
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 22 June 2022
Sec. Addictive Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.915440
This article is part of the Research Topic Novel Treatment Approaches and Future Directions in Substance Use Disorders View all 21 articles
The current study aimed to evaluate the effect of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex (DLPFC) on behavioral impulsivity in methamphetamine addicts. Forty-five methamphetamine addicts were recruited and randomly divided into active tDCS and sham tDCS groups to receive a daily tDCS intervention for 5 days, with the intensity set to 2 mA for the active group and 0 mA for the sham group. Anodal and cathodal electrodes were, respectively, placed over the right and left DLPFC. Behavioral impulsivity in methamphetamine addicts was examined by the 2-choice oddball task at 3-time points: before tDCS intervention (baseline), after the first intervention (day 1), and after 5 repeated interventions (day 5). Besides, twenty-four healthy male participants were recruited as the healthy controls who completed a 2-choice oddball task. Analysis of accuracy for the 2-choice oddball task showed that behavioral impulsivity was counterproductively increased in the active group, which was shown by the decreased accuracy for the deviant stimulus. The results suggested that the present protocol may not be optimal and other protocols should be considered for the intervention of methamphetamine addicts in the future.
Substance use disorders are prevalent health problems that are accompanied by mental disorders (1) and physical dysfunction (2), even underlying factors in criminal behavior (3). Individuals who chronically use methamphetamine exhibit higher behavioral impulsivity (4) which may result in constant drug use and relapse (5, 6). Behavioral impulsivity or behavioral disinhibition refers to the inability to inhibit a prepotent action (7). Previous studies have found that methamphetamine addicts exhibit higher behavioral impulsivity than healthy controls (8, 9), which persisted about 10 months after methamphetamine addicts abstained naturally (10). Behavioral inhibition is associated with most current therapies for methamphetamine addiction, which treat individuals by increasing their behavioral inhibition ability (11). Therefore, it is expected that a robust therapy outcome can be obtained by decreasing the behavioral impulsivity of methamphetamine addicts.
Methamphetamine addicts have shown structural (12, 13), metabolic (14), and functional (15) abnormalities in the frontal cortex, such as the dorsolateral prefrontal cortex (DLPFC). The DLPFC plays a primary role in the execution and inhibition of behavior, and its impairment decreased the ability to inhibit behavior (16). Notably, recent evidence suggested that using transcranial direct current stimulation (tDCS) to stimulate the DLPFC decreases behavioral impulsivity in individuals with attention deficit hyperactivity disorder (17), Gambling Disorder (18), and healthy individuals (19). However, it is unclear whether tDCS may effectively decrease behavioral impulsivity in methamphetamine addicts.
Transcranial direct current stimulation is a method of non-invasive brain stimulation, which has been used in the intervention of various psychiatric disorders (20) and the enhancement of cognitive function (21). The protocol of tDCS is crucial to the effectiveness of the technique (22). Previous studies have found a variety of tDCS protocols effective in decreasing craving in methamphetamine addicts (23–25), such as bilateral tDCS over the DLPFC (right anodal/left cathodal). This protocol has been shown to be effective in decreasing the symptoms of addiction, impulsivity in some substance addictions (e.g., tobacco and cocaine), or psychiatric disorders (18, 26, 27). In addition, multi-session of tDCS intervention has been found more effective than one session (28). Therefore, it can be expected that multi-session bilateral tDCS over the DLPFC (right anodal/left cathodal) can effectively decrease impulsivity in methamphetamine addicts.
Based on the evidence above, we hypothesized bilateral tDCS over the DLPFC (right anodal/left cathodal) may decrease behavioral impulsivity in methamphetamine addicts. To test this hypothesis, the current study used a 2-choice oddball task to examine behavioral impulsivity, as it has been shown to be effective in measuring behavioral impulsivity (29). The 2-choice oddball task requires participants to respond to two types of stimuli accurately and then quickly: one is standard and the other is deviant. The ratio of standard to deviant stimuli is 4 to 1, which means participants would be more habitual to respond to the standard stimulus; when a deviant stimulus presents, participants would inhibit their habitual response. Therefore, the accuracy and response time (RT) for deviant stimulus can be served as indicators of behavioral impulsivity (30).
According to a priori computation of the required sample size in the current design using G*Power statistical software (31), 36 individuals are necessary for 0.95 statistical power, and 45 individuals were used in the current study. The effect size was set to a threshold of medium (i.e., 0.25), according to previous meta-analysis reports regarding the effect of tDCS on drug addiction (28, 32), and the alpha was set to 0.05.
