- 1Department of Radiology, Yanan University Affiliated Hospital, Yanan, China
- 2MR Research China, GE Healthcare, Beijing, China
- 3MR Scientific Marketing, Siemens Healthineers, Shanghai, China
Background: Patients with temporal lobe epilepsy (TLE) frequently complain of poor sleep quality, which is a condition that clinicians are typically neglecting. In this study, Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), and Athens Insomnia Scale (AIS) were used to assess the sleep status of patients with temporal lobe epilepsy (TLE). Simultaneously diffusion kurtosis imaging (DKI) was applied to examine the white matter microstructure abnormalities in patients with TLE and sleep disorders.
Methods: TLE patients who have been diagnosed in the cardio-cerebrovascular ward of the Yanan University Affiliated Hospital from October 2020 to August 2021 were recruited. Finally, 51 patients and 30 healthy controls were enrolled in our study, with all subjects completing the sleep evaluation questionnaire and undergoing a DKI examination. Using independent sample t-test, analysis of variance (ANOVA), and Mann-Whitney U test to compare groups.
Results: Thirty patients (58.82%) complained of long-term sleep difficulties. The overall differences among the evaluation of AIS, ESS, and PSQI are significant (P = 0.00, P = 0.00, P = 0.03). The scores of AIS, ESS in Left and Right-TLE (L/R-TLE) with sleep disorders, as well as PSQI in L-TLE, are statistically higher than the control group (P = 0.00, P = 0.00, P = 0.00, P = 0.00, P = 0.02). L-TLE with sleep disorders showed decreased MK on affected sides (P = 0.01). However, statistical differences in MD and FA have not been observed (P = 0.34, P = 0.06); R-TLE with sleep disorders showed significantly decreased MK and increased MD on affected sides (P = 0.00, P = 0.00), but FA's statistical difference has not been observed (P = 0.20).
Conclusions: TLE patients with sleep disorders have different DKI parameters than individuals who do not have sleep issues. During this process, the kurtosis parameter (MK) was more sensitive than the tensor parameters (MD, FA) in detecting the patient's aberrant white matter diffusion. DKI may be a better choice for in vivo investigation of anomalous craniocerebral water diffusion.
Introduction
The most prevalent intractable focal epilepsy is temporal lobe epilepsy (TLE), which is frequently linked with hippocampal sclerosis (HS) (1). Moreover, the location of TLE tissue damage followed a specific anatomical and functional pattern, with the sections directly or indirectly related to the medial temporal lobe being the most affected (2). Seizures affect patients' cognitive abilities and quality of life (3) and negatively affect sleep structure. Previous studies discovered that poor sleep quality would affect peoples' memory formation and life wellbeing (4). Although the interaction between seizures and sleep issues is still under research, sleep disorders have become a common but often overlooked problem in patients with epilepsy by neurologists. Sleep disorders are common comorbidities in epilepsy, such as obstructive sleep apnea syndrome (OSA), restless legs syndrome, and chronic insomnia. Sleep deficit, poor sleep quality, and daytime sleepiness are common complaints among clinical TLE patients (5). According to statistics, about 50% of patients have chronic insomnia (6).
Early identification of the comorbidity can help ameliorate patients' epilepsy burden and improve their quality of life. Yaranagula (7) found that although surgery can control the frequency of seizures, the sleep quality of the patients did not improve. The sleep quality scores before and after surgery were lower, so the purpose of epilepsy treatment should not be limited to controlling the frequency, but also to treat the disease on the adverse effects on the patient, such as sleep, are minimized. Rapid eye movement (REM) has a protective effect on seizures (8). Previous studies have used polysomnography (PSG) to investigate the sleep structure of TLE patients and compared the sleep status of patients without seizures during the day with the patients after seizures, finding that nighttime REM sleep was significantly reduced after seizures and that reductions were more pronounced after nighttime seizures than daytime seizures, patients also experienced significantly lower sleep efficiency, and the effect of L-TLE on REM was more significant after nighttime seizures than daytime seizures (9).
The anomalous diffusion of cerebral water molecules was discovered in researches that used diffusion tensor imaging (DTI) to examine patients with primary insomnia (PI) (10). Jensen first introduced diffusional kurtosis imaging (DKI) in 2005, which expands traditional DTI by estimating the kurtosis of the water diffusion probability distribution function. DKI can provide both kurtosis parameters and tensor parameters. Many researchers have used DKI to assess microscopic white matter abnormalities in patients with various types of epilepsy using the region of interest (ROI), voxel analysis, and automated fiber quantification (AFQ) and have discovered abnormal brain regions and extensive anomalies in brain networks (1, 11, 12). Nonetheless, this approach has hardly been used in studies to identify subtle structural abnormalities in TLE patients with sleep disturbances. Furthermore, in clinical work, sleep questionnaires are frequently used to examine the subjective sleep experience of TLE patients.
Our purpose is to use the DKI to analyze microstructural abnormalities in white matter in TLE patients with subjective sleep issues and to measure patients' subjective sleep quality by delivering sleep questionnaires. We also looked into the differences in sleep scores between sick and healthy controls.
