- 1Shenzhen Hospital of Integrated Traditional Chinese and Western Medicine, Guangzhou University of Chinese Medicine, Shenzhen, China
- 2Gaozhou Hospital of Traditional Chinese Medicine, Gaozhou, China
The Mediterranean diet (MED), a dietary pattern rich in fruits and vegetables, whole grains, legumes, nuts, fish, and olive oil, has anti-oxidative and anti-inflammatory effects. Although some data suggest that MED adherence is associated with decreased manifestation of depressive symptoms, it remains necessary to further analyze this apparent non-linear association as well as the influence of different factors on the relationship between MED and depression. Here, we investigated associations between the alternate MED (aMED) score and depressive symptom via multivariate logistic regression, weighted generalized additive (GAM) and two-step linear regression models, analyzing data from the 2005–2018 National Health and Nutrition Examination Survey (NHANES). The most important factor relevant to aMED score that contributed to the prevalence of depressive symptom was assessed using random forest. Furthermore, we examined whether the relationship between aMED score and depressive symptom differs by age, race, sex, socioeconomic variables, lifestyle- and health-related variables, and chronic medical conditions, via subgroup analyses. A total of 19,477 participants (20–80 years of age) were included in this cross-sectional study. In crude and adjusted (1–5) multivariate logistic regression models, increased aMED score was noted to associate with non-depressive status, as defined using the Patient Health Questionnaire-9 (P < 0.05). Data analyses via GAM and two-piecewise linear regression revealed a non-linear association between aMED and depressive symptom, which had an inflection point of 3. Random forest results revealed that vegetable score contributes greatest to the relationship between aMED and depressive symptom. Subgroup analyses revealed that aMED score is significantly negatively related with depressive symptom in most different populations (P < 0.05) with the exception of high annual income, diabetes, borderline blood glucose level and Parkinson's disease (PD) (P > 0.05). In conclusion, we observed a non-linear association between aMED score and depressive symptom. Further studies are needed to validate our results.
Introduction
Depression, a common mental disorder, is characterized by persistent sadness and anhedonia in the context of previously rewarding or enjoyable activities (1). It is estimated that 5% of adults suffer from depression worldwide (2). The effectiveness of current prevention and treatment methods for depression, however, is limited and frequently associated with side effects. Interestingly, plant-based dietary patterns have been reported to greatly influence mental health, suggesting that diet has great use as an important adjunct therapy in the clinical management of depression (3).
Adherence to healthy diets is important to reduce the risk of illness throughout life. The Mediterranean diet (MED), which is rich in fruits and vegetables, whole grains, legumes, nuts, fish, and olive oil, is widely considered to beneficially impact health and longevity (4, 5). Not only has MED been incorporated into the national dietary guidelines set forth by the United States Department of Agriculture (USDA) but it has also attracted attention as a lifestyle modification with significant potential to reduce risks of menopausal metabolic syndrome, polycystic ovary syndrome, type 2 diabetes and cognitive decline (6–9). Furthermore, several studies reported that MED is associated with a decreased risk of depression (10–12).
However, there are several unaddressed issues in previous studies exploring MED and depression. Firstly, the alternate Mediterranean Diet (aMED) score, an important indicator for assessing adherence to MED, is a continuous variable that warrants further study to confirm its non-linear relationship with depression. Secondly, the etiology of depression is complicated and includes social, genetic and psychological factors (13, 14). Previous studies reported that the manifestation and severity of depression are not only affected by factors such as gender, educational level, marital status, and income but also depend on other comorbid conditions including stroke, thyroid problems and Parkinson's disease (PD) (15–20). Importantly, a single nucleotide variation in the oxoglutarate dehydrogenase-like gene rs2293239 (p.Asn725Ser) was identified as a major genetic predictor of familial depression (21). Thus, it is necessary to further analyze the influence of different factors on the relationship between MED and depression. Finally, prior reports warrant more detailed clarification of dietary habit influences on mental health in order to provide a foundation for effective lifestyle intervention guidelines (22).
In this study, we hypothesized aMED score and depressive symptom may have a non-linear relationship. We investigated associations between aMED score and depressive symptom via multivariate logistic regression, weighted generalized additive model (GAM) and two-step linear regression models, analyzing data from a large sample compiled in the National Health and Nutrition Examination Survey (NHANES) 2005–2018. Then, we identified the most important aMED score factor that contributed to depressive symptom prevalence utilizing random forest. We also examined whether the relationship between aMED score and depressive symptom differs by age, race, sex, socioeconomic variables, lifestyle- and health-related variables, and chronic medical conditions, utilizing subgroup analyses.
