- 1School of Mental Health, Wenzhou Medical University, Wenzhou, China
- 2Department of Neurosurgery, Affiliated Cixi Hospital, Wenzhou Medical University, Ningbo, China
- 3Department of Social Sciences, Chatham University, Pittsburgh, PA, United States
- 4The Affiliated Kangning Hospital, Wenzhou Medical University, Wenzhou, China
- 5Department of Psychology, College of Liberal Arts, Wenzhou-Kean University, Wenzhou, China
- 6Beijing Hui-Long-Guan Hospital, Peking University, Beijing, China
- 7Xinjiang Key Laboratory of Neurological Disorder Research, The Second Affiliated Hospital of Xinjiang Medical University, Urumqi, China
Objective: Alcohol dependence can increase the level of anxiety. A growing body of research has identified a link between anxiety symptoms of problem drinkers and their genetic or environment factors, respectively. However, to date few studies have directly examined gene-environment (G × E) interaction on their anxiety symptoms during the acute alcohol withdrawal. The present study aims to examine the interaction between the proopiomelanocortin (POMC) rs2071345 polymorphism and alcohol dependence on anxiety symptoms of male problem drinkers, and further test the exact form of interaction on two competing models: the diathesis-stress model vs. the differential susceptibility model.
Methods: A total of 440 male problem drinkers (Mage = 44.5 years, SD = 9.45) were recruited from nine main psychiatric hospitals of northern China during acute alcohol withdrawal. Blood samples were collected for genotyping, self-reported anxiety symptoms, and levels of alcohol dependence were assessed.
Results: Results indicated that the POMC rs2071345 polymorphism significantly moderated anxiety symptoms associated with alcohol dependence. A region of significance (RoS) test showed that male problem drinkers with T allele were more likely to experience more anxiety symptoms than those with CC homozygote when the standardized score of concurrent alcohol dependence was above 0.31. Confirmatory model evaluation indicated that the interaction effect involving POMC gene polymorphism conformed to the diathesis-stress model rather than differential-susceptibility model of person × environment interaction.
Conclusions: This study suggested that the SNP in POMC rs2071345 was associated with alcohol dependence in anxiety symptoms of male problem drinkers and further provided evidence in support of the diathesis-stress hypothesis of alcohol dependence in terms of anxiety symptoms.
Introduction
Alcohol dependence is a chronic, relapsing neuropsychiatric disorder that results from a variety of genetic, psychosocial, and environmental factors, causing physical and mental diseases such as anxiety and depression (1). A global prevalence of alcohol dependence among 2.4 billion alcohol drinkers was up to 39.60%, which represents a considerable public health burden (2). Moreover, alcohol dependence shares a high co-morbidity with anxiety-related disorders (3, 4). Furthermore, those with comorbid anxiety and alcohol dependence typically have a poorer psychosocial functioning, physical health, and ultimately cause serious consequences including divorce, crime, self-harm, and suicide tendency, especially in the context of alcohol withdrawal (5, 6). Therefore, it is an urgent issue to investigate the association between alcohol dependence and anxiety in the context of alcohol withdrawal among problem drinkers.
However, the presence and extent of anxiety under the context of alcohol withdrawal, varies greatly among problem drinkers, which points out that these external stressors are neither a necessary nor a sufficient cause for psychopathology. A meta-analysis of twin studies demonstrated that the genetic influences could explain 0.32–0.43 of the variance in anxiety (7). Thus, genetic vulnerability that may influence the environmental contributors on anxiety has attracted more attention. A previous study has demonstrated that participants with FKBP5 polymorphism were more likely to exhibit anxiety when exposed to childhood trauma (8). Moreover, another study had found that SLC1A1, GSTZ1, and CALCRL gene polymorphisms, in association with harsh punitive parenting, may contribute to social anxiety in adolescence (9). Furthermore, existing G × E research has largely focused on the modulator role of gene in negative emotions caused by early stressful experiences (i.e., childhood abuse, ignoring and maltreatment) (10–13), whereas few studies have examined the interaction of gene and current stressful experiences (acute alcohol withdrawal). These findings highlight that the interaction between genetic vulnerability and adverse environmental factors (G × E) is increasingly emphasized as an important mechanism in understanding the link between alcohol dependence and anxiety.
