmet that facilitates feeling immersed in the proposed virtual scenarios and headphones, enhance auditory immersion as well.
This device allows seeing a virtual world with an optical visor which, thanks to new “room scale” technology, transforms the environment into a 3D space in which the user can freely move. This technology, associated with precise head tracking and controls that simulate hand movements, transforms VR into a particularly immersive experience.
The development platform Unity allows to design and build highly immersive VR scenarios that are compatible with HTC-VIVE.
Procedure
Each subject will be asked to sit in the VR station and will be given all the necessary information regarding the experiment. After signing the informed consent, the subject will give demographic data and will fill out the questionnaires. Before the VR session begins, the participant will be administered the POMS and the VAS on craving. The experimenter will then instruct the participant on how to move in the virtual environment and how to use the HTC-VIVE VR device. The first scenario will be a 3-min baseline simulation during which the subject will familiarize themselves with VR, learn the controls to move around the virtual environment, and practice with the device. In a fixed sequence, the other three scenarios will be shown: house entrance (neutral), bedroom without BDZs, and bedroom with a medicine bottle similar to commercially available BDZ bottles. The subjects will not have to undertake a specific task but will be allowed to freely explore the environment by using the HTC-VIVE PRO Full Kit directional joystick and by moving their head. At the end of each scenario, the subject will remove the visor and headphones and fill out the VAS to report BDZ craving and the modified AAS scale.
Every scenario, including the baseline, will last 3 min. At the end of the last one, the subject will be administered the VAS craving scale, the POMS, the modified AAS, the SSQ, and the PQ.
Questionnaires
Anamnesis schedule (Supplementary Appendix 1) with 10 questions.
VAS scale (Supplementary Appendix 2) with a question relative to BDZ craving. The instrument is a single-item visual analog scale with a score ranging from 0 (absent) to 9 (extreme).
The POMS (52) (Supplementary Appendix 3) is widely used to assess mood and to identify possibly problematic affective states. It is a self-report questionnaire, and it is mainly used in clinical psychology, psychotherapy, and medicine. It comprises 58 adjectives that define six mood states: tension-anxiety (T), which describes an overt or covert increase in somatic tension; depression (D), which indicates a depressed mood accompanied by a sense of inadequacy, hopelessness, emotional isolation, melancholy, and guilt; aggression-anger (A), which describes anger and dislike toward others; vigor-activity (V), comprising adjectives that suggest exuberance, energy, euphoria, and optimism; tiredness-indolence (TI), which represents boredom, low energy, and physical fatigue; confusion (C), characterized by a sense of disturbance and linked to the organization-disorganization dimension, including anxiety and the feeling of cognitive inefficiency.
The adjectives are rated on a 5-point Likert scale (0 = not at all, 1 = a little bit, 2 = moderately, 3 = quite a bit, and 4 = extremely). A Total Mood Disturbance score (TMD) can be calculated by adding the scores for tension, depression, anger, tiredness, and confusion and then subtracting the score for vigor. The POMS showed good reliability both concerning the TMD score (α = 0.85) and the T, D, A, V, S, and C subscales (α = 0.89; α = 0.94; α = 0.71; α = 0.69; α = 0.62; and α = 0.77, respectively).
The SSQ (53) is widely used to measure symptoms of cyber sickness. It comprises 16 items and allows the computation of a total score assessing the severity of the reported symptoms, as well as three subscales for Nausea, Oculomotor Disturbances, and Disorientation (Supplementary Appendix 4).
To measure the attention given to BDZ-related cues, a modified version of the AAS questionnaire (54) will be used, with BDZ-themed questions. Responses are given on a Likert scale ranging from 0 to 10 (Supplementary Appendix 5).
Participants’ sense of presence will be assessed with the PQ (55), comprising 24 items rated on a 7-point Likert scale (Supplementary Appendix 6).
