
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 14 July 2022
Sec. Mood Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.899318
 Elisabeth Schramm1*
Elisabeth Schramm1* Christoph Breuninger1
Christoph Breuninger1 Rainer Wohlfarth2
Rainer Wohlfarth2 Moritz Elsaesser1
Moritz Elsaesser1 Hannah Piosczyk1
Hannah Piosczyk1 Thomas Fangmeier1
Thomas Fangmeier1Background: For relapse prevention in depression, conventional mindfulness programs such as the mindfulness-based cognitive therapy proved to be useful. However, early life trauma is a risk factor for having adverse experiences during meditation. Thus, for this patient group mindfulness skills are often difficult to learn and may be facilitated by using animals and a nature setting.
Methods: The aim of the study was to evaluate the preventative efficacy of a nature- and animal assisted mindfulness program (NAM) over the course of 1 year in unstable or partially remitted depressed patients with a history of early life trauma. NAM included 8 group sessions of 150 min each over 8 weeks plus one booster session. Sixty-seven participants were randomized to either NAM combined with treatment-as-usual (TAU; guideline oriented treatment) or TAU alone. The primary outcome was depression diagnosis over the course of 12 months after end of treatment. Secondary outcomes included clinician- and self-rated depressive symptoms, quality of life, mindfulness skills, and rumination post, and 12 months after the intervention. In addition, we evaluated the participants' satisfaction with the program.
Results: Analyses revealed significant differences in relapse rates and number of weeks depressed throughout the course in favor of NAM. Furthermore, global quality of life improved significantly more in the NAM group. There was no significant difference for other secondary outcomes. Satisfaction with the program was high with a low drop-out rate of 6%. The vast majority of the participants felt safe practicing mindfulness in nature and found sheep for assistance helpful and motivating.
Conclusions: A nature- and animal assisted mindfulness program proved to be feasible, highly acceptable, and more effective than standard treatment in preventing relapses in recurrently depressed patients with childhood maltreatment. Nature and animals can facilitate the engagement in the treatment process for individuals with a history of early trauma. However, further evidence in multicenter trials is necessary.
Relapse prevention is one of the most important goals in depression treatment as major depression is usually a recurrent disorder with a relapsing course. After remission from a depressive episode, continuing (1) or sequentially introducing psychotherapy as add-on to antidepressants (2, 3), can reduce the relapse and recurrence rate in the further course. Of the psychotherapy programs specifically developed for relapse prevention, Mindfulness Based Cognitive Therapy [MBCT; (4)] is effective in remitted patients, particularly in those with frequent depressive episodes (5, 6). However, research and clinical observations raised some concerns with providing mindfulness practices to patients with trauma histories (7) while controversial findings are reported if early trauma could be a risk factor for having adverse experiences during meditation (8). There is evidence that mindfulness practices can be particularly efficacious in preventing depression relapse among people with histories of childhood maltreatment (9, 10), but could be done with more care to reduce risk of adverse events and increase engagement (11–13). While in most meditation studies adverse effects were not systematically assessed, patients with a trauma history often spontaneously report difficulties such as heightened emotional reactions or re-experiencing traumatic memories (7). This kind of passive monitoring of adverse events is thought to underestimate the prevalence of meditation-related negative effects by far (14). Furthermore, there is no consensus on what can be considered an adverse effect in mindfulness trainings. The main challenges with extended sitting practice, body scan or breath awareness for patients with posttraumatic stress disorder (15) or histories of trauma including childhood maltreatment (16) are attributed to over-arousal, distress due to embodied traumatic memories, or feeling overwhelmed with relaxation-induced anxiety and loss of structure. In addition, early maltreated patients reported to feel unsafe lying down with eyes closed in a closed room with other unfamiliar participants (17). Under these conditions, it is difficult to engage in the treatment process and learn mindfulness skills. Therefore, corresponding adaptations and more flexibility in teaching mindfulness have been proposed for this patient group.
Less activating options in a per se stabilizing environment such as nature may offer smoother access to painful memories or dysregulative experiences arising from early trauma. Contact with nature has a beneficial effect on mental wellbeing, facilitates positive affect, and reduces anxiety and depressive symptoms (18–20). A recent meta-analytical review suggests that nature-based mindfulness is moderately superior to mindfulness conducted in non-natural settings (21). Moreover, animal-assisted therapy has been shown in several meta-analyses to be effective in reducing stress reactions, depressive and anxiety symptoms, and in increasing motivation (22, 23). There is also evidence for increasing treatment comfort, engagement, and completion by using animals in therapy (24). These findings are underlined by numerous studies indicating that the presence of an animal leads to diminished fear (25–28) and promotes calmness (29, 30).
For teaching mindfulness skills, sheep as flight and social animals proved to be particularly suitable (31) since they show several mindfulness skills themselves. Sheep as intelligent, complex, and feeling individuals sense emotions and moods of human beings and react correspondingly (32). By nature, sheep are non-judgmental and show full awareness in presence. This mirrors the attitude prompted in mindfulness of being present, non-judgmental, accepting, and compassionate which can be especially beneficial for patients with a trauma history. To focus on the here and now facilitates coping with rumination and worries, which are common among maltreated individuals (33). To practice a non-judgmental attitude can be an antidote against feelings of shame, guilt, and anger (34, 35). The cultivation of compassion and particularly of self-compassion (36) decreases self-blame and low self-esteem (37). Animals do not create a therapeutic process per se, but they rather support the change processes intended by the therapist (38). In an open pilot study (31), a nature- and animal assisted mindfulness program based on MBCT proved to be feasible and highly accepted by depressed patients. Despite methodological limitations, the results were encouraging and warranted further study with a larger randomized sample.
The aim of the present study was to evaluate the preventative efficacy of the NAM program over the course of 1 year. The primary hypothesis was that participants with a history of depression and childhood trauma randomized to NAM would report significantly less relapses and recurrences during the 1-year assessment period after the intervention. Primary outcome was defined as time to relapse (fulfillment of criteria for Major Depressive Disorder according to DSM-IV-TR) for at least 2 weeks. Secondary outcomes included self-rated [Beck Depression Inventory-II, BDI-II; (39)] and clinician-rated [Hamilton Rating Scale for Depression, HRSD-17; (40)] depressive symptoms, quality of life [WHOQoL; (41)], mindfulness skills [Freiburger Fragebogen zur Achtsamkeit, FFA; (42)], and rumination [Responsiveness Scale Questionnaire, RSQ-D; (43)].
