
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry, 24 June 2022
Sec. Addictive Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.883517
This article is part of the Research TopicCommunity Series - Purple Haze: Issues on Cannabis Legalization, volume IIView all 11 articles
 Caroline A. MacCallum1*
Caroline A. MacCallum1* Lindsay A. Lo2
Lindsay A. Lo2 Carly A. Pistawka3
Carly A. Pistawka3 April Christiansen4
April Christiansen4 Michael Boivin5
Michael Boivin5 Melissa Snider-Adler6
Melissa Snider-Adler6Clinicians play an important role in promoting safe and responsible medical cannabis use. One essential component to safe use is considering a patient's risk of neurocognitive impairment. However, there remains a lack of practical guidance on how clinicians can evaluate this risk for medical cannabis patients. Here, a practical framework is presented for clinicians to assess and stratify cannabis-associated impairment risk. The proposed framework is intended to practically guide healthcare providers in gaining a more comprehensive review of a patient's impairment-related factors. This framework can be used to assess impairment risk for patients currently using or considering medical cannabis and is recommended for all patients who perform safety-sensitive duties. Healthcare providers (HCP) managing patient's medical cannabis or those conducting assessments to determine risk of impairment for safety-sensitive workplaces can utilize this framework to stratify patients' risk of impairment. Such assessments can inform patient-specific needs for support, education, and guidance, to ensure cannabis is used safely and responsibly.
As medical cannabis use increases worldwide, concerns have arisen over the potential for cannabis impairment during safety-sensitive work or activities (1). Currently, medical cannabis is most strongly indicated for chronic pain, spasticity associated with multiple sclerosis, chemotherapy-induced nausea and vomiting, and treatment of intractable seizures in Dravet and Lennox-Gastaut syndromes (2). Although evidence is less clear, medical cannabis is also commonly used to treat symptoms associated with neuropathic pain, fibromyalgia, arthritis, sleep disorders, anxiety, and depression (3–6). There are several routes of administration for cannabis, the most common for medical use are inhalation (e.g., smoking or vaporizing) and oral ingestion (e.g., oils or capsules) (7–9). Each route of administration has unique pharmacokinetic and pharmacodynamic properties, leading to different times of onset and duration of action (10, 11). Dosing and administration of medical cannabis is complicated by not only having multiple methods of administration, but also a wide variety of product types and chemovars. Cannabis products vary in their composition of the two primary cannabinoids, tetrahydrocannabinol (THC) and cannabidiol (CBD). Typically, cannabis treatment protocols are tailored to the individual patient, with the exact dose and administration protocol being dictated by patient-specific needs and goals of treatment (8). All of these factors influence the potential of cannabis-related impairment.
Cannabis has the potential to impair multiple domains of neurocognitive function (12, 13). Evidence to date supports that THC is the primary psychoactive component in cannabis responsible for causing impairment (14). THC is a partial agonist for Cannabinoid receptor type 1 (CB1) and binds to CB1 receptors in regions of the brain involved with cognition, memory, anxiety, sensory perception, and motor coordination (15). This pharmacological action is what causes the dose-dependent disruption of cognitive and psychomotor domains important for safety-sensitive work or activities, such as driving motor vehicles (16, 17). In contrast, CBD, the other primary cannabinoid in cannabis, is generally considered non-impairing at low and moderate doses (See Figure 1) (18). Current evidence suggests CBD may cause sedation in some individuals at higher doses (19, 20). However, evidence is inconclusive and dose ranges are unclear. Some studies and reviews report no sedation at higher doses of 1,000–1,500 mg of CBD (11, 19, 21, 22), while others, primarily in pediatric epilepsy populations, report sedation at more moderate doses of 5–10 mg/kg/day CBD (20, 23, 24). Further investigation is needed to assess if there is a true dose-dependent effect or if sedation is due to the co-administration of other drugs such as antiepileptics or CNS depressants, which may lead to drug interactions resulting in increased sedation (20, 25, 26). As such, when discussing impairment there are a myriad of other factors that are important to consider beyond just the dose of THC that can contribute to an individual's risk (12).