Development and creation of the virtual environments
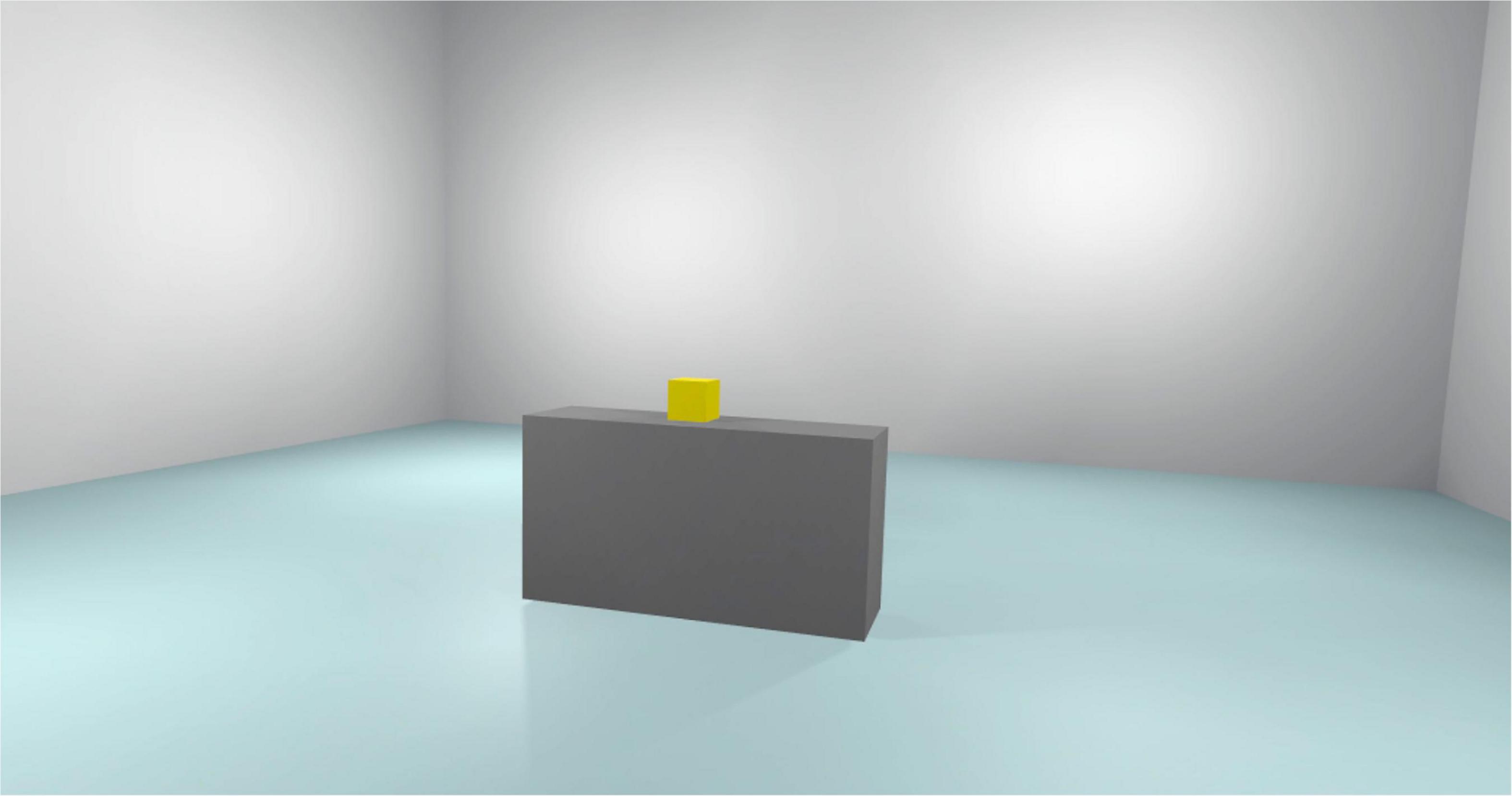
The virtual environments that will be used have been created in photorealistic quality (Figures 1–4) and in “cybersickness-free” mode to allow participants to have a comfortable virtual experience, without any unpleasant side effects. Indeed, cybersickness is a feeling of malaise comprising headaches, vomiting, dizziness, and/or nausea, and it is triggered by a mismatch between visual inputs and those responding to actual movements (56). To achieve this, patients will be allowed to move within the virtual environment through “real” steps whose movement will be faithfully reproduced in the virtual environment. Also, subjects may use teleportation to reach distant positions and beyond the play area. Through the joystick, participants will be able to point to the place they wish to reach, with the virtual experience resuming exactly from the desired spot. The VR environments run on the following VR hardware requirements: (a) HTC-VIVE PRO Full Kit (Figure 5); (b) Gaming PC, Intel Core i7-9700K—GeForce RTX 2070 8GB–16GB DDR4–480GB SSD—Windows 10—Wi-Fi; and (c) a 49“ or 55” TV monitor.
The software has been developed by Hybrid Reality (Padova, Italy1), an innovative start-up that developed the scenarios and provides optimization support.
Virtual environments
Figure 2 reported the tutorial scenario. Participants are exposed to this virtual environment for 3 min. The role of this scenario is to increase familiarity with VR and this is the only scenario in which subjects can interact with the experimenter. In this step of the experiment, the experimenter gives the subjects some instructions and tips on how to better interact with the virtual environment.
