
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 09 June 2022
Sec. Sleep Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.898600
This article is part of the Research TopicCircadian Rhythm Sleep-wake Disorders: Pathophysiology, Comorbidity, and ManagementView all 9 articles
A correction has been applied to this article in:
Corrigendum: Decrease in social zeitgebers is associated with worsened delayed sleep-wake phase disorder: findings during the pandemic in Japan
 Rei Otsuki1,2,3
Rei Otsuki1,2,3 Kentaro Matsui1,2*
Kentaro Matsui1,2* Takuya Yoshiike2
Takuya Yoshiike2 Kentaro Nagao2,4
Kentaro Nagao2,4 Tomohiro Utsumi2,5
Tomohiro Utsumi2,5 Ayumi Tsuru1,2
Ayumi Tsuru1,2 Naoko Ayabe2,6
Naoko Ayabe2,6 Megumi Hazumi2,7
Megumi Hazumi2,7 Michio Fukumizu2,8
Michio Fukumizu2,8 Kenichi Kuriyama2
Kenichi Kuriyama2Background: Delay in sleep-wake rhythms was observed in the general population during the coronavirus disease 2019 (COVID-19) pandemic. Patients with delayed sleep-wake phase disorder (DSWPD) may have also experienced exacerbation of symptoms, but no studies have investigated this topic. In this study, we aimed to retrospectively examine the changes in symptoms of outpatients with DSWPD both before and during the pandemic and to identify the factors associated with the exacerbation of sleep-wake rhythms.
Methods: We included outpatients with DSWPD aged 16 years or older who visited the outpatient clinic due to sleep disorders between January and September 2020. Decreased social zeitgebers was defined as a reduction of 50% or more in the frequency of commuting to school or work during the COVID-19 pandemic. The severity of DSWPD was assessed using the clinical global impressions - severity of illness (CGI-S) at two points: before and during the pandemic. We defined the worsened, unchanged, and improved groups as those whose CGI-S scores worsened by at least one point, remained unchanged, and improved by at least one point, respectively. Multivariate logistic regression analysis was performed to determine the factors associated with worsened DSWPD symptoms.
Results: Sixty patients with DSWPD were eligible for this study. Even before the pandemic, patients who were unemployed or did not attend school tended to show more severe DSWPD symptoms. During the pandemic, 27 patients belonged to the worsened group; 28 patients, unchanged group; and 5 patients, improved group. Decreased social zeitgebers (odds ratio [OR] = 6.668, 95% confidence interval [CI]: 1.653–26.891, p < 0.05) and comorbid mood disorders (OR = 8.876, 95% CI: 1.714–45.974, p < 0.05) showed independent significant associations with the worsening of DSWPD symptoms.
Conclusions: During the pandemic, the symptoms of DSWPD tended to worsen. The obtained findings emphasize the importance of social zeitgebers, suggesting the need for external motivation in DSWPD treatment.
Circadian rhythm sleep-wake disorders (CRSWDs) are characterized by an inability to synchronize the endogenous circadian rhythm with the daily sleep-wake schedule required for social life, resulting in distress and social disadvantage for the individual (1). CRSWDs consist of several disorders, and delayed sleep-wake phase disorder (DSWPD) is the most common. The prevalence of DSWPD in the general population ranges from 0.1 to 5.1% (2–4), and a higher prevalence (1.1 to 15.9%) has been reported in adolescents and young adults (5–7). DSWPD is known to have serious negative social consequences due to pronounced difficulty falling asleep and waking up at the desired time. These symptoms result in difficulty going to work or school tardiness and absence, as well as falling asleep or poor performance during working hours, with mental health problems, including anxiety and depression (8–11). DSWPD is often comorbid with mood disorders, schizophrenia, and developmental disorders. Moreover, it has been suggested that these comorbidities are associated with worsened symptoms of their own disorders (12–15). DSWPD comorbid with psychiatric or developmental disorders has been reported to have different clinical characteristics compared with primary DSWPD, such as older age, higher rates of unemployment, and poorer treatment responsiveness (10, 16).
Since the intrinsic cycle of the human circadian rhythm is slightly longer than 24 h (17–20), synchronizing factors that advance the circadian phase are considered essential to keep the sleep-wake rhythms synchronized to 24 h. Not only by exposure to high-intensity light immediately after waking (21), non-photic social zeitgebers, such as work and school during the daytime, contribute to preventing delayed sleep-wake phase rhythms (22). However, some people have been in confinement due to the coronavirus disease (COVID-19)-related lockdown, which may have disrupted their sleep-wake rhythms due to the loss of social zeitgebers (23). Delay in bed time and waking time of workers and students as a result of the lockdown has already been reported (24). A tendency toward delayed sleep-wake rhythms during the pandemic has also been indicated in the general population (25–28); thus, the possibility of increased DSWPD development has been pointed out (29).
