
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 21 September 2022
Sec. Schizophrenia
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.884828
This article is part of the Research Topic Negative Symptoms and Cognitive Impairment in Schizophrenia-Spectrum Disorders View all 15 articles
 Amir Valizadeh1*†
Amir Valizadeh1*† Mathew Mbwogge2†
Mathew Mbwogge2† Anita Rasouli Yazdi3†
Anita Rasouli Yazdi3† Nazanin Hedayati Amlashi3†
Nazanin Hedayati Amlashi3† Ainaaz Haadi3†
Ainaaz Haadi3† Monir Shayestefar1†
Monir Shayestefar1† Mana Moassefi1†
Mana Moassefi1†Background: Mirror neuron system (MNS) consists of visuomotor neurons that are responsible for the mirror neuron activity (MNA), meaning that each time an individual observes another individual performing an action, these neurons encode that action, and are activated in the observer's cortical motor system. Previous studies report its malfunction in autism, opening doors to investigate the underlying pathophysiology of the disorder in a more elaborate way and coming up with new rehabilitation methods. The study of MNA function in schizophrenia patients has not been as frequent and conclusive as in autism. In this research, we aimed to evaluate the functional integrity of MNA and the microstructural integrity of MNS in schizophrenia patients.
Methods: We included case-control studies that have evaluated MNA in schizophrenia patients compared to healthy controls using a variety of objective assessment tools. In August 2022, we searched Embase, PubMed, and Web of Science for eligible studies. We used an adapted version of the NIH Quality Assessment of Case-Control Studies tool to assess the quality of the included studies. Evidence was analyzed using vote counting methods of the direction of the effect and was tested statistically using the Sign test. Certainty of evidence was assessed using CERQual.
Results: We included 32 studies for the analysis. Statistical tests revealed decreased MNA (p = 0.002) in schizophrenia patients. The certainty of the evidence was judged to be moderate. Investigations of heterogeneity revealed a possible relationship between the age and the positive symptoms of participants in the included studies and the direction of the observed effect.
Discussion: This finding contributes to gaining a better understanding of the underlying pathophysiology of the disorder by revealing its possible relation to some of the symptoms in schizophrenia patients, while also highlighting a new commonality with autism.
Systematic review registration: PROSPERO identifier: CRD42021236453.
The mirror neuron system (MNS), which is a physiological substrate that may subserve certain mechanisms underlying social cognition has recently gained a lot of attention from the research community. MNS is a system consisting of visuomotor neurons that are responsible for the mirror mechanisms, meaning that each time an individual observes another individual performing an action, these neurons which encode that action, are activated in the observer's cortical motor system (1). Observed activations of this system are referred to as mirror neuron activity (MNA). MNA is considered a subdomain of social cognition (2). Several important functions beyond the action domain have been theorized for MNS, such as being a fundamental building block for understanding others' actions (3), encoding the intentions of the actor (4, 5), facilitating imitation (6, 7), and playing a role in human infants' ability to map similarities between self and others (8). Additionally, there has been an emphasis on the possible ties between MNA and empathy (9), and language (10). Mirror neurons were first discovered in the premotor area F5 of macaque monkeys (11). Later, similar neurons were found in the inferior parietal lobule, area PF, of macaque monkeys, and the concept of MNS was established (12). Some studies have claimed the discovery of similar neurons in various regions of the human brain, including the ventral premotor cortex (PMv) (13, 14), inferior frontal gyrus (IFG) (15–17), superior temporal sulcus (STS) (18–20), and inferior parietal lobule (IPL) (14, 21). In the meantime, some counter-arguments exist that question the function and even the very existence of the human MNS, with the strongest argument being the absence of single-cell recording data for human subjects (22). These counter-arguments were assuaged following single-cell recording studies in pre-surgical patients (23), the repetition suppression functional magnetic resonance imaging (fMRI) procedure in healthy volunteers (17), and lesion study in the human brain regions that have been proposed to be associated with human MNS (24). Nevertheless, MNS has been one of the most widely investigated domains of social cognition in psychiatric disorders within human beings. Even a recent paper by Heyes and Catmur (25) has called for more research on this phenomenon.
Other regions have also been proposed as an extension to MNS, one of the most important of them being the Brodmann area 2 (BA2) (26), which is the strongest generator of the mu rhythm (27). Mu rhythm (oscillations from 8 to 13 Hz) suppression has been proposed to be an indication of the MNA, as it is seen both when an individual performs and observes an action (28–30). A meta-analysis has demonstrated that there might also be other brain regions that do not have mirror properties but may convey necessary information to MNS including the primary visual cortex, supplementary motor area, dorsal premotor cortex, superior parietal lobe, cerebellum, and parts of the limbic system (31).
In the context of psychological disorders, MNA has been mostly investigated in autism. This is due to the “broken mirror” hypothesis and its role in explaining the social and language deficits of this disorder (32). However, research has produced insufficient evidence to support this hypothesis in its pure form, and instead, two alternative models have been proposed: the EP-M model and the social top-down response modulation (STORM) model. The STORM model proposes that autism symptoms originate from abnormalities within the top-down regulation of the MNS, rather than within the MNS itself, while the EP-M model proposes that imitation behavior in autistic individuals is served by the pathways between brain areas associated with MNS (33). Nevertheless, both these alternative models also suggest that there might be some possible dysfunction in the MNA in these patients, either within the MNS itself or within the systems that regulate MNA (32, 33). The discovery of such dysfunction has opened the doors to investigate and explain the underlying pathophysiology of the disorder in a more elaborate way and to come up with new rehabilitation methods (34–36).
Schizophrenia is one of the most debilitating and common neuropsychiatric disorders, with an estimated prevalence between 0.28 and 0.75% in the population worldwide (37–40). Deficits in a variety of cognitive domains are well-known for this disorder, such as impaired attention, verbal memory, and social cognition, and these are listed as specifiers for schizophrenia in the 11th revision of the International Classification of Diseases (ICD-11) (41). There are several reports of individuals with both autism and schizophrenia (42–45), which reveal that deficits in the theory of mind (ToM) exist in both disorders. Also, there are reports that both disorders share several genetic signals (46). A previous meta-analysis (47) of fMRI studies on autism and schizophrenia patients during ToM tasks revealed hypoactivation in the STS area, one of the main brain regions associated with MNA, in both groups, yet again emphasizing the deficits in ToM in both disorders, and possibly, hypothesizing the presence of MNA impairments in schizophrenia similar to the already known MNA impairments in autism patients.
MNA dysfunction might be another commonality between these disorders. Investigations of MNA in schizophrenia have not been as profound and conclusive as in autism. Based on a recent review (48) that partly examined this subject, findings of the state of MNA function in schizophrenia are mixed, with some studies suggesting impaired MNA function in the patients, while others did not find such a phenomenon. If such dysfunction is proven to be present in schizophrenia patients, it might potentially serve for implementing new rehabilitation treatments based on MNA training, as such treatment options have been previously found in multiple reports to be beneficial for autism patients (49–53).
To date, there has not been a systematic review with a qualitative analysis of studies that examine MNA/MNS in schizophrenia patients. Although a previous systematic review of the studies exists (54), that paper is a review of the evidence with little data analysis, and thus, considering the importance of the functions theorized to be associated with MNA, and the new studies published since that review, a new systematic examination of studies on this subject with a more in-depth analysis of the findings seems necessary. Results of such investigations may also help in gaining a more in-depth understanding of the mechanisms underlying schizophrenia and its possible common pathogenesis with autism. Specifically, we aim to evaluate the following:
• Primary objective: The functional integrity of MNA in schizophrenia patients. By functional integrity, we mean evaluating MNA using brain function measurement methods to investigate if it is identical to those in healthy control subjects.
• Secondary objective: The microstructural integrity of MNS in schizophrenia patients. By microstructural integrity, we mean evaluating MNS using brain microstructure evaluation methods to investigate if it is identical to those in healthy control subjects.
The design and methods used for this review comply with the Center for Reviews and Dissemination (CRD) Guidance for Undertaking Reviews in Healthcare (55), a guideline that presents rigor methods for undertaking systematic reviews, and Meta-analyses of Observational Studies in Epidemiology (MOOSE) (56), a guide on methods for conducting systematic reviews and meta-analyses specifically on observational studies. This review is reported in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (57) guideline. The review protocol has been published elsewhere (58).
Eligibility criteria for including studies were informed using the SPIDER (Sample, Phenomenon of Interest, Design, Evaluation, Research type) framework (59):
Sample: Patients of any age and sex diagnosed with schizophrenia or schizoaffective disorder confirmed by a physician in line with the International Classification of Diseases (ICD) or Diagnostic and Statistical Manual of Mental Disorders (DSM), irrespective of the severity or duration of illness, compared to healthy controls. Participants with any other macrostructural or functional neurologic disorders were excluded.
Phenomenon of interest: MNA and microstructural integrity of main brain regions (PMv, IFG, STS, IPL, and BA2) that are theorized to be associated with MNS.
Design: Observational case-control studies.
Evaluation:
- Functional methods: Electroencephalography (EEG), Magnetoencephalography (MEG), Transcranial magnetic stimulation (TMS), Electromyography (EMG), Proton Emission Tomography (PET), and Functional magnetic resonance imaging (fMRI). These methods are indirect measurements of what may reflect MNA, based on prior literature, as we cannot directly measure MNA in humans yet.
- Microstructural methods: Diffusion Tensor Imaging (DTI), Diffusion-Weighted Imaging (DWI), and Diffusion Spectrum Imaging (DSI). Only studies that specifically aimed to evaluate the microstructural integrity of the MNS were included.
Research type: Qualitative, quantitative, and mixed-methods.