Materials and methods
Data source
The NHANES was a nationwide, cross-sectional survey conducted by the National Center for Health Statistics (NCHS). The survey included demographic, socioeconomic, dietary and health-related questions and assessed medical, dental, and physiological parameters. Laboratory tests were evaluated by appropriately trained medical personnel. Further details regarding the data collection process and analytical guidelines are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). All studies involving human participants were reviewed and approved by the Research Ethics Committee of the National Public Health Institute. Written informed consent to participate in this study was provided by the participants' legal guardian or next of kin (https://www.cdc.gov/nchs/nhanes/irba98.htm accessed on 09 December 2021).
Study population
The 2005–2018 NHANES assessed for depressive symptom and dietary patterns among survey participants. Seven biennial datasets were combined for subsequent analysis. In this study, we excluded participants with missing dietary (N = 16,677) or depressive symptom (N = 32,730) data. In total, 19,477 participants aged over 20 years were eligible for study inclusion (Supplementary Figure 1).
Mediterranean diet index
Dietary intake was assessed via structured interview focused on 24-h diet recall. The first diet recall was obtained during in-person assessment at a mobile examination center (day 1) and the second over the telephone (day 2) within 10 days of the in-person assessment. For primary analyses, we used the 24-h diet recall obtained during the in-person interview (day 1 and day 2 recalls). The MED index was determined in two steps. First, average 24-h diet recall data was linked to the USDA Food Patterns Equivalents Database to convert foods and beverages to equivalent USDA food pattern components (23). All dietary intake data from both 24-h recalls were aggregated as an average intake over 2 days for each participant. A MED index was subsequently calculated using the aMED score (4, 5). The aMED score (total score = 18) includes nine components: vegetables, legumes, fruits, nuts, whole grains, red and processed meats, fish, alcohol and olive oil (5).
Depressive symptom
Depressive symptom was assessed using the Patient Health Questionnaire-9 (PHQ-9), a reliable and valid screening instrument composed of nine items assessing for presence and severity of clinical depressive symptoms over the past 2 weeks (24). Each of the nine items can be scored from 0 (“not at all”) to 3 (“nearly every day”), with a total score ranging from 0 to 27. Depressive symptom was dichotomized based on a PHQ-9 score ≥10. This cutoff point, frequently used in prior studies, has been noted to have a sensitivity of 88% and a specificity of 88% for detecting major depression (12, 16, 24, 25).
Potential covariates
Baseline sociodemographic data, including demographic and questionnaire data, were obtained from NHANES. These data included sex (male or female), age (continuous; NHANES coded individuals over the age of 80 years as simply 80 years old), body mass index (BMI; <25, 25 to <30, ≥30), race (Hispanic, non-Hispanic), education (less than secondary and secondary, higher than secondary), marital status (married/living with a partner, widowed/divorced/separated, never married), annual income (< $75,000, ≥$75,000), health insurance (yes or no), self-reported health (excellent/very good, good, fair/poor), smoking history (yes or no) and insomnia (yes or no). Metabolic equivalent for task (MET) was used to assess the leisure time physical activities of participants. Self-reported time spent in moderate and vigorous leisure time exercise in a typical week was multiplied by the respective assigned MET score and values from both activities summed up to yield a total MET in mins/week. Participants who had MET-mins/week scores of ≥600 were classified as active while those with scores <600 were classified as inactive (16). Sedentary behavior was assessed by self-reported hours spent sitting at a desk, traveling in a car or bus, reading, playing cards, watching television, or using a computer on a typical day.
Medical record data, including data concerning hypertension, diabetes, stroke, thyroid illness, malignancy, cardiovascular diseases (CVD; including congestive heart failure, coronary heart disease, angina pectoris and heart attack), respiratory diseases (including emphysema, chronic bronchitis, and asthma) and PD was also collected. Many patients with PD suffered depression at the time of diagnosis. To explore whether PD was a potential covariate that affected the relationship between aMED and depressive symptom, patients suffering PD were identified amongst our participant pool (26, 27). Here, PD cases were identified if clinical history was remarkable for treatment with any of the following PD-specific medications: benztropine (generic drug code: d00175), carbidopa (generic drug code: d03473), levodopa (generic drug code: d03473), ropinirole (generic drug code: d04215), methyldopa (generic drug code: d00133), entacapone (generic drug code: d04460), and amantadine (generic drug code: d00086) (26, 27).
Statistical analysis
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) cross-sectional checklist was considered in this study (Supplementary Table 1) (28). Continuous variables were reported as mean ± standard deviation while categorical variables were reported as frequencies or percentages. Moreover, a complex and multi-level probability sampling design was implemented for NHANES data. As such, appropriate weighting methodology was applied in our study. Weighted Student's t (continuous variables included age and aMED score) and weighted chi-square (categorical variables included race, education, marital status, annual income, health insurance, self-reported health history, smoking history and insomnia, hypertension, CVD, respiratory diseases, diabetes, stroke, thyroid illness, malignancy and PD) tests were performed to assess for significant differences among means and proportions of the two groups.
