
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Psychiatry, 11 May 2022
Sec. Addictive Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.884605
This article is part of the Research TopicCommunity Series in Neurobiological Biomarkers for Developing Novel Treatments of Substance and Non-Substance Addiction, volume IIView all 16 articles
Background: In recent years, much research has examined the effects of various interventions and treatments for smoking cessation. The results suggest that interventions targeting changes of nicotine content can help smokers reduce tobacco use or quit smoking. A number of clinical studies show that smokers who received an immediate reduction in nicotine content to very low levels have significantly greater reductions in the number of cigarettes smoked and toxic substance exposure compared to those with gradual reductions. However, from the perspective of smoking craving, whether the immediate and gradual reduction in nicotine content reduce smoking by reducing cravings needs further investigation.
Methods: 74 eligible Participants were randomly allocated to one of the two experimental conditions: (1) immediate reduction to 0.1 mg of nicotine per cigarette (n = 40); (2) gradual reduction from 1.0 (0.8 g ~ 1.2 mg) to 0.1 mg of nicotine per cigarette (n = 34). All participants completed 1-week baseline period during which they smoked their usual cigarette, followed by 16-week of interventions. The primary outcomes included cigarette cravings and number of cigarettes smoked per day (CPD); secondary outcomes included the number of cigarette-free day and emotional states.
Results: Among the 52 participants [51 (98.1%) men; mean (SD) age, 33.44 (6.71) years; mean (SD) CPD, 16.83 (9.94)] who completed the trial, significantly lower cravings for cigarettes were observed in the immediate (n = 25) vs. gradual nicotine reduction group (n = 27) in the morning (t = −2.072, p = 0.039) and after dinner (t = –2.056, p = 0.041). Compared with the baseline daily smoking, the number of cigarettes smoked per day was significantly reduced at the beginning of week 12 in the immediate nicotine reduction group (p = 0.001) and at week 16 in the gradual nicotine reduction group (p < 0.001). The number of participants with any cigarette-free day was not significantly different between the groups (p = 0.198). The number of cigarette-free days was significantly more in the immediate vs. gradual nicotine reduction group (p = 0.027).
Conclusions: The significantly lower cravings were observed in the immediate vs. gradual nicotine reduction group, and led to faster reduction in the number of CPD, and a significant increase in the number of cigarette-free days. These findings add to the evidence base for reduced nicotine content in cigarettes.
Clinical Trial Registration: ClinicalTrials.gov, identifier: ChiCTR2100048216.
Smoking remains one of the leading causes of morbidity and premature death worldwide (1–5). Long-term smoking can affect many systems of the body, resulting in serious and life-threatening diseases, such as ischemic heart disease, chronic obstructive pulmonary disease, tracheal cancer, bronchial cancer, lung cancer, stroke, etc., (5–11). According to data from the Global Burden of Disease (GBD), in 2019, the number of smokers worldwide increased to 1.1 billion, and smoking caused 7.7 million deaths worldwide. In China, the number of smokers reached 341 million (30%), and smoking causes around 2.4 million deaths a year (5). The harmful lifelong consequences of smoking lead to huge public health costs. It is estimated that smoking causes economic losses of over US $500 billion annually worldwide (12).
Nicotine is the main addictive component of cigarettes (2, 13–15). The essence of smoking addiction is nicotine dependence (16) characterized in DSM-IV, by impulsive use, discontinuation difficulty, and withdrawal symptoms after chronic use, and craving which is one of the core symptoms of nicotine addiction (17–22). Craving is common among smokers (23). Long-term use of nicotine can induce changes in the neuroplasticity of the cortex and striatum, thus forming a strong and lasting memory of nicotine addiction, resulting in a continuous craving for cigarettes (24). The existence of craving directly leads to a series of adverse consequences such as smokers' failure to quit smoking or susceptibility to relapse (25, 26). Craving is an important indicator for maintaining addictive behavior and predicting relapse after withdrawal (17, 27–35). Moreover, some studies have found that craving can significantly predict the withdrawal rate after treatment (36, 37), and therefore nicotine craving has become a criterion for estimating the effectiveness of treatment (38). Although the mechanism of craving is not completely clear, it has become an important target for the treatment of smoking addicts (39). The pain point of smoking addiction is mainly manifested in the high relapse rate, and craving is the key factor in precipitating relapse (40, 41). Thus, reducing craving has become the main target in clinical smoking cessation.
Since craving is the main precipitator of relapse, creating new intervention content that targets cravings could greatly enhance the effectiveness of the treatment. In recent years, some researchers have proposed that reducing the content of nicotine in cigarettes is an effective strategy that reduces smoking and improves public health (2, 42–48). A gradual reduction in nicotine content is a potential way to reduce the addiction to cigarettes and promote smokers to quit smoking (42, 44, 49–51). Multiple studies have shown that gradual reduction in nicotine content reduces nicotine intake, without increased exposure to tobacco toxins, and without significant “compensatory” smoking (43, 52–55). The gradual reduction in nicotine content is considered a possible smoking cessation approach (42, 44, 49–51), but it may take a long time to realize the potential health benefits (42, 56). Recent studies have found that reducing nicotine levels faster may be the same even more effective than gradual reduction (45). There is growing evidence showed that immediate reduction in nicotine content reduces the number of cigarettes smoked per day (45, 47, 57, 58), reduces exposure to toxic substances (43, 45, 52, 57–59), reduces nicotine dependence (45, 47, 57, 58), increases smoking cessation attempts (43, 45, 52, 57–59), and “compensatory” smoking is rare compared with the use of traditional nicotine cigarettes (43, 45, 52, 57, 60, 61). A comparison of the two reduction methods showed that the immediate reduction in nicotine content has a significant advantage, as it results in less exposure to toxic substances (60–64), less smoking per day (61), less nicotine dependence (56, 61), and more cigarette-free days (60, 61, 64) over time. The answer to the question of whether the gradual and immediate reduction in nicotine content reduces smoking by reducing craving is still unknown and thus has to be answered. Especially the dynamic changes in cravings shall be assessed, and therefore it is necessary to examine the relationship between daily craving changes and smoking behavior in real-time.