Figure 3 reported the neutral scenario. Subjects are exposed to this virtual environment for 3 min. This scenario represents a house entryway. In this scenario, the subject can move freely in the virtual environment, but there are no interactive objects. Every interaction between the subjects and the experimenter is forbidden.
Figure 4 reported the No Cue scenario. Subjects are exposed to this virtual environment for 3 min. This scenario represents a bedroom.
In this scenario, the subject can move freely in the virtual environment, but there are no interactive objects. Every interaction between the subjects and the experimenter is forbidden.
Figure 5 reported the Cue scenario. Subjects are exposed to this virtual environment for 3 min. This scenario represents the same bedroom as the No Cue Scenario. In this scenario, the subject can move freely in the virtual environment. There are only BDZ-related interactive objects. Every interaction between the subjects and the experimenter is forbidden.
Given the COVID-19 pandemic, appropriate accessories will also be employed: disposable, non-woven, breathable face masks for HTC-VIVE PRO, waterproof and hygienic replaceable foam rubber for HTC-VIVE, sanitizing spray, hand sanitizer gel, and surgical masks will be used to guarantee appropriate hygiene of the instruments and the patient’s safety (Figures 6, 7).
Participants
During the recruitment phase, subjects will be given clear and easy-to-understand information regarding the rationale and purpose of the study, as well as information concerning the possible consequences related to their participation in the study. We will give out informative pamphlets, with detailed information about the research as well as the informed consent, and the subjects will be required to carefully read, fill out, and sign. Both documents will be written in simple language and the name of the person who gave the information to the patient will be listed. The experimenter will further need to sign the informed consent and write the date to validate the document. All procedures will be carried out in accordance with the Declaration of Helsinki.
Inclusion criteria
Experimental group:
1. Males and females aged 18–65 years.
2. Subjects with BDZ-use disorder asked to be treated at the Addiction Medicine Unit due to their inability to autonomously quit using BDZs.
3. High-dose BDZ abusers. Although the definition of what constitutes a “high dose” is still controversial and no real consensus exists about the appropriate clinical criteria necessary to define it, we will consider a patient a high-dose user if their BDZ intake will be at least five times higher than the maximum daily defined dose (DDD)
Control group:
1. Males and females aged 18–65 years.
2. Subjects without SUDs (including a BDZ-use disorder) according to ICD 10 F10-F19.
Exclusion criteria
At least one of the following:
1. A history of epilepsy or having a first-degree relative with a history of epilepsy.
2. Serious chronic or cardiovascular diseases.
3. Being pregnant.
4. Having a pacemaker or other metal devices on the head and neck, with the exception of piercings and dental braces.
5. Taking psychoactive substances which may interfere with the results of the study.
The presence or absence of each criterion will be assessed by the experimenter before the study begins.
Safety and hygiene measures
To ensure participant safety and hygiene in the experimental setting, we will comply with the guidelines provided by the Italian Superior Institute of Health (Istituto Superiore di Sanità, ISS), which were approved and implemented in the study by Giordano et al. (57). These include the following procedures:
1. Cleaning of the hands. All staff that will handle the VR devices must use an alcohol-based hand sanitizer. Before a user touches a device, they must wash their hands for at least 40 s, following a specific sequence as illustrated on the Ministry of Health’s website; also, they must rub their hands with an alcohol-based cleaning gel for at least 20 s. This will be done for both operators and participants.
2. The protective waterproof foam guards must be in place on the visor to ensure sanitization of the device.
3. Inserting the waterproof single-use masks in the device before use and substituting them for each subject or operator.
4. Disinfecting all objects that the participants and operators may touch.
5. Surgical masks will be mandatory at all times.
6. Each hygiene measure must be repeated between one participant and the next. At the end of each daily session, all procedures must be enacted one last time to ensure the correct sanitization of the devices.
7. Hospital cleaning personnel will thoroughly clean the room and hospital environment.
8. Trisept Complex, which is a sanitizing product that is available at the internal pharmacy of the University Hospital, will be used to disinfect the VR devices.
Statistical analyses
Primary endpoint
To evaluate the association between environmental features and craving, we will use the VAS scale (10 levels) in the experimental group after exposure to the three scenarios: neutral, bedroom without BDZ bottles, and bedroom with bottles similar to the ones containing BDZs.
Secondary endpoints
To evaluate the association between BDZ craving and mood, affective state, attention, sense of presence, and VR-induced sickness, we will use the total scores and subscales (if present) of the following questionnaires: VAS, POMS, AAS, SSQ, and PQ as measured at the specific timepoints (see flow chart) in the experimental group. The temporal course of the scores will also be compared between the two groups.