Multivariate logistic regression models were weighted and generated to evaluate the association between aMED score and depressive symptom. Considering STROBE, we simultaneously obtained results of crude (no adjustment for covariates) and model 1 (only adjustment for age and sex), 2 (adjustment for socioeconomic variables), 3 (adjustment for lifestyle- and health-related variables), 4 (adjustment for chronic medical conditions) and 5 (adjustment for all covariates) adjusted analyses (28).
To evaluate the potential non-linear relationship between exposure and outcome, a GAM was generated. If a non-linear relationship was detected, a two-step linear regression model was generated to assess aMED score threshold effect and depressive symptom based on a smoothing plot. The threshold level of the aMED score at which the association between depressive symptom began to change and become significantly different was evaluated using a recurrence method. The inflection point was moved along a predefined interval with the inflection point showing maximum model likelihood investigated (29).
Random forest, a common machine learning method, was applied to analyze the contribution of various elements comprising the aMED score to depressive symptom. The learning method only allows a random sample of predictor variables was considered at each tree split. This model derived consecutive decision trees using random samples of training data to predict the residuals of previous models, thus creating a combination of trees that weighted difficulty to predict events to a greater degree (30). Based on the Gini splitting index, the optimal number of splits for each individual tree, the total number of trees, and an additional shrinkage factor—which reweights the prediction contribution from each individual tree—were determined using 10-fold cross-validation.
Subgroup analysis was conducted using a stratified multivariate logistic regression model. Participants with missing data for any other covariates were excluded. All analyses were conducted using the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Boston, MA, USA). P-values <0.05 (two-sided) were considered significantly different.
Consent to participate
All participants in this study signed written informed consent and all research procedures were approved by the National Center for Health Statistics Research Ethics Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm). As these data are public, approval of an institutional review board was not required for this study.
Results
Baseline participant characteristics
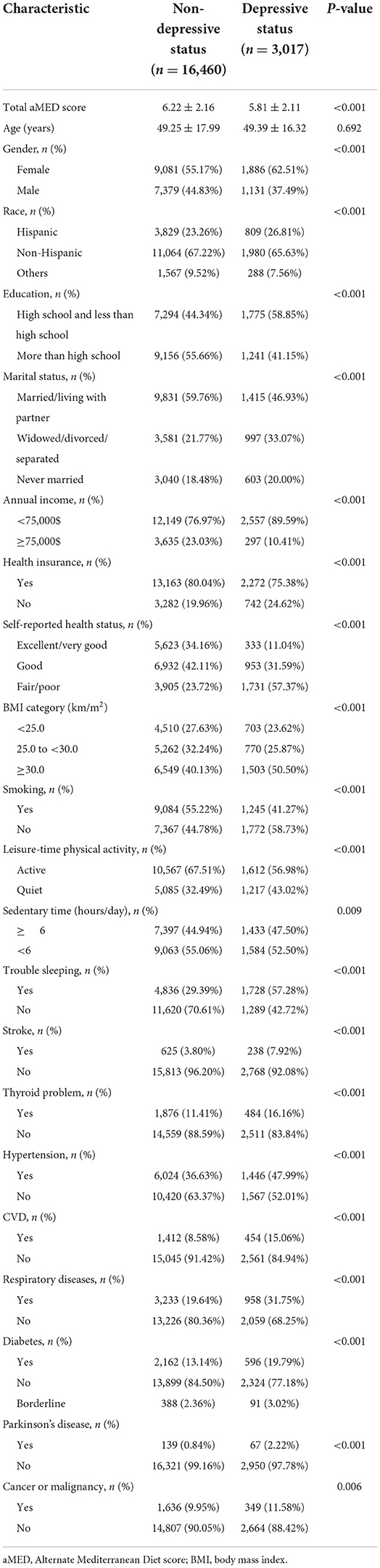
In total, 19,477 participants over 20 years of age were eligible for inclusion in this study. According to PHQ-9 scoring, 16,460 participants were classified as non-depressive and 3,017 participants depressive. Baseline characteristics are list in Table 1. Depressive participant aMED score (5.81 ± 2.11) was lower compared to that of non-depressive participants (6.22 ± 2.16) (P < 0.001). Participants who were female, Hispanic or Other, less educated, single (widowed/divorced/separated/never married), low earners, without health insurance, with a poor self-reported health status, overweight, non-smoking, inactive, with an extended period of daily sedentary behavior, as well as a history of suffering insomnia, stroke, thyroid illness, hypertension, CVD, respiratory diseases, diabetes, PD, or malignancy, were found to be more likely to suffer depression (P < 0.05). However, there was no difference in the average age of participants with depressive symptom compared to those who were non-depressive.
