
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 24 June 2022
Sec. Addictive Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.927075
This article is part of the Research Topic Insights Into Structural and Functional Organization of the Brain: Evidence From Neuroimaging and Non-Invasive Brain Stimulation Techniques View all 7 articles
 Jinghan Dang
Jinghan Dang Qiuying Tao
Qiuying Tao Xiaoyu Niu
Xiaoyu Niu Mengzhe Zhang
Mengzhe Zhang Xinyu Gao
Xinyu Gao Zhengui Yang
Zhengui Yang Miaomiao Yu
Miaomiao Yu Weijian Wang
Weijian Wang Shaoqiang Han*
Shaoqiang Han* Jingliang Cheng*
Jingliang Cheng* Yong Zhang*
Yong Zhang*Background: Previous voxel-based morphometric (VBM) and functional magnetic resonance imaging (fMRI) studies have shown changes in brain structure and function in cocaine addiction (CD) patients compared to healthy controls (HC). However, the results of these studies are poorly reproducible, and it is unclear whether there are common and specific neuroimaging changes. This meta-analysis study aimed to identify structural, functional, and multimodal abnormalities in CD patients.
Methods: The PubMed database was searched for VBM and task-state fMRI studies performed in CD patients between January 1, 2010, and December 31, 2021, using the SEED-BASE d MAP software package to perform two independent meta-groups of functional neural activation and gray matter volume, respectively. Analysis, followed by multimodal analysis to uncover structural, functional, and multimodal abnormalities between CD and HC.
Results: The meta-analysis included 14 CD fMRI studies (400 CD patients and 387 HCs) and 11 CD VBM studies (368 CD patients and 387 controls). Structurally, VBM analysis revealed significantly lower gray matter volumes in the right superior temporal gyrus, right insula, and right retrocentral gyrus than in the HC. On the other hand, the right inferior parietal gyrus increased in gray matter (GM) volume in CD patients. Functionally, fMRI analysis revealed activation in the right temporal pole, right insula, and right parahippocampal gyrus. In the right inferior parietal gyrus, the left inferior parietal gyrus, the left middle occipital gyrus, and the right middle frontal gyrus, the degree of activation was lower.
Conclusion: This meta-analysis showed that CD patients had significant brain GM and neural changes compared with normal controls. Furthermore, multi-domain assessments capture different aspects of neuronal alterations in CD, which may help develop effective interventions for specific functions.
Cocaine is an alkaloid that is produced biosynthetically by Erythroxylum coca, a shrub native to the Andean Highlands and northern parts of the Amazon in South America (1). This psychostimulant drug has become an essential part of the world drug scene and is also the world’s most trafficked drug after cannabis (resin or marijuana) (2). The worldwide prevalence of cocaine use was estimated at 0.3–0.4% of the population aged 15–64 years (between 13 and 20 million users) (3). Recent epidemiological data indicate that the prevalence of cocaine use is increasing (2).
Addiction is a chronic relapsing disorder characterized by the loss of inhibitory control over drug-seeking and taking, and maintenance of drug use despite negative consequences (4). Cocaine addiction (CD) is a worldwide public health problem, which has somatic, psychological, psychiatric, socio-economic, and judicial complications (5). It’s short half-life and strong dopaminergic precursor activity make it the most addictive of the psychostimulants (6). Numerous studies have shown that cocaine causes irreversible structural changes in the brain, heart, lungs, and other organs such as the liver and kidneys, and that many mechanisms are involved in the occurrence of these damages (7). Compared with the general population, cocaine use is associated with a significantly increased risk of schizophrenia (8) and may also induce transient psychotic symptoms such as paranoid beliefs and paranoia, hallucinations, and stereotyped actions (9). Psychosis develops during substance use and may not resolve even after withdrawal or withdrawal (10). Stroke risk appears to be significantly increased with cocaine use (11–13). The addictive nature of this drug can cause significant acute and long-term psychological effects in humans (14). It is worth mentioning that crack cocaine users have more family problems than other drug users, and previous research has shown that this population has higher rates of living on the streets and coming from broken homes (15). In light of the significant prevalence and negative consequences of CD, proposed diagnostic criteria have been included in the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and have been widely used to diagnose addiction and evaluate its treatment (16).
With the development of modern imaging technology, functional magnetic resonance imaging, and voxel-based morphometric analysis are widely used to study the brain characteristics of CD. However, these studies often provide inconsistent results, in part because of the differences in the methods and sample characteristics of most studies and the limited number of samples. Therefore, the persistent neurological changes associated with CD are still largely unknown. It is of great significance to conduct meta-analysis to draw a consistent conclusion. In addition to functional neurological changes, structural markers such as gray matter volume are also important because they may be relatively stable over time and can be used as the basis for functional neural activity (17). Voxel-based morphometry (VBM) analysis is a standardized method for measuring the volume of gray matter, and has been widely used in the study of CD. Therefore, CD’s VBM study can provide additional information and complement the findings of fMRI research. In previous studies using VBM gray matter (GM) volume, almost identical reductions in insular and temporal GM volume compared with controls have been reported (18–21). However, studies using functional magnetic resonance (fMRI) reported more significant changes in prefrontal cortex function compared with controls (22–26).
