
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 29 September 2022
Sec. Neuroimaging
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.944520
This article is part of the Research Topic The Neurobiology of Suicide: The 'Suicidal Brain' View all 11 articles
 Xiao Li1
Xiao Li1 Xiaolu Chen2
Xiaolu Chen2 Renqiang Yu3
Renqiang Yu3 Linqi Dai1
Linqi Dai1 Ming Ai1
Ming Ai1 Qian Huang1
Qian Huang1 Yi Zhou1
Yi Zhou1 Wanjun Chen1
Wanjun Chen1 Jiamei Guo1
Jiamei Guo1 Anhai Zheng1
Anhai Zheng1 Li Kuang1*
Li Kuang1*Objective: We aimed to investigate changes in whole-brain gray matter volumes (GMVs) before and after electroconvulsive therapy (ECT) in adolescents with major depressive disorder (MDD) and suicidal ideation (SI).
Methods: Thirty adolescents with MDD and SI were observed, and structural magnetic resonance imaging (sMRI) was performed at baseline and after ECT for each patient. But Twenty-five healthy controls (HCs) were scanned only at baseline. The voxel-based morphometry (VBM) techniques were used to examine GMVs.
Results: Compared with HCs, MDDs at baseline showed decreased GMVs in the left middle temporal gyrus, right superior temporal gyrus, right middle temporal gyrus, left precuneus, right precuneus, and left superior frontal gyrus. After ECT, MDDs showed increased GMVs in the right superior frontal gyrus and right superior temporal gyrus. Pearson’s correlation found that Beck Scale for Suicide Ideation (BSSI) scores at baseline were negatively correlated with GMVs in the left superior frontal gyrus and HAMD and BSSI scores after ECT were negatively correlated with GMVs in the right superior temporal gyrus.
Conclusion: Frontal–temporal–precuneus structure changes may be a potential cause of depressive and suicidal symptoms in adolescents. ECT may improve depressive and suicidal symptoms in adolescents by regulating brain structures to compensate original defects.
Suicide is the third leading cause of death in adolescents (1) and is associated with major depressive disorder (MDD) (2). In addition to suicide deaths, suicidal ideation (SI) and suicide attempts also warrant attention, the development of SI to SA is a distinct phenomenon with different explanations, such as depressive symptoms, hopelessness, or even impulsivity that can predict ideation, but they struggle to distinguish those who have SA from those who only have SI (3). Klonsky et al. found the combination of pain and hopelessness, especially when pain exceeds connectedness, was the main factor causing SI and dispositional, acquired, and practical contributors to increased capacity for suicide were the main factors causing progression from ideation to attempts (4). Globally, lifetime prevalence rates were approximately 9.2% for SI and 2.7% for SA (5), and the prevalence rate of SA in adolescents was 4.1% in the United States and 4.2% in Europe (6). SI is defined as “thoughts of death, dying, plans for suicide, or desire for death” (7, 8), and it is thought to be the first step leading to suicide (3). Tan et al. found 32.09% of 12,733 adolescents in China reported SI (9), and Liu et al found the rate of SI was 20.6% in adolescents in Shandong province in China (10). Additionally, MDD adolescents with SI always have worse clinical outcomes (11). Therefore, measuring SI in MDD adolescents is necessary and may help determine the risk of suicide.
Previous structural magnetic resonance imaging (sMRI) studies showed that abnormalities gray matter volumes (GMVs) were associated with suicide in mood disorders. For example, reduced GMVs in the left and right dorsolateral prefrontal cortex (DLPFC) and right ventral lateral prefrontal cortex (VLPFC) were detected in patients with MDD and SI compared with MDD patients without SI and HCs (12). A longitudinal follow-up study on adolescents found a reduction in dorsal striatal GMVs in MDD adolescents with SI and may predict SI (13). Generally, understanding of alterations in GMVs in MDD adolescents with SI is still limited but of necessity and significance.
Electroconvulsive therapy (ECT) is a quick method to reduce depressive and suicidal symptoms, but for MDD adolescents with SI, the treatment effect of ECT was not so good compared with that adults (14), and due to the side effect, the caregivers always do not accept ECT as the first treatment option, a survey of 7,469 Australian psychiatric patients who received ECT showed only 0.2% of them were younger than 18 years (15), and another study containing 12,608 participants who received ECT from China showed only 3.2% (406) of them were adolescents (16). However, there were still some studies that demonstrated ECT was a good treatment option for MDD adolescents (17, 18). Previous studies demonstrated ECT could alternate GMVs in MDD patients. Qiu et al. (19) found GMVs were increased in bilateral amygdala and hippocampus in MDDs after ECT. Nordanskog et al. (20) found increased GMVs in bilateral hippocampus after 6–12 ECT sessions in MDD patients, and the increased GMVs in bilateral hippocampus correlate with clinical symptoms. However, how ECT changes GMVs in MDD adolescents is still unclear.
To our knowledge, few studies have explored the changes in GMVs in MDD adolescents with SI after ECT; therefore, we have hypothesized that (1) the adolescents with MDD and SI will show changed GMVs compared with HCs and (2) ECT would make GMVs changed in MDD adolescents with SI.
Thirty adolescents with MDD and SI aged 12–17 years were recruited, and the diagnosis was confirmed by senior psychiatrists using the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). The severity of symptoms was accessed by both 17-item Hamilton Depression Rating Scale (21) and the Beck Scale for Suicide Ideation (22) at baseline and after ECT. The Chinese version of HAMD and BSSI has been found to be reliable and valid (23, 24). The inclusion criteria were as follows: (1) patients who fulfilled the MDD diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; (2) patients with a total score of ≥ 17 points on the Hamilton Depression Rating Scale (HAMD-17); (3) first-onset MDD or diagnosed before but without antidepressants in recent 8 weeks, no history of ECT treatment; and (4) patients with suicidal ideation (SI) and Beck Scale for Suicide Ideation (BSSI) scores >11 points in recent one week. Patients were excluded if they had: (1) a neurological or serious physical condition, any history of alcohol or drug abuse, any other somatic diseases, or morphological anomalies of the brain; (2) any surgically placed electronic or metal materials that might interfere with MRI assessment; or (3) head motion exceeding 2.5 mm in translation or 2.5° in rotation.
Healthy controls were volunteers matched with the MDDs in sex, age, and educational level. The controls reported neither lifetime psychiatric disorder nor a family history of psychosis in their first-degree relatives. Otherwise, the exclusion criteria remained the same as MDDs.
The study protocol was approved by the Human Research and Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (no. 2017-157). All adolescents and their caregivers gave written informed consent after being informed about the details of the study.