Multiple logistic regression analyses of aMED score and depressive symptom
Multivariable logistic regression was applied to evaluate the relationship between aMED score and the prevalence of depressive symptom (Table 2). In the crude model, the prevalence of depressive symptom and aMED score were negatively associated (OR: 0.915, 95% CI: 0.899–0.932; P < 0.001). In the adjusted model 1 (adjusted for age, sex and race), results did not differ from those obtained using crude analysis (OR: 0.905, 95% CI: 0.888–0.922; P < 0.001). After adjusting for different covariate categories in models 2–5, a negative association between depressive symptom and aMED score remained (Table 2). Our findings indicated that higher aMED score was associated with lower odds of depressive symptom; results were stable and robust.
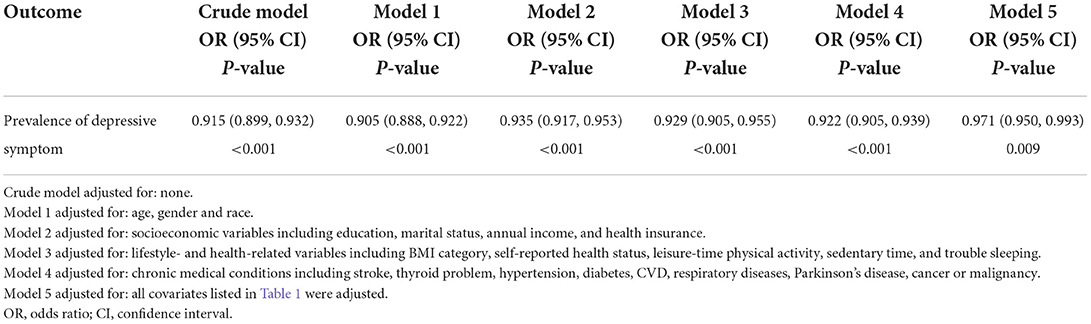
Table 2. Multivariable logistic regression of the association between aMED score and the prevalence of depressive symptom.
Analysis of non-linear relationships between aMED scores and depressive symptom
Because aMED score is a continuous variable, we considered that it may have a non-linear relationship with depressive symptom. Via GAM, we found a non-linear relationship between aMED score and depressive symptom (Figure 1). Using two-step linear regression analysis, we calculated an inflection point of 3. To the left of the inflection point, effect size, 95% CI and P-values were 1.079, 0.924–1.259, and 0.337, respectively. A negative relationship, however, was also observed between aMED score and depressive symptom to the right of the inflection point (0.907, 0.889–0.926, and <0.001; (Supplementary Table 2).
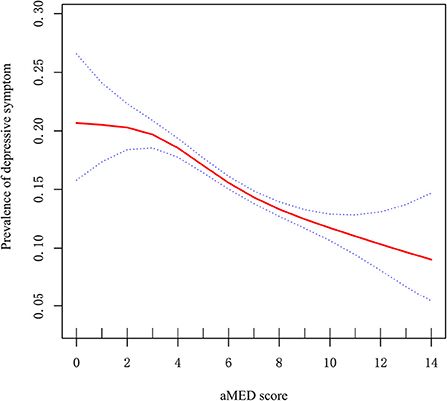
Figure 1. Non-linear relationship between aMED score and prevalence of depressive symptom. X-axis: aMED score; Y-axis: prevalence of depressive symptom. Red: OR; blue circles: 95% CI.
Random forest analysis of aMED score component influence on depressive symptom
To explore which aMED score components contributed most to the prevalence of depressive symptom, we used a random forest method for further analysis. High to low component contributions were as follows: vegetable, fruit, fish, meat, alcohol, legume, dairy, and cereal scores (Supplementary Figure 2). These results underscored a stronger association between high-fiber foods and a lower prevalence of depressive symptom.
Results of subgroup analyses
Because many factors are associated with depression, we performed a stratified analysis of populations with different characteristics to identify factors that influence the relationship between aMED score and depressive symptom. As shown in Supplementary Table 3, the negative relationship between aMED score and depressive symptom does not change in most characteristic populations irrespective of factors such as age, sex, race, education level, marital status, health insurance, BMI, smoking, leisure time physical activity, sedentary behavior, insomnia, stroke, thyroid illness, hypertension, CVD, respiratory diseases or malignancy (P < 0.001). Interestingly, no significant associations between aMED score and depressive symptom were observed among high annual income earners (P = 0.093), diabetics (P = 0.063), individuals with borderline blood glucose levels (P = 0.093), and patients suffering PD (P = 0.474).
