
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 26 May 2022
Sec. Child and Adolescent Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.880496
Objectives: Although several studies have reviewed the suicidal risk of antidepressants, the conclusions remain inconsistent. We, therefore, performed a meta-analysis of observational studies to address the association between exposure to antidepressants, especially selective serotonin reuptake inhibitors (SSRIs) and the risk of suicide and suicide attempt in children and adolescents.
Methods: MEDLINE and Embase were searched from January 1990 to April 2021. Seventeen cohort and case-control studies were identified that reported suicide or suicide attempt in children and young adults (aged 5–25 years) who were exposed to any antidepressants. We extracted the estimates and corresponding 95% confidence intervals (CIs) from each publication.
Results: The results showed that antidepressant exposure significantly increased the risk of suicide and suicide attempt when compared with no antidepressant usage among children and adolescents. The pooled relative risk (RR) was 1.38 (95% CI: 1.16–1.64; I2 = 83.1%). Among the antidepressants, SSRI use was associated with an increased risk of suicide and suicide attempt, and the pooled RR was 1.28 (95% CI: 1.09–1.51; I2 = 68.8%). In subgroup analysis, the attempted suicidal risk of antidepressant and SSRI was significantly increased (RR = 1.35, 95% CI: 1.13–1.61; I2 = 86.2% for all antidepressants; and RR = 1.26, 95% CI: 1.06–1.48; I2 = 73.8% for SSRIs), while the completed suicidal risk of antidepressant and SSRI was not statistically significant (RR = 2.32, 95% CI: 0.82–6.53; I2 = 6.28% for all antidepressants; and RR = 1.88, 95% CI: 0.74–4.79; I2 = 52.0% for SSRIs). In addition, the risk of suicide and suicide attempt between SSRIs and other antidepressants was similar (RR 1.13, 95% CI: 0.87–1.46, I2 = 32.4%).
Conclusion: The main findings of this meta-analysis provide some evidence that antidepressant exposure seems to have an increased suicidal risk among children and young adults. Since untreated depression remains one of the largest risk factors for suicide and the efficacy of antidepressants is proven, clinicians should evaluate carefully their patients and be cautious with patients at risk to have treatment emergence or worsening of suicidal ideation (TESI/TWOSI) when prescribing antidepressants to children and young patients.
Accumulating evidence suggests that antidepressant use in children and adolescents is substantially increased in recent years (1), though the FDA issued a “black box” warning for selective serotonin reuptake inhibitors (SSRIs) of suicidal thinking and behavior in children and adolescents in 2004 (2) and expanded to include young adults (aged 18–24 years) in 2007 (3). SSRIs are the most commonly prescribed antidepressants for the treatment of depressive and anxiety disorders (1) and the relationship between SSRIs and other antidepressants, and the risk of suicidal behavior (including completed suicide and suicide attempt) has been subject to considerable public attention since 2004, especially in young people. Likewise, clinical guidelines covering the use of antidepressants in children and adolescents have taken the suicidal risk into consideration and recommended careful monitoring for suicidal behaviors after initiation of antidepressant treatment (4). Suicide is one of the major causes of death with a rate of 534.3 per 100,000 person-years in patients with major depression (5), and suicide clusters occur more frequently in young people than in adults, especially through social media (6, 7); thus, it is of great importance to identify the suicidal risk of antidepressants.
Suicidal ideation and behavior developed during depression treatment are called treatment emergence or worsening of suicidal ideation (TESI/TWOSI). The incidence of TESI varies from 3.2 to 17% among different studies, depending on the studied population and the threshold used for TESI (8). Predictors associated with TESI and TWOSI include sociodemographic and clinical risk factors (i.e., the preadult onset of depression, gender, depression severity, physical pain, and poor response to antidepressants) (8, 9) and also genetic risk factors (10, 11). Also, effective temperament types were independently and strongly associated with lifetime suicide attempts (12). However, whether antidepressants would increase the suicidal ideation and behavior in children and adolescents remain controversial, and an updated review of new evidence is needed as the prevalence of antidepressant use in children and adolescents is growing in the last decade (13–15), for example, the prevalence of antidepressant use in children and adolescents increased from 13 to 16% in the USA and from 07 to 11% in the United Kingdom in 2005–2012 (15).
Meta-analysis of the randomized controlled trials (RCTs), the highest level of evidence, produced inconsistent findings. Unlike the meta-analysis (16) conducted a decade ago, which found that the overall risk ratio for SSRIs in depressed pediatric patients was 1.66 (95% CI: 1.02–2.68), the recent meta-analysis of RCTs (17, 18) gives the conclusion that only venlafaxine was found to be associated with an increased risk of suicidal behavior or ideation in the young population, and the suicidal risk of other antidepressants remains unclear due to the absence of reliable data. As most trials on antidepressants excluded patients with suicidal ideation and behavior (19, 20), RCTs only provided limited assessments of antidepressant use on suicidal risk, leading to uncertainty about medication safety and efficacy in this specific population. However, it is possible to overcome the barriers to include suicidal participants and conduct the clinical trials safely, as seen with recent research on esketamine for example (9, 21), and inclusion of this specific population into psychiatric clinical trials in the future is critical to efforts to reduce suicidal rates. In addition, observational studies, which were conducted in the real world and included a large, broad spectrum of individuals with long duration, might also offer a reliable suggestion for clinical services and could be complementary to that provided by clinical trials.
A recent umbrella review (22) of observational studies evaluated the adverse outcomes of antidepressants, and the only convincing evidence found is the association between antidepressant use and the risk of suicide attempt or completion among children and adolescents, which was published in 2009 (23). Many studies were published since then, which might affect the conclusion. Therefore, we conducted an updated meta-analysis of observational studies to further address the association between antidepressant exposure and the risk of suicide and suicide attempt in children and adolescents.
We followed the checklist of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for background, design, analysis, and interpretation. We conducted a literature search of MEDLINE/PubMed, Embase, and Cochrane Library from January 1990 to April 2021 for relevant studies assessing the association between antidepressants and the risk of suicide. Various combinations of keywords were utilized, including but not limited to (“antidepressant,” “antidepressive,” and “SSRI”) and (“suicide,” “suicide attempt,” and “suicidal behavior”). We also scrutinized the reference lists of relevant major reviews. No language restrictions were imposed. The detailed search strategy and the PubMed search term are shown in Supplementary Table 1.
Eligible studies were included in this meta-analysis if they satisfied the following criteria: (1) the study design was observational cohort or case-control studies, (2) the study population include children or young patients (age < 25 years), (3) the exposure of interest was antidepressant use, and (4) the outcomes were suicidal behaviors (i.e., completed suicide or suicide attempt) or deliberate self-harm (with a clear definition to include suicidal behavior). One reviewer assessed the titles and abstracts of all studies identified through electronic searches. Potentially eligible studies were reviewed independently by a second reviewer, with discrepancies resolved by discussion.