Ecological Momentary Assessment (EMA) is an innovative approach developed for real-time data collection, which greatly improves the field's understanding of the cognition, emotion, and behavior of smokers as they occur in the natural environment (65). The advantages of the EMA approach over retrospective self-reporting include more accurate tracking of smoking frequency and patterns, more detailed capturing of smoking cravings, and high ecological validity of the data (65–67). Since both craving and substance use are situational phenomena related to emotion and environment (68–70), measuring these variables in daily life may lead to more reliable answers. Therefore, in this study, EMA was used to assess daily craving changes and smoking behavior in real-time.
The main goal of this study was to examine the effects of the immediate and gradual reduction in nicotine content in cigarettes on cigarette craving, as well as to observe changes in smoking behavior. The main hypothesis of this research was that significantly lower cravings and lowered number of cigarettes smoked per day will be observed in the immediate vs. gradual nicotine reduction group for craving.
This study has been approved by the Medical Ethics Committee of Shougang Hospital of Peking University and has been registered in the Chinese Clinical Trial Registry. All participants provided informed consent after they were qualified to participate.
To avoid the problem that offering free cigarettes increases smoking or increases the use of cigarettes with regular nicotine content, cigarettes consumed in the study were purchased by participants at designated regular tobacco companies. Study cigarettes, both menthol and non-menthol, are all of the same brand. In a study, researchers examined commercial low yield cigarettes and found that little change was seen in plasma cotinine concentration from 0.9 to the 0.4 mg nicotine yield cigarettes, suggesting compensation in smoking behavior. However, significant decreases plasma cotinine concentration and carcinogen exposure biomarker levels were observed when smokers were switched to 0.1 mg nicotine cigarettes, most likely due to the extensive filter ventilation of these “ultra-low yield” cigarettes, too much to be overcome by compensation. In addition, the 0.1 mg nicotine cigarette also produced non-significantly greater withdrawal (54). Other related studies have shown that 0.1 mg nicotine cigarettes can reduce the amount of smoking and exposure to harmful substances (52, 71, 72). Thus, the cigarette with a nicotine content of 0.1 mg was used in the immediate reduction group. Cigarettes with nicotine content of 0.6 mg (43, 53, 73–77), 0.3 mg (53, 57, 73, 75–77) and 0.1 mg (52, 71, 72) were selected in the gradual reduction group.
Participants were recruited from Daxing District, Beijing, by handing out flyers and advertising on WeChat moments. Inclusion criteria included participants meeting the legal age for buying cigarettes (18 years old); the average daily smoking amount ≥5 cigarettes for at least 1 year; no intention to quit smoking in the past 30 days; and stable mental and psychiatric conditions. Exclusion criteria included participants who intend to quit smoking within the next 30 years; regular use of tobacco products other than cigarettes; current use of nicotine replacement or other tobacco products for cessation; symptoms of severe mental or medical illness during the past 3 months; and being pregnant or breastfeeding. A total of 94 people applied for participation, of which 74 eligible participants were included. One blinded researcher (XJC) who had no direct contact with the participants did a computer-generated randomization to assign participants to one of the two groups.
This study was a randomized parallel experiment (Figure 1). Participants (N = 74) were randomly assigned to 1 of 2 experimental conditions: (1) immediate reduction to 0.1 mg of nicotine per gram of tobacco cigarettes (n = 40); (2) gradual reduction from 1.0 (0.8 ~ 1.2 mg) to 0.1 mg of nicotine per gram of tobacco cigarettes (n = 34).
Cigarette smokers were contacted by the researcher and were screened for eligibility over the telephone. Participants were told that the goal of the study was to examine how changes in nicotine content in cigarettes affect smoking behavior over time. They were also told that if they participate in the research, they would need to buy their own cigarettes during the course of the study. Eligible participants completed a baseline period of 1 week and then were randomly assigned to 1 of 2 experimental conditions for 16 weeks. Participants smoked their usual brand of cigarettes during the baseline period and used the cigarettes they were assigned to purchase during the 16-week experimental phase. In the immediate reduction group, the participants were required to smoke 0.1 mg of nicotine per gram of tobacco cigarettes for 16 weeks. In the gradual reduction group, the participants were asked to reduce the nicotine intake once every 4 weeks (0.6 mg of nicotine per gram of tobacco cigarettes were used from week 1 to week 4; 0.3 mg of nicotine per gram of tobacco cigarettes were used from week 4 to week 8; and 0.1 mg of nicotine per gram of tobacco cigarettes were used from week 8 to week 16). During the experiment, participants were told to use the designated brands of cigarettes (Study cigarettes) and try not to use their usual brand of cigarettes (Non-study cigarettes). If both types of cigarettes are used, record them and inform the researchers. Participants were required to buy the designated cigarettes during the experiment to avoid the problem that providing free cigarettes would increase smoking or use of more cigarettes with regular nicotine content. At the end of the experiment, participants were paid according to their compliance with participating in the experiment, everyone was paid for ¥200—¥400.
During the baseline and experimental periods, this study used a score table to record the emotional state and craving degree of the participants before and after smoking in the morning and evening. The scoring method was 1–9 points to record emotional state (78), when the degree of craving was assessed, 1 indicated not wanting to smoke at all, and 9 indicated being very eager to smoke (Table 1). At the same time, the number of cigarettes smoked per day (study cigarettes and non-study cigarettes) was recorded on the score table.
All participants were asked to put a score table in the cigarette packs (Table 1). In the morning when they have their first cigarette, they were asked to timely estimate their emotional state and craving values before and after smoking; in the evening, when smoking their last cigarette, participants were requested to timely estimate their emotional state and craving values before and after smoking and write down the number of cigarettes they smoked that day. The following three ways were used to provide feedback on the daily smoking to the research staff. The first one was to directly send the information on the score table to the staff through WeChat or SMS on the same day; the second one required the information on the score table to be filled into the questionnaire and submitted to the staff before going to bed every night. The third one required filling in the information on their own score table in an Excel file every day and sending it to the staff regularly.