Patients with DSWPD who are already receiving treatment may also have experienced a delay in their sleep-wake rhythms during the pandemic. Although the vulnerability to circadian rhythm dysregulation in patients with DSWPD has been widely reported (30–32), the association of decreased social zeitgebers during the pandemic with changes in the sleep-wake schedule in patients with DSWPD has not yet been elucidated. Furthermore, no study has investigated how comorbidity with psychiatric or developmental disorders has affected sleep-wake schedules in patients with DSWPD during the COVID-19 pandemic. We hypothesized that worsened DSWPD symptoms would be more pronounced in patients who experienced a significant change in lifestyle, particularly the loss of social zeitgebers due to suspension of commuting to school or work during the pandemic. We also speculated that patients with comorbid psychiatric and developmental disorders would be more likely to experience worsened DSWPD symptoms than those without such disorders, considering their possible vulnerability during the pandemic. We aimed to investigate the changes in the symptoms of DSWPD in outpatients before and during the COVID-19 pandemic to examine the aforementioned hypothesis, as well as to identify the factors associated with the changes in severity among these patients.
We retrospectively reviewed the medical records of patients with DSWPD aged 16 years or older who visited the outpatient clinic of the National Center Hospital of Neurology and Psychiatry for sleep disorders between January and September 2020. Patients with DSWPD who stopped visiting the hospital or had insufficient information were excluded from the analysis. DSWPD diagnosis was confirmed by board-certified sleep medicine physicians of the Japanese Society of Sleep Research (KM, TY, AT, MF, and KK) according to the International Classification of Sleep Disorders, 3rd edition (1).
The following demographic and clinical data were retrospectively collected from the medical records: age, sex, body mass index (BMI), school/work status, presence of cohabitants, comorbidity of psychiatric disorders and sleep disorders (except DSWPD), introduction of chronobiological interventions including melatonin agonists (ramelteon and melatonin) and bright light therapy (33), and pharmacological treatment using psychotropic medications other than melatonin agonists. The introduction of chronobiological interventions was investigated at baseline and during the pandemic. However, for psychotropic medications other than melatonin agonists, only the baseline status was assessed due to insufficient information in the medical records. For social zeitgebers, we assessed the frequency of commuting to school and work before and during the COVID-19 pandemic and defined decreased social zeitgeber as a reduction of 50% or more in the frequency of commuting to school or work under the COVID-19 pandemic than before.
The severity of DSWPD symptoms was retrospectively evaluated using the clinical global impressions - severity of illness (CGI-S) (34) for two periods: baseline, from January to March 2020 (before the COVID-19 pandemic), and endpoint, from April to September 2020 (during the COVID-19 pandemic). CGI-S was scored as follows: 1 = normal, not sick at all; 2 = borderline psychosis; 3 = mild illness; 4 = moderate illness; 5 = marked illness; 6 = severe illness; and 7 = most severe illness. We used the CGI-S because there has been no internationally accepted severity scale for DSWPD. In addition, the CGI-S is simple, can be examined retrospectively using the same criteria, and has been used to rate the severity of DSWPD in some previous studies (35, 36). When determining the CGI-S score, two board-certified psychiatrists of the Japanese Society of Psychiatry and Neurology (RO and KM, KM is also a board-certified sleep medicine physician of the Japanese Society of Sleep Research) evaluated the severity of DSWPD, according to the difference between the desired time and the actual time of sleep onset and awakening and the percentage of days when the patient could fall asleep and wake up at the desired time. In some patients for whom sleep logs and actigraphs were recorded, those results were also accounted. Using the median value of the baseline CGI-S scores, we defined a baseline CGI-S score of 4 or higher as moderate-to-severe DSWPD and a baseline CGI-S score of less than four as mild DSWPD. For the change in symptoms during the pandemic, we used the difference in CGI-S scores between baseline and endpoint: an increase of one or more points as worsened, no change as unchanged, and a decrease of one or more points as improved.
Based on baseline severity, a comparison between mild DSWPD and moderate-to-severe DSWPD was made using the χ2 test for categorical variables and the Mann–Whitney U-test for the following continuous variables: age, sex, BMI, being a student, presence of cohabitants, comorbidity of psychiatric disorders, comorbidity of sleep disorders other than DSWPD, introduction of chronobiological interventions before the pandemic, and use of psychotropic medications other than melatonin agonists. To investigate the factors associated with worsened DSWPD, logistic regression analysis was conducted for age, sex, BMI, student (yes/no), cohabitation (yes/no), coexistence of psychiatric disorders (schizophrenia/mood disorders/anxiety disorders/intellectual disability, and developmental disorders), coexistence of sleep disorders other than DSWPD (yes/no), and introduction of chronobiological interventions during the COVID-19 pandemic (yes/no). Multiple regression analysis was performed with worsened DSWPD symptoms (i.e., increase of one or more points in CGI-S score) as the dependent variable. All variables were first examined in a univariate model. Then, a multiple regression model was performed on all variables that showed significant correlation in the univariate model to determine the main correlations, controlling for confounding factors. SPSS version 27.0J (SPSS Japan, Inc., Tokyo, Japan) was used for statistical analysis, and the statistical significance level was set at less than 5%.