Two reviewers (ZGB and XY) independently extracted and collected the data. The following information was extracted from each eligible study: name of the first author, year of publication, study design, study location, mean follow-up time, participants’ characteristics, number of participants, outcomes, outcome assessment methods, and adjustment for potential confounders. We extracted crude and adjusted estimates and corresponding 95% confidence intervals (CIs). When studies had several adjustment models, we used the most comprehensively adjusted estimates in multivariable-adjusted models. Crude relative risks (RRs) or odds ratios (ORs) were calculated when the case numbers in the exposure group and comparison group were shown but the risk estimates remain unknown.
Quality assessment was performed using the Newcastle-Ottawa Scale (NOS) (24). The maximum NOS score for an observational study is 9 points (4 points for selection, 2 points for comparability, and 3 points for outcome).
Since the incidence of suicide and suicide attempt was low, we regarded OR as close approximations of RR and combined them with a hazard ratio (HR), resulting in a common estimate of RR. Potential heterogeneity among studies was estimated using the Cochran Q test and I2 statistic (25). I2 can be interpreted as the proportion of the total variance due to heterogeneity. Where the I2 estimate was greater than or equal to 50%, we interpreted this as indicating the presence of high levels of heterogeneity. We used a fixed-effect model (Mantel-Haenszel method) when heterogeneity was negligible, and a random effect model (DerSimonian and Laird method) when heterogeneity was significant (26). In addition, we used restricted maximum likelihood random-effects meta-regression to explore heterogeneity by the publication year of study, length of follow-up, the outcome, and the diagnosis of depression. The possibility of publication bias was evaluated using the Egger regression asymmetry test (27) and the Begg test. Sensitivity analyses for the influence of single studies on the pooled RR were conducted by omitting studies one by one and reestimating the pooled RR. All statistical analyses were performed using Stata version 12 (Stata Corp).
Figure 1 shows a flow diagram for the study selection process, the literature search identified 4,340 references that included antidepressant exposure and suicide or suicide attempt, which were reduced to 3,638 after removal of duplicates, and 3,578 references were excluded as not being relevant by review of the title and abstract. After evaluating the full texts of these 60 publications, 43 studies were excluded (Supplementary Table 2), and 17 independent studies (28–44) meeting the inclusion criteria were assessed in this meta-analysis.
The characteristics of 17 identified studies are shown in Supplementary Table 3, 10 studies (28, 30, 33, 34, 38–40, 42–44) were conducted in the United States and seven studies (29, 31, 32, 35–37) in Europe. Of the included studies, 13 were cohort studies and 4 were case-control studies. Overall follow-up time ranged from 4 months to 17.7 years, with a weighted mean of 2.1 years. Two studies reported estimates on more than one outcome (30, 36) (suicide and suicide attempt), and fifteen studies reported only one outcome (suicide or suicide attempt). Fourteen studies used ICD-9 or ICD-10 codes to define suicide and suicide attempt and the other three used medical records. One study reported the results of two databases separately (40). The results of the study quality assessment (score 0–9) yielded scores of 6 or above (high quality) for fifteen studies and 5 for two studies, with an average score of 7.1.
Among 11 studies that examined the association between any antidepressants (including SSRIs) use and completed or attempted suicide among adolescents (13 estimates), antidepressant exposure significantly increased the risk of completed or attempted suicide when compared with no antidepressant exposure (Figure 2), and the overall RR for incidence of suicide or suicide attempt was 1.38 (95% CI: 1.16–1.64, I2 = 83.1%, P < 0.001). To explore the potential source of heterogeneity across studies, a sensitivity analysis was performed by removing one study at a time, and the corresponding pooled RR was not materially altered after the removal of any study (Supplementary Figure 1). We performed a meta-regression with publication year and length of follow-up as covariates in the meta-analysis. The results showed that the publication year of the study explains 50.98% of heterogeneity (P = 0.026). Neither the Begg test nor the Egger regression test for publication bias reached significance (P > 0.05 for both tests).
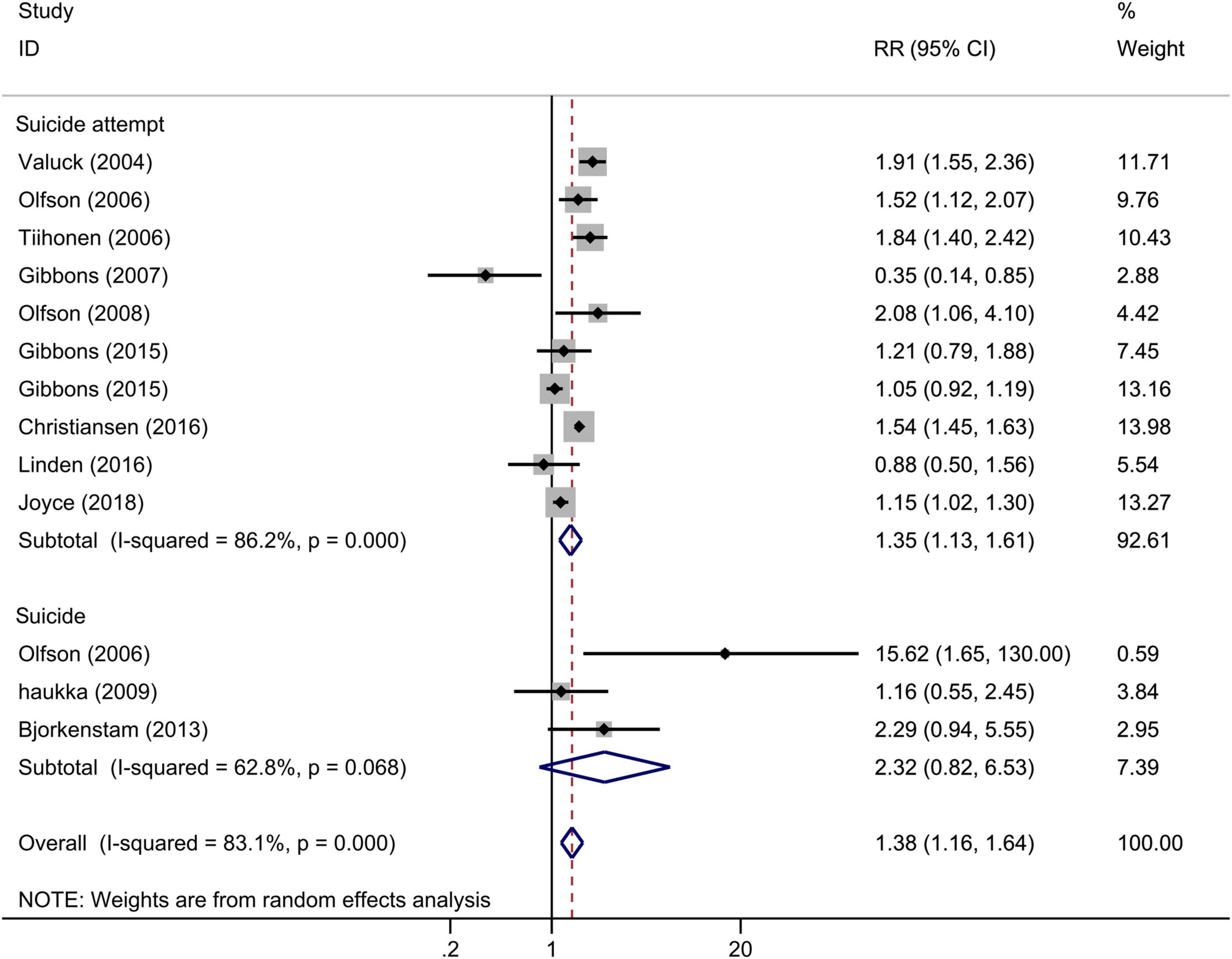
Figure 2. Forest plot of antidepressant exposure and the risk of suicidal behavior among children and adolescents.