To sum up, the purpose of this study is to explore the common GM abnormalities and functional deficits of CD individuals, which are very important for the development of specific intervention for CD or transdiagnostic treatment. We conducted two meta-analyses including all VBM and fMRI studies separately, and further performed a joint analysis between the two main meta-analyses. Based on previous studies, we hypothesized that the Insula in CD subjects would develop disorder-specific GMV abnormalities. As for fMRI, we hypothesized that CD patients exhibit abnormal under activation of the prefrontal cortex (PFC).
We did our Meta-Analyses in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Systematic and comprehensive searches of VBM and fMRI studies of CD from Jan 1, 2010, to Dec 31, 2021 was performed using the PubMed, Web of Science and Scopus database, combined with the following keywords: (“cocaine” or “cocaine related disorders” or “cocaine addiction” or “CD”) and (“voxel-based morphometry” or “VBM” or “gray matter” or “functional magnetic resonance imaging” or “fMRI”) and “brain.” Studies were included if the following inclusion criteria were met: (1) they reported whole brain results; (2) they compared CDs and HCs; (3) they were a task-related fMRI or VBM study; (4) they provided peak coordinates in Montreal Neurological Institute (MNI) or Talairach spaces; (5) the diagnoses of each study were based on DSM. (6) They used consistent thresholds in different regions. Studies were excluded if (1) the patient group included other diseases; (2) they did not use VBM; (3) peak coordinates were not reported; (4) only region of interest results were available; (5) inconsistent thresholds were applied in different regions. (6) they had fewer than 10 patients.
Two investigators (Jinghan Dang and Qiuying Tao) independently assessed the quality of each included study using the Newcastle-Ottawa Scale (NOS) (27). NOS consist of three quality parameters for cohort studies: selection, comparability, and outcome, which are assigned with a maximum of four, two, and three stars, respectively. Therefore, nine stars reflect the highest quality. Studies with more than six stars are considered high quality (28). Any discrepancy was resolved through a joint revaluation of the original study with a third author (Xiaoyu Niu).
We performed a coordinate-based meta-analysis (29) using the anisotropic effect size version of the SDM software package (Version 5.15) for GM volumes between long-term cocaine use and healthy controls Variety. The SDM method has been well validated and described in detail in many studies. The data processing procedure is briefly summarized here (30). For each meta-analysis, AES-SDM converted peak coordinates to Hedge’s effect size and recreated voxel-level maps for each study based on an anisotropic unnormalized Gaussian kernel. Mean plots were then calculated using a random effects model, weighted to account for sample size, within-study variability, and between-study heterogeneity (31). Here are the steps: (1) p-values or z-values in some studies need to be converted to t-values online (32); (2) the peak coordinates are converted to normalized MNI space; (3) set the full width at half maximum (FWHM) to 20 mm as this will maintain a balance between sensitivity and specificity and other parameters including voxel p < 0.005, peak height threshold > 1 and cluster extent threshold > 10 voxels; (4) After excluding one study at a time, a jack-knife sensitivity analysis was performed by repeating the meta-analysis to verify the stability and reliability of the results. If a brain region survives most repetitions, we can conclude that the abnormality is stable (33). (5) To check for possible publication bias, the effect size at the peak point for each significant cluster of each study was extracted and funnel plots were constructed with the standard error of the effect as the vertical axis and the effect size as the horizontal axis (31). Additionally, Egger’s test, a regression of standard normal deviation (defined as effect size divided by its standard error) on precision (defined as the inverse of the standard error of effect size), was used to quantitatively test the asymmetry of each funnel plot. A funnel plot is considered asymmetric if the intercept of the regression deviates significantly from 0 (34, 35).
As demonstrated in Figure 1, the final dataset contains 14 fMRI contrasts covering 400 CDs and 387 HCs, and 11 VBM contrasts covering 368 CDs and 387 HCs. See Table 1 for more demographic, clinical, and other characteristics.