Discussion
Over the past several decades, interest concerning the prevention and treatment of diseases via dietary modification has shifted from studying the effects of single nutrients and foods to studying dietary patterns (31). A meta-analysis of randomized controlled trials suggested that dietary interventions hold promise for effectively reducing symptoms of depression (22). Adherence to MED in particular appears to reduce manifestation of chronic illnesses and premature mortality (32). Although evidence of benefits concerning a lower prevalence of depression in the setting of proper MED adherence is scarce, some studies have reported such findings (10–12). Our results similarly suggest a negative association between aMED score and the prevalence of depressive symptom. On the basis of multivariate logistic regression analysis, we applied GAM to further evaluate the non-linear association between aMED score and the prevalence of depressive symptom. Importantly, GAM can not only address non-parametric smoothing but also fit a regression spline to data, which is obviously advantageous in analysis of non-linear associations (33). Here, we noted a negative association between aMED score and the prevalence of depressive symptom at an aMED score of 3 points or greater. These findings suggest that individuals with depressive symptoms should ideally strive for aMED scores of >3, thus increasing the likelihood that MED alleviates clinical depression.
The beneficial effects of the MED can be mainly attributed to its numerous components rich in anti-oxidants and possessing anti-inflammatory properties (34). Positive effects in the setting of depression are likely mainly due to the activity of unsaturated fatty acids and polyphenols (2018). Moreover, our findings suggest that high-fiber food, including vegetables and fruit, plays a greater role than foods such as fish, meat, alcohol, legumes, dairy and cereals, with respect to the negative association between aMED score and depressive symptom. These results demonstrate MED is a dietary pattern based on plant-derived foods (32). Importantly, vegetables and fruits are rich in phytochemicals, phenolic compounds and flavonoids. Most of these compounds possess significant anti-oxidant properties which likely improve depressive symptoms via inhibition of oxidative stress and alleviation of chronic inflammation. For example, lutein, a dietary phytochemical capable of crossing the blood-brain barrier, has been demonstrated to exert an antidepressant-like effect and alleviate neurochemical imbalances (35). Curcumin, a polyphenol extracted from the rhizome of the turmeric plant, has been demonstrated to exert antidepressant effects in both animal and human trials (36, 37). Similarly, S-equol, a metabolite of dietary soy isoflavones, was reported to alleviate depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity (38). Moreover, omega-3 polyunsaturated fatty acids may reduce inflammation and benefit mood by fostering the production of the anti-inflammatory prostaglandin E3 (as opposed to the pro-inflammatory prostaglandin E2) (39). Although vegetable and fruit scores were noted to exert a greater effect on the association between aMED score and the prevalence of depressive symptom, other ingredients also contribute to the relationship. As such, the anti-inflammatory and mood-related benefits of MED adherence result due to a synergistic action of nutrients composing this dietary pattern.
Due to the complex etiology of depression, there is currently no universal and effective treatment. In order to make the case for clinical application of MED in effective prevention and management of depression, we used subgroup analyses to explore the association between aMED score and the prevalence of depressive symptom in different populations. Results revealed that aMED score has a significantly negative relationship with depressive symptom in most populations, except among patients earning a high annual income or suffering diabetes, borderline blood glucose levels or PD. Previous study reported that high annual income earners generally have higher aMED scores (5). In addition, most American adults do not adhere to dietary patterns resembling MED. This phenomenon may explain the lack of a statistically significant association between aMED score and depressive symptom among high annual income earners. Furthermore, cognitive impairment is one key risk factor of depression (40). Cognitive impairment is also known to be a main complication of diabetes and PD (41, 42). This may similarly explain the lack of a statistically significant association between aMED score and depressive symptom in patients suffering diabetes or PD. Interestingly, the protective effect of MED on depressive symptoms was noted to gradually decrease as BMI increased. The prevalence of depression is known to be increased in the setting of obesity, a condition that promotes inflammation as well as resistance to insulin and leptin (43, 44). Thus, a combination of MED adherence and proper weight control is guaranteed to improve the effectiveness of MED adherence in regard to alleviating depression. No or little association of MED score with depression among younger women, however, has also been reported due to genetic factors (45). We found that age and sex do not influence the relationship between aMED score and depressive symptom. Therefore, further research is needed to confirm the aforementioned discrepancies.
There were some limitations in our study. First, due to the nature of cross-sectional design of present study, residual confounding from unmeasured confounders should be a concern. Second, dietary intake was assessed by asking subjects to recall what was eaten over the prior 2 days and thus may not accurately reflect the typical dietary patterns of subjects. Third, due to missing data, some psychiatric comorbidities were not considered as covariates in our analyses. For example, eating disorders and attention deficit hyperactivity disorder were not considered in this study (46, 47). Moreover, the state of mental health cannot be ruled out in regard to exerting a significant influence on the association between aMED score and depression as cognitive function and prescription medications were not assessed in this study. In addition, possible misclassification of dietary behavior due to memory bias may have occurred during data collection. Finally, although the PHQ-9 is a validated screening tool designed to assess depressive symptoms, it is not intended for use in the diagnosis of clinical depression. Furthermore, as diagnostic delay of depression in clinical practice is common, our findings might be caused by reversion causation; we were unable to consider this phenomenon when performing analyses due to a lack of relevant data (48). Future studies are warranted to clarify the impact of diagnostic delay on the relationship between MED and depression.