In the subgroup analysis according to the outcomes (Figure 2), antidepressant exposure significantly increased the risk of suicide attempt (RR = 1.35, 95% CI: 1.13–1.61, I2 = 86.2%, P < 0.001), with no significant effect on completed suicide (RR = 2.32, 95% CI: 0.82–6.53, I2 = 62.8%, P = 0.068). We also performed subgroup analyses according to the adjustment, and the pooled adjusted and crude RR showed similar results (Supplementary Figure 2). The result of pooled adjusted estimate suggested that the results of the overall meta-analysis were robust.
Across 15 studies that examined the association between SSRIs and completed or attempted suicide among adolescents (18 estimates), SSRI exposure significantly increased the risk of completed and attempted suicide (Figure 3), and the pooled RR for incidence of suicide or suicide attempt was 1.28 (95% CI: 1.09–1.51, I2 = 68.8%, P < 0.001). The control group was no antidepressant use or any other antidepressant use. This result was robust in the sensitivity analysis (Supplementary Figure 3). Results from separate meta-regression models suggested that the publication year (P > 0.05) and follow-up duration (P > 0.05) were not the sources of heterogeneity. We found no evidence of publication bias (P > 0.05 for Begg and Egger tests).
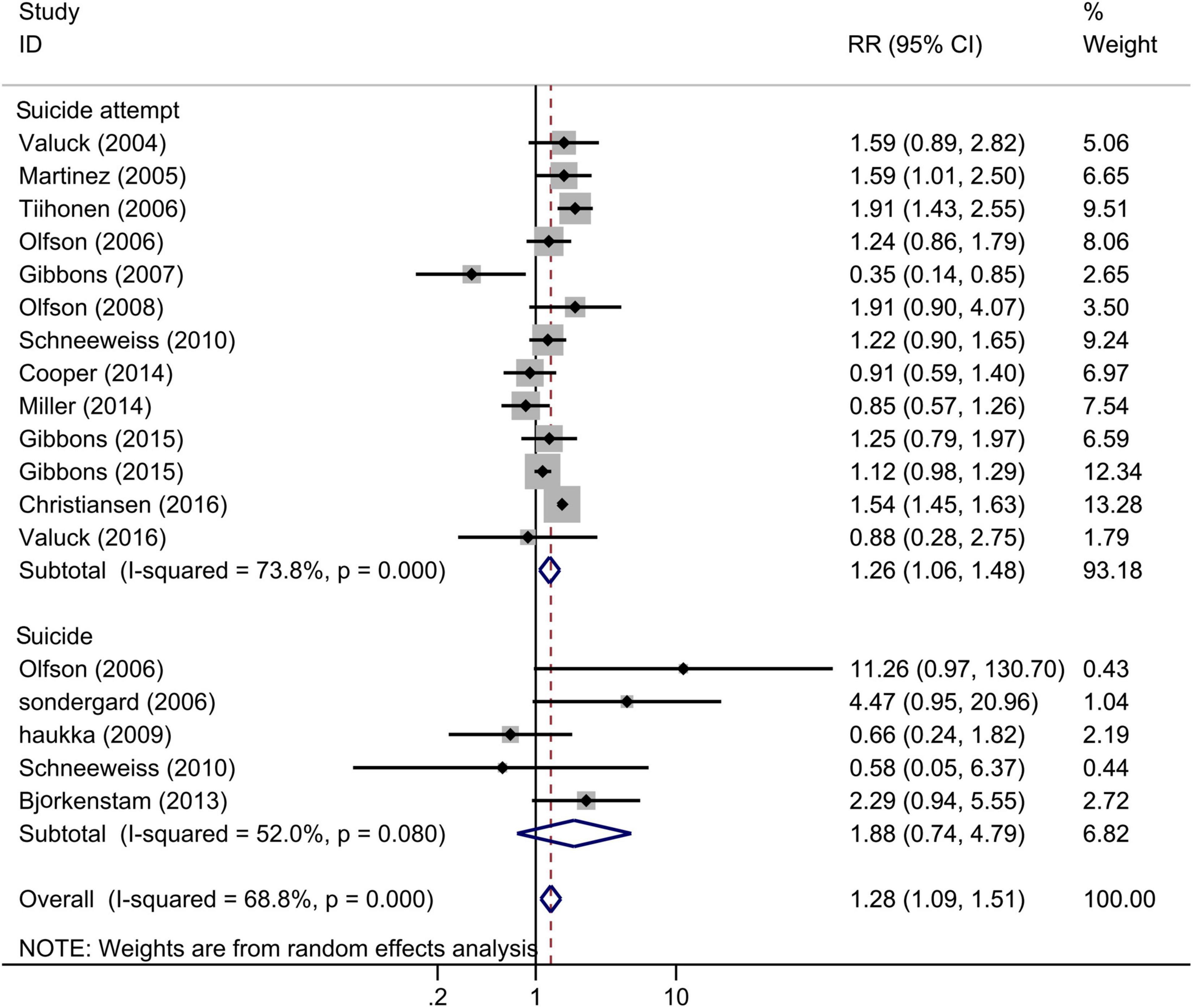
Figure 3. Forest plot of selective serotonin reuptake inhibitor (SSRI) exposure and the risk of suicidal behavior among children and adolescents.