Subjects and Methods
Participants
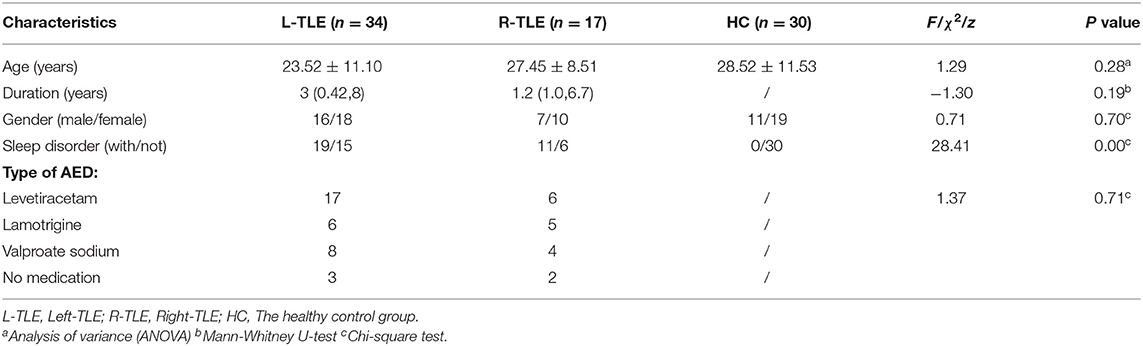
The local institutional review board of the Yanan University Affiliated Hospital approved this prospective study (number: YAS-S01-202106001), and informed consent was obtained from all participants. From October 2020 to August 2021, patients suspected of having unilateral TLE were recruited for this study. Inclusion criteria were as follows: (1) in line with the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) (13) diagnostic criteria for TLE; (2) younger than 50 years old, since previous research have suggested that adults over 50 had different sleep habits than younger people (14); (3) had no seizures within 24 h before MR examination and (4) had complete DKI-MRI data and sleep status data available. Of the 57 patients initially enrolled, 6 were excluded (2 with obvious image artifacts, 1 with concomitant white matter lesions grade III, 1 is encephalitis secondary epilepsy, and 2 with concomitant traumatic brain injury). Finally, 51 patients with unilateral TLE were enrolled. Another 30 healthy volunteers with no neurological abnormalities were also recruited to form a control group.
Neurologists referred to the International Classification of Sleep Disorders-third edition (ICSD-3) and screened patients for underlying sleep disorders, such as insomnia, somnolence, sleep-related breathing disorders or movement disorders (restless legs syndrome), etc. (15), based on their main complaints. Then allocated subjects to 3 subgroups, consisting of TLE patients with sleep disorders, without sleep disorders and the control group. A flow chart is shown in Figure 1.
Sleep Questionnaires Evaluation
Epworth Sleepiness Scale
Excessive daytime sleepiness (EDS) is described as a recent three-month inability to stay awake during the primary awake part of the day. The Epworth Sleepiness Scale (ESS) (16) is a questionnaire that assesses a person's daytime sleepiness. Participants were asked to rate their chances of nodding off in various situations. It can determine the likelihood of dozing off in eight situations that we encounter daily, which spans from 0 (never dozing off) points to 3 (frequently dozing off). Previous research (17) validated the construct validity and internal consistency of the score. An ESS score of more than ten is seen as abnormal (7, 18).
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) (19) was compiled in 1989 to assess sleep quality during the previous month. It consists of 19 self-evaluated questions and five roommate-evaluated questions, with the last five items being used solely for clinical data. These 19 self-assessment questions look at a variety of sleep-related characteristics, such as sleep length and latency estimates, as well as the frequency and severity of specific sleep-related issues. These 19 items are separated into seven components, and the scores from the seven components are summed together to provide a PSQI score (0–21). The higher the score, the poorer the sleep quality. The sleep quality scale measures subjective sleep quality, sleep latency, and sleep length, among other things. A PSQI score of more than five is considered abnormal (7).
Athens Insomnia Scale
The Athens Insomnia Scale (AIS) (20) is an eight-item self-assessment of sleep status with a total score of 0–24, with 1–5 questions evaluating sleep status and 6–8 items evaluating daytime mental state, an AIS score of more than six are considered abnormal (21). The Chinese version (22) of AIS has been proven to be reliable and effective in adolescents and adults.
Magnetic Resonance Imaging Acquisition and Data Analysis
Images acquisition were performed on a 3T MRI scanner (Magnetom Verio, Siemens Healthineers, Erlangen, Germany) equipped with an 8-channel head coil. The conventional MRI protocols included the following sequences: axial T1-weighted, axial T2-weighted, and axial FLAIR images. The detail parameters about DKI imaging were: TR = 11000 ms, TE = 96 ms, field- of- view = 230 × 230 mm2, matrix size = 100 × 100, scanning slice thickness = 2 mm, b = 0, 1,000 and 2,000 s/mm2, using 30 different diffusion coding directions totally.
The images were double-blind analyzed by two neuroimaging physicians who have worked for over 10 years and the average of the two was taken as the final result. The diffusional kurtosis estimator software (https://www.nitrc.org/projects/dke/Version2.6) was used to analyze raw diffusion images and calculate DKI's parametric maps, specifically maps of mean diffusivity (MD), fractional anisotropy (FA), and mean kurtosis (MK). Figure 2 shows a representation of DKI's major parametric maps. Using the T1WI image as a reference, one circular ROI was drawn in the white matter of the temporal lobe on the affected side of patients and matched side of control group through MRIcron software (https://www.nitrc.org/projects/mricron/Version1.0.20190902), avoiding the sulci, split-brain, and ventricle. The ROI would be no <25 mm2, and the average would be taken by measuring at the same point on three consecutive levels of MK, MD, and FA graphs (23).
