- 1Department of Urology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 2Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Background: The burden of depression in the elderly is increasing worldwide with global aging. However, there is still a lack of research on the relationship between depressive symptoms and the progression of renal function. Our aim is to evaluate the longitudinal association between baseline depressive symptoms and the changes in serum cystatin C levels over 10 years' follow-up period.
Methods: We used longitudinal data from the Health and Retirement Study (HRS), an existing community based nationally representative aging cohort study which enrolled individuals over age 50 in the USA. Depressive symptoms were determined using an eight-item version of the Center for Epidemiologic Studies Depression Scale (CESD) at wave 7 (2004) and wave 8 (2006). Persistent depressive symptoms were defined as both CESD scores measured at waves 7 and 8 were ≥3; episodic depressive symptoms were defined as CESD scores ≥3 at wave 7 or wave 8. A linear mixed model was used to evaluate the correlation between baseline depressive symptoms and future changes in cystatin C levels.
Results: The mean age of the 7,642 participants was 63.8 ± 10.8 years, and 60.9% were women. Among the participants, 1,240 (16.2%) had episodic depressive symptoms and 778 (10.2%) had persistent depressive symptoms. Compared with participants with no depressive symptoms at both waves, a significant increase in serum cystatin C levels was found among those with persistent depressive symptoms.
Conclusions: Our results showed that baseline persistent depressive symptoms were significantly associated with an increased rate of serum cystatin C levels. The level of serum cystatin C should be monitored in the elderly with persistent depressive symptoms.
Introduction
In worldwide, all the governments need to face the social problem of depression in older people, as it might lead to increasing burdens and costs to individuals and society. To explore the modifiable risk factors and effective prevention for depression is needed. Accumulating evidences from studies suggest that depression can regulate the response of angiogenesis and immune state, which may cause increased mortality (1). Although depression is a common health problem in the middle-aged and elderly (2, 3), it is reported that depression is also common in patients with Chronic Kidney Disease (CKD) (4).
However, in recent years, there is limited research on the progress of renal function failure and depression in the elderly. Recently, a cross-sectional study reported the association between depression and renal function measured by estimated glomerular filtration rate (eGFR) (5). In addition, the manifestations of depression in various comorbidities are obesity, diabetes, and cardiovascular diseases. It may further predict poor renal prognosis (6, 7). However, the longitudinal relationship between baseline depression or depressive symptoms and future age-related renal impairment (from mild to moderate) is unclear.
Although serum or plasma creatinine have become the most commonly used serum marker of renal function, these are often misleading in the elderly, because muscle mass declines with aging, creatinine metabolism could be also changed (8). Cystatin C is a member of the type 2 cystatin superfamily of cysteine protease inhibitors. It is a small protein produced by almost all human cells and can be obtained in all body fluids (9). With freely filtered through the glomerulus, then reabsorbed and completely catabolized without secretion or subsequent reabsorption into the circulation (10), the characteristic is less dependent on muscle mass than creatinine, so that Cystatin C may be more accurate in kidney function's evaluation (9, 11).
The objectives of the current analysis were 1) to investigate whether depressive symptoms are associated with the levels of serum cystatin C at the baseline; and 2) to develop a better understanding of the potential relevance between baseline depressive symptoms and future changes of cystatin C levels in the elderly.
Methods
Study Population
We used longitudinal data from the Health and Retirement Study (HRS), an existing community based nationally representative aging cohort study which is freely available to all researchers. Since 1992, HRS has randomly recruited individuals over the age of 50 in the United States through a multistage regional probability sampling design (12). The cohort was in accordance with the Helsinki Declaration and approved by the Institutional Review Committee for Health Sciences and behavioral sciences at the University of Michigan (HUM00061128). Written informed consent was obtained from all participants.
