- 1Center for Mental Health Research in School of Management, Zunyi Medical University, Zunyi, China
- 2The Institute of Ethnology and Anthropology, Chinese Academy of Social Sciences, Beijing, China
- 3School of Criminal Justice, China University of Political Science and Law, Beijing, China
- 4Psychological Guidance Center, Zunyi Medical University, Zunyi, China
According to the social-cognitive theory and the social-information-processing theory, individuals with conduct disorder, a persistent and repetitive pattern of problematic behavior, might have cognitive biases toward hostile facial expressions. However, according to the optimal stimulation/arousal theory, the stimulation-seeking theory and the fearlessness theory, individuals with conduct disorder might have less fear and show less response to hostile or threatening facial expressions. To reconcile the discrepancy, we examined the cognitive biases including attentional processing and working memory processing to emotional faces among adolescents with conduct disorder. 35 male adolescent delinquents with conduct disorder and 35 age-matched delinquents without conduct disorder completed a visual search task and a delayed-match-to-sample task to examine their attentional processing and working memory processing for sad, angry, happy, and fearful faces, respectively. It was found that conduct disordered individuals searched angry and fearful faces, rather than sad and happy faces, more slowly than individuals without conduct disorder. However, no difference in mnemonic processing for facial emotions was found between groups. The results indicated that male adolescent delinquents with conduct disorder showed deficits in attentional orientation to hostile and threatening faces, supporting the optimal stimulation/arousal theory, the stimulation-seeking theory and the fearlessness theory, but not the social-cognitive theory.
Introduction
Conduct disorder (CD) is a highly impairing psychiatric disorder that usually manifests in childhood or adolescence. It is defined as a persistent and repetitive pattern of problematic behavior that violates others’ rights or that violates age-appropriate social norms or rules, and is characterized by a pattern of severe antisocial and aggressive behavior (1). CD is a psychiatric disorder associated with delinquency or crime. Previous studies revealed that delinquent or criminal youth were more likely to show CD symptoms or had more severe CD symptoms (2, 3). Additionally, CD could predict future antisocial outcomes and increase the risk of future crime (4–6).
Aggression may be developed and shaped by the manners that humans percept, process, store and retrieve information (7). According to the social-cognitive theory and the social-information-processing theory, the schema is the knowledge structure shaped by one’s unique experience and can automatically guide his/her social information processing (8–11). Previous studies revealed that aggressive or violent individuals were more likely to hold or endorse aggression-related schema (12–14). They usually showed a pattern of biased processing, such as the negative social-cognitive bias (15), the attentional bias toward hostile social cues (16–18), selective recall for hostile social cues (19), and the hostile attribution bias (19, 20). Moreover, attention to antisocial semantic cues could predict high aggression (21). Regarding CD individuals, as they often experience exposure to childhood abuse or maltreatment (22–26) and exposure to aggressive/deviant models or peers (27–29), they may develop a maladapted or distorted schema, which leads them to preferentially encode hostile cues, to mentally represent social cues as threats, to easily access aggressive responses and finally to engage in aggressive behavior.
Among social cues, facial expressions are one of the most frequently encountered in daily life and most effective in conveying one’s emotions and hostility. For example, an angry face indicates hostility and aggression, while a sad face implies the need for help and social support. CD individuals may thus develop biased cognitive processing on emotional faces. Behavioral studies demonstrated that 7- to 13-year-old children with CD interpreted emotions less accurately than controls and tended to misinterpret emotions as anger (30), and adolescents with CD were more likely to confuse fear with anger relative to healthy controls (31). Another study revealed that children with CD showed a stronger association between the hostile attribution bias and the attentional bias to angry faces compared with controls (32). On the neurophysiological level, CD showed stronger mismatch negativity (MMN) induced by fearful rather than sad syllables in an auditory oddball paradigm (33). A recent study revealed that aggressive males showed selectively attentional bias to angry faces, and undifferentiated P3 amplitude between angry and neutral faces (34). Another study found that aggression was associated with enhanced amygdala reactivity to angry faces (21). Taken together, evidence suggested that individuals with CD held the aggressive or hostile schema and may thus show biased processing of hostile or threatening information.
However, other perspectives support that CD may show reduced response or avoidance to hostile or threatening information. According to the optimal stimulation/arousal theory (35–37), the stimulation-seeking theory (38, 39), and the fearlessness theory (40, 41), individuals with CD have a lower level of physical arousal which reflects less fear and thus seek exaggerated external stimulation to lead their physical activity to reach the optimal level, and in turn show less response to affective information. Researchers observed a lower level of emotional response to unpleasant slides (42) and reduced corrugator muscle response to angry faces among CDs (43). Neuroimaging evidence revealed reduced activations in the amygdala to negative pictures or angry faces among CDs (44–46). Furthermore, studies also revealed that adolescents with CD showed attentional avoidance and difficulty in attentional disengagement from facial expressions including angry, fearful and happy faces compared to controls (47). An eye-movement tracking study revealed that adolescents with CD fixated less on fearful and sad faces (48). Therefore, it is still in debate whether individuals with CD show larger or less cognitive bias to emotional stimuli, especially to hostile or threatening faces.
To reconcile the discrepancy among previous studies and perspectives, we examined the cognitive biases including attentional processing and working memory processing to emotional faces among CD adolescents by adopting classical paradigms. Dot-probe paradigm (47) and emotional stroop paradigm (32) have been adopted to assess attentional processing of emotional faces among individuals with CD. The results were mixed. Compared to controls, adolescents with CD showed attention bias to facial expressions in a dot-probe task (47), while children with CD did not show any attentional biases to facial expressions in a pictorial emotional Stroop task (32). In the present study, we adopted the visual search paradigm which has a high ecological validity as it mimics everyday situations in which one attempts to find a target face among distractive faces. In this task, participants were asked to detect, locate or identify the target among distractors as quickly as possible and the results may reveal how attention suppresses irrelevant distractors as well as shifts/orients attention to the target. The performance in the visual search task could be modulated by facial emotions. Some researchers found a superiority effect on angry faces (49–53), while others found a superiority effect on happy faces (54–56). Nevertheless, the visual search paradigm is an effective and stable task to reveal the visual attentional processing of facial emotions. Relative to attentional processing, visual working memory processing on emotional faces among individuals with CD has been largely known. Working memory, which is a fundamental cognitive function of human, is usually characterized as the ability to maintain and manipulate perceptual information in a short period of time (57). Three subsystems were identified, including a central executive system to process information, and two slave systems of visuospatial sketch pad and phonological loop to store visual and verbal information, respectively. N-back task has been used to assess the working memory for object (e.g., letter) and spatial position among individuals with CD (58–60), which revealed that CD group performed worse than controls did, and CD symptoms were correlated with reduced P3 amplitude in the context of low working memory load. However, n-back task mainly reveals the updating mechanism in working memory processing and some researchers indicate its inefficiency in measuring working memory (61). In the present study, we adopted the delayed-match-to-sample (DMTS) paradigm which is one of the most common tasks to study visual working memory. The DMTS task consists of three phases, including a sample (encoding) phase, a delay (maintenance) phase, and a test (retrieval) phase. It is mainly used to examine the accuracy and capacity in encoding and maintaining visual stimuli. Previous studies showed stable test-retest reliability in DMTS task (62) and stable brain structures associated with the task (i.e., dorsolateral prefrontal cortex, fusiform gyrus and posterior parietal cortex) (63). Taken together, if the social-cognitive theory and the social-information-processing theory are critical mechanisms in the development of CD, we may predict that CD adolescents show higher attentional and working memory biases to frightening and hostile faces; if the optimal stimulation/arousal theory, the stimulation-seeking theory and the fearlessness theory are critical mechanisms, we may predict the opposite trend, that is, CD adolescents show lower attentional and working memory biases to threatening and hostile faces.
Materials and methods
Participants
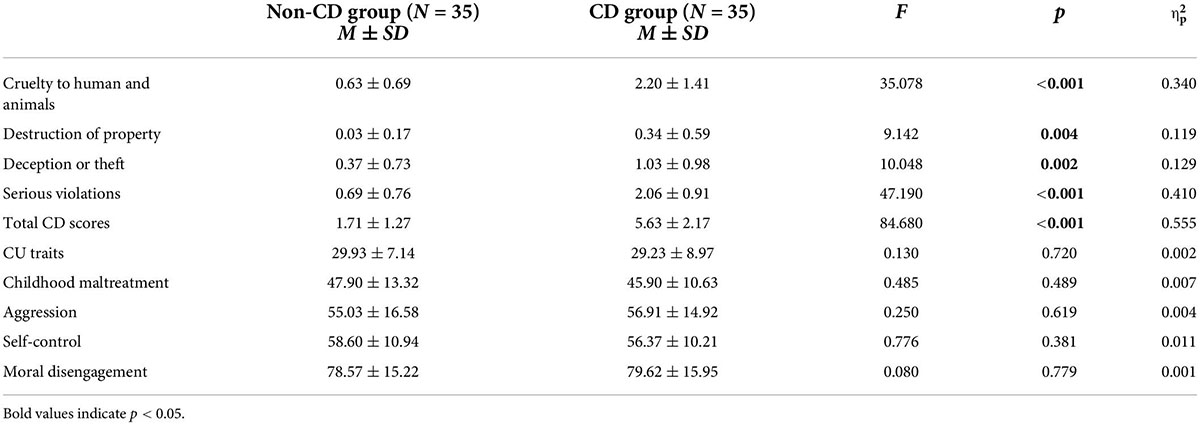
Male adolescent delinquents in a reform school and a reformatory in Guizhou province of China underwent a structured clinical interview with the Diagnostic and Statistical Manual of Mental Disorders from DSM-IV-TR Axis I Disorders (64). The diagnosis of CD and its severity was established on this 15-item screening questionnaire which consists of four factors, including cruelty to humans and animals (e.g., bullying, fighting, and physical injury to pets), destruction of property (e.g., arson and vandalism), deception or theft (e.g., lying for self-interest), and serious violations (e.g., playing truant). If individuals who meet three of these criteria in one year and meanwhile meet at least one criterion within half a year are diagnosed to have conduct disorder. Furthermore, individuals meeting five or more criteria were assigned a “moderate-to-severe” conduct disorder diagnosis (65).