Sample size
The appropriate sample size for this study was computed with the software G*Power 3.1.5.1 (58). We chose to base the computation on the difference between the mean VAS craving scores measured within the subjects after the neutral scenario vs. the BDZ-related scenario. Since we expect this difference to be medium-large, we chose a 0.7 effect size (59). Alpha was set to 0.017 considering multiple comparisons among the three scenarios. Since it will be a pilot study, we choose a two-tailed test with 80% power. The resulting sample size was 25 subjects.
To test if the VR scenarios can appropriately distinguish between BDZ abusers and controls, we will also recruit 25 healthy subjects, bringing the total sample size to 50.
Data analysis
All the variables considered in the study will be analyzed by using their most appropriate descriptive statistic. In particular, we will use mean and standard deviation for normally distributed continuous variables, median and interquartile range for non-normally distributed variables, and frequency distribution for categorical variables.
In the experimental group, we will perform a one-way ANOVA for VAS craving at each study timepoint, meaning after exposure to each scenario.
After the repeated-measures ANOVA, we will conduct Bonferroni’s multiple comparison correction to compute post-hoc comparisons and test significant contrasts among the various timepoints for VAS craving. Should the ANOVA assumptions be violated, a Friedman test will be performed. In the experimental group, we will also compute Pearson’s correlations or Spearman’s rank coefficients among VAS, POMS, AAS, SSQ, and PQ scores measured at the same timepoints. We will also explore the correlations between VAS score variations in the three scenarios (Δ VAS neutral—bedroom without BDZ; Δ VAS neutral—bedroom with BDZs; Δ VAS bedroom without BDZs—bedroom with BDZs) and the POMS, AAS, SSQ, and PQ score variations (Δ).
Finally, we will use multilevel linear models to assess group differences in the VAS, POMS, AAS, SQ, and PQ scores as repeatedly measured in the same subjects at specific timepoints. All statistical analyses will be conducted using the PRISM6 software (GraphPad, CA, USA).
Study plan
Flow chart of the study for the experimental and control groups.
Ethics statement
Approval for the research was obtained from the Ethics Committee for Clinical Trials (CESC) of the Provinces of Verona and Rovigo based at the Integrated University Hospital of Verona, Italy (approval code: 3624CESC with Protocol No. 16883 of 09-03-2022). The latest revision of the Declaration of Helsinki as well as the Oviedo Declaration is the basis for the ethical conduct of the study. The study protocol is designed and will be conducted to ensure adherence to the principles and procedures of Good Clinical Practice and to comply with Italian law, as described in the following documents and accepted, by signature, by the study investigators: ICH Harmonized Tripartite Guidelines for Good Clinical Practice 1996; Directive 91/507/EEC, The Rules Governing Medicinal Products in the European Community; D. L.vo n. 211 of 24 June 2003; D. L.vo n. 200, 6 November 2007; Ministerial Decree of 21 December 2007; AIFA Determination, 20 March 2008. All essential clinical records will be retained to demonstrate the validity of the study and the integrity of the data collected. The promoter of this study, in accordance with the responsibilities required by the rules of good clinical practice (Legislative Decree 211/2003) and in accordance with the laws and regulations regarding data protection (including the European Regulation on the protection of personal data 2016/679), will process the personal data that will be collected exclusively for the implementation of the study and for device surveillance.
Discussion
Benzodiazepines are among the most widely used psychotropic medications worldwide, but the chronic use of BDZs can cause several deficits. The risk of dependence after long-term use has been widely reported, and abrupt withdrawal of the drug causes several unpleasant symptoms. In addition to subjects that begin using BDZs to treat anxiety and insomnia and end up using them inappropriately, some subjects deliberately abuse BDZs. In this case, BDZs are taken to counter anxiety or to enhance the effects of other drugs, such as alcohol or opioids, in what becomes a polydrug use pattern (15, 60). Withdrawal syndrome, even from therapeutic doses of BDZs, can be severe and, in some cases, may preclude the patient from ceasing the use of the drug (32, 61). Notwithstanding the important presence of BDZs in clinical practice, no studies have analyzed CR in the context of BDZ addiction yet. VR is a promising research tool since it creates a state of immersion closer to reality, but that still allows the measure of neuropsychological and behavioral responses in a more controlled way (62). For this reason, VR has been extensively used in addiction to drugs and tobacco, for example, to explore smoking withdrawal, craving, and cue reactivity (62). These VR reports, while confirming the findings that were demonstrated in previous, traditional laboratory studies (i.e., cues presented as pictures or videos), still need to better characterize VR-triggered cue reactivity. Environmentally induced craving has been described for various SUDs, and especially for alcohol and tobacco, in which the subjects who were exposed to abuse-related VR stimuli manifested increased craving (47, 63, 64). This has not been investigated in BDZ dependence, but looking at literature concerning other substances, we expect that CR may also be involved in BDZ addiction. Therefore, we expect increased craving in BDZ abusers exposed to BDZ-like stimuli in VR settings, and also significant differences in craving between the experimental group and the control group, with the former exhibiting higher levels of overall craving. One of the most critical issues regard the design of complex and personalized experimental sessions that would also allow measuring and standardizing the variables and parameters of interest (65).