In the subgroup analysis according to the outcomes (Figure 3), SSRI exposure significantly increased the risk of suicide attempt (RR = 1.26, 95% CI: 1.06–1.48, I2 = 73.8%, P < 0.001), with no significant effect on completed suicide (RR = 1.88, 95% CI: 0.74–4.79, I2 = 52.0%, P = 0.08). In the subgroup analysis of adjustment (Supplementary Figure 4), the pooled adjusted RR was 1.42 (95% CI 1.18–1.72; I2 = 69.7%), similar to analysis that included all studies, while the pooled crude RR was 1.11 (95% CI 0.83–1.50; I2 = 55.1%).
The comparisons of antidepressant exposure were no antidepressant exposure in all included studies, and the comparisons of SSRI exposure were any other antidepressant use or no antidepressant use. Thus, we performed the subgroup analysis based on the comparisons, and the results indicated that the risk of suicide/suicide attempt of SSRI use was significantly increased compared with no antidepressant use (Figure 4, RR = 1.37, 95% CI: 1.12–1.67, I2 = 73.8%, P < 0.001), but similar to other antidepressants (Figure 4, RR = 1.13, 95% CI: 0.87–1.46, I2 = 32.4%, P = 0.18). This subgroup analysis indicated that the increased risk of suicidal behavior for SSRIs and other antidepressants was similar among children and adolescents. Since the subgroup finding was based on a small number of studies, the results should be interpreted with caution.
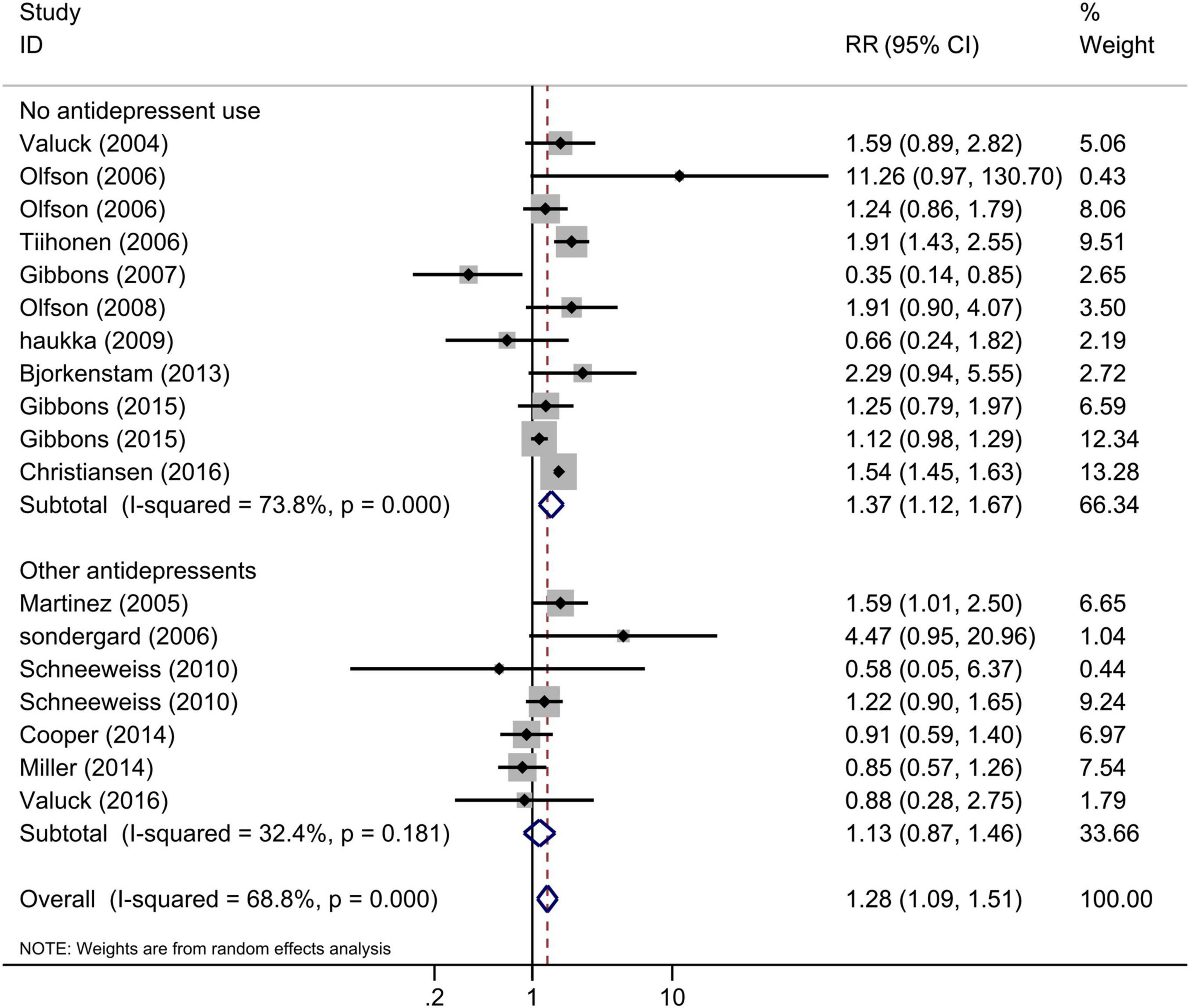
Figure 4. Subgroup analysis of SSRI exposure and risk of suicidal behavior according to the comparisons.
Our meta-analysis identified 17 observational studies and provided a comprehensive review and quantitative estimates of the association between antidepressant exposure and the risk of suicide and suicide attempt. The results showed that antidepressant use in children and young populations increased the risk of suicidal behavior compared with no antidepressant use. Especially, the exposure to only SSRIs also increased the risk of the suicide attempt. These results were consistent with the main conclusion of the previous review of clinical trial data (23, 45, 46). Similar results were obtained in subgroup analyses of a suicide attempt; however, the increased risk of completed suicide exposed to antidepressants was not significant among adolescents, suggesting that the risk of completed suicide might be discordant with attempted suicide. Together, these results suggested that adolescents and young adults might be at increased risk for suicidal behaviors following antidepressant therapy, including SSRIs.
A few studies have investigated clinical and genetic risk factors of TESI/TWOSI in adults that can contribute to the increased risk of suicidal behavior at the antidepressant onset. The results showed that the severity of depression, the first few weeks of treatment, drug abuse, poor response to antidepressants, physical pain, and previous history of suicidal behavior or ideation was associated with the emergence of TESI/TWOSI (41, 47–51). In addition, some of the few available studies about genetic risk factors of TESI/TWOSI reported associations with single nucleotide polymorphisms of genes involved in the neurotropic and synaptic plasticity systems (52, 53), noradrenergic system (52), glutamatergic system (54), stress and inflammatory responses (11, 55), and opioid system (56). Those clinical and genetic risk factors reported in adults may also contribute to the TESI/TWOSI in children and adolescents and, thus, help to monitor the TESI/TWOSI during treatment.