We performed a power analysis to determine the sample size using the G-Power 3.1.9.7 software (66). In order to find a significant interaction effect between emotion and group, at the level of ηp2 = 0.1, α = 0.05, power = 0.95, the required total sample size is 22. We recruit 35 subjects for both groups, resulting in a total sample size of 70. 35 delinquents who met five or more criteria were assigned to the CD group. And 35 age-matched delinquents, who didn’t meet three of these criteria in one year nor meet at least one criterion within half a year, were assigned to the non-CD group. No significant difference in age was found between CD (M = 16.51, SD = 1.46, range from13 to 20) and non-CD (M = 16.80, SD = 1.37, range from 13 to 20) groups (t = 0.84, df = 68, p = 0.402). All participants are right-handed and their vision or corrected vision is normal. Exclusion criteria included a history of mental illnesses or a family member’s history of mental illness. All procedures performed in this study involving human participants were in accordance with the ethical standards of the Ethical Committee of Human Research at a medical university and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was given by all adolescent delinquents and their legal guardians. All the participants completed the visual search experiment. However, three delinquents in the CD group quit the DMTS experiment for personal reasons. The order of the tasks was randomized across subjects.
Measurements
The Inventory of Callous-Unemotional Traits (ICU) The 24-item scale consists of three subscales including callousness, uncaring and unemotional traits (67, 68). Callousness refers to the callous attitude toward others, uncaring is characterized by the lack of caring about performance and the unemotional trait is characterized by the lack of emotional expression. Response options range from 0 = not at all true to 3 = definitely true. Higher scores indicate a higher level of CU traits.
The Aggression Questionnaire (AQ) The 29-items scale can be used to assess aggression and consists of four factors: physical aggression, verbal aggression, anger, and hostility (69). Participants rate each item on a Likert scale from 1 = extremely uncharacteristic of me to 5 = extremely characteristic of me. Higher scores indicate a higher level of aggression.
The Short Form of the Childhood Trauma Questionnaire (CTQ-SF) This version of the CTQ contains 28 items (25 clinical items and 3 validity items) assessing childhood maltreatment, which consists of five factors: emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect (70). Each item is rated on a Likert scale from 1 = never to 5 = always. A higher score implies more frequent exposure to maltreatment in childhood.
The Self-Control Scale (SCS) This 36-items scale consists of five subscales: self-discipline, deliberate/non-impulsive action, healthy habits, work ethic, and reliability (71). Participants rate each item on a 5-point Likert scale ranging from 1 = not at all like me to 5 = very much like me. The Chinese version of the SCS consists of five factors: impulse control, resistance to temptation, healthy habits, concentration on work and abstinence from entertainment. Confirmatory factor analysis (CFA) indicated a good construct validity for the revised SCS (72). Higher scores indicate better self-control.
The Moral Disengagement Scale (MDS) This 32-item scale consists of eight factors, including euphemistic labeling, distortion and minimization of consequences, moral justification, diffusion of responsibility, displacement of responsibility, disadvantageous comparisons, dehumanization, and victim-blaming (73, 74). Participants rate each item on a Likert scale from 1 = extremely disagree to 5 = extremely agree. Higher scores suggest a higher level of moral disengagement.
Visual search task
Stimuli
The experimental stimuli were selected from the Chinese Affective Picture System (CAPS). Search targets included 16 sad faces, 16 angry faces, 16 happy faces, and 16 fearful faces, half of which were female faces. 58 neutral faces (29 female faces) were selected as distractors. All pictures were grayscaled and an oval mask was used to remove non-facial features (e.g., hair, neck, ears) from each face. Then, all the images were cropped into a uniform size (130 × 150 pixels), and the brightness and contrast were matched. A repeated-measures ANOVA found that the valences were significantly different among categories [F(3, 45) = 294.80, p < 0.001, ηp2 = 0.952). Post-hoc tests suggested that the valence of happy faces (M = 6.75, SD = 0.51) was significantly higher than angry (M = 2.62, SD = 0.40), sad (M = 2.84, SD = 0.54), and fearful (M = 2.80, SD = 0.42) faces (ps < 0.001), while there were no differences among the three negative faces (ps > 0.999). Moreover, no significant difference in arousal was found [F(3, 45) = 1.68, p = 0.185, ηp2 = 0.101) (M(SD): happy 5.71(1.11), angry 6.44(1.47), sad 5.67(1.37), and fearful 6.38(1.00)].
Procedure
Participants sat in a quiet room during the experiment while visual stimuli were presented on a 17-inch liquid crystal display on a Lenovo desktop with a resolution of 1,600 × 900 and a refresh rate of 60 Hz (75). The computer screen was placed 60 cm in front of the participants. As Figure 1A illustrated, each trial of the task began with a white fixation cross presented in the center of the black screen for a random period of 500∼1,500 ms. Afterward, an array of eight faces or two faces appeared until a response was made. Participants were asked to press one key (F) if they found the target (an emotional face) among the distractors (neutral faces) and another key (J) if they didn’t find the target as quickly and accurately as possible. After the response, a blank screen was presented for 500 ms. In total, eight blocks, consisting of 512 trials, were included in this experiment. In four blocks, participants were asked to find the target from seven distractors (set size = 8; high load condition). In the other four blocks, they were asked to find the target from one distractor (set size = 2; low load condition). In each block, the target emotion was fixed. 48 out of 64 trials in each block contained a target, while the other 16 trials did not. The sequence of the eight blocks was randomized among participants.
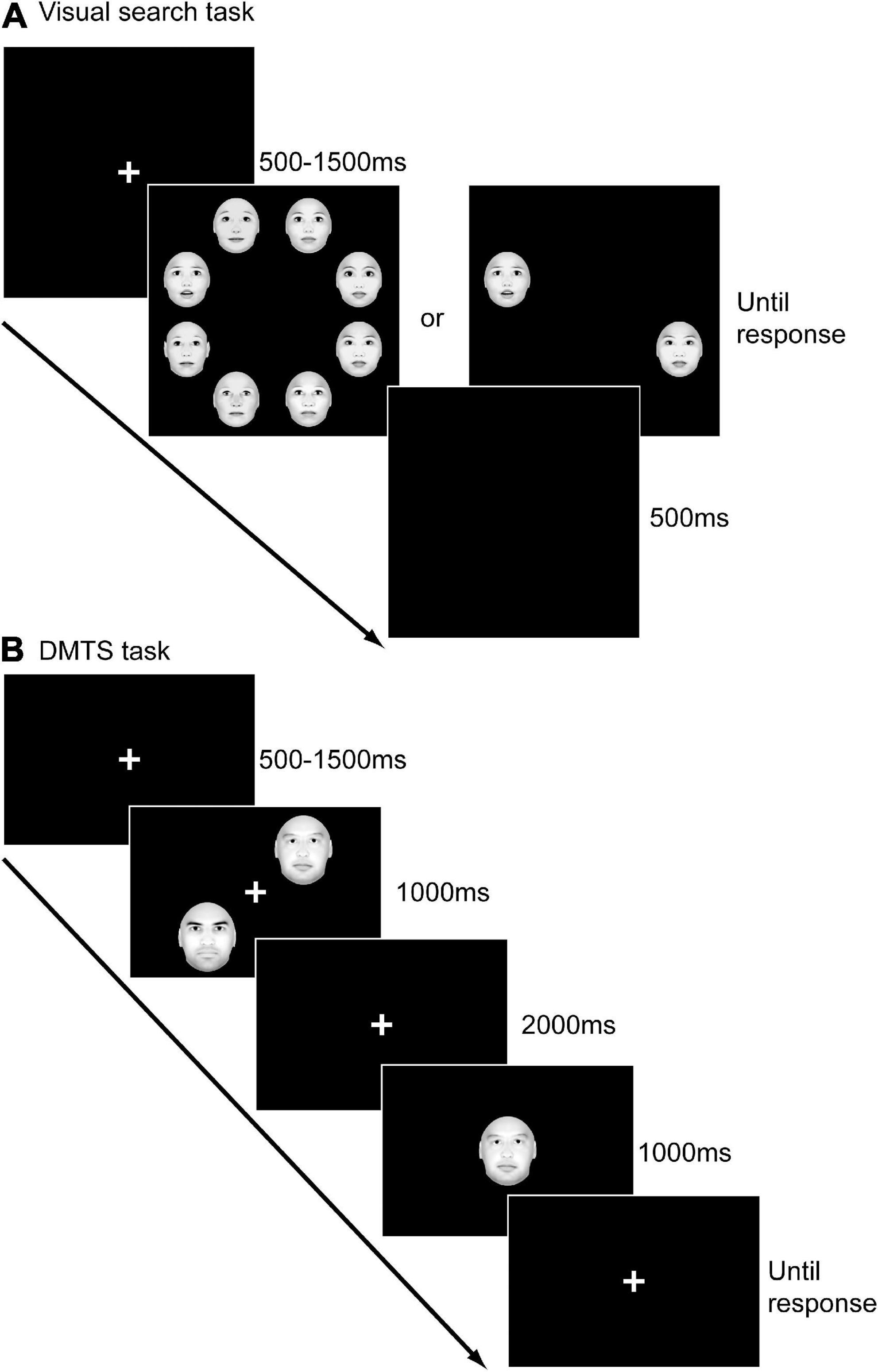
Figure 1. The experimental procedures of the visual search task (A) and the delayed-match-to-sample (DMTS) task (B).