We conducted a monocentric, randomized controlled trial between July 2016 and October 2019 comparing NAM vs. TAU in a group format in outpatients with unstable or partially remitted depression and a history of childhood maltreatment. Participants were recruited from July 2016 through June 2018, intervention groups were conducted between September 2016 and June 2018, and the last follow-up assessments were taken in September 2019. The Research Ethics Board of the University of Freiburg approved the trial. In accordance with the Declaration of Helsinki, participants were informed in detail about the purpose and design of the trial and provided written informed consent prior to randomization. This trial was registered prospectively with the German Clinical Trials Register (registry number: DRKS00010800).
In total, 67 patients were randomized to either NAM combined with TAU or TAU alone (Figure 1). Randomization was conducted in 5 groups of 10–16 participants, resulting in one NAM and one TAU group each time, according to a central computerized randomization schedule (using random sequences from random.org). Some participants thus had to wait after screening and study inclusion for their randomization group to be complete, which explains the loss of participants prior to baseline assessment and intervention as detailed in Figure 1.
Eligible patients were between 18 and 70 years of age and had a partial or unstable remission of a major depressive episode [i.e., one or more HRSD-17 scores >7 during the 4-week interval before screening, as established by retrospective week-by-week assessment; the criteria of a MDD [Major Depressive Disorder] according to DSM-IV-TR are no longer fulfilled; (44)]. Eligible patients also had at least 3 episodes of a MDD (the last one no longer than 2 years ago) and early trauma experiences according to the self-rated Childhood Trauma Questionnaire [CTQ-SF; (45)] in at least one of the five trauma domains with moderate to severe intensity. All participants had to be in the care of a primary care physician or a psychiatrist throughout the study.
Exclusion criteria comprised a current depressive episode, an acute high risk of suicide, a primary diagnosis of another Axis I mental disorder (46); a diagnosis of antisocial, schizotypal, or borderline personality disorder; a serious medical condition; a history of schizophrenia, schizoaffective disorder, bipolar disorder, substance abuse, and organic brain disorders.
Clinical diagnoses were assessed at screening using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders [SCID-I; (46)] and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders [SCID-II; (47)].
Analogous to the MBCT program (48), the NAM program consists of eight weekly group sessions of 2.5 h duration and one booster session 3 months after termination. The program was conducted with 4–8 participants in a nature setting. NAM is described in a structured guideline (31). The focus was on formal and informal mindfulness and compassion practices in nature and assisted by tamed sheep, psychoeducation on relapse prevention of depressive episodes, and contemplation of practice experiences in the group. The meditation practices included breath awareness, body scan, open awareness, loving-kindness meditation, walking meditation, and mindful eating as well as daily, formal (e.g., body scan), and informal homework (e.g., mindfulness in daily life while walking in nature). An overview of the session contents can be found in the Appendix.
Several modifications to the conventional MBCT program were made to adapt the intervention to our study population (Table 1).
More flexibility on the length of the meditation practices for individuals with a history of childhood maltreatment was also reported in other studies (36, 49). A previous investigation (17) suggested that patients with a trauma history had difficulties practicing over longer periods of time, and found that shorter practices were very helpful for those individuals.
The NAM group program was conducted by a licensed psychotherapist (E. Schramm) with comprehensive training in mindfulness as well as experience with depression treatment and educational work with the sheep participating in the study. The program was accompanied by an animal caretaker from Mundenhof (animal farm run by the city of Freiburg, Germany). Eight tamed “Coburger foxsheep,” a breed of Ovis gmelini aries, participated in the program. They are familiar with interacting with human beings and trained to walk with or without a leash. The animals' stress level during the meetings was continuously monitored by the animal caretaker joining the program. No signs of stress (e.g., restlessness, frequent urination, accelerated breathing, etc.) were detected in the animals. On the contrary, they actively sought contact with the participants. All training sessions took place outside at the Mundenhof farm which is surrounded by forests and meadows.
Patients randomized to TAU were encouraged to continue with guideline-oriented treatment (including psychotherapy and/or pharmacotherapy by a primary care physician, psychiatrist, or licensed psychotherapist) throughout the study period. In order to compensate for participation in the study assessments, patients in the TAU condition were offered to participate in the NAM program after their follow-up evaluation.
The following assessments were taken pre-treatment, post-treatment and at one-year follow-up (with parallel assessments in the TAU group), with the exception of the Beck Depression Inventory-Fast Screening [BDI-FS (50)]. All clinician ratings were conducted by blinded, trained, and experienced raters. The primary outcome was the course of depression 12 months after termination of the NAM program measured with a stepped diagnostic process of monthly BDI-FS and subsequent phone interviews.
BDI-FS (50): The screening of self-assessed depressive symptoms with the BDI-FS consists of seven items out of the BDI-II. This screening tool is designed to keep the repeated assessments of depressive symptoms as short as possible. The calculated transformation of the total score of the BDI-FS into an equivalent BDI-II score is described in the BDI-FS manual (50) (p. 11). In different studies, a high correlation between both instruments could be found (r = 0.85 to r = 0.92) (51, 52). Through a combination of a number of specific items and a minimum total score, sufficiently high sensitivity and specificity is reached. In an American sample, major depression was correctly classified with a sensitivity of 87% and a specificity of 85% with this algorithm (53). Participants were prompted monthly (and if necessary, reminded) by e-mail and completed the BDI-FS form online.
SCID & HRSD-17 telephone interview: participants with a sum score of at least five points (and at least one point on either the depressed mood or anhedonia items) were contacted by telephone, and a HRSD-17 (40) rating and SCID-I (46) assessment (affective disorders section) were performed.
Beck Depression Inventory-II [BDI-II; (39)]: The self-rated questionnaire includes 21 items to assess depressive symptoms from the patient's perspective. The test quality criteria, such as internal consistency, validity, and test-retest reliability, are most satisfactory in both clinical and non-clinical subjects (Cronbach's alpha = 0.89).