In August 2022, AV searched Embase (via Ovid), PubMed, and Web of Science for eligible studies. We also carried out a “snowball” search through forward-citation and backward-citation tracking using Scopus on all of the included studies. Our search strategy is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses literature search extension (PRISMA-S) (60). No restriction or search filter was used. The search strategy is presented in Data sheet 1.
Records were imported to EndNote version X9. NH and AH independently reviewed the titles and abstracts of the retrieved records. AV was consulted to make the final decision in cases of disagreements. The full texts of all potentially eligible records were retrieved. MMb and AR independently screened full-text studies. A study was included when both reviewers independently assessed it as satisfying the inclusion criteria.
A data extraction form was developed, pilot tested, and then refined. After finalizing the data extraction form, MMb, AR, and MSh independently used it to extract data from eligible studies. Extracted data were compared, with any discrepancies being resolved through discussion. AV entered data into Microsoft Excel, double-checking them for accuracy. When information regarding any data was unclear, we contacted the authors of the reports to provide further details.
We extracted the following information from the included studies:
• Sample size and characteristics such as age, gender, handedness, and ethnicity;
• Inclusion and exclusion criteria of the study;
• Assessment tool information (paradigm class and equipment properties);
• Ethical considerations;
• Severity score of the disease;
• Brain regions with different activation patterns between patients and controls (for task-based fMRI, MEG, and PET);
• Results and conclusions of the study; and
• Funding sources and conflicts of interest.
Ethnicities were categorized according to the NIH Racial and Ethnic Categories (61). Severity scores included the Positive and Negative Syndrome Scale (PANSS) (62), the Scale for the Assessment of Negative Symptoms (SANS) (63), and the Scale for the Assessment of Positive Symptoms (SAPS) (64). To achieve a better comparison state, SANS and SAPS were converted to PANSS (65) scores.
We used an adapted version of the NIH Quality Assessment Tool for Case-Control Studies (66). This tool is originally developed to evaluate the internal validity of case-control studies, is consisted of 11 questions, and assesses the following factors: risk of potential for selection bias, information bias, measurement bias, confounding, exposures occurring before outcomes, evaluation of a dose-response gradient, accuracy of measurement of both exposure and outcome, sufficient time frame to see an effect, and appropriate control for confounding. Using this tool, the overall methodological and reporting quality of a study should be judged as either poor, fair, or good.
After consensus, we made some changes to the tool, so it better suits our review. As sample size justification does not apply to our topic, we changed the third question to check if the authors included a considerable sample size. Considering the multimodal nature of this review, it was not possible to use power analysis to calculate the minimum required sample size for each study. Considering a recent analytical study (67), a sample size of 34 participants is required to surpass 80% power to detect an effect size of D = 0.5 at α = 0.05 (though usually in functional neuroimaging studies α = 0.001 is the standard). Nevertheless, investigations revealed that 90% of the highly cited fMRI papers had a sample size smaller than that (67). Considering these facts, by consensus, we decided to define the minimum required sample size as at least 34 participants (17 for each group). We changed the fourth question to address one of the most important possible confounders in our review, unrelated concurrent psychiatric and neurologic disorders. We considered the minimum required inclusion/exclusion criteria to address substance dependence, and other possible medical disorders, and having specified the diagnostic criteria used to diagnose patients. Also, as the 8th and 9th (concurrent control and exposures occurring before outcomes) questions don't apply to our subject, we changed them to address if controls were matched with cases for age, gender, and handedness because they might be important confounders in our study. We defined matching for age as having a p > 0.05 for the difference between groups, while for gender, we defined it as having a p > 0.5. We modified the 10th question to assess the validity and sufficient report of the paradigm used in the study. We considered a paradigm valid if at least some methodological studies have previously confirmed its reliability for the assessment of MNA. In the case of methodological innovations, the validity of the paradigm was assessed subjectively by discussion among the reviewers. Also, in cases of the inadequate report of paradigm parameters (e.g., not reporting acquisition parameters of an fMRI experiment), the study was ranked poor for this domain. The reviewers' arguments for each subjective decision behind the validity and report of the paradigms used in each study are presented in detail in Data sheet 2. We also removed the 11th question which addressed blinding of outcome assessors, since interventional methods (where blinding is of paramount concern) do not apply to our subject. Finally, we changed the last question to check if ethical issues were considered in the study design.
The adapted version of the tool was pilot-tested before use. MMb and AR independently evaluated included studies and recorded supporting information and justifications for their judgments. In cases of disagreements, AV was consulted.
As we included data from multiple paradigms, with different outcomes, quantitative analysis was not feasible. Thus, we aimed for qualitative analysis. The full texts of the included studies were read and evaluated by AV and MMo. We determined the direction of the effect based on the studies' results, as either “decreased,” “intact,” or “increased MNA” for the primary outcome and “intact” or “altered MNS” for the secondary outcome. Regarding the primary outcome, we only included studies that have directly evaluated MNA. We did not consider studies that assessed other cognitive domains hypothesized to be related to MNA (e.g., empathy, etc.) as eligible for analysis. For the secondary outcome, we did not perform any analysis as there were very few studies for this purpose.
AV analyzed the data using Microsoft Excel and dmetar (68) package for R version 4. A qualitative meta-analysis was performed for the primary outcome based on vote counting of the direction of the effect. Vote counting, a simple method for analyzing evidence from multiple evaluations, involves comparing the number of studies showing benefit (reduced MNA in the case of our study) with the number of studies showing harm (intact/increased MNA in the case of our study) (69). A harvest plot was designed to present results from the analysis. We also designed graphics to represent evaluated domains of methodological and reporting quality for each study and the quality across all studies.
To test for the statistical significance of the vote counting analyses, we used the sign test. The sign test is a non-parametric test that uses a binary measure of either a positive or a negative effect to test whether there is sufficient evidence to reject the null hypothesis of an equal number of positive and negative results (70). The P-value from a sign test represents the probability of observing the given number of positive and negative results if the null hypothesis was true. To perform the test, we counted the number of studies in each effect direction for the outcome. Also, to explore the results of the most commonly used paradigms, we conducted separate analyses on paradigms with more than 5 studies, which were EEG with 7 studies and task-based fMRI with 9 studies. We used GraphPad (Link) to calculate the two-tailed P-value for the sign test. We considered a p < 0.05 as significant (alpha error).
To explore heterogeneity in the results, we compared the outcome between subgroups. We conducted a test for subgroup differences between studies that evaluated MNA in “drug-naïve/drug-free for at least 1 month” patients, against studies on “medicated” patients. To check for this difference, we conducted Fisher's exact test (Link). Also, knowing that gray matter volumes atypically decline with age in schizophrenia patients (71), we conducted a logistic meta-regression test by comparing the mean age of the participants in each study, against the direction of the effect. We used the weighted least squares (WLS) method for this regression, with the weight associated with each study being the square root of its sample size (). Similar subgroup analyses were done for the gender of the participants (female to male ratio), mean positive PANSS scores of patients, and mean negative PANSS scores of the patients against the direction of the effect.
To evaluate the robustness of our results, we conducted a sensitivity analysis by excluding studies that were judged to be of poor methodological and reporting quality. We used the same previous methods above for this analysis.
The strength of the overall body of evidence was assessed using the Confidence in Evidence from Reviews of Qualitative Research Methods (CERQual) (72). This approach evaluates four components to score confidence in the review findings. These include methodological limitations, relevance, coherence, and adequacy. Each finding starts with a “high confidence” score which could be downgraded to “moderate confidence,” “low confidence,” or “very low confidence” if the CERQual process revealed concerns. AV and MMo evaluated each finding using the tool and attributed a score to it based on the four-point scoring system. We resolved discrepancies through discussion.
We identified 486 records through database searching. After deduplication and screening titles and abstracts of the records, 424 records were excluded. After reviewing the full texts of these reports, 28 were found to be eligible for inclusion in the review. Following citation searching of these studies, 6 more eligible studies were found. In the end, 32 studies (34 reports) were included in this review, 29 for the main outcome (functional integrity of MNA) and 3 for the secondary outcome (microstructural integrity of MNS). A detailed report of the study selection process is presented in Figure 1. It is of special notice that the three papers of Horan et al. were considered as one study for the statistical analyses (since they were performed on the same patients in the same setting), but were assessed for methodological quality separately (because they reported three different phases of a study).
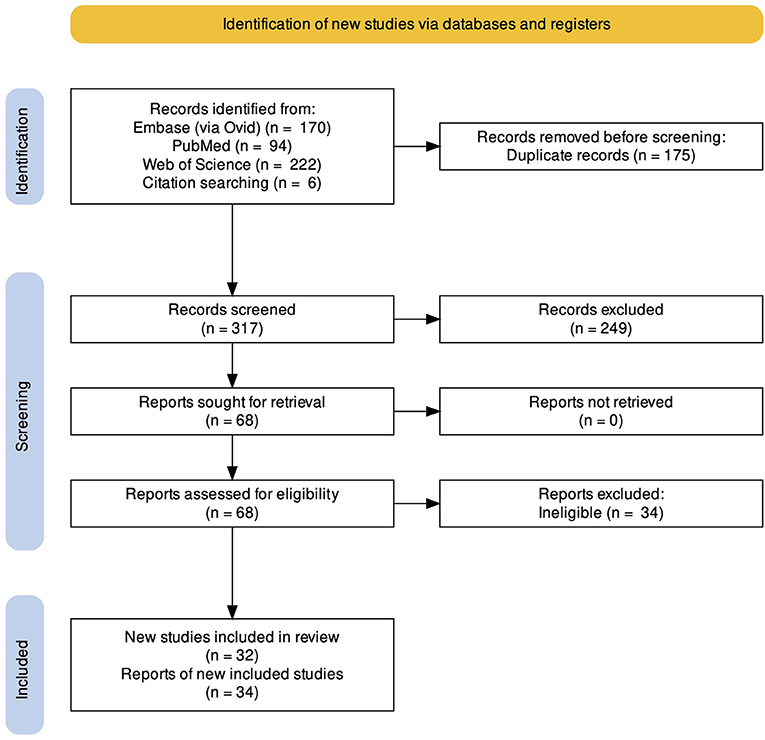
Figure 1. PRISMA Flow diagram of the study. We identified 486 records through database searching and six records through citation searching. Following deduplication, 317 records were screened, from which, 32 relevant studies (34 reports) were found and included in the review.