Design and analysis
The visual search experiment is a 4 (Facial emotion: sad, angry, fearful, and happy) × 2 (Load: high and low) × 2 (Group: CD and non-CD) mixed design. The dependent variables included accuracy rate, reaction time and search slope. First, we excluded outliers of trials defined as RTs outside M ± 3SD. Then, we calculated the search slope for each emotional target. The search slope is the slope of the linear fitting line with reaction time versus set size (76). It is an indicator of search efficiency, as a smaller search slope indicates more effective searching and a higher sensitivity to the target. Next, two 4 (emotion) × 2 (load) × 2 (group) repeated measures ANOVAs were conducted on the accuracy and the reaction time respectively, while a 4 (emotion) × 2 (group) repeated measures ANOVA was conducted on the search slope.
Delayed-match-to-sample task
Stimuli
Faces were also selected from the CAPS, including 24 sad, 24 angry, 24 happy, 24 fearful, and 24 neutral faces. Each emotional category included 12 male and 12 female faces. All images were processed similar to the visual search task, except that the image size was set to 185 × 200 pixels. Repeated measures ANOVA showed that the valences were different among emotional categories [F(4, 92) = 303.14, p < 0.001, ηp2 = 0.929). Post-hoc tests suggested that the valence of happy faces (M = 6.46, SD = 0.59) was significantly higher than angry (M = 2.70, SD = 0.43), sad (M = 2.97, SD = 0.67), and fearful (M = 2.79, SD = 0.39) faces (ps < 0.001), while there were no differences among the negative emotions (ps > 0.843). Moreover, no significant difference in arousal was found [F(3, 45) = 1.68, p = 0.185,ηp2 = 0.101) (M(SD): happy 5.51(1.23), angry 6.18(1.25), sad 5.64(1.39), and fearful 6.32(1.23)].
Procedure
As Figure 1B illustrated, each trial of the task began with a white cross on a black background for a random period of 500∼1,500 ms. Subsequently, two faces (sample) with the same expression appeared for 1,000 ms at two of the locations of upper left, lower left, upper right and lower right. Afterward, a blank screen was presented for 2,000 ms, and participants were asked to maintain the two faces they just saw in their minds. Next, a test face was presented in the center of the screen for 1,000 ms. After the disappearance of the test stimulus, the fixation appeared again until participants made a response. Participants were asked to determine whether the test face was one of the two sample faces. All faces presented in a block had the same emotion. The experiment consisted of five blocks. Each block included 48 trials, in half of which the test face matched the sample face. The sequence of blocks was randomized among participants.
Design and analysis
The present experiment is a five (Facial emotion: sad, angry, fearful, happy, and neutral) × 2 (Group: CD and non-CD) mixed design. Accuracy, RT, and indicators in signal detection theory (SDT) were treated as dependent variables. Outliers of trials were first excluded in the same way as the visual search task. Discriminability (d’) and reporting criterion (C) were then calculated based on the SDT. In the present experiment, a signal is defined as the matched trials while noise is defined as the unmatched trials. Hit rate (H) is calculated as the rate of the trials in that participants made a yes response in a matched trial, while false alarm rate (FA) is the rate of the trials in that participants made a yes response in an unmatched trial. Then, the hit rate and the false alarm rate are transformed into Z-scores, respectively [i.e., Z(H) and Z(F)]. d’ and C were calculated as: d’ = Z(H)–Z(F) and C = 0.5 × [Z(H)+Z(F)]. A larger d’ indicates stronger discriminability between signal and noise, which reflects stronger working memory capacity. A larger C implies a stricter reporting criterion, which reflects participants are conservative to report signals. Four 5 (emotion) × 2 (group) repeated measures ANOVAs were conducted on the accuracy, the RT, the d’ and the C.
Results
Scale results
Scores of CD screening, ICU, CTQ-SF, AQ, SCS, and MDS in each group were displayed in Table 1. MANOVA revealed that CDs showed higher scores on each dimension of, and the total CD scores compared with non-CDs. However, on the other scales, we did not find any significant difference between groups.
Visual search task results
For accuracies (Figure 2), we performed a 4 (emotion) × 2 (load) × 2 (group) repeated measures ANOVA and found a significant interaction effect between emotion and load [F(3, 204) = 5.699, p = 0.001, ηp2 = 0.077). A simple effect analysis indicated that the accuracy for sad faces was higher than for angry faces only when the load was high (p = 0.043). All other interaction effects were not significant (all Fs < 2.3, ps > 0.08). The main effects of emotion [F(3, 204) = 109.839, p < 0.001, ηp2 = 0.618] and load [F(1, 68) = 40.587, p < 0.001, ηp2 = 0.374] were significant. However, we did not find any significant interaction or main effect related to the group, indicating that CD may not affect the accuracy of the visual search.
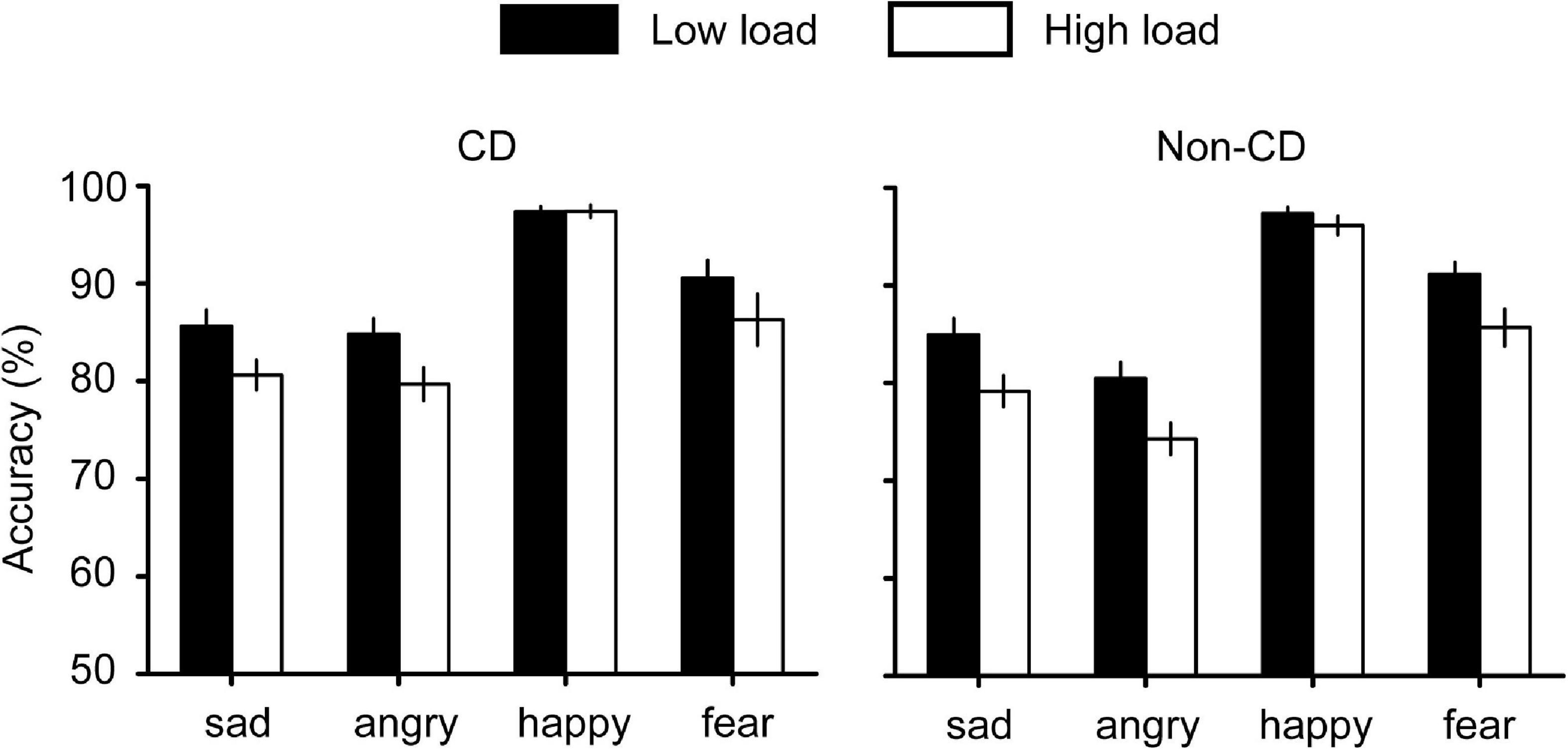
Figure 2. Accuracy results for the visual search task. In low load condition, the search array contains two faces. In high load condition, the search array contains eight faces.