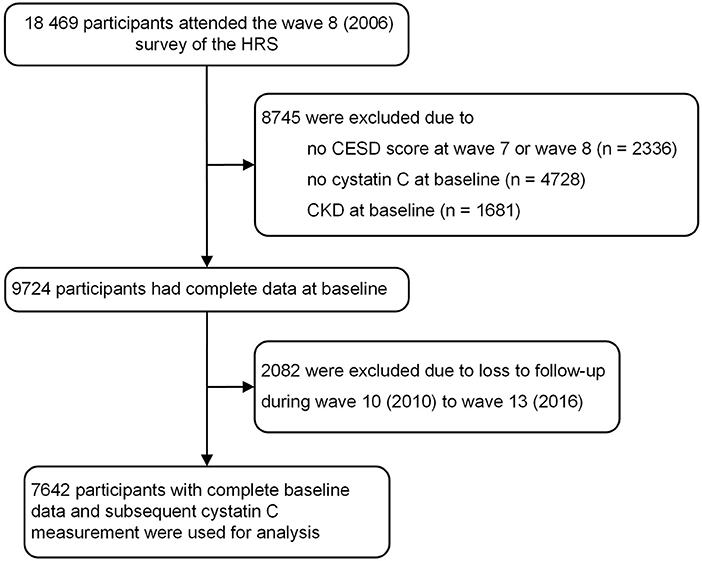
Figure 1 shows the flow chart of the current participant analysis. In our study, wave 8 (2006) in HRS was regarded as the baseline. A total of 18,469 participants participated in the wave 8 survey of HRS. The exclusion standard included: (1) missing completion at the wave 7 or wave 8 of the Center for Epidemiologic Studies Depression Scale (CESD) scores, (2) no data of serum cystatin C at the baseline, (3) history of CKD at baseline, or (4) additional 2,082 individuals without follow-up during wave 10 to wave 13.
Depressive Symptoms
Depressive symptoms were measured by the eight-item version of the Center for Epidemiological Studies Depression scale (CESD-8). This is a widely used self-report measure of depressive symptoms, which has been used in many population-based studies to identify people at risk of depression (13). Participants were asked to think about the past time and feelings, and then distinguish whether the following statements are more similar to them: you feel depressed; You find everything difficult; Your sleep is disturbed; You are happy; You feel lonely; You enjoy life; You feel sad; You can't go. To select Yes or No. The CESD version has internal consistency and factor structure, which is equivalent to the longer version of the scale (14). Although there were scores ≥4, according to the relevant survey, people were considered to have depressive symptoms (15, 16). In our study, depressive symptoms were defined as three or more of eight, which is a threshold used to indicate the clinical diagnosis of depression (17). People with depressive symptoms at wave 7 or wave 8 were classified as having episodic depressive symptoms, and those with depressive symptoms in both waves were classified as persistent depressive symptoms.
Measurement of Serum Cystatin C
In the wave 8 (2006) survey of HRS, half of the participants were randomly preselected for blood collection, wave 9 (2008) included the other half. So that both wave surveys decided the baseline serum cystatin C data. Blood specimens were collected by dried blood spot. Serum cystatin C was detected in these two waves using a BNII nephelometer (Siemens, Inc., Deerfield, IL) at the University of Vermont. The equivalent value of serum cystatin C in the national health and Nutrition Examination Survey recommended by HRS researchers was used in our analysis.
Definition of CKD and All-Cause Death
Renal function was defined by using the CKD Epidemiology Collaboration (CKD-EPI) equation, the new CKD-EPI equation was first published in 2012 with cystatin C alone or combined with creatinine (10). The most basic application of cystatin C is for GFR estimation. The 2012 Kidney Disease Improving Global Outcomes (KDIGO) recommended that cystatin C be measured in adults with creatinine eGFR from 45–60 mL/min/1.73m2 but lack of other markers of renal damage to confirm the diagnosis of CKD (18). The mortality data were taken from the tracker file with verification from family members.
Assessment of Covariates
Covariates included clinical and demographic characteristics. Clinical characteristics included hypertension and diabetes. As for demographic characteristics, sex, race, age (years), and body mass index (kg/m2) were selected for our analysis.