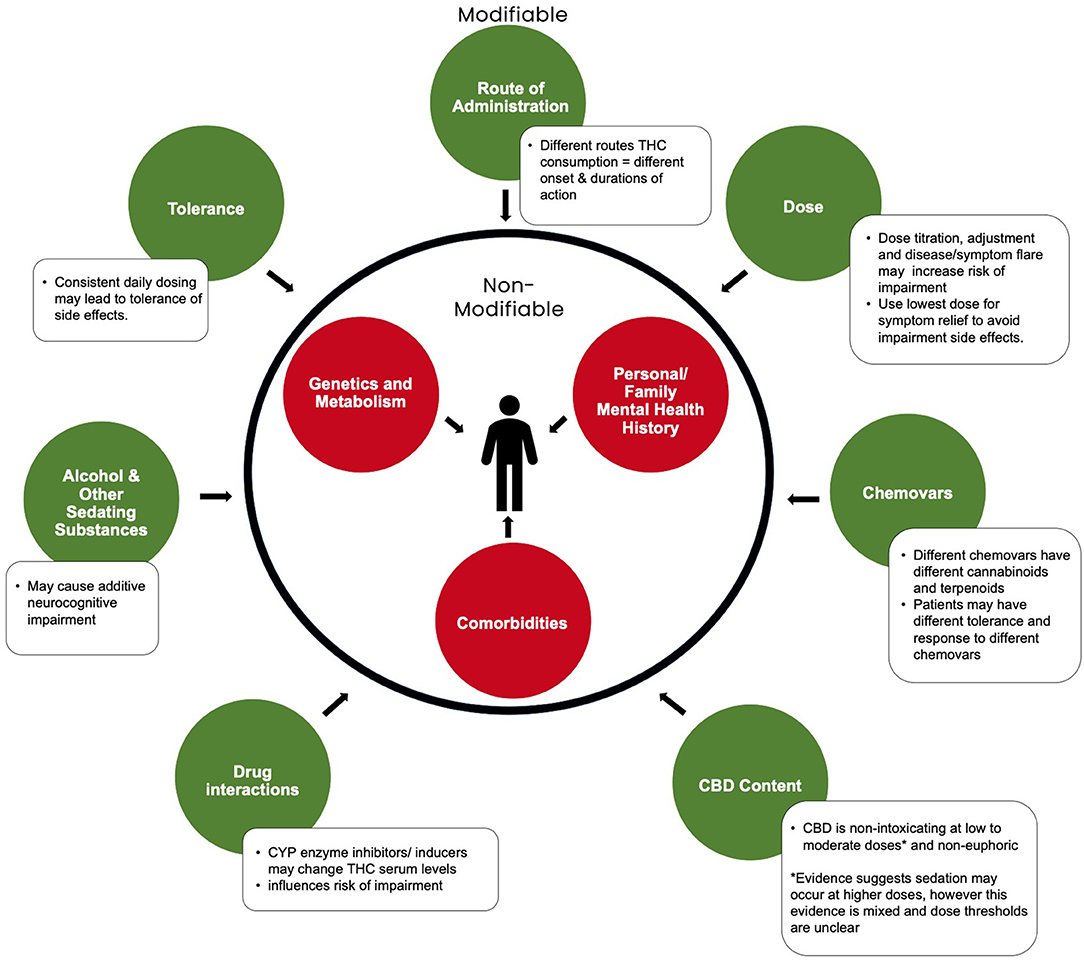
Figure 1. Modifiable and non-modifiable factors influencing cannabis-related neurocognitive impairment. Adapted from Eadie et al. (12)2.
Education and risk mitigation are important components of a clinician's role in promoting the safe and responsible medical cannabis use. Determining impairment risk has been a significant challenge for many clinicians. There is a lack of suitable testing metrics for determining cannabis impairment with a lack of established correlation between measurement of bodily fluids and level of impairment. Additionally, there is a lack of available well-rounded guidance or consensus recommendations to assess a patient's impairment risk. An additional challenge is the lack of literature available specifically focused on medical cannabis-related impairment. Here, we present a practical framework for clinicians to assess and stratify cannabis-associated impairment risk. Current evidence is interwoven within this practical framework.
This impairment framework has been developed to help guide healthcare providers (HCPs) assessing a patient's impairment risk (Table 1). The idea for this practical guide was born from a needs assessment conducted by author CM for continuing education programs, as well as recent published reports revealing a HCP need for practical guidance on assessing the many aspects of cannabis-related impairment (27, 28). This framework was developed through a combination of expert clinical opinion, reviewing common questions in medical education sessions conducted by the authors, and reviewing the available literature. The first step in developing this framework was translating the clinical processes used by authors CM, MB, and MSA when assessing patient impairment risk in-clinic into a step by step framework. The next step was a collaborative discussion reviewing common questions and points of concerns asked during medical education run by authors, these were then incorporated in the framework. A practical overview of the literature was then conducted to elaborate on each framework component and make final adjustments to content. Finally, author consensus based on expert clinical opinion and relevant literature categorized factors into higher, moderate, and lower risk of impairment. The outcome of this process resulted in a practical framework that can help guide clinicians when assessing their patients' potential risk of cannabinoid-related impairment. It is best practice to complete an assessment of impairment risk for patients being considered for or who are currently using medical cannabis, especially those in safety-sensitive occupations (e.g., driving, operating heavy machinery, dealing with hazardous materials, or working in a safety-sensitive workplace).
Clinicians should engage with their patients to understand the reasons why they are using cannabis. Medical and recreational cannabis have different goals of use (29, 30). In a strictly medical context, cannabis and certain cannabinoids are used to manage symptoms associated with a medical condition and improve an individual's ability to function (31). Patients with HCP authorizations for medical cannabis should have a formal diagnosis and documentation of their medical condition. In clinical settings, it has been observed that these patients typically titrate to the lowest dose required to obtain symptom relief, with acceptable side effects, and follow consistent and standardized dosing procedures (8). This pattern often leads to lower cannabinoid doses, thus reducing impairment risk and may support side effect tolerance development (8, 32). It is important to determine if cannabis was initiated by a knowledgeable, licensed HCP and if there is regular ongoing monitoring and support, as lack of education and guidance can increase the risk of misuse and possible impairment. Additionally, individuals reporting the use of medical cannabis, but are not under the guidance and monitoring of a knowledgeable HCP, may have use patterns more similar to recreational users (31).
Recreational cannabis is generally used by those seeking relaxation, euphoria and/or impairment. Recreational users often consume larger THC doses over a shorter period of time in order to obtain the desired effect. This pattern is associated with an increased risk of adverse effects and impairment (15, 33, 34). Recreational use also tends to be more inconsistent in product type and pattern of use (31, 35). This can lead to unpredictable effects, thus increasing the risk of impairment.