We included 29 studies (73–103) with 1,542 participants for the primary outcome and 3 studies (104–106) with 126 participants for the secondary outcome. Overall, 32 studies were included in this systematic review. For a detailed summary of the characteristics of the included studies, see Data sheet 3.
Sixteen studies (14 for the primary outcome, 2 for the secondary outcome) were judged to have good methodological and reporting quality, eight (7 for the primary outcome, 1 for the secondary outcome) were judged to have fair quality, and ten (all for the primary outcome) were judged to have poor quality. For more information on the quality domains for each study, please check Data sheets 2, 3. Figure 2 shows the judgments for each domain in each included study for each outcome. Judgments for each domain and each outcome across all studies are presented in Figure 3.
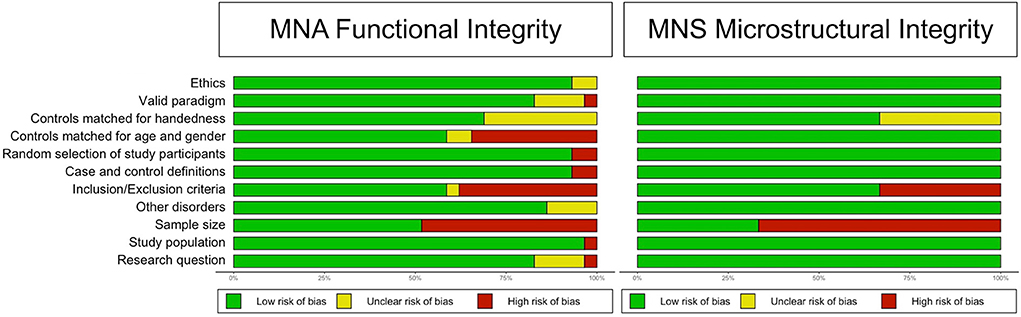
Figure 2. Methodological and reporting quality graph: Review authors' judgments about each methodological and reporting quality item presented as percentages across all included studies.
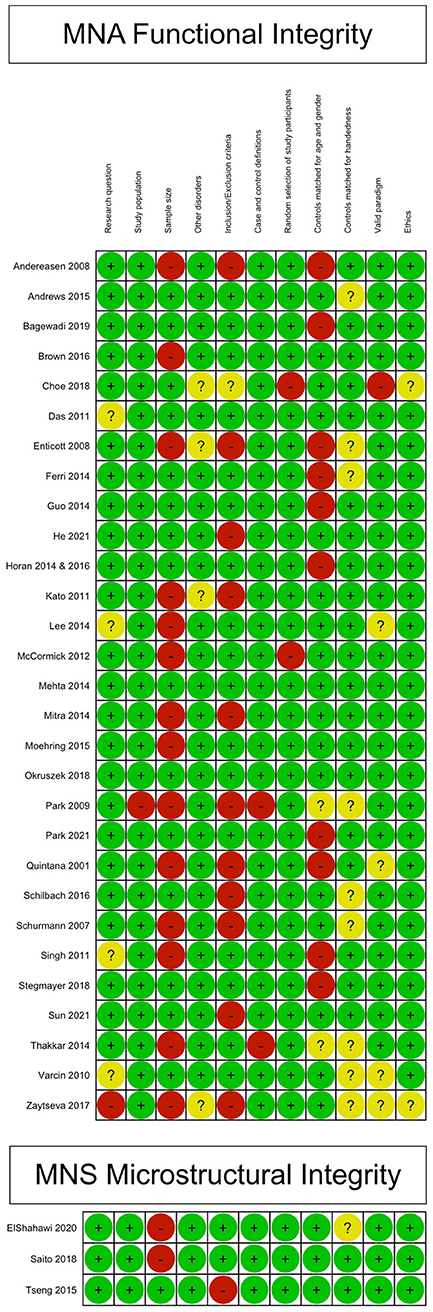
Figure 3. Methodological and reporting quality summary: review authors' judgments about each methodological and reporting quality item for each included study.
Regarding the primary outcome, the direction of the effect in most studies was toward decreased MNA. Four studies concluded that MNA in schizophrenia patients was not different from healthy controls, while two studies indicated that they detected increased MNA in these patients. For a detailed summary of the results of individual studies for this outcome, see the harvest plot in Figure 4. All three studies that evaluated the secondary outcome concluded that MNS microstructural integrity was altered in patients.
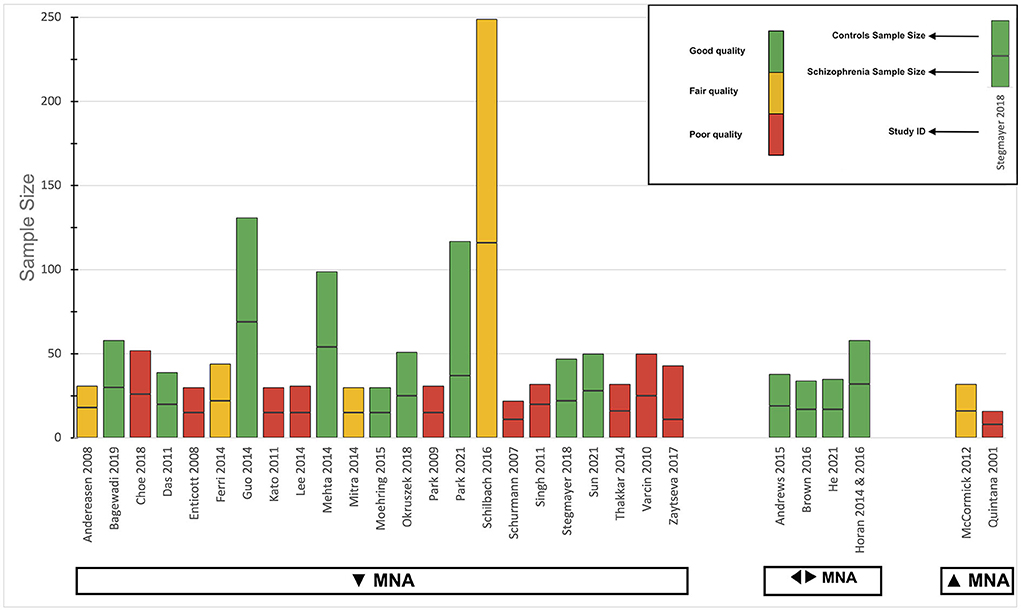
Figure 4. Harvest plot of the overall analysis for the primary outcome. The height of each bar represents the sample size, divided by a line into two sections to represent the sample size of each group (case and control). The methodological and reporting quality of each study is presented by the color of the bar; green for good, yellow for fair, and red for poor. The direction of the effect for the studies is mentioned below the bars: ▾ for decreased MNA, ◂▸ for intact MNA, and ▴ for increased MNA. MNA, Mirror neuron activity.
A summary of the characteristics of the included studies is presented in Table 1. The comments section for this table is built upon the comments provided in a similar table in the systematic review of Mehta et al. (54). For a more detailed report of the characteristics of contributing studies, see Data sheet 3.
MEG, fMRI, and PET are known to provide good spatial resolutions. The different patterns of activity in MNA-specific brain regions between cases and controls in the included studies are provided in Table 2.
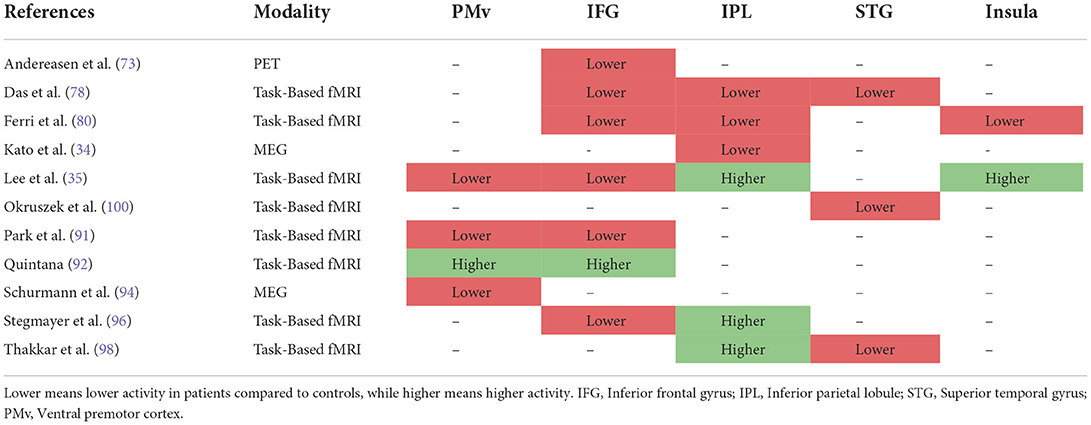
Table 2. The difference in the pattern of activation of different mirror neuron activity (MNA)-specific brain regions between schizophrenia and healthy control participants in task-based fMRI, MEG, and PET studies of MNA.