Hamilton Rating Scale for Depression (HRSD-17): The HRSD-17 is a clinician administered clinical interview for the assessment of the severity of depressive symptoms. Scores range from 0 to 51, with higher scores reflecting more depressive symptoms. A score of 0–8 is qualified as “normal,” 9–16 as “mild,” 17–24 as “moderate,” and a score ≥ 25 as “severe.” Detailed information on psychometric properties of the HRSD-17 can be found elsewhere (54).
Freiburger Fragebogen zur Achtsamkeit [FFA; (42)] is a one-dimensional self-rated questionnaire with 14 items for the evaluation of different aspects of mindfulness and associated skills. The measure has satisfactory test quality criteria.
Responsiveness Scale Questionnaire [RSQ-D German version; (43)] includes 32 items distributed on two dimensions: rumination and distraction. The measure has satisfactory test quality criteria.
Quality of Life [WHOQoL-Bref; (41)] contains 26 items related to 4 different dimensions: The physical domain, the mental domain, social relationships, the environment domain. The global domain includes items to assess life quality and health satisfaction. The measure has satisfactory test quality criteria.
In addition, early trauma experiences were assessed at baseline with the short form of the Childhood Trauma Questionnaire [CTQ-SF; (45)]. The CTQ is a 28-item retrospective self-report questionnaire evaluating the dimensions emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect that occurred during childhood and/or adolescence (“when they were growing up”). Exposure frequencies using a five-point Likert scale were summed up for domain scores. Cutoff scores for each domain were used to assign categories of “none to minimal,” “low to moderate,” “moderate to severe,” and “severe to extreme.” The German version of the CTQ has been shown to be a reliable and valid instrument (55).
To assess the participants' satisfaction with the NAM intervention as well as their experiences with practicing mindfulness in nature and with animal assistance, we used a self-designed questionnaire with 23 items. Of these, 18 items (5- point-scale ranging from 1 = “fully disagree” to 5 = “fully agree”) were averaged into three scales: nature helpful (6 items, e.g., “The mindfulness exercises in nature were easy for me to carry through.”, “The practice setting in nature allowed me to get involved in the mindfulness exercises.”), animals helpful (10 items, e.g., “The presence of the animals allowed me to get involved in the mindfulness exercises.”, “The ‘mindfulness' of the animals served as a model for me.”, “I found sheep suitable animals for the mindfulness program.”), and flooded/agitated (2 items: “I was flooded with images, feelings, or early memories during mindfulness practice.”, “At times I was agitated by the mindfulness practice.”).
The sample size calculation was based on the relapse rates in a meta-analysis of MBCT for relapse prevention in MDD (5), specifically in the subgroup with 3 or more prior depressive episodes, reported as 63% for TAU and 35% for MBCT. Assuming this reported relapse rate in our TAU group and a somewhat improved relapse rate of 31% in the NAM group resulted in a sample size of 64 patients for the primary survival analysis (with a power of 80% and the conventional alpha error of 5%), calculated with Stata 13.1.
The primary efficacy analysis was performed according to the intention-to-treat (ITT) principle and therefore was based on the full analysis set (FAS), assuming data missing at random. The FAS included all randomized patients, and patients were analyzed according to their randomized arm regardless of protocol deviations. Patients dropping out before the baseline assessment were excluded (compare Figure 1). Statistical analyses were performed in R version 4.1.0 (56).
The primary endpoint (time to first relapse of MDD during the course of 12 months after termination of the NAM program, as determined by SCID-I by telephone if monthly BDI-FS ratings indicated elevated depressive symptoms) was analyzed via log-rank test of the two survival curves (NAM vs. TAU) using the R survival package (57).
Missing data (and thus censoring of survival data) was an issue in the monthly BDI-FS and SCID data (compare Figure 2), with a mean (SD) of 3.87 (3.50) months of missing data in NAM and 3.33 (3.61) in TAU group. Thus, after performing the pre-specified ITT analysis on the raw data, we imputed the MDD status for the missing months using the R Amelia package (58). Only cases with at least 3 of the 11 monthly measurements were used (excluding n = 4/5 cases from NAM/TAU groups, respectively, and leaving n = 27/25 for the imputed analysis). The available monthly measurement data was included in the imputation model as lags and leads (thus preserving the information on the sequential order), as well as monthly BDI-FS status when only the depression diagnosis was missing because the SCID-I interview couldn't be conducted in time. The model further included baseline CTQ and baseline, post and follow-up BDI-II, HRSD-17, and WHOQoL global and mental domains. The resulting continuous predictions of MDD status were dichotomized such that the percentage of MDDs in observed and missing months was similar (~10%). Imputation diagnostics by over-imputation and visual inspection of predicted values in the timelines indicated successful prediction. The primary analysis was repeated on 100 imputed datasets, combining the resulting Chi-Square values and testing for significance with the R miceadds package (59). Missing items in questionnaire data were rare (<1%) and were imputed with iterative robust model-based imputation from the VIM R package (60) for observations with <20% missing items. Visual inspection of raw data and model residuals indicated that no outliers were present in the data.