Proopiomelanocortin (POMC), a gene that located in the arcuate nucleus, responds to metabolic stress, such as food deprivation and glucoprivation (14, 15), and psychological stress (16), which appears to be a strong candidate for this interaction. The POMC processes many functionally different peptides, and among these biologically active peptides, ACTH and β-endorphin (β-END) are two principal components of the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis is known as the major brain circuitry that regulates the neuroendocrine response to stress (17). β-END is a member of opioid peptides that are widely and differentially distributed throughout the nervous system, which has been implicated in a variety of behaviors including the regulation of pain and reward, as well as processes associated with stress, fear, or anxiety (18). In the central nervous system, β-END contributes to the positive reinforcement and motivational properties of drugs of abuse. In addition, there is an evidence that lowered plasma β-END during alcohol withdrawal may contribute to their experienced anxiety (19, 20). Moreover, it is reported that the POMC gene expression is associated with anxiety-like behavior in those that experienced maternal deprivation (21). Another study found that POMC gene polymorphisms related to alcohol dependence (22). In addition, Chang et al. (23) investigated the role of gene–environment interaction between POMC rs2071345 polymorphism and stressful life events and found that POMC rs2071345 polymorphism, via an interaction with stressful life events, are associated with antidepressant treatment outcomes in major depressive disorder patients.
To date, few studies have examined the exact form of the interaction between the environment and POMC gene polymorphisms. There are two models can explain the potential role of genetic factors in G × E interactions: the diathesis-stress model and the differential susceptibility model. In the diathesis-stress model, carriers of ‘risk’ genotype variants when exposed to adverse environmental experiences would be more likely to develop the negative outcome (24, 25). While the differential susceptibility model suggests that ‘risk’ genotypes would be better considered ‘plasticity' or ‘susceptibility’ genotypes, and that carriers would be susceptible to both adverse and enriched environments, for better and for worse (24, 26, 27).
Therefore, this study aimed to examine the moderating role of POMC rs2071345 polymorphism on the association of alcohol dependence and anxiety among problem drinkers, and further explored the nature of POMC rs2071345 × alcohol dependence by testing two competing models: diathesis-stress vs. differential susceptibility model.
Methods
Participants and Procedure
Participants were 440 male problem drinkers (18 years and above) recruited from Psychiatric Hospitals in northern China. All the male problem drinkers were hospitalized for alcohol dependence, meeting the criteria according to the DSM-IV. Moreover, general mental assessments including anxiety symptoms were carried out by the admitting physician before recruiting into hospital. Among the participants, no outstanding anxiety symptoms were initially found. All participants were of Chinese Han ethnicity. The mean age of the participants was 44.15 years (SD = 9.45, range = 20–67 years). Most of the participants (65.45%) had earned a junior high school education level, and the average time in schooling was 10.64 years (SD = 2.87, range = 5–17 years).
Exclusion criteria for participants included a history of other substance use disorders beyond nicotine, which was allowed, presence of serious liver or kidney disease, history of serious neuropsychic diseases illness, or lacking a clear understanding of informed consent.
All study procedures were approved by the Ethics Committee of Peking University Health Science Center. First, participants were provided with a detailed description of the study procedures by the trained research investigators. Second, written informed consent from participants was obtained prior to data collection (98.89% agreed to participate in our study). Then, participants were asked to complete a series of questionnaires in a quiet ward. Finally, participants provided a blood sample for DNA extraction, checked one by one on location by research investigators. Genomic DNA was extracted from peripheral blood.
Measures
Assessment of Alcohol Dependence
A modified Chinese version of the Michigan Alcoholism Screening Test (MAST) (28) was used to measure the severity of symptoms associated with disordered alcohol use. Each of 24 items on the MAST is rated on a 4-point scale ranging from “not at all” (value = 0) to “extremely” (value = 4). The sum of the response scores can range from 0 to 96. Higher scores indicate more severe alcohol dependence. The Cronbach's alpha for the whole scale was 0.90 (29).
Assessment of Anxiety
The 20-item Self-Rating Anxiety Scale (SAS) (30) was used to assess the severity of depression anxiety. In this scale, participants are asked to respond how often he has experienced each symptom on a 4-point scale ranging from 1 (none or a little of the time) to 4 (most or all the time). The total sum of all the items was used in the analyses; higher scores indicate greater severity of anxiety. The SAS has high internal-consistency reliability, with alpha values of 0.82 (31).