IFG was the most investigated area across the literature where 6/7 studies reported the detection of a decreased MNA in that region. IPL was the second most investigated area, but interestingly, it was also the one with the most controversial results. Of the 6 studies that evaluated this area, 3 reported the detection of decreased MNA and the other 3 reported the detection of increased MNA. Also, MNA was reported to be decreased in PMv in 3 of the 4 studies that investigated this area. Results for the STG area were pretty consistent with 3 of 3 studies reporting decreased MNA. Only 2 studies reported a difference in the insula activation, where their results were in the opposite direction.
The results of the analysis for the primary outcome are presented as a harvest plot in Figure 4. Most studies concluded that MNA was significantly reduced in schizophrenia patients, compared to controls (23/29, 79.3%). The two-tailed sign test P-value was calculated to be 0.002, meaning that the chance of observing either 23 or more studies, or 6 or fewer studies in 29 studies, in that direction, is 0.2%. Only two studies (87, 92) found significant results in the opposite direction (2/29, 7.9%). Four studies (74, 75, 82–84, 102) concluded that there was no significant difference between patients and healthy controls (4/29, 13.8%).
We also conducted a vote-counting analysis for the direction of the effect for studies that only used task-based fMRI as their assessment tool. In this group, seven studies concluded that MNA was reduced in cases, although this finding was not statistically significant (7/10, 70.0%; P = 0.344). A similar analysis was also conducted for studies that only used EEG. In this group, four studies concluded that MNA was reduced in cases (4/7, 57.1%; P = 1.000), showing almost no statistical significance. Also, two studies showed an intact MNA and one concluded that MNA was increased in cases. Results were very contradictory for the EEG group and demanded explicit evaluation.
Regarding the primary outcome, for the patients in the “drug-naïve/free for at least 1 month” subgroup, 4/4 studies, and the patients in the “medicated” subgroup, 14/20 studies were in the direction of decreased MNA, while 6 studies were in the direction of either intact or increased MNA. The test for subgroup differences revealed no significant difference between them (P = 0.539).
Results for the logistic meta-regression analyses for age, gender (female to male ratio), positive PANSS scores, and negative PANSS scores against the direction of the effect for the primary outcome are presented in Table 3 and Figure 5. There seems to be a relationship between the age of participants and the direction of the effect. A similar relationship was observed between the positive PANSS scores and the direction of the effect. Studies that found intact/increased MNA, were performed on patients of higher age and higher positive PANSS scores. These relationships were found to be statistically significant (P < 0.001 for age and P = 0.004 for positive PANSS scores).
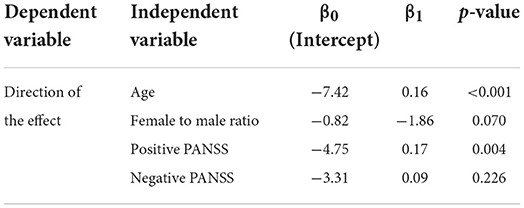
Table 3. Results of the logistic meta-regression analyses for investigating the possible causes of heterogeneity.
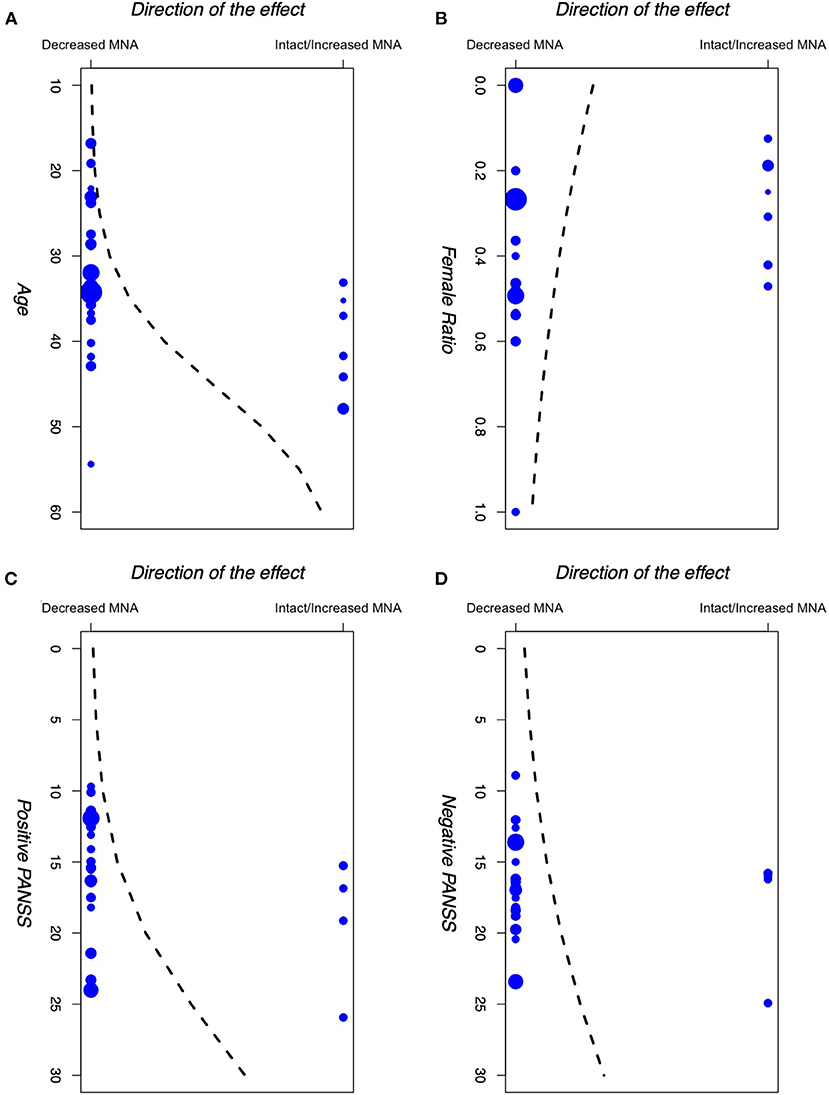
Figure 5. Logistic meta-regression analyses for (A) age, (B) gender (female to male ratio), (C) +PANSS scores, and (D) −PANSS scores of participants in the included studies against the direction of the effect of those studies. PANSS: Positive and Negative Syndrome Scale.
To check the robustness of our results for the primary outcome, we performed an analysis on studies that were judged to have fair or good methodological and reporting quality. Most of these studies were in favor of decreased MNA in cases, although this finding was not statistically significant (13/18; P = 0.096).
Given the multi-modal nature of the included studies, it was not possible to use statistical tests or funnel plots to check for the possible role of publication bias in our results. We figured the best way for checking any potential publication bias in our review is to check for the time-lag phenomenon, defined as “an initial wave of studies reporting positive or expected results, followed by a secondary wave of negative results” which is an indicator of possible publication bias (107). Our investigations on a quarter of the most recent included studies [8 studies (75, 96, 97, 100, 102–105), from 2018 to 2021] revealed that 87.5% (7/8 studies) were in the same direction as the results of our main analysis (reduced MNA, altered MNS). Although this finding does not rule out the publication bias for certain, it ascertains the absence of it to some considerable degree.
For the primary outcome, we believed there were some concerns for the “methodological limitations” domain as the considerable presence of bias across the included studies might have affected our results. We also believed there were minor concerns for the “coherence” domain because some studies reported contradictory results. No concerns were identified for the “adequacy” and “relevance” domains. Overall, given that the frequent presence of bias across the included studies might have affected our results, we decided to downgrade the certainty of the evidence by one level because of the “methodological limitations” domain. Thus, we believe there was moderate confidence in our findings.
Mehta et al. (54) conducted a systematic review on the same subject in 2014. However, to our knowledge, this is the first systematic review that has also incorporated qualitative data analysis to evaluate the mirror mechanism in schizophrenia patients and identify some of the possible sources of heterogeneity in the findings.
Some hypothesize that schizophrenia might be a disorder of the “social brain” (108). Mirror neurons are collections of neurons that are believed to be part of this social brain network (109). From this point of view, it has been hypothesized that MNA is impaired in schizophrenia. Our study reveals, with moderate confidence, that there is indeed an impaired MNA system in these patients.
Although most findings were in the same direction, one might question why others found contradictory results. We aim to describe here some of the potential causes underlying those results.
First, our meta-regression analyses found a statistically significant relationship between the mean positive PANSS scores and age of the participants in each study and the direction of the observed effect in that study. Studies that demonstrated intact/increased MNA enrolled patients with higher positive PANSS scores and age, compared to the studies that demonstrated decreased MNA.
Regarding the relationship between the positive PANSS scores and the direction of the effect, our results indicate that patients with higher positive PANSS scores are more likely to have intact/increased MNA. Positive PANSS measures the severity of the positive symptoms of the disorder, such as delusions, conceptual disorganization, hallucinations, and hostility (62). McCormick et al. (87) found a similar pattern in their study, suggesting that MNS may be overactive when positive symptoms are most prevalent (especially hallucinations). Mitra et al. (89) reported a negative correlation between the mu wave suppression and the thought disturbance cluster on PANSS, proposing that according to the theory that dopamine levels in the brain and the performance of the brain circuits have an “inverted-U” shaped relationship, an increase in brain dopamine levels during schizophrenia possibly disrupts the MNS circuit, leading to psychopathology manifestations. Other studies did not find a significant correlation between positive PANSS scores and MNA (74, 78, 80, 84, 86, 88, 90, 96, 99, 102). These contradictory results could be due to differences in experimental conditions, stage of disease, or the measures used to assess symptoms. Nevertheless, the idea of MNA correlating with patients' symptoms seems to be a plausible hypothesis. Indeed, this hypothesis was previously mentioned by Mehta et al. (54), making it an explanation worth further investigation. This is also in line with Frith and Corcoran's theoretical model of the relationship between social cognitive processes and psychotic symptoms (110).