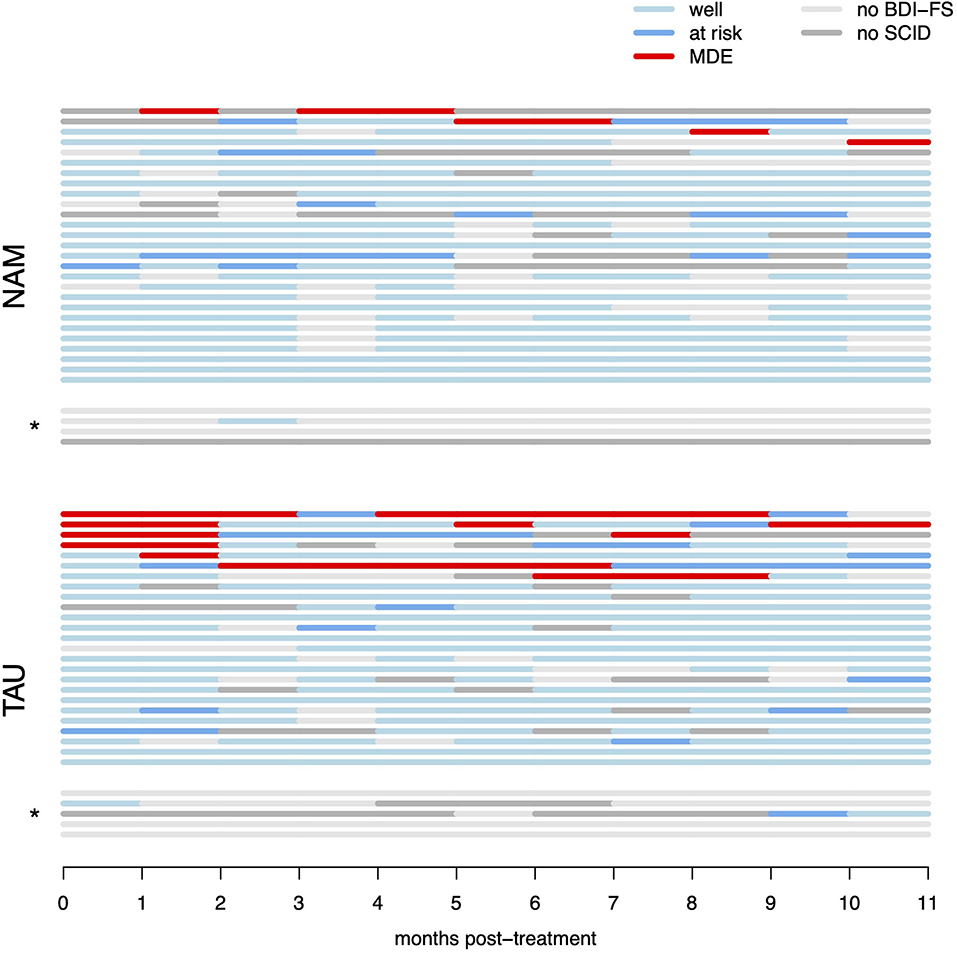
Figure 2. Raw survival data (monthly per-participant data regarding recurrence of MDD). “Well” denotes months with sub-threshold BDI-FS scores (and no diagnostic interview performed), “at risk” denotes months with elevated BDI-FS scores, but criteria for MDE were not met in the diagnostic interview, “MDE” denotes months with elevated BDI-FS scores and diagnosis of major depressive episode in the diagnostic interview by telephone. “No BDI-FS” denotes months for which BDI-FS data could not be obtained, “no SCID” denotes months with elevated BDI-FS scores, but participants could not be reached for the diagnostic interview. *indicates participants with <3 months of available monthly data, which were excluded from the multiple imputation analysis (but included in the raw data ITT survival analysis).
Secondary outcomes were analyzed as linear mixed models with R package lme4 (61), with fixed effects for time (pre-treatment, post-treatment and 1 year follow-up) and group (NAM vs. TAU) as well as their interaction effect, using a random intercept for each participant to account for the repeated measurements. The main test of interest was the chi-squared test of the interaction effect. Further, contrasts of interest were tested using R package lmerTest (62). Treatment contrasts of the interaction between time and group were performed with pre-treatment values as baseline, thus testing for group differences changes from pre- to post-treatment as well as pre-treatment to follow-up.
Participants were predominantly female, middle aged, and with rather high educational status (Table 2). Only a minority worked full-time, with high rates of marginal employment and unemployment.
Due to difficulties in recruiting enough participants for timely randomization into the required groups, 19 participants with elevated, yet less than moderate CTQ scores and early onset of depression were included. Similarly, 13 participants with HRSD-17 scores of 7 or lower during screening were included, when the clinical judgement indicated partial or instable remission. The degree of childhood maltreatment ranged from having suffered mild abuse in only few dimensions, to extreme abuse and neglect in several dimensions (Table 3). Most common were emotional abuse and neglect by about three quarters of the participants. In both groups, about 30% reported no dimensions with moderate or higher severity, about 25% reported 1 dimension, and 45% reported 2–5 dimensions. HRSD-17 scores were in the subthreshold range as expected, with considerable variation even between the four weekly assessments obtained during screening. Only half of participants had prior experience with mindfulness exercises, mostly from in-patient settings.
The test for the primary hypothesis indicated a significant difference between the survival curves in NAM vs. TAU groups [ = 6.62, p = 0.010]. This suggests, that participants in the NAM group experienced fewer relapses and fewer weeks in MDD compared to the TAU group. However, this result has to be interpreted cautiously because of a high level of censoring resulting from missing measurements in the monthly assessments (see Statistical Methods above). All four of the MDD relapses observed in the NAM group (13% relapse, nNAM = 31) are not captured in the raw survival model because of missing data occurring first (i.e., censoring). In contrast, only one of the seven MDD relapses observed in the TAU group (23% relapse, nTAU = 30) is missed (censored) for the same reason. This is partly due to MDD relapses occurring later in the NAM group as hypothesized (with four of the TAU relapses occurring in the first month of observation already), but nonetheless this reduces the robustness of the raw survival analysis (compare Figure 2). The repeated primary analysis on 100 imputed datasets failed to reach statistical significance [F(1, 2496.21) = 0.915, p = 0.339].
The number of observed months in depression added up across all participants was 7 months in the NAM and 27 months in the TAU group in the raw data, with an individual mean time in depression of 0.23 months (SD = 0.67) per NUM participant and 0.90 months (SD = 1.97) per TAU participant. However, the between-groups t-test was on the border to statistical significance [t(35.4) = 1.77, p = 0.084]. Repeating the between-groups test with the multiply imputed dataset and pooling with the R package Zelig (63) also failed to reach statistical significance (z = 1.41, p = 0.158). In the imputed datasets, each patient in NAM group was in depression for 0.54 months on average, patients in TAU group for 1.28 months.
For the HRSD-17, neither the effect of time [ = 2.52, p = 0.284] nor treatment group [ = 0.16, p = 0.692), nor the hypothesized group-by-time interaction ( = 0.73, p = 0.696] were statistically significant. For the BDI-II, the effect of time was statistically significant [ = 11.39, p = 0.003], indicating an improvement over time in self-rated depressive symptoms, which appears especially pronounced in the NAM group (Figure 3). But neither the main effect of group [ = 0.22, p = 0.642] nor the hypothesized group-by-time interaction [ = 3.39, p = 0.183] were statistically significant.