The following measures were taken at the baseline and after the intervention: Fagerström Test for Nicotine Dependence (FTND) (57), WHO Quality of Life-BREF (WHOQOL-BREF) (79); Self Rating Anxiety Scale (SAS) (80); Self-rating Depression Scale (SDS) (81). Profile of Mood States (POMS) (82). Demographic data and smoking history were collected at baseline.
FTND: A total of 6 items, and the score of the scale ranges from 0 to 10, with higher values indicating greater dependence. The degree of nicotine dependence can be divided into five levels: very low dependence (0–2), low dependence (3–4), medium dependence (5), high dependence (6–7), and very high dependence (8–10).
WHOQOL-BREF: An international scale developed by the World Health Organization to measure an individual's health-related quality of life. 5-point scale was used to measure the quality of life from four aspects: physical health, psychological, social relationships and environment. The higher the score in each area, the better the quality of life, and the most likely area scores are 35 (physical health), 30 (psychological), 15 (social relationships) and 40 (environment).
SAS: It is used to evaluate the anxiety symptoms of adults. Compiled by W.K.Zung in 1971, there were 20 items and four grades.
SDS: It is used to evaluate the depressive symptoms of adults. Compiled by WilliamW.K.Zung in 1965, with 20 items and four grades. The severity of depression was measured from 4 aspects: psycho-emotional symptoms (2 items), somatic disorders (8 items), depressive psychological disorders (8 items) and psychomotor disorders (2 items).
POMS: The Chinese Profile of Mood States (POMS) revised by Zhu Beili (83) contains 40 items with a grade of 5. It contains seven dimensions: tension, anger, fatigue, depression, energy, panic and self-related emotions. The reliability is between 0.60 and 0.82.
SPSS 21.0 data analysis software was used for statistical analysis. The primary end points of this study need to be evaluated continuously every day, and the intervention time is as long as 16 weeks. Most of the dropouts dropped out of the experiment in the first few days of the intervention period, and the real-time data of the primary end points were greatly missing, and there was no late follow-up data. Thus, the analysis method used in this study is per-protocol (PP) analysis, that is, participants with good compliance and completion of the study were analyzed. Chi-square test and independent-sample T-test were used to analyze the differences demographic characteristics of the two groups of participants. A Chi-square test was used to compare the completion rates at week 16. Changes in cigarette craving and emotion were analyzed using a generalized linear mixed model, and the number of cigarettes was analyzed using repeated-measures analysis of variance. A Chi-square test and negative binomial regression analysis were used to analyze the number of participants with any cigarette-free day, the number of cigarette-free days among all participants. Subjective reports were analyzed by an independent sample T-test.
A total of 74 participants (40 in the immediate reduction group and 34 in the gradual reduction group) were eligible, and 52 participants completed the experiment (25 in the immediate reduction group and 27 in the gradual reduction group). The completion rate of the immediate reduction group was 62.5%, and the completion rate of the gradual reduction group was 79.4%. The dropout rate of the immediate group is 37.5% (n = 15), and that of the gradual group is 20.6% (n = 7). In the later stage of follow-up, the dropout of participants in the immediate group was mainly due to the poor adaptability of some participants to very low nicotine cigarettes, and adverse events such as dizziness and nausea occurred in the first few days of using very low nicotine cigarettes, resulting in negative emotions of participants, which resulted in participants quit the intervention. The gradual group being resistant to changing cigarettes during the nicotine content change from 0.6 mg to 0.3 mg nicotine cigarettes, resulting in more dropout.
Table 2 shows the demographics and smoking history of the two groups. There is no significant difference between the two groups in demographics and smoking history, indicating that the participants in the two groups were similar (Table 2).
The changes of cigarette craving after getting up in the morning and after dinner (before smoking-after smoking) were analyzed by generalized linear mixed model. The results showed that the smoking cravings after getting up in the morning were significantly lower in the immediate vs. gradual nicotine reduction group (12 week, t = –2.091, p = 0.038; 16 week, t = –2.072, p = 0.039) after 12 weeks of intervention; and the smoking cravings after dinner were significantly lower in the immediate vs. gradual nicotine reduction group (16 week, t = –2.056, p = 0.041) after 16 weeks of intervention. Smoking cravings were significantly lower in the immediate reduction group at 4 week (t = 5.789, p < 0.001), 8 week (t = 6.386, p < 0.001), 12 week (t = 5.227, p < 0.001), and 16 week (t = 4.861, p < 0.001) of intervention vs. baseline smoking cravings after getting up in the morning. Smoking cravings were not significantly different in the gradual reduction group at 4 week (t = 1.593, p = 0.112) of intervention vs. baseline smoking cravings after getting up in the morning, but were significantly reduced at 8 week (t = 3.440, p = 0.001), 12 week (t = 2.442, p = 0.015), and 16 week (t = 2.464, p = 0.014) of intervention. Smoking cravings were significantly lower in the immediate reduction group at 8 week (t = 2.385, p = 0.018) of intervention vs. baseline smoking cravings after dinner, but no significant difference were observed at other time periods (4 week, t = 1.837, p = 0.068; 12 week, t = 1.559, p = 0.120; 16 week, t = 1.865, p = 0.063). Smoking cravings were significantly lower in the gradual reduction group at 8 week (t = 2.183, p = 0.030) of intervention vs. baseline smoking cravings after dinner, but no significant difference were observed at other time periods (4 week, t = 1.545, p = 0.124; 12 week, t = 1.435, p = 0.153; 16 week, t = 1.358, p = 0.0176) (Figures 2A,B).