Regarding age, similar results were found previously in autism, suggesting that individuals with autism may outgrow any mirror neuron deficit after a certain age (111, 112), although some other studies question these results (113, 114). One recent study indicates that in general, there might be some differences in the MNA between younger and older adults (115), where older adults showed Mu suppression in frontal and frontotemporal regions during a memory task, in contrast with young adults who showed Mu enhancement. Besides these, some studies have also shown that the social cognitive performance of schizophrenia patients may actually increase by age (116). Linke et al. (117) found a similar pattern in their study as well, but after including the patient's age at onset in their models, they concluded that this observed increase in social cognitive performance is not really due to the patients' age, but it is actually due to their later onset of the disease, as older patients are usually those with a later onset of psychosis as well. This is in line with previous studies that revealed age at the onset of the disease is negatively correlated with patients' cognitive performance (118, 119).
In the study of Horan et al. (82–84), they used a mask before group-level analyses, which might “bias against finding significant between-group differences,” as stated by the authors. In the study of Andrews et al. (73), they used a combination of TMS and EEG that may have reduced the quality of the EEG signals from some participants. Also, the baseline stimulus used to directly compare the EEG and TMS measures (blank screen) was not the same for the two measures. The studies of McCormick et al. (87) and Quintana (92) found increased MNA in patients. Quintana (91) study made a controversial decision by excluding BOLD signal changes during incorrect responses. The authors proposed that patients may have a compensatory increase in MNA while correctly performing the task. In the study of McCormick et al. (87), subgroup analyses showed only a subgroup of patients had greater mu suppression, the active psychosis subgroup. These findings indicate the need for more research on these subgroups of schizophrenia patients.
Most notably, we found the results of EEG studies to be very contradictory. Some possible explanations for such results have been previously mentioned in the study of Hobson and Bishop (120). First, they suggested that because the mu frequency band overlaps with the alpha frequency band (which is sensitive to attentional fluctuation), mu suppression could potentially be confounded by changes in attentional engagement. They also report that there is little consistency in how the specific baseline against which mu suppression is assessed should be defined. Finally, they examined mu suppression in 61 typical adults and reported that even in an optimal evaluation condition, 16–21% of participants showed no mu suppression to action observation task. Overall, they concluded that mu suppression can be used to index the human MNS, but the effect seems to be weak and unreliable, and it may also easily be confounded by alpha rhythm suppression. More interestingly, a recent study found that observation tasks may sometimes elicit mu rhythm enhancement rather than suppression (121). All these results question the reliability of the EEG paradigm for assessing MNA. Also, the validity of the TMS/EEG paradigm has been seriously questioned by another recent study (122). With all of those in mind, we still didn't consider these paradigms as invalid in our bias assessment process, as this domain required subjective judgments (where we tried to be conservative) and there are still some counter-arguments supporting the possible reliability of these paradigms.
Negative symptoms account for a substantial portion of the morbidity associated with schizophrenia (123). Empirical research has argued for an association between negative symptoms and anomalous MNA (124). We found the same association in some of the studies included in this review (85, 86, 95, 98). The study of Singh et al. (95) found lower mu wave suppression to positively correlate with negative PANSS scores, suggesting MNS may be underactive when negative symptoms predominate. However, the study of Brown et al. (76) found a statistically significant correlation between mu wave suppression and negative PANSS scores in the opposite direction of Singh's et al. Also, the study of Kato et al. (85) reported a negative correlation between the amplitudes of root-mean-square (RMS) of MEG responses and negative PANSS scores. Finally, Park et al. (91) reported the presence of a negative correlation between the functional deficits in MNS and negative PANSS scores. Although we didn't find any significant correlation between MNA and negative PANSS scores (P = 0.226), future studies should provide an in-depth assessment of the relationship between these two factors.
Deficits in communications skills have been previously documented in schizophrenia (125), but there has not been a comprehensive explanation for the etiology of this phenomenon up to this date. Indeed, MNS has been linked to developing communication skills via integrating auditory, visual, and motor stimulation (126). A study by Cantisani et al. reported a negative linear association between resting-state cerebral blood flow in the left inferior and middle frontal gyrus of schizophrenia patients with their communication skills, measured through the Social and Occupational Functioning Assessment Scale (SOFAS) (127). Our results indicate that across the literature, the inferior frontal gyrus (IFG) was the most investigated area for MNA in schizophrenia patients, where most studies indicated decreased MNA detection in this area. Putting these findings together, the disruption in MNA might be suggested as a possible explanation for communication skills in schizophrenia patients. Further studies are required to validate this hypothesis.
Echopraxia is the pathological repetition by imitation of the movements of another person. In the context of schizophrenia, it has been mostly associated with the catatonic form (128). A previous speculative paper by Pridmore et al. suggested that pathologically handled MNS-generated representations, especially in IFG, might be involved in this dysfunction (129). Indeed, this was in line with the findings of the study of Zaytseva et al. (101) where the authors reported altered mu rhythm suppression in the right frontal and central brain regions in patients with catatonic schizophrenia. More studies on catatonic patients in the future are suggested to further evaluate the validity of this finding.
Previously, some have argued that MNS might have a plastic feature (130), meaning that after receiving treatment, disruptions in this system might at least partially resolve. However, a study by Mitra et al. (131) found that following 8 weeks of antipsychotic treatment, no significant changes took place in the MNA of patients. This is partly in line with our results that revealed there was no significant MNA difference between medicated and drug-free patients. These indicate that even though antipsychotic medications may improve cognitive deficits for some schizophrenia patients, they may not affect MNA significantly.
Our results indicate that MNA is altered in schizophrenia patients, similar to the individuals with autism. This finding contributes to the efforts of exploring the dimensions of mental disorders to integrate many levels of information to understand the nature of mental health and illness, such as efforts taking place in the projects of RdoC (132).
Deriving clinical impact from such results could be an existing area of research. In a pilot study in 2020 (133), Hadoush et al. evaluated the effect of bilateral anodal transcranial direct current stimulation (tDCS) applied over the MNS of autism patients. They concluded that this intervention has a moderate therapeutic effect on children with autism in terms of their sociability, behavior, health, and even physical conditions. This pilot study reveals the potential of new rehabilitation methods through MNS-based training, which might benefit patients. It might be interesting to evaluate if similar results could be obtained for schizophrenia patients.
Another study that evaluated the effect of add-on yoga therapy on schizophrenia patients, revealed that MNA increased in the intervention group following 6 weeks of yoga therapy. They also found significant improvements in social cognition composite score (SCCS), negative symptoms (SANS), and positive symptoms (SAPS). One hypothesis is that the improvements in those clinical symptoms might have been achieved through the training of the MNS. Indeed, a previous study on yoga therapy for 2 years on 12 autism patients (6 in the interventional arm and 6 in the comparator arm) revealed improvements in imitation and other social skills of the participants (134). The authors hypothesized that guided imitation of therapist body positions might have stimulated MNA, resulting in an improved sense of self. Investigating the causal relationship between such findings might benefit future research.
All the included studies used indirect measures of MNA. It is known that intracranial electrodes give the most reliable evidence of MNA, but understandably, such procedures cannot be used for research on humans. Nevertheless, the indirect nature of the assessment tools used in the included studies, compared to the definitive direct sell recording techniques, should be considered as a limitation of the evidence. Also, a considerable proportion of the included studies had a sample size of < 34, which decreased the power of their statistical analysis. By the way, some studies did not use valid and comprehensive inclusion/exclusion criteria, which might increase the chance of confounding in their results. Finally, a proportion of the included studies did not report if controls were matched with cases for handedness.
We acknowledge several limitations in our study. Firstly, we used a weak statistical test for our analyses. Although it requires mentioning that considering the wide range of assessment paradigms we included in this review, more powerful statistical tests were not feasible. Secondly, we didn't assess the same outcome in other populations with almost the same pathology (i.e., schizoaffective disorder). Finally, some important information was not reported in the included studies. We tried to reach out to the authors to ask for that information but did not get any response. Nevertheless, we believe that possible none of these methodological limitations would significantly change the overall conclusions of this review.
Overall, we acknowledge that presenting a quantified summary for such a highly debated and controversial topic, given so few studies with vastly different modalities, would have its challenges and may require some methodological innovations. With that in mind, we still believe that our study managed to provide a clearer picture of the current state of knowledge on this subject, while also pointing to some of the existing biases and limitations in the literature.
From our findings, one can claim, with moderate confidence, that MNA is altered in schizophrenia patients. This finding provides clues for a more in-depth understanding of the disorder and helps find a more comprehensive revision of the underlying pathophysiology of psychosis spectrum disorders. As more findings are being discovered that help to achieve a more in-depth understanding of psychiatric disorders, adjustments to our definitions for these illnesses seem necessary. Future researchers may evaluate the same deficits in patients with other disorders (e.g., bipolar disorder, depression, etc.) to come up with a better understanding of the common features across these disorders and facilitate the process of finding new semantic definitions for psychiatric illnesses.
We also urge future researchers on this subject to try to compensate for the existing biases and limitations in the literature. This may include conducting studies with larger sample sizes, using rigor eligibility criteria to minimize confounding effects, and utilizing valid paradigms to ensure the reliability of the results. Also, research on deriving potential clinical impact using MNS-based training methods could be an exciting topic for future investigations.
The original contributions presented in the study are included in Supplementary material. Further inquiries can be directed to the corresponding author.