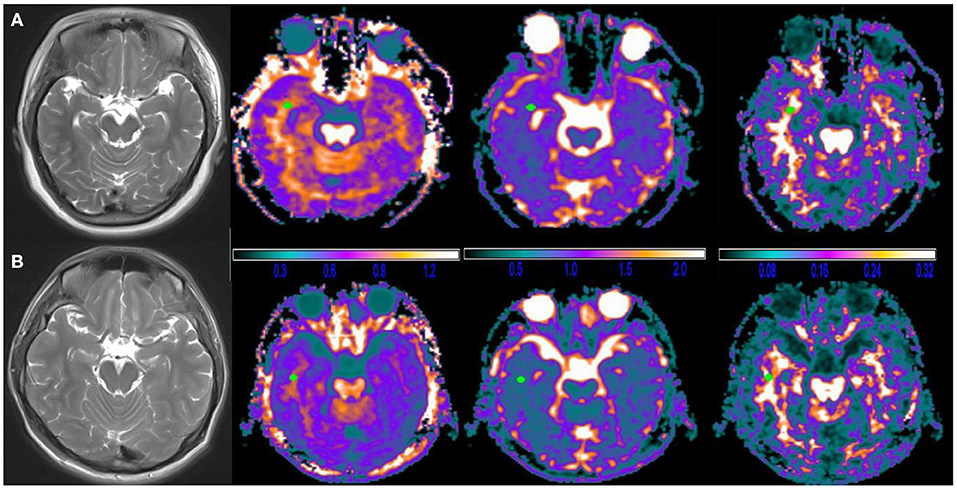
Figure 2. Typical diffusional kurtosis imaging (DKI)-derived parametric maps in a case brain. (A) Female, 27 years old, R-TLE with sleep disorder, (B) Male, 35 years old, R-TLE without sleep disorder; from left to right are T2WI images, mean kurtosis (MK) maps, mean diffusivity (MD) maps and fractional anisotropy (FA) maps. No clear abnormalities were seen on T2WI images and DKI images in both patients, which could be detected by measurement of DKI parameters. Note: the unit of MD is μm2/ms, and FA, MK are dimensionless.
Statistical Analysis
SPSS software was used to analyze the data (version 20.0). Continuous variables utilize mean±standard deviation (m±SD), while categorical variables use frequency. An independent sample t-test, analysis of variance (ANOVA), and Mann-Whitney U test were used to compare between groups, depending on the normality of the data. Post-hoc pairwise comparisons were performed using LSD-t test. The Chi-square test and Fisher-Freeman-Halton test were used to study the association between categorical variables. A P < 0.05 is considered significant.
Results
Demographic and Clinical Characteristics of Subjects
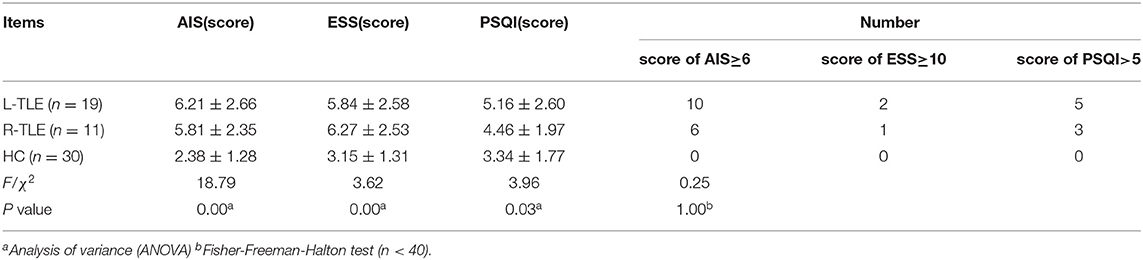
After screening, 51 TLE were finally enrolled (23 males and 28 females). Video EEG revealed unusual temporal lobe discharge on one side, including 34 cases of Left-TLE (L-TLE) and 17 cases of Right-TLE (R-TLE). Table 1 shows demographic information and clinical traits. Thirty patients (58.82%) complained of long-term sleep difficulties, while 21 thought sleep quality was satisfactory. Five patients (9.80%) did not take any medications; the others took the single antiepileptic therapy (AED).
Results of Self-Evaluation Sleep Questionnaire
Table 2 compares the overall differences in AIS, ESS, and PSQI scores between TLE with sleep disorders and the control group. The AIS, ESS, and PSQI total differences are statistically significant (P = 0.00, P = 0.00, P = 0.03). Figure 3 shows the pairwise comparison: the scores of L/R-TLE in AIS, ESS, and PSQI in L-TLE are statistically higher than the control group (P = 0.00, P = 0.00, P = 0.00, P = 0.00, P = 0.02). Although the difference in PSQI between R-TLE and the control group was not statistically significant, the mean value was higher than the control group. The number of patients with abnormal scores (AIS≥6, ESS≥10, and PSQI>5) was few, and no statistical difference was detected.
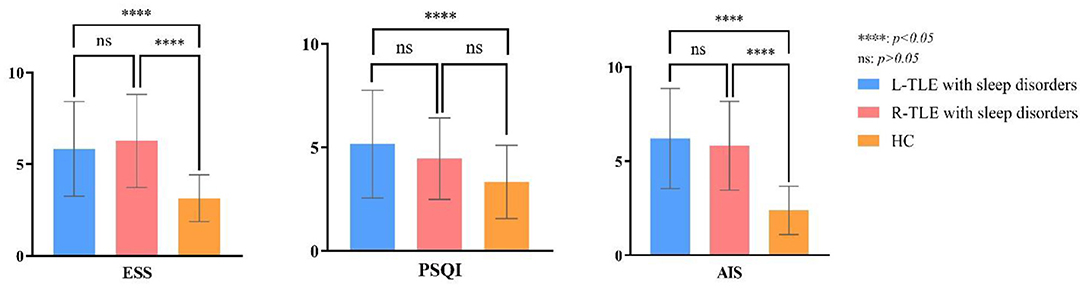
Figure 3. Post-hoc multiple comparison in sleep scores of TLE with sleep disorders and the healthy controls.