The MDDs were treated by bi-temporal ECT using Thymatron DGx (Somatics, LLC, Lake Bluff, IL, USA) at the First Affiliated Hospital of Chongqing Medical University. The first three sessions of ECT took place on continuous days; the remaining sessions of ECT were performed every 2 days, with a break on weekends, and each patient would take eight times of ECT. We used low 0.25 mode, and the initial energy for ECT was determined according to the patient’s age: energy percent = age × 50%. The stimulation energy was adjusted based on the seizure time, and subsequent dosing was determined by seizure morphology adequacy. Anesthesia was induced with succinylcholine (0.5–1 mg/kg) and diprivan (1.5–2 mg/kg).
All MDDs received antidepressants during ECT sessions, with sertraline (n = 18, 60%), fluoxetine (n = 11, 36.7%), and escitalopram (n = 1, 0.03%). Twenty-six patients received antipsychotics, with quetiapine (n = 13, 43.3%), olanzapine (n = 8, 26.7%), aripiprazole (n = 4, 13.3%), and risperidone (n = 1, 0.03%). One patient received trihexyphenidyl (n = 1, 0.03%).
MR images were obtained using a 3T GE Signa HDxt scanner (General Electric Healthcare, Chicago, IL, USA) with an eight-channel head coil. During scanning, subjects were told to keep their eyes closed and awake, not to focus their thoughts on anything in particular. Foam pads and earplugs were used to fix their heads to minimize head motion and reduce machine noise, respectively. The settings used to obtain the 3D T1-weighted anatomical images were as follows: a repetition time of 8.4 ms, a flip angle of 12°, an echo time of 3.3 ms, a field of view of 24 cm × 24 cm, a matrix of 256, a field of view of 24-cm slices, 158 axial slices, and a slice thickness of 1 mm.
The T1-weighted images were processed with Statistical Parametric Mapping 12 (SPM12) software package using voxel-based morphometry (VBM) toolbox. All T1 images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using the “new segment” tool implemented in SPM12. During spatial normalization, inter-subject registration was achieved using respective registration based on group assignment. A modulation step was used to ensure that the overall amount of tissue in a class was unaltered. The segmented images were normalized to the Montreal Neurological Institute (MNI) template and were smoothed with an 8-mm full-width at half-maximum (FWHM) Gaussian filter. The voxel size of data acquisition was 1 mm3, and the voxel size of normalized data was 1.5 mm3.
Demographic data and symptom scores were analyzed using SPSS (version 26.0; IBM Corp., Armonk, NY, USA). A two-sample t-test was performed in SPM12 to compare GMVs between MDDs at baseline and HCs, and paired t-tests were performed between MDDs pre- and post-ECT treatment. The GMV voxel was p < 0.001, the significance level was p < 0.05 with Gaussian random field (GRF) correction, and the cluster size was > 170.
At baseline, Pearson’s correlation analyses were performed to examine the correlations between GMVs in the brain regions with significant differences and clinical symptoms (HAMD/BSSI). After ECT, Pearson’s correlation analyses were performed to examine whether the changes in these measures were correlated with the changes in clinical symptoms.
The psychological measurements and demographic data are listed in Table 1. There were no significant differences between MDDs and HCs in age, sex, and educational level (p > 0.05). After ECT sessions, the total scores on the HAMD and BSSI were significantly decreased (p < 0.05) (Table 2).
Compared with HCs, MDDs showed decreased GMVs in the left middle temporal gyrus, right superior temporal gyrus, right middle temporal gyrus, left precuneus, right precuneus, and left superior frontal gyrus (p < 0.05 with GRF correction) (Figure 1 and Table 3) and the bar graph showed decreased GMVs in the left superior frontal gyrus in MDDs (p < 0.01) (Figure 2).
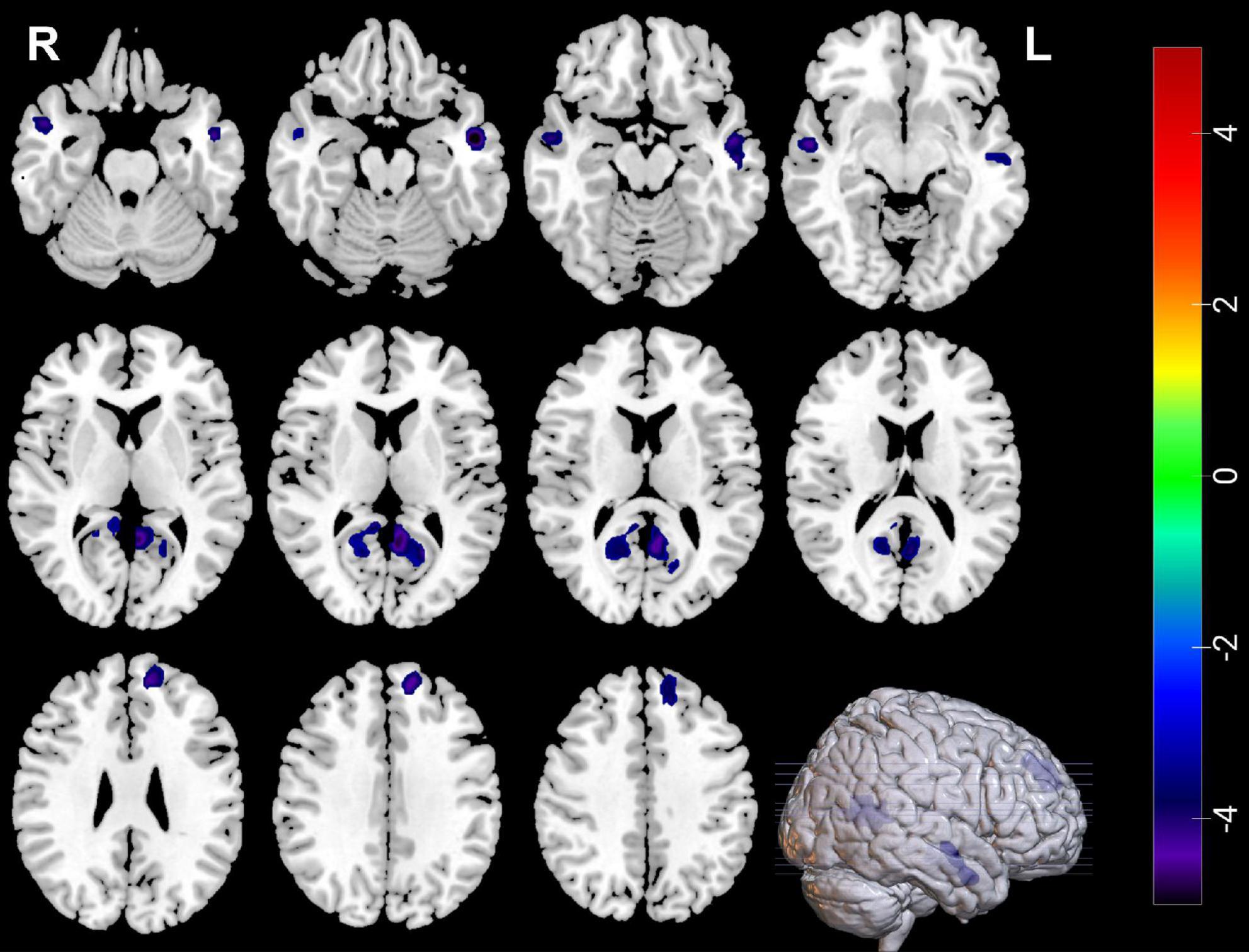
Figure 1. Blue regions mean decreased GMVs in the left middle temporal gyrus, right superior temporal gyrus, right middle temporal gyrus, left precuneus, right precuneus, and left superior frontal gyrus of MDDs compared with HCs (GMV voxel was p < 0.001; the significance level was p < 0.05 with GRF correction).