Of the 108 patients with DSWPD, 48 were excluded because they had interrupted outpatient visits (n = 15), were referred to other outpatient clinics (n = 3), and could not be rated for severity due to insufficient information (n = 30). Finally, 60 patients with DSWPD were included in the analysis. Table 1 shows the demographic and clinical data of the study subjects. Accordingly, the median age of the total cohort (range) was 24 (16–71) years, and 56.7% of the subjects were male. The median baseline CGI-S score (range) was 3 (2–6), and the median endpoint CGI-S score (range) was 4 (2–6). A total of 26 patients (43.3%) were students, and 20 patients (33.3%) were unemployed or did not attend school at baseline. Notably, 38 patients (63.3%) experienced decreased social zeitgebers during the COVID-19 pandemic. Psychiatric or developmental disorders were comorbid in 26 patients (43.3%): 1 had schizophrenia, 13 had mood disorders (including 9 with major depressive disorder and 4 with bipolar disorder), 6 had anxiety disorders (including 1 with generalized anxiety disorder, 3 with social anxiety disorder, and 2 with obsessive-compulsive disorder), and 11 had developmental disorders (including 2 with attention-deficit/hyperactivity disorder, 8 with autism spectrum disorder, and 1 with both, with some overlap to other psychiatric disorders). The number of patients with sleep disorders other than DSWPD was 18 (30%), including 12 with obstructive sleep apnea, 3 with central hypersomnia (including 2 with narcolepsy type 2, 1 with idiopathic hypersomnia), 3 with sleep-related movement disorders (including 1 with restless legs syndrome and 2 with periodic limb movement disorder), and 1 with parasomnia (sleep-related eating disorder). Forty-four (73.3%) patients received chronobiological interventions and 33 (55%) took psychotropic medications at baseline.
Before the pandemic, 32 and 28 patients were considered to have mild DSWPD and moderate-to-severe DSWPD, respectively, according to the baseline CGI-S score. Comparison between the two groups showed that the moderate-to-severe DSWPD group had significantly more patients who were unemployed or did not attend school (p = 0.001) than the mild DSWPD group. The patients in the mild DSWPD group tended to be older, have a higher BMI, and were more likely to have comorbidities of other sleep disorders than moderate-to-severe DSWPD, but these differences were not significant (p = 0.061, p = 0.064, and p = 0.055, respectively) (Table 2).
Baseline and endpoint CGI-S scores showed that 27 patients worsened, 28 remained unchanged, and 5 improved with regard to DSWPD symptoms. For patients with worsened DSWPD symptoms, univariate logistic regression analysis showed that decreased social zeitgebers (odds ratio [OR] = 4.675, 95% confidence interval [CI]: 1.427–15.321, p < 0.05) and comorbidity of mood disorders (OR = 5.882, 95% CI: 1.421–24.355, p < 0.05) were significantly associated. In the multiple logistic regression model, both decreased social zeitgebers (OR = 6.668, 95% CI: 1.653–26.891, p < 0.05) and comorbidity of mood disorders (OR = 8.876, 95% CI: 1.714–45.974, p < 0.05) exhibited independent significant associations (Table 3).
During the observation period, only 1 out of the 44 patients discontinued chronobiological interventions. A total of 43 patients received chronobiological interventions during the pandemic: 36 patients received melatonin agonists (melatonin, ramelteon) only, 1 patient received bright light therapy only, and 6 patients received combination therapy of melatonin agonists and bright light therapy. The use of melatonin agonists, bright light therapy, and combinations of these two therapies in these three groups are shown separately in the mild and moderate-to-severe DSWPD groups (Table 4). All five patients in the improvement group received chronobiological interventions (four, ramelteon or melatonin only; one, combination therapy with melatonin agonists and bright light therapy). Even under treatment, 14 of the 20 patients in the mild DSWPD group worsened. Meanwhile, the majority of the patients in the moderate-to-severe DSWPD group showed no change in symptoms.