For the overall quality of life (WHOQOL-BREF), the hypothesized group-by-time interaction [ = 7.08, p = 0.029] was statistically significant, The corresponding contrasts indicate that the group difference in improvement from pre- to post-treatment was not statistically significant [t(107.9) = 0.35; p = 0.727], but the improvement from pre-treatment to follow-up was [t(109.9) = 2.42; p = 0.017], indicating an improvement over time in quality of life in the NAM group particularly in the long run (Figure 4). Neither the main effect of time [ = 5.03, p = 0.081] nor group [ = 0.53, p = 0.465] were statistically significant. The hypothesized group-by-time interaction did not reach statistical significance for any of the other secondary outcomes [WHOQOL subscales, mindfulness skills (FFA), and rumination (RSQ), ps > 0.15].
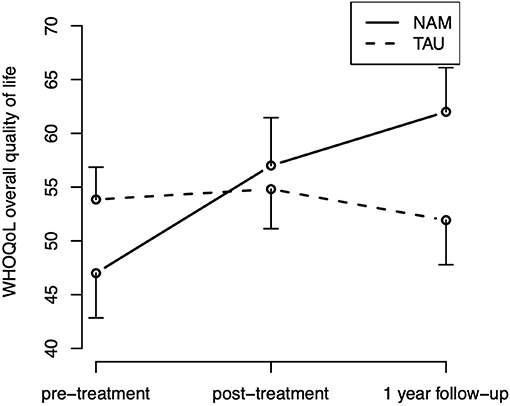
Figure 4. Overall quality of life (WHOQOL) time course by treatment group (means and standard errors).
Twenty-four (of 29) participants filled in an evaluation form after completing the program. Overall satisfaction was high, with 18 (75%) reporting being very satisfied with the program, 5 (21%) being satisfied, and 1 person (4%) neutral (on a 5-point scale from very satisfied to very unsatisfied). A majority of 18 participants (75%) experienced the duration with 8 weekly sessions as too short, the remaining 6 (25%) rating the duration as just right (on a 3-point scale from too long to too short).
Participants rated nature as very helpful, with a mean (SD) score of 4.72 (0.33) on the scale from 1 to 5. Similarly, the involvement of animals was rated as very helpful with a mean score of 4.67 (0.37). The amount of agitation experienced during exercises was rated as moderate, with a mean score of 3.08 (1.09). Figure 5 displays the responses to individual items of interest from these scales.

Figure 5. Participants experiences with the NAM intervention (percentages of responses on each 5-point scale, total n = 24 for all scales). (A) “I felt safe and grounded because of the natural environment” (B) “I prefer a therapy program in nature rather than clinic rooms” (C) “Contact with the animals was helpful for me” (D) “At times I was agitated by the mindfulness practice.”
The aim of the present study was to evaluate the preventative efficacy of a nature- and animal assisted mindfulness program over the course of 1 year in high-risk patients (i.e., unstable or partially remitted with a history of early life trauma and three or more depressive episodes). There was a significant difference between the survival curves in NAM vs. standard treatment. The results suggest fewer relapses and months in depression in the NAM group. While relapses were relatively rare in our sample overall, both the number of participants with relapses as well as the individual duration and number of relapses appear reduced in the NAM group (compare Figure 2), resulting in only about a quarter of observed months in depression compared to TAU. Self-rated depressive symptoms and rumination decreased, and mindfulness skills increased in the course of the program in both groups with no significant difference. The global quality of life improved significantly more in the NAM group.
Another goal of this study was to determine the feasibility and acceptance of the program by the participants. Compared to attrition rates of 29.2% (64) in other studies using mindfulness approaches, we observed a low dropout rate of only 6% in the NAM group suggesting high compliance with the intervention among the patients. However, the specific retention rate among patients with childhood maltreatment is largely unknown (8), but expected to be higher than in patients without childhood maltreatment. In addition, the satisfaction with the NAM program was very high: The vast majority of participants in the intervention group were “very satisfied” or “satisfied” with the program, although wishing for a longer duration of the intervention. Most patients felt safe and grounded practicing mindfulness in nature. While almost all patients fully agreed that sheep were helpful and motivating as assistance throughout the sessions, only a few (17%) still felt sometimes agitated during the exercises. The use of nature and animals facilitated the engagement in the treatment process and prevented participants from dropping out. This might have also been influenced by the fact that almost all participants rated themselves as animal and nature lovers, a question that is often discussed as a limitation in the literature, however rarely assessed.
Since increasing mindfulness skills did not seem to be the main driver of effects, we assume as potential mechanisms of NAM that the assistance of nature and animals led to a decrease of arousal and enabled the patients to feel safe enough to get fully involved in the program. Thus, by increasing treatment engagement, the learning of general coping skills was facilitated and in this way might have reduced depression symptoms and relapses in the long term.
Our results are in line with a recent meta-analytical review suggesting that nature-based mindfulness shows advantages compared to mindfulness programs in non-natural settings (21). Mindfulness in wild nature—as practiced in our study—seems to be more beneficial than mindfulness in more cultivated settings such as parks or gardens, but the importance of the setting needs further investigation. To our knowledge, this is the first study using a nature- as well as an animal assisted setting in a mindfulness program. There is very limited prior empirical research on the clinical effects of conventional mindfulness-based interventions in adult individuals with childhood maltreatment (8). The wide variety of animals used, the duration of the intervention, outcome measures, control conditions, and methodological quality make it difficult to compare across studies utilizing animal assisted therapy. None of the studies used monthly assessments of relapses which is one of the strengths of our trial. It is criticized in the literature that despite hundreds of published studies on this issue, there is only limited evidence for the efficacy of the incorporation of animals in the treatment of mental disorders as these studies frequently suffer from lack of methodological rigor. Even though in recent years the quality of animal assisted therapy research has gotten better, most studies still use very small sample sizes, no appropriate control groups, no blinding of raters, no assessments of long-term effectiveness, no manualization of animal interventions, selective reporting of outcome measures or other weaknesses in the research designs (65–67). In our study, most of those quality characteristics were considered.