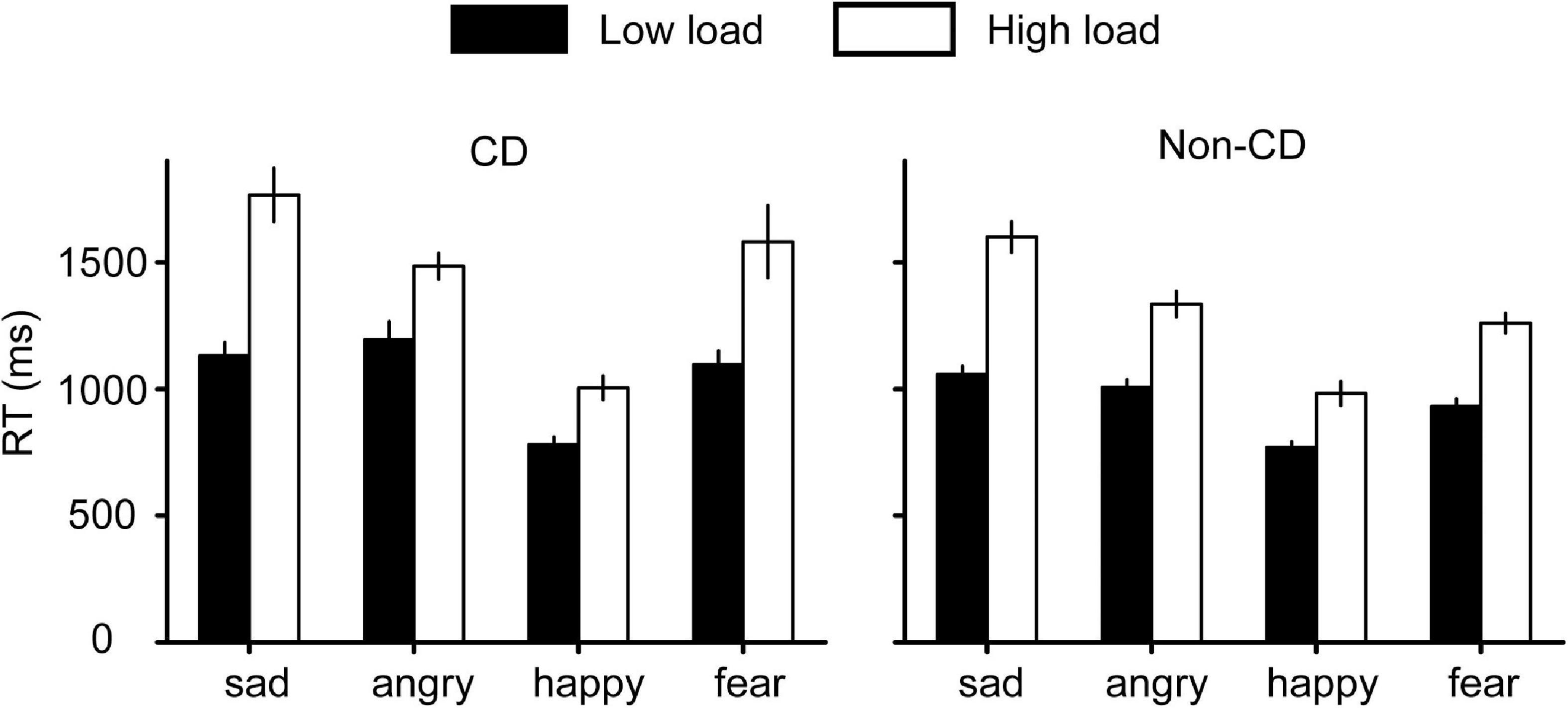
For RTs (Figure 3), a similar 4 (emotion) × 2 (load) × 2 (group) repeated measures ANOVA was conducted. First, the interaction effect between emotion and group was significant [F(3, 204) = 3.389, p = 0.019, ηp2 = 0.047]. A simple analysis revealed that RTs for angry and fearful faces were larger in the CD group compared with the non-CD group (angry: p = 0.012, fearful: p = 0.012), but there were no group differences for sad (p = 0.131) and happy faces (p = 0.742). Second, the interaction effect between emotion and the load was significant [F(3, 204) = 11.178, p < 0.001, ηp2 = 0.141]. A simple effect showed that, when the load was high, the RT for sad faces was longer than that for angry faces (p < 0.001), but the trend disappeared when the load was low (p > 0.999). Other interaction effects were non-significant (all Fs < 1.3, ps > 0.26). Third, the main effects for emotion [F(3, 204) = 69.076, p < 0.001, ηp2 = 0.504], load [F(1, 68) = 237.956, p < 0.001, ηp2 = 0.778], and group [F(1, 68) = 5.440, p = 0.023, ηp2 = 0.074] were all significant. Taken together, these results indicated attentional deficits in CDs for angry and fearful faces.
For search slopes (Table 2), a 4 (emotion) × 2 (group) repeated measures ANOVA was performed. The interaction effect and the main effect of the group were non-significant. The main effect of emotion was significant [F(3, 204) = 11.178, p < 0.001, ηp2 = 0.141]. A post-hoc test demonstrated that the slope for sad faces was larger than those for angry (p = 0.002) and happy (p < 0.001) faces. The slope for happy faces was smaller than that for fearful faces (p = 0.026). These results revealed a happy superiority effect in visual search, but CD may not affect the search efficiencies for emotional faces.
Delayed-match-to-sample task results
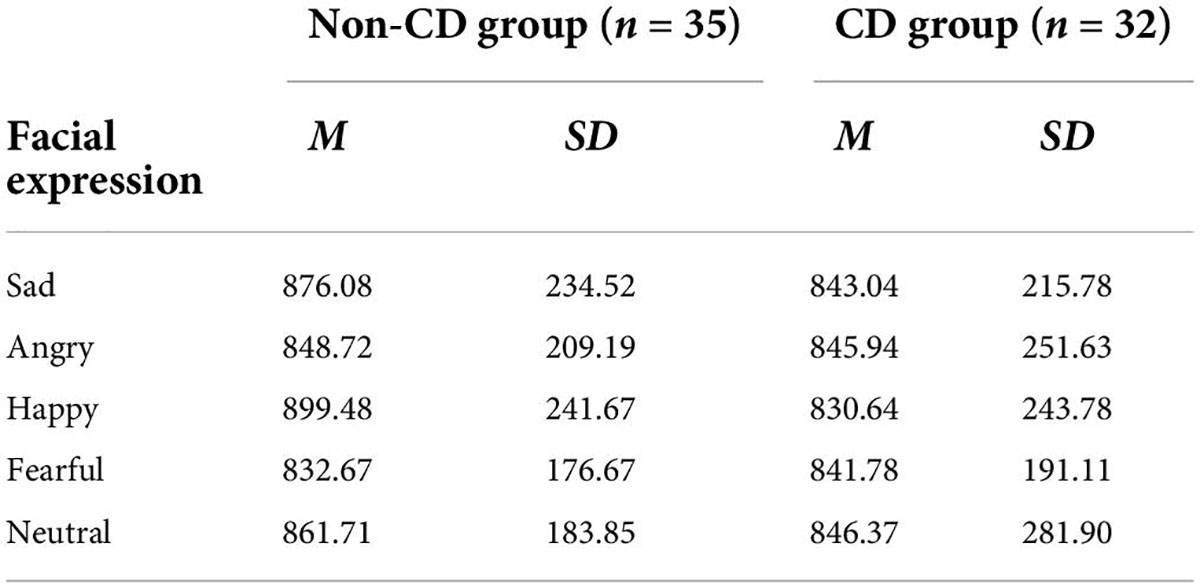
For accuracies (Table 3), a 5 (emotion) × 2 (group) repeated measures ANOVA was performed. The interaction effect and the main effect of the group were non-significant (all Fs < 0.5, ps > 0.83). The main effect of emotion was significant [F(4, 260) = 20.456, p < 0.001, ηp2 = 0.239]. A post-hoc test suggested that the accuracies for neutral and happy faces were lower than those for negative affective faces (ps < 0.006). However, there was no difference between neutral and happy faces (p > 0.999), nor among three negative affective faces (all ps > 0.36).
For RTs (Table 4), a similar 5 (emotion) × 2 (group) repeated measures ANOVA was performed. We did not find any significant interaction or main effects (all Fs < 0.8, ps > 0.82).
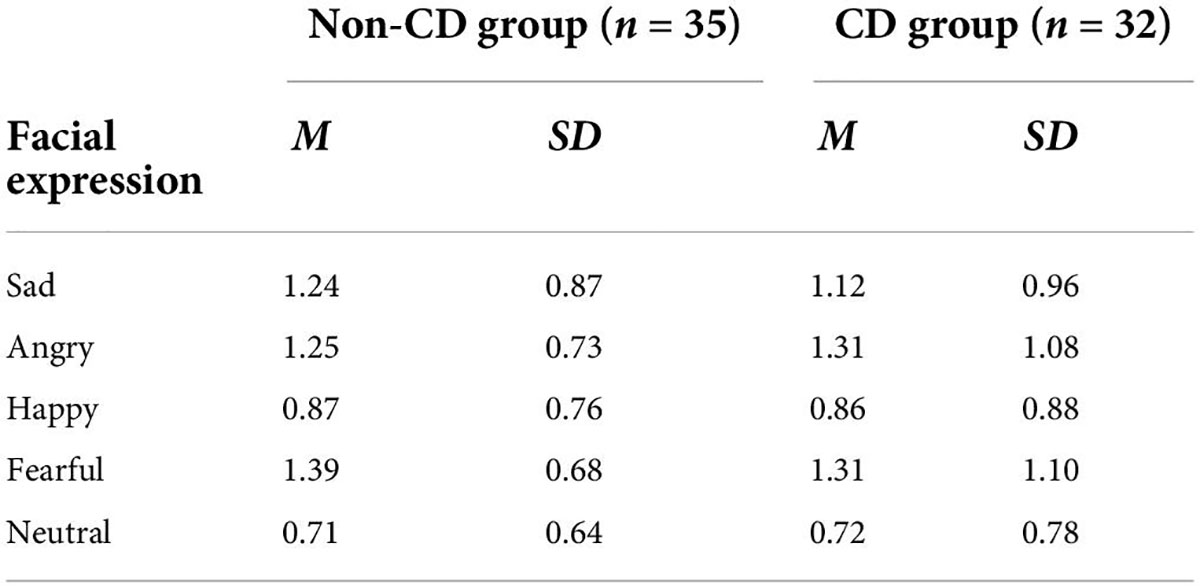
For d’ (Table 5), 5 × 2 repeated measures ANOVA showed a non-significant interaction effect and the main effect of the group (all Fs < 0.4, ps > 0.85). The main effect of emotion was significant [F(4, 260) = 18.202, p < 0.001, ηp = 0.219]. A post-hoc test suggested that the d’ for neutral and happy faces were lower than those for negative affective faces (ps < 0.025). However, there was no difference between neutral and happy faces (p > 0.999), nor among three negative affective faces (all ps > 0.34).