Forty-five individuals with methamphetamine addiction were recruited from Sichuan Ziyang Drug Rehabilitation Center, Sichuan Province, China. They were found by the police when they took drugs for the last time, and then they received unified management and treatment in the drug rehabilitation center, and have no chance to take drugs for 2 years. Inclusion criteria included meeting the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, never using drugs other than methamphetamine, and no acute physical or mental illness. Exclusion criteria included history of multiple drug use, current methamphetamine use or medication, history of acute physical and mental problems (e.g., epilepsy, stroke, cardiovascular disease), presence of metal implants (e.g., electrodes, pacemakers, heart bypass), and history of brain stimulation interventions. Each methamphetamine addict was randomly assigned to an active tDCS group (n = 23) with a 2 mA current intensity or a sham tDCS group (n = 22) with a 0 mA current intensity, according to a computer-generated randomization sequence. The overall mean age of the methamphetamine addicts was 24.1 (SD = 2.13) years, 24.3 (SD = 1.57) years in the active group, and 24 (SD = 2.62) years in the sham group. Additionally, 24 healthy male participants were recruited as healthy controls. Their mean age was 25.2 (SD = 4.14) years. The three groups were matched in age, F(2,66) = 1.129, p = 0.33, ηp2 = 0.033.
All participants were right-handed and had normal or corrected-to-normal vision. They voluntarily participated in the study and signed written informed consent before receiving the intervention. The current study has been registered on the platform of the China Trial Registration Center (Registration number: ChiCTR2100046112) and has been approved by the Ethical Committee of the Institute of Brain and Psychological Sciences, Sichuan Normal University in China. The experimental procedure was in line with principles of the Declaration of Helsinki and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines; see Figure 1.
“Direct currents of 2 mA generated by an electrical stimulator (Brain Premier tDCS Device; China) were applied through a pair of saline-soaked 1.5” round sponge electrodes for 20 min. In both active and sham groups, anodal and cathodal electrodes were placed over the right and left DLPFC, respectively (F4-F3), which was determined via the standard tDCS navigation system provided by the NeuStim NSS18 equipment of Neuracle Company (Changzhou, China). For the sham group, the direct current intensity is set to 0 mA and the intervention time is the same as the active group. To test the effectiveness of the sham protocol, after the intervention, participants were randomly selected and asked orally about their feelings. The tDCS intervention was performed for 5 sessions over 5 consecutive days. The experimenter who applied tDCS was blind to the study hypothesis but not to the setting of two groups (active vs. sham).
The two-choice oddball task contained 200 trials, including 160 standard stimuli (“W”) and 40 deviation stimuli (“M”). Each trial started with a jittered fixation cross appearing at the center of the screen and varying from 500 to 1,500 ms. For the participants in each group, if the standard stimulus was presented, they were to press the “F” key with their left index finger as quickly as possible. If the stimulus was deviant, they were to press the “J” key with their right index finger. Before the task started, each participant completed 15 practice trials to familiarize themself with the procedure. To avoid the practice effect, the formal experiment did not start until participants achieved 100% accuracy in both standard and deviant stimuli during practice. At the end of the experiment, the accuracy was given as feedback to participants.
Behavioral impulsivity was primarily indicated by the accuracy of the deviant stimulus. In the methamphetamine addicts group, behavioral impulsivity was examined at 3-time points: before tDCS intervention (baseline), after the first intervention (day 1), and after 5 repeated interventions (day 5). Besides, healthy controls completed a 2-choice oddball task as the baseline impulsivity level of healthy individuals.
To verify the effectiveness of the manipulation, we collected the RT and accuracy from the 2-choice oddball task and used the baseline data to conduct a 2 × 2 mixed-design ANOVA, with stimulus (standard, deviant) as within-subject factor and group (healthy controls, methamphetamine addicts) as between-subject factor. To analyze the effect of tDCS on behavioral impulsivity in methamphetamine addicts, a 2 × 3 × 2 mixed-design ANOVA was used, with group (sham, active) as between-subject variable, session (baseline, day 1, day 5), and stimulus (standard, deviant) as within-subject variables. Potential group differences in demographic data and questionnaires were analyzed using independent samples t-tests, ANOVA, and Kruskal–Wallis tests, as appropriate.
According to the Greenhouse–Geisser method, the degrees of freedom for F-ratios that violate the spherical assumption are corrected. The false discovery rate (FDR) correction was used for post-hoc comparisons if statistically significant main or interaction effects appeared. All statistical analyses were performed in R (33). A 2-sided p < 0.05 was considered statistically significant, and the effect size was reported as partial η2 (ηp2).