Some medical patients will also use cannabis recreationally. This too may increase risk of impairment as the effects and risks of THC are additive due to its highly lipophilic properties and accumulation of THC in adipose tissue (14). Clinicians are encouraged to approach the topic non-judgmentally. Consider one of the following approaches: “A number of my patients also use cannabis recreationally; do you use cannabis recreationally as well?” or “How often do you also use cannabis recreationally?”.
Different routes of cannabis administration have unique pharmacokinetic properties that dictate the duration of potential impairment and will when it is safe to engage in safety-sensitive activities (10, 36). It is important to understand the timeframe where a patient may be at risk in order to determine when cannabis can be used safely. Oral ingestion is a long-acting dosage form, with an onset of action within 1–2 h, lasting an average of 6–8 h (10, 37). Oral formulations are often ideal for medical use but there is also a greater period of potential impairment, and a risk for delayed impairment (38).
Inhalation is a short-acting dosage form, with an onset of 5–10 min, lasting an average of 1–4 h (14, 39). As a result, inhaled medical cannabis is commonly used for acute symptoms and presents a shorter period for potential impairment. However, there can be difficulties with accurate dosing, since length (time) and depth of inhalation significantly impact the cannabinoid dose consumed. This may increase risk of unintentional impairment.
We advise against the use of concentrated dosage cannabis forms for medical use (e.g., dabbing) as they are commonly associated with excessive impairment and health risks (40, 41). To date, local application of topical cannabinoids to intact skin does not appear to be associated with central effects, and thus can be used without risk of impairment (42).
Ensuring the cannabis product being used is from a regulated, third party tested supplier is important. Products from illicit sources may have mislabeled cannabinoid contents, presenting a risk of unexpected impairment. One study evaluating CBD products sold online, found that 21% of these products contained sufficient THC to produce impairment (43). Further, non-regulated products, especially purchased online, may contain synthetic cannabinoids or be more likely to be highly potent, increasing risk of impairment (40). Regulated products can provide some confidence that the label matches the product's cannabinoid content. Regulated products normally have strict regional requirements (state, provincial, or federal) for labeling and testing (40, 44).
Different chemovars (strains) will have different cannabinoid content. Cannabis dosing takes into consideration the THC and/or CBD content of each plant chemovar. In dried cannabis flower it is labeled as a percentage of cannabinoid in the total weight (%/g), or by concentration in cannabis oils (mg/ml). The majority of impairing adverse events are THC-dose dependent (12, 45). Of note, tetrahydrocannabinolic acid (THCA) is the carboxylic acid form of THC in the “raw” plant. THCA is non-intoxicating and non-impairing (46) unless decarboxylation through heating occurs (47, 48).
There is increasing evidence to support that CBD is non-impairing. High oral doses of 100 mg of CBD up to supratherapeutic doses of 1,500 and 4,500 mg of CBD have not produced detectable effects on cognitive or motor function (11, 21, 22).
Determining what THC dose will elicit impairment remains highly patient-specific, regardless of the method of administration. Given the multiple factors responsible for impairment (Figure 1), it is challenging to separate effects of THC dose, specifically in determining a “safe” dose that will be non-impairing for all patients. Experimental studies utilizing neuropsychological battery tests, simulator or on-road testing, were conducted to assess the influence of cannabis on driving, cognitive, and psychomotor ability. In healthy, infrequent cannabis users, acute oral THC doses of 7.5 and 15 mg slightly impaired time perception, therefore also affecting motor response preparation and execution processes, impulsivity and inhibition (49), as well as episodic memory and learning (50). However, these same doses did not significantly alter performance on the Digit Symbol Substitution Test, Hopkins Verbal Learning Task, Digit Span Forward, Go/no-go, or the Delay or Probability discounting tasks (49). Other studies report that relative to placebo, 10 mg of oral THC did not appear to alter cognitive or psychomotor performance among healthy, infrequent cannabis users (51). Importantly, participants of these studies would not have been on stable doses of medical cannabis. A recent randomized, controlled trial found low, single doses of 0.5–1.0 mg inhaled THC did not result in impairment in processing speed (Reaction Time Test, RTI), episodic memory (Paired Associates Learning Task, PAL), working memory (Spatial Working Memory Test, SWM) or sustained attention (Rapid Visual Information Processing Test, RVP) in patients with chronic pain (52). While doses above 40 mg of THC are considered high and carry a substantial risk of impairment (32, 37). The risk of impairment for doses between these ranges strongly depends on patient-specific factors. In alignment with previous literature (53), we believe stable doses below 10 mg/day generally carry a lower risk of impairment.
For dried product, evidence supports that most medical cannabis patients have therapeutic benefit from between 1 and 3 g of cannabis per day (44). Consuming over 5 g/day of dried cannabis flower is a potential flag of problematic use (37). Problematic use is associated with a high risk for cannabis impairment and should be intervened for a variety of health-related reasons.
Frequency and pattern of use are important in determining the total daily dose and the times of the day for which a patient may be at the highest risk of impairment. Greater frequency of use results in longer periods of potential impairment and less time between cannabis use and engaging in driving or safety-sensitive duties. Daytime THC use may present a greater safety risk, especially if the patient engages in safety-sensitive activities during the day. The pattern of use will depend on patient-specific goals. Assessing the timeframe between use of cannabis and driving or engaging in safety-sensitive positions/workplaces is imperative when assessing risk. If the frequency of use is such that an individual is using inhaled cannabis within 4–6 h prior to driving or 8–12 h prior to engaging in safety-sensitive positions/workplaces respectively, then the individual would be considered higher risk based on the frequency and time of day cannabis is taken. Given the longer duration of action of orally ingested cannabis, longer timeframes are recommended (Table 3).