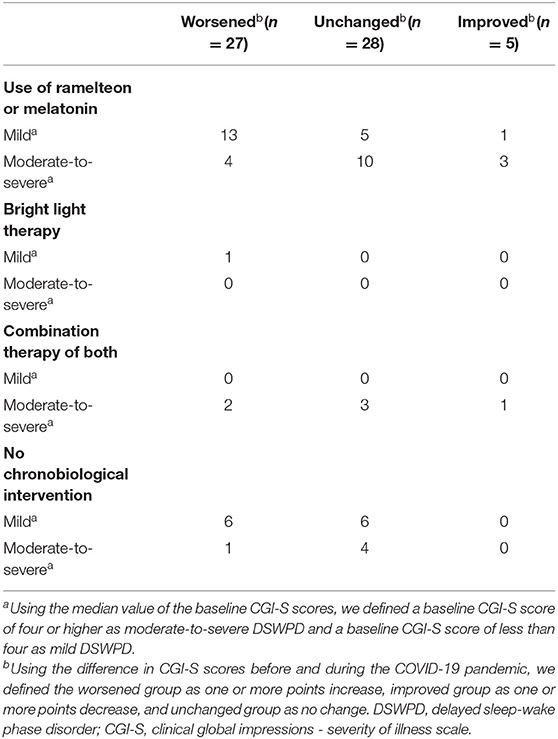
Table 4. Breakdown of chronobiological intervention during the COVID-19 pandemic, baseline severity of DSWPD, and changes in symptoms.
The present study examined the changes in severity among patients with DSWPD symptoms during the COVID-19 pandemic. As we hypothesized, reduced social zeitgeber showed an association with worsened severity of DSWPD. Environmental, social, and behavioral factors have already been identified as potentially important in DSWPD treatment (37, 38). However, to the best of our knowledge, this study is the first to address the possible effect of social zeitgebers on symptom severity and course of treatment in DSWPD, even when reviewing the literature prior to the COVID-19 pandemic.
Although bright light exposure in the morning advances the sleep-wake phase and stabilizes sleep-wake rhythms (21), whether the loss of social zeitgebers resulted in delayed sleep-wake rhythms via reduced opportunities for morning light exposure or whether it simply reduced the motivation to wake up in the morning, regardless of light exposure, remains unclear. However, regardless of whether bright light therapy was demonstrated, hospitalization is effective in modulating the sleep-wake rhythms of patients with CRSWD, including DSWPD (39), suggesting the importance of enforcement to wake up in DSWPD treatment. In addition, the moderate-to-severe DSWPD group had more patients who were unemployed or did not attend school than the mild DSWPD group at baseline in the present study. This finding suggests that even before the COVID-19 pandemic, the lack of social zeitgebers may have been associated with the severity of DSWPD or poor response to the intervention. However, poor response to treatment itself might have led to a decrease in social zeitgebers; thus, the causal relationship between the two remains unclear. Recent case reports of DSWPD by Epstein and colleagues indicated that being free from work during the pandemic had delayed sleep rhythms but improved sleep regularity and daytime sleepiness (40). For the two cases presented by them, environmental adjustments that allow the patient to work with a delayed sleep-wake rhythm would be more desirable than enforcing a morning-type sleep-wake rhythm. In the treatment of DSWPD, the main focus has been on advancing the sleep-wake rhythm to meet social needs (33), and the present study suggests that the introduction of factors that motivate or enforce getting up may be effective in this regard. However, given the findings that DSWPD or extreme evening chronotype can be derived from genetic vulnerability (41–44), another goal of treatment, i.e., to improve daily functioning, including employment, while the sleep-wake rhythm remains delayed, can also be an option. Future studies should examine this proposed goal setting in DSWPD treatment, as there is currently no consensus on this methodology that is not necessarily aimed at modifying the sleep-wake rhythm.
Comorbid mood disorders were significantly associated with worsened DSWPD symptoms independently from decreased social zeitgebers. Comorbidity of DSWPD to mood disorders is common; moreover, it has been suggested that patients with mood disorders are vulnerable to the disruption of sleep-wake schedules (45, 46). During the COVID-19 pandemic, symptoms of depression and anxiety in patients with major depressive disorder and bipolar disorder worsened (47, 48). Particularly for bipolar disorder, CRSWD is considered to be closely related to its pathogenesis (49); lockdown may have led to the recurrence of depressive symptoms in patients with bipolar disorder through the dysregulation of sleep-wake rhythms (50). Considering that psychiatric symptoms, such as anxiety and depression, are known to cause disruptions in sleep-wake rhythms (13), such co-occurrence of exacerbation in mood symptoms and sleep-wake rhythm dysregulations during the pandemic may have occurred not only in patients with bipolar disorder but also in those with mood disorders in general. The subjects in the present study may have also had worsened DSWPD symptoms combined with exacerbation of mood episodes. However, due to the lack of information on changes in the severity of psychiatric symptoms, we could not examine the association between mood episodes and sleep-wake rhythm disorders. To evaluate the relationship between mood symptoms and sleep-wake rhythms, future studies should investigate the severity of mood symptoms as well as changes in sleep-wake rhythms.