The mean abstinence duration was 153 (SD = 78.3) days in the active group, and 123 (SD = 58.6) in the sham group, and the 2 groups were overall matched in the duration of abstinence (p = 0.15). The three groups were matched on other demographic variables, demographic data are presented in Table 1.
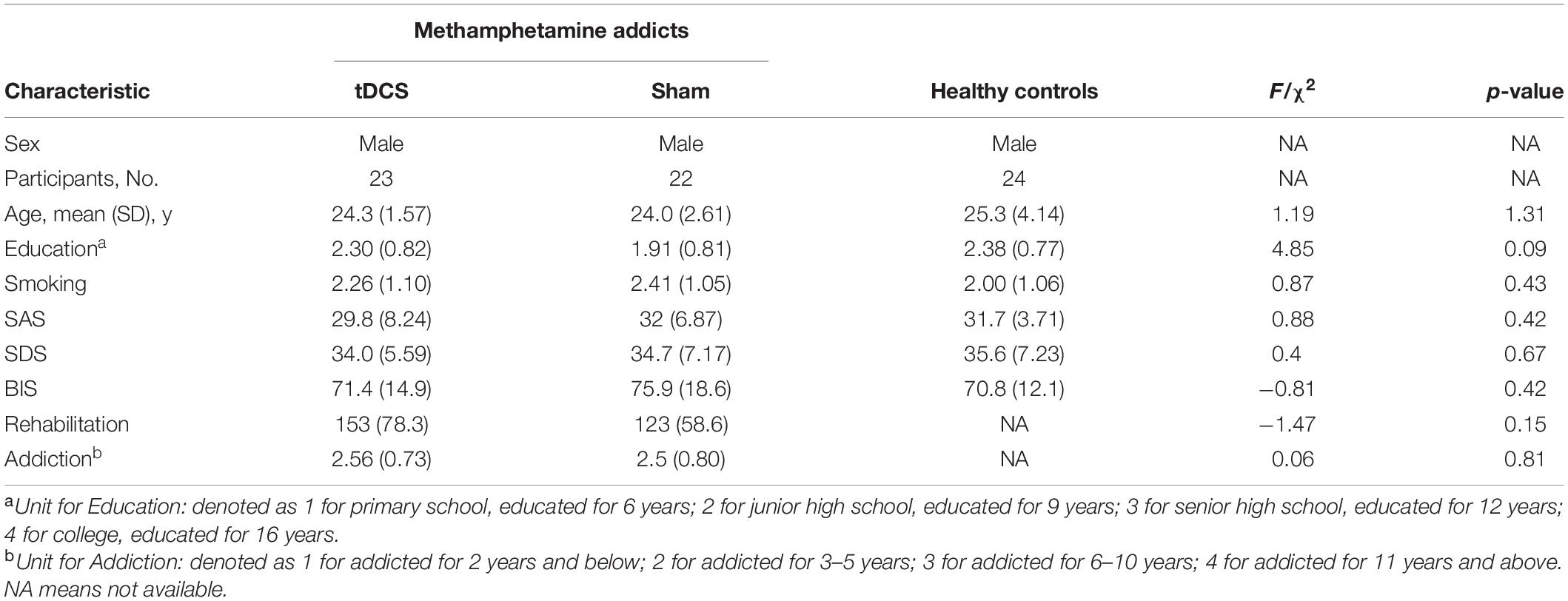
Table 1. Demographic data of methamphetamine addicts undergoing active or sham tDCS and healthy controls.
Baseline data were used to check the effectiveness of manipulation. The mixed-design ANOVA of accuracy and RT in the 2-choice oddball task showed statistically significant group-by-stimulus interaction effects, accuracy, F(1,67) = 4.204, p = 0.044, ηp2 = 0.059; RT, F(1,67) = 12.016, p < 0.001, ηp2 = 0.152. The deviant stimulus had lower accuracy and longer RT relative to the standard stimulus in both samples (ps < 0.001), indicating that the experimental manipulation was effective and that the 2-choice oddball task could successfully measure behavioral impulsivity. Notably, although methamphetamine addicts and healthy controls showed similar accuracy in the standard stimulus (p = 0.606) and RT for the deviant stimulus (p = 0.479), the accuracy of the deviant stimulus (p = 0.033) and the RT for the standard stimulus (p = 0.003) was significantly lower in methamphetamine addicts compared with healthy controls, indicating that methamphetamine addicts showed higher behavioral impulsivity relative to healthy controls; see Figure 2.