There are also several limitations of the present study. First, patients were heterogeneous in terms of baseline severity of depressive symptoms and severity of childhood trauma. Aggravating this issue, we had to allow for an extended use of inclusion criteria (severity of HRSD score and CTQ-score) for recruitment reasons. Furthermore, it proved difficult to obtain monthly assessments from patients in this chronically impaired clinical group who had to respond both to monthly e-mail invitations for an online questionnaire and be contacted by telephone in case of elevated values. The multiple imputation of missing values provides important context, but plausibly made it harder to detect meaningful differences in combination with the primary survival analysis, because the purposeful introduction of random variation in multiple imputation runs counter to the high importance the survival analysis places on singular events. In combination with a relapse rate generally lower than anticipated, this probably led to a dilution of treatment effects in the multiple imputation procedure through the introduction of “random” relapses. At the same time, the analysis of the raw data is affected by the survival analysis' sensitivity to missing data, resulting in the loss of information after missing months (i.e., “censoring”). As noted in the methodological literature, a perspective of clinical improvement and mental health as continuous variables might be more helpful (68, 69).
In conclusion, in a study environment including mainly individuals who like animals and nature, a nature- and animal assisted mindfulness program for relapse prevention proved to be feasible and highly acceptable among patients with trauma histories. NAM turned out to be more effective than standard treatment in preventing relapses and weeks in MDD in our study population. Further evidence should routinely include measurement of early trauma to increase the amount of empirical data on this topic. We found that nature and animals can facilitate the engagement in the treatment process for individuals with a history of childhood maltreatment. However, more research is needed on trauma sensitive mindfulness-based protocols including nature- and animal assistance. Since this form of nature- and animal-assisted mindfulness program was never investigated before, the first step was to compare the approach with treatment-as-usual. Future work could directly compare the effects to conventional MBCT programs among patients with and without childhood maltreatment histories.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the University of Freiburg. The patients/participants provided their written informed consent to participate in this study.
ES and CB had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ES, TF, and RW: study concept and design. ES, CB, TF, HP, and ME: acquisition, analysis, or interpretation of data and drafting of the manuscript. CB: statistical analysis. ES: obtained funding and administrative, technical, or material support and study supervision. All authors: critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
This study was funded by the Stiftung zur Förderung der Erforschung von Zivilisationserkrankungen, Baden-Baden, Germany. The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the Mundenhof KonTiKi team, Freiburg, Germany for the support of the study and Angelika Fischhaber and Barbara Schäfer for conducting the mindfulness program as co-trainer and taking care of the animals welfare. The supporters played no role in the study design, or in collecting, analyzing, and interpreting our data, in writing the report, or in the decision to submit the article for publication. We acknowledge support by the Open Access Publication Fund of the University of Freiburg.
1. Karyotaki E, Smit Y, Holdt Henningsen K, et al. Combining pharmacotherapy and psychotherapy or monotherapy for major depression? A meta-analysis on the long-term effects. J Affect Disord. (2016) 194:144–52. doi: 10.1016/j.jad.2016.01.036
2. Guidi J, Fava GA. Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: a systematic review and meta-analysis. JAMA Psychiatry. (2021) 78:261–9. doi: 10.1001/jamapsychiatry.2020.3650
3. Schramm E, Elsaesser M, Guidi J. The role of psychological interventions in the maintenance treatment of depression. Psychother Psychosom. (2022) 91:212–3. doi: 10.1159/000522014
4. Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. (2000) 68:615–23. doi: 10.1037/0022-006X.68.4.615
5. Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clin Psychol Rev. (2011) 31:1032–40. doi: 10.1016/j.cpr.2011.05.002
6. Kuyken W, Warren FC, Taylor RS, Whalley B, Crane C, Bondolfi G, et al. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse: an individual patient data meta-analysis from randomized trials. JAMA Psychiatry. (2016) 73:565–74. doi: 10.1001/jamapsychiatry.2016.0076
7. Lindahl JR, Fisher NE, Cooper DJ, Rosen RK, Britton WB. The varieties of contemplative experience: a mixed-methods study of meditation-related challenges in Western Buddhists. PLoS ONE. (2017) 12:e0176239. doi: 10.1371/journal.pone.0176239
8. Joss D, Teicher MH. Clinical effects of mindfulness-based interventions for adults with a history of childhood maltreatment: a scoping review. Curr Treat Options Psych. (2021) 8:31–46. doi: 10.1007/s40501-021-00240-4
9. Williams JMG, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJ, et al. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: a randomized dismantling trial. J Consult Clin Psychol. (2014) 82:275–86. doi: 10.1037/a0035036
10. Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. (2015) 386:63–73. doi: 10.3310/hta19730
11. Beshai S, Parmar P. Trait mindfulness may buffer against the deleterious effects of childhood abuse in recurrent depression: a retrospective exploratory study. Clin Psychol. (2019) 23:26–36. doi: 10.1111/cp.12147
12. Beshai S, Dobson KS, Bockting CL, Quigley L. Relapse and recurrence prevention in depression: current research and future prospects. Clin Psychol Rev. (2011) 31:1349–60. doi: 10.1016/j.cpr.2011.09.003
13. Bockting CL, Hollon SD, Jarrett RB, Kuyken W, Dobson K. A lifetime approach to major depressive disorder: the contributions of psychological interventions in preventing relapse and recurrence. Clin Psychol Rev. (2015) 41:16–26. doi: 10.1016/j.cpr.2015.02.003
14. Bent S, Padula A, Avins AL. Brief communication: better ways to question patients about adverse medical events: a randomized, controlled trial. Ann Intern Med. (2006) 144:257–61. doi: 10.7326/0003-4819-144-4-200602210-00007
15. King AP, Erickson TM, Giardino ND, Favorite T, Rauch SA, Robinson E, et al. A pilot study of group mindfulness-based cognitive therapy (mbct) for combat veterans with posttraumatic stress disorder (ptsd). Depress Anxiety. (2013) 30:638–45. doi: 10.1002/da.22104
16. Vallejo Z, Amaro H. Adaptation of mindfulness-based stress reduction program for addiction relapse prevention. Humanistic Psychol. (2009) 37:192–206. doi: 10.1080/08873260902892287
17. Treleaven DA. Trauma-Sensitive Mindfulness: Practices for Safe and Transformative Healing. New York, NY; London: W. W. Norton & Company (2018).