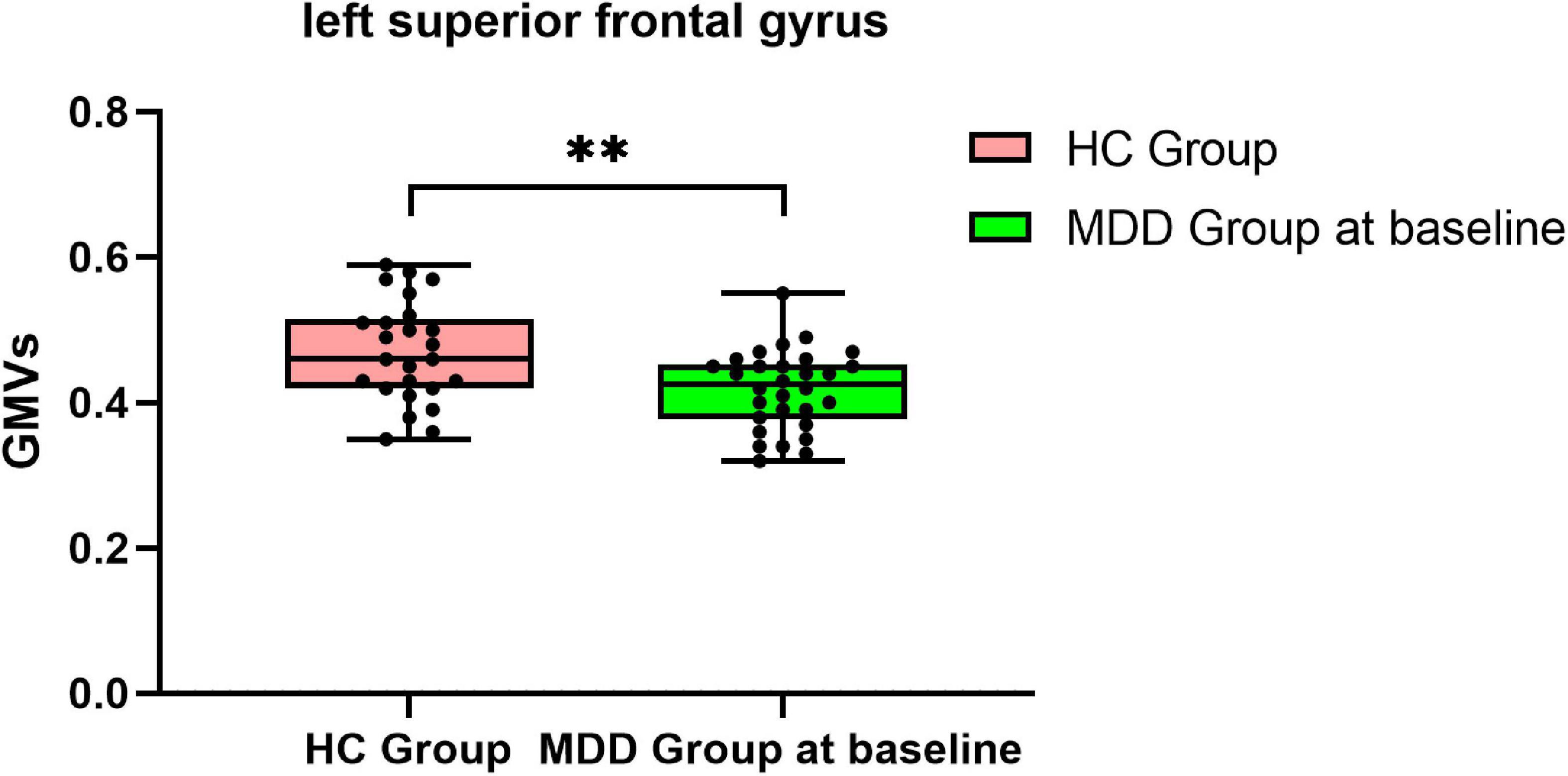
Figure 2. Bar graph showing decreased GMVs in the left superior frontal gyrus in MDDs compared with HCs, **p < 0.01.
Compared with pre-ECT, patients showed increased GMVs in the right superior frontal gyrus and right superior temporal gyrus (p < 0.05 with GRF correction) (Figure 3 and Table 4) and the bar graph showed increased GMVs in the right superior temporal gyrus in MDDs after ECT (p < 0.05) (Figure 4).