AV: conception and coordination of the review, designing the protocol, search, study selection, data extraction, methodological and reporting quality assessment, analysis of evidence, interpretation of the results, assessing the certainty of the evidence, and writing the review. MMb: data extraction, methodological, and reporting quality assessment. AR: data extraction, methodological, and reporting quality assessment. NH and AH: study selection. MS: data extraction. MMo: data extraction and assessing the certainty of the evidence. All authors contributed to the article and approved the submitted version.
Special thanks to Prof. Giacomo Rizzolatti for his comments on the protocol.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.884828/full#supplementary-material
1. Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. (2004) 27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230
2. Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. (2013) 11:126. doi: 10.1186/1741-7015-11-126
3. Bonini L, Rozzi S, Serventi FU, Simone L, Ferrari PF, Fogassi L. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cerebral Cortex. (2010) 20:1372–85. doi: 10.1093/cercor/bhp200
4. Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. (2005) 308:662–7. doi: 10.1126/science.1106138
5. Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. (2010) 11:264–74. doi: 10.1038/nrn2805
6. Brass M, Heyes C. Imitation: is cognitive neuroscience solving the correspondence problem? Trends Cogn Sci. (2005) 9:489–95. doi: 10.1016/j.tics.2005.08.007
7. Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, et al. Neural circuits underlying imitation learning of hand actions. Neuron. (2004) 42:323–34. doi: 10.1016/S0896-6273(04)00181-3
8. Marshall PJ, Meltzoff AN. Neural mirroring mechanisms and imitation in human infants. Philos Trans R Soc B Biol Sci. (2014) 369:20130620. doi: 10.1098/rstb.2013.0620
9. Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. (2009) 60:653–70. doi: 10.1146/annurev.psych.60.110707.163604
10. Théoret H, Pascual-Leone A. Language acquisition: do as you hear. Curr Biol. (2002) 12:R736–7. doi: 10.1016/S0960-9822(02)01251-4
11. di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. (1992) 91:176–80. doi: 10.1007/BF00230027
12. Gallese V. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. (1998) 2:493–501. doi: 10.1016/S1364-6613(98)01262-5
13. Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. J Neurophysiol. (2007) 98:1415–27. doi: 10.1152/jn.00238.2007
14. Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex. (2009) 19:1239–55. doi: 10.1093/cercor/bhn181
15. Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Euro J Neurosci. (2001) 13:400–4. doi: 10.1111/j.1460-9568.2001.01385.x
16. Grèzes J, Armony JL, Rowe J, Passingham RE. Activations related to “mirror” and “canonical” neurones in the human brain: an fMRI study. Neuroimage. (2003) 18:928–37. doi: 10.1016/S1053-8119(03)00042-9
17. Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD. Evidence of mirror neurons in human inferior frontal gyrus. J Neurosci. (2009) 29:10153–9. doi: 10.1523/JNEUROSCI.2668-09.2009
18. Buccino G, Binkofski F, Riggio L. The mirror neuron system and action recognition. Brain Lang. (2004) 89:370–6. doi: 10.1016/S0093-934X(03)00356-0
19. Iacoboni M. Neurobiology of imitation. Curr Opin Neurobiol. (2009) 19:661–5. doi: 10.1016/j.conb.2009.09.008
20. de la Rosa S, Schillinger FL, Bülthoff HH, Schultz J, Uludag K. fMRI adaptation between action observation and action execution reveals cortical areas with mirror neuron properties in human BA 44/45. Front Hum Neurosci. (2016) 10:78. doi: 10.3389/fnhum.2016.00078
21. Chong TTJ, Cunnington R, Williams MA, Kanwisher N, Mattingley JB. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr Biol. (2008) 18:1576–80. doi: 10.1016/j.cub.2008.08.068
22. Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J Cogn Neurosci. (2009) 21:1229–43. doi: 10.1162/jocn.2009.21189
23. Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-Neuron responses in humans during execution and observation of actions. Curr Biol. (2010) 20:750–6. doi: 10.1016/j.cub.2010.02.045
24. Binder E, Dovern A, Hesse MD, Ebke M, Karbe H, Saliger J, et al. Lesion evidence for a human mirror neuron system. Cortex. (2017) 90:125–37. doi: 10.1016/j.cortex.2017.02.008
25. Heyes C, Catmur C. What happened to mirror neurons? Perspect Psychol Sci. (2022) 17:153–68. doi: 10.1177/1745691621990638
26. Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. (2010) 50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112
27. Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. (1994) 60:537–50. doi: 10.1016/0306-4522(94)90263-1
28. Oberman LM, McCleery JP, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron activity during the observation of human and robot actions: toward an analysis of the human qualities of interactive robots. Neurocomputing. (2007) 70:2194–203. doi: 10.1016/j.neucom.2006.02.024
29. Muthukumaraswamy SD, Johnson BW, McNair NA. Mu rhythm modulation during observation of an object-directed grasp. Cogn Brain Res. (2004) 19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001
30. Muthukumaraswamy SD, Johnson BW. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology. (2004) 41:152–6. doi: 10.1046/j.1469-8986.2003.00129.x
31. Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. (2012) 36:341–9. doi: 10.1016/j.neubiorev.2011.07.004
32. Ramachandran VS, Oberman LM. Broken mirrors: a theory of autism. Sci Am. (2006) 295:62–9. doi: 10.1038/scientificamerican1106-62
33. Yates L, Hobson H. Continuing to look in the mirror: a review of neuroscientific evidence for the broken mirror hypothesis, EP-M model and STORM model of autism spectrum conditions. Autism. (2020) 24:1945–59. doi: 10.1177/1362361320936945
34. Iacoboni M, Mazziotta JC. Mirror neuron system: basic findings and clinical applications. Ann Neurol. (2007) 62:213–8. doi: 10.1002/ana.21198
35. Ramachandran VS, Seckel EL. Synchronized dance therapy to stimulate mirror neurons in autism. Med Hypotheses. (2011) 76:150–1. doi: 10.1016/j.mehy.2010.10.047
36. Khalil R, Tindle R, Boraud T, Moustafa AA, Karim AA. Social decision making in autism: on the impact of mirror neurons, motor control, and imitative behaviors. CNS Neurosci Ther. (2018) 24:669–76. doi: 10.1111/cns.13001
37. Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull. (2018) 44:1195–203. doi: 10.1093/schbul/sby058
38. Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. (2005) 2:e141. doi: 10.1371/journal.pmed.0020141
39. Moreno-Küstner B, Martín C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS ONE. (2018) 13:e0195687. doi: 10.1371/journal.pone.0195687
40. Pacific W, Hasan SAW. Magnitude and Impact. World Health Organization (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/schizophrenia
41. World Health Organization. International Statistical Classification of Diseases and Related Health Problems: Alphabetical Index. World Health Organization; International Statistical Classification of Diseases and Related Health Problems (2004).
42. Mouridsen SE, Rich B, Isager T. Psychiatric disorders in adults diagnosed as children with atypical autism. A case control study. J Neural Transm. (2008) 115:135–8. doi: 10.1007/s00702-007-0798-1
43. Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. (2009) 48:10–8. doi: 10.1097/CHI.0b013e31818b1c63
44. Solomon M, Olsen E, Niendam T, Ragland JD, Yoon J, Minzenberg M, et al. From lumping to splitting and back again: atypical social and language development in individuals with clinical-high-risk for psychosis, first episode schizophrenia, and autism spectrum disorders. Schizophr Res. (2011) 131:146–51. doi: 10.1016/j.schres.2011.03.005
45. Stahlberg O, Soderstrom H, Rastam M, Gillberg C. Bipolar disorder, schizophrenia, and other psychotic disorders in adults with childhood onset AD/HD and/or autism spectrum disorders. J Neural Transm. (2004) 111:891–902. doi: 10.1007/s00702-004-0115-1
46. King BH, Lord C. Is schizophrenia on the autism spectrum? Brain Res. (2011) 1380:34–41. doi: 10.1016/j.brainres.2010.11.031
47. Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS ONE. (2011) 6:e25322. doi: 10.1371/journal.pone.0025322
48. Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. (2015) 16:620–31. doi: 10.1038/nrn4005
49. Field T, Field T, Sanders C, Nadel J. Children with autism display more social behaviors after repeated imitation sessions. Autism. (2001) 5:317–23. doi: 10.1177/1362361301005003008
50. Escalona A, Field T, Nadel J, Lundy B. Brief report: imitation effects on children with autism. J Autism Dev Disord. (2002) 32:141–4. doi: 10.1023/A:1014896707002
51. Ingersoll B, Lewis E, Kroman E. Teaching the imitation and spontaneous use of descriptive gestures in young children with autism using a naturalistic behavioral intervention. J Autism Dev Disord. (2007) 37:1446–56. doi: 10.1007/s10803-006-0221-z