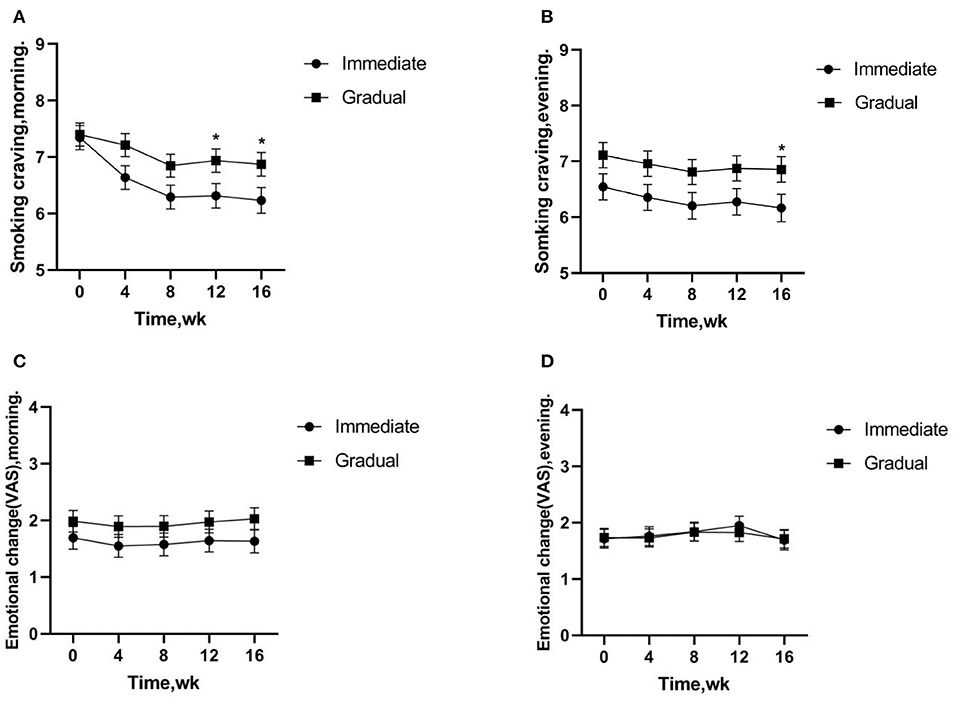
Figure 2. (A,B) Smoking cravings indicate changes in cravings before-after smoking after getting up in the morning and after dinner, respectively. (A,B) Significantly lower cravings were observed in the immediate vs. gradual nicotine reduction group. (C,D) The emotional changes of smoking indicate changes in cravings before-after smoking after getting up in the morning and after dinner, (C,D) there was no significant difference between the immediate reduction group and the gradual reduction group. *p < 0.05.
The emotional changes of smoking after getting up in the morning (before-after smoking) were analyzed. The results showed that there was no significant difference between the immediate reduction group and the gradual reduction group (t = –1.285, p = 0.200). The emotional changes of smoking after dinner (before-after smoking) were analyzed. The results showed a similar effect pattern (t = 0.121, p = 0.904) (Figures 2C,D).
Two-factor repeated measurement ANOVA was used to compare the reduction effect of different intervention groups after 16 weeks of intervention. Significantly fewer numbers of total CPD were smoked in the immediate reduction group at weeks 12 (p = 0.001) and 16 (p < 0.001) vs. baseline smoking. Significantly increased numbers of total CPD were smoked in the gradual reduction group at weeks 4 (p = 0.006) and 8 (p = 0.025) vs. baseline smoking, but significantly fewer at weeks 16 (p < 0.001). The same effect pattern was observed in the study of cigarettes for several weeks.The number of cigarettes significantly fewer in both the immediate (p < 0.001) and gradual reduction group (p < 0.001) at the weeks 16 (Figure 3).
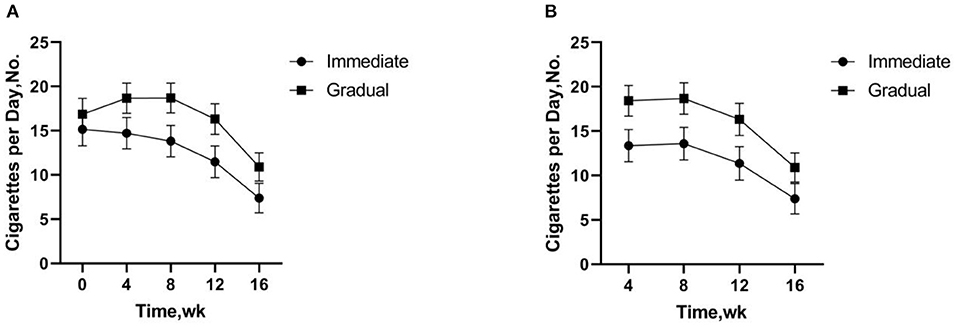
Figure 3. (A) Total cigarettes per day includes study cigarettes and non-study cigarettes. (B) Study Cigarettes are low nicotine content cigarettes used in the experiment. (A,B) There was no significant difference in the number of cigarettes per day between the immediate and gradual reduction group, but the number of cigarettes per day fewer more quickly in the immediate reduction group compared to baseline.
A Chi-square test was used to analyze the number of participants with any cigarette-free day. The number of participants with any cigarette-free day was not significantly different between the immediate vs. gradual reduction group (p = 0.198). Negative binomial regression analysis was used to analyze the number of cigarette-free days among all participants. The number of cigarette-free days among all participants was significantly higher in the immediate vs. gradual reduction group (p = 0.027) (Table 3).
At week 16, significantly lower FTND scores were observed in the immediate vs. gradual reduction group (4.629, p = 0.000). There were no significant differences between the immediate vs. the gradual nicotine reduction group in four areas of WHOQOL-BREF scores: physical health (t = 1.324, p = 0.191), psychological (t = –0.723, p = 0.473), social relationships (t = 0.093, p = 0.926), and environment (t = –0.966, p = 0.339) at week 16. Total scores of emotional disturbances as assessed by the POMS were not significantly different for the immediate vs. gradual reduction group at week 16 (t = 0.817, p = 0.418). Anxiety symptoms scores as assessed by the SAS (t = –1.622, p = 0.111) and depression symptoms scores as assessed by the SDS (t = –0.687, p = 0.495) were not significantly different for the immediate vs. gradual reduction group at week 16 (Table 4).