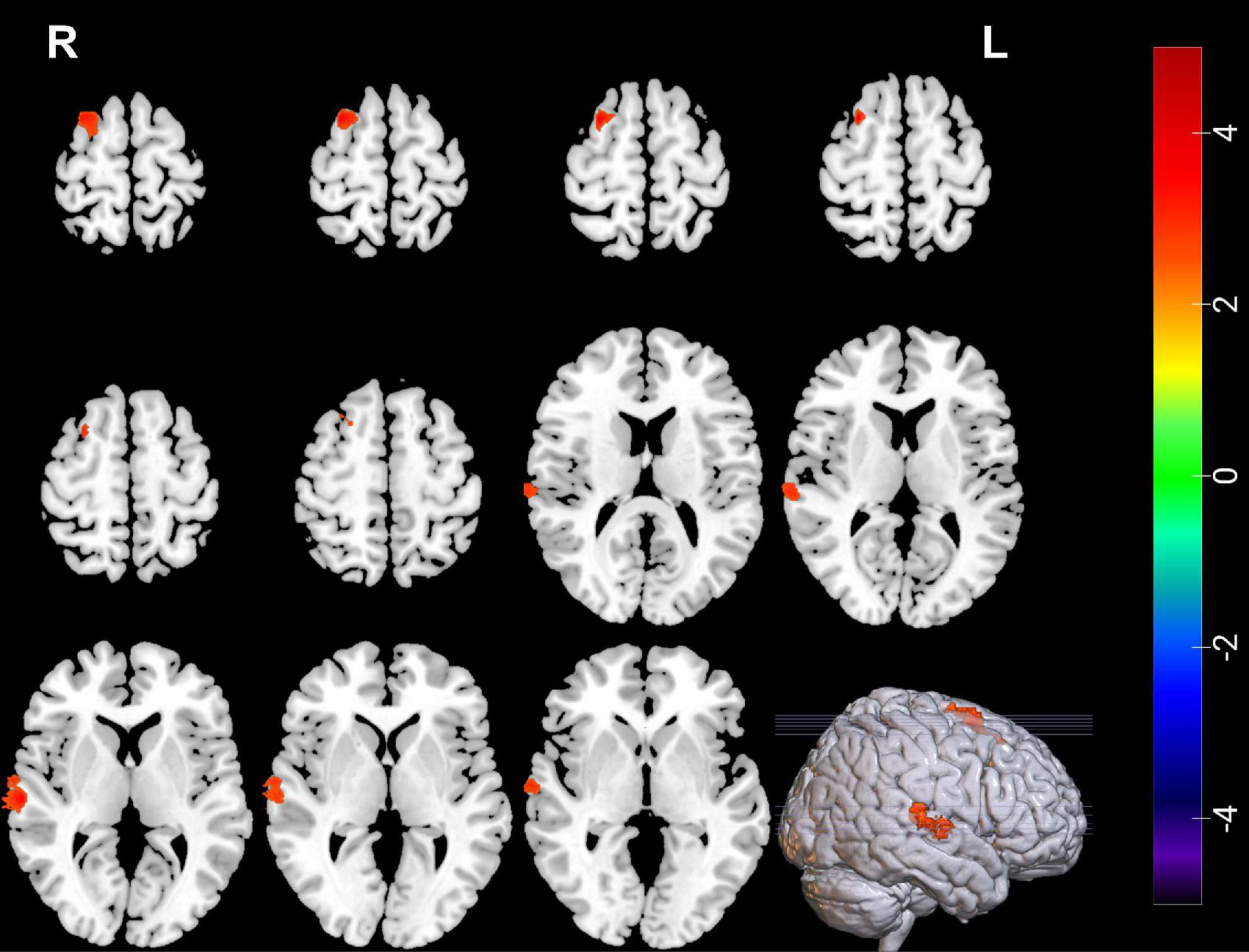
Figure 3. Red regions mean increased GMVs in the right superior temporal gyrus and right superior frontal gyrus of MDDs after ECT (GMV voxel was p < 0.001; the significance level was p < 0.05 with GRF correction).
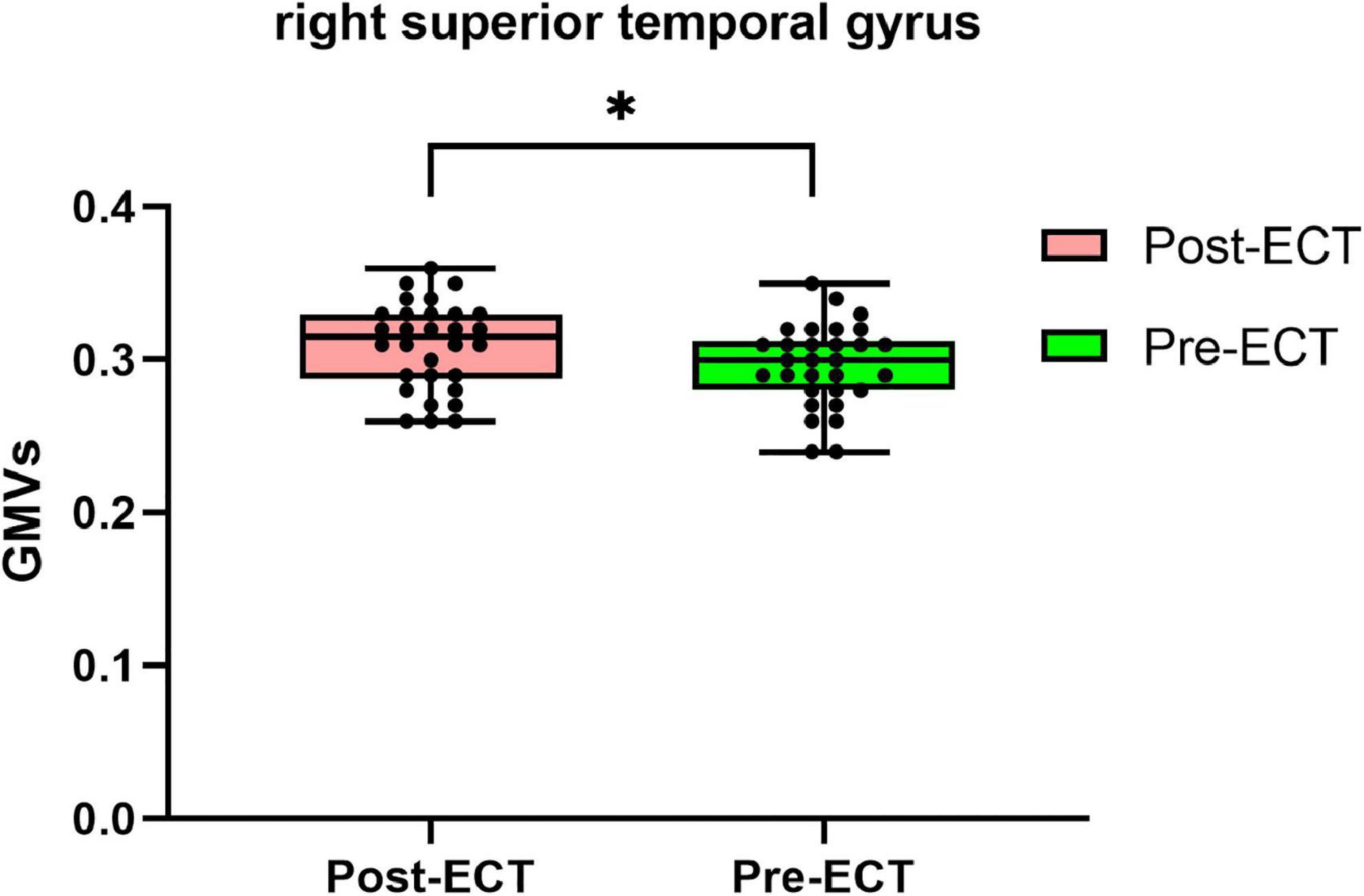
Figure 4. Bar graph showing increased GMVs in the right superior temporal gyrus in MDDs after ECT, *p < 0.05.
Pearson’s correlation found that the BSSI scores at baseline were negatively correlated with GMVs in the left superior frontal gyrus (r = −0.5234, p = 0.003) (Figure 5), the HAMD scores after ECT were negatively correlated with GMVs in the right superior temporal gyrus (r = −0.4076, p = 0.0254) (Figure 6), and the BSSI scores after ECT were negatively correlated with GMVs in the right superior temporal gyrus (r = −0.4326, p = 0.0170) (Figure 7). There was no correlation between changed HAMD/BSSI scores and GMVs.