Hypertension was identified as a patient's systolic blood pressure ≥ 140 mmHg and / or diastolic blood pressure ≥ 90 mmHg, or if the participant was currently using relative antihypertensive drugs. Body mass index was defined as weight (kg)/height2 (m2). The definition for Diabetes as hemoglobin A1c >6.5%, fasting blood glucose >7 mmol/L, or currently used for anti-diabetic treatment (16).
Statistical Analysis
Characteristics of the overall sample were described using means and standard deviations (SD) or median (quartile range) for continuous data, and n (%) for categorical data.
Linear regression models were used to evaluate the cross-sectional associations between baseline status of depressive symptoms and serum cystatin C levels. Then, we used linear mixed model to perform longitudinal associations between sum of CES-D scores and the changes of serum cystatin C levels. We also ran longitudinal analyses to explore the association between baseline status of depressive symptoms and future changes of serum cystatin C levels, using participants without depressive symptoms as the reference. Linear mixed models use all available data during follow-up, considering that repeated measurements of the same participant are interrelated, and can deal with missing data (16). Cox regression models were used to analyze the longitudinal associations of baseline persistent depressive symptoms with incident CKD and all-cause mortality. Model 1 was adjusted for age and gender, and Model 2 was further adjusted for race, body mass index, hypertension, and diabetes.
All analyses were performed by using SAS (version 9.4; SAS Institute Inc). All tests were bilateral, the value is 0.05 as the threshold of statistical significance.
Results
Participant Selection and Characteristics
A total of 18,469 participants were included. Detailed process of participant selection was shown in Figure 1. Among these, 7,642 participants with complete baseline data and subsequent cystatin C measurement were used for our analysis. 778 participants (10.2%) were classified as experiencing persistent depressive symptoms, the other 1,240 participants (16.2%) were classified as experiencing episodic depressive symptoms.
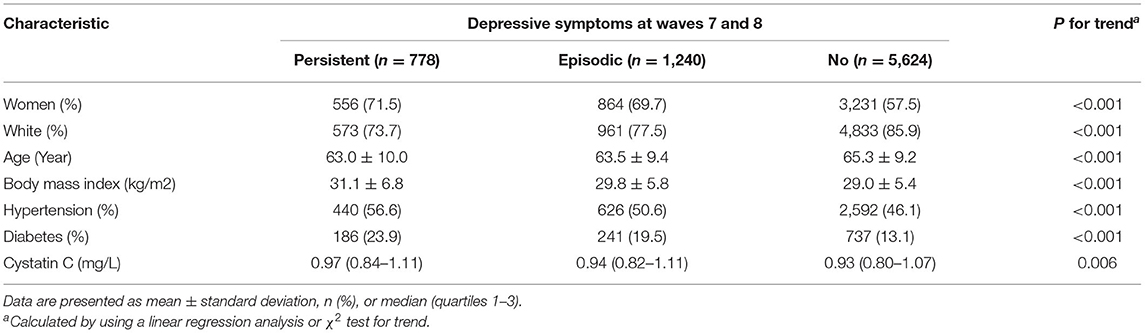
Table 1 shows the baseline characteristics of participants according to the baseline status of depressive symptoms. The mean age of included participants for the HRS was 63.8 ± 10.8 years. In generally speaking, participants who reported a longer duration of depressive symptoms were significantly less well characterized than those who did not. Study participants reporting longer duration had higher body mass index, were more likely to be female, and had a higher prevalence of hypertension and diabetes mellitus.
Cross-Sectional Association Between Depressive Symptoms and Serum Cystatin C
As seen in Figure 2, solid lines represent mean differences in serum cystatin C (mg/L) after adjusting for age, sex, race, body mass index, hypertension, and diabetes. The shadows represent the 95% confidence intervals (CI). Compared with participants with no depressive symptoms at both waves, a significant increase in serum cystatin C levels was found among those with persistent depressive symptoms.