Conclusions
In this study, the relationship between aMED score and depressive symptom was demonstrated to be non-linear. Our findings confirm that MED alleviates depressive symptom at aMED scores of 3 or greater. However, the association between aMED score and depressive symptom in populations earning a high annual income, as well as suffering diabetes, borderline blood glucose levels or PD, requires further evaluation.
Data availability statement
The data presented in the study are deposited in the the NHANES website, accession link: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the National Public Health Institute. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin (https://www.cdc.gov/nchs/nhanes/irba98.htm accessed on December 9, 2021).
Author contributions
Conceptualization: WX and MZ. Methodology, validation, and writing—original draft preparation: YF. Software and visualization: ZD. Formal analysis, data curation, and writing—review and editing: LZ. Investigation: ML. Resources: ZH. Supervision and funding acquisition: MZ. Project administration: WX. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by the Traditional Chinese Medicine Bureau of Guangdong Province (20211345), the Natural Science Foundation of Guangdong Province (2022A151510450), the Shenzhen Science and Technology Innovation Committee Subject (JCYJ20210324123614040), Bao'an TCM Development Foundation (2020KJCX-KTYJ-130 and 2020KJCX-KTYJ-133), and Sanming Project of Medicine in Shenzhen (SZZYSM202106009).
Acknowledgments
The authors thank Si Chen for her technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.936283/full#supplementary-material
References
1. Feng L, Xing H, Zhang K. The therapeutic potential of traditional Chinese medicine in depression: targeting adult hippocampal neurogenesis. Phytomedicine. (2022) 98:153980. doi: 10.1016/j.phymed.2022.153980
2. Wold Health Organization (2022). Depression. Available online at: https://www.who.int/health-topics/depression#tab=tab_1 (accessed 30 April, 2022].
3. Jacka FN, O'Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the 'SMILES' trial). BMC Med. (2017) 15:23. doi: 10.1186/s12916-017-0791-y
4. Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. (2014) 17:2769–82. doi: 10.1017/S1368980013003169
5. Taylor MK, Mahnken JD, Sullivan DK. NHANES 2011-2014 reveals cognition of US older adults may benefit from better adaptation to the Mediterranean diet. Nutrients. (2020) 12:929. doi: 10.3390/nu12071929
6. DeSalvo KB, Olson R, Casavale KO. Dietary Guidelines for Americans. JAMA. (2016) 315:457–8. doi: 10.1001/jama.2015.18396
7. Marini HR. Mediterranean diet and soy isoflavones for integrated management of the menopausal metabolic syndrome. Nutrients. (2022) 14:550. doi: 10.3390/nu14081550
8. Mei S, Ding J, Wang K, Ni Z, Yu J. Mediterranean diet combined with a low-carbohydrate dietary pattern in the treatment of overweight polycystic ovary syndrome patients. Front Nutr. (2022) 9:876620. doi: 10.3389/fnut.2022.876620
9. Sarsangi P, Salehi-Abargouei A, Ebrahimpour-Koujan S, Esmaillzadeh A. Association between adherence to the Mediterranean diet and risk of type 2 diabetes: an updated systematic review and dose-response meta-analysis of prospective cohort studies. Adv Nutr. (2022) doi: 10.1093/advances/nmac046. [Epub ahead of print].