The mechanisms of the increased suicidal risk of antidepressants among adolescents were not quite clear; however, some studies in animal models have found that treatment with SSRIs could exert potent anxiogenic behavioral effects, in particular during the acute phase of treatment (57–59). The inhibitory circuit of corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis may contribute to the aversive behavior following acute exposure to SSRIs (58). Furthermore, another research (60) on an animal model reported that brief administration of paroxetine in young rats can promote, rather than reduce, the depressive state, contrary to the therapeutic changes observed in adult rats. Those findings might partially explain the adverse effects of antidepressants in adolescents.
Recently, a few studies reported that intranasal esketamine or ketamine may result in significantly rapid improvement in depressive symptoms and suicidal ideation among depressed patients at imminent risk for suicide (21, 61), and as previous history of suicidal behavior or ideation and poor response to antidepressants are both risk factors for TESI/TWOSI, treatment with esketamine or ketamine may be an option to be further explored among children and adolescents with those risk factors. In addition, dialectical behavior therapy is reported to have beneficial effects on self-harm in children and adolescents, and still, further evaluation for dialectical behavior therapy and cognitive behavior therapy is warranted (62).
In line with our results, several RCTs found a significant increase in the incidence of suicide attempts for adolescents and young adults receiving antidepressants compared with placebo (63, 64), including the analyses conducted by the FDA (16, 65). Bridge (66) analyzed 27 RCTs of second-generation antidepressants in participants younger than 19 years with major depressive disorder, obsessive-compulsive disorder (OCD), or non-OCD anxiety disorders and found an overall increased risk of suicidal ideation/suicide attempt associated with antidepressant treatment. Also, Hetrick et al. (45) performed a systematic review of published and unpublished randomized controlled trials comparing newer generation antidepressants (mainly SSRIs) with placebo in children and adolescents aged 6–18 years. Statistical analysis revealed an increased risk (58%) of suicide-related outcomes for those on antidepressants compared with placebo (RR = 1.58, 95% CI: 1.02–2.45). Moreover, our result among adolescents was similar to the previous systemic review (23) of observational studies which found that exposure to SSRIs almost doubled the risk of suicide among adolescents.
Counter to the evidence showing an increase in suicide-related behaviors, some arguments have been mounted. Several recent meta-analyses of RCTs (17, 18, 67) found no statistically significant risk between suicide-related outcomes and antidepressant use, except venlafaxine. But children and adolescents considered at risk of suicide were frequently excluded from RCTs so the proportions of suicide-related outcomes in those trials were low for most included studies and 95% CIs were wide for all comparisons. Otherwise, a review (68) aggregated data from six population studies of adolescent suicides that contained individual data on SSRIs at or around the time of death, and the results found no evidence that SSRIs were associated with increased suicide in young people. Wijlaars (69) performed a self-control study and revealed no systematic differences between the association of SSRIs and the incidence risk ratios for attempted suicide or intentional self-harm. But some important confounders such as depression severity were not controlled in both studies. In addition, numerous population-based studies (70–72) have shown an inverse association between antidepressant use and suicide rates. Another study (73) found suicide in the 10–19-year age group increased for five consecutive years (60.5%), and the increase occurred among individuals not treated with antidepressants. However, this negative association does not allow conclusion to be drawn regarding causality, and it is crucial to control for potential confounding factors. Consequently, the balance between risks and benefits will need to be considered for individual patients.
It is unclear whether the risk for antidepressant use is discrepant between completed suicide and attempted suicide. Consistent with our subgroup analysis, a meta-review (74) of a systematic review study found that SSRIs may increase suicidal thoughts, but not actual suicide in early phase therapy. Tiihonen (32) suggested that antidepressant use was associated with an increased risk of non-fatal suicidal behavior and a decreased risk of fatal suicidal behavior. The possible explanations for this finding include that: (i) the subgroup analysis on completed suicide was based on a small number of studies (three for antidepressants and five for SSRIs, respectively); (ii) the number of completed suicidal cases is limited in most included studies. Thus, this result that the increased risk of completed suicide exposed to antidepressants was not significant among adolescents should be interpreted with caution.
Our analysis has several strengths. We did sensitivity analyses for the influence of single studies on the pooled RR which suggested that no individual study had excessive influences on the main results. We pooled adjusted estimates in the subgroup analysis, using models adjusting for most established risk factors. Also, most studies adjusted for potential risk for suicide such as age, sex, and psychiatric characteristics (e.g., psychiatric contact or hospitalization, history of suicidal behavior, severity of depression, and multiple antidepressant medications), and the adjustments of each study are listed in Supplementary Table 3. Additionally, almost all studies used ICD-9 or ICD-10 codes to define suicide and suicide attempt, making sure that the standards of the outcomes stayed consistent. However, we found a great level of heterogeneity, and this may be due to the publication year of the study according to the meta-regression since the estimate of suicidal risk would be affected after the “black box” warning issued by FDA.
Several limitations of our study should also be acknowledged. First, a time-dependent decline in the suicidal rate for antidepressant recipients was identified (75); however, we did not analyze the association between prescription time and suicidal risk owing to the absence of reliable data. Second, a meta-analysis of observational data has limited ability to adjust for baseline differences and specific risk factors and is prone to bias and confounding. We made an attempt to allow for multiple confounding by including adjusted estimates from multivariate models in each contributing study. However, due to limited data from the original studies, we were unable to stratify studies by sex, severity of illness, species of SSRIs, or the history of the suicide attempt. Third, not all studies made enough adjustments for confounders. For example, some risk factors for suicidal behavior (e.g., personality disorder, physical disease, and childhood irritability) are not adjusted in the included studies (76–78), also, not all the studies adjusted their results on lifetime history of suicide attempts and other suicidal behavior characteristics. Thus, results from the main analyses were affected by various confounders. It is important that future studies specify those confounding factors. Fourth, no inference can be made about newer antidepressants (e.g., vortioxetine hydrobromide) that have not been assessed in any of the included analyses. Fifth, although we carried out several subgroup analyses and meta-regression, the substantial heterogeneity present in most analyses remains unexplained. Finally, observational studies cannot provide causal evidence of the effect of antidepressant use on suicidal behaviors; they can describe only associations.