Conclusion
Studies on BDZ abuse are not many, especially concerning high-dose abusers. There are still many relatively unknown variables that would nevertheless be important to investigate, such as craving.
BDZ craving is clinically characterized as an incontrollable urge to take the target substance when it is not readily available. Unlike what has been done for alcohol and tobacco, environmentally induced craving is often considered absent in BDZ abuse and, therefore, it has not yet been investigated in BDZ users. Its role in BDZ abuse, however, has never been tested in scientific research, and neither has its actual presence or absence in this SUD. Virtual reality, therefore, enables the study of this phenomenon without exposing the subjects to real risks. This study protocol aims to fill the current gaps in the scientific literature concerning BDZ-evoked craving in high-dose BDZ users and to better characterize the possible role of the environment in this important mechanism.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee for Clinical Trials (CESC) of the Provinces of Verona and Rovigo based at the Integrated University Hospital of Verona, Italy (approval code: 3624CESC with Protocol No. 16883 of 09-03-2022). The patients/participants provided their written informed consent to participate in this study.
Author contributions
LZ, CC, and FL: conceptualization and writing review and editing. LZ: data curation and investigation. GV: statistical analysis. CC and LZ: methodology. FL and CC: supervision. SC, FF, ST, AC, VM, MM, and VS: writing original draft. All authors have read and agreed to the published version of the manuscript.
Funding
The study described in this manuscript was funded by the Veneto Region (Italy) connected to the funding assigned by DGR no. 2154/2015, with the deliberation of the General Manager 1216 of 30/12/2016 to the Unit of Addiction Medicine in the Department of Internal Medicine in G.B. Rossi Hospital, Verona, Italy. The regional funding concerns the prevention and contrast of pathological gambling. The funding agency had no role in the design of the study or in writing this manuscript and will not influence the collection, analysis, and interpretation of data, apart from their financial contribution.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.956892/full#supplementary-material
Footnotes
References
1. APA. Diagnostic and Statistical Manual of Mental Disorders, DSM-5. 5th ed. Arlington, VA: APA (2013).
2. Hone-Blanchet A, Wensing T, Fecteau S. The use of virtual reality in craving assessment and cue-exposure therapy in substance use disorders. Front Hum Neurosci. (2014) 8:844. doi: 10.3389/fnhum.2014.00844
3. Keyes KM, Krueger RF, Grant BF, Hasin DS. Alcohol craving and the dimensionality of alcohol disorders. Psychol Med. (2011) 2011:629–40. doi: 10.1017/S003329171000053X
4. Stohs ME, Schneekloth TD, Geske JR, Biernacka JM, Karpyak VM. Alcohol craving predicts relapse after residential addiction treatment. Alcohol Alcohol. (2019) 54:167–72. doi: 10.1093/alcalc/agy093
5. Drummond DC. Theories of drugs craving, ancient and modern. Addiction. (2001) 96:33–46. doi: 10.1046/j.1360-0443.2001.961333.x
6. Hartwell EE, Ray LA. Craving as a DSM-5 symptom of alcohol use disorder in non-treatment seekers. Alcohol Alcohol. (2018) 53:235–40. doi: 10.1093/alcalc/agx088
7. van Lier HG, Noordzij ML, Pieterse ME, Postel MG, Vollenbroek-Hutten MMR, de Haan HA, et al. An ideographic study into physiology, alcohol craving and lapses during one hundred days of daily life monitoring. Addict Behav Rep. (2022) 16:100443.
8. Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. (2019) 394:781–92. doi: 10.1016/S0140-6736(19)31775-1
9. Grodin EN, Sussman L, Sundby K, Brennan GM, Diazgranados N, Heilig M, et al. Neural correlates of compulsive alcohol seeking in heavy drinkers. Biol Psychiatry Cogn Neurosci. (2018) 3:1022–31. doi: 10.1016/j.bpsc.2018.06.009
10. Soyka M. Treatment of benzodiazepine dependence. N Engl J Med. (2017) 376:1147–57. doi: 10.1056/NEJMra1611832
11. Lader M. Benzodiazepine harm: How can it be reduced? Br J Clin Pharmacol. (2014) 77:295–301. doi: 10.1111/j.1365-2125.2012.04418.x
12. López-Muñoz F, Alamo C, García-García P. The discovery of chlordiazepoxide and the clinical introduction of benzodiazepines: Half a century of anxiolytic drugs. J Anxiety Disord. (2011) 25:554–62. doi: 10.1016/j.janxdis.2011.01.002
13. Sirdifield C, Anthierens S, Creupelandt H. General practitioners’ experiences and perceptions of benzodiazepine prescribing: Systematic review and meta-synthesis. BMC Fam Pract. (2013) 14:191. doi: 10.1186/1471-2296-14-191
14. Baldwin DS, Aitchison K, Bateson A. Benzodiazepines: Risks and benefits. A reconsideration. J Psychopharmacol. (2013) 27:967–71. doi: 10.1177/0269881113503509
15. Faccini M, Leone R, Opri S, Casari R, Resentera C, Morbioli L, et al. Slow subcutaneous infusion of flumazenil for the treatment of long-term, high-dose benzodiazepine users: A review of 214 cases. J Psychopharmacol. (2016) 30:1047–53. doi: 10.1177/0269881116647505
16. Tamburin S, Faccini M, Casari R, Federico A, Morbioli L, Franchini E, et al. Low risk of seizures with slow flumazenil infusion and routine anticonvulsant prophylaxis for high-dose benzodiazepine dependence. J Psychopharmacol. (2017) 31:1369–73. doi: 10.1177/0269881117714050
17. Lugoboni F, Mirijello A, Faccini M, Casari R, Cossari A, Musi G, et al. Quality of life in a cohort of high-dose benzodiazepine dependent patients. Drug Alcoh Depend. (2014) 142:105–9. doi: 10.1016/j.drugalcdep.2014.06.020
18. Tamburin S, Federico A, Faccini M, Casari R, Morbioli L, Sartore V. Determinants of quality of life in high-dose benzodiazepine misusers. Int J Environ Res Public Health. (2017) 14:38. doi: 10.3390/ijerph14010038
19. Ohayon MM, Lader MH. Use of psychotropic medication in the general population of France, Germany, Italy, and the United Kingdom. J Clin Psychiatry. (2002) 63:817–25. doi: 10.4088/jcp.v63n0912
20. Petitjean S, Ladewig D, Meier CR, Amrein R, Wiesbeck GA. Benzodiazepine prescribing to the Swiss adult population: Results from a national survey of community pharmacies. Int Clin Psychopharmacol. (2007) 22:292–8. doi: 10.1097/YIC.0b013e328105e0f2
21. Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: A meta-analysis. CNS Drugs. (2004) 18:37–48. doi: 10.2165/00023210-200418010-00004
22. Boeuf-Cazou O, Bongue B, Ansiau D, Marquiè JC, Lapeyre-Mestre M. Impact of long-term benzodiazepine use on cognitive functioning in young adults: The VISAT cohort. Eur J Clin Pharmacol. (2011) 67:1045–52. doi: 10.1007/s00228-011-1047-y
23. Finkle WD, Der JS, Greenland S, Adams JL, Ridgeway G, Blaschke T, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. (2011) 59:1883–90. doi: 10.1111/j.1532-5415.2011.03591.x
24. Fond G, Berna F, Boyer L, Godin O, Brunel L, Andrianarisoa M. Benzodiazepine long-term administration is associated with impaired attention/working memory in schizophrenia: Results from the national multicentre FACE-SZ data set. Eur Arch Psychiatry Clin Neurosci. (2018) 268:17–26. doi: 10.1007/s00406-017-0787-9
25. Helmes E, Østbye T. Associations between benzodiazepine use and neuropsychological test scores in older adults. Can J Aging. (2015) 34:207–14. doi: 10.1017/S0714980815000082
26. Kok L, Slooter AJ, Hillegers MH, van Dijk D, Veldhuijzen DS. Benzodiazepine use and neuropsychiatric outcomes in the ICU: A systematic review. Crit Care Med. (2018) 46:1673–80. doi: 10.1097/CCM.0000000000003300
27. Picton JD, Marino AB, Nealy KL. Benzodiazepine use and cognitive decline in the elderly. Am J Health Syst Pharm. (2018) 75:e6–12. doi: 10.2146/ajhp160381
28. Puustinen J, Lähteenmäki R, Polo-Kantola P, Salo P, Vahlberg T, Lyles A, et al. Effect of withdrawal from long-term use of temazepam, zopiclone or zolpidem as hypnotic agents on cognition in older adults. Eur J Clin Pharmacol. (2014) 70:319–29. doi: 10.1007/s00228-013-1613-6
29. van der Sluiszen NNJJM, Vermeeren A, Jongen S, Jongen S, Vinckenboschm F, Ramaekers JG. Influence of long-term benzodiazepine use on neurocognitive skills related to driving performance in patient populations: A review. Pharmacopsychiatry. (2017) 50:189–96. doi: 10.1055/s-0043-112755
30. Wedmann F, Himmel W, Nau R. Medication and medical diagnosis as risk factors for falls in older hospitalized patients. Eur J Clin Pharmacol. (2019) 75:1117–24. doi: 10.1007/s00228-019-02668-3
31. Ashton H. The diagnosis and management of benzodiazepine dependence. Curr Opin Psychiatry. (2005) 18:249–55. doi: 10.1097/01.yco.0000165594.60434.84
32. Lader M. Benzodiazepines revisited–will we ever learn? Addiction. (2011) 106:2086–109. doi: 10.1111/j.1360-0443.2011.03563.x
33. Jones JD, Mogali S, Comer SD. Polydrug abuse: A review of opioid and benzodiazepine combination use. Drug Alcohol Depend. (2012) 125:8–18.