Although chronobiological treatments, including bright light therapy and pharmacological treatment with melatonin agonists, have been recognized as effective for sleep-wake dysregulation in patients with DSWPD (51–54), the protective effect of chronobiological interventions on the exacerbation of DSWPD symptoms during the pandemic was not suggested in the present study. In this study, 23 out of the 28 patients in the moderate-to-severe DSWPD group had already received chronobiological interventions before the COVID-19 pandemic. This result suggests that a certain subset of patients with DSWPD does not respond well to the treatments. Moreover, 14 of the 21 patients in the mild DSWPD group who received chronobiological interventions also had worsening of DSWPD symptoms during the pandemic. These results indicate that the maintenance of social synchronization factors may be a prerequisite for the effectiveness of chronobiological interventions. However, of note, all of the five patients who improved during the COVID-19 pandemic (including three patients with decreased social zeitgebers) received chronobiological interventions. Therefore, although its efficacy rate may not be high, the use of melatonin agonists or bright light therapy could still be beneficial in patients with DSWPD. To date, the treatment of DSWPD, particularly interventions for refractory cases, has not been well established (33). Therefore, the development and evaluation of efficacious chronobiological interventions are warranted in future research.
This study had several limitations that should be addressed. First, it was a retrospective study at a single institution that has a specialized outpatient clinic for sleep disorders. Furthermore, only the CGI-S was used to assess severity, and the detailed records of sleep habits were not examined. We also did not use the CGI-I score because we had limited medical record information, and it was difficult to assess the level of improvement in detail on a seven-point scale. In addition, a multinomial logistic analysis using the unchanged group as a reference would have been preferable given that some of the subjects had improved in their DSWPD symptoms. However, we did not employ that analysis in the present study due to the small sample size. Second, changes in sleep-wake rhythms during the lockdown could be explained by several factors, including increased screen exposure time, loss of regularity in dietary rhythms, decreased physical activity, and changes in daytime light exposure (55). In particular, exposure to blue light can delay the sleep onset latency (25, 56); yet, the effect of increased exposure to blue light due to longer screen time during the pandemic (57) was not examined in this study. Future studies should comprehensively investigate the living conditions potentially affecting sleep-wake rhythms. Third, the levels of psychiatric and physical symptoms were not assessed. Furthermore, although various stressors due to the pandemic may have affected sleep-wake rhythms, either directly or through depressive symptoms, these stress factors were not controlled for in this study.
The symptoms of DSWPD tended to worsen during the pandemic. The decrease in social zeitgebers following the COVID-19 pandemic was suggested to be a major factor in the exacerbation of symptoms in patients with DSWPD. The importance of social zeitgebers, i.e., external enforcement to waking up, has been emphasized; it may have been linked to stabilizing sleep-wake rhythms more than the treatments that are conventionally considered effective for DSWPD. Hence, the active implementation of social zeitgebers could be a novel intervention in DSWPD treatment. Further studies should clarify the impact of patient characteristics or mood symptoms on therapeutic response and the effectiveness of external enforcement in DSWPD treatment.
The clinical datasets of participants presented in this article are not readily available due to ethical reasons. Requests to access the datasets should be directed to KM, bWF0c3VpLmtlbnRhcm9AbmNucC5nby5qcA==.
This study was approved by the Ethics Committee of the National Center for Neurology and Psychiatry Hospital (A2020-092). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the National Legislation and the Institutional Requirements.
RO, KM, TY, KN, TU, AT, NA, MH, MF, and KK contributed to the study design, data collection, result interpretation, and manuscript preparation. RO and KM performed the statistical analysis of the data. RO, KM, and KK drafted the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by JSPS KAKENHI Grant-in-Aid for Young Scientists (Grant No. 19K17098).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine (2014).