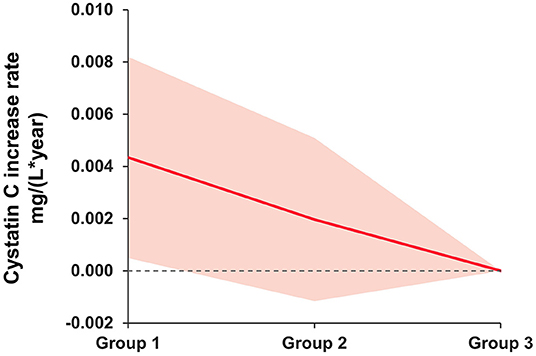
Figure 2. Mean difference rate of change in serum cystatin C (mg/L) between baseline depressive symptom groups. Group 1 as persistent depressive symptoms. Group 2 as episodic depressive symptoms. Group 3 as no depressive symptoms. The group 3 was the reference group. Solid lines represent mean differences in serum cystatin C (mg/L) after adjusting for age, sex, race, body mass index, hypertension, and diabetes. The shadows represent the 95% CIs. The detailed results are presented in Table 3.
Longitudinal Association Between Depressive Symptoms and Serum Cystatin C
As shown in Table 2, the longitudinal correlation between the sum of CESD scores and the rate of change in serum cystatin C. After multivariable adjustment, an increase of one unit in the total score of CESD was associated with a faster increase in serum cystatin C levels in patients with persistent and episodic depressive symptoms (0.004 mg/L, 95% CI: 0.001 to 0.008, P = 0.025; 0.002 mg/L, 95% CI: −0.001 to 0.005, P = 0.288), respectively, compared with participants without depressive symptoms. Besides, our analyses found that per 3-point increase in CESD scores was associated with a higher rate of change in serum cystatin C levels (0.003 mg/L, 95% CI: 0.001 to 0.005, P = 0.003), after multivariable adjustment.
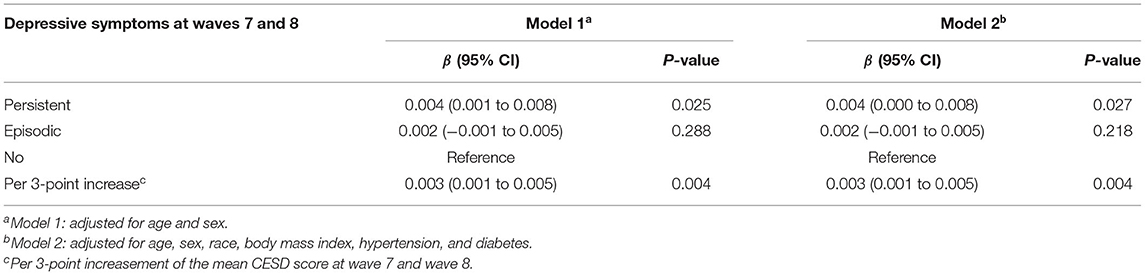
Table 2. Association between baseline depressive symptoms and rate of change in serum cystatin C (mg/L): longitudinal analyses using linear mixed models.
Longitudinal Associations of Depressive Symptoms With Incident CKD and All-Cause Death
As shown in Table 3, the risks of CKD and all-cause death in participants with persistent depressive were significantly or marginally significantly higher, compared with those without depressive symptoms. Similarly, CESD scores, as a continuous variable, was always significantly correlated with new onset CKD and all-cause death after multivariate adjustment.