MK, MD and FA Values in White Matter of the Temporal Lobe on Subjects
Nineteen combined with sleep disorders and 15 without in L-TLE, as illustrated in Table 3. We compared the DKI parameters in three groups: L-TLE with sleep disorders, L-TLE without sleep disorders, and the control group. We manually delineated the ROI and quantified the MK, MD, and FA values on the afflicted side (left) of a patient and matched the side of the control group (left). Table 3 and Figure 4 demonstrate the results.
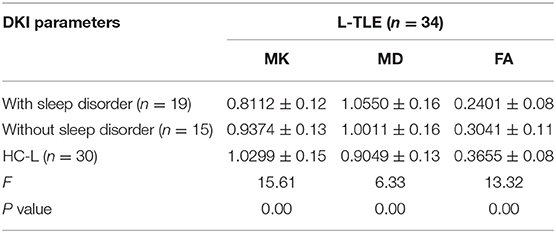
Table 3. ANOVA of DKI parameters between L-TLE with or without sleep disorders and the healthy controls.
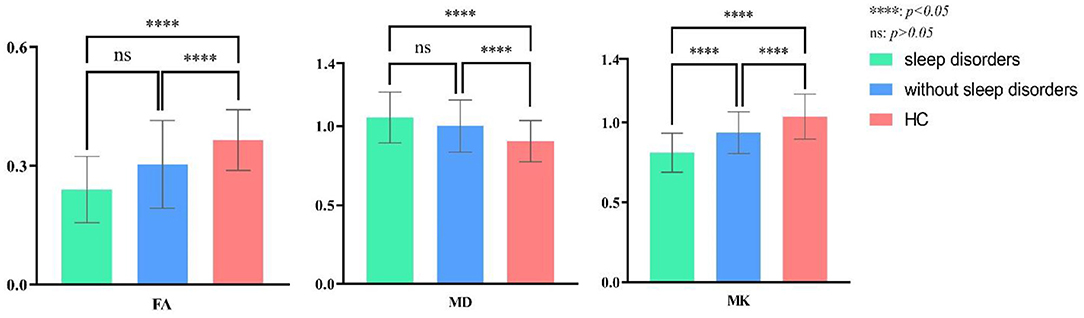
Figure 4. Post-hoc multiple comparison in DKI parameters of L-TLE with or without sleep disorders and the healthy controls.
The overall difference in MK, MD, and FA between these three groups was statistically significant (P = 0.00). The FA, MK of TLE decreased, and the MD increased compared to the control group, regardless of whether sleep disturbance was combined (FA: P = 0.00, P = 0.01; MD: P = 0.00, P = 0.04; MK: P = 0.00, P = 0.03). Especially MK was significantly lower in the sleep disorder group than without sleep disorder (P = 0.01). FA was also lower, though not statistically significant (P = 0.06). We did not observe a significant difference in MD between the sleep disorder group and those without (P = 0.30).
In R-TLE, eleven combined with sleep disorders and six without, as illustrated in Table 4. As with L-TLE, we compared DKI parameters between these three groups in the patient's afflicted side (right) and matched side of the control group (right): R-TLE with sleep disorders, R-TLE without sleep disorders, and the control group. Table 4 and Figure 5 demonstrate the results.
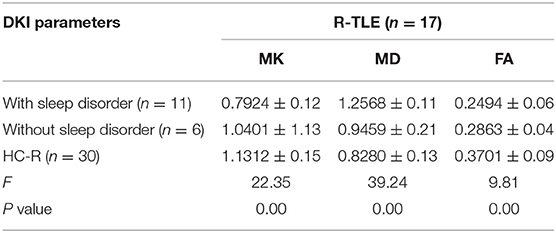
Table 4. ANOVA of DKI parameters between R-TLE with or without sleep disorders and the healthy controls.
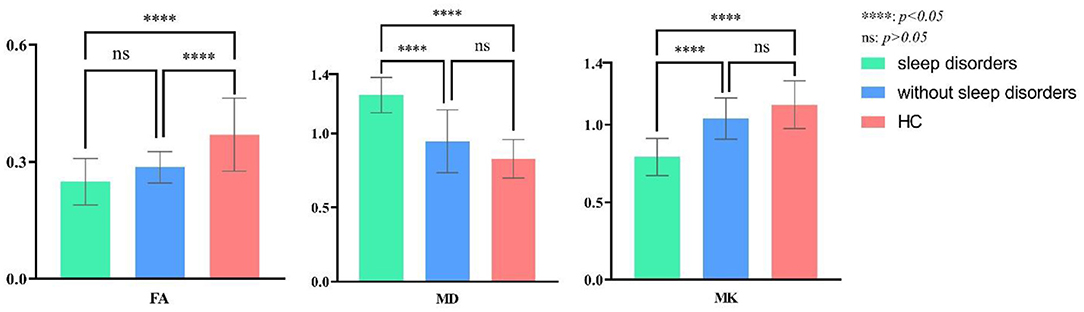
Figure 5. Post-hoc multiple comparison in DKI parameters of R-TLE with or without sleep disorders and the healthy controls.