2. Schrader H, Bovim G, Sand T. The prevalence of delayed and advanced sleep phase syndromes. J Sleep Res. (1993) 2:51–5. doi: 10.1111/j.1365-2869.1993.tb00061.x
3. Yazaki M, Shirakawa S, Okawa M, Takahashi K. Demography of sleep disturbances associated with circadian rhythm disorders in Japan. Psychiatry Clin Neurosci. (1999) 53:267–8. doi: 10.1046/j.1440-1819.1999.00533.x
4. Frangopoulos F, Nicolaou I, Zannetos S, Economou NT, Adamide T III, Georgiou A, et al. Setting objective clinical assessment tools for circadian rhythm sleep-wake disorders - A Community-based cross-sectional epidemiological study. Nat Sci Sleep. (2021) 13:791–802. doi: 10.2147/NSS.S308917
5. Yamadera W, Sasaki M, Itoh H, Ozone M, Ushijima S. A multicenter study of sleep-wake rhythm disorders: clinical features of sleep-wake rhythm disorders. Psychiatry Clin Neurosci. (1996) 50:195–201. doi: 10.1111/j.1440-1819.1996.tb02742.x
6. Kamei Y, Urata J, Uchiyaya M, Hayakawa T, Ozaki S, Shibui K, et al. Clinical characteristics of circadian rhythm sleep disorders. Psychiatry Clin Neurosci. (1998) 52:234–5. doi: 10.1111/j.1440-1819.1998.tb01049.x
7. Dagan Y, Eisenstein M. Circadian rhythm sleep disorders: toward a more precise definition and diagnosis. Chronobiol Int. (1999) 16:213–22. doi: 10.3109/07420529909019087
8. Reis C, Paiva T. Delayed sleep-wake phase disorder in a clinical population: gender and sub-population diferences. Sleep Sci. (2019) 12:203–13. doi: 10.5935/1984-0063.20190086
9. Kayaba M, Matsushita T, Enomoto M, Kanai C, Katayama N, Inoue Y, et al. Impact of sleep problems on daytime function in school life: a cross-sectional study involving Japanese university students. BMC Public Health. (2020) 20:e371. doi: 10.1186/s12889-020-08483-1
10. Sivertsen B, Harvey AG, Pallesen S, Hysing M. Mental health problems in adolescents with delayed sleep phase: results from a large population-based study in Norway. J Sleep Res. (2015) 24:11–8. doi: 10.1111/jsr.12254
11. Saxvig IW, Pallesen S, Wilhelmsen-Langeland A, Molde H, Bjorvatn B. Prevalence and correlates of delayed sleep phase in high school students. Sleep Med. (2012) 13:193–9. doi: 10.1016/j.sleep.2011.10.024
12. Baker EK. Richdale AL. Examining the behavioral sleep-wake rhythm in adults with autism spectrum disorder and no comorbid intellectual disability. J Autism Dev Disord. (2017) 47:1207–22. doi: 10.1007/s10803-017-3042-3
13. Bron TI, Bijlenga D, Kooij JJ, Vogel SW, Wynchank D, Beekman AT, et al. Attention-deficit hyperactivity disorder symptoms add risk to circadian rhythm sleep problems in depression and anxiety. J Affect Disord. (2016) 200:74–81. doi: 10.1016/j.jad.2016.04.022
14. Takaesu Y, Inoue Y, Murakoshi A, Komada Y, Otsuka A, Futenma K, et al. Prevalence of circadian rhythm sleep-wake disorders and associated factors in euthymic patients with bipolar disorder. PLoS ONE. (2016) 11:e0159578. doi: 10.1371/journal.pone.0159578
15. Matsui K, Inada K, Kuriyama K, Yoshiike T, Nagao K, Oshibuchi H, et al. Prevalence of circadian rhythm sleep-wake disorder in outpatients with schizophrenia and its association with psychopathological characteristics and psychosocial functioning. J Clin Med. (2021) 10:1513. doi: 10.3390/jcm10071513
16. Yamadera W, Sasaki M, Itoh H, Ozone M, Ushijima S. Clinical features of circadian rhythm sleep disorders in outpatients. Psychiatry Clin Neurosci. (1998) 52:311–6. doi: 10.1046/j.1440-1819.1998.00395.x
17. Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. (1999) 284:2177–81. doi: 10.1126/science.284.5423.2177
18. Middleton B, Arendt J, Stone BM. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J Sleep Res. (1996) 5:69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x
19. Klerman EB, Dijk DJ, Kronauer RE, Czeisler CA. Simulations of light effects on the human circadian pacemaker: implications for assessment of intrinsic period. Am J Physiol. (1996) 270:R271–82. doi: 10.1152/ajpregu.1996.270.1.R271
20. Kitamura S, Hida A, Enomoto M, Watanabe M, Katayose Y, Nozaki K, et al. Intrinsic circadian period of sighted patients with circadian rhythm sleep disorder, free-running type. Biol Psychiatry. (2013) 73:63–9. doi: 10.1016/j.biopsych.2012.06.027
21. Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. (2003) 549(Pt. 3):945–52. doi: 10.1113/jphysiol.2003.040477