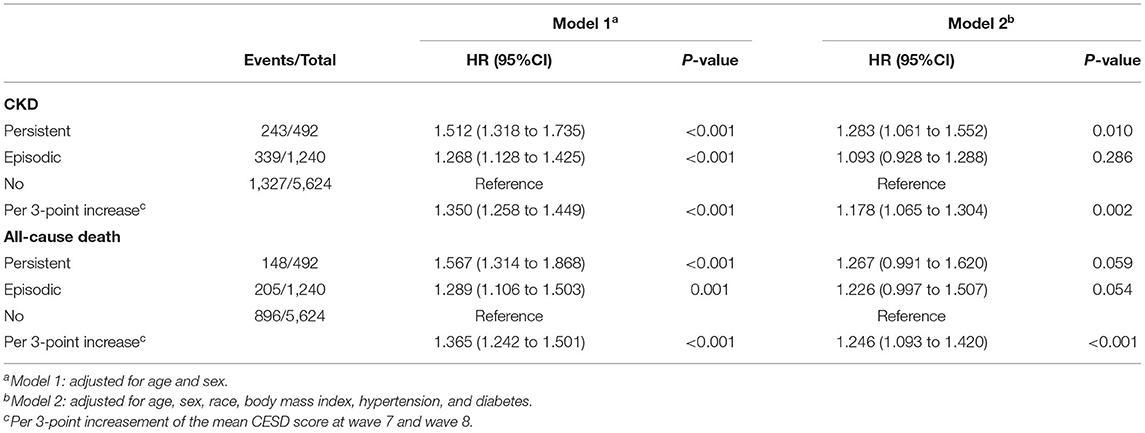
Table 3. Association between baseline depressive symptoms and risk of incident CKD and all-cause mortality.
Discussion
This ongoing cohort study of an elderly American populations investigate the temporal association between episodic or persistent depressive symptoms and the level of cystatin C in a large general population based on previous assessments of depressive symptoms. After extensive adjustment for covariates, persistent depressive symptoms were significantly associated with the change of kidney dysfunction, incident CKD, and all-cause mortality. These associations were not related to gender, age, race, body mass index, hypertension, and diabetes.
Emerging evidences suggest that depression and CKD may both affect most of the world's population, depression is more common in CKD patients than without CKD (19). In general, depressive symptoms can cause active inflammation and impaired immune response which may lead to poor clinical outcomes (20). Even if all patients with depression receive the best treatment of evidence-based intervention, residual symptoms and dysfunction are still common (21). Therefore, some previous studies have evaluated relationship between depressive symptoms and rapid eGFR decrease or progression to renal failure (22–26). Considering the adverse clinical results caused by depressive symptoms, the high prevalence of these symptoms in elderly maintained the importance of our study.
There are many mechanisms for the relationship between depressive symptoms and serum cystatin C. Although etiology and pathogenesis of depression are not fully understood, there are consistent and strong empirical evidences that neuronal injury and immune inflammation are important factors related to depression (27–31). Cystatin C is an important endogenous inhibitor of cysteine protease activity (32), which is also closely related to the nerve injury and immune inflammation (33, 34). Another possible mechanism is that cystatin C is close to oxidative stress, which can play a significant role in the process of depression (35). Last but not least, apoptosis may be an important risk factor for depression, it can also influence on the level of Cystatin C by changing the concentration of active caspinase-9 protein (36, 37). However, most investigations by now laid the emphasis on the relationship about higher cystatin C concentration appear to present with high risk of depression (38, 39). Different from previous studies, in this large cohort study of older Americans, persistent depressive symptoms were significantly associated with elevated serum cystatin C levels.
To our knowledge, this study is the largest general population-based investigation exploring the relationship between depressive symptoms and level of serum cystatin C with a long-term follow-up of 10 years. In our research, we showed that patients with higher depression events correlated with higher serum levels of cystatin C, then had an increased CES-D score at baseline. Our results imply that the prospective association between depressive symptoms and rapid kidney function decline in the general population, serum cystatin C might be a more sensitive biomarker for CKD.
What is more, we guaranteed comprehensive follow-up and strict adjudication of depression events, so that the results are reliable. Then, the determination method of serum cystatin C assay chosen for this study is both widely available and stable (coefficient of variation of precision <5%). The serum index is simple, reliable, and convenient for clinical promotion.
Several potential limitations need to be mentioned. The study sample comes from a single cohort, and the sample size may be larger. Additionally, the time courses of cystatin C levels were not evaluated and amended, especially the information for serum cystatin C level was absent in wave 7. By evaluating the time course of cystatin C, further research is necessary to consolidate the clinical significance of depressive symptoms. In addition, HRS enrolled only middle-aged and older persons, and as such, we do not know whether this association is suitable for younger person.