The main findings of this meta-analysis provide some evidence that antidepressant exposure seems to have an increased suicidal risk among children and young adults. Since untreated depression remains one of the largest risk factors for suicide and the efficacy of antidepressants is proven, clinicians should evaluate carefully their patients and be cautious with patients at risk to have TESI and TWOSI when prescribing antidepressants to children and young patients (8). The most advantageous treatment or combination of non-pharmacological interventions should be considered for this specific population. Moreover, esketamine and ketamine are reported to transiently decrease suicidal ideation in patients with serious suicidal thoughts or actions, yet the long-term outcomes and safety in children and young adults are still not clear (79). Given the potential for life-threatening events in young children and adolescents, it is essential to seek and evaluate new strategies for young patients at risk.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
KL and JW conceived and designed this study. JG and QC conducted the databases search. GZ and YX selected the studies, extracted the data, and assessed the risk of bias. KL was in charge of writing-reviewing of the manuscript. SX and JW took supervision. All authors contributed to this study and approved the final manuscript.
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81903581), the Shenzhen Science and Technology Program (Grant Nos. JCYJ20190807150005699 and RCBS20200714115000009), the Shenzhen Key Medical Discipline Construction Fund (Grant No. SZXK059), and the Shenzhen Key Laboratory of Prevention and Treatment of Severe Infections (Grant No. ZDSYS20200811142804014).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.880496/full#supplementary-material
1. Hartz I, Skurtveit S, Hjellvik V, Furu K, Nesvåg R, Handal M. Antidepressant drug use among adolescents during 2004-2013: a population-based register linkage study. Acta Psychiatr Scand. (2016) 134:420–9. doi: 10.1111/acps.12633
2. U.S. Food and Drug Administration.Suicidality in Children and Adolescents Being Treated with Antidepressant Medications. Silver Spring, MD: US FDA (2004).
4. Lawton A, Moghraby OS. Depression in children and young people: identification and management in primary, community and secondary care (Nice Guideline Cg28). Arch Dis Child Educ Pract Ed. (2016) 101:206–9. doi: 10.1136/archdischild-2015-308680
5. Fu X, Qian Y, Jin X, Yu H, Wu H, Du L, et al. Suicide rates among people with serious mental illness: a systematic review and meta-analysis. Psychol Med. (2021) 1–11. doi: 10.1017/s0033291721001549
6. Hawton K, Hill NTM, Gould M, John A, Lascelles K, Robinson J. Clustering of suicides in children and adolescents. Lancet Child Adolesc Health. (2020) 4:58–67. doi: 10.1016/s2352-4642(19)30335-9
7. Solano P, Ustulin M, Pizzorno E, Vichi M, Pompili M, Serafini G, et al. A google-based approach for monitoring suicide risk. Psychiatry Res. (2016) 246:581–6. doi: 10.1016/j.psychres.2016.10.030
8. Courtet P, Nobile B, Lopez-Castroman J. Antidepressants and suicide risk: harmful or useful? In: U Kumar editor. Handbook of Suicidal Behaviour. Singapore: Springer Singapore (2017). p. 329–47. doi: 10.1007/978-981-10-4816-6_18
9. McCall W, Benca R, Rosenquist P, Youssef N, McCloud L, Newman J, et al. Reducing suicidal ideation through insomnia treatment (Rest-It): a randomized clinical trial. Am J psychiatry. (2019) 176:957–65. doi: 10.1176/appi.ajp.2019.19030267
10. Menke A, Domschke K, Czamara D, Klengel T, Hennings J, Lucae S, et al. Genome-wide association study of antidepressant treatment-emergent suicidal ideation. Neuropsychopharmacology. (2012) 37:797–807. doi: 10.1038/npp.2011.257
11. Nobile B, Ramoz N, Jaussent I, Dubois J, Guillaume S, Gorwood P, et al. Polymorphisms of stress pathway genes and emergence of suicidal ideation at antidepressant treatment onset. Transl psychiatry. (2020) 10:320. doi: 10.1038/s41398-020-01003-0
12. Baldessarini R, Innamorati M, Erbuto D, Serafini G, Fiorillo A, Amore M, et al. Differential associations of affective temperaments and diagnosis of major affective disorders with suicidal behavior. J Affect Disord. (2017) 210:19–21. doi: 10.1016/j.jad.2016.12.003
13. Lagerberg T, Molero Y, D’Onofrio B, Fernández de la Cruz L, Lichtenstein P, Mataix-Cols D, et al. Antidepressant prescription patterns and CNS Polypharmacy with antidepressants among children, adolescents, and young adults: a population-based study in sweden. Eur Child Adolesc Psychiatry. (2019) 28:1137–45. doi: 10.1007/s00787-018-01269-2
14. Jack RH, Hollis C, Coupland C, Morriss R, Knaggs RD, Butler D, et al. Incidence and prevalence of primary care antidepressant prescribing in children and young people in England, 1998-2017: a population-based cohort study. PLoS Med. (2020) 17:e1003215. doi: 10.1371/journal.pmed.1003215
15. Bachmann C, Aagaard L, Burcu M, Glaeske G, Kalverdijk L, Petersen I, et al. Trends and patterns of antidepressant use in children and adolescents from five western countries, 2005-2012. Eur Neuropsychopharmacol. (2016) 26:411–9. doi: 10.1016/j.euroneuro.2016.02.001
16. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. (2006) 63:332–9. doi: 10.1001/archpsyc.63.3.332
17. Cipriani A, Zhou X, Del Giovane C, Hetrick S, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. (2016) 388:881–90. doi: 10.1016/s0140-6736(16)30385-3
18. Zhou X, Teng T, Zhang Y, Del Giovane C, Furukawa T, Weisz J, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry. (2020) 7:581–601. doi: 10.1016/s2215-0366(20)30137-1
19. Iltis AS, McCall WV, Deria R. Suicidality, depression, and the FDA: health inequities and the ethical conduct of research. J Clin Psychiatry. (2020) 81:19m13050. doi: 10.4088/JCP.19m13050
20. Courtet P, Nobile B. Inclusion of suicidal individuals in research studies. J Clin Psychiatry. (2020) 81:20com13276. doi: 10.4088/JCP.20com13276
21. Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. (2018) 175:620–30. doi: 10.1176/appi.ajp.2018.17060720
22. Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry. (2019) 76:1241–55. doi: 10.1001/jamapsychiatry.2019.2859
23. Barbui C, Esposito E, Cipriani A. Selective serotonin reuptake inhibitors and risk of suicide: a systematic review of observational studies. CMAJ. (2009) 180:291–7. doi: 10.1503/cmaj.081514
24. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Health Research Institute (2000).