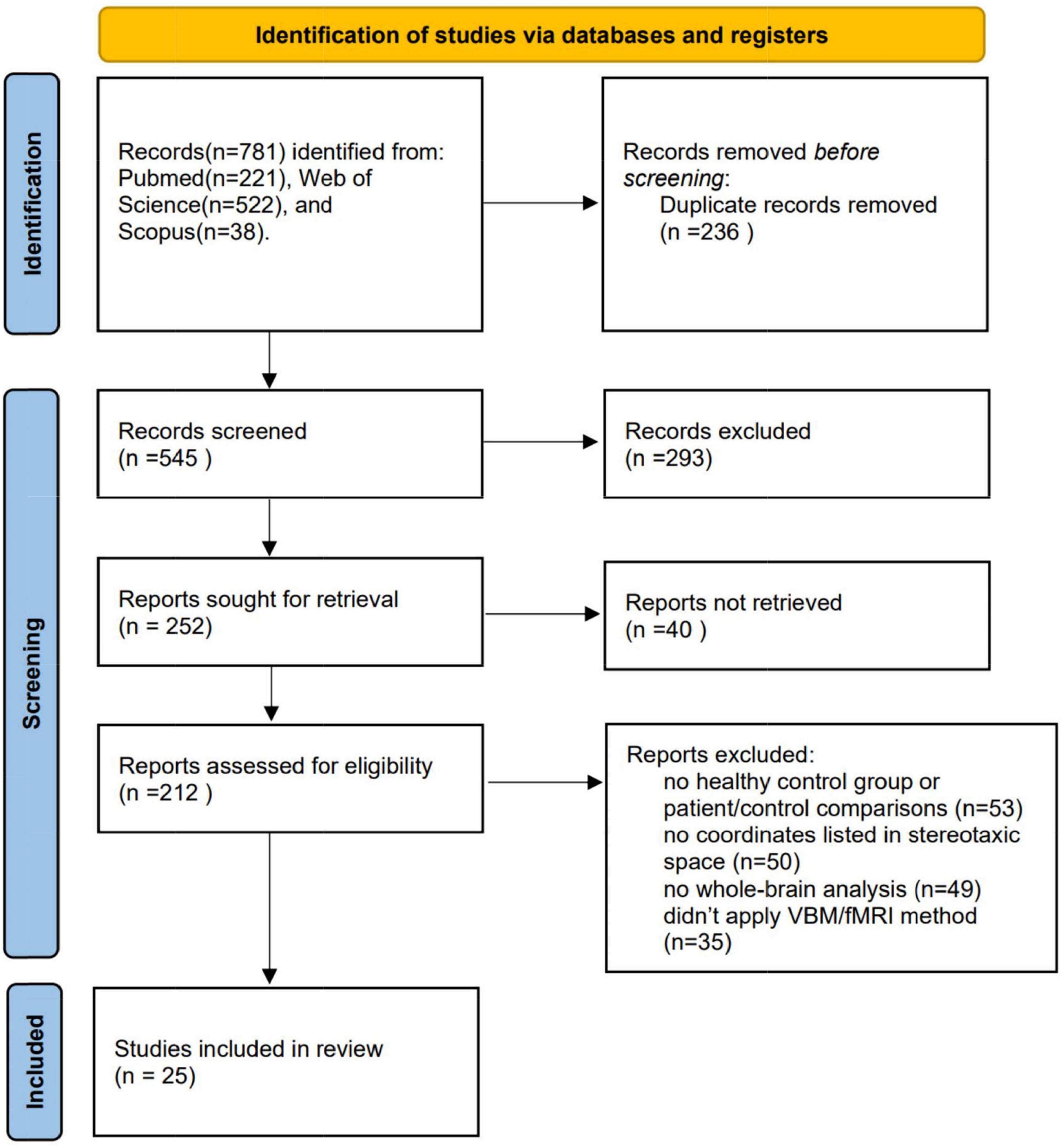
Figure 1. Procedure for including eligible studies in the meta-analysis. VBM, voxel- based morphometry; fMRI, functional magnetic resonance imaging.
The 11 studies of VBM had an average NOS score of 6.36, and the 14 studies of fMRI had an average NOS score of 6.36. They were all of high quality (NOS score ≥ 6) (36) (Supplementary Table 1).
Combining all fMRI studies, CDs showed activation in the right inferior temporal gyrus (ITG.R), right insula (INS.R), right parahippocampal gyrus (HIP.R), and right temporal pole: superior temporal gyrus (TPOsup.R) compared to HCS. However, CDS activation was lower in the right inferior parietal gyrus (IPL.R), left inferior parietal gyrus (IPL.L), left middle occipital gyrus (MOG.L), and right middle frontal gyrus (MFG.L) (Figure 2 and Table 2).
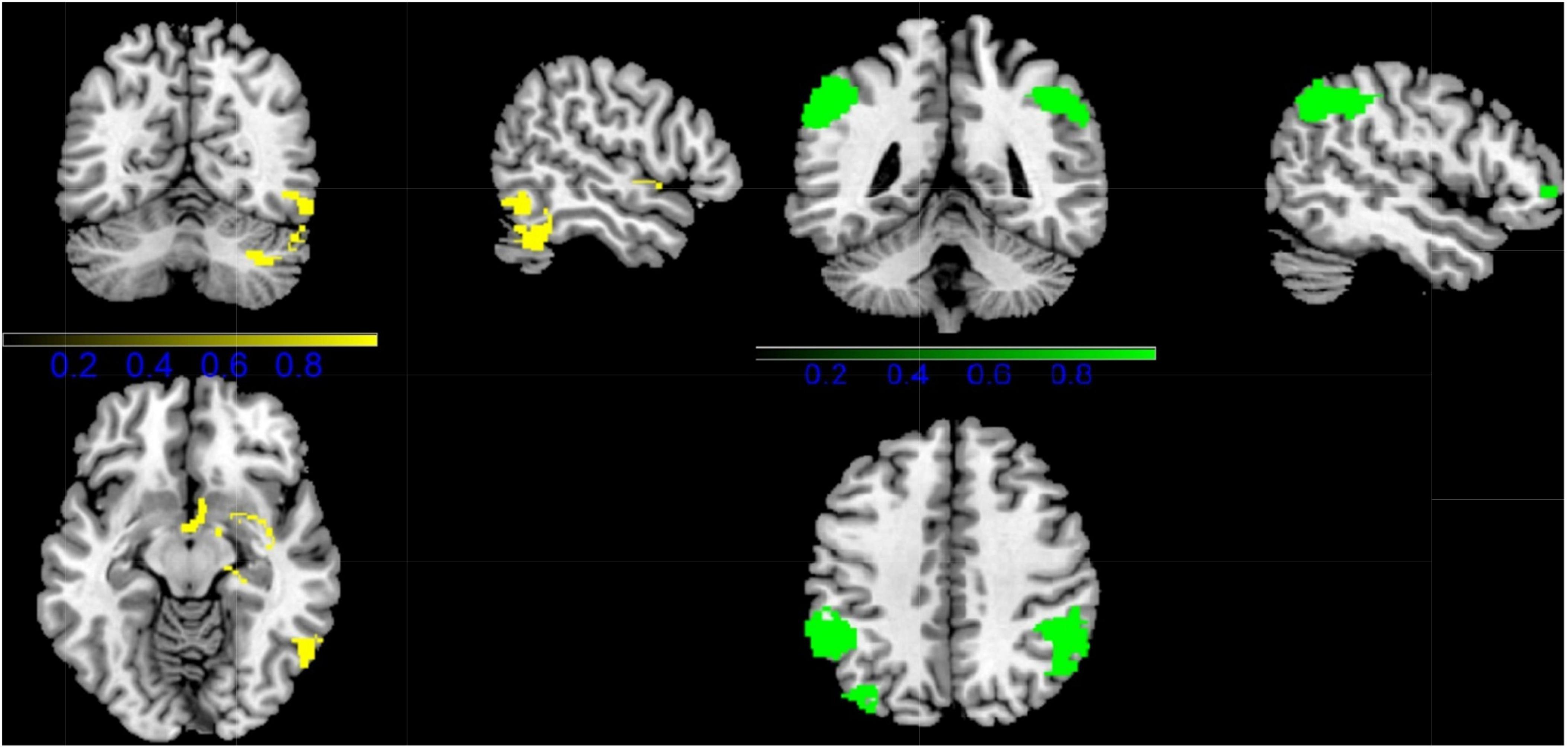
Figure 2. Results of Functional Magnetic Resonance Imaging (fMRI) for CD. (Yellow, CD increased; Green: CD decreased).
Regarding the VBM study, CD showed significantly lower gray-matter volume in right temporal pole: superior temporal gyrus (TPOsup.R), right insula (INS.R), and right postcentral gyrus (POCG.R), compared with HC. While people with CD showed increased GM volume in right inferior parietal gyri (ILF.R) (Figure 3 and Table 2).
In patients with CD, the Right inferior parietal (excluding supramarginal and angular) gyri was increased in volume and decreased in function connection relative to controls (MNI coordinates, 48, –56, 50, 265 voxels) (Figure 4 and Table 2).
To assess the reliability of the findings, Jackknife sensitivity analysis was performed. In major fMRI meta-analyses these results were highly reproducible, as at least 11 of the 14 combinations could be identified. For the VBM meta-analysis, changes in the right insula were preserved in all study combinations. Furthermore, the other results were remarkably robust as at least 8 of the 11 combinations were identifiable.
Egger’s tests were performed to examine potential publication bias. The results of the Egger tests were non-significant (P > 0.05 for all comparisons, Bonferroni corrected), suggesting that there was no publication bias.
The purpose of our meta-analysis is to explore the changes of brain GM and functional abnormalities between CD and HC. The main fMRI Meta-analysis showed that CD was related to the overactivation of ITG.R, INS.R, HIP.R, and TPOsup.R, but to the insufficient activation of IPL, MOG.L, and MFG.L. In addition, VBM meta-analysis showed that the gray matter volume of CD in TPOsup.R, INS.R, and PoCG.R decreased, while that of IPL.R increased. Through multimodal analysis, the gray matter volume of IPL.R was abnormally increased, but the function was not activated. The whole brain jack-knife sensitivity analysis of the system provides a reliable result.