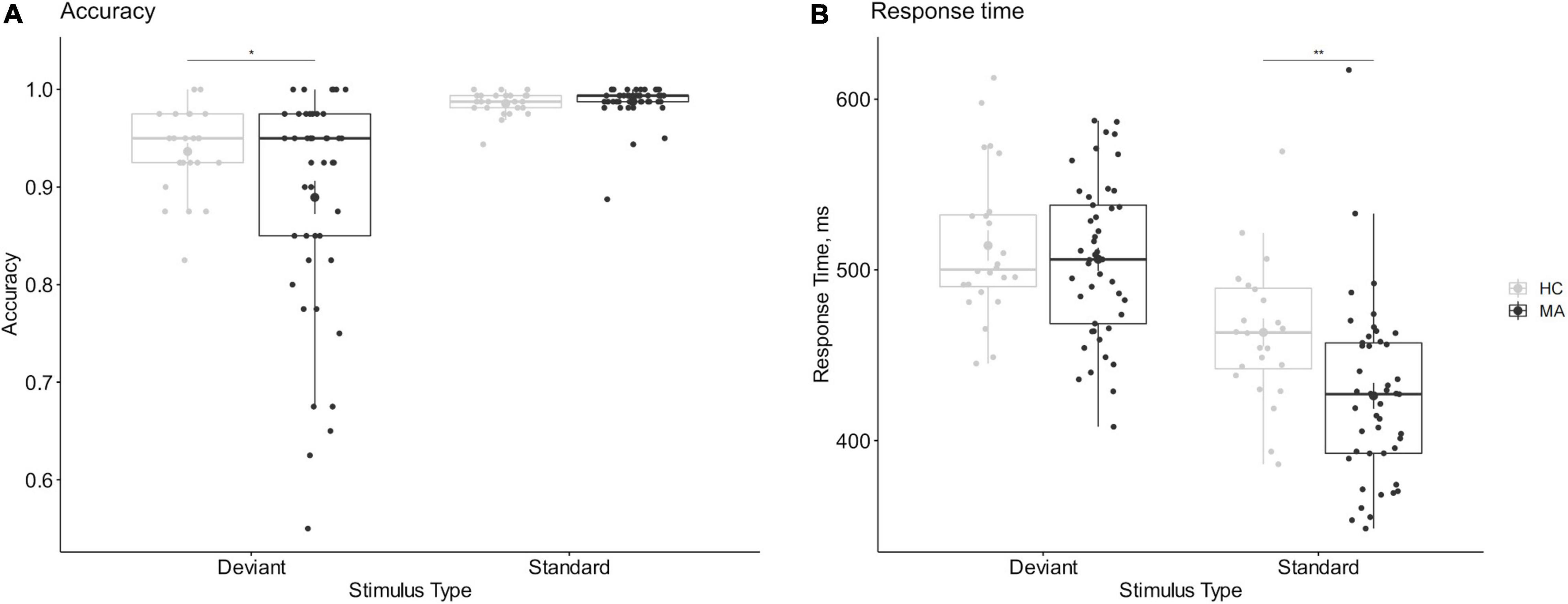
Figure 2. Comparison of behavioral impulsivity between methamphetamine addicts and healthy controls. (A) The accuracy of 2-choice oddball task. (B) The response time of 2-choice oddball task. (A) The accuracy of 2-hoice oddball. (B) The response time of 2-choice oddball. MA refers to methamphetamine addicts, HC refers to healthy controls. *Refers to 2-sided p < 0.05. **Refers to 2-sided p < 0.01. Error bars show the standard error of the mean. Enlarged dots refer to means.
The mixed-design ANOVA of accuracy showed a statistically significant 3-way interaction among stimulus, session and group, F(1.62,69.51) = 5.96, p = 0.007, ηp2 = 0.122. The simple effects analysis found a significantly decreasing deviant stimulus accuracy (p < 0.035) and no significantly different standard stimulus (p > 0.296) after 5 days of interventions in the active group. Importantly, this significant stimulus-by-time interaction only found in the active group, F(1.46,32.13) = 6.354, p = 0.009, ηp2 = 0.224, but not in the sham group, F(2,42) = 0.686, p = 0.509, ηp2 = 0.032. These results indicated a significantly increased behavioral impulsivity after 5 consecutive days of interventions in the active group, and no difference in the sham group; see Figure 3.