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. (1997) 127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008
27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
28. Valuck RJ, Libby AM, Sills MR, Giese AA, Allen RR. Antidepressant treatment and risk of suicide attempt by adolescents with major depressive disorder: a propensity-adjusted retrospective cohort study. CNS Drugs. (2004) 18:1119–32. doi: 10.2165/00023210-200418150-00006
29. Martinez C, Rietbrock S, Wise L, Ashby D, Chick J, Moseley J, et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ. (2005) 330:389. doi: 10.1136/bmj.330.7488.389
30. Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults: a case-control study. Arch Gen Psychiatry. (2006) 63:865–72. doi: 10.1001/archpsyc.63.8.865
31. Sondergard L, Kvist K, Andersen PK, Kessing LV. Do antidepressants precipitate youth suicide?: a nationwide pharmacoepidemiological study. Eur Child Adolesc Psychiatry. (2006) 15:232–40. doi: 10.1007/s00787-006-0527-6
32. Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Tanskanen A, Haukka J. Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry. (2006) 63:1358–67. doi: 10.1001/archpsyc.63.12.1358
33. Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Mann JJ. Relationship between antidepressants and suicide attempts: an analysis of the veterans health administration data sets. Am J Psychiatry. (2007) 164:1044–9. doi: 10.1176/appi.ajp.164.7.1044
34. Olfson M, Marcus SCA. Case-control study of antidepressants and attempted suicide during early phase treatment of major depressive episodes. J Clin Psychiatry. (2008) 69:425–32. doi: 10.4088/jcp.v69n0313
35. Haukka J, Arffman M, Partonen T, Sihvo S, Elovainio M, Tiihonen J, et al. Antidepressant use and mortality in finland: a register-linkage study from a nationwide cohort. Eur J Clin Pharmacol. (2009) 65:715–20. doi: 10.1007/s00228-009-0616-9
36. Schneeweiss S, Patrick AR, Solomon DH, Dormuth CR, Miller M, Mehta J, et al. Comparative safety of antidepressant agents for children and adolescents regarding suicidal acts. Pediatrics. (2010) 125:876–88. doi: 10.1542/peds.2009-2317
37. Bjorkenstam C, Moller J, Ringback G, Salmi P, Hallqvist J, Ljung R. An association between initiation of selective serotonin reuptake inhibitors and suicide – a nationwide register-based case-crossover study. PLoS One. (2013) 8:e73973. doi: 10.1371/journal.pone.0073973
38. Cooper WO, Callahan ST, Shintani A, Fuchs DC, Shelton RC, Dudley JA, et al. Antidepressants and suicide attempts in children. Pediatrics. (2014) 133:204–10. doi: 10.1542/peds.2013-0923
39. Miller M, Pate V, Swanson SA, Azrael D, White A, Sturmer T. Antidepressant class, age, and the risk of deliberate self-harm: a propensity score matched cohort study of SSRI and SNRI users in the USA. CNS Drugs. (2014) 28:79–88. doi: 10.1007/s40263-013-0120-8
40. Gibbons RD, Coca Perraillon M, Hur K, Conti RM, Valuck RJ, Brent DA. Antidepressant treatment and suicide attempts and self-inflicted injury in children and adolescents. Pharmacoepidemiol Drug Saf. (2015) 24:208–14. doi: 10.1002/pds.3713
41. Christiansen E, Agerbo E, Bilenberg N, Stenager E. SSRIs and risk of suicide attempts in young people – a danish observational register-based historical cohort study, using propensity score. Nord J Psychiatry. (2016) 70:167–75. doi: 10.3109/08039488.2015.1065291
42. Linden S, Bussing R, Kubilis P, Gerhard T, Segal R, Shuster JJ, et al. Risk of suicidal events with atomoxetine compared to stimulant treatment: a cohort study. Pediatrics. (2016) 137:e20153199. doi: 10.1542/peds.2015-3199
43. Valuck RJ, Libby AM, Anderson HD, Allen RR, Strombom I, Marangell LB, et al. Comparison of antidepressant classes and the risk and time course of suicide attempts in adults: propensity matched, retrospective cohort study. Br J Psychiatry. (2016) 208:271–9. doi: 10.1192/bjp.bp.114.150839
44. Joyce NR, Schuler MS, Hadland SE, Hatfield LA. Variation in the 12-month treatment trajectories of children and adolescents after a diagnosis of depression. JAMA Pediatr. (2018) 172:49–56. doi: 10.1001/jamapediatrics.2017.3808
45. Hetrick SE, McKenzie JE, Cox GR, Simmons MB, Merry SN. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev. (2012) 11:CD004851. doi: 10.1002/14651858.CD004851.pub3
46. Umetsu R, Abe J, Ueda N, Kato Y, Matsui T, Nakayama Y, et al. Association between selective serotonin reuptake inhibitor therapy and suicidality: analysis of U.S. food and drug administration adverse event reporting system data. Biol Pharm Bull. (2015) 38:1689–99. doi: 10.1248/bpb.b15-00243
47. Zisook S, Trivedi MH, Warden D, Lebowitz B, Thase ME, Stewart JW, et al. Clinical correlates of the worsening or emergence of suicidal ideation during SSRI treatment of depression: an examination of citalopram in the star*D study. J Affect Disord. (2009) 117:63–73. doi: 10.1016/j.jad.2009.01.002
48. Courtet P, Jaussent I, Lopez-Castroman J, Gorwood P. Poor response to antidepressants predicts new suicidal ideas and behavior in depressed outpatients. Eur Neuropsychopharmacol. (2014) 24:1650–8. doi: 10.1016/j.euroneuro.2014.07.007
49. Seemüller F, Riedel M, Obermeier M, Bauer M, Adli M, Mundt C, et al. The controversial link between antidepressants and suicidality risks in adults: data from a naturalistic study on a large sample of in-patients with a major depressive episode. Int J Neuropsychopharmacol. (2009) 12:181–9. doi: 10.1017/s1461145708009139
50. Termorshuizen F, Palmen SJ, Heerdink ER. Suicide behavior before and after the start with antidepressants: a high persistent risk in the first month of treatment among the young. Int J Neuropsychopharmacol. (2015) 19:yv081. doi: 10.1093/ijnp/pyv081
51. Calati R, Laglaoui Bakhiyi C, Artero S, Ilgen M, Courtet P. The impact of physical pain on suicidal thoughts and behaviors: meta-analyses. J Psychiatr Res. (2015) 71:16–32. doi: 10.1016/j.jpsychires.2015.09.004
52. Perroud N, Aitchison KJ, Uher R, Smith R, Huezo-Diaz P, Marusic A, et al. Genetic predictors of increase in suicidal ideation during antidepressant treatment in the gendep project. Neuropsychopharmacology. (2009) 34:2517–28. doi: 10.1038/npp.2009.81