According to our results, neural changes in the right insula were present in both the main VBM and fMRI meta-analyses. Previous studies have found that the gray matter value of the insular cortex is lower in drug-dependent patients (37–40). The insula is connected to several regions of the brain, such as the orbitofrontal cortex, frontal opercular structures, lateral premotor cortex, somatosensory area, parietal lobe, superior temporal sulcus, cingulate gyrus, amygdala, peri-olfactory, and entorhinal cortex. The insula projects and provides cortical input to components of the ventral striatum, a structure that plays a major role in addiction. This structure of the ventral striatum plays a role in the formation of stimulus-drug associations (41). Abnormal volumetric development of insular gray matter may lead to abnormal input to the ventral striatum leading to facilitation of addictive behaviors. Addiction, on the other hand, results from an imbalance between the unconscious impulse system and the conditioning system of conscious and cognitive control (42). When the balance is disrupted, the inhibitory function does not work, which results in people being unable to help with medication (43). Insula, the structural basis of the reflex system, which are responsible for impulsive control, decision making, and emotional regulation (44). GM atrophy of the insula was found in our meta-analysis, which may suggest that this abnormality contributes to poor impulse control, manifested by constant drug seeking and repetitive behaviors. In a recent review, Naqvi and Bechara (45) reviewed the existing literature on the role of the insula in drug addiction. Within their theoretical framework, it has been suggested that the insula modulates the reciprocal sensory effects of drugs, which then become available for consciousness, memory, and executive function, supporting a central role for this neural structure in addiction. Enhanced function of the insula in CD may indicate that they are accustomed to cocaine stimuli and insensitive to other conventional stimuli. Taken together, fMRI and VBM may reflect different aspects of neural alterations, with evidence focusing on the important role of the insula in CD.