The overall difference in MK, MD, and FA between these three groups was statistically significant (P = 0.00). The FA, MK of TLE with sleep disorders reduced, and the MD increased than that of the control group (FA: P = 0.00; MD: P = 0.00; MK: P = 0.00). There were only six individuals in R-TLE without sleep disorders, so only FA was found to be reduced in without sleep disorder group than the control group (P = 0.00), and we did not found difference in MK and MD between them (MD: P = 0.06; MK: P = 0.17).
The sleep disorder group found significantly higher MD and lower MK patients (MD: P = 0.00; MK: P = 0.00). The difference in FA between the sleep disorder group and those without has not been observed (P = 0.39).
Discussion
Our study's combination of sleep disorders with DKI is a significant advantage, which previous investigations hardly used. Another advantage is the homogeneity of subjects; we controlled for factors that might affect sleep quality, such as age and the amount of medication.
Mechanisms of the interaction between seizures and sleep have been under investigation. Abnormal electrical discharges between seizures can lead to sleep disruption (6), and the disruption of a patient's normal sleep would exacerbate the seizures. Sleep quality also affects the type and likelihood of a patient's seizures, and the association between sudden unexpected death in epilepsy (SUDEP) and sleep is also gradually being recognized.
Our research grouped the sleep condition of patients with TLE and investigated the microstructure through DKI. Meanwhile, we evaluated the sleep quality scores between patients with sleep disorders and the control group by sleep questionnaires. We discovered that TLE with sleep difficulties are pretty common (58.82% in this study), and the values of DKI's parameters also have anomalies.
The diffusion environment of water molecules is affected in TLE due to injured nerve fiber bundles, disrupted white matter integrity, and proliferation of irregular glial cells. Therefore, differences exist in DKI parameters between TLE and the controls. The preliminary results of the present study are also close to our previous works on the microstructure of TLE. However, we aimed to apply the DKI technique to subgroup further comparisons between comorbid sleep disorders and non-comorbid sleep disorders. We want to obtain more information about comorbidities and support individualized treatment of patients with sleep problems.
TLE patients frequently experience insomnia, poor sleep quality, and excessive daytime drowsiness. The prescription of AEDs mainly focused on reducing seizures, and it is easy to overlook other mental and sleep disorders that patients may be experiencing. Our study only included individuals who used one AED to minimize the effects of AEDs on sleep. Drugs have a significant impact on the sleep quality of PWE, prior investigations have reported that combining numerous AEDs can result in a decrease in sleep quality in patients (24).
Twenty-three patients (50%) utilized Levetiracetam in our investigation. Studies (25) have shown that large-dose levetiracetam use caused excessive drowsiness during the day. Patients with sleep disorders had a higher ESS score could be related to their medication. Furthermore, daytime sleepiness in patients may be related to other multiple factors, such as nocturnal seizures, sedative effects of AEDs, and sleep deprivation at night. However, the number of abnormal ESS scale scores was lower, probably because excessive daytime sleepiness was more common in frontal lobe epilepsy than TLE (26). Lamotrigine and valproate sodium were the other two medicines the patients took in this study. Foldvary (27) compared lamotrigine to several older antiepileptic medicines and concluded that lamotrigine users rarely have significant daytime sleepiness or night sleeplessness. As Foldvary's article indicates, valproate sodium also has no substantial effect on sleep structure (28).
White matter plays a vital role in regulating brain activity and the coupling between brain regions and behavioral regulation (29). Previous studies have found that the proportion of fiber bundles in the left striatum and hippocampus of insomnia patients reduced through DTI technology (30), which may mean the abnormal reduction of white matter fiber tracts in insomnia patients. To further explore the microstructure abnormalities of TLE with sleep disorders, our study used DKI for patients' brain imaging and analysis. Compared with DTI, DKI can describe higher-order diffusion dynamics and more complex diffusion distribution. DKI could detect complex and crossed white matter fiber bundles and provide more comprehensive quantitative parameters, which will help improve the disease detection rate of white matter abnormalities (1). Although the number of studies related to abnormal white matter structure in sleep disorders is lacking, it has confirmed that abnormal changes in microstructure and brain networks exist in the brain of this group, and this abnormality spreads from the limbic system to the entire brain (31).
Numerous previous studies have demonstrated that sleep plays an essential role in the brain. Neuroimaging found sleep disturbances associated with functional deficits as measured by fMRI or atrophy of the cerebral cortex, and the integrity of the white matter microstructure may underlie these associations. Poor sleep may disrupt axonal integrity and degenerate white matter, but white matter pathology may also precede sleep disturbance (32).
Voldsbekk et al. (33) combined spherical mean technique (SMT) and diffusion imaging to inquire into differences between sleep deprivation and normal sleep-wake-cycle (SWC) group. He discovered that sleep deprivation was associated with extensive white matter changes and had an abnormal intracranial diffusion coefficient. Vyas (34) utilized DKI to brain microstructural changes in OSA and detected abnormalities in kurtosis parameters in several brain regions of the patients. They indicated that kurtosis parameters are more sensitive to abnormalities shown at the microcosmic structure level before detectable abnormalities appear in conventional MRI or other imaging modalities.