53. Voegeli G, Ramoz N, Shekhtman T, Courtet P, Gorwood P, Kelsoe JR. Neurotrophin genes and antidepressant-worsening suicidal ideation: a prospective case-control study. Int J Neuropsychopharmacol. (2016) 19:yw059. doi: 10.1093/ijnp/pyw059
54. Menke A, Lucae S, Kloiber S, Horstmann S, Bettecken T, Uhr M, et al. Genetic markers within glutamate receptors associated with antidepressant treatment-emergent suicidal ideation. Am J Psychiatry. (2008) 165:917–8. doi: 10.1176/appi.ajp.2008.08020274
55. Laje G, Allen AS, Akula N, Manji H, John Rush A, McMahon FJ. Genome-wide association study of suicidal ideation emerging during citalopram treatment of depressed outpatients. Pharmacogenet Genomics. (2009) 19:666–74. doi: 10.1097/FPC.0b013e32832e4bcd
56. Nobile B, Ramoz N, Jaussent I, Gorwood P, Olié E, Castroman JL, et al. Polymorphism A118g of opioid receptor Mu 1 (OPRM1) is associated with emergence of suicidal ideation at antidepressant onset in a large naturalistic cohort of depressed outpatients. Sci Rep. (2019) 9:2569. doi: 10.1038/s41598-019-39622-3
57. Yohn CN, Gergues MM, Samuels BA. The role of 5-Ht receptors in depression. Mol Brain. (2017) 10:28. doi: 10.1186/s13041-017-0306-y
58. Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. (2016) 537:97–101. doi: 10.1038/nature19318
59. Jaggar M, Banerjee T, Weisstaub N, Gingrich JA, Vaidya VA. 5-Ht(2a) receptor loss does not alter acute fluoxetine-induced anxiety and exhibit sex-dependent regulation of cortical immediate early gene expression. Neuronal Signal. (2019) 3:Ns20180205. doi: 10.1042/ns20180205
60. West CH, Ritchie JC, Weiss JM. Paroxetine-induced increase in activity of locus coeruleus neurons in adolescent rats: implication of a countertherapeutic effect of an antidepressant. Neuropsychopharmacology. (2010) 35:1653–63. doi: 10.1038/npp.2010.34
61. Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. (2018) 175:150–8. doi: 10.1176/appi.ajp.2017.17040472
62. Witt KG, Hetrick SE, Rajaram G, Hazell P, Taylor Salisbury TL, Townsend E, et al. Interventions for self-harm in children and adolescents. Cochrane Database Syst Rev. (2021) 3:Cd013667. doi: 10.1002/14651858.CD013667.pub2
63. Aursnes I, Tvete IF, Gaasemyr J, Natvig B. Suicide attempts in clinical trials with paroxetine randomised against placebo. BMC Med. (2005) 3:14. doi: 10.1186/1741-7015-3-14
64. Apter A, Lipschitz A, Fong R, Carpenter DJ, Krulewicz S, Davies JT, et al. Evaluation of suicidal thoughts and behaviors in children and adolescents taking paroxetine. J Child Adolesc Psychopharmacol. (2006) 16:77–90. doi: 10.1089/cap.2006.16.77
65. Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US food and drug administration. BMJ. (2009) 339:b2880. doi: 10.1136/bmj.b2880
66. Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. (2007) 297:1683–96. doi: 10.1001/jama.297.15.1683
67. Hetrick S, McKenzie J, Bailey A, Sharma V, Moller C, Badcock P, et al. New generation antidepressants for depression in children and adolescents: a network meta-analysis. Cochrane Database Syst Rev. (2021) 5:CD013674. doi: 10.1002/14651858.CD013674.pub2
68. Dudley M, Goldney R, Hadzi-Pavlovic D. Are adolescents dying by suicide taking SSRI antidepressants? A review of observational studies. Australas Psychiatry. (2010) 18:242–5. doi: 10.3109/10398561003681319
69. Wijlaars LP, Nazareth I, Whitaker HJ, Evans SJ, Petersen I. Suicide-related events in young people following prescription of ssris and other antidepressants: a self-controlled case series analysis. BMJ Open. (2013) 3:e003247. doi: 10.1136/bmjopen-2013-003247
70. Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry. (2005) 62:165–72. doi: 10.1001/archpsyc.62.2.165
71. Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant prescription rates and rate of early adolescent suicide. Am J Psychiatry. (2006) 163:1898–904. doi: 10.1176/ajp.2006.163.11.1898
72. Isacsson G, Holmgren A, Osby U, Ahlner J. Decrease in suicide among the individuals treated with antidepressants: a controlled study of antidepressants in suicide, Sweden 1995-2005. Acta Psychiatr Scand. (2009) 120:37–44. doi: 10.1111/j.1600-0447.2009.01344.x
73. Isacsson G, Ahlner J. Antidepressants and the risk of suicide in young persons–prescription trends and toxicological analyses. Acta Psychiatr Scand. (2014) 129:296–302. doi: 10.1111/acps.12160
74. Cipriani A, Geddes JR, Furukawa TA, Barbui C. Metareview on short-term effectiveness and safety of antidepressants for depression: an evidence-based approach to inform clinical practice. Can J Psychiatry. (2007) 52:553–62. doi: 10.1177/070674370705200903
75. Hopkins K, Crosland P, Elliott N, Bewley S. Diagnosis and management of depression in children and young people: summary of updated nice guidance. BMJ. (2015) 350:h824. doi: 10.1136/bmj.h824
76. Orri M, Galera C, Turecki G, Forte A, Renaud J, Boivin M, et al. Association of childhood irritability and depressive/anxious mood profiles with adolescent suicidal ideation and attempts. JAMA Psychiatry. (2018) 75:465–73. doi: 10.1001/jamapsychiatry.2018.0174
77. Grilo C, Udo T. Association of borderline personality disorder criteria with suicide attempts among US adults. JAMA Netw Open. (2021) 4:e219389. doi: 10.1001/jamanetworkopen.2021.9389
78. Oh K, Van Dam N, Doucette J, Murrough J. Effects of chronic physical disease and systemic inflammation on suicide risk in patients with depression: a hospital-based case-control study. Psychol Med. (2020) 50:29–37. doi: 10.1017/s0033291718003902
Keywords: antidepressants, SSRIs, suicide, suicide attempt, children and adolescents
Citation: Li K, Zhou G, Xiao Y, Gu J, Chen Q, Xie S and Wu J (2022) Risk of Suicidal Behaviors and Antidepressant Exposure Among Children and Adolescents: A Meta-Analysis of Observational Studies. Front. Psychiatry 13:880496. doi: 10.3389/fpsyt.2022.880496
Received: 24 February 2022; Accepted: 26 April 2022;
Published: 26 May 2022.
Edited by:
Roberto Canitano, Siena University Hospital, ItalyReviewed by:
Gianluca Serafini, San Martino Hospital (IRCCS), ItalyCopyright © 2022 Li, Zhou, Xiao, Gu, Chen, Xie and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shouxia Xie, c3pzaG91eGlhQDE2My5jb20=; Junyan Wu, d3VqdW55YW5AbWFpbC5zeXN1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.