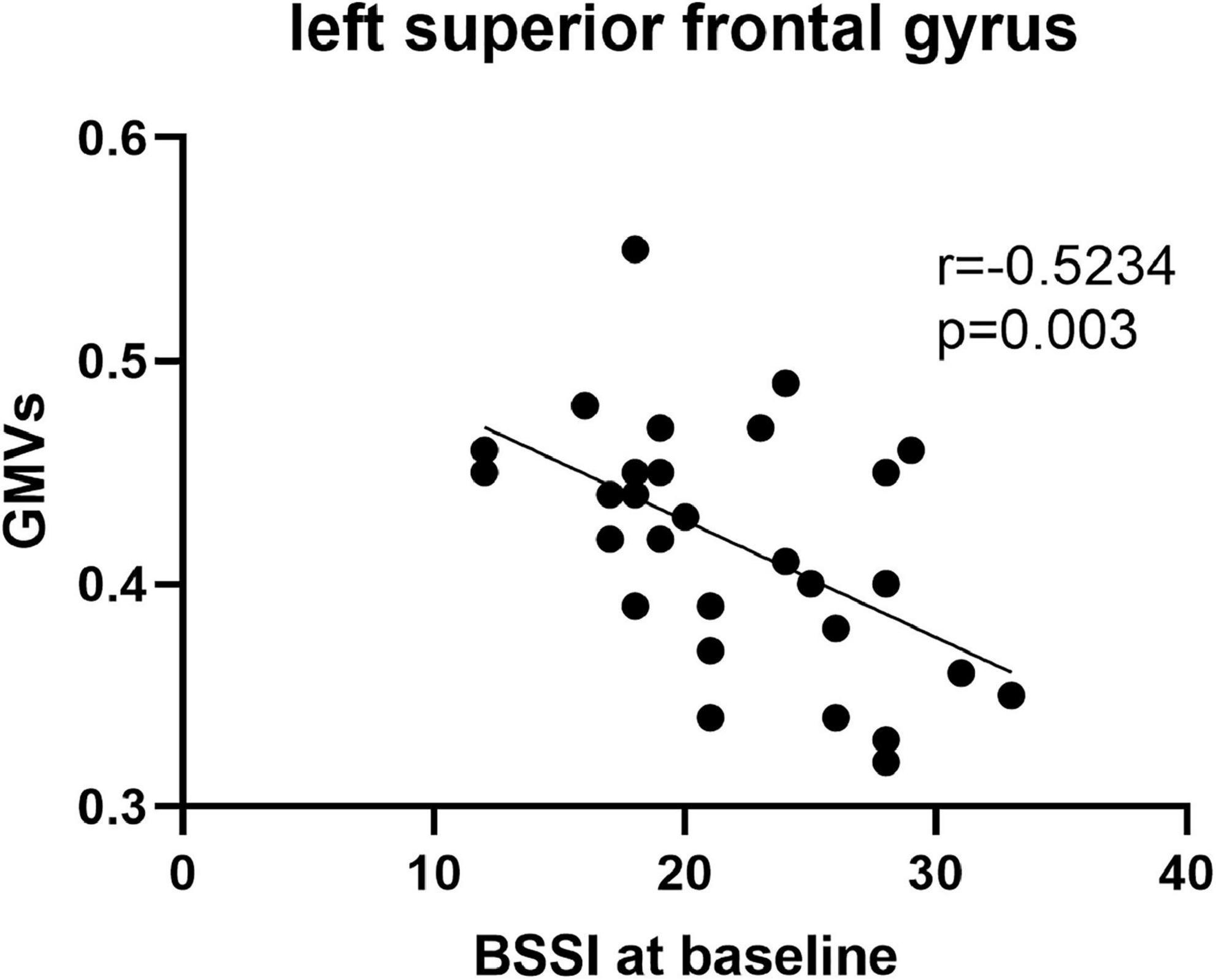
Figure 5. Negative correlations between the BSSI at baseline and GMVs in the left superior frontal gyrus (r = –0.5234, p = 0.003).
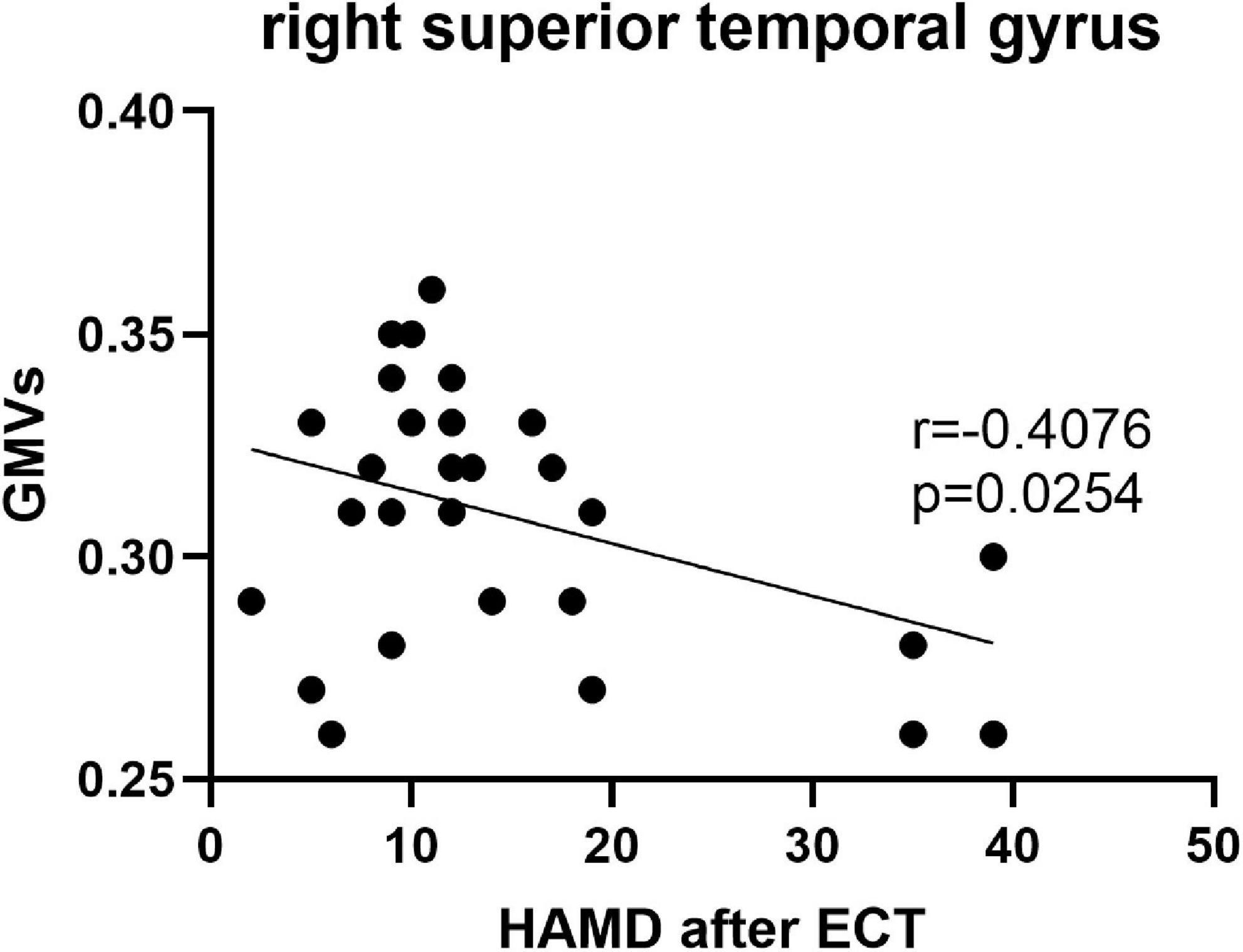
Figure 6. Negative correlations between the HAMD after ECT and GMVs in the right superior temporal gyrus (r = –0.4076, p = 0.0254).