10. Sánchez-Villegas A, Martínez-González MA, Estruch R, Salas-Salvadó J, Corella D, Covas MI, et al. Mediterranean dietary pattern and depression: the PREDIMED randomized trial. BMC Med. (2013) 11:208. doi: 10.1186/1741-7015-11-208
11. Sánchez-Villegas A, Henríquez-Sánchez P, Ruiz-Canela M, Lahortiga F, Molero P, Toledo E, et al. A longitudinal analysis of diet quality scores and the risk of incident depression in the SUN Project. BMC Med. (2015) 13:197. doi: 10.1186/s12916-015-0428-y
12. Oddo VM, Welke L, McLeod A, Pezley L, Xia Y, Maki P, et al. Adherence to a Mediterranean diet is associated with lower depressive symptoms among U.S. adults. Nutrients. (2022) 14:278. doi: 10.3390/nu14020278
13. Won E, Kim YK. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr Neuropharmacol. (2016) 14:665–73. doi: 10.2174/1570159X14666151208113006
14. Penninx B, Eikelenboom M, Giltay EJ, van Hemert AM, Riese H, Schoevers RA, et al. Cohort profile of the longitudinal Netherlands Study of Depression and Anxiety (NESDA) on etiology, course and consequences of depressive and anxiety disorders. J Affect Disord. (2021) 287:69–77. doi: 10.1016/j.jad.2021.03.026
15. Dinh Le T, Huy Duong H, Thi Nguyen L, Phi Thi Nguyen N, Tien Nguyen S, Van Ngo M. The relationship between depression and multifactorial control and microvascular complications in vietnamese with type 2 diabetes mellitus aged 30-60 years. Diabetes Metab Syndr Obes. (2022) 15:1185–95. doi: 10.2147/DMSO.S354443
16. Fan Z, Gong X, Xu H, Wang H, Zeng N, Li L, et al. Gender differences in the associations between tobacco smoke exposure and depressive symptoms among U.S. adults: NHANES 2007-2018. J Psychiatr Res. (2022) 146:249–57. doi: 10.1016/j.jpsychires.2021.11.013
17. Hsu MY, Huang SC, Liu PL, Yeung KT, Wang YM, Yang HJ. The interaction between exercise and marital status on depression: a cross-sectional study of the Taiwan biobank. Int J Environ Res Public Health. (2022) 19:1876. doi: 10.3390/ijerph19031876
18. Lozupone M, D'Urso F, Copetti M, Sardone R, Arcuti S, Castellana F, et al. The diagnostic accuracy of late-life depression is influenced by subjective memory complaints and educational level in an older population in Southern Italy. Psychiatry Res. (2022) 308:114346. doi: 10.1016/j.psychres.2021.114346
19. Su D, Cui Y, Liu Z, Chen H, Fang J, Ma H, et al. Altered brain activity in depression of Parkinson's disease: a meta-analysis and validation study. Front Aging Neurosci. (2022) 14:806054. doi: 10.3389/fnagi.2022.806054
20. Zhao L, Sun Q, Guo Y, Yan R, Lv Y. Mediation effect of perceived social support and resilience between physical disability and depression in acute stroke patients in China: a cross-sectional survey. J Affect Disord. (2022) 308:155–9. doi: 10.1016/j.jad.2022.04.034
21. Pan Z, Tian H, Fang T, Liu Z, Liu X, Dou G, et al. OGDHL Variant rs2293239: a potential genetic driver of chinese familial depressive disorder. Front Psychiatry. (2022) 13:771950. doi: 10.3389/fpsyt.2022.771950
22. Firth J, Marx W, Dash S, Carney R, Teasdale SB, Solmi M, et al. The effects of dietary improvement on symptoms of depression and anxiety: a meta-analysis of randomized controlled trials. Psychosom Med. (2019) 81:265–80. doi: 10.1097/PSY.0000000000000673
23. Service, UAR. Food and Nutrient Data Base for Dietary Studies. Available online at: http://www.ars.usda.gov/Services/docs.htm?docid=12085 (accessed 15, April 2022).
24. Kroenke K, Spitzer RL, Williams JB, Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. (2010) 32:345–59. doi: 10.1016/j.genhosppsych.2010.03.006
25. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
26. Botelho J, Lyra P, Proença L, Godinho C, Mendes JJ, Machado V. Relationship between blood and standard biochemistry levels with periodontitis in Parkinson's disease patients: data from the NHANES 2011-2012. J Pers Med. (2020) 10:69. doi: 10.3390/jpm10030069
27. Lyra P, Machado V, Proença L, Mendes JJ, Botelho J. Tooth loss and blood pressure in Parkinson's disease patients: an exploratory study on NHANES data. Int J Environ Res Public Health. (2021) 18:32. doi: 10.3390/ijerph18095032
28. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med. (2007) 147:W163–194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1
29. Liu S, Wang X, Lu Y, Li T, Gong Z, Sheng T, et al. The effects of intraoperative cryoprecipitate transfusion on acute renal failure following orthotropic liver transplantation. Hepatol Int. (2013) 7:901–9. doi: 10.1007/s12072-013-9457-9