As with any pharmacotherapy, periods of medication titration or dose adjustment increases the risk of adverse events. Chronic and continuous medical cannabis use can lead to tolerance to many potential adverse side effects such as fatigue, dizziness, and acute intoxication (54). This is similar to other prescription medications used in this patient population.
A recent systematic review and meta-analysis found that regular cannabis users experienced less impairment in discrete driving-related cognitive skills compared to occasional users following acute consumption of a single dose of THC (~20 mg) (55). Other studies have corroborated these findings, reporting that frequent cannabis users (smoking ≥ 4 days/week) demonstrated less acute impairment across several neuropsychological tests compared to occasional users (smoking ~1 day/week) as a potential consequence of tolerance (56). However, another recent systematic review of meta-analyses concluded that acute and non-acute, residual impairment (within minutes to hours post-acute intoxication phase) in executive function, processing speed, verbal learning and memory, and attention may occur with regular, mostly heavy, consumption despite potential tolerance (13). It is important to note that this low-to-moderate quality evidence was extracted from a heterogeneous group of studies which varied in the operationalization of cannabis use history (frequency), cognitive tests used, cannabis dose, and control variables employed. As evidence is still varied on whether regular consumption of cannabis can lessen the risk of acute impairment as a result of developed tolerance, it cannot be assumed that patients frequently using cannabis, even at medically appropriate doses, are not at risk of impairment.
Clinicians should actively discuss dose stability with patients to determine if tolerance is developing. HCPs should be cautious in recommending safety-sensitivity activities even in a patient with potential tolerance. Tolerance to cannabis, as with other substances, may not equate to complete lack of impairment.
Adverse effects are a common sign of an excessive cannabis dose. Common cannabis-related impairment adverse effects are not experienced by the majority of patients using medical cannabis when the THC starting dose is low and titration is slow. The presence of certain adverse effects may result in impairment (Table 2). Generally, if a patient experiences these adverse effects, safety-sensitive activities should be refrained from and adjustments to the cannabis regimen are recommended.
Patients with comorbidities that result in fatigue, dizziness, or cognitive slowing may compound impairment (8, 12). Notable conditions to consider include, but are not limited to, older age, concurrent mental health conditions, substance use disorders, neurodegenerative disorders, sleep disorders, and chronic pain conditions (8, 57–59). These conditions alone, and in combination with cannabis, may impair an individual's ability to be alert and engage in normal cognitive or motor function. Additional patient factors that are important to consider are concurrent medications and driving/safety-sensitive occupations, which are discussed below (8, 12, 58, 59). Patients with factors that may cause additive impairment should be monitored more closely to ensure absence of adverse effects.
Drug interactions may increase risk of impairment. Medical cannabis patients commonly take other impairing medications to manage their condition(s). While cannabis is believed to be safe to use with most medications, clinicians should assess all other medications for potential interactions (60). Common prescription or over-the-counter medications that may pose a risk for additive impairment or sedation when combined with THC include antiepileptics, antipsychotics, benzodiazepines, opioids, tricyclic antidepressants, dimenhydrinate, diphenhydramine, or muscle relaxants (61). The use of recreational substances such as alcohol as well as other illicit substances can also cause increased impairment.
Since cannabis is metabolized in the liver by CYP 450 isoenzymes (THC: CYP2C9, CYP2C19, CYP3A4, and CBD: CYP2C19, CYP3A4), CYP inhibitors or inducers may cause pharmacokinetic drug interactions, which can impact the blood serum levels of cannabinoids or the interacting medication (61). It should be noted that there is an indirect potential for impairment with moderate to high doses of CBD when taken with other CYP3A4 inhibitors (e.g., anti-seizure medications such as clobazam) (62). Additionally, drug interactions that increase or prolong the availability of THC may lead to prolonged impairment. In patients with potential drug interactions, increased monitoring and drug levels, when appropriate, should be carried out until absence of impairment or adverse effects are ruled out.
The patient's specific lifestyle should be considered when determining risk of impairment. If a patient does not drive or work in a safety-sensitive position or workplace, the outcomes of impairment are generally less serious. Safety-sensitive activities can include such tasks as operating transportation, use of heavy machinery, and dealing with hazardous materials. The consequences of even mild impairment can be more profound in these circumstances, impacting the worker, their colleagues, the community, and the environment. Extra precaution and focus on mitigating impairment risk should be taken for those who work in a safety-sensitive position or workplace.
Although driving a personal motor vehicle is considered a safety-sensitive activity, those who work in safety-sensitive occupations, where impairment may lead to catastrophic consequences in the workplace, may require more stringent restrictions in dose and timing of administration of cannabis. The more complex the task, the less likely individuals can compensate for the mild to moderate impairments associated with cannabis use. Due to the significant hazard associated with any impairment, tighter restrictions for those in safety-sensitive occupations should be considered and an abundance of caution is reasonable and recommended (63).
Regarding driving a car, a patient is generally considered low risk when driving the morning after inhaling a stable dose of THC the previous evening. Educating patients on windows of impairment in which driving should be avoided is critical. The 2021 Canadian Cannabis Survey revealed that 21% of people reporting cannabis use in the last 12 months had driven within 2 h of smoking or vaporizing. Of individuals reporting driving within 2 h, 78% reported they did not feel impaired and 22% reported that they thought they could drive carefully (64). This highlights the importance of HCP guidance to mitigate potential harms.
It is important to know the route of administration as each has a different duration of action and periods of potential impairment. This should be considered in the context of when cannabis is being used and when an individual is safe to operate a motor vehicle or performs any safety-sensitive duty.