Several researchers have already evaluated the potential link between cystatin C levels and depression (38–40). A study which enrolled 1,440 Chinese elders (>60 years old) found a harmful relationship between high serum cystatin C levels and risk of depression (38). Another prospective cohort study of 11,847 Chinese people (>45 years old) demonstrated that high levels of serum cystatin C were associated with an increased risk of depression (40). They computed the risk of depression by using modified Poission regression models, found that the association between serum cystatin C levels and the risk of depression remained significant after adjusting for multiple covariance. Consistent with the above findings, our results show that serum cystatin C not only is associated with depression, but also severity of depressive symptoms is closely related to change of serum cystatin C. This association persists after controlling for multiple potentially confounding variables. We indicate that cystatin C should be monitored in the pathogenesis of depression among all the kidney function markers.
Conclusions
Our results showed that baseline persistent depressive symptoms were significantly correlated with an increased rate of serum cystatin C levels. The level of serum cystatin C should be monitored in the elderly with persistent depressive symptoms. Potential mechanisms of the relationship between kidney dysfunction and depression need to be further characterized. If further confirmed, our data could be the evidence for depressive symptoms' screening and effective psychosocial intervention to improve the primary prevention of CKD and all-cause death.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Committee for Health Sciences and Behavioral Sciences at the University of Michigan (HUM00061128). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
TH and LZ conceived the study and designed the statistical analyses. TH, LZ, and LW did the statistical analyses and prepared the draft of the manuscript. TH, WJ, LZ, and LW substantively revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The National Natural Science Foundation of China (81901125) have contributed to analysis and interpretation of data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the HRS research team and the field team for collecting and providing the data. The authors also thank all volunteers and staff involved in this research.
References
1. Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. (2010) 28:4094–9. doi: 10.1200/JCO.2009.26.9357
2. Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS MED. (2013) 10:e1001547. doi: 10.1371/journal.pmed.1001547
3. Lei X, Sun X, Strauss J, Zhang P, Zhao Y. Depressive symptoms and ses among the mid-aged and elderly in china: evidence from the china health and retirement longitudinal study national baseline. Soc Sci Med. (2014) 120:224–32. doi: 10.1016/j.socscimed.2014.09.028
4. Pu L, Zou Y, Wu SK, Wang F, Zhang Y, Li GS, et al. Prevalence and associated factors of depressive symptoms among chronic kidney disease patients in China: results from the Chinese cohort study of chronic kidney disease (C-stride). J Psychosom Res. (2020) 128:109869. doi: 10.1016/j.jpsychores.2019.109869
5. Wang WL, Liang S, Zhu FL, Liu JQ, Wang SY, Chen XM, et al. The prevalence of depression and the association between depression and kidney function and health-related quality of life in elderly patients with chronic kidney disease: a multicenter cross-sectional study. Clin Interv Aging. (2019) 14:905–13. doi: 10.2147/CIA.S203186
6. Novak M, Mucsi I, Rhee CM, Streja E, Lu JL, Kalantar-Zadeh K, et al. Increased risk of incident chronic kidney disease, cardiovascular disease, and mortality in patients with diabetes with comorbid depression. Diabetes Care. (2016) 39:1940–7. doi: 10.2337/dc16-0048