As a classic parameter of DKI, MK is related to the microstructure changes of many diseases and is sensitive to more subtle brain changes. The increase in the myelin sheath of white matter fibers, dense accumulation of axons and fiber bundles, and decreases in the permeability of the axon membrane caused increases in kurtosis parameters. MK decreased in the sleep disorder comorbidity group in our study. It could be due to a net loss of microscopic tissue complexity caused by damage to the myelin barrier and other microscopic cell structures in these areas, such as the loss of nerve synapses. Which results in decreased cell connections, decreased tissue integrity, and increased extracellular gap (35), and is also consistent with pathological denervation (1). Epilepsy activity and long-term parasomnia may make water molecules in the brain more susceptible to spreading toward synchrony. In an immunohistochemistry investigation of Huntington's disease (36), the number of fibers that can be stained and arranged orderly reduced in regions with decreased kurtosis values.
The present study differs from Tummala's study on OSA (37), which found increased kurtosis parameters in several intracranial regions. They suggested that the hypoxic and ischemic damage caused by OSA may cause swelling of neurons and axons, leading to acute axonal and myelin tissue damage. This study also proposed that the mechanism for the increased kurtosis parameter in acute disease may be the incremental extracellular fluid due to degeneration of axons and myelin sheaths, the more pronounced the non-Gaussian nature of water molecule diffusion. In contrast, in chronic disease, for instance, epilepsy, the diffusion of water molecules tends to be more Gaussian in distribution due to the decrease in axons, myelin, neurons, and glial cells and the increase in extracellular gaps.
MD variations linked to the activity dynamics of epileptic seizures according to the previous research (38, 39): in the hyper-acute phase after protracted convulsions or status epilepticus, MD decreases due to cytotoxic edema. MD gradually develops during the sub-acute attack period (~5 days), which is linked to angioedema, while the chronic phase of neuron loss and gliosis leading to an increase in interstitial water content will further lead to an increase in MD.
In our study, the MD of TLE with sleep disorders also tended to higher than patients without sleep disorders. This difference is significant in R-TLE, which may also be consistent with reports of increased MD in the sub-acute state because all patients included in this study had no seizures within 24 h before the scanning. The finding also supports the notion of extensive axonal and neuronal damage. Our results were consistent with research on Rapid eye movement (REM) sleep behavior disorder (RBD), which found the increased MD value in many brain regions (40).
FA is the most often used parameter in daily work and scientific research in neuroimaging and represents water molecules' degree of anisotropic diffusion. The degree of dispersion more noticeable, the bigger the FA. Although the difference in FA in our study was not significant, a downward trend can still be seen, particularly in L-TLE. A decrease in FA reflects the blockage of water molecule transport in the lesion area. The variations in axon density and the integrity of the myelin membrane frequently disrupted water diffusion, the transient increase in diffusion rate generated by the start of angioedema may enhance the probability of water molecules encountering the barrier (41, 42). Our results are similar to Kang's article. He discovered differences in white matter tract spread measures on white matter connections in the left thalamus and inferior frontal gyrus in insomniacs. Insomniacs had compromised white matter integrity, and FA reduced compared with healthy controls. The results may also provide new evidence for decreased connectivity in the thalamic-frontal regions of insomnia patients in functional imaging studies (43). In the future, increasing the sample size may result in more statistically significant findings.
We did not overemphasize the causal relationship between TLE and sleep disorders, which may lead to inappropriate treatment of sleep disorders. Some neurologists may focus on resolving sleep disorders by controlling the primary disease, thus neglecting to correct the patient's sleep problems alone. However, treating the primary disease and sleep disorders should be carried out simultaneously and curing sleep problems also promotes the effectiveness of treatment of the primary disease (15).
However, we still have some limitations: First and foremost, since we have no obtained histology specimens, we must rely on past research to make assumptions. We should collect more longitudinal data from patients to investigate the disease's unique mechanisms. Second, to avoid drug effects on sleep quality, the patients we studied all used only one AED, which may leave out the group of patients treated with a mix of medicines and could have more specific abnormalities in diffusion parameters. Third, our study is a cross-sectional study, and we have not yet obtained a causal relationship from it. So it is unclear whether the abnormality in the observed white matter microstructure is the cause or the result. We hope that future studies with longitudinal designs will identify causal directionality. Fourth, the study's sample size was small (especially in R-TLE), and future continued expansion of the sample size will hopefully lead to more meaningful results.
Conclusions
To summarize, our research discovered the abnormal DKI parameters in TLE with sleep disorders. The kurtosis parameter (MK) is more sensitive than the tensor parameters (MD, FA) in detecting aberrant white matter diffusion in the patient during this procedure. The DKI properties revealed in our study may represent the underlying pathophysiological mechanism of TLE with sleep disorders, which will need verifying in future investigations.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Yanan University Affiliated Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
MG and YL proposed the article concept and wrote the manuscript. BS, JH, and JL conducted data collection and analysis. RY and CH conducted the statistical analysis. XW and SW supported the MR technology for this article. XH, MG, and YL supervised the whole research and received funding. All authors have read and approved the final version of the article.
Funding
The Red Cross Foundation of China funded the study (approval number: XM_HR_ICON_2020_10_07).
Conflict of Interest
XW was employed by GE Healthcare. SW was employed by Siemens Healthineers.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Sincere thanks to all the patients and volunteers who participated in this study, and the engineers of Siemens AG of Germany who provided technical support, the study was completed with the cooperation of everyone.