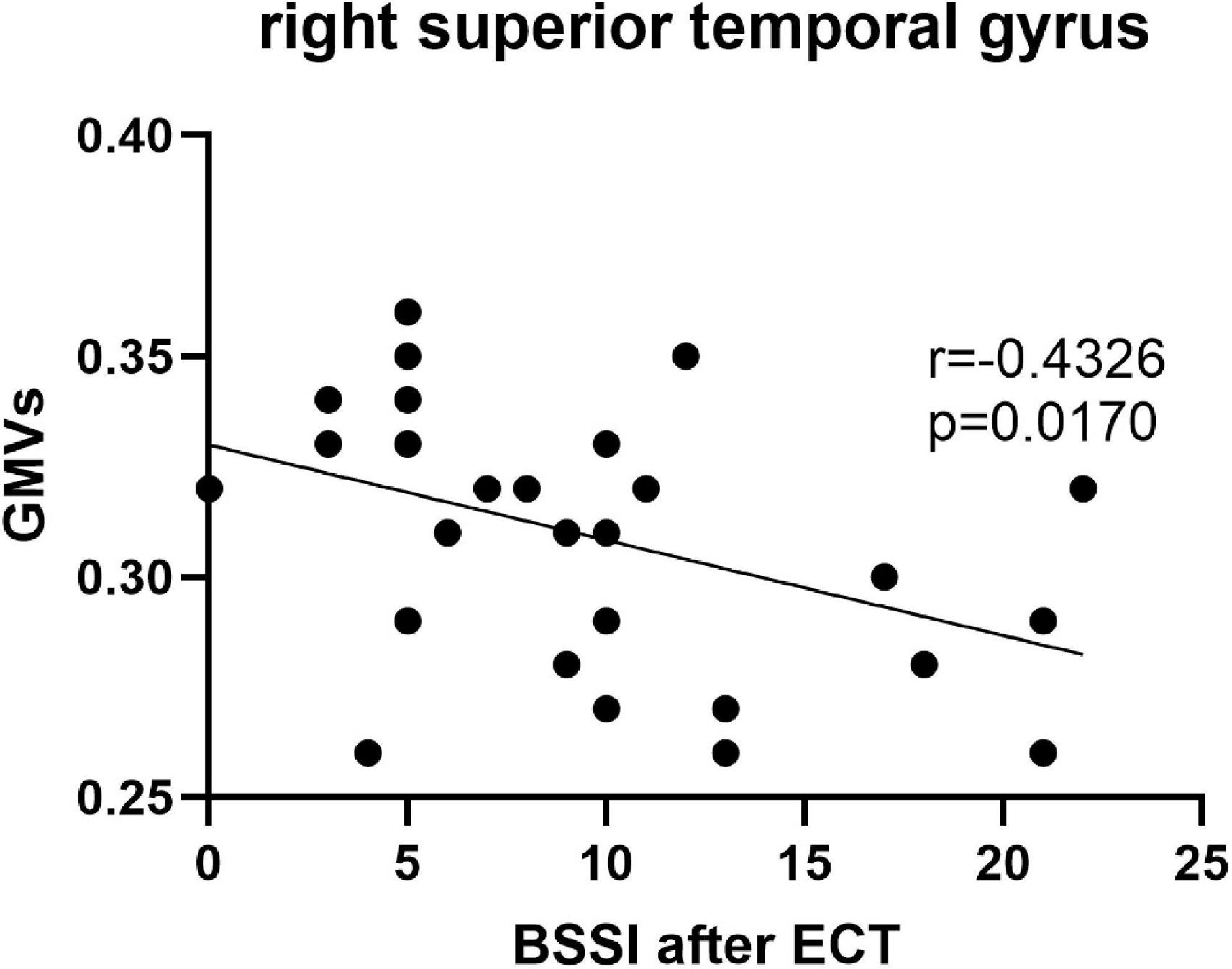
Figure 7. Negative correlations between the BSSI after ECT and GMVs in the right superior temporal gyrus (r = –0.4326, p = 0.0170).
This study found the HAMD score and BSSI score of MDD adolescents with SI significantly reduced after ECT, indicating that ECT could quickly decrease the depressive and suicidal symptoms of adolescent patients. In our study, we used VBM techniques and found decreased GMVs in the left middle temporal gyrus, right superior temporal gyrus, right middle temporal gyrus, left precuneus, right precuneus, and left superior frontal gyrus compared with HCs and increased GMVs in the right superior frontal gyrus and right superior temporal gyrus after ECT.
The frontal lobe locates in the front of the brain and is one of the most important areas, which is closely related to the individual’s emotional regulation, thinking process, attention function, problem-solving, demand motivation, behavior planning, and other advanced cognitive activities. In this study, GMVs in the superior frontal gyrus changed in patients at baseline compared with HCs and also showed in MDDs before and after ECT, indicating that the superior frontal gyrus was an important area for the onset of depression and might be the area for the ECT response. Ma et al. found decreased GMV in the medial part of the left superior frontal gyrus in patients with depressive symptoms (25), which was consistent with our results. Seok et al. found decreased GMV in the right superior frontal gyrus in adolescents with irritability compared with healthy youths (26). Interestingly, we found ECT increased GMV in the right superior frontal gyrus, but not in the left superior frontal gyrus, and this inconsistency of two comparison results may be related to that ECT could trigger both efficacy and side effect. The precuneus is involved in many high-level cognitive functions of the brain, including episodic memory, self-attention, and information-related processing. Therefore, changes in brain structure in the region reflect abnormalities in emotional control, thought processes, and negative behaviors in patients with depression and suicide. In the current study, GMVs in precuneus in the MDDs at baseline significantly reduced, suggesting the abnormality of precuneus might be related to symptom onset of MDD adolescents with SI.