34. Chiamulera C. Cue reactivity in nicotine and tobacco dependence: A “dual-action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking -associated stimuli. Brain Res Rev. (2005) 48:74–97. doi: 10.1016/j.brainresrev.2004.08.005
35. Betts JM, Dowd AN, Forney M, Hetelekides E, Tiffany ST. A meta-analysis of cue reactivity in tobacco cigarette smokers. Nicotine Tob Res. (2021) 23:249–58. doi: 10.1093/ntr/ntaa147
36. Shiffman S, Hickox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: Prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. (1996) 64:993–1002. doi: 10.1037//0022-006x.64.5.993
37. Gilpin EA, Messer K, Pierce JP. Population effectiveness of pharmaceutical aids for smoking cessation: What is associated with increased success? Nicotine Tob Res. (2006) 8:661–9. doi: 10.1080/14622200600910801
38. Chiamulera C, Ferrandi E, Benvegnù G, Ferraro S, Tommasi F, Maris B, et al. Virtual reality for neuroarchitecture: Cue reactivity in built spaces. Front Psychol. (2017) 13:185. doi: 10.3389/fpsyg.2017.00185
39. Ulrich RS. Effects of interior design on wellness: Theory and recent scientific research. J Health Care Inter Des. (1991) 3:97–109.
40. Ghiţă A, Gutiérrez-Maldonado J. Applications of virtual reality in individuals with alcohol misuse: A systematic review. Addict Behav. (2018) 81:1–11. doi: 10.1016/j.addbeh.2018.01.036
41. Segawa T, Baudry T, Bourla A, Blanc JV, Peretti CS, Mouchabac S, et al. Virtual reality (VR) in assessment and treatment of addictive disorders: A systematic review. Front Neurosci. (2019) 13:1409. doi: 10.3389/fnins.2019.01409
42. Bohil CJ, Alicea B, Biocca FA. Virtual reality in neuroscience research and therapy. Nat Rev Neurosci. (2011) 12:752–62. doi: 10.1038/nrn3122
43. Gutiérrez-Maldonado J, Ferrer-García M, Dakanalis A, Riva G. Virtual reality: Applications to eating disorders. In: Agras WS, Robinson A editors. The Oxford handbook of eating disorders. Oxford: Oxford University Press (2018). p. 470–91.
44. Iachini T, Maffei L, Masullo M, Senese VP, Rapuano M, Pascale A, et al. The experience of virtual reality: Are individual differences in mental imagery associated with sense of presence? Cogn Process. (2019) 20:291–8. doi: 10.1007/s10339-018-0897-y
45. Riva G. Virtual reality: An experiential tool for clinical psychology. Br J Guid Counc. (2009) 37:335–43. doi: 10.1080/03069880902957056
46. Lee J, Kim M, Kim J. A study on immersion and VR sickness in walking interaction for immersive virtual reality applications. Symmetry. (2017) 9:78–95.
47. Bordnick PS, Traylor A, Copp HL, Graap KM, Carter B, Ferrer M, et al. Assessing reactivity to virtual reality alcohol based cues. Addict Behav. (2008) 33:743–56. doi: 10.1016/j.addbeh.2007.12.010
48. Culbertson C, Nicolas S, Zaharovits I, London ED, Garza R, Brody AL, et al. Methamphetamine craving induced in an online virtual reality environment. Pharmacol Biochem Behav. (2010) 96:454–60. doi: 10.1016/j.pbb.2010.07.005
49. Saladin ME, Brady KT, Graap K, Rothbaum BO. A preliminary report on the use of virtual reality technology to elicit craving and cue reactivity in cocaine dependent individuals. Addict Behav. (2006) 31:1881–94. doi: 10.1016/j.addbeh.2006.01.004
50. Ryan JJ, Kreiner DS, Chapman MD, Stark-Wroblewski K. Virtual reality cues for binge drinking in college students. Cyberpsychol Behav Soc Netw. (2010) 13:159–62.