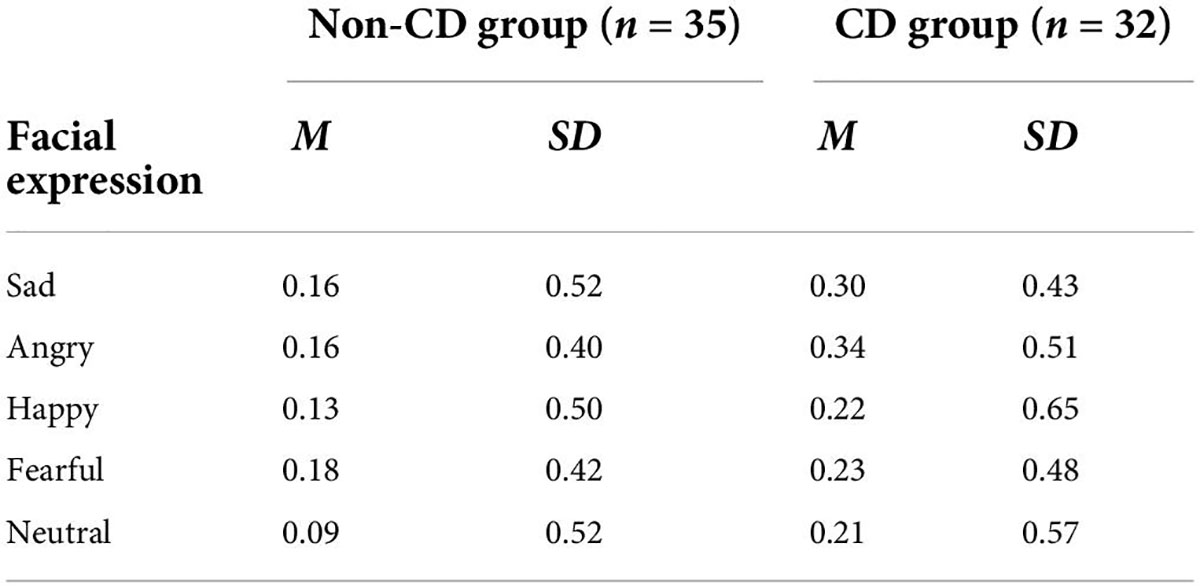
For C (Table 6), 5 × 2 repeated measures ANOVA revealed that there were no significant interactions or main effects (all Fs < 1.4, ps > 0.25).
Discussion
Facial emotion conveys rich social information and plays an important role in social interaction. The present study explored how CD affects the cognitive processing of emotional faces, including visuospatial attention and visual working memory, among male adolescent delinquents. We introduced a strict experimental control, as the CDs and non-CDs were all adolescent delinquents and lived in the same environment. As a result, the differences between the two groups may largely attribute to the disorder. The results thus provide critical empirical evidence for relevant theories on CD. Specifically, our results mainly support the optimal stimulation/arousal theory, the stimulation-seeking theory and the fearlessness theory, but not the social-cognitive theory.
The core finding of our study is that adolescents with CD searched angry and fearful faces more slowly compared with non-CDs, which was not found on sad and happy faces. These deficiencies were independent of the load, indicating global deficits in attentional orientation to hostile and threatening faces among CDs. It was partly consistent with a previous study which revealed that adolescents with CD fixated less on fearful and sad faces (48). The results support the optimal stimulation/arousal theory (35–37), the stimulation-seeking theory (38, 39) and the fearlessness theory (40, 41). According to these theories, individuals with CD may experience less fear and have a lower level of physical arousal. As a result, they would show less response to affective faces. Physiological measurements revealed that individuals with CD showed reduced baseline or resting heart rate, skin conductance and electrodermal activity (77, 78), reduced amplitudes of startle reflex (79–81), and reduced response in the hypothalamus-pituitary-adrenal axis (77, 82–84). In addition, individuals with CD also showed deficits in fear conditioning, suggesting that they are fearless (80, 85). Angry and fearful faces convey hostility or threatening signals, and thus may draw attention automatically. For example, in some studies, people found a larger attentional bias toward fearful faces relative to happy and sad faces, even the fearful face was unaware (86–91). Similarly, other studies also showed attentional bias toward angry faces (49–53). Correspondingly, larger P1 amplitude was found elicited by angry faces relative to happy and sad faces (92–95). In the present study, we showed that adolescents with CD might not be as sensitive as the non-CDs to experience hostile or threatening signals. It may be a crucial reason for their aggressive behaviors.
Our study provided a strict control on the environmental variables, as both groups were selected from the same facilities, and all participants were delinquents. It’s very important to control the living environment between groups, for the environment and partnership may largely affect the behavior and cognition of an individual (15, 96–108). From the scale results, we found that the two groups were not significantly different from each other in CU traits, childhood maltreatment, aggression, self-control and moral disengagement, indicating that the groups were well matched for aspects other than CD. Therefore, our results provide convincing evidence and make us reconsider previous theories and findings. For example, a previous study adopting the dot-probe paradigm found that adolescents with CD showed attentional avoidance of angry, fearful and happy faces compared to typically developing adolescents recruited from schools and colleges (47). Another study revealed that youths with CD showed impairments in recognition of anger, disgust, fear, happiness, sadness and surprise, compared with typically developing controls (109, 110). One possible reason for the difference between these results and ours might be the influence of peers and the environment. Further studies are required to examine these influential factors more detailed.
Compared with attentional processing, the working memory processing was found hardly affected by CD. We only found higher accuracies and discriminability for negative affective faces, indicating a general negative mnemonic bias among adolescent delinquents. It should be noted that this mnemonic bias could not be attributed to higher sensitivity to these emotional faces, as we showed a happy superiority effect in the visual search task. These results also indicated that the attentional processing and mnemonic processing on emotional faces may not share the same underlying mechanisms, and adolescent delinquents may preserve negative faces more stably and accurately. A larger number of previous studies revealed that, compared with happy and neutral faces, angry faces significantly improved the working memory capacity for facial identity (111–115), and the working memory sensitivity for fearful faces was higher than that for neutral faces (116). Working memory is an ability that preserves and updates information in a short period of time (117–119). The stronger working memory processing on negative emotional faces among adolescent delinquents may imply difficulty in refreshing negative information, and thus cause aggressive behaviors. Nevertheless, more evidence is needed to further elucidate the relationship between working memory processing and aggression.
Finally, we noticed that there were several limitations in the present study. First, we did not include a typically developing group. As we discussed above, the difference between delinquents and typically developing individuals may largely attribute to the difference in environment and partnership, and thus the comparison does not help understand the effect of CD. Further study concerning the effect on the environment may include such a group to provide more information on the development of CD. Second, we only investigated the cognitive processing with two typical tasks. Although these tasks are pervasively adopted to assess cognitive processing, there are also limitations in these tasks. For example, visual search task could mainly examine the attentional bias but not attentional disengagement. Regarding working memory, DMTS may mainly assess the maintaining of working memory, which is a part of the common working memory ability (120). Future studies may adopt n-back task to examine the updating process of emotional faces (121, 122). In addition, memory load may impact the performance (123–125). In the present study, we set the memory load at a moderate level to avoid any ceiling effect and floor effect. Nevertheless, working memory load should be manipulated as an independent variable in future studies. In summary, other tasks and paradigms may be further included to reveal the effect of CD on more aspects of cognitive processing. Third, only male adolescents were recruited in the present study, as there were few female delinquents in the local facilities. Further studies may focus on the effect of CD on the cognitive processing among female adolescents and reveal common and different mechanisms between male and female delinquents.
Conclusion
Male adolescent delinquents with CD showed deficits in attentional orientation to hostile and threatening faces (e.g., angry and fearful faces), partly supporting the optimal stimulation/arousal theory, the stimulation-seeking theory and the fearlessness theory.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Committee of Human Research at Zunyi Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
HK: conception, design, acquisition of data, analysis of data, interpretation of data, funding acquisition, writing-original draft, and writing-review and editing. WL: conception, design, data acquisition, data analysis, data interpretation, and writing-review and editing. XL: conception, design, and analysis of data. YY and MX: acquisition of data and writing-review and editing. BS: acquisition of data. QX: conception, design, analysis of data, funding acquisition, and writing-review and editing. TB: conception, design, analysis of data, interpretation of data, funding acquisition, and writing-review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the New Scientist Training Program of Zunyi Meidical University (CK-1233-038), the National Natural Science Foundation of China (32060191), and the Scientific Research Startup Foundation of Zunyi Medical University (F-990).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association (2013).