18. Coventry PA, Brown JenniferVE, Pervin J, Brabyn S, Pateman R, Breedvelt J, et al. Nature-based outdoor activities for mental and physical health: systematic review and meta-analysis. SSM - Population Health. (2021) 16:100934. doi: 10.1016/j.ssmph.2021.100934
19. van den Berg M, van Poppel M, van Kamp I, Andrusaityte S, Balseviciene B, Cirach M, et al. Visiting green space is associated with mental health and vitality: a cross-sectional study in four european cities. Health Place. (2016) 38:8–15. doi: 10.1016/j.healthplace.2016.01.003
20. Ulrich RS, Simons RF, Losito BD, Fiorito E, Miles MA, Zelson M. Stress recovery during exposure to natural and urban environments. J Environ Psychol. (1991) 11:201–30. doi: 10.1016/S0272-4944(05)80184-7
21. Djernis D, Lerstrup I, Poulsen D, Stigsdotter U, Dahlgaard J, O'Toole M. A systematic review and meta-analysis of nature-based mindfulness: effects of moving mindfulness training into an outdoor natural setting. Int J Environ Res Public Health. (2019) 16:3202. doi: 10.3390/ijerph16173202
22. Virués-Ortega J, Pastor-Barriuso R, Castellote JM, Población A, de Pedro-Cuesta J. Effect of animal-assisted therapy on the psychological and functional status of elderly populations and patients with psychiatric disorders: a meta-analysis. Health Psychol Rev. (2012) 6:197–221. doi: 10.1080/17437199.2010.534965
23. Waite TC, Hamilton L, O'Brien W. A meta-analysis of Animal Assisted Interventions targeting pain, anxiety and distress in medical settings. Complement Ther Clin Pract. (2018) 33:49–55. doi: 10.1016/j.ctcp.2018.07.006
24. Fine AH. Handbook on Animal-Assisted Therapy: Foundations and Guidelines for Animal-Assisted Interventions. London: Academic Press (2019).
25. Barker SB, Pandurangi AK, Best AM. Effects of animal-assisted therapy on patients' anxiety, fear, and depression before ECT. J ECT. (2003) 19:38–44. doi: 10.1097/00124509-200303000-00008
26. Hoffmann AOM, Lee AH, Wertenauer F, Ricken R, Jansen JJ, Gallinat J, et al. Dog-assisted intervention significantly reduces anxiety in hospitalized patients with major depression. Eur J Integr Med. (2009) 1:145–8. doi: 10.1016/j.eujim.2009.08.002
27. Lang UE, Jansen JB, Wertenauer F, Gallinat J, Rapp MA. Reduced anxiety during dog assisted interviews in acute schizophrenic patients. Eur J Integr Med. (2010) 2:123–7. doi: 10.1016/j.eujim.2010.07.002
28. Wohlfarth R, Mutschler B, Bitzer E. Der Epilepsiehund – traumtänzerei, tierquälerei oder sinnvoller einsatz? Z Epileptol. (2013) 26:90–7. doi: 10.1007/s10309-013-0313-7
29. Crowley-Robinson P, Fenwick DC, Blackshaw JK. A long-term study of elderly people in nursing homes with visiting and resident dogs. Appl Anim Behav Sci. (1996) 47:137–48. doi: 10.1016/0168-1591(95)01017-3
30. Perkins J, Bartlett H, Travers C, Rand J. Dog-assisted therapy for older people with dementia: a review. Australas J Ageing. (2008) 27:177–82. doi: 10.1111/j.1741-6612.2008.00317.x
31. Schramm E, Hediger K, Lang UE. From animal behavior to human health: an animal-assisted mindfulness intervention for recurrent depression. Zeitschrift für Psychol. (2015) 223:192–200. doi: 10.1027/2151-2604/a000220
32. Marino L, Merskin D. Intelligence, complexity, and individuality in sheep. Anim Sentience. (2019) 4:1–26. doi: 10.51291/2377-7478.1374
33. Kim JS, Jin MJ, Jung W, Hahn SW, Lee SH. Rumination as a mediator between childhood trauma and adulthood depression/anxiety in non-clinical participants. Front Psychol. (2017) 8:1597. doi: 10.3389/fpsyg.2017.01597
34. Stuewig J, McCloskey LA. The relation of child maltreatment to shame and guilt among adolescents: psychological routes to depression and delinquency. Child Maltreat. (2005) 10:324–36. doi: 10.1177/1077559505279308
35. Dorahy MJ, Clearwater K. Shame and guilt in men exposed to childhood sexual abuse: a qualitative investigation. J Child Sex Abus. (2012) 21:155–75. doi: 10.1080/10538712.2012.659803
36. Joss D, Khan A, Lazar SW, Teicher MH. Effects of a mindfulness-based intervention on self-compassion and psychological health among young adults with a history of childhood maltreatment. Front Psychol. (2019) 10:2373. doi: 10.3389/fpsyg.2019.02373
37. Vettese LC, Dyer CE, Li WL, Wekerle C. Does self-compassion mitigate the association between childhood maltreatment and later emotion regulation difficulties? A preliminary investigation. Int J Ment Health Addiction. (2011) 9:480–91. doi: 10.1007/s11469-011-9340-7
38. Fredrickson M, Howie AR. Methods, standards, guidelines, and considerations in selecting animals for animal-assisted therapy: Part B: guidelines and standards for animal selection in animal-assisted activity and therapy programs. In: Heidenreich T, Michalak J, editors. Handbook on Animal-Assisted Therapy: Theoretical Foundations and Guidelines for Practice. Tübingen: Academic Press (2000). p. 99–114. doi: 10.1016/B978-012369484-3/50008-6
39. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corperation (1996). doi: 10.1037/t00742-000
40. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56. doi: 10.1136/jnnp.23.1.56
41. The WHOQoL Group. Development of the World Health Organization WHOQOL-BREF Quality of Life Assessment. Psychol Med. (1998) 28:551–8. doi: 10.1017/S0033291798006667
42. Walach H, Buchheld N, Buttenmüller V, Kleinknecht N, Grossmann P, & Schmidt S. Empirische Erfassung der Achtsamkeit - Die Konstruktion des Freiburger Fragebogens zur Achtsamkeit (FFA) und weitere Validierungsstudien. In Heidenreich T, Michalak J, editors. Achtsamkeit und Akzeptanz in der Psychotherapie. Ein Handbuch. Tübingen: dgvt-Verlag (2004). p. 755–800.
43. Kühner C, Huffziger S.&, Nolen-Hoeksema S. Response Styles Questionnaire - Deutsche Version (RSQ-D). Göttingen: Hogrefe (2007).
44. Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. (2010) 40:41–50. doi: 10.1017/S0033291709006011
45. Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. (2003) 27:169–90. doi: 10.1016/S0145-2134(02)00541-0
46. First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Clinical Trials Version (SCID-CT). New York, NY: Biometrics Research, New York State Psychiatric Institute (2002).
47. First MB, Gibbon M. The structured clinical interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In: Hilsenroth MJ, Segal DL, Hersen M, editors. Comprehensive Handbook of Psychological Assessment, Vol. 2: Personality Assessment. Hoboken, NJ: John Wiley & Sons, Inc (2004). p. 134–43.
48. Segal ZV, Teasdale JD, Williams JM, Gemar MC. The mindfulness-based cognitive therapy adherence scale: inter-rater reliability, adherence to protocol and treatment distinctiveness. Clin Psychol Psychother. (2002) 9:131–8. doi: 10.1002/cpp.320
49. Joss D, Lazar SW, Teicher MH. Nonattachment predicts empathy, rejection sensitivity, and symptom reduction after a mindfulness-based intervention among young adults with a history of childhood maltreatment. Mindfulness. (2020) 11:975–90. doi: 10.1007/s12671-020-01322-9
50. Beck AT, Steer RA, Brown GK, Kliem S, Bräler E. Beck Depressions-Inventar - Fast Screen (BDI-FS). Frankfurt am Main: Pearson Publisher(2013).
51. Benedict RHB, Fishman I, McClellan MM, Bakshi R, Weinstock-Guttman B. Validity of the Beck Depression Inventory-Fast Screen in multiple sclerosis. Mult. Scler. (2003) 9:393–6. doi: 10.1191/1352458503ms902oa
52. Poole H, Bramwell R, Murphy P. The utility of the Beck Depression Inventory Fast Screen (BDI-FS) in a pain clinic population. Eur J Pain. (2009) 13:865–9. doi: 10.1016/j.ejpain.2008.09.017
53. Neitzer A, Sun S, Doss S, Moran J, Schiller B. Beck Depression Inventory-Fast Screen (BDI-FS): an efficient tool for depression screening in patients with end-stage renal disease. Hemodial Int. (2012) 16:207–13. doi: 10.1111/j.1542-4758.2012.00663.x
54. Vindbjerg E, Makransky G, Mortensen EL, Carlsson J. Cross-cultural psychometric properties of the hamilton depression rating scale. Can J Psychiatry. (2019) 64:39–46. doi: 10.1177/0706743718772516
55. Wingenfeld K, Spitzer C, Mensebach C, Grabe HJ, Hill A, Gast U, et al. The German version of the Childhood Trauma Questionnaire (CTQ): preliminary psychometric properties. Psychother Psychosom Med Psychol. (2010) 60:442–50. doi: 10.1055/s-0030-1247564
56. R Core Team. R: A Language Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2021). Available online at: https://www.R-project.org/
57. Therneau T. _A Package for Survival Analysis in R_. R package version 3.2-11. Available online at: https://CRAN.R-project.org/package=survival> (2021).
58. Honaker J, King G, Blackwell M. Amelia II: a program for missing data. J Stat Softw. (2011) 45:1–47. doi: 10.18637/jss.v045.i07
59. Robitzsch A, Grund S. miceadds: Some Additional Multiple Imputation Functions, Especially for ‘mice'. R package version 3.11-6 (2021).
60. Kowarik A, Templ M. Imputation with the R Package VIM. J Stat Softw. (2016) 74:1–16. doi: 10.18637/jss.v074.i07
61. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. (2015) 67:1–48. doi: 10.18637/jss.v067.i01
62. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. (2017) 82:1–26. doi: 10.18637/jss.v082.i13
63. Choirat C, Honaker J, Imai K, King G, Lau O. _Zelig: Everyone's Statistical Software. Version 5.1.7. Available online at: https://zeligproject.org/>(2020).
64. Nam S, Toneatto T. The influence of attrition in evaluating the efficacy and effectiveness of mindfulness-based interventions. Int J Ment Health Addiction. (2016) 14:969–81. doi: 10.1007/s11469-016-9667-1
65. Koukourikos K, Georgopoulou A, Kourkouta L, Tsaloglidou A. Benefits of animal assisted therapy in mental health. Int J Caring Sci. (2019) 12:1898–905. Available online at: http://www.internationaljournalofcaringsciences.org/docs/64_koukorikos_review_12_3.pdf
66. Santaniello A, Dicé F, Claudia Carratú R, Amato A, Fioretti A, Menna LF. Methodological and terminological issues in animal-assisted interventions: an umbrella review of systematic reviews. Animals. (2020) 10:759. doi: 10.3390/ani10050759
67. Turner DC, Rowan AN, Herzog H, et al. A glimpse at the future of animal-assisted interventions: Selected commentaries. In: Fine A, editor. Handbook on Animal-Assisted Therapy: Foundations and Guidelines for Animal-Assisted Interventions, 4th Ed. Pomona, CA: Elsevier Academic Press (2015). p. 391–414. doi: 10.1016/B978-0-12-801292-5.00028-6
68. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. (2006) 332:1080. doi: 10.1136/bmj.332.7549.1080
Keywords: mindfulness based intervention, nature based, animal assisted, psychotherapy, depression prevention, randomized controlled trial, early life trauma, childhood maltreatment
Citation: Schramm E, Breuninger C, Wohlfarth R, Elsaesser M, Piosczyk H and Fangmeier T (2022) Effectiveness of Nature- and Animal Assisted Mindfulness for Relapse Prevention in Depressed Patients With a History of Childhood Maltreatment. Front. Psychiatry 13:899318. doi: 10.3389/fpsyt.2022.899318
Received: 18 March 2022; Accepted: 09 June 2022;
Published: 14 July 2022.
Edited by:
Arndt Büssing, Witten/Herdecke University, GermanyReviewed by:
Christina Metcalf, University of Colorado Anschutz Medical Campus, United StatesCopyright © 2022 Schramm, Breuninger, Wohlfarth, Elsaesser, Piosczyk and Fangmeier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabeth Schramm, ZWxpc2FiZXRoLnNjaHJhbW1AdW5pa2xpbmlrLWZyZWlidXJnLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.