The middle temporal gyrus is involved in cue-directed attention and working memory (27, 28). Previous studies showed MDD patients had reduced GMVs in the temporal cortex (29, 30). In this study, GMVs in bilateral middle temporal gyrus of the patient group at baseline reduced compared with HCs, which might reflect depressive symptoms. Previous studies found that ECT increased GMVs in the temporal cortex in patients with depression (31, 32). In addition to the middle temporal gyrus, the right superior temporal gyrus is also an important brain region for emotion processing. Previous studies showed that the superior temporal gyrus and middle temporal gyrus were related not only to recall of personal experiences, but also to perception of intentional behavior and memory (33, 34). In addition, these two brain regions were also involved in the regulation of emotional information and cognition (35–37). Therefore, aberrations of GMV in these two regions may lead to abnormal spontaneous brain activity and might contribute to emotional dysregulation, which would increase the risk of suicide in depression. Soloff et al. (38) found that the superior/middle temporal gyrus was negatively correlated with the impulsivity of the low-fatality suicide attempters, but positively correlated with the aggressiveness of the high-fatality suicide attempters. Therefore, suicide attempts might be related to impulsivity personality traits mediated by the superior/middle temporal gyrus.
In addition to sMRI, fMRI also focused on the relationship between the superior temporal gyrus and suicide. A previous study (39) showed the ALFF activity in the right superior temporal gyrus enhanced significantly in depressed patients with SA, but no similar results were found in the comparison between depressed patients without SA and HCs. Van Heeringen et al. (40) also found increased blood perfusion in the temporal lobe of MDD patients. As the superior temporal gyrus is involved in the regulation of emotion and cognition, researchers believe that the abnormal ALFF activity in depressed patients with suicide may lead to suicidal behavior through weakened decision making and increased impulsivity. In addition, studies showed that the right superior temporal gyrus was activated in healthy people when performing mind tasks. Suicide attempters were considered to have impaired cognitive control and decision-making ability, and cognitive rigidity and impaired decision-making ability were particularly pronounced in high-fatality suicide attempters.
We found ECT changed GMVs in the right superior temporal gyrus, and previous studies paid more attention to alteration in the brain function in this area after ECT. Wang et al. found the changes in local FCD of the right superior temporal gyrus were significantly correlated with the changes in Hamilton Rating Scale for Depression (HRSD) scores in MDD patients before and after ECT (41). Previous studies also focused on the superior temporal gyrus changed in MDD adolescents with suicidal behaviors. Pan et al. (42) found GMVs in the right superior temporal gyrus reduced in MDD adolescents with a history of suicidal symptoms, and abnormal temporal–parietal GMVs were associated with an increased risk of suicide in youth with depression (43). So, we considered ECT could decrease the depressive and suicide symptoms by regulating the right superior temporal gyrus of brain.
Several limitations of our current study should be noted. First, our sample size was small, and further studies with larger samples are needed to verity our findings. Second, we did not assess childhood trauma or maltreatment in the MDDs and HCs, which could influence the brain structure. Third, we did not evaluate the side effect caused by ECT. Fourth, patients received antidepressants during ECT sessions, and though the duration of medications is only 2 weeks and antidepressants were always thought not to work (44, 45), we still could not exclude the impact on the brain structure.
Frontal–temporal–precuneus structural changes may be a potential cause of depressive and suicidal symptoms in adolescents. ECT may improve depressive and suicidal symptoms in adolescents by regulating brain structures to compensate original defects.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Human Research and Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
XL conceived the structure of the manuscript and wrote the manuscript. RY, YZ, QH, and LD prepared the samples and performed the fMRI. XC analyzed the data. JG, AZ, and WC performed the ECT in patients. MA and LK critically reviewed the manuscript. All authors have read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (No. 81971286).
We sincerely appreciate all the participants and their families for participating in our study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zalsman G. Genetics of suicidal behavior in children and adolescents. In: Y Dwivedi editor. The Neurobiological Basis of Suicide. Boca Raton, FL: CRC Press (2012). p. 297–314.
2. Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry. (2006) 47:372–94. doi: 10.1111/j.1469-7610.2006.01615.x
3. Klonsky ED, May AM, Safer BY. Suicide, suicide attempts, and suicidal ideation. Annu Rev Clin Psychol. (2016) 12:307–30. doi: 10.1146/annurev-clinpsy-021815-093204
4. Klonsky ED, May AM. The Three-Step Theory (3ST): a new theory of suicide rooted in the “ideation-to-action” framework. Int J Cogn Ther. (2015) 8:114–29. doi: 10.1521/ijct.2015.8.2.114
5. Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. (2008) 192:98–105. doi: 10.1192/bjp.bp.107.040113
6. Carli V, Hoven CW, Wasserman C. A newly identifi ed group of adolescents at “invisible” risk for psychopathology and suicidal behavior: findings from the SEYLE study. World Psychiatry. (2014) 13:78–86. doi: 10.1002/wps.20088
7. Miller AB, McLaughlin KA, Busso DS, Brueck S, Peverill M, Sheridan MA. Neural correlates of emotion regulation and adolescent suicidal ideation. Biol Psychiatry Cogn Neurosci Neuroimag. (2018) 3:125–32. doi: 10.1016/j.bpsc.2017.08.008
8. Levi-Belz Y, Gavish-Marom T, Barzilay S, Apter A, Carli V, Hoven C, et al. Psychosocial factors correlated with undisclosed suicide attempts to significant others: findings from the adolescence SEYLE study. Suicide Life Threat Behav. (2019) 49:759–73. doi: 10.1111/sltb.12475
9. Tan L, Xia T, Reece C. Social and individual risk factors for suicide ideation among Chinese children and adolescents: A multilevel analysis. Int J Psychol. (2018) 53:117–25. doi: 10.1002/ijop.12273
10. Liu XC, Chen H, Liu ZZ, Wang JY, Jia CX. Prevalence of suicidal behaviour and associated factors in a large sample of Chinese adolescents. Epidemiol Psychiatr Sci. (2019) 28:280–9. doi: 10.1017/S2045796017000488
11. Szanto K, Mulsant BH, Houck P, Dew MA, Reynolds CF. Occurrence and course of suicidality during short-term treatment of late-life depression. Arch Gen Psychiatry. (2003) 60:610–7. doi: 10.1001/archpsyc.60.6.610
12. Zhang R, Wei S, Chang M, Jiang X, Tang Y, Wang F. Dorsolateral and ventrolateral prefrontal cortex structural changes relative to suicidal ideation in patients with depression. Acta Neuropsychiatr. (2020) 32:84–91. doi: 10.1017/neu.2019.45
13. Ho TC, Cichocki AC, Gifuni AJ, Camacho MC, Ordaz SJ, Singh MK, et al. Reduced dorsal striatal gray matter volume predicts implicit suicidal ideation in adolescents. Soc Cogn Affect Neurosci. (2018) 13:1215–24. doi: 10.1093/scan/nsy089
14. Ghaziuddin N, Shamseddeen W, Gettys G, Ghaziuddin M. Electroconvulsive therapy for the treatment of severe mood disorders during adolescence: A retrospective chart review. J Child Adolesc Psychopharmacol. (2020) 30:235–43. doi: 10.1089/cap.2019.0054
15. Chanpattana W. A questionnaire survey of ECT practice in Australia. J ECT. (2007) 23:89–92. doi: 10.1097/YCT.0b013e318031bc50
16. Zhang QE, Wang ZM, Sha S, Ng CH, Seiner SJ, Welch CA, et al. Common use of electroconvulsive therapy for chinese adolescent psychiatric patients. J ECT. (2016) 32:251–5. doi: 10.1097/YCT.0000000000000327
17. Puffer CC, Wall CA, Huxsahl JE, Frye MA. A 20-year practice review of electroconvulsive therapy in adolescents. J Child Adolesc Psychopharmacol. (2016) 26:632–6. doi: 10.1089/cap.2015.0139
18. Mitchell S, Hassan E, Ghaziuddin N. A follow-up study of electroconvulsive therapy in children and adolescents. J ECT. (2018) 34:40–4. doi: 10.1097/YCT.0000000000000452
19. Qiu H, Li X, Zhao W, Du L, Huang P, Fu Y, et al. Electroconvulsive therapy-induced brain structural and functional changes in major depressive disorders: A longitudinal study. Med Sci Monit. (2016) 22:4577–86. doi: 10.12659/msm.898081
20. Nordanskog P, Dahlstrand U, Larsson MR, Larsson EM, Knutsson L, Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT. (2010) 26:62–7.
21. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
22. Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: The scale for suicide ideation. J Consult Clin Psychol. (1979) 47:343–52. doi: 10.1037//0022-006x.47.2.343
23. Zhao JP, Zheng YP. Reliability and validity of Hamilton Depression Scale assessed in 329 Chinese depression patients. Chin Ment Health J. (1992) 5:214–6. doi: 10.1016/0010-440x(88)90063-6
24. Li XY, Phillips MR, Tong YS, Li KJ, Zhang YL, Zhang YP, et al. Reliability and validity of the Chinese version of Beck Suicide Ideation Scale (BSI-CV) in adult community reMDDents. Chin Ment Health J. (2010) 24:250–5. doi: 10.3969/j.issn.1000-6729.2010.04.003
25. Ma T, Ji YY, Yan LF, Lin JJ, Li ZY, Wang W, et al. Gray matter volume abnormality in chronic pain patients with depressive symptoms: A systemic review and meta-analysis of voxel-based morphometry studies. Front Neurosci. (2022) 16:826759. doi: 10.3389/fnins.2022.826759
26. Seok JW, Bajaj S, Soltis-Vaughan B, Lerdahl A, Garvey W, Bohn A, et al. Structural atrophy of the right superior frontal gyrus in adolescents with severe irritability. Hum Brain Mapp. (2021) 42:4611–22. doi: 10.1002/hbm.25571
27. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. (2002) 3:201–15. doi: 10.1038/nrn755
28. Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. (2006) 103:10046–51. doi: 10.1073/pnas.0604187103
29. Yuan Y, Zhu W, Zhang Z, Bai F, Yu H, Shi Y, et al. Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: an optimized voxel-based morphometry study. Biol Psychiatry. (2008) 64:541–4. doi: 10.1016/j.biopsych.2008.04.032
30. Peng J, Liu J, Nie B, Li Y, Shan B, Wang G, et al. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur J Radiol. (2011) 80:395–9. doi: 10.1016/j.ejrad.2010.04.006
31. Tendolkar I, van Beek M, van Oostrom I, Mulder M, Janzing J, Oude Voshaar R, et al. Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: a longitudinal pilot study. Psychiatry Res. (2013) 214:197–203. doi: 10.1016/j.pscychresns.2013.09.004
32. Nordanskog P, Larsson MR, Larsson EM, Johanson A. Hippocampal volume in relation to clinical and cognitive outcome after electroconvulsive therapy in depression. Acta Psychiatr Scand. (2014) 129:303–11. doi: 10.1111/acps.12150
33. Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. (2003) 7:77–83. doi: 10.1016/s1364-6613(02)00025-6
34. Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. Neuroimage. (2003) 19:1835–42. doi: 10.1016/s1053-8119(03)00230-1
35. Ellison A, Schindler I, Pattison LL, Milner AD. An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain. (2004) 127:2307–15. doi: 10.1093/brain/awh244
36. Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. (2001) 411:950–3. doi: 10.1038/35082075
37. Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. (1998) 121:47–57. doi: 10.1093/brain/121.1.47
38. Soloff P, White R, Diwadkar VA. Impulsivity, aggression and brain structure in high and low lethality suicide attempters with borderline personality disorder. Psychiatry Res. (2014) 222:131–9. doi: 10.1016/j.pscychresns.2014.02.006
39. Fan TT, Wu X, Yao L, Dong J. Abnormal baseline brain activity in suicidal and non-suicidal patients with major depressive disorder. Neurosci Lett. (2013) 534:35–40. doi: 10.1016/j.neulet.2012.11.032
40. Van Heeringen K, Van den Abbeele D, Vervaet M, Soenen L, Audenaert K. The functional neuroanatomy of mental pain in depression. Psychiatry Res. (2010) 181:141–4. doi: 10.1016/j.pscychresns.2009.07.011
41. Wang J, Wei Q, Yuan X, Jiang X, Xu J, Zhou X, et al. Local functional connectivity density is closely associated with the response of electroconvulsive therapy in major depressive disorder. J Affect Disord. (2018) 225:658–64. doi: 10.1016/j.jad.2017.09.001
42. Pan LA, Ramos L, Segreti AM, Brent DA, Phillips ML. Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br J Psychiatry. (2015) 206:339–40. doi: 10.1192/bjp.bp.114.151316
43. Peng H, Wu K, Li J, Qi H, Guo S, Chi M, et al. Increased suicide attempts in young depressed patients with abnormal temporal–parietal–limbic gray matter volume. J Affect Disord. (2014) 165:69–73. doi: 10.1016/j.jad.2014.04.046
44. Wang G. Onset of action of antidepressants. Chin J Psychiatry. (2011) 44:49–50. doi: 10.3760/cma.j.issn.1006-7884.2011.01.021
Keywords: MDD, ECT (electroconvulsive therapy), adolescent, Suicide Ideation, structural MRI (sMRI)
Citation: Li X, Chen X, Yu R, Dai L, Ai M, Huang Q, Zhou Y, Chen W, Guo J, Zheng A and Kuang L (2022) Changes in gray matter volume following electroconvulsive therapy in adolescent depression with suicidal ideation: A longitudinal structural magnetic resonance imaging study. Front. Psychiatry 13:944520. doi: 10.3389/fpsyt.2022.944520
Received: 15 May 2022; Accepted: 29 August 2022;
Published: 29 September 2022.
Edited by:
Ali Saffet Gonul, Ege University, TurkeyReviewed by:
Jie Yang, Central South University, ChinaCopyright © 2022 Li, Chen, Yu, Dai, Ai, Huang, Zhou, Chen, Guo, Zheng and Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Kuang, a3VhbmdsaTAzMDhAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.