2. Rosenblatt JA, Rosenblatt A, Biggs EE. Criminal behavior and emotional disorder: Comparing youth served by the mental health and juvenile justice systems. J Behav Health Serv Res. (2000) 27:227–37. doi: 10.1007/BF02287315
3. Bowen KL, Morgan JE, Moore SC, Van Goozen SH. Young offenders’ emotion recognition dysfunction across emotion intensities: Explaining variation using psychopathic traits, conduct disorder and offense severity. J Psychopathol Behav Assess. (2014) 36:60–73. doi: 10.1007/s10862-013-9368-z
4. Pardini DA, Fite PJ. Symptoms of conduct disorder, oppositional defiant disorder, attention-deficit/hyperactivity disorder, and callous-unemotional traits as unique predictors of psychosocial maladjustment in boys: Advancing an evidence base for DSM-V. J Am Acad Child Adolesc Psychiatry. (2010) 49:1134–44. doi: 10.1016/j.jaac.2010.07.010
5. Mordre M, Groholt B, Kjelsberg E, Sandstad B, Myhre AM. The impact of ADHD and conduct disorder in childhood on adult delinquency: A 30 years follow-up study using official crime records. BMC Psychiatry. (2011) 11:57. doi: 10.1186/1471-244X-11-57
6. Frick PJ, Ray JV, Thornton LC, Kahn RE. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol Bull. (2014) 140:1–57. doi: 10.1037/a0033076
7. Sestir MA, Bartholow B. Theoretical explanations of aggression and violence. In: Gannon TA, Ward T, Beech AR, Fisher D editors. Aggressive Offenders Cognition. Theory, Research and Practice. (Hoboken, NJ: John Wiley & Sons Ltd) (2007). p. 157–78.
8. Dodge KA. Social-cognitive mechanisms in the development of conduct disorder and depression. Annu Rev Psychol. (1993) 44:559–84. doi: 10.1146/annurev.ps.44.020193.003015
9. Crick NR, Dodge KA. A review and reformulation of social information-processing mechanisms in children’s social adjustment. Psychol Bull. (1994) 115:74–101. doi: 10.1093/deafed/enw030
10. Gannon TA. Social cognition in violent and sexual offending: An overview. Psychol Crime Law. (2009) 15:97–118. doi: 10.1080/10683160802190822
11. Dodge KA. A social information processing model of social competence in children. In: Perlmutter L editor. Cognitive Perspectives on Children’s Social and Behavioral Development. (New York, NY: Psychology Press) (2014). p. 85–134. doi: 10.4324/9781315802343-7
12. Archer J, Haigh AM. Beliefs about aggression among male and female prisoners. Aggress Behav. (1997) 23:405–15. doi: 10.1002/(SICI)1098-2337(1997)23:6<405::AID-AB1>3.0.CO;2-F
13. Archer J, Haigh AM. Do beliefs about aggressive feelings and actions predict reported levels of aggression? Br J Soc Psychol. (1997) 36:83–105. doi: 10.1111/j.2044-8309.1997.tb01120.x
14. Polaschek DLL, Collie RM, Walkey FH. Criminal attitudes to violence: Development and preliminary validation of a scale for male prisoners. Aggress Behav. (2004) 30:484–503. doi: 10.1002/ab.20081
15. Bradshaw CP, Rodgers CRR, Ghandour LA, Garbarino J. Social–cognitive mediators of the association between community violence exposure and aggressive behavior. Sch Psychol Q. (2009) 24:199–210. doi: 10.1037/a0017362
16. Gouze KR. Attention and social problem solving as correlates of aggression in preschool males. J Abnorm Child Psychol. (1987) 15:181–97. doi: 10.1007/BF00916348
17. Smith P, Waterman M. Processing bias for sexual material: The emotional stroop and sexual offenders. Sex Abuse. (2004) 16:163–71. doi: 10.1177/107906320401600206
18. Cima M, Vancleef L, Lobbestael J, Meesters C, Korebrits A. Don?t You dare look at me, or else: Negative and aggressive interpretation bias, callous unemotional traits and type of aggression. J Child Adolesc Behav. (2014) 2:1–9.
19. Dodge KA, Frame CL. Social cognitive biases and deficits in aggressive boys. Child Dev. (1982) 53:620–35. doi: 10.2307/1129373
20. Dodge KA, Newman JP. Biased decision-making processes in aggressive boys. J Abnorm Psychol. (1981) 90:375–9. doi: 10.1037/0021-843X.90.4.375
21. Buades-Rotger M, Krämer UM. From words to action: Implicit attention to antisocial semantic cues predicts aggression and amygdala reactivity to angry faces in healthy young women. Aggress Behav. (2018) 44:624–37. doi: 10.1002/ab.21787
23. Young SE, Smolen A, Hewitt JK, Haberstick BC, Stallings MC, Corley RP, et al. Interaction between MAO-A genotype and maltreatment in the risk for conduct disorder: Failure to confirm in adolescent patients. Am J Psychiatry. (2006) 163:1019–25. doi: 10.1176/ajp.2006.163.6.1019
24. Afifi TO, Mcmillan KA, Asmundson GJG, Pietrzak RH, Sareen J. An examination of the relation between conduct disorder, childhood and adulthood traumatic events, and posttraumatic stress disorder in a nationally representative sample. J Psychiatr Res. (2011) 45:1564–72. doi: 10.1016/j.jpsychires.2011.08.005
25. Maniglio R. Significance, nature, and direction of the association between child sexual abuse and conduct disorder: A systematic review. Trauma Violence Abuse. (2015) 16:241–57. doi: 10.1177/1524838014526068
26. Dargis M, Newman J, Koenigs M. Clarifying the link between childhood abuse history and psychopathic traits in adult criminal offenders. Pers Disord. (2016) 7:221–8. doi: 10.1037/per0000147
28. Miller GE, Prinz RJ. Enhancement of social learning family interventions for childhood conduct disorder. Psychol Bull. (1990) 108:291–307. doi: 10.1037/0033-2909.108.2.291
29. Kendler KS, Jacobson K, Myer JM, Eaves LJ. A genetically informative developmental study of the relationship between conduct disorder and peer deviance in males. Psychol Med. (2008) 38:1001–11. doi: 10.1017/S0033291707001821
30. Cadesky EB, Mota VL, Schachar RJ. Beyond words: How do children with ADHD and/or conduct problems process nonverbal information about affect? J Am Acad Child Adolesc Psychiatry. (2000) 39:1160–7. doi: 10.1097/00004583-200009000-00016
31. Airdrie JN, Langley K, Thapar A, Van Goozen SHM. Facial emotion recognition and eye gaze in attention-deficit/hyperactivity disorder with and without comorbid conduct disorder. J Am Acad Child Adolesc Psychiatry. (2018) 57:561–70. doi: 10.1016/j.jaac.2018.04.016
32. Hartmann D, Ueno K, Schwenck C. Attributional and attentional bias in children with conduct problems and callous-unemotional traits: A case–control study. Child Adolesc Psychiatry Ment Health. (2020) 14:9. doi: 10.1186/s13034-020-00315-9
33. Hung A-Y, Ahveninen J, Cheng Y. Atypical mismatch negativity to distressful voices associated with conduct disorder symptoms. J Child Psychol Psychiatry. (2013) 54:1016–27. doi: 10.1111/jcpp.12076
34. Crago RV, Renoult L, Biggart L, Nobes G, Satmarean T, Bowler JO. Physical aggression and attentional bias to angry faces: An event related potential study. Brain Res. (2019) 1723:146387. doi: 10.1016/j.brainres.2019.146387
35. Quay HC. Psychopathic personality as pathological stimulation-seeking. Am J Psychiatry. (1965) 122:180–3. doi: 10.1176/ajp.122.2.180
36. Zentall SS. Optimal stimulation as theoretical basis of hyperactivity. Am J Orthopsychiatry. (1975) 45:549–63. doi: 10.1111/j.1939-0025.1975.tb01185.x
37. Zentall SS, Zentall TR. Optimal stimulation: A model of disordered activity and performance in normal and deviant children. Psychol Bull. (1983) 94:446–71. doi: 10.1037/0033-2909.94.3.446
38. Zuckerman M. Psychobiology of Personality. Cambridge, MA: Cambridge University Press (1991). doi: 10.1097/00004850-199100640-00009
39. Raine A, Venables PH, Mednick SA. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: Evidence from the mauritius child health project. J Am Acad Child Adolesc Psychiatry. (1997) 36:1457–64. doi: 10.1097/00004583-199710000-00029
40. Raine A. The Psychopathology of Crime: Criminal Behavior as a Clinical Disorder. San Diego, CA: Academic Press (1993).
41. Raine A. Psychophysiology and antisocial behavior: A biosocial perspective and a prefrontal dysfunction hypothesis. In: Stoff D, Breiling J, Maser J editors. Handbook of Antisocial Behavior. New York, NY: Wiley (1997). p. 289–304.