Genotyping
Genomic DNA was extracted from 5 ml of peripheral blood of each participant using the salting-out method. The POMC rs2071345 were conducted using the Taqman SNP genotyping assay (ABI: Applied Biosystems Inc., Foster City, CA, USA). The primers and probes of SNPs were analyzed from ABI assay on demand kit. Reactions were carried out according to the manufacturer's protocol. All laboratory procedures were carried out in a manner blind to case-control status. The conditions of PCR were as follows: 50°C for 2 min, 95°C for 10 min, followed by 50 cycles of 95°C for 15 s and 60°C for 1 min. Ten percent of the DNA samples were duplicated randomly and tested, and no-fault genotyping was found.
Statistical Analysis
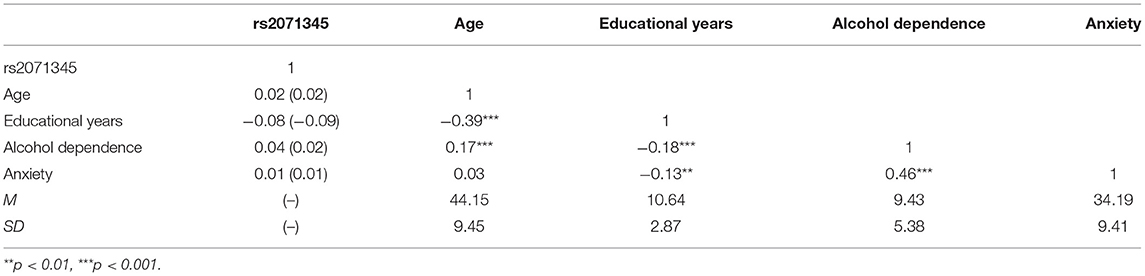
Firstly, we tested the genotype distributions of POMC rs2071345 genotyping for Hardy-Weinberg equilibrium (HWE) proportions by using the χ2 test (32) and Pearson correlation analyses were conducted to examine correlations between POMC rs2071345, age, educational years, alcohol dependence and anxiety. Consistent with other research, CT and TT genotypes was collapsed into T-allele group and coded as 1, CC genotype was coded as 0.
Secondly, we conducted the traditional linear regression to examine the interactive effect between the POMC rs2071345 polymorphism and alcohol dependence on male problem drinkers' anxiety. When significant interactions were found, post-hoc probing of significant interactions is conducted using regions of significance (RoS) analysis (33). RoS analysis provides the lower and higher bound where the association between POMC rs2071345 and alcohol dependence is significant for estimating the forms of G × E interaction. Thirdly, re-parameterized regression model, a newly developed approach proposed by Widaman et al. (34), was conducted to examine the nature of G × E interaction. The models were as follows:
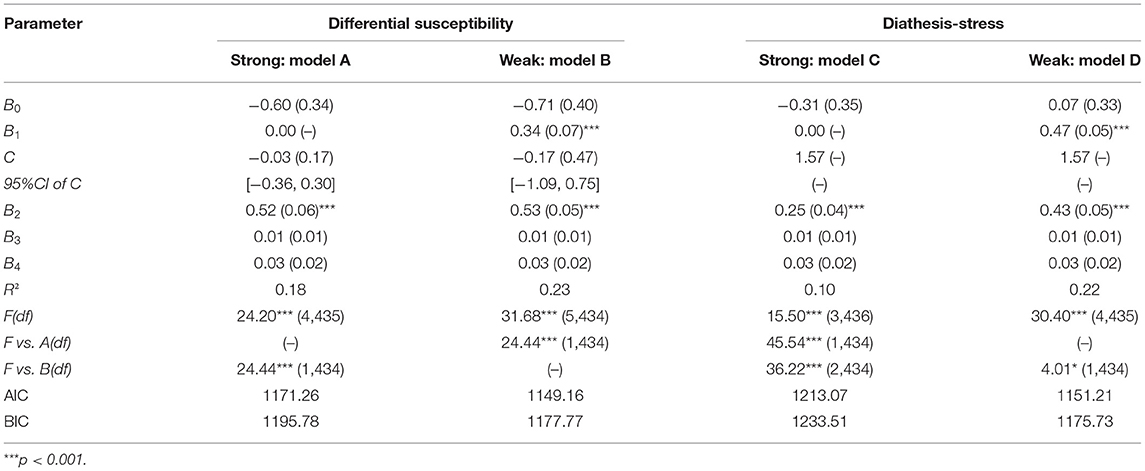
Here Y is the dependent variable of anxiety, X represents alcohol dependence, X2 and X3 are controlled variables: age and educational years, group is the different allelic group; C is the crossover point where the slopes of two genotype groups cross. The crossover point C estimate and confidence interval estimate can be determined whether the interaction between the POMC rs2071345 polymorphism and alcohol dependence is consistent with the differential susceptibility model or the diathesis-stress model. If the point estimation and 95% confidential interval of C fall at the maximum value of alcohol dependence, the interaction is consistent with diathesis stress model. In contrast, if the estimate of C is within the range of alcohol dependence, the form of interaction is consistent with differential susceptibility model. As diathesis-stress model and differential