The right middle frontal gyrus is less active in CD compared to HC, and the dmPFC in the supplementary motor area plays a key role in performance monitoring and cognitive control (46) and is associated with impaired inhibitory control in addicts (47). Our results show that attenuated PFC responses to stimuli fit the Impaired Response Inhibition and Significant Attribution (IRSA) model (48, 49). The IRSA model argues that drug dependence is mediated by dysfunction of the ACC and PFC, which involves a marked response to drug-related reward and is attributed to a high response to drug-related reward, and a markedly high response to non-drug-related stimuli Impairment (26), therefore, inactivation of the PFC in cocaine addicts may indicate that they are accustomed to the associated rewards brought on by cocaine and are insensitive to other non-drug rewards. Clinically, anhedonia is an important factor in cocaine relapse (50). Recent studies have demonstrated that Repetitive transcranial magnetic stimulation (rTMS) appears to have a unique therapeutic application for directly targeting and remodeling dysfunction in brain circuits altered by chronic cocaine exposure (51). Moreover, continuous rTMS regimen is safe and feasible in CD patients as a potential treatment (52). Our results may provide research directions for this method of brain stimulation.
According to the primary meta-analysis and multimodal analysis, the GM of the right inferior parietal gyrus increased but the activity decreased, and the activity of the left inferior parietal gyrus decreased. The bilateral inferior parietal lobules (IPL) mainly include the supramarginal and angular gyri. The inferior parietal lobule is a core node of the default mode network, a group of brain regions preferentially involved in mind formation (53). These regions were strongly deactivated in the goal orientation task compared to resting or passive baselines. It is thought to be involved in internal mental states that become salient when people do not engage in external interactions (53) and it forms the most consistent rest-state network (54). Inactivation of the inferior parietal cortex, including bilateral AGs, is highly reliable (55) and consistent across tasks, paradigms, subjects and studies (56, 57). Disruptions in connections between the DMN and cortical areas involved in executive function, memory and mood may be critical to drug use, research has shown (58). These findings suggest that CDs may lead to poor impulse control by reducing neural activity in the IPL and predispose people to some degree of addiction. As for the supramarginal gyrus, this may be an area involved in the visual memory system. In particular, these regions have been shown to be involved in the processing of visually presented stimuli and the extraction of spatial locations (59, 60). Evidence from these studies suggests that the right inferior parietal lobe, particularly the supramarginal gyrus, appears to play a role in processing visually presented information. Importantly, visual memory ability has been shown to be associated with treatment engagement as well as substance relapse (61). Whether the increase in GM in the inferior parietal gyrus of CD has an effect on visual memory ability can be a direction for future research.
In addition, previous studies have shown that some individuals with personality disorders, mainly those with social cognitive deficits, also have reduced temporal pole volume, such as those with cocaine-dependent personality disorders (62). And the temporal pole with right dominance is involved in various functions of social cognitive network, mainly emotion processing, empathy, and insight, which is consistent with our results. Furthermore, the superior temporal gyrus is implicated in impulsivity and craving (63) and is involved in the regulatory control of reward-seeking behavior (64), an important component of the addiction process. However, the exact mechanism of temporal pole abnormalities in CD-related diseases remains to be fully explored, and more attention should be paid in future studies.
This meta-analysis has certain limitations. First, a method based on peak coordinates was used in this study, rather than raw statistical brain maps, so it may be difficult to detect some results with small or moderate effects. Second, the heterogeneity of VBM research methods cannot be avoided, such as differences in MRI machines, slice thicknesses, preprocessing schemes (traditional or optimized), smoothing kernel sizes, and statistical thresholds may be responsible for inconsistent results. Third, this study focuses on the findings of task-based fMRI and VBM. Although studies using other techniques (e.g., diffusion tensor imaging, resting-state fMRI) may also provide valuable information on the neural mechanisms of CD, due to insufficient numbers of studies or limitations of coordinate-based methods, these Meta-analyses of modalities; therefore, future systematic reviews and meta-analyses of other modalities of CD are encouraged. Longitudinal studies could be conducted in the future to explore whether these brain regions may be potential neural targets for the treatment of cocaine addiction.
In conclusion, possibly due to the limitations of the current meta-analysis, the results of this study showed that cocaine addicts had increased GM volume in the right inferior parietal gyrus and significantly decreased GM volume in the right superior temporal gyrus and right insula. There was increased activation in the right insula and right inferior temporal gyrus, and decreased activation in the bilateral inferior parietal gyrus and right middle frontal gyrus. The main evidence from brain function and gray matter volume comes together, suggesting that CD is associated with core neural changes in the right inferior parietal gyrus. In the future, we hope to further study the relationship between the right insula and the right inferior parietal gyrus and cocaine dependence. Future research using multimodal, multidomain research will further demonstrate and complement our foundation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JD and YZ designed the experiment. JD, QT, and ZY performed the experiment. XN, XG, MZ, ZY, MY, WW, JC, SH, and YZ modified the experiment and manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.927075/full#supplementary-material
1. Drake LR, Scott PJH. DARK classics in chemical neuroscience: cocaine. ACS Chem Neurosci. (2018) 9:2358–72. doi: 10.1021/acschemneuro.8b00117
2. Karila L, Petit A, Lowenstein W, Reynaud M. Diagnosis and consequences of cocaine addiction. Curr Med Chem. (2012) 19:5612–8. doi: 10.2174/092986712803988839
3. United Nations Office on Drugs and Crime.World Drug Report 2015. Vienna: United Nations Office on Drugs and Crime (2022).
4. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. (2016) 3:760–73. doi: 10.1016/S2215-0366(16)00104-8
5. Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat. (2001) 21:111–7. doi: 10.1016/s0740-5472(01)00192-1
6. Kishi T, Matsuda Y, Iwata N, Correll CU. Antipsychotics for cocaine or psychostimulant dependence: systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. (2013) 74:e1169–80. doi: 10.4088/JCP.13r08525
7. Riezzo I, Fiore C, De Carlo D, Pascale N, Neri M, Turillazzi E, et al. Side effects of cocaine abuse: multiorgan toxicity and pathological consequences. Curr Med Chem. (2012) 19:5624–46. doi: 10.2174/092986712803988893
8. Callaghan RC, Cunningham JK, Allebeck P, Arenovich T, Sajeev G, Remington G, et al. Methamphetamine use and schizophrenia: a population-based cohort study in California. Am J Psychiatry. (2012) 169:389–96. doi: 10.1176/appi.ajp.2011.10070937
9. Roncero C, Daigre C, Grau-López L, Barral C, Pérez-Pazos J, Martínez-Luna N, et al. An international perspective and review of cocaine-induced psychosis: a call to action. Subst Abus. (2014) 35:321–7. doi: 10.1080/08897077.2014.933726
10. Martinotti G, De Risio L, Vannini C, Schifano F, Pettorruso M, Di Giannantonio M. Substance-related exogenous psychosis: a postmodern syndrome. CNS Spectr. (2021) 26:84–91. doi: 10.1017/S1092852920001479
11. Lappin JM, Darke S, Farrell M. Stroke and methamphetamine use in young adults: a review. J Neurol Neurosurg Psychiatry. (2017) 88:1079–91. doi: 10.1136/jnnp-2017-316071
12. Sordo L, Indave BI, Barrio G, Degenhardt L, de la Fuente L, Bravo MJ. Cocaine use and risk of stroke: a systematic review. Drug Alcohol Depend. (2014) 142:1–13. doi: 10.1016/j.drugalcdep.2014.06.041
13. Muntan CD, Tuckler V. Cerebrovascular accident following MDMA ingestion. J Med Toxicol. (2006) 2:16–8. doi: 10.1007/BF03161008
14. Ryan SA. Cocaine use in adolescents and young adults. Pediatr Clin North Am. (2019) 66:1135–47. doi: 10.1016/j.pcl.2019.08.014
15. Moura HF, Benzano D, Pechansky F, Kessler FH. Crack/cocaine users show more family problems than other substance users. Clinics (Sao Paulo). (2014) 69:497–9. doi: 10.6061/clinics/2014(07)10
16. American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, TX: American Psychiatric Association (2013).
17. Honey CJ, Kötter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA. (2007) 104:10240–5. doi: 10.1073/pnas.0701519104
18. Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, et al. Reduced striatal volume in cocaine-dependent patients. NeuroImage. (2011) 56:1021–6. doi: 10.1016/j.neuroimage.2011.02.035
19. Gardini S, Venneri A. Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. Brain Res Bull. (2012) 87:205–11. doi: 10.1016/j.brainresbull.2011.11.021
20. Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl). (2011) 218:681–92. doi: 10.1007/s00213-011-2360-y
21. Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M, et al. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. (2012) 125:208–14. doi: 10.1016/j.drugalcdep.2012.02.012
22. Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, et al. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict Biol. (2010) 15:504–16. doi: 10.1111/j.1369-1600.2010.00230.x
23. Kaag AM, Reneman L, Homberg J, van den Brink W, van Wingen GA. Enhanced amygdala-striatal functional connectivity during the processing of cocaine cues in male cocaine users with a history of childhood trauma. Front Psychiatry. (2018) 9:70. doi: 10.3389/fpsyt.2018.00070
24. Tobler PN, Preller KH, Campbell-Meiklejohn DK, Kirschner M, Kraehenmann R, Stämpfli P, et al. Shared neural basis of social and non-social reward deficits in chronic cocaine users. Soc Cogn Affect Neurosci. (2016) 11:1017–25. doi: 10.1093/scan/nsw030
25. Verdejo-Garcia A, Clark L, Verdejo-Román J, Albein-Urios N, Martinez-Gonzalez JM, Gutierrez B, et al. Neural substrates of cognitive flexibility in cocaine and gambling addictions. Br J Psychiatry. (2015) 207:158–64. doi: 10.1192/bjp.bp.114.152223
26. Canterberry M, Peltier MR, Brady KT, Hanlon CA. Attenuated neural response to emotional cues in cocaine-dependence: a preliminary analysis of gender differences. Am J Drug Alcohol Abuse. (2016) 42:577–86. doi: 10.1080/00952990.2016.1192183
27. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
28. Gu WJ, Wang F, Tang L, Liu JC. Single-dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials and observational studies. Chest. (2015) 147:335–46. doi: 10.1378/chest.14-1012
29. Seed-Based D Mapping.Neuroimaging Software Library Including Meta-Analytic Methods for fMRI, VBM, DTI and PET and Other Tools. (2022). Available online at: https://www.sdmproject.com (accessed January 15, 2022).
30. SDM-PSI Tutorial.SDM-PSI Tutorial, Version Jan 2019. (2019). Available online at: https://www.sdmproject.com/software/tutorial.pdf (accessed January 15, 2022).