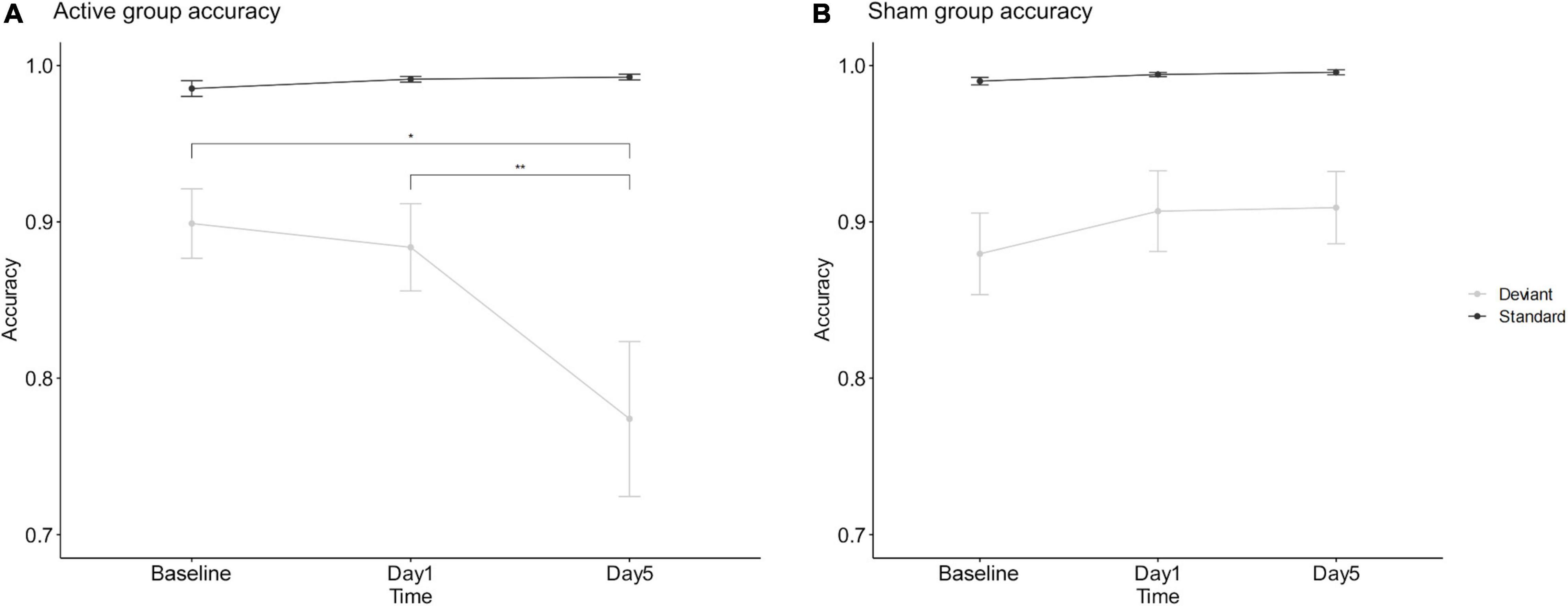
Figure 3. Mean accuracy of active tDCS group (A) and sham group (B). *Refers to 2-sided p < 0.05. **Refers to 2-sided p < 0.01. Error bars show the standard error of the mean.
The analysis of RT found no statistically significant three-way interaction among stimulus, time and group, F(2,86) = 2.826, p = 0.065, ηp2 = 0.062, except for a significant stimulus by time interaction, F(2,86) = 21.876, p < 0.001, ηp2 = 0.337, and a significant group by stimulus interaction, F(1,43) = 6.105, p = 0.018, ηp2 = 0.124. Further analysis revealed that, regardless of the active group or the sham group, the RT for the standard stimulus significantly decreased over time (both: ps < 0.006), while the RT for the deviant stimulus was not significantly different before and after the interventions (both: ps > 0.124).
The current study aimed to evaluate the effect of bilateral tDCS (right anodal/left cathodal) over DLPFC in decreasing behavioral impulsivity in methamphetamine addicts. Inconsistent with our hypothesis, the results suggested that the current protocol of bilateral tDCS counterproductively increased impulsivity in methamphetamine addicts. Specifically, after 5 consecutive days of intervention, the accuracy for deviant stimulus was significantly decreased in the active group, while the sham group did not.
Mounting evidence indicated that the DLPFC is closely linked to behavioral control. Previous studies have found that individuals with impaired DLPFC generally performed worse on executive measures relative to healthy individuals and individuals with damage in other brain regions (34). Moreover, some studies have found that stimulating the DLPFC of individuals through tDCS decreased the impulsivity of healthy (19), ADHD individuals (35), and gambling addicts (18), and suggested that using an appropriate tDCS protocol to stimulate DLPFC may effectively improve individuals’ impulse control or behavioral inhibition ability. Based on these findings, the current study selected the DLPFC as a tDCS target and expected this protocol to significantly decrease behavioral impulsivity in methamphetamine addicts. However, counterproductive results were observed, with a significant increase in behavioral impulsivity of methamphetamine addicts. These results indicated that using tDCS to stimulate the DLPFC does an effective method to modulate behavioral impulsivity, but the protocol that used bilateral tDCS (right anodal/left cathodal) over DLPFC may lead to up-modulation.