7. Hedayati SS, Jiang W, O'Connor CM, Kuchibhatla M, Krishnan KR, Cuffe MS, et al. The association between depression and chronic kidney disease and mortality among patients hospitalized with congestive heart failure. Am J Kidney Dis. (2004) 44:207–15. doi: 10.1053/j.ajkd.2004.04.025
8. Anand S, Johansen KL, Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci. (2014) 69:315–22. doi: 10.1093/gerona/glt109
9. Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. (2001) 37:79–83. doi: 10.1053/ajkd.2001.20628
10. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
11. Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. (2005) 352:2049–60. doi: 10.1056/NEJMoa043161
12. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. (2014) 43:576–85. doi: 10.1093/ije/dyu067
13. Zheng F, Xie W. High-sensitivity C-reactive protein and cognitive decline: the English longitudinal study of aging. Psychol Med. (2018) 48:1381–9. doi: 10.1017/S0033291717003130
14. Turvey CL, Wallace RB, Herzog R. A revised ces-D measure of depressive symptoms and a dsm-based measure of major depressive episodes in the elderly. Int Psychogeriatr. (1999) 11:139–48. doi: 10.1017/S1041610299005694
15. Li C, Miles T, Shen L, Shen Y, Liu T, Zhang M, et al. Early-life exposure to severe famine and subsequent risk of depressive symptoms in late adulthood: the China health and retirement longitudinal study. Br J Psychiatry. (2018) 213:579–86. doi: 10.1192/bjp.2018.116
16. Zheng F, Zhong B, Song X, Xie W. Persistent depressive symptoms and cognitive decline in older adults. Br J Psychiatry. (2018) 213:638–44. doi: 10.1192/bjp.2018.155
17. Schane RE, Walter LC, Dinno A, Covinsky KE, Woodruff PG. Prevalence and risk factors for depressive symptoms in persons with chronic obstructive pulmonary disease. J Gen Intern Med. (2008) 23:1757–62. doi: 10.1007/s11606-008-0749-z
18. Andrassy KM. Comments on “kdigo 2012 clinical practice guideline for the evaluation and management of chronic kidney disease”. Kidney Int. (2013) 84:622–3. doi: 10.1038/ki.2013.243
19. Lin M, Huang H, Yao J, Liang J, Li L, Lin W, et al. Association between depression and renal hyperfiltration in a general Chinese population. Kidney Blood Press Res. (2019) 44:1441–52. doi: 10.1159/000503922
20. Cukor D, Cohen SD, Peterson RA, Kimmel PL. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. (2007) 18:3042–55. doi: 10.1681/ASN.2007030345
21. Jia F, Li X, Liu F, Shi X, Liu H, Cao F. Association of renal function and depressive symptoms: evidence from the china health and retirement longitudinal study. J Psychosom Res. (2020) 137:110224. doi: 10.1016/j.jpsychores.2020.110224
22. Fischer MJ, Kimmel PL, Greene T, Gassman JJ, Wang X, Brooks DH, et al. Elevated depressive affect is associated with adverse cardiovascular outcomes among African Americans with chronic kidney disease. Kidney Int. (2011) 80:670–8. doi: 10.1038/ki.2011.153
23. Chiang HH, Guo HR, Livneh H, Lu MC, Yen ML, Tsai TY. Increased risk of progression to dialysis or death in ckd patients with depressive symptoms: a prospective 3-year follow-up cohort study. J Psychosom Res. (2015) 79:228–32. doi: 10.1016/j.jpsychores.2015.01.009
24. Tsai YC, Chiu YW, Hung CC, Hwang SJ, Tsai JC, Wang SL, et al. Association of symptoms of depression with progression of ckd. Am J Kidney Dis. (2012) 60:54–61. doi: 10.1053/j.ajkd.2012.02.325
25. Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. (2010) 303:1946–53. doi: 10.1001/jama.2010.619
26. Kop WJ, Seliger SL, Fink JC, Katz R, Odden MC, Fried LF, et al. Longitudinal association of depressive symptoms with rapid kidney function decline and adverse clinical renal disease outcomes. Clin J Am Soc Nephrol. (2011) 6:834–44. doi: 10.2215/CJN.03840510