31. Yao YW, Liu L, Ma SS, Shi XH, Zhou N, Zhang JT, et al. Functional and structural neural alterations in Internet gaming disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2017) 83:313–24. doi: 10.1016/j.neubiorev.2017.10.029
32. Seed-based d Mapping.Statistics Converter. (2022). Available online at: https://www.sdmproject.com/utilities/?show=Statistics (accessed January 16, 2022).
33. Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. (2009) 195:393–402. doi: 10.1192/bjp.bp.108.055046
34. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. (1997) 315:1533–7. doi: 10.1136/bmj.315.7121.1533
35. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. (2001) 54:1046–55. doi: 10.1016/s0895-4356(01)00377-8
36. Moldovan D, Racasan S, Kacso IM, Rusu C, Potra A, Bondor C, et al. Survival after parathyroidectomy in chronic hemodialysis patients with severe secondary hyperparathyroidism. Int Urol Nephrol. (2015) 47:1871–7. doi: 10.1007/s11255-015-1106-x
37. Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. (2002) 51:134–42. doi: 10.1016/s0006-3223(01)01269-0
38. Lyoo IK, Pollack MH, Silveri MM, Ahn KH, Diaz CI, Hwang J, et al. Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology (Berl). (2006) 184:139–44. doi: 10.1007/s00213-005-0198-x
39. Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry. (2007) 78:610–4. doi: 10.1136/jnnp.2006.095869
40. Verdejo-García A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. (2009) 56:48–62. doi: 10.1016/j.neuropharm.2008.07.035
41. Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. (2002) 137:75–114. doi: 10.1016/s0166-4328(02)00286-3
42. Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. (2013) 38:2363–72. doi: 10.1038/npp.2013.134
43. David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and non-smokers: a functional magnetic resonance imaging study. Biol Psychiatry. (2005) 58:488–94. doi: 10.1016/j.biopsych.2005.04.028
44. Jastreboff AM, Sinha R, Lacadie CM, Balodis IM, Sherwin R, Potenza MN. Blunted striatal responses to favorite-food cues in smokers. Drug Alcohol Depend. (2015) 146:103–6. doi: 10.1016/j.drugalcdep.2014.09.006
45. Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. (2010) 214:435–50. doi: 10.1007/s00429-010-0268-7
46. Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. (2004) 306:443–7. doi: 10.1126/science.1100301
47. Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. (2011) 12:652–69. doi: 10.1038/nrn3119
48. Hall SM, Muñoz RF, Reus VI, Sees KL. Nicotine, negative affect, and depression. J Consult Clin Psychol. (1993) 61:761–7. doi: 10.1037//0022-006x.61.5.761
49. Keltner D, Gross JJ. Functional accounts of emotions. Cogn Emot. (1999) 13:467–80. doi: 10.1080/026999399379140
50. Carelli RM, West EA. When a good taste turns bad: neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine. Neuropharmacology. (2014) 76:360–9. doi: 10.1016/j.neuropharm.2013.04.025
51. Pettorruso M, Spagnolo PA, Leggio L, Janiri L, Di Giannantonio M, Gallimberti L, et al. Repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex may improve symptoms of anhedonia in individuals with cocaine use disorder: a pilot study. Brain Stimul. (2018) 11:1195–7. doi: 10.1016/j.brs.2018.06.001
52. Martinotti G, Pettorruso M, Montemitro C, Spagnolo PA, Acuti Martellucci C, Di Carlo F, et al. Repetitive transcranial magnetic stimulation in treatment-seeking subjects with cocaine use disorder: a randomized, double-blind, sham-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. (2022) 116:110513. doi: 10.1016/j.pnpbp.2022.110513
53. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. (2008) 1124:1–38. doi: 10.1196/annals.1440.011
54. Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. (2009) 106:13040–5. doi: 10.1073/pnas.0905267106
55. Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. (2009) 19:2209–29. doi: 10.1093/cercor/bhn256
56. Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. (2009) 29:14496–505. doi: 10.1523/JNEUROSCI.4004-09.2009
57. Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. (2009) 21:489–510. doi: 10.1162/jocn.2008.21029
58. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. NeuroImage. (2019) 200:313–31. doi: 10.1016/j.neuroimage.2019.06.036
59. Melrose RJ, Harwood D, Khoo T, Mandelkern M, Sultzer DL. Association between cerebral metabolism and rey-osterrieth complex figure test performance in Alzheimer’s disease. J Clin Exp Neuropsychol. (2013) 35:246–58. doi: 10.1080/13803395.2012.763113
60. Moscovitch C, Kapur S, Köhler S, Houle S. Distinct neural correlates of visual long-term memory for spatial location and object identity: a positron emission tomography study in humans. Proc Natl Acad Sci USA. (1995) 92:3721–5. doi: 10.1073/pnas.92.9.3721
61. Desfosses M, Meadows H, Jackson M, Crowe SF. The relationship between neuropsychological functioning and mental health outcomes of chronic alcohol users involved in counselling: prediction of treatment outcome. Aust Psychol. (2014) 49:287–96. doi: 10.1111/ap.12071