52. Ingersoll B, Schreibman L. Teaching reciprocal imitation skills to young children with autism using a naturalistic behavioral approach: effects on language, pretend play, and joint attention. J Autism Dev Disord. (2006) 36:487–505. doi: 10.1007/s10803-006-0089-y
53. Ingersoll B, Gergans S. The effect of a parent-implemented imitation intervention on spontaneous imitation skills in young children with autism. Res Dev Disabil. (2007) 28:163–75. doi: 10.1016/j.ridd.2006.02.004
54. Mehta UM, Thirthalli J, Aneelraj D, Jadhav P, Gangadhar BN, Keshavan MS. Mirror neuron dysfunction in schizophrenia and its functional implications: a systematic review. Schizophr Res. (2014) 160:9–19. doi: 10.1016/j.schres.2014.10.040
55. Tacconelli E. Systematic reviews: CRD's guidance for undertaking reviews in health care. Lancet Infect Dis. (2010) 10:226. doi: 10.1016/S1473-3099(10)70065-7
56. Stroup DF. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
57. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
58. Valizadeh A, Amlashi NH, Rasooli A, Mbwogge M, Haadi A. The mirror mechanism in schizophrenia spectrum disorders: protocol for a systematic review and meta-synthesis. Res Square. (2021). doi: 10.21203/rs.3.rs-264432/v3
59. Cooke A, Smith D, Booth A. Beyond PICO. Qual Health Res. (2012) 22:1435–43. doi: 10.1177/1049732312452938
60. Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. (2021) 10:39. doi: 10.1186/s13643-020-01542-z
61. National Institutes of Health. Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes. National Institutes of Health (2015). Available online at: https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html
62. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
63. Andreasen NC. The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry. (1989) 155:49–52. doi: 10.1192/S0007125000291496
64. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa (1984)
65. van Erp TGM, Preda A, Nguyen D, Faziola L, Turner J, Bustillo J, et al. Converting positive and negative symptom scores between PANSS and SAPS/SANS. Schizophr Res. (2014) 152:289–94. doi: 10.1016/j.schres.2013.11.013
66. National Institute of Health. NIH Quality Assessment of Case-Control Studies. National Institute of Health (2014). Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
67. Szucs D, Ioannidis JPA. Sample size evolution in neuroimaging research: an evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. Neuroimage. (2020) 221:117164. doi: 10.1016/j.neuroimage.2020.117164
68. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-Analysis With R. Boca Raton, FL: Chapman and Hall/CRC (2021). doi: 10.1201/9781003107347
69. Lewis JJ, Pattanayak SK. Who adopts improved fuels and cookstoves? A systematic review. Environ Health Perspect. (2012) 120:637–45. doi: 10.1289/ehp.1104194
70. Starnes DS, Yates D, Moore DS. The Practice of Statistics. Macmillan (2010). Available online at: https://www.google.com/books/edition/The_Practice_of_Statistics/r0Z-AN_T2hIC?hl=en&gbpv=0
71. Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, et al. Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. (2017) 174:286–95. doi: 10.1176/appi.ajp.2016.16050610
72. Lewin S, Glenton C, Munthe-Kaas H, Carlsen B, Colvin CJ, Gülmezoglu M, et al. Using qualitative evidence in decision making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual). PLoS Med. (2015) 12:e1001895. doi: 10.1371/journal.pmed.1001895
73. Andereasen N, Calage C, O'Leary D. Theory of mind and schizophrenia: a positron emission tomography study of medication-free patients. Schizophr Bull. (2009) 35:1030. doi: 10.1093/schbul/sbp069
74. Andrews SC, Enticott PG, Hoy KE, Thomson RH, Fitzgerald PB. No evidence for mirror system dysfunction in schizophrenia from a multimodal TMS/EEG study. Psychiatry Res. (2015) 228:431–40. doi: 10.1016/j.psychres.2015.05.067
75. Bagewadi VI, Mehta UM, Naik SS, Govindaraj R, Varambally S, Arumugham SS, et al. Diminished modulation of motor cortical reactivity during context-based action observation in schizophrenia. Schizophr Res. (2019) 204:222–9. doi: 10.1016/j.schres.2018.07.043
76. Brown EC, Gonzalez-Liencres C, Tas C, Brüne M. Reward modulates the mirror neuron system in schizophrenia: a study into the mu rhythm suppression, empathy, and mental state attribution. Soc Neurosci. (2016) 11:175–86. doi: 10.1080/17470919.2015.1053982
77. Choe E, Lee TY, Kim M, Hur JW, Yoon YB, Cho KIK, et al. Aberrant within- and between-network connectivity of the mirror neuron system network and the mentalizing network in first episode psychosis. Schizophr Res. (2018) 199:243–9. doi: 10.1016/j.schres.2018.03.024
78. Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: a functional MRI study. Schizophr Res. (2012) 134:158–64. doi: 10.1016/j.schres.2011.08.019
79. Enticott P, Hoy K, Herring S, Johnston P, Daskalakis Z, Fitzgerald P. Reduced motor facilitation during action observation in schizophrenia: a mirror neuron deficit? Schizophr Res. (2008) 102:116–21. doi: 10.1016/j.schres.2008.04.001
80. Ferri F, Costantini M, Salone A, Ebisch S, de Berardis D, Mazzola V, et al. Binding action and emotion in first-episode schizophrenia. Psychopathology. (2014) 47:394–407. doi: 10.1159/000366133
81. Guo S, Kendrick KM, Yu R, Wang HLS, Feng J. Key functional circuitry altered in schizophrenia involves parietal regions associated with sense of self. Hum Brain Mapp. (2014) 35:123–39. doi: 10.1002/hbm.22162
82. Horan WP, Iacoboni M, Cross KA, Korb A, Lee J, Nori P, et al. Self-reported empathy and neural activity during action imitation and observation in schizophrenia. NeuroImage Clin. (2014) 5:100–8. doi: 10.1016/j.nicl.2014.06.006
83. Horan WP, Jimenez AM, Lee J, Wynn JK, Eisenberger NI, Green MF. Pain empathy in schizophrenia: an fMRI study. Soc Cogn Affect Neurosci. (2016) 11:783–92. doi: 10.1093/scan/nsw002
84. Horan WP, Pineda JA, Wynn JK, Iacoboni M, Green MF. Some markers of mirroring appear intact in schizophrenia: evidence from mu suppression. Cogn Affect Behav Neurosci. (2014) 14:1049–60. doi: 10.3758/s13415-013-0245-8
85. Kato Y, Muramatsu T, Kato M, Shibukawa Y, Shintani M, Mimura M. Magnetoencephalography study of right parietal lobe dysfunction of the evoked mirror neuron system in antipsychotic-free schizophrenia. PLoS ONE. (2011) 6:e28087. doi: 10.1371/journal.pone.0028087
86. Lee JS, Chun JW, Yoon SY, Park HJ, Kim JJ. Involvement of the mirror neuron system in blunted affect in schizophrenia. Schizophr Res. (2014) 152:268–74. doi: 10.1016/j.schres.2013.10.043
87. McCormick LM, Brumm MC, Beadle JN, Paradiso S, Yamada T, Andreasen N. Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res Neuroimaging. (2012) 201:233–9. doi: 10.1016/j.pscychresns.2012.01.004
88. Mehta UM, Thirthalli J, Basavaraju R, Gangadhar BN, Pascual-Leone A. Reduced mirror neuron activity in schizophrenia and its association with theory of mind deficits: evidence from a transcranial magnetic stimulation study. Schizophr Bull. (2014) 40:1083–94. doi: 10.1093/schbul/sbt155
89. Mitra S, Nizamie SH, Goyal N, Tikka SK. Mu-wave activity in schizophrenia: evidence of a dysfunctional mirror neuron system from an indian study. Indian J Psychol Med. (2014) 36:276–81. doi: 10.4103/0253-7176.135380
90. Möhring N, Shen C, Hahn E, Ta TMT, Dettling M, Neuhaus AH. Mirror neuron deficit in schizophrenia: evidence from repetition suppression. Schizophr Res. (2015) 168:174–9. doi: 10.1016/j.schres.2015.07.035
91. Park KM, Kim JJ, Ku J, Kim SY, Lee HR, Kim SI, et al. Neural basis of attributional style in schizophrenia. Neurosci Lett. (2009) 459:35–40. doi: 10.1016/j.neulet.2009.04.059
92. Quintana J. A compensatory mirror cortical mechanism for facial affect processing in schizophrenia. Neuropsychopharmacology. (2001) 25:915–24. doi: 10.1016/S0893-133X(01)00304-9
93. Schilbach L, Derntl B, Aleman A, Caspers S, Clos M, Diederen KMJ, et al. Differential patterns of dysconnectivity in mirror neuron and mentalizing networks in schizophrenia. Schizophr Bull. (2016) 42:1135–48. doi: 10.1093/schbul/sbw015
94. Schürmann M, Järveläinen J, Avikainen S, Cannon TD, Lönnqvist J, Huttunen M, et al. Manifest disease and motor cortex reactivity in twins discordant for schizophrenia. Br J Psychiatry. (2007) 191:178–9. doi: 10.1192/bjp.bp.106.024604
95. Singh F, Pineda J, Cadenhead KS. Association of impaired EEG mu wave suppression, negative symptoms and social functioning in biological motion processing in first episode of psychosis. Schizophr Res. (2011) 130:182–6. doi: 10.1016/j.schres.2011.04.004
96. Stegmayer K, Bohlhalter S, Vanbellingen T, Federspiel A, Wiest R, Müri RM, et al. Limbic interference during social action planning in schizophrenia. Schizophr Bull. (2018) 44:359–68. doi: 10.1093/schbul/sbx059
97. Sun F, Zhao Z, Lan M, Xu Y, Huang M, Xu D. Abnormal dynamic functional network connectivity of the mirror neuron system network and the mentalizing network in patients with adolescent-onset, first-episode, drug-naïve schizophrenia. Neurosci Res. (2021) 162:63–70. doi: 10.1016/j.neures.2020.01.003