27. Zeng Q, Huang Z, Wei L, Fang J, Lin K. Correlations of serum cystatin C level and gene polymorphism with vascular cognitive impairment after acute cerebral infarction. Neurol Sci. (2019) 40:1049–54. doi: 10.1007/s10072-019-03777-8
28. Kim JW, Szigethy EM, Melhem NM, Saghafi EM, Brent DA. Inflammatory markers and the pathogenesis of pediatric depression and suicide: a systematic review of the literature. J Clin Psychiatry. (2014) 75:1242–53. doi: 10.4088/JCP.13r08898
29. Nishuty NL, Khandoker MMH, Karmoker JR, Ferdous S, Shahriar M, Qusar M, et al. Evaluation of serum interleukin-6 and c-reactive protein levels in drug-naïve major depressive disorder patients. Cureus. (2019) 11:e3868. doi: 10.7759/cureus.3868
30. Anjum S, Qusar M, Shahriar M, Islam SMA, Bhuiyan MA, Islam MR. Altered serum interleukin-7 and interleukin-10 are associated with drug-free major depressive disorder. Ther Adv Psychopharmacol. (2020) 10:2045125320916655. doi: 10.1177/2045125320916655
31. Das R, Emon MPZ, Shahriar M, Nahar Z, Islam SMA, Bhuiyan MA, et al. Higher levels of serum il-1 beta and tnf-alpha are associated with an increased probability of major depressive disorder. Psychiatry Res. (2021) 295:113568. doi: 10.1016/j.psychres.2020.113568
32. Abrahamson M, Alvarez-Fernandez M, Nathanson CM. Cystatins. Biochem Soc Symp. (2003) 70:179–99. doi: 10.1042/bss0700179
33. Nagai A, Terashima M, Sheikh AM, Notsu Y, Shimode K, Yamaguchi S, et al. Involvement of cystatin C in pathophysiology of cns diseases. Front Biosci. (2008) 13:3470–9. doi: 10.2741/2941
34. Wilson ME, Boumaza I, Bowser R. Measurement of cystatin C functional activity in the cerebrospinal fluid of amyotrophic lateral sclerosis and control subjects. Fluids Barriers CNS. (2013) 10:15. doi: 10.1186/2045-8118-10-15
35. Islam MR, Ali S, Karmoker JR, Kadir MF, Ahmed MU, Nahar Z, et al. Evaluation of serum amino acids and non-enzymatic antioxidants in drug-naïve first-episode major depressive disorder. BMC Psychiatry. (2020) 20:333. doi: 10.1186/s12888-020-02738-2
36. Xu Y, Ding Y, Li X, Wu X. Cystatin C is a disease-associated protein subject to multiple regulation. Immunol Cell Biol. (2015) 93:442–51. doi: 10.1038/icb.2014.121
37. Le-Niculescu H, Levey DF, Ayalew M, Palmer L, Gavrin LM, Jain N, et al. Discovery and validation of blood biomarkers for suicidality. Mol Psychiatry. (2013) 18:1249–64. doi: 10.1038/mp.2013.95
38. Wu L, Yan Z, Jiang H, Xing H, Li H, Qiu C. Serum cystatin C, impaired kidney function, and geriatric depressive symptoms among older people living in a rural area: a population-based study. BMC Geriatr. (2018) 18:265. doi: 10.1186/s12877-018-0957-2
39. Minev E, Unruh M, Shlipak MG, Simsonick E, Yaffe K, Leak TS, et al. Association of cystatin C and depression in healthy elders: the health, aging and body composition study. Nephron Clin Pract. (2010) 116:c241–6. doi: 10.1159/000317205
Keywords: persistent depressive symptoms, serum cystatin C, longitudinal cohort study, the HRS, renal function
Citation: Han T, Zhang L, Jiang W and Wang L (2022) Persistent Depressive Symptoms and the Changes in Serum Cystatin C Levels in the Elderly: A Longitudinal Cohort Study. Front. Psychiatry 13:917082. doi: 10.3389/fpsyt.2022.917082
Received: 10 April 2022; Accepted: 05 May 2022;
Published: 03 June 2022.
Edited by:
Wuxiang Xie, Peking University, ChinaReviewed by:
Dae Jong Oh, Seoul Metropolitan Government—Seoul National University Boramae Medical Center, South KoreaYueqin Hu, Beijing Normal University, China
Copyright © 2022 Han, Zhang, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, emhhbmdsaTg4MjhAMTYzLmNvbQ==
 Tiandong Han
Tiandong Han Li Zhang2*
Li Zhang2*