A review containing six RCT's in medical cannabis populations found impairment resolved within 2–4 h post dose,2 in line with several other clinical trials (56, 65, 66). However, until there is more robust literature for medical cannabis populations, a cautious approach of consuming THC at least 4–6 h, if inhaled, and 6–8 h, if ingested, prior to operating a personal motor vehicle is suggested (6, 29).
Longer duration between timing of dose and the start of work, as well as tighter restrictions on dosing of THC may be required for patients who work in a safety-sensitive position or workplace. We advise waiting at least 8–12 h, if inhaled, and 12 h, if ingested, prior to engaging in safety-sensitive positions or workplaces.
Certain medical conditions can increase the risk of impairment. Studies have shown conditions such as multiple sclerosis, insomnia, epilepsy, anxiety, and depression have an increased risk of motor vehicle accidents (67–69). Reducing or eliminating the symptoms associated with these medical conditions can therefore decrease risk of impairment. If medical cannabis is successful in controlling symptoms that may impact motor or cognitive function on their own, individuals may actually have a lower risk of impairment (70).
Evidence is still varied on whether or not CBD can lessen the impact of THC-associated side effects (71), but using products that contain CBD may allow for a reduced THC dose required due to synergistic effects (72). THC and CBD combinations were also associated with positive effects on symptoms, while experiencing significantly less paranoia and anxiety than THC-only products (72). From a clinical and safety standpoint, CBD is a preferred choice for individuals that engage in safety-sensitive activities. It is important to note that many CBD-dominant products still contain low levels of THC.
Many individuals consume medical cannabis without proper safety education (73). As per best practice standards, HCPs should provide education on side effects, product/chemovar selection, activity limitations, dosing and titration, method of administration, and treatment monitoring to reduce the risk of patient harm (8, 32). The frequency of monitoring will depend on patient specific circumstances, clinician experience, and guidelines by local regulatory bodies. HCPs are advised to tailor the frequency of monitoring to reflect the benefit and risk considerations for the individual patient.
The lack of suitable testing metrics poses a challenge in determining cannabis-related impairment. The proposed framework is intended as a practical guide for HCP's to comprehensively assess and stratify the potential risk of impairment in their patients. This information guides discussion and patient education regarding these potential risks and allows for adjustments to mitigate or reduce the risk of impairment. This is especially important for individuals who perform any safety-sensitive activities.
Whether it be returning to work, driving, or working in a safety-sensitive position or workplace, the potential for cannabis impairment should be evaluated. Factors associated with different levels of impairment risk are summarized in Table 3. To stratify risk for any patient, each factor must be considered and assessed. If any considerations fall under higher risk for impairment, the individual is considered higher risk, regardless of the number of risk factors in the moderate or lower risk of impairment categories. Similarly, if any considerations fall under moderate risk, with no higher risk of impairment considerations, the individual is considered at moderate risk of impairment. An individual can only be considered to be at lower risk of impairment if all considerations fall under the lower risk category.
The framework presented in this piece is intended as a proposed guide to help clinicians assess risk of cannabinoid-related impairment in their patients. However, it is not without limitations. Although the framework discussed is commonly used in-clinic by authors, it has not been formally evaluated. Thus, we cannot formally speak to its reliability or validity. Despite this, the current lack of available guidance on the topic gives merit to share available guidance while more standardized processes are developed. Second, cannabis-related impairment is a complex topic, as there is a wide range of domains through which impairment may occur and there is notable variability between patients. While this framework is meant to provide a general overview, it should not be forgotten that each patient requires an individualized assessment and may have unique factors that influence impairment risk. Third, using this framework relies on patients providing honest and complete information. Without this, the guidance could be misinformed and could cause liability for HCPs and those relying on the risk assessment (employers for example). This stresses the importance of developing good rapport and trust with the patient to promote open and honest conversation. Additionally, taking the time to educate the patients on the danger of engaging in safety sensitive activities or work and how to mitigate this risk is key.
Future directions in this work should look at the reliability and validity of this framework more formally. Developing a points system may be a useful avenue to pursue to help consider all risk factors more clearly. Medical cannabis patients are a heterogenous population, thus another avenue would be investigating how cannabis-related impairment differs between medical populations, and if there are differing key factors that may promote or mitigate impairment.
Factors discussed in the framework can impact the degree and duration of impairment. Although this framework is guided by the current evidence, more research in this area can provide stronger guidance on potential risk factors for cannabis-related impairment. Each patient will have unique considerations. Proper screening and evaluation of a patient can help promote the safe and responsible use of medical cannabis.
CM was primarily responsible for the conceptualization and overall intellectual leadership of this project. In collaboration with CM, LL wrote the first draft of this manuscript with additional support from CP. AC, MB, and MS-A contributed to revising the manuscript with additional intellectual input. All authors contributed to the article and approved the submitted version.
CM is the Medical Director of Greenleaf Medical Clinic and Chief Medical Officer for Translational Life Sciences. She is on the Board of Directors for The Green Organic Dutchman. She is an advisor to PreveCeutical, Pinnacle Care, Africanna, EO Care, Andira Medicine, Active Patch Technologies, Syqe Medical, and Dosist. Additionally, she has provided medical consultation and/or received support for industry sponsored continuing medical education from: Aleafia, Aurora, Canopy, Spectrum, Tilray, Emerald Health, and CCRN. MB has received financial support as a speaker and consultant for CHE activities from Teva, Pfizer, Novo Nordisk, Khiron, Tilray, mdBriefcase, J&J, Abbvie, Ascensia, Astra Zeneca, Biosynt, and Emergent BioSolutions. MS-A has received financial support from Syqe Medical and is on the advisory board of Syqe Medical and Indivior Canada.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Authors would like to acknowledge the medical cannabis patients whose experiences have enhanced our clinical understanding of impairment.