62. Albein-Urios N, Martinez-Gonzalez JM, Lozano Ó, Moreno-López L, Soriano-Mas C, Verdejo-Garcia A. Negative urgency, disinhibition and reduced temporal pole gray matter characterize the comorbidity of cocaine dependence and personality disorders. Drug Alcohol Depend. (2013) 132:231–7. doi: 10.1016/j.drugalcdep.2013.02.008
63. Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, et al. Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res. (2009) 43:739–47. doi: 10.1016/j.jpsychires.2008.09.012
64. Chiamulera C. Cue reactivity in nicotine and tobacco dependence: a “multiple-action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli. Brain Res Brain Res Rev. (2005) 48:74–97. doi: 10.1016/j.brainresrev.2004.08.005
65. Meade CS, Bell RP, Towe SL, Hall SA. Cocaine-related alterations in fronto-parietal gray matter volume correlate with trait and behavioral impulsivity. Drug Alcohol Depend. (2020) 206:107757. doi: 10.1016/j.drugalcdep.2019.107757
66. Rabin RA, Parvaz MA, Alia-Klein N, Goldstein RZ. Emotion recognition in individuals with cocaine use disorder: the role of abstinence length and the social brain network. Psychopharmacology (Berl). (2021) 239:1019–33. doi: 10.1007/s00213-021-05868-x
67. Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, et al. Gene × disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry. (2011) 68:283–94. doi: 10.1001/archgenpsychiatry.2011.10
68. Crunelle CL, Kaag AM, van Wingen G, van den Munkhof HE, Homberg JR, Reneman L, et al. Reduced frontal brain volume in non-treatment-seeking cocaine-dependent individuals: exploring the role of impulsivity, depression, and smoking. Front Hum Neurosci. (2014) 8:7. doi: 10.3389/fnhum.2014.00007
69. Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CR. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend. (2014) 134:51–62. doi: 10.1016/j.drugalcdep.2013.09.004
70. Matuskey D, Bhagwagar Z, Planeta B, Pittman B, Gallezot JD, Chen J, et al. Reductions in brain 5-HT1B receptor availability in primarily cocaine-dependent humans. Biol Psychiatry. (2014) 76:816–22. doi: 10.1016/j.biopsych.2013.11.022
71. Yip SW, Worhunsky PD, Xu J, Morie KP, Constable RT, Malison RT, et al. Gray-matter relationships to diagnostic and transdiagnostic features of drug and behavioral addictions. Addict Biol. (2018) 23:394–402. doi: 10.1111/adb.12492
72. Konova AB, Moeller SJ, Tomasi D, Parvaz MA, Alia-Klein N, Volkow ND, et al. Structural and behavioral correlates of abnormal encoding of money value in the sensorimotor striatum in cocaine addiction. Eur J Neurosci. (2012) 36:2979–88. doi: 10.1111/j.1460-9568.2012.08211.x
73. Moeller SJ, Konova AB, Parvaz MA, Tomasi D, Lane RD, Fort C, et al. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry. (2014) 71:61–70.
74. Bedi G, Hao X, Van Dam NT, Cooper ZD, Rubin E, Vadhan NP, et al. Social motivational processing and interpersonal function in aging cocaine smokers. Addict Biol. (2019) 24:1044–55. doi: 10.1111/adb.12669
75. Moeller SJ, Froböse MI, Konova AB, Misyrlis M, Parvaz MA, Goldstein RZ, et al. Common and distinct neural correlates of inhibitory dysregulation: stroop fMRI study of cocaine addiction and intermittent explosive disorder. J Psychiatr Res. (2014) 58:55–62. doi: 10.1016/j.jpsychires.2014.07.016
76. Ide JS, Hu S, Zhang S, Mujica-Parodi LR, Li CR. Power spectrum scale invariance as a neural marker of cocaine misuse and altered cognitive control. NeuroImage Clin. (2016) 11:349–56. doi: 10.1016/j.nicl.2016.03.004
77. Verdejo-Garcia A, Verdejo-Román J, Albein-Urios N, Martínez-González JM, Soriano-Mas C. Brain substrates of social decision-making in dual diagnosis: cocaine dependence and personality disorders. Addict Biol. (2017) 22:457–67. doi: 10.1111/adb.12318
78. Zhang S, Zhornitsky S, Le TM, Li CR. Hypothalamic responses to cocaine and food cues in individuals with cocaine dependence. Int J Neuropsychopharmacol. (2019) 22:754–64. doi: 10.1093/ijnp/pyz044
Keywords: cocaine addiction, voxel-based morphometry, gray matter, meta-analysis, functional magnetic resonance imaging
Citation: Dang J, Tao Q, Niu X, Zhang M, Gao X, Yang Z, Yu M, Wang W, Han S, Cheng J and Zhang Y (2022) Meta-Analysis of Structural and Functional Brain Abnormalities in Cocaine Addiction. Front. Psychiatry 13:927075. doi: 10.3389/fpsyt.2022.927075
Received: 23 April 2022; Accepted: 07 June 2022;
Published: 24 June 2022.
Edited by:
Giovanni Martinotti, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Andrea Miuli, University of Studies G. d’Annunzio Chieti and Pescara, ItalyCopyright © 2022 Dang, Tao, Niu, Zhang, Gao, Yang, Yu, Wang, Han, Cheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhang, enp1emhhbmd5b25nMjAxM0AxNjMuY29t; Jingliang Cheng, ZmNjY2hlbmdqbEB6enUuZWR1LmNu; Shaoqiang Han, U2hhb3FpYW5nSGFuQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.