22. Honma K, Hashimoto S, Nakao M, Honma S. Period and phase adjustments of human circadian rhythms in the real world. J Biol Rhythms. (2003) 18:261–70. doi: 10.1177/0748730403018003008
23. Snoeijer BT, Burger M, Sun S, Dobson RJB, Folarin AA. Measuring the effect of Non-pharmaceutical interventions (Npis) on mobility during the COVID-19 pandemic using global mobility data. NPJ Digit Med. (2021) 4:81. doi: 10.1038/s41746-021-00451-2
24. Cellini N, Canale N, Mioni G, Costa S. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J Sleep Res. (2020) 29:e13074. doi: 10.1111/jsr.13074
25. Salfi F, Amicucci G, Corigliano D, D'Atri A, Viselli L, Tempesta D, et al. Changes of evening exposure to electronic devices during the COVID-19 lockdown affect the time course of sleep disturbances. Sleep. (2021) 44:zsab080. doi: 10.1101/2020.10.20.20215756
26. Staller N, Randler C. Changes in sleep schedule and chronotype due to COVID-19 restrictions and home office. Somnologie. (2020):25:131-7. doi: 10.1007/s11818-020-00277-2
27. Lee PH, Marek J, Nálevka P. Sleep pattern in the US and 16 European countries during the COVID-19 outbreak using crowdsourced smartphone data. Eur J Public Health. (2021) 31:23–30. doi: 10.1093/eurpub/ckaa208
28. Sinha M, Pande B, Sinha R. Impact of COVID-19 lockdown on sleep-wake schedule and associated lifestyle related behavior: a national survey. J Public Health Res. (2020) 9:1826. doi: 10.4081/jphr.2020.1826
29. Bryson WJ. Circadian rhythm sleep-wake disorders and the COVID-19 pandemic. J Clin Sleep Med. (2020) 16:1423. doi: 10.5664/jcsm.8540
30. Uchiyama M, Okawa M, Shibui K, Liu X, Hayakawa T, Kamei Y, et al. Poor compensatory function for sleep loss as a pathogenic factor in patients with delayed sleep phase syndrome. Sleep. (2000) 23:553–8. doi: 10.1093/sleep/23.4.1h
31. Micic G, de Bruyn A, Lovato N, Wright H, Gradisar M, Ferguson S, et al. The endogenous circadian temperature period length (tau) in delayed sleep phase disorder compared to good sleepers. J Sleep Res. (2013) 22:617–24. doi: 10.1111/jsr.12072
32. Micic G, Lovato N, Gradisar M, Burgess HJ, Ferguson SA, Lack L. Circadian melatonin and temperature taus in delayed sleep-wake phase disorder and Non-24-hour sleep-wake rhythm disorder patients: an ultradian constant routine study. J Biol Rhythms. (2016) 31:387–405. doi: 10.1177/0748730416650069
33. Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: Advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), Non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. (2015) 11:1199-236. doi: 10.5664/jcsm.5100
34. Guy W. Ecdeu Assessment Manual for Psychopharmacology. Rockville, MD: United States Department of Health, Education and Welfare, Public Health Service (1976). doi: 10.1037/e591322011-001
35. Murray JM, Sletten TL, Magee M, Gordon C, Lovato N, Bartlett DJ, et al. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. (2017) 40:1–10. doi: 10.1093/sleep/zsw002
36. Takeshima M, Shimizu T, Echizenya M, Ishikawa H, Kanbayashi T. Inpatient phase-advance therapy for delayed sleep-wake phase disorder: a retrospective study. Nat Sci Sleep. (2018) 10:327. doi: 10.2147/NSS.S179264
37. Kalak N, Gerber M, Kirov R, Mikoteit T, Pühse U, Holsboer-Trachsler E, et al. The relation of objective sleep patterns, depressive symptoms, and sleep disturbances in adolescent children and their parents: a sleep-EEG study with 47 families. J Psychiatr Res. (2012) 46:1374–82. doi: 10.1016/j.jpsychires.2012.07.006
38. Wilhelmsen-Langeland A, Dundas I, Saxvig IW, Pallesen S, Nordhus IH, Bjorvatn B. Psychosocial challenges related to delayed sleep phase disorder. Open Sleep J. (2012) 5:51–8. doi: 10.2174/1874620901205010051
39. Iwamitsu Y, Ozeki Y, Konishi M, Murakami J, Kimura S, Okawa M. Psychological characteristics and the efficacy of hospitalization treatment on delayed sleep phase syndrome patients with school refusal. Sleep Biol Rhythms. (2007) 5:15–22. doi: 10.1111/j.1479-8425.2006.00252.x
40. Epstein LJ, Cai A, Klerman EB, Czeisler CA. Resolving delayed sleep-wake phase disorder with a pandemic: two case reports. J Clin Sleep Med. (2022) 18:315–8. doi: 10.5664/jcsm.9526