Methamphetamine addicts have severely impaired DLPFC relative to healthy individuals (36). Specifically, methamphetamine addicts had significantly lower gray matter thickness in the DLPFC region (12, 37, 38) and lower activation during behavioral inhibition tasks (39). One recent study observed that bilateral tDCS (right anodal/left cathodal) over DLPFC increased the activation of executive control networks in the resting state of methamphetamine addicts and decrease the craving of methamphetamine addicts (25). However, given the extent of damage to the DLPFC in methamphetamine addicts, it is possible that activating this region may overdraw their DLPFC activity and subsequently decrease their impulse control. For example, a warm-up usually improves performance in healthy people, but the same warm-up may deplete sick person and his/her subsequent performance. This may be one potential reason why a similar protocol decreased impulsivity in healthy individuals (40) and individuals with other psychological disorders (18) but led to counterproductive results in methamphetamine addicts.
Several limitations should be addressed in future work. First, although the current study used a 2-choice oddball task to assess impulsivity, no functional neuroimaging with tDCS intervention was collected, which led us unable to examine possible functional changes in the DLPFC. Therefore, future research can examine the current findings using neuroimaging techniques. Second, the current study measured baseline behavioral impulsivity in the HC group but they did not undergo tDCS intervention, leaving it not possible to examine how tDCS affects behavioral impulsivity in healthy people. In addition, the current study employed simple letters as stimuli (i.e., M and W) to ensure experimental control. However, previous studies have found that methamphetamine addicts have higher impulsivity to meth-related information (41, 42), so future work should consider selecting drug-related images as stimuli to improve the ecological validity of the study. Furthermore, the current study used only one active tDCS protocol (right anodal/left cathodal), which prevented us from exploring the effects of unilateral stimulation of the DLPFC on impulsivity in methamphetamine addicts. However, because the anodes and cathodes of tDCS may be associated with opposing neural effects (20) and the anodal tDCS may have different effects on the left DLPFC and the right DLPFC in methamphetamine addicts (22), additional tDCS protocols are needed in future studies to further investigate the lateralizing effects of tDCS on DLPFC function.
The current study evaluated the effect of bilateral tDCS (right anodal/left cathodal) over the DLPFC on behavioral impulsivity in methamphetamine addicts and found a counterproductively increased impulsivity after the 5-day intervention in methamphetamine addicts. The results suggested that using tDCS to stimulate the DLPFC is an effective method to modulate behavioral impulsivity, but as it is counterproductive, the current protocol may not be optimal and other protocols should be considered for the intervention of methamphetamine addicts in the future.
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethical Committee of the Institute of Brain and Psychology Science, Sichuan Normal University. The patients/participants provided their written informed consent to participate in this study.
XJ and JY conceived and designed the current study. XJ and CZ collected the data and statistical analysis. XJ, YT, and JY interpreted the data. XJ, YT, ZZ, and JY wrote the final manuscript. All authors contributed to reviewed and approved the final manuscript.
This study was funded by the National Natural Science Foundation of China through grants 31971018 to JY.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Rufang Wang, Jingzhen He, and Ting Luo for their assistance in interviewing the participants.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.915440/full#supplementary-material
1. Köck P, Meyer M, Elsner J, Dürsteler KM, Vogel M, Walter M. Co-occurring mental disorders in transitional aged youth with substance use disorders – a narrative review. Front Psychiatry. (2022) 13:827658. doi: 10.3389/fpsyt.2022.827658
2. Sommers I, Baskin D, Baskin-Sommers A. Methamphetamine use among young adults: health and social consequences. Addict Behav. (2006) 31:1469–76. doi: 10.1016/j.addbeh.2005.10.004