References
1. Glenn GR, Jensen JH, Helpern JA, Spampinato MV, Kuzniecky R, Keller SS, et al. Epilepsy-related cytoarchitectonic abnormalities along white matter pathways. J Neurol Neurosurg Psychiatry. (2016) 87:930–6. doi: 10.1136/jnnp-2015-312980
2. Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology. (2009) 73:834–42. doi: 10.1212/WNL.0b013e3181b783dd
3. Hampton T. Experts describe “spectrum” of epilepsy. JAMA. (2010) 303:313–4. doi: 10.1001/jama.2009.1977
4. van Schalkwijk FJ, Ricci M, Nikpour A, Miller LA. The impact of sleep characteristics and epilepsy variables on memory performance in patients with focal seizures. Epilepsy Behav. (2018) 87:152–8. doi: 10.1016/j.yebeh.2018.06.034
5. de Weerd A, de Haas S, Otte A, Trenité DK, van Erp G., Cohen A, et al. Subjective sleep disturbance in patients with partial epilepsy: a questionnaire-based study on prevalence and impact on quality of life. Epilepsia. (2004) 45:1397–404. doi: 10.1111/j.0013-9580.2004.46703.x
6. Moore JL, Carvalho DZ, St Louis EK, Bazil C. Sleep and epilepsy: a focused review of pathophysiology, clinical syndromes, co-morbidities, and therapy. Neurotherapeutics. (2021) 18:170–80. doi: 10.1007/s13311-021-01021-w
7. Yaranagula SD, Asranna A, Nagappa M, Nayak CS, Pratyusha PV, Mundlamuri RC, et al. Sleep profile and Polysomnography in patients with drug-resistant temporal lobe epilepsy (TLE) due to hippocampal sclerosis (HS) and the effect of epilepsy surgery on sleep-a prospective cohort study. Sleep Med. (2021) 80:176–83. doi: 10.1016/j.sleep.2020.12.016
8. Ng M, Pavlova M. Why are seizures rare in rapid eye movement sleep? Review of the frequency of seizures in different sleep stages. Epilepsy Res Treat. (2013) 2013:932790. doi: 10.1155/2013/932790
9. Nakamura M, Jin K, Kato K, Itabashi H, Iwasaki M, Kakisaka Y, et al. Differences in sleep architecture between left and right temporal lobe epilepsy. Neurol Sci. (2017) 38:189–92. doi: 10.1007/s10072-016-2731-6
10. Sanjari Moghaddam H, Mohammadi E, Dolatshahi M, Mohebi F, Ashrafi A, Khazaie H, et al. White matter microstructural abnormalities in primary insomnia: a systematic review of diffusion tensor imaging studies. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 105:110132. doi: 10.1016/j.pnpbp.2020.110132
11. Gao J, Feng ST, Wu B, Gong N, Lu M, Wu PM, et al. Microstructural brain abnormalities of children of idiopathic generalized epilepsy with generalized tonic-clonic seizure: a voxel-based diffusional kurtosis imaging study. J Magn Reson Imaging. (2015) 41:1088–95. doi: 10.1002/jmri.24647
12. Sinha N, Peternell N, Schroeder GM, de Tisi J, Vos SB, Winston GP, et al. Focal to bilateral tonic-clonic seizures are associated with widespread network abnormality in temporal lobe epilepsy. Epilepsia. (2021) 62:729–41. doi: 10.1111/epi.16819
13. Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. (2005) 46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x
14. Reyner LA, Horne JA, Reyner A. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep. (1995) 18:127–34.
15. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. (2014) 146:1387–94. doi: 10.1378/chest.14-0970
16. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
17. Sap-Anan N, Pascoe M, Wang L, Grigg-Damberger M, Andrews N, Foldvary-Schaefer NJE, et al. The Epworth Sleepiness Scale in epilepsy: Internal consistency and disease-related associations. Epilepsy Behav. (2021) 121:108099. doi: 10.1016/j.yebeh.2021.108099
18. Miner B, Gill TM, Yaggi HK, Redeker NS, Van Ness PH, Han L, et al. The Epidemiology of Patient-Reported Hypersomnia in Persons With Advanced Age. J Am Geriatr Soc. (2019) 67:2545–52. doi: 10.1111/jgs.16107
19. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
20. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/s1389-9457(00)00065-4
21. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res. (2003) 55:263–7. doi: 10.1016/S0022-3999(02)00604-9
22. Chung KF, Kan KK, Yeung WF. Assessing insomnia in adolescents: comparison of insomnia severity index, athens insomnia scale and sleep quality index. Sleep Med. (2011) 12:463–70. doi: 10.1016/j.sleep.2010.09.019
23. Park CH, Ohn SH. A challenge of predicting seizure frequency in temporal lobe epilepsy using neuroanatomical features. Neurosci Lett. (2019) 692:115–21. doi: 10.1016/j.neulet.2018.11.005
24. Chakravarty K, Shukla G, Poornima S, Agarwal P, Gupta A, Mohammed A, et al. Effect of sleep quality on memory, executive function, and language performance in patients with refractory focal epilepsy and controlled epilepsy versus healthy controls - a prospective study. Epilepsy Behav. (2019) 92:176–83. doi: 10.1016/j.yebeh.2018.12.028
25. Jain SV, Glauser TA. In response: Effects of epilepsy treatments on sleep architecture and daytime sleepiness: an evidence-based review of objective sleep metrics. Epilepsia. (2014) 55:778. doi: 10.1111/epi.12597