42. Herpertz SC, Mueller B, Qunaibi M, Lichterfeld C, Konrad K, Herpertz-Dahlmann B. Response to emotional stimuli in boys with conduct disorder. Am J Psychiatry. (2005) 162:1100–7. doi: 10.1176/appi.ajp.162.6.1100
43. De Wied M, Van Boxtel A, Zaalberg R, Goudena PP, Matthys W. Facial EMG responses to dynamic emotional facial expressions in boys with disruptive behavior disorders. J Psychiatr Res. (2006) 40:112–21. doi: 10.1016/j.jpsychires.2005.08.003
44. Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. (2005) 57:7–15. doi: 10.1016/j.biopsych.2004.10.008
45. Stadler C, Sterzer P, Schmeck K, Krebs A, Kleinschmidt A, Poustka F. Reduced anterior cingulate activation in aggressive children and adolescents during affective stimulation: Association with temperament traits. J Psychiatr Res. (2007) 41:410–7. doi: 10.1016/j.jpsychires.2006.01.006
46. Ewbank MP, Passamonti L, Hagan CC, Goodyer IM, Calder AJ, Fairchild G. Psychopathic traits influence amygdala-anterior cingulate cortex connectivity during facial emotion processing. Soc Cogn Affect Neurosci. (2018) 13:525–34. doi: 10.1093/scan/nsy019
47. Short RML, Adams WJ, Garner M, Sonuga-Barke EJS, Fairchild G. Attentional biases to emotional faces in adolescents with conduct disorder, anxiety disorders, and comorbid conduct and anxiety disorders. J Exp Psychopathol. (2016) 7:466–83. doi: 10.5127/jep.053915
48. Martin-Key NA, Graf EW, Adams WJ, Fairchild G. Facial emotion recognition and eye movement behaviour in conduct disorder. J Child Psychol Psychiatry. (2018) 59:247–57. doi: 10.1111/jcpp.12795
49. Hansen CH, Hansen RD. Finding the face in the crowd: An anger superiority effect. J Pers Soc Psychol. (1988) 54:917–24. doi: 10.1037/0022-3514.54.6.917
50. Williams MA, Mattingley JB. Do angry men get noticed? Curr Biol. (2006) 16:R402–4. doi: 10.1016/j.cub.2006.05.018
51. Gerritsen C, Frischen A, Blake A, Smilek D, Eastwood JD. Visual search is not blind to emotion. Percept Psychophys. (2008) 70:1047–59. doi: 10.3758/PP.70.6.1047
52. Pinkham AE, Griffin M, Baron R, Sasson NJ, Gur RC. The face in the crowd effect: Anger superiority when using real faces and multiple identities. Emotion. (2010) 10:141–6. doi: 10.1037/a0017387
53. Huang S-L, Chang Y-C, Chen Y-J. Task-irrelevant angry faces capture attention in visual search while modulated by resources. Emotion. (2011) 11:544–52. doi: 10.1037/a0022763
54. Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial expressions of emotion: Are angry faces detected more efficiently? Cogn Emot. (2000) 14:61–92. doi: 10.1080/026999300378996
55. Williams MA, Moss S, Bradshaw J, Mattingley J. Look at me, I’m smiling: Visual search for threatening and nonthreatening facial expressions. Visual Cogn. (2005) 12:29–50. doi: 10.1080/13506280444000193
56. Calvo MG, Nummenmaa L. Detection of emotional faces: Salient physical features guide effective visual search. J Exp Psychol. (2008) 137:471–94. doi: 10.1037/a0012771
58. Baskin-Sommers AR, Krusemark EA, Curtin JJ, Lee C, Vujnovich A, Newman JP. The impact of cognitive control, incentives, and working memory load on the P3 responses of externalizing prisoners. Biol Psychol. (2014) 96:86–93. doi: 10.1016/j.biopsycho.2013.12.005
59. Saarinen S, Fontell T, Vuontela V, Carlson S, Aronen ET. Visuospatial working memory in 7- to 12-year-old children with disruptive behavior disorders. Child Psychiatry Hum Dev. (2015) 46:34–43. doi: 10.1007/s10578-014-0449-3
60. Jamshidi S, Farrokhi H, Seyedzadeh Dalooyi SI. Comparison of sustained attention, working memory and response inhibition in children with conduct disorder and normal. Rooyesh. (2020) 8:159–68.
61. Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. (2010) 18:394–412. doi: 10.1080/09658211003702171
62. Borghans L, Princen M. The stability of memory performance using an adapted version of the Delayed Matching To Sample task: An ERP study. Maastricht Stud J Psychol Neurosci. (2012) 1:9–18.
63. Daniel TA, Katz JS, Robinson JL. Delayed match-to-sample in working memory: A BrainMap meta-analysis. Biol Psychol. (2016) 120:10–20. doi: 10.1016/j.biopsycho.2016.07.015
64. APA. Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association (1994).
65. Kim-Cohen J, Arseneault L, Caspi A, Tomás MP, Taylor A, Moffitt TE. Validity of DSM-IV conduct disorder in 41/2-5-year-old children: A longitudinal epidemiological study. Am J Psychiatry. (2005) 162:1108–17. doi: 10.1176/appi.ajp.162.6.1108
66. Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. (2009) 41:1149–60. doi: 10.3758/BRM.41.4.1149
67. Frick PJ. The Inventory of Callous-Unemotional Traits. New Orleans, LA: University of New Orleans (2004). doi: 10.1037/t62639-000
68. Essau CA, Sasagawa S, Frick PJ. Callous-unemotional traits in a community sample of adolescents. Assessment. (2006) 13:454–69. doi: 10.1177/1073191106287354
69. Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. (1992) 63:452–9. doi: 10.1037/0022-3514.63.3.452
70. Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl. (2003) 27:169–90. doi: 10.1016/S0145-2134(02)00541-0
71. Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. (2004) 72:271–324. doi: 10.1111/j.0022-3506.2004.00263.x
72. Tan S-H, Guo Y-Y. Revision of self-control scale for chinese college students. Chin J Clin Psychol. (2008) 16:468–70.
73. Bandura A, Barbaranelli C, Caprara G, Pastorelli C. Mechanisms of moral disengagement in the exercise of moral agency. J Pers Soc Psychol. (1996) 71:364–74. doi: 10.1037/0022-3514.71.2.364
74. Bandura A, Caprara GV, Barbaranelli C, Pastorelli C, Regalia C. Sociocognitive self-regulatory mechanisms governing transgressive behavior. J Pers Soc Psychol. (2001) 80:125–35. doi: 10.1037/0022-3514.80.1.125
75. Zhang G-L, Li A-S, Miao C-G, He X, Zhang M, Zhang Y. A consumer-grade LCD monitor for precise visual stimulation. Behav Res Methods. (2018) 50:1496–502. doi: 10.3758/s13428-018-1018-7
76. Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. (1980) 12:97–136. doi: 10.1016/0010-0285(80)90005-5
77. Van Goozen SH, Matthys W, Cohen-Kettenis PT, Buitelaar JK, Van Engeland H. Hypothalamic-pituitary-adrenal axis and autonomic nervous system activity in disruptive children and matched controls. J Am Acad Child Adolesc Psychiatry. (2000) 39:1438–45. doi: 10.1097/00004583-200011000-00019
78. Cappadocia MC, Desrocher M, Pepler D, Schroeder JH. Contextualizing the neurobiology of conduct disorder in an emotion dysregulation framework. Clin Psychol Rev. (2009) 29:506–18. doi: 10.1016/j.cpr.2009.06.001
79. Van Goozen SH, Snoek H, Matthys W, Van Rossum I, Van Engeland H. Evidence of fearlessness in behaviourally disordered children: A study on startle reflex modulation. J Child Psychol Psychiatry. (2004) 45:884–92. doi: 10.1111/j.1469-7610.2004.00280.x
80. Fairchild G, Stobbe Y, Van Goozen SHM, Calder AJ, Goodyer IM. Facial expression recognition, fear conditioning, and startle modulation in female subjects with conduct disorder. Biol psychiatry. (2010) 68:272–9. doi: 10.1016/j.biopsych.2010.02.019
81. Syngelaki EM, Fairchild G, Moore SC, Savage JC, Van Goozen SHM. Affective startle potentiation in juvenile offenders: The role of conduct problems and psychopathic traits. Soc Neurosci. (2013) 8:112–21. doi: 10.1080/17470919.2012.712549
82. Van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-De Wied C, Wiegant VM, Van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biol Psychiatry. (1998) 43:531–9. doi: 10.1016/S0006-3223(97)00253-9
83. Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Arch Gen Psychiatry. (2001) 58:297–302. doi: 10.1001/archpsyc.58.3.297
84. Shoal GD, Giancola PR, Kirillova GP. Salivary cortisol, personality, and aggressive behavior in adolescent boys: A 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry. (2003) 42:1101–7. doi: 10.1097/01.CHI.0000070246.24125.6D
85. Fairchild G, Van Goozen SH, Stollery SJ, Goodyer IM. Fear conditioning and affective modulation of the startle reflex in male adolescents with early-onset or adolescence-onset conduct disorder and healthy control subjects. Biol Psychiatry. (2008) 63:279–85. doi: 10.1016/j.biopsych.2007.06.019
86. Eimer M, Kiss M. Attentional capture by task-irrelevant fearful faces is revealed by the N2pc component. Biol Psychol. (2007) 74:108–12. doi: 10.1016/j.biopsycho.2006.06.008
87. Carlson JM, Reinke KS. Masked fearful faces modulate the orienting of covert spatial attention. Emotion. (2008) 8:522–9. doi: 10.1037/a0012653
88. Peltola MJ, Leppänen JM, Palokangas T, Hietanen JK. Fearful faces modulate looking duration and attention disengagement in 7-month-old infants. Dev Sci. (2008) 11:60–8. doi: 10.1111/j.1467-7687.2007.00659.x