98. Thakkar KN, Peterman JS, Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am J Psychiatry. (2014) 171:539–48. doi: 10.1176/appi.ajp.2013.13040498
99. Varcin KJ, Bailey PE, Henry JD. Empathic deficits in schizophrenia: the potential role of rapid facial mimicry. J Int Neuropsychol Soc. (2010) 16:621–9. doi: 10.1017/S1355617710000329
100. Okruszek Ł, Wordecha M, Jarkiewicz M, Kossowski B, Lee J, Marchewka A. Brain correlates of recognition of communicative interactions from biological motion in schizophrenia. Psychol Med. (2018) 48:1862–71. doi: 10.1017/S0033291717003385
101. Zaytseva Y, Morozova A, Bendova M, Garakh Z. Is motor imagery different in catatonic schizophrenia? Psych J. (2017) 6:137–8. doi: 10.1002/pchj.155
102. He Y, Steines M, Sammer G, Nagels A, Kircher T, Straube B. Modality-specific dysfunctional neural processing of social-abstract and non-social-concrete information in schizophrenia. NeuroImage Clin. (2021) 29:102568. doi: 10.1016/j.nicl.2021.102568
103. Park SH, Kim T, Ha M, Moon SY, Lho SK, Kim M, et al. Intrinsic cerebellar functional connectivity of social cognition and theory of mind in first-episode psychosis patients. NPJ Schizophr. (2021) 7:59. doi: 10.1038/s41537-021-00193-w
104. ElShahawi HH, Sakr HM, Hashim MA, Mohamed HH, Abdeen MS. Social cognition correlation to white matter integrity alteration in mirror neurons of schizophrenic patients: DTI study. Neurol Psychiatry Brain Res. (2020) 38:65–73. doi: 10.1016/j.npbr.2020.10.004
105. Saito Y, Kubicki M, Koerte I, Otsuka T, Rathi Y, Pasternak O, et al. Impaired white matter connectivity between regions containing mirror neurons, and relationship to negative symptoms and social cognition, in patients with first-episode schizophrenia. Brain Imaging Behav. (2018) 12:229–37. doi: 10.1007/s11682-017-9685-z
106. Tseng CEJ, Chien YL, Liu CM, Wang HLS, Hwu HG, Tseng WYI. Altered cortical structures and tract integrity of the mirror neuron system in association with symptoms of schizophrenia. Psychiatry Res Neuroimaging. (2015) 231:286–91. doi: 10.1016/j.pscychresns.2015.01.010
107. Ioannidis JPA. Why most published research findings are false. PLoS Med. (2005) 2:e124. doi: 10.1371/journal.pmed.0020124
109. Spunt RP, Lieberman MD. The busy social brain. Psychol Sci. (2013) 24:80–6. doi: 10.1177/0956797612450884
110. Frith CD, Corcoran R. Exploring ‘theory of mind' in people with schizophrenia. Psychol Med. (1996) 26:521–30. doi: 10.1017/S0033291700035601
111. Bastiaansen JA, Thioux M, Nanetti L, van der Gaag C, Ketelaars C, Minderaa R, et al. Age-Related increase in inferior frontal gyrus activity and social functioning in autism spectrum disorder. Biol Psychiatry. (2011) 69:832–8. doi: 10.1016/j.biopsych.2010.11.007
112. Chan MMY, Han YMY. Differential mirror neuron system (MNS) activation during action observation with and without social-emotional components in autism: a meta-analysis of neuroimaging studies. Mol Autism. (2020) 11:72. doi: 10.1186/s13229-020-00374-x
113. Nedelko V, Hassa T, Hamzei F, Weiller C, Binkofski F, Schoenfeld MA, et al. Age-independent activation in areas of the mirror neuron system during action observation and action imagery. A fMRI study. Restor Neurol Neurosci. (2010) 28:737–47. doi: 10.3233/RNN-2010-0542
114. Enticott PG, Kennedy HA, Rinehart NJ, Tonge BJ, Bradshaw JL, Taffe JR, et al. Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biol Psychiatry. (2012) 71:427–33. doi: 10.1016/j.biopsych.2011.09.001
115. Kladi A, Iliadou P, Tsolaki M, Moraitou D. Age-related differences in Mu rhythm during emotional destination memory task. Curr Aging Sci. (2022) 15:26–36. doi: 10.2174/1874609814666210607154838
116. Rajji TK, Voineskos AN, Butters MA, Miranda D, Arenovich T, Menon M, et al. Cognitive performance of individuals with schizophrenia across seven decades: a study using the MATRICS consensus cognitive battery. Am J Geriatr Psychiatry. (2013) 21:108–18. doi: 10.1016/j.jagp.2012.10.011
117. Linke M, Jankowski KS, Ciołkiewicz A, Jedrasik-Styła M, Parnowska D, Gruszka A, et al. Age or age at onset? Which of them really matters for neuro and social cognition in schizophrenia? Psychiatry Res. (2015) 225:197–201. doi: 10.1016/j.psychres.2014.11.024
118. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. (2009) 195:286–93. doi: 10.1192/bjp.bp.108.060723
119. Smeets-Janssen MMJ, Meesters PD, Comijs HC, Eikelenboom P, Smit JH, de Haan L, et al. Theory of mind differences in older patients with early-onset and late-onset paranoid schizophrenia. Int J Geriatr Psychiatry. (2013) 28:1141–6. doi: 10.1002/gps.3933
120. Hobson HM, Bishop DVM. Mu suppression – a good measure of the human mirror neuron system? Cortex. (2016) 82:290–310. doi: 10.1016/j.cortex.2016.03.019
121. Krivan SJ, Caltabiano N, Cottrell D, Thomas NA. I'll cry instead: Mu suppression responses to tearful facial expressions. Neuropsychologia. (2020) 143:107490. doi: 10.1016/j.neuropsychologia.2020.107490
122. Bekkali S, Youssef GJ, Donaldson PH, Hyde C, Do M, He JL, et al. Is there a relationship between EEG and sTMS neurophysiological markers of the putative human mirror neuron system? J Neurosci Res. (2021) 99:3238–49. doi: 10.1002/jnr.24969
123. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Vol. 5. Washington, DC: American Psychiatric Association (2013). doi: 10.1176/appi.books.9780890425596
124. Gur RE, Kohler CG, Ragland JD, Siegel SJ, Lesko K, Bilker WB, et al. Flat affect in schizophrenia: relation to emotion processing and neurocognitive measures. Schizophr Bull. (2006) 32:279–87. doi: 10.1093/schbul/sbj041
125. Dickinson D, Bellack AS, Gold JM. Social/Communication skills, cognition, and vocational functioning in schizophrenia. Schizophr Bull. (2007) 33:1213–20. doi: 10.1093/schbul/sbl067
126. le Bel RM, Pineda JA, Sharma A. Motor–auditory–visual integration: the role of the human mirror neuron system in communication and communication disorders. J Commun Disord. (2009) 42:299–304. doi: 10.1016/j.jcomdis.2009.03.011
127. Cantisani A, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Walther S. Blood perfusion in left inferior and middle frontal gyrus predicts communication skills in schizophrenia. Psychiatry Res Neuroimaging. (2018) 274:7–10. doi: 10.1016/j.pscychresns.2018.02.002
128. Wong E, Ungvari GS, Leung SK, Tang WK. Rating catatonia in patients with chronic schizophrenia: Rasch analysis of the bush-francis catatonia rating scale. Int J Methods Psychiatr Res. (2007) 16:161–70. doi: 10.1002/mpr.224
129. Pridmore S, Brüne M, Ahmadi J, Dale J. Echopraxia in schizophrenia: possible mechanisms. Austral N Z J Psychiatry. (2008) 42:565–71. doi: 10.1080/00048670802119747
130. Mehta UM, Waghmare A v., Thirthalli J, Venkatasubramanian G, Gangadhar BN. Is the human mirror neuron system plastic? Evidence from a transcranial magnetic stimulation study. Asian J Psychiatr. (2015) 17:71–7. doi: 10.1016/j.ajp.2015.06.014
131. Mitra S, Nizamie SH, Goyal N. Putative mirror neuron activity in patients with schizophrenia remains unchanged after 8 weeks of antipsychotic treatment. Asian J Psychiatr. (2018) 38:70–1. doi: 10.1016/j.ajp.2017.10.016
132. Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. (2010) 167:748–51. doi: 10.1176/appi.ajp.2010.09091379
133. Hadoush H, Nazzal M, Almasri NA, Khalil H, Alafeef M. Therapeutic effects of bilateral anodal transcranial direct current stimulation on prefrontal and motor cortical areas in children with autism spectrum disorders: a pilot study. Autism Res. (2020) 13:828–36. doi: 10.1002/aur.2290
Keywords: mirror neuron activity, schizophrenia, meta-analysis, systematic review, schizophrenia spectrum disorder, mirror neuron system, mirror neurons
Citation: Valizadeh A, Mbwogge M, Rasouli Yazdi A, Hedayati Amlashi N, Haadi A, Shayestefar M and Moassefi M (2022) The mirror mechanism in schizophrenia: A systematic review and qualitative meta-analysis. Front. Psychiatry 13:884828. doi: 10.3389/fpsyt.2022.884828
Received: 27 February 2022; Accepted: 16 August 2022;
Published: 21 September 2022.
Edited by:
Joseph Ventura, UCLA Department of Psychiatry, United StatesReviewed by:
Katharine Thakkar, Michigan State University, United StatesCopyright © 2022 Valizadeh, Mbwogge, Rasouli Yazdi, Hedayati Amlashi, Haadi, Shayestefar and Moassefi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir Valizadeh, dGhpc2lzYW1pcnZAZ21haWwuY29t
†ORCID: Amir Valizadeh orcid.org/0000-0001-5983-8527
Mathew Mbwogge orcid.org/0000-0003-0594-1937
Anita Rasouli Yazdi orcid.org/0000-0002-5963-1419
Nazanin Hedayati Amlashi orcid.org/0000-0003-0804-0018
Ainaaz Haadi orcid.org/0000-0002-6613-1877
Monir Shayestefar orcid.org/0000-0001-7280-3900
Mana Moassefi orcid.org/0000-0002-0111-7791
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.