26. Oldani A, Ferini-Strambi L, Zucconi M. Symptomatic nocturnal frontal lobe epilepsy. Seizure. (1998) 7:341–3. doi: 10.1016/S1059-1311(98)80030-7
27. Foldvary N, Perry M, Lee J, Dinner D, Morris HH. The effects of lamotrigine on sleep in patients with epilepsy. Epilepsia. (2001) 42:1569–73. doi: 10.1046/j.1528-1157.2001.46100.x
28. Foldvary-Schaefer N, Grigg-Damberger M. Sleep and epilepsy: what we know, don't know, and need to know. J Clin Neurophysiol. (2006) 23:4–20. doi: 10.1097/01.wnp.0000206877.90232.cb
29. Bi Y, Yuan K, Yu D, Wang R, Li M, Li Y, et al. White matter integrity of central executive network correlates with enhanced brain reactivity to smoking cues. Hum Brain Mapp. (2017) 38:6239–49. doi: 10.1002/hbm.23830
30. Chen L, Shao Z, Xu Y, Wang S, Zhang M, Liu S, et al. Disrupted frontostriatal connectivity in primary insomnia: a DTI study. Brain Imaging Behav. (2021) 15:2524–31. doi: 10.1007/s11682-021-00454-3
31. Spiegelhalder K, Regen W, Prem M, Baglioni C, Nissen C, Feige B, et al. Reduced anterior internal capsule white matter integrity in primary insomnia. Hum Brain Mapp. (2014) 35:3431–8. doi: 10.1002/hbm.22412
32. Kocevska D, Cremers LGM, Lysen TS, Luik AI, Ikram MA, Vernooij MW, et al. Sleep complaints and cerebral white matter: A prospective bidirectional study. J Psychiatr Res. (2019) 112:77–82. doi: 10.1016/j.jpsychires.2019.02.002
33. Voldsbekk I, Groote I, Zak N, Roelfs D, Geier O, Due-Tønnessen P, et al. Sleep and sleep deprivation differentially alter white matter microstructure: A mixed model design utilising advanced diffusion modelling. Neuroimage. (2021) 226:117540. doi: 10.1016/j.neuroimage.2020.117540
34. Vyas S, Singh P, Khandelwal N, Govind V, Aggarwal AN, Mohanty M. Evaluation of cerebral microstructural changes in adult patients with obstructive sleep apnea by MR diffusion kurtosis imaging using a whole-brain atlas. Indian J Radiol Imaging. (2019) 29:356–63. doi: 10.4103/ijri.IJRI_326_19
35. Garza-Villarreal EA, Chakravarty MM, Hansen B, Eskildsen SF, Devenyi GA, Castillo-Padilla D, et al. The effect of crack cocaine addiction and age on the microstructure and morphology of the human striatum and thalamus using shape analysis and fast diffusion kurtosis imaging. Transl Psychiatry. (2017) 7:e1122. doi: 10.1038/tp.2017.92
36. Blockx I, De Groof G, Verhoye M, Van Audekerke J, Raber K, Poot D, et al. Microstructural changes observed with DKI in a transgenic Huntington rat model: evidence for abnormal neurodevelopment. Neuroimage. (2012) 59:957–67. doi: 10.1016/j.neuroimage.2011.08.062
37. Tummala S, Roy B, Vig R, Park B, Kang DW, Woo MA, et al. Non-Gaussian Diffusion Imaging Shows Brain Myelin and Axonal Changes in Obstructive Sleep Apnea. J Comput Assist Tomogr. (2017) 41:181–189. doi: 10.1097/RCT.0000000000000537
38. Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Prolonged febrile seizures are associated with hippocampal vasogenic edema and developmental changes. Epilepsia. (2006) 47:1493–8. doi: 10.1111/j.1528-1167.2006.00621.x
39. Concha L, Kim H, Bernasconi A, Bernhardt BC, Bernasconi N. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology. (2012) 79:455–62. doi: 10.1212/WNL.0b013e31826170b6
40. Lee DA, Lee HJ, Kim HC, Park KM. Application of machine learning analysis based on diffusion tensor imaging to identify REM sleep behavior disorder. Sleep Breath. (2021) 1–8. doi: 10.1007/s11325-021-02434-9
41. Wu Q, Butzkueven H, Gresle M, Kirchhoff F, Friedhuber A, Yang Q, et al. MR diffusion changes correlate with ultra-structurally defined axonal degeneration in murine optic nerve. Neuroimage. (2007) 37:1138–47. doi: 10.1016/j.neuroimage.2007.06.029
42. Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci. (2010) 30:996–1002. doi: 10.1523/JNEUROSCI.1619-09.2010
Keywords: diffusional kurtosis imaging (DKI), temporal lobe epilepsy (TLE), sleep disorders, sleep questionnaires, abnormal white matter structure
Citation: Guo M, Shen B, Li J, Huang X, Hu J, Wei X, Wang S, Yuan R, He C and Li Y (2022) Diffusion Abnormality in Temporal Lobe Epilepsy Patients With Sleep Disorders: A Diffusion Kurtosis Imaging Study. Front. Psychiatry 13:885477. doi: 10.3389/fpsyt.2022.885477
Received: 28 February 2022; Accepted: 13 April 2022;
Published: 26 May 2022.
Edited by:
Yuanqiang Zhu, Fourth Military Medical University, ChinaReviewed by:
Haihua Bao, Affiliated Hospital of Qinghai University, ChinaGuihua Jiang, Guangdong Second Provincial General Hospital, China
Guanmin Quan, Second Hospital of Hebei Medical University, China
Copyright © 2022 Guo, Shen, Li, Huang, Hu, Wei, Wang, Yuan, He and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjing Li, bGl4aWFuZ18wNjhAMTYzLmNvbQ==
 Min Guo
Min Guo Boxing Shen1
Boxing Shen1