30. Churpek MM, Yuen TC, Winslow C, Meltzer DO, Kattan MW, Edelson DP. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. (2016) 44:368–74. doi: 10.1097/CCM.0000000000001571
31. Jacobs DR Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr. (2009) 89:1543s−8s. doi: 10.3945/ajcn.2009.26736B
32. Dominguez LJ, Di Bella G, Veronese N, Barbagallo M. Impact of Mediterranean diet on chronic non-communicable diseases and longevity. Nutrients. (2021) 13:28. doi: 10.3390/nu13062028
33. Ravindra K, Rattan P, Mor S, Aggarwal AN. Generalized additive models: building evidence of air pollution, climate change and human health. Environ Int. (2019) 132:104987. doi: 10.1016/j.envint.2019.104987
34. Gantenbein KV, Kanaka-Gantenbein C. Mediterranean Diet as an antioxidant: the impact on metabolic health and overall wellbeing. Nutrients. (2021) 13:1951. doi: 10.3390/nu13061951
35. Zeni ALB, Camargo A, Dalmagro AP. Lutein prevents corticosterone-induced depressive-like behavior in mice with the involvement of antioxidant and neuroprotective activities. Pharmacol Biochem Behav. (2019) 179:63–72. doi: 10.1016/j.pbb.2019.02.004
36. Lamanna-Rama N, Romero-Miguel D, Desco M, Soto-Montenegro ML. An update on the exploratory use of curcumin in neuropsychiatric disorders. Antioxidants. (2022) 11:353. doi: 10.3390/antiox11020353
37. Lopresti AL. Potential role of curcumin for the treatment of major depressive disorder. CNS Drugs. (2022) 36:123–41. doi: 10.1007/s40263-022-00901-9
38. Lu C, Gao R, Zhang Y, Jiang N, Chen Y, Sun J, et al. S-equol, a metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. (2021) 12:5770–8. doi: 10.1039/D1FO00547B
39. Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. (2003) 100:1751–6. doi: 10.1073/pnas.0334211100
40. Orgeta V, Leung P, Del-Pino-Casado R, Qazi A, Orrell M, Spector AE, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev. (2022) 4:Cd009125. doi: 10.1002/14651858.CD009125.pub3
41. Fan Y, Han J, Zhao L, Wu C, Wu P, Huang Z, et al. Experimental models of cognitive impairment for use in Parkinson's disease research: the distance between reality and ideal. Front Aging Neurosci. (2021) 13:745438. doi: 10.3389/fnagi.2021.745438
42. Yu W, Yin H, Sun Y, Shi S, Li J, Wang X. The attenuation effect of potassium 2-(1-hydroxypentyl)-benzoate in a mouse model of diabetes-associated cognitive decline: the protein expression in the brain. CNS Neurosci Ther. (2022) 28:1108–23. doi: 10.1111/cns.13847
43. Lorena FB, do Nascimento BPP, Camargo E, Bernardi MM, Fukushima AR, do NP, et al. Long-term obesity is associated with depression and neuroinflammation. Arch Endocrinol Metab. (2021) 65:537–48. doi: 10.20945/2359-3997000000400
44. Fulton S, Décarie-Spain L, Fioramonti X, Guiard B, Nakajima S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol Metab. (2022) 33:18–35. doi: 10.1016/j.tem.2021.10.005
45. Yin W, Löf M, Chen R, Hultman CM, Fang F, Sandin S. Mediterranean diet and depression: a population-based cohort study. Int J Behav Nutr Phys Act. (2021) 18:153. doi: 10.1186/s12966-021-01227-3
46. Robinson LR, Bitsko RH, O'Masta B, Holbrook JR, Ko J, Barry CM, et al. A systematic review and meta-analysis of parental depression, antidepressant usage, antisocial personality disorder, and stress and anxiety as risk factors for attention-deficit/hyperactivity disorder (ADHD) in children. Prev Sci. (2022). doi: 10.1007/s11121-022-01383-3. [Epub ahead of print].
47. Robison R, Lafrance A, Brendle M, Smith M, Moore C, Ahuja S, et al. A case series of group-based ketamine-assisted psychotherapy for patients in residential treatment for eating disorders with comorbid depression and anxiety disorders. J Eat Disord. (2022) 10:65. doi: 10.1186/s40337-022-00588-9
Keywords: Mediterranean diet, depressive symptom, weighted generalized additive model, random forest, multivariate logistic regression models
Citation: Fan Y, Zhao L, Deng Z, Li M, Huang Z, Zhu M and Xu W (2022) Non-linear association between Mediterranean diet and depressive symptom in U.S. adults: A cross-sectional study. Front. Psychiatry 13:936283. doi: 10.3389/fpsyt.2022.936283
Received: 05 May 2022; Accepted: 27 June 2022;
Published: 15 July 2022.
Edited by:
Jiangwei Sun, Karolinska Institutet (KI), SwedenCopyright © 2022 Fan, Zhao, Deng, Li, Huang, Zhu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhua Xu, eHdodWEyMDAyQDE2My5jb20=; Meiling Zhu, bWVpbGluZ3podTIwMjBAMTI2LmNvbQ==
 Yaohua Fan
Yaohua Fan Lijun Zhao
Lijun Zhao Zhiyuan Deng2
Zhiyuan Deng2 Mengzhu Li
Mengzhu Li Zifeng Huang
Zifeng Huang Meiling Zhu
Meiling Zhu