The present study aimed to examine (1) smoking behavior, cravings and emotional change among smokers in their daily life using Ecological Momentary Assessment (EMA) (84–86); (2) compare the effectiveness of immediate vs. gradual reduction intervention; and (3) explore whether two different nicotine reduction methods affect smoking behavior by reducing craving from a mechanism point of view.
In this study, the immediate nicotine reduction compared with gradual nicotine reduction was associated with a faster decrease in cigarette cravings, lowered cigarette cravings, faster reduction in the CPD, and more cigarette-free days over time. However, the immediate nicotine reduction caused a higher dropout rate. The use of low nicotine cigarettes had no effect on the quality of life and emotional state of the participants.
When these 2 methods were compared in this study, the results demonstrated that with immediate nicotine reduction, the smoking craving reduction could be realized sooner than gradual nicotine reduction. Therefore, the immediate reduction method is possible to facilitate cessation of cigarettes as quickly as possible. Immediate reduction method is more effective than gradual reduction method, because nicotine immediate reduction method is more conducive to promote smokers to quit smoking, faster to achieve potential public health effects. In a large clinical trial involving 1,250 smokers from 10 academic institutions, immediate reduction in nicotine may achieve positive public health effects more quickly (61).
The results of the comparison of both approaches have shown that cigarette cravings were reduced faster and significantly in the immediate reduction group. This is consistent with other research (61, 87). However, previous studies used the smoking craving scale to assess cravings periodically during the intervention, rather than continuously assessing the dynamic changes of cravings during the intervention (61). Some studies suggested that craving is an instantaneous state that changes constantly, so it may be inaccurate to assess craving over a long period of time (88). In addition, some researchers suggest that craving is a measurable continuous state (89). Therefore, in this research, we utilized EMA, to continuously assess the dynamic changes of participants' cravings over the course of intervention. The results demonstrated that cravings were significantly lower and decreased faster in the immediate nicotine reduction group.
There was no significant difference in the number of CPD between the immediate and the gradual reduction group in the study. The results of this study are not consistent with those of previous studies (61, 64). Previous studies have shown that significantly fewer numbers of CPD in the immediate than the gradual reduction group (61, 64). One possible explanation is that the duration of this study was only 16 weeks, so there were no significant differences in smoking reduction between both interventions. Another possible explanation is that the sample size of this study was too small. Compared with the baseline, both the immediate and the gradual reduction groups were able to significantly reduce the number of CPD after 16 weeks of intervention, with a faster reduction in the number of CPD in the immediate reduction group. The results have shown that the number of CPD in the immediate reduction group was reduced during the intervention and significantly reduced at week 12, while the number of CPD in the gradual reduction group increased at weeks 4 and 8 and significantly reduced at week 16. There was a temporary increase in smoking in the gradual reduction group, possibly due to compensatory smoking in moderate nicotine cigarettes (45, 52, 53, 61).
In the comparison between the immediate vs. gradual reduction group, the results demonstrated significantly more cigarette-free days among all participants in the immediate reduction group. The results are consistent with those of Hatsukami et al. (61). Both intervention methods had no effect on the quality of life and emotional state of the participants, indicating that switching to low nicotine cigarettes may be more acceptable by participants who participated in the entire intervention.
There was a higher drop-out in the immediate group. However, the withdrawal rate was no different between the immediate vs. the gradual reduction group. Other studies have shown that immediate nicotine reduction is less satisfying (61, 87), leading to more severe withdrawal symptoms (56, 61) and a higher subjects' attrition rate (61, 87) than gradual nicotine reduction. The reason why there was no difference in compliance between the immediate and the gradual reduction group may be due to the gradual nicotine reduction group being resistant to changing cigarettes during the nicotine content change from 0.6 mg to 0.3 mg nicotine cigarettes, resulting in more subjects dropout. In this study, participants were free to choose blended / flue-cured 0.6 mg nicotine cigarettes. However, when switched to 0.3 mg nicotine cigarettes, only the blend cigarettes were available. 75.0% of the participants in this study were flue-cured cigarette users, and discomfort caused by different types of cigarettes made it easier for participants to drop out during this process.
This study has several limitations. Firstly, the duration of this study was only 16 weeks, and the long-term effects of the two nicotine reduction methods were uncertain (61). Secondly, there was no follow-up at the end of the study. Third, the relatively small number of participants could limit the universality of the findings (43, 52). Furthermore, the average level of education of the participants was higher, and the universality of the findings is limited. Likewise, the selectivity of cigarette types was limited, which may affect the measurement of various outcomes. Moreover, the monitoring of adverse events (any negative changes in physical or mental health) and the measurement of withdrawal reaction were not carried out during the study. Seventh, in this study, most of the participants were male and there was only one female who completed the study, the study didn't compare the effectiveness of the two methods in male and in female, future studies could further compare the effectiveness of the two methods in male and in female. In addition, in this study, there is a lack of objective indicators to measure the intervention effect, and it may not be very comprehensive and objective to evaluate the intervention effect only from the self-report. in the future study, the intervention effect can be measured from multiple perspectives, and the mode of combining physiology with self-report can be adopted. Biomarkers such as cotinine can be used for physiological indicators. As well, in terms of efficacy analysis, per-protocol (PP) analysis may overestimate the efficacy. The interpretation of the results of this study is more applicable to participants who are more compliant with low nicotine cigarettes.
Among smokers, the immediate nicotine reduction group led to a faster and significant decrease in cigarette cravings, a faster reduction in the number of CPD, and a significant increase in the number of cigarette-free days among all participants in the gradual reduction group.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Shougang Hospital of Peking University. The patients/participants provided their written informed consent to participate in this study.
QL and YL contributed in conceptualization and methodology. YL supervised the study design and implementation. QL designed the experiment, analyzed data, wrote the paper, and revised the article. ZL designed experiment and collected data. XC provided methodological and substantive support throughout the manuscript process. MG, XC, and XL revised the article. All authors contributed to the article and approved the submitted version.