1. Bowles N, Herzig M, Shea S. Recent legalization of cannabis use: effects on sleep, health, and workplace safety. Nat Sci Sleep. (2017) 9:249–51. doi: 10.2147/NSS.S152231
2. National National Academies of Sciences Engineering Medicine Health Medicine Division Board Board on Population Health Public Health Practice Committee Committee on the Health Effects of Marijuana. An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press (US) (The National Academies Collection: Reports funded by National Institutes of Health) (2017). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK423845/ (accessed April 15, 2017).
3. Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse. (2014) 40:23–30. doi: 10.3109/00952990.2013.821477
4. Ko GD, Bober SL, Mindra S, Moreau JM. Medical cannabis – the Canadian perspective. J Pain Res. (2016) 9:735–44. doi: 10.2147/JPR.S98182
5. Walsh Z, Callaway R, Belle-Isle L, Capler R, Kay R, Lucas P, et al. Cannabis for therapeutic purposes: patient characteristics, access, and reasons for use. Int J Drug Policy. (2013) 24:511–6. doi: 10.1016/j.drugpo.2013.08.010
6. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, ON: College of Family Physicians of Canada (2014).
7. Shiplo S, Asbridge M, Leatherdale ST, Hammond D. Medical cannabis use in Canada: vapourization and modes of delivery. Harm Reduct J. (2016) 13:30. doi: 10.1186/s12954-016-0119-9
8. MacCallum CA, Lo LA, Boivin M. “Is medical cannabis safe for my patients?” a practical review of cannabis safety considerations. Eur J Int Med. (2021) 89:10–8. doi: 10.1016/j.ejim.2021.05.002
9. Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T. (2017) 42:180–8.
10. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. (2018) 84:2477–82. doi: 10.1111/bcp.13710
11. Spindle TR, Cone EJ, Goffi E, Weerts EM, Mitchell JM, Winecker RE, et al. Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users. Drug Alcohol Depend. (2020) 211:107937. doi: 10.1016/j.drugalcdep.2020.107937
12. Eadie L, Lo LA, Christiansen A, Brubacher JR, Barr AM, Panenka WJ, et al. Duration of neurocognitive impairment with medical cannabis use: a scoping review. Front Psychiatry. (2021) 12:638962. doi: 10.3389/fpsyt.2021.638962
13. Dellazizzo L, Potvin S, Giguère S, Dumais A. Evidence on the acute and residual neurocognitive effects of cannabis use in adolescents and adults: a systematic meta-review of meta-analyses. Addiction. (2022). doi: 10.1111/add.15764. [Epub ahead of print].
14. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. (2007) 4:1770–804. doi: 10.1002/cbdv.200790152
15. Ashton CH. Adverse effects of cannabis and cannabinoids. Br J Anaesth. (1999) 83:637–49. doi: 10.1093/bja/83.4.637
16. Brubacher JR, Chan H, Staples JA. Cannabis-impaired driving and Canadian youth. Paediatr Child Health. (2020) 25:S21–5. doi: 10.1093/pch/pxaa017
17. Busardò FP, Pellegrini M, Klein J, di Luca NM. Neurocognitive correlates in driving under the influence of cannabis. CNS Neurol Disord Drug Targets. (2017) 16:534–40. doi: 10.2174/1871527316666170424115455
18. Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor: negative allosteric modulation of CB1 by cannabidiol. Br J Pharmacol. (2015) 172:4790–805. doi: 10.1111/bph.13250
19. Bergamaschi MM, Queiroz RHC, Zuardi AW, Crippa JAS. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. (2011) 6:237–49. doi: 10.2174/157488611798280924
20. Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardò FP. Cannabidiol adverse effects and toxicity. Curr Neuropharmacol. (2019) 17:974–89. doi: 10.2174/1570159X17666190603171901
21. Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, et al. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend. (2017) 172:9–13. doi: 10.1016/j.drugalcdep.2016.11.030
22. Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav. (2018) 88:162–71. doi: 10.1016/j.yebeh.2018.07.027
23. Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. (2016) 15:270–8. doi: 10.1016/S1474-4422(15)00379-8
24. Devinsky O, Patel AD, Thiele EA, Wong MatthewH, Appleton R, Harden CL, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. (2018) 90:e1204–11. doi: 10.1212/WNL.0000000000005254
25. Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M. Clinical and preclinical evidence for functional interactions of cannabidiol and Δ9-tetrahydrocannabinol. Neuropsychopharmacology. (2018) 43:142–54. doi: 10.1038/npp.2017.209
26. Dos Santos RG, Guimarães FS, Crippa JAS, Hallak JEC, Rossi GN, Rocha JM, et al. Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol. (2020) 16:517–26. doi: 10.1080/17425255.2020.1754793
27. Chin G, Etiz BAF, Nelson AM, Lim PK, Scolaro JA. Knowledge and opinion on cannabinoids among orthopaedic traumatologists. J Am Acad Orthop Surg Glob Res Rev. (2021) 5:e21.00047. doi: 10.5435/JAAOSGlobal-D-21-00047
28. Szaflarski M, McGoldrick P, Currens L, Blodgett D, Land H, Szaflarski JP, et al. Attitudes and knowledge about cannabis and cannabis-based therapies among US neurologists, nurses, and pharmacists. Epilepsy Behav. (2020) 109:107102. doi: 10.1016/j.yebeh.2020.107102
29. Capler NR, Bilsker D, Van Pelt K, MacPherson D. Cannabis Use and Driving: Evidence Review. Canadian Drug Policy Coalition (CDPC) (2017).