89. Lobue V. More than just another face in the crowd: Superior detection of threatening facial expressions in children and adults. Dev Sci. (2009) 12:305–13. doi: 10.1111/j.1467-7687.2008.00767.x
90. Peltola MJ, Leppänen JM, Vogel-Farley VK, Hietanen JK, Nelson CA. Fearful faces but not fearful eyes alone delay attention disengagement in 7-month-old infants. Emotion. (2009) 9:560–5. doi: 10.1037/a0015806
91. Carlson JM, Reinke KS. Spatial attention-related modulation of the N170 by backward masked fearful faces. Brain Cogn. (2010) 73:20–7. doi: 10.1016/j.bandc.2010.01.007
92. Aguado L, Valdes-Conroy B, Rodríguez S, Román FJ, Dieguez T, Fernandez Cahill M. Modulation of early perceptual processing by emotional expression and acquired valence of faces: An ERP study. J Psychophysiol. (2012) 26:29–41. doi: 10.1027/0269-8803/a000065
93. Blechert J, Sheppes G, Di Tella C, Williams H, Gross JJ. See what you think: Reappraisal modulates behavioral and neural responses to social stimuli. Psychol Sci. (2012) 23:346–53. doi: 10.1177/0956797612438559
94. Pfabigan DM, Alexopoulos J, Sailer U. Exploring the effects of antisocial personality traits on brain potentials during face processing. PLoS One. (2012) 7:e50283. doi: 10.1371/journal.pone.0050283
95. Leleu A, Godard O, Dollion N, Durand K, Schaal B, Baudouin JY. Contextual odors modulate the visual processing of emotional facial expressions: An ERP study. Neuropsychologia. (2015) 77:366–79. doi: 10.1016/j.neuropsychologia.2015.09.014
96. Slocum LA, Simpson SS, Smith DA. Strained lives and crime: Examining intra-individual variation in strain and offending in a sample of incarcerated women. Criminology. (2005) 43:1067–110. doi: 10.1111/j.1745-9125.2005.00033.x
97. Hay C, Evans MM. Violent victimization and involvement in delinquency: Examining predictions from general strain theory. J Crim Justice. (2006) 34:261–74. doi: 10.1016/j.jcrimjus.2006.03.005
98. Baron SW, Forde DR. Street youth crime: A test of control balance theory. Justice Q. (2007) 24:335–55. doi: 10.1080/07418820701294870
99. Cullen FT, Unnever JD, Hartman JL, Turner MG, Agnew R. Gender, bullying victimization, and juvenile delinquency: A test of general strain theory. Vict Offender. (2008) 3:346–64. doi: 10.1080/15564880802338468
100. Moon B, Blurton D, Mccluskey JD. General strain theory and delinquency:focusing on the influences of key strain characteristics on delinquency. Crime Delinq. (2008) 54:582–613. doi: 10.1177/0011128707301627
101. Church WT, Wharton T, Taylor JK. An examination of differential association and social control theory:family systems and delinquency. Youth Violence Juv Justice. (2009) 7:3–15. doi: 10.1177/1541204008324910
102. Moon B, Morash M, Mccluskey CP, Hwang H-W. A comprehensive test of general strain theory:key strains, situational- and trait-based negative emotions, conditioning factors, and delinquency. J Res Crime Delinq. (2009) 46:182–212. doi: 10.1177/0022427808330873
103. Lin W-H, Cochran JK, Mieczkowski T. Direct and vicarious violent victimization and juvenile delinquency: An application of general strain theory*. Sociol Inq. (2011) 81:195–222. doi: 10.1111/j.1475-682x.2011.00368.x
104. Tittle C, Antonaccio O, Botchkovar E. Social learning, reinforcement and crime: Evidence from three European cities. Soc Forces. (2012) 90:863–90. doi: 10.1093/sf/sor020
105. Fontaine RG, Fida R, Paciello M, Tisak MS, Caprara GV. The mediating role of moral disengagement in the developmental course from peer rejection in adolescence to crime in early adulthood. Psychol Crime Law. (2014) 20:1–19. doi: 10.1080/1068316X.2012.719622
106. Baron SW. Differential social support, differential coercion, and organized criminal activities. Justice Q. (2015) 32:1089–117. doi: 10.1080/07418825.2014.887760
107. Stockdale LA, Morrison RG, Kmiecik MJ, Garbarino J, Silton RL. Emotionally anesthetized: Media violence induces neural changes during emotional face processing. Soc Cogn Affect Neurosci. (2015) 10:1373–82. doi: 10.1093/scan/nsv025
108. Cochran JK, Maskaly J, Jones S, Sellers CS. Using structural equations to model akers’ social learning theory with data on intimate partner violence. Crime Delinq. (2017) 63:39–60. doi: 10.1177/0886260517710486
109. Sully K, Sonuga-Barke EJS, Fairchild G. The familial basis of facial emotion recognition deficits in adolescents with conduct disorder and their unaffected relatives. Psychol Med. (2015) 45:1965–75. doi: 10.1017/S0033291714003080
110. Kleine Deters R, Naaijen J, Rosa M, Aggensteiner PM, Banaschewski T, Saam MC, et al. Executive functioning and emotion recognition in youth with oppositional defiant disorder and/or conduct disorder. World J Biol Psychiatry. (2020) 21:539–51.
111. Jackson MC, Wolf C, Johnston SJ, Raymond JE, Linden DE. Neural correlates of enhanced visual short-term memory for angry faces: An FMRI study. PLoS One. (2008) 3:e3536. doi: 10.1371/journal.pone.0003536
112. Jackson MC, Wu CY, Linden DE, Raymond JE. Enhanced visual short-term memory for angry faces. J Exp Psychol Hum Percept Perform. (2009) 35:363–74. doi: 10.1037/a0013895
113. Jackson MC, Linden DEJ, Raymond JE. Angry expressions strengthen the encoding and maintenance of face identity representations in visual working memory. Cogn Emot. (2014) 28:278–97. doi: 10.1080/02699931.2013.816655
114. Thomas PM, Jackson MC, Raymond JE. A threatening face in the crowd: Effects of emotional singletons on visual working memory. J Exp Psychol Hum Percept Perform. (2014) 40:253–63. doi: 10.1037/a0033970
115. Stiernströmer ES, Wolgast M, Johansson M. Effects of facial expression on working memory. Int J Psychol. (2016) 51:312–7. doi: 10.1002/ijop.12194
116. Sessa P, Luria R, Gotler A, Jolicśur P, Dell’acqua R. Interhemispheric ERP asymmetries over inferior parietal cortex reveal differential visual working memory maintenance for fearful versus neutral facial identities. Psychophysiology. (2011) 48:187–97. doi: 10.1111/j.1469-8986.2010.01046.x
117. Morris N, Jones DM. Memory updating in working memory: The role of the central executive. Br J Psychol. (1990) 81:111–21. doi: 10.1111/j.2044-8295.1990.tb02349.x
118. Ericsson KA, Kintsch W. Long-term working memory. Psychol Rev. (1995) 102:211–45. doi: 10.1037/0033-295X.102.2.211
119. Semprini M, Bonassi G, Barban F, Pelosin E, Iandolo R, Chiappalone M, et al. Modulation of neural oscillations during working memory update, maintenance, and readout: An hdEEG study. Hum Brain Mapp. (2021) 42:1153–66. doi: 10.1002/hbm.25283
120. Schmiedek F, Hildebrandt A, Lövdén M, Wilhelm O, Lindenberger U. Complex span versus updating tasks of working memory: The gap is not that deep. J Exp Psychol Learn Mem Cogn. (2009) 35:1089–96. doi: 10.1037/a0015730
121. Khorasani A, Aguilar M, Nejati V, Abadi H. Role of working memory updating and working memory capacity in moderating the relationship between impulsivity with propensity of risk taking behaviors and decision making in boy adolescents. Asian Soc Sci. (2016) 12:37. doi: 10.5539/ass.v12n11p37
122. Harden KP, Engelhardt LE, Mann FD, Patterson MW, Grotzinger AD, Savicki SL, et al. Genetic associations between executive functions and a general factor of psychopathology. J Am Acad Child Adolesc Psychiatry. (2020) 59:749–58. doi: 10.1016/j.jaac.2019.05.006
123. Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, et al. An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-to-sample task. Cogn Brain Res. (2005) 23:207–20. doi: 10.1016/j.cogbrainres.2004.10.010
124. Rissman J, Gazzaley A, D’esposito M. Dynamic adjustments in frontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cereb cortex. (2008) 18:1618–29. doi: 10.1093/cercor/bhm195
Keywords: conduct disorder, male adolescent delinquents, attentional bias, working memory, facial expressions
Citation: Kou H, Luo W, Li X, Yang Y, Xiong M, Shao B, Xie Q and Bi T (2022) Cognitive deficits for facial emotions among male adolescent delinquents with conduct disorder. Front. Psychiatry 13:937754. doi: 10.3389/fpsyt.2022.937754
Received: 06 May 2022; Accepted: 01 August 2022;
Published: 23 August 2022.
Edited by:
Qinghua He, Southwest University, ChinaReviewed by:
Yang Zhang, Soochow University, ChinaShenglin She, Guangzhou Medical University, China
Copyright © 2022 Kou, Luo, Li, Yang, Xiong, Shao, Xie and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinhong Xie, OTkyOTU2NkBxcS5jb20=; Taiyong Bi, Yml0YWl5b25nQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Hui Kou
Hui Kou Wei Luo2†
Wei Luo2† Qinhong Xie
Qinhong Xie Taiyong Bi
Taiyong Bi