3. Hoffman WF, Jacobs MB, Dennis LE, McCready HD, Hickok AW, Smith SB, et al. Psychopathy and corticostriatal connectivity: the link to criminal behavior in methamphetamine dependence. Front Psychiatry. (2020) 11:90. doi: 10.3389/fpsyt.2020.00090
4. Andres T, Ernst T, Oishi K, Greenstein D, Nakama H, Chang L. Brain microstructure and impulsivity differ between current and past methamphetamine users. J Neuroimmune Pharmacol. (2016) 11:531–41. doi: 10.1007/s11481-016-9675-8
5. Adinoff B, Rilling LM, Williams MJ, Schreffler E, Schepis TS, Rosvall T, et al. Impulsivity, neural deficits, and the addictions: the “Oops” factor in relapse. J Addict Dis. (2007) 26:25–39. doi: 10.1300/J069v26S01_04
6. Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. (2008) 320:1352–5. doi: 10.1126/science.1158136
7. Nguyen R, Brooks M, Bruno R, Peacock A. Behavioral measures of state impulsivity and their psychometric properties: a systematic review. Personal Individ Differ. (2018) 135:67–79. doi: 10.1016/j.paid.2018.06.040
8. Potvin S, Pelletier J, Grot S, Hébert C, Barr AM, Lecomte T. Cognitive deficits in individuals with methamphetamine use disorder: a meta-analysis. Addict Behav. (2018) 80:154–60. doi: 10.1016/j.addbeh.2018.01.021
9. Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. (2005) 79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002
10. Liu W, Tian Y, Yang J. Impulse inhibition ability with methamphetamine dependents varies at different abstinence stages. Front Psychiatry. (2021) 12:169. doi: 10.3389/fpsyt.2021.626535
11. Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. (2011) 194:287–95. doi: 10.1016/j.pscychresns.2011.04.010
12. Huang S, Dai Y, Zhang C, Yang C, Huang Q, Hao W, et al. Higher impulsivity and lower grey matter volume in the bilateral prefrontal cortex in long-term abstinent individuals with severe methamphetamine use disorder. Drug Alcohol Depend. (2020) 212:108040. doi: 10.1016/j.drugalcdep.2020.108040
13. Huang S, Yang W, Luo J, Yan C, Liu J. White matter abnormalities based on TBSS and its correlation with impulsivity behavior of methamphetamine addicts. Front Psychiatry. (2020) 11:452. doi: 10.3389/fpsyt.2020.00452
14. Bakhshinezhad H, Darharaj M, Feyzi YF, Babaei S, Ahadi R, Jamei B, et al. The relationship between brain metabolites alterations and neuropsychological deficits in patients with methamphetamine use disorder: a proton magnetic resonance spectroscopy study. Arch Clin Neuropsychol. (2021) 37:160–72. doi: 10.1093/arclin/acab033
15. Khajehpour H, Mohagheghian F, Ekhtiari H, Makkiabadi B, Jafari AH, Eqlimi E, et al. Computer-aided classifying and characterizing of methamphetamine use disorder using resting-state EEG. Cogn Neurodyn. (2019) 13:519–30. doi: 10.1007/s11571-019-09550-z
16. Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. (2018) 98:886–903. doi: 10.1016/j.neuron.2018.03.048
17. Allenby C, Falcone M, Bernardo L, Wileyto EP, Rostain A, Ramsay JR, et al. Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul. (2018) 11:974–81. doi: 10.1016/j.brs.2018.04.016
18. Salatino A, Miccolis R, Gammeri R, Ninghetto M, Belli F, Nobili M, et al. Improvement of impulsivity and decision making by transcranial direct current stimulation of the dorsolateral prefrontal cortex in a patient with gambling disorder. J Gambl Stud. (2021) 38:627–34. doi: 10.1007/s10899-021-10050-1
19. Beeli G, Casutt G, Baumgartner T, Jäncke L. Modulating presence and impulsiveness by external stimulation of the brain. Behav Brain Funct. (2008) 4:33. doi: 10.1186/1744-9081-4-33
20. Chase HW, Boudewyn MA, Carter CS, Phillips ML. Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol Psychiatry. (2020) 25:397–407. doi: 10.1038/s41380-019-0499-9
21. Tong J, Kong C, Wang X, Liu H, Li B, He Y. Transcranial direct current stimulation influences bilingual language control mechanism: evidence from cross-frequency coupling. Cogn Neurodyn. (2020) 14:203–14. doi: 10.1007/s11571-019-09561-w
22. Shahbabaie A, Hatami J, Farhoudian A, Ekhtiari H, Khatibi A, Nitsche MA. Optimizing electrode montages of transcranial direct current stimulation for attentional bias modification in early abstinent methamphetamine users. Front Pharmacol. (2018) 9:907. doi: 10.3389/fphar.2018.00907
23. Xu X, Ding X, Chen L, Chen T, Su H, Li X, et al. The transcranial direct current stimulation over prefrontal cortex combined with the cognitive training reduced the cue-induced craving in female individuals with methamphetamine use disorder: a randomized controlled trial. J Psychiatr Res. (2021) 134:102–10. doi: 10.1016/j.jpsychires.2020.12.056