Funding for this study was provided by Scientific and Technological Innovation 2030-Brain Science and Brain-Inspired Technology Project (2021ZD0202100).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the participants and researchers.
1. Mokdad A, Marks J, Stroup D, Gerberding J. Actual causes of death in the United States, 2000. JAMA. (2004) 291:1238–45. doi: 10.1001/jama.291.10.1238
2. Botvin GJ. The Health Consequences of Smoking-−50 Years of Progress: A Report of the Surgeon General. Bethesda, MD: USnational Library of Medicine (2014).
3. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. (2004) 43:1731–7. doi: 10.1016/j.jacc.2003.12.047
4. Carter B, Abnet C, Feskanich D, Freedman N, Hartge P, Lewis C, et al. Smoking and mortality–beyond established causes. N Engl J Med. (2015) 372:631–40. doi: 10.1056/NEJMsa1407211
5. Reitsma MB, Kendrick PJ, Ababneh E, Abbafati C, Abbasi-Kangevari M, Abdoli A, et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the global burden of disease study 2019. Lancet. (2021) 397:2337–60. doi: 10.1016/S0140-6736(21)01169-7
6. Imamura M, Waseda Y, Marinova G, Ishibashi T, Obayashi S, Sasaki A, et al. Alterations of NOS, arginase, and DDAH protein expression in rabbit cavernous tissue after administration of cigarette smoke extract. Am J Physiol Regul Integr. (2007) 293:R2081–9. doi: 10.1152/ajpregu.00406.2007
7. Ayo-Yusuf OA, Olutola BG. Epidemiological association between osteoporosis and combined smoking and use of snuff among South African women. Niger J Clin Pract. (2014) 17:174–7. doi: 10.4103/1119-3077.127542
8. Mahapatra S, Kamath R, Shetty BK, Binu VS. Risk of oral cancer associated with gutka and other tobacco products: a hospital-based case-control study. J Cancer Res Ther. (2015) 11:199–203. doi: 10.4103/0973-1482.143332
9. (CDC) CfDCaP. Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000-2004. MMWR Morb Mortal Wkly Rep. (2008) 57:1226–8. Available online at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a3.htm
10. Koks G, Fischer K, Koks S. Smoking-related general and cause-specific mortality in Estonia. BMC Public Health. (2017) 18:34. doi: 10.1186/s12889-017-4590-3
11. Li J, Yang F, Li X, Zhang M, Fu R, Yin X, et al. Characteristics, survival, and risk factors of Chinese young lung cancer patients: the experience from two institutions. Oncotarget. (2017) 8:89236–44. doi: 10.18632/oncotarget.19183
12. Varghese C, Onuma O, Johnson W, Brainin M, Hacke W, Norrving B. Organizational update: World Health Organization. Stroke. (2017) 48:e341. doi: 10.1161/STROKEAHA.117.016941
13. Smith TT, Rupprecht LE, Denlinger-Apte RL, Weeks JJ, Panas RS, Donny EC, et al. Animal research on nicotine reduction: current evidence and research gaps. Nicotine Tob Res. (2017) 19:1005–15. doi: 10.1093/ntr/ntx077
14. Fowler CD, Gipson CD, Kleykamp BA, Rupprecht LE, Harrell PT, Rees VW, et al. Basic science and public policy: informed regulation for nicotine and tobacco products. Nicotine Tob Res. (2018) 20:789–99. doi: 10.1093/ntr/ntx175
15. Orellana JA, Busso D, Ramirez G, Campos M, Rigotti A, Eugenin J, et al. Prenatal nicotine exposure enhances Cx43 and Panx1 unopposed channel activity in brain cells of adult offspring mice fed a high-fat/cholesterol diet. Front Cell Neurosci. (2014) 8:403. doi: 10.3389/fncel.2014.00403
16. Wang L, Liu Z, Gambardella L, Delacour A, Shapiro R, Yang J, et al. Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol. (2000) 114:901–8. doi: 10.1046/j.1523-1747.2000.00951.x
17. Killen JD. Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol. (1997) 5:137–42. doi: 10.1037/1064-1297.5.2.137
18. Poling A, Methot LL, Lesage MG. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). American Psychiatric Association (2000).
19. Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. J Brain Res Brain Res Rev. (1993) 18:247–91. doi: 10.1016/0165-0173(93)90013-P
20. Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FA, Nitsche MA, et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. (2008) 51:34–41. doi: 10.1016/j.appet.2007.09.016
21. Kassel JD, Shiffman S. What can hunger teach us about drug craving? A comparative analysis of the two constructs. Adv Behav Res Ther. (1992) 14:141–67. doi: 10.1016/0146-6402(92)90006-A
23. Tiffany ST, Warthen MW, Goedeker KC. The functional significance of craving in nicotine dependence. Nebr Symp Motiv. (2009) 55:171–97. doi: 10.1007/978-0-387-78748-0_10
24. Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. (1994) 22:1–18. doi: 10.3758/BF03199951
25. Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. (2007) 32:2301–9. doi: 10.1038/sj.npp.1301371
26. Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. (2004) 7:211–4. doi: 10.1038/nn1200
27. Al“Absi M, Carr SB, Bongard S. Anger and psychobiological changes during smoking abstinence and in response to acute stress: Prediction of smoking relapse. Int J Psychophysiol. (2007) 66:109–15. doi: 10.1016/j.ijpsycho.2007.03.016
28. Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad. (2012) 1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x
29. Van Zundert RM, Ferguson SG, Shiffman S, Engels R. Dynamic effects of craving and negative affect on adolescent smoking relapse. Health Psychol. (2012) 31:226–34. doi: 10.1037/a0025204
30. West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. (2008) 197:371–7. doi: 10.1007/s00213-007-1041-3
31. Nakajima M. al'Absi M. Predictors of risk for smoking relapse in men and women: a prospective examination. Psychol Addict Behav. (2012) 26:633–7. doi: 10.1037/a0027280