30. Osborne GB, Fogel C. Understanding the motivations for recreational marijuana use among adult Canadians. Subst Use Misuse. (2008) 43:539–72. doi: 10.1080/10826080701884911
31. Turna J, Balodis I, Munn C, Van Ameringen M, Busse J, MacKillop J. Overlapping patterns of recreational and medical cannabis use in a large community sample of cannabis users. Compr Psychiatry. (2020) 102:152188. doi: 10.1016/j.comppsych.2020.152188
32. Bhaskar A, Bell A, Boivin M, Briques W, Brown M, Clarke H, et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: results of a modified Delphi process. J Cannabis Res. (2021) 3:22. doi: 10.1186/s42238-021-00073-1
33. Subritzky T, Lenton S, Pettigrew S. Practical Lessons Learned From the First Years of the Regulated Recreational Cannabis Market in Colorado. In: Legalizing Cannabis Routledge (2020). doi: 10.4324/9780429427794-4
34. Gilman JM, Schuster RM, Potter KW, Schmitt W, Wheeler G, Pachas GN, et al. Effect of medical marijuana card ownership on pain, insomnia, and affective disorder symptoms in adults: a randomized clinical trial. JAMA Netw Open. (2022) 5:e222106. doi: 10.1001/jamanetworkopen.2022.2106
35. Goulet-Stock S, Rueda S, Vafaei A, Ialomiteanu A, Manthey J, Rehm J, et al. Comparing medical and recreational cannabis users on socio-demographic, substance and medication use, and health and disability characteristics. Eur Addict Res. (2017) 23:129–35. doi: 10.1159/000475987
36. Meyer P, Langos M, Brenneisen R. Human pharmacokinetics and adverse effects of pulmonary and intravenous THC-CBD formulations. Med Cannabis Cannabinoids. (2018) 1:36–43. doi: 10.1159/000489034
37. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. (2018) 49:12–9. doi: 10.1016/j.ejim.2018.01.004
38. Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use – basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy. (2018) 52:87–96. doi: 10.1016/j.drugpo.2017.11.008
39. Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther. (2007) 82:572–8. doi: 10.1038/sj.clpt.6100200
40. MacCallum CA, Lo LA, Pistawka CA, Boivin M. A clinical framework for evaluating cannabis product quality and safety. Cannabis Cannabinoid Res. (2022). doi: 10.1089/can.2021.0137. [Epub ahead of print].
41. Loflin M, Earleywine M. A new method of cannabis ingestion: the dangers of dabs? Addict Behav. (2014) 39:1430–3. doi: 10.1016/j.addbeh.2014.05.013
42. Peters J, Chien J. Contemporary routes of cannabis consumption: a primer for clinicians. J Osteopath Med. (2018) 118:67–70. doi: 10.7556/jaoa.2018.020
43. Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling accuracy of cannabidiol extracts sold online. JAMA. (2017) 318:1708. doi: 10.1001/jama.2017.11909
44. Canada Health Canada. Information for Health Care Professionals: Cannabis (Marihuana, Marijuana) and the Cannabinoids : Dried or Fresh Plant and Oil Administration by Ingestion or Other Means Psychoactive Agent. (2018). Available online at: http://publications.gc.ca/collections/collection_2018/sc-hc/H129-19-2018-eng.pdf (accessed November 8, 2021).
45. Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. (2004) 73:109–19. doi: 10.1016/j.drugalcdep.2003.10.008
46. Russo EB, Marcu J. Cannabis Pharmacology: The Usual Suspects a Few Promising Leads. In: Advances in Pharmacology. Elsevier (2017) p. 67–134. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1054358917300273 (accessed June 13, 2020).
47. Wang M, Wang YH, Avula B, Radwan MM, Wanas AS, van Antwerp J, et al. Decarboxylation study of acidic cannabinoids: a novel approach using ultra-high-performance supercritical fluid chromatography/photodiode array-mass spectrometry. Cannabis Cannabinoid Res. (2016) 1:262–71. doi: 10.1089/can.2016.0020
48. Zaharia LS, Trofin I, Vaireanu DI, Dabija G. Influence of Temperature and Heating Time on the Decarboxylation OF Δ9-THCA and CBDA in the cannabis inflorescences, 82, 74–84.
49. McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacol. (2003) 28:1356–65. doi: 10.1038/sj.npp.1300176
50. Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology. (2002) 164:61–70. doi: 10.1007/s00213-002-1169-0
51. Schlienz NJ, Spindle TR, Cone EJ, Herrmann ES, Bigelow GE, Mitchell JM, et al. Pharmacodynamic dose effects of oral cannabis ingestion in healthy adults who infrequently use cannabis. Drug Alcohol Depend. (2020) 211:107969. doi: 10.1016/j.drugalcdep.2020.107969
52. Almog S, Aharon-Peretz J, Vulfsons S, Ogintz M, Abalia H, Lupo T, et al. The pharmacokinetics, efficacy, and safety of a novel selective-dose cannabis inhaler in patients with chronic pain: a randomized, double-blinded, placebo-controlled trial. Eur J Pain. (2020) 24:1505–16. doi: 10.1002/ejp.1605
53. Vandrey R, Herrmann ES, Mitchell JM, Bigelow GE, Flegel R, LoDico C, et al. Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J Anal Toxicol. (2017) 41:83–99. doi: 10.1093/jat/bkx012
54. Ramaekers JG, Mason NL, Theunissen EL. Blunted highs: Pharmacodynamic and behavioral models of cannabis tolerance. Eur Neuropsychopharmacol. (2020) 36:191–205. doi: 10.1016/j.euroneuro.2020.01.006
55. McCartney D, Arkell TR, Irwin C, McGregor IS. Determining the magnitude and duration of acute Δ9-tetrahydrocannabinol (Δ9-THC)-induced driving and cognitive impairment: a systematic and meta-analytic review. Neurosci Biobehav Rev. (2021) 126:175–93. doi: 10.1016/j.neubiorev.2021.01.003
56. Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. (2009) 23:266–77. doi: 10.1177/0269881108092393
57. Abuhasira R, Ron A, Sikorin I, Novack V. Medical cannabis for older patients—treatment protocol and initial results. J Clin Med. (2019) 8:1819. doi: 10.3390/jcm8111819
58. Gottschling S, Ayonrinde O, Bhaskar A, Blockman M, D'Agnone O, Schecter D, et al. Safety considerations in cannabinoid-based medicine. Int J Gen Med. (2020) 13:1317–33. doi: 10.2147/IJGM.S275049
59. Minerbi A, Häuser W, Fitzcharles MA. Medical cannabis for older patients. Drugs Aging. (2019) 36:39–51. doi: 10.1007/s40266-018-0616-5
60. MacCallum CA, Freitas L, Lo LA, Eadie L, Brubacher JR. Cannabinoid-Related Adverse Events and Impairment. In: Cannabinoids and Pain, Cham: Springer. (2021). doi: 10.1007/978-3-030-69186-8_36
61. Alsherbiny MA, Li CG. Medicinal cannabis-potential drug interactions. Medicines. (2018) 6:3. doi: 10.3390/medicines6010003
62. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. (2015) 56:1246–51. doi: 10.1111/epi.13060
63. Beckson M, Hagtvedt R, Els C. Cannabis use before safety-sensitive work: what delay is prudent? Neurosci Biobehav Rev. (2022) 133:104488. doi: 10.1016/j.neubiorev.2021.12.011
65. Arkell TR, Vinckenbosch F, Kevin RC, Theunissen EL, McGregor IS, Ramaekers JG. Effect of cannabidiol and Δ 9 -Tetrahydrocannabinol on driving performance: a randomized clinical trial. JAMA. (2020) 324:2177. doi: 10.1001/jama.2020.21218
66. Newmeyer MN, Swortwood MJ, Abulseoud OA, Huestis MA. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol Depend. (2017) 175:67–76. doi: 10.1016/j.drugalcdep.2017.02.003
67. Lings S. Driving accident frequency increased in patients with multiple sclerosis: driving and multiple sclerosis. Acta Neurol Scand. (2002) 105:169–73. doi: 10.1034/j.1600-0404.2002.1o165.x
68. Aduen PA, Kofler MJ, Sarver DE, Wells EL, Soto EF, Cox DJ, et al. depression, and motor vehicle crashes: a prospective cohort study of continuously-monitored, real-world driving. J Psychiatr Res. (2018) 101:42–9. doi: 10.1016/j.jpsychires.2018.02.026
69. Garbarino S, Magnavita N, Guglielmi O, Maestri M, Dini G, Bersi FM, et al. Insomnia is associated with road accidents. further evidence from a study on truck drivers. PLoS ONE. (2017) 12:e0187256. doi: 10.1371/journal.pone.0187256
70. Celius EG, Vila C. The influence of THC:CBD oromucosal spray on driving ability in patients with multiple sclerosis-related spasticity. Brain Behav. (2018) 8:e00962. doi: 10.1002/brb3.962
71. Arkell TR, Lintzeris N, Kevin RC, Ramaekers JG, Vandrey R, Irwin C, et al. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology. (2019) 236:2713–24. doi: 10.1007/s00213-019-05246-8
72. Gibson LP, Karoly HC, Ellingson JM, Klawitter J, Sempio C, Squeri JE, et al. Effects of cannabidiol in cannabis flower: implications for harm reduction. Addict Biol. (2022) 27:e13092. doi: 10.1111/adb.13092
73. Arboleda MF, Prosk E. Practical recommendations for the use of medical cannabis. In: Narouze SN, editor. Cannabinoids and Pain. Cham: Springer International Publishing (2021). p. 153–65. Available online at: https://link.springer.com/10.1007/978-3-030-69186-8_21 (accessed April 26, 2022).
Keywords: cannabinoids, medical cannabis, THC, impairment, occupational safety, driving
Citation: MacCallum CA, Lo LA, Pistawka CA, Christiansen A, Boivin M and Snider-Adler M (2022) A Clinical Framework for Assessing Cannabis-Related Impairment Risk. Front. Psychiatry 13:883517. doi: 10.3389/fpsyt.2022.883517
Received: 25 February 2022; Accepted: 30 May 2022;
Published: 24 June 2022.
Edited by:
Yasser Khazaal, University of Lausanne, SwitzerlandReviewed by:
Raffaele Giorgetti, Marche Polytechnic University, ItalyCopyright © 2022 MacCallum, Lo, Pistawka, Christiansen, Boivin and Snider-Adler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline A. MacCallum, aW5mb0BkcmNhcm9saW5lbWFjY2FsbHVtLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.