24. Rezvanian S, Saraei MA, Mohajeri H, Hassani Abharian P. The effect of Different tDCS protocols on drug craving and cognitive functions in methamphetamine addicts. Basic Clin Neurosci. (2022). 13:0–0. doi: 10.32598/bcn.2021.1929.1
25. Shahbabaie A, Ebrahimpoor M, Hariri A, Nitsche MA, Hatami J, Fatemizadeh E, et al. Transcranial DC stimulation modifies functional connectivity of large-scale brain networks in abstinent methamphetamine users. Brain Behav. (2018) 8:e00922. doi: 10.1002/brb3.922
26. Perri RL, Perrotta D. Transcranial direct current stimulation of the prefrontal cortex reduces cigarette craving in not motivated to quit smokers: a randomized, sham-controlled study. Addict Behav. (2021) 120:106956. doi: 10.1016/j.addbeh.2021.106956
27. Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM. A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int J Neuropsychopharmacol. (2015) 18:yv066. doi: 10.1093/ijnp/pyv066
28. Song S, Zilverstand A, Gui W, Li H, Zhou X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimul. (2019) 12:606–18. doi: 10.1016/j.brs.2018.12.975
29. Yuan J, He Y, Qinglin Z, Chen A, Li H. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology. (2008) 45:986–93. doi: 10.1111/j.1469-8986.2008.00693.x
30. Yuan J, Liu W, Liang Q, Cao X, Lucas MV, Yuan T-F. Effect of low-frequency repetitive transcranial magnetic stimulation on impulse inhibition in abstinent patients with methamphetamine addiction: a randomized clinical trial. JAMA Netw Open. (2020) 3:e200910. doi: 10.1001/jamanetworkopen.2020.0910
31. Faul F, Erdfelder E, Lang A-G, Buchner AG. *Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
32. Chen J, Qin J, He Q, Zou ZA. Meta-analysis of transcranial direct current stimulation on substance and food craving: what effect do modulators have? Front Psychiatry. (2020) 11:598. doi: 10.3389/fpsyt.2020.00598
33. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2021).
34. Barbey AK, Colom R, Grafman J. Dorsolateral prefrontal contributions to human intelligence. Neuropsychologia. (2013) 51:1361–9. doi: 10.1016/j.neuropsychologia.2012.05.017
35. Soltaninejad Z, Nejati V, Ekhtiari H. Effect of anodal and cathodal transcranial direct current stimulation on DLPFC on modulation of inhibitory control in ADHD. J Atten Disord. (2019) 23:325–32. doi: 10.1177/1087054715618792
36. Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. (2002) 26:53–63. doi: 10.1016/S0893-133X(01)00334-7
37. Aoki Y, Orikabe L, Takayanagi Y, Yahata N, Mozue Y, Sudo Y, et al. Volume reductions in frontopolar and left perisylvian cortices in methamphetamine induced psychosis. Schizophr Res. (2013) 147:355–61. doi: 10.1016/j.schres.2013.04.029
38. Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. (2011) 106:1474–83. doi: 10.1111/j.1360-0443.2011.03433.x
39. Salo R, Fassbender C, Buonocore MH, Ursu S. Behavioral regulation in methamphetamine abusers: an fMRI study. Psychiatry Res Neuroimaging. (2013) 211:234–8. doi: 10.1016/j.pscychresns.2012.10.003
40. Gilmore CS, Dickmann PJ, Nelson BG, Lamberty GJ, Lim KO. Transcranial direct current stimulation (tDCS) paired with a decision-making task reduces risk-taking in a clinically impulsive sample. Brain Stimul. (2018) 11:302–9. doi: 10.1016/j.brs.2017.11.011
41. Dakhili A, Sangchooli A, Jafakesh S, Zare-Bidoky M, Soleimani G, Batouli SAH, et al. Cue-induced craving and negative emotion disrupt response inhibition in methamphetamine use disorder: behavioral and fMRI results from a mixed Go/No-Go task. Drug Alcohol Depend. (2022) 233:109353. doi: 10.1016/j.drugalcdep.2022.109353
Keywords: impulsivity, methamphetamine, transcranial direct current stimulation, dorsolateral prefrontal cortex, two-choice oddball
Citation: Jiang X, Tian Y, Zhang Z, Zhou C and Yuan J (2022) The Counterproductive Effect of Right Anodal/Left Cathodal Transcranial Direct Current Stimulation Over the Dorsolateral Prefrontal Cortex on Impulsivity in Methamphetamine Addicts. Front. Psychiatry 13:915440. doi: 10.3389/fpsyt.2022.915440
Received: 08 April 2022; Accepted: 30 May 2022;
Published: 22 June 2022.
Edited by:
Marc Walter, University of Basel, SwitzerlandReviewed by:
Ana Sánchez-Kuhn, University of Almería, SpainCopyright © 2022 Jiang, Tian, Zhang, Zhou and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiajin Yuan, eXVhbmppYWppbjE2OEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.