32. Roche DJ, Bujarski S, Moallem NR, Guzman I, Shapiro JR, Ray LA. Predictors of smoking lapse in a human laboratory paradigm. Psychopharmacology. (2014) 231:2889–97. doi: 10.1007/s00213-014-3465-x
33. al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. (2004) 73:267–78. doi: 10.1016/j.drugalcdep.2003.10.014
34. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. (2009) 36:235–43. doi: 10.1016/j.jsat.2008.06.005
35. Rohsenow DJ, Martin RA, Eaton C, Monti PM. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J Stud Alcohol Drugs. (2007) 68:641–48. doi: 10.15288/jsad.2007.68.641
36. Hoving EF, Mudde AN, Vries HD. Predictors of smoking relapse in a sample of Dutch adult smokers; the roles of gender and action plans. Addict Behav. (2006) 31:1177–89. doi: 10.1016/j.addbeh.2005.09.002
37. Abdullah AS, Lam TH, Chan SS, Hedley AJ. Smoking cessation among Chinese young smokers: does gender and age difference matters and what are the predictors? Addict Behav. (2006) 31:913–21. doi: 10.1016/j.addbeh.2005.08.009
38. Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine Tob Res. (2004) 6:327–48. doi: 10.1080/1462220042000202481
39. BROUSSE, Chazeron DE. Le craving : des clés pour comprendre. Alcool Addictol. (2014) 36:105–15. Available online at: http://www.eurotox.org/images/stories/docs/RapportFWB/eurotox_2013-2014
40. Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. (2014) 38:1–16. doi: 10.1016/j.neubiorev.2013.10.013
41. Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol. (2004) 55:463–91. doi: 10.1146/annurev.psych.55.090902.142054
42. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. (1994) 331:123–25. doi: 10.1056/NEJM199407143310212
43. Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P 3rd. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. (2007) 16:2479–85. doi: 10.1158/1055-9965.EPI-07-0393
44. Hatsukami DK, Perkins KA, Lesage MG, Ashley DL, Henningfield JE, Benowitz NL, et al. Nicotine reduction revisited: science and future directions. Tob Control. (2010) 19:e1–10. doi: 10.1136/tc.2009.035584
45. Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. (2010) 105:343–55. doi: 10.1111/j.1360-0443.2009.02780.x
46. Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. (2013) 22(Suppl. 1):i14–7. doi: 10.1136/tobaccocontrol-2012-050860
47. Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. (2007) 102:324–34. doi: 10.1111/j.1360-0443.2006.01670.x
48. Zeller M, Hatsukami D. Strategic Dialogue on Tobacco Harm Reduction G. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. Tob Control. (2009) 18:324–32. doi: 10.1136/tc.2008.027318
49. Henningfield J, Benowitz N, Slade J, Houston T, Davis R, Deitchman S. Reducing the addictiveness of cigarettes. Council on Scientific Affairs, American Medical Association. Tob Control. (1998) 7:281–93. doi: 10.1136/tc.7.3.281
50. Walker N, Bullen C, McRobbie H. Reduced-nicotine content cigarettes: Is there potential to aid smoking cessation? Nicotine Tob Res. (2009) 11:1274–9. doi: 10.1093/ntr/ntp147
51. Tengs TO, Ahmad S, Savage JM, Moore R, Gage E. The AMA proposal to mandate nicotine reduction in cigarettes: a simulation of the population health impacts. Prev Med. (2005) 40:170–80. doi: 10.1016/j.ypmed.2004.05.017
52. Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. (2012) 21:761–9. doi: 10.1158/1055-9965.EPI-11-0644
53. Mercincavage M, Souprountchouk V, Tang KZ, Dumont RL, Wileyto EP, Carmella SG, et al. A randomized controlled trial of progressively reduced nicotine content cigarettes on smoking behaviors, biomarkers of exposure, and subjective ratings. Cancer Epidemiol Biomarkers Prev. (2016) 25:1125–33. doi: 10.1158/1055-9965.EPI-15-1088
54. Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Progressive commercial cigarette yield reduction: biochemical exposure and behavioral assessment. Cancer Epidemiol Biomarkers Prev. (2009) 18:876–83. doi: 10.1158/1055-9965.EPI-08-0731
55. Benowitz NL, Nardone N, Dains KM, Hall SM, Stewart S, Dempsey D, et al. Effect of reducing the nicotine content of cigarettes on cigarette smoking behavior and tobacco smoke toxicant exposure: 2-year follow up. Addiction. (2015) 110:1667–75. doi: 10.1111/add.12978
56. Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev. (2015) 24:472–6. doi: 10.1158/1055-9965.EPI-14-0739
57. Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. (2015) 373:1340–9. doi: 10.1056/NEJMsa1502403
58. Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, Hecht SS, et al. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. (2013) 22:1015–24. doi: 10.1158/1055-9965.EPI-12-1439
59. Hatsukami DK, Luo X, Dick L, Kangkum M, Allen SS, Murphy SE, et al. Reduced nicotine content cigarettes and use of alternative nicotine products: exploratory trial. Addiction. (2017) 112:156–67. doi: 10.1111/add.13603
60. Smith TT, Hatsukami DK, Benowitz NL, Colby SM, McClernon FJ, Strasser AA, et al. Whether to push or pull? Nicotine reduction and non-combusted alternatives - two strategies for reducing smoking and improving public health. Prev Med. (2018) 117:8–14. doi: 10.1016/j.ypmed.2018.03.021
61. Hatsukami DK, Luo X, Jensen JA., al'Absi M, Allen SS, Carmella SG, et al. Effect of immediate vs. gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA. (2018) 320:880–91. doi: 10.1001/jama.2018.11473
62. Hatsukami DK, Luo X, Heskin AK, Tang MK, Carmella SG, Jensen J, et al. Effects of immediate versus gradual nicotine reduction in cigarettes on biomarkers of biological effects. Addiction. (2019) 114:1824–33. doi: 10.1111/add.14695
63. Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res. (2013) 15:1918–25. doi: 10.1093/ntr/ntt082