51. Traylor AC, Parrish DE, Copp HL, Bordnick TS. Using virtual reality to investigate complex and contextual cue reactivity in nicotine dependent problem drinkers. Addict Behav. (2011) 36:1068–75. doi: 10.1016/j.addbeh.2011.06.014
52. Grove JR, Prapavessis H. Preliminary evidence for the reliability and validity of an abbreviated profile of mood states. Int J Sport Psychol. (1992) 23:93–109.
53. Robert SK, Norman EL, Kevin SB, Michael GL. Simulator sickness questionnaire: an enhanced method for quantifying simulator sickness. Int J Aviat Psychol. (1993) 3:203–20.
54. Monti PM, Rohsenow D, Rubonis S, Niaura R, Sirota A, Colby S, et al. Alcohol cue-reactivity: Effects of detoxification and extended exposure. J Stud Alcohol. (1993) 54:235–45.
55. Witmer BG, Singer MJ. Measuring presence in virtual environments: a presence questionnaire. Presence. (1998) 7:225–40.
56. Weech S, Kenny S, Barnett-Cowan M. Presence and cybersickness in virtual reality are negatively related: A review. Front Psychol. (2019) 10:158. doi: 10.3389/fpsyg.2019.00158
57. Giordano R, Donati MA, Zamboni L, Fusina F, Primi C, Lugoboni F. Alter game: A study protocol on a virtual “serious game” for relapse prevention in patients with gambling disorder. Front Psychiatry. (2022) 13:854088. doi: 10.3389/fpsyt.2022.854088
58. Faul F, Erdfelder E, Lang AL, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91.
59. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers (1988).
60. Cloos JM, Bocquet V, Rolland-Portal I, Koch P, Chouinard G. Hypnotics and triazolobenzodiazepines-best predictors of high-dose benzodiazepine use: Results from the Luxembourg national health insurance registry. Psychother Psychosom. (2015) 84:273–83. doi: 10.1159/000434755
61. Janhsen K, Roser P, Hoffmann K. The problems of long-term treatment with benzodiazepines and related substances. Dtsch Arztebl Int. (2015) 112:1–7. doi: 10.3238/arztebl.2015.0001
62. Pericot-Valverde I, Germeroth LJ, Tiffany ST. The use of virtual reality in the production of cue-specific craving for cigarettes: A meta-analysis. Nicotine Tob Res. (2015) 18:538–46. doi: 10.1093/ntr/ntv216
63. Simon J, Etienne A-M, Bouchard S, Quertemont E. Alcohol craving in heavy and occasional alcohol drinkers after cue exposure in a virtual environment: The role of the sense of presence. Front Hum Neurosci. (2020) 14:124. doi: 10.3389/fnhum.2020.00124
64. Benvegnù G, Tommasi F, Ferraro S, Libener E, Di Chio M, Bosi S, et al. Smokers “context reactivity” in virtual domestic environments. Eur Addict Res. (2021) 27:439–46. doi: 10.1159/000515301
Keywords: cue reactivity, benzodiazepine, addiction, virtual reality, abuse
Citation: Zamboni L, Toldo S, Fusina F, Mattiello M, Mannari V, Campagnari S, Schiavone V, Congiu A, Verlato G, Chiamulera C and Lugoboni F (2022) Study protocol—Evoked craving in high-dose benzodiazepine users. Front. Psychiatry 13:956892. doi: 10.3389/fpsyt.2022.956892
Received: 30 May 2022; Accepted: 12 September 2022;
Published: 13 October 2022.
Edited by:
Lais F. Berro, University of Mississippi Medical Center, United StatesReviewed by:
Antonio Mirijello, Home for Relief of Suffering (IRCCS), ItalyLiana Fattore, CNR Neuroscience Institute (IN), Italy
Copyright © 2022 Zamboni, Toldo, Fusina, Mattiello, Mannari, Campagnari, Schiavone, Congiu, Verlato, Chiamulera and Lugoboni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenzo Zamboni, bG9yZW56by56YW1ib25pODhAZ21haWwuY29t
†These authors have contributed equally to this work
 Lorenzo Zamboni
Lorenzo Zamboni Silvia Toldo3
Silvia Toldo3 Matteo Mattiello
Matteo Mattiello Vanessa Mannari
Vanessa Mannari Valentina Schiavone
Valentina Schiavone Alessio Congiu
Alessio Congiu Cristiano Chiamulera
Cristiano Chiamulera Fabio Lugoboni
Fabio Lugoboni