41. Archer SN, Carpen JD, Gibson M, Lim GH, Johnston JD, Skene DJ, et al. Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep. (2010) 33:695–701. doi: 10.1093/sleep/33.5.695
42. Lane JM, Vlasac I, Anderson SG, Kyle SD, Dixon WG, Bechtold DA, et al. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK biobank. Nat Commun. (2016) 7:10889. doi: 10.1038/ncomms10889
43. Patke A, Murphy PJ, Onat OE, Krieger AC, Özçelik T, Campbell SS, et al. Mutation of the human circadian clock gene cry1 in familial delayed sleep phase disorder. Cell. (2017) 169:203-15.e13. doi: 10.1016/j.cell.2017.03.027
44. Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. (2019) 10:343. doi: 10.1038/s41467-018-08259-7
45. Takaesu Y, Inoue Y, Ono K, Murakoshi A, Futenma K, Komada Y, et al. Circadian rhythm sleep-wake disorders as predictors for bipolar disorder in patients with remitted mood disorders. J Affect Disord. (2017) 220:57–61. doi: 10.1016/j.jad.2017.05.041
46. Zaki NFW, Spence DW, BaHammam AS, Pandi-Perumal SR, Cardinali DP, Brown GM. Chronobiological theories of mood disorder. Eur Arch Psychiatry Clin Neurosci. (2018) 268:107–18. doi: 10.1007/s00406-017-0835-5
47. Pellegrina U, Quaglino V, Deligne H. [Covid-19, Impacts on the mental health of people suffering from anxiety and depression]. Soins Psychiatr. (2020) 41:29–33. doi: 10.1016/S0241-6972(20)30123-7
48. Dalkner N, Wagner-Skacel J, Ratzenhofer M, Fellendorf F, Lenger M, Maget A, et al. Psychological symptoms during and after Austrian first lockdown in individuals with bipolar disorder? A follow-up control-group investigation. Int J Bipolar Disord. (2021) 9:16. doi: 10.1186/s40345-021-00222-8
49. Takaesu Y. Circadian rhythm in bipolar disorder: a review of the literature. Psychiatry Clin Neurosci. (2018) 72:673–82. doi: 10.1111/pcn.12688
50. Carta MG, Ouali U, Perra A, Ben Cheikh Ahmed A, Boe L, Aissa A, et al. Living with bipolar disorder in the time of Covid-19: biorhythms during the severe lockdown in Cagliari, Italy, and the moderate lockdown in Tunis, Tunisia. Front Psychiatry. (2021) 12:e634765. doi: 10.3389/fpsyt.2021.634765
51. Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, Johnston SH, Allen R, Kelly KA, et al. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. (1990) 13:354–61. doi: 10.1093/sleep/13.4.354
52. Kayumov L, Brown G, Jindal R, Buttoo K, Shapiro CM. A randomized, double-blind, placebo-controlled crossover study of the effect of exogenous melatonin on delayed sleep phase syndrome. Psychosom Med. (2001) 63:40–8. doi: 10.1097/00006842-200101000-00005
53. Richardson GS, Zee PC, Wang-Weigand S, Rodriguez L, Peng X. Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J Clin Sleep Med. (2008) 4:456–61. doi: 10.5664/jcsm.27282
54. Zee PC, Wang-Weigand S, Wright KP Jr, Peng X, Roth T. Effects of ramelteon on insomnia symptoms induced by rapid, eastward travel. Sleep Med. (2010) 11:525–33. doi: 10.1016/j.sleep.2010.03.010
55. Bertrand L, Schröder C, Bourgin P, Maruani J, Atoui Y, d'Ortho MP, et al. Sleep and circadian rhythm characteristics in individuals from the general population during the French COVID-19 full lockdown. J Sleep Res. (2021) 31:e13480. doi: 10.1111/jsr.13480
56. Christensen MA, Bettencourt L, Kaye L, Moturu ST, Nguyen KT, Olgin JE, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS ONE. (2016) 11:e0165331. doi: 10.1371/journal.pone.0165331
Keywords: delayed sleep-wake phase disorder, coronavirus disease 2019, COVID-19, state of emergency, Japan, social zeitgeber, bipolar disorder, depression
Citation: Otsuki R, Matsui K, Yoshiike T, Nagao K, Utsumi T, Tsuru A, Ayabe N, Hazumi M, Fukumizu M and Kuriyama K (2022) Decrease in Social Zeitgebers Is Associated With Worsened Delayed Sleep-Wake Phase Disorder: Findings During the Pandemic in Japan. Front. Psychiatry 13:898600. doi: 10.3389/fpsyt.2022.898600
Received: 17 March 2022; Accepted: 17 May 2022;
Published: 09 June 2022.
Edited by:
Takashi Kanbayashi, University of Tsukuba, JapanReviewed by:
Thomas Dye, Cincinnati Children's Hospital Medical Center, United StatesCopyright © 2022 Otsuki, Matsui, Yoshiike, Nagao, Utsumi, Tsuru, Ayabe, Hazumi, Fukumizu and Kuriyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kentaro Matsui, bWF0c3VpLmtlbnRhcm9AbmNucC5nby5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.