64. Dmc A, Brl B, Ssd C, Da D, Ae B, Rnc E, et al. Impact of nicotine reduction in cigarettes on smoking behavior and exposure: Are there differences by race/ethnicity, educational attainment, or gender? Drug Alcohol Depend. (2021) 225:108756. doi: 10.1016/j.drugalcdep.2021.108756
65. Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. (2008) 4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415
66. Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, Time-Line Follow-Back, and ecological momentary assessment. Health Psychol. (2009) 28:519–26. doi: 10.1037/a0015197
67. Griffith SD, Shiffman S, Heitjan DF. A method comparison study of timeline followback and ecological momentary assessment of daily cigarette consumption. Nicotine Tob Res. (2009) 11:1368–73. doi: 10.1093/ntr/ntp150
68. Monk RL, Qureshi AW, Mcneill A, Erskine-Shaw M, Heim D. Perfect for a gin and tonic: how context drives consumption within a modified bogus taste test. Alcohol Alcohol. (2017) 53:1–7. doi: 10.1093/alcalc/agx084
69. Wall A-M, McKee SA, Hinson RE. Assessing variation in alcohol outcome expectancies across environmental context: an examination of the situational-specificity hypothesis. Psychol Addict Behav. (2000) 14:367–75. doi: 10.1037/0893-164X.14.4.367
70. Dvorak RD, Waters AJ, MacIntyre JM, Gwaltney CJ. Affect, craving, and cognition: An EMA study of ad libitum adolescent smoking. Psychol Addict Behav. (2018) 32:583–94. doi: 10.1037/adb0000392
71. Gori GB, Lynch CJ. Smoker intake from cigarettes in the 1-mg Federal Trade Commission tar class. Regul Toxicol Pharmacol. (1983) 3:110–20. doi: 10.1016/0273-2300(83)90035-1
72. Benowitz NL, Jacob PI, Yu L, Talcott R, Jones RT. Reduced Tar, Nicotine, and carbon monoxide exposure while smoking ultralow- but not low-yield cigarettes. JAMA. (1986) 256:241–46. doi: 10.1001/jama.256.2.241
73. Macqueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Evans DE, Drobes DJ. Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology. (2012) 223:47–54. doi: 10.1007/s00213-012-2685-1
74. Donny EC, Hatsukami DK, Benowitz NL, Sved AF, Tidey JW, Cassidy RN. Reduced nicotine product standards for combustible tobacco: building an empirical basis for effective regulation. Prev Med. (2014) 68:17–22. doi: 10.1016/j.ypmed.2014.06.020
75. Hammond D, O'Connor RJ. Reduced nicotine cigarettes: smoking behavior and biomarkers of exposure among smokers not intending to quit. Cancer Epidemiol Biomarkers Prev. (2014) 23:2032–40. doi: 10.1158/1055-9965.EPI-13-0957
76. Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced-nicotine cigarettes for smoking cessation. Nicotine Tob Res. (2008) 10:1139–48. doi: 10.1080/14622200802123294
77. Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, et al. The combined effect of very low nicotine content cigarettes, used as an adjunct to usual Quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: a randomized controlled trial. Addiction. (2012) 107:1857–67. doi: 10.1111/j.1360-0443.2012.03906.x
78. Johnson BA, Chen YR, Schmitz J, Bordnick P, Shafer A. Cue reactivity in cocaine-dependent subjects: effects of cue type and cue modality. Addict Behav. (1998) 23:7–15. doi: 10.1016/S0306-4603(97)00014-2
79. Health WHODoM. WHOQOL-BREF: Introduction, Administration, Scoring and Generic Version of the Assessment : Field Trial Version, December 1996. Geneva: World Health Organization (1996).
80. Zung W. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12:371–79. doi: 10.1016/S0033-3182(71)71479-0
81. ZUNG, William WK. A Self-Rating Depression Scale. Arch Gen Psychiatry. (1965) 12:63. doi: 10.1001/archpsyc.1965.01720310065008
82. Grove JR, Prapavessis H. Preliminary evidence for the reliability and validity of an abbreviated profile of mood states. Int J Sport Psychol. (1992) 23:93–109.
83. Zhu BL. Brief introduction of POMS scale and its model for China. J Teach Phys Educ. (1995) 10:36–7. doi: 10.13297/j.cnki.issn1005-0000.1995.01.007
84. Shiffman S. Conceptualizing analyses of ecological momentary assessment data. Nicotine Tob Res. (2014) 16 Suppl 2:S76–87. doi: 10.1093/ntr/ntt195
85. Morgenstern J, Kuerbis A, Muench F. Ecological momentary assessment and alcohol use disorder treatment. Alcohol Res Current Rev. (2014) 36:101–10.
86. Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. (2009) 66:88–94. doi: 10.1001/archgenpsychiatry.2008.509
87. Smith TT, Donny EC, Luo X, Allen AM, Carroll DM, Denlinger-Apte RL, et al. The impact of gradual and immediate nicotine reduction on subjective cigarette ratings. Nicotine Tob Res. (2019) 21:S73–80. doi: 10.1093/ntr/ntz158
88. Davies GM, Willner P, Morgan MJ. Smoking-related cues elicit craving in tobacco “chippers”: a replication and validation of the two-factor structure of the Questionnaire of Smoking Urges. Psychopharmacology. (2000) 152:334–42. doi: 10.1007/s002130000526
Keywords: immediate nicotine reduction, gradual nicotine reduction, craving, smoking behavior, ecological momentary assessment
Citation: Li Q, Chen X, Li X, Gorowska M, Li Z and Li Y (2022) The Effects of Immediate vs Gradual Reduction in Nicotine Content of Cigarettes on Smoking Behavior: An Ecological Momentary Assessment Study. Front. Psychiatry 13:884605. doi: 10.3389/fpsyt.2022.884605
Received: 26 February 2022; Accepted: 20 April 2022;
Published: 11 May 2022.
Edited by:
Yanhui Liao, Zhejiang University School of Medicine, ChinaCopyright © 2022 Li, Chen, Li, Gorowska, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghui Li, bGl5b25naHVpQHBzeWNoLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.