susceptibility model can be further subdivided into “strong” and “weak” version. Strong versions assume that only individuals with “risk/plasticity allele” are affected by environment, while the weak versions assume that both allele carriers are affected by environment but “non-risk/non-plasticity allele” carriers are less affected by environment than “risk/plasticity allele” carriers (35). These models are nested within each other. Thus, we used an F test to examine whether one model explained significantly more variance than another one. In addition, for non-nested models, Akaike information criterion (AIC) and Bayesian information criterion (BIC) was used to evaluate which model fits better. Lower scores indicated better fitting.
Results
Descriptive Statistics
Of the 440 male problem drinkers, 202 (45.91 %) were CC homozygotes, 186 (42.27%) were CT heterozygotes, and 52 (11.82 %) were TT homozygotes. Genotype distribution for POMC rs2071345 was consistent with Hardy–Weinberg equilibrium (χ2= 0.83, p > 0.05). We conducted a series of t-tests to examine whether male problem drinkers differed by genotype between alcohol dependence and anxiety symptoms. Results indicated that no significant differences were found (alcohol dependence: t = 0.85; anxiety: t = 0.14, p(s) > 0.05).
The descriptive statistics of research variables are shown in Table 1. Anxiety (r = 0.46, p < 0.01) was positively correlated with alcohol dependence, while the education year (r = −0.18, p < 0.01) was negatively correlated with alcohol dependence. Besides, there were no significant relationships between the polymorphism POMC rs2071345 and all the other variables.
Interactions Between POMC rs2071345 Genotype, Alcohol Dependence, and Anxiety
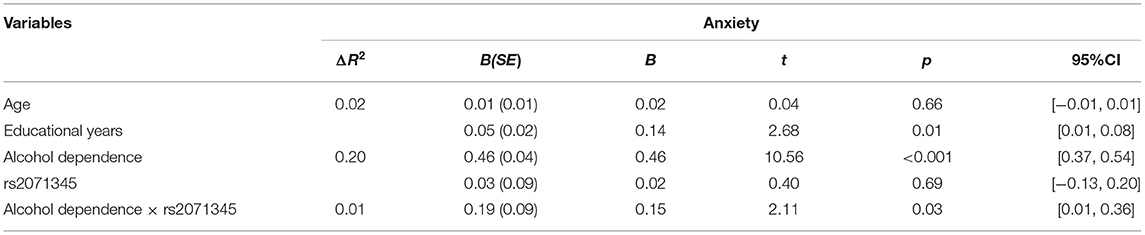
We conducted traditional hierarchical regression analysis to identify the interaction between the POMC rs2071345 genotype and alcohol dependence on anxiety. There was a main effect of alcohol dependence on anxiety (p < 0.001), such that more alcohol dependence was associated with higher levels of anxiety. There were no significant main effects of POMC rs2071345 genotype on anxiety (p > 0.05). The interaction between the POMC rs2071345 genotype and alcohol dependence was significant (p = 0.03).
Furthermore, the RoS test was conducted to interpret the interaction effect. The slopes for alcohol-dependence on anxiety were as follows: T allele carriers, β = 0.61, t = 13.01, p < 0.001; CC homozygote carriers, β = 0.46, t = 14.55, p < 0.001 (Table 2). The lower and upper bounds of regions of significance were −0.59 and 0.31. That is, subjects with T allele were more likely to experience more anxiety symptoms than subjects with CC homozygote when the standardized score of concurrent alcohol dependence was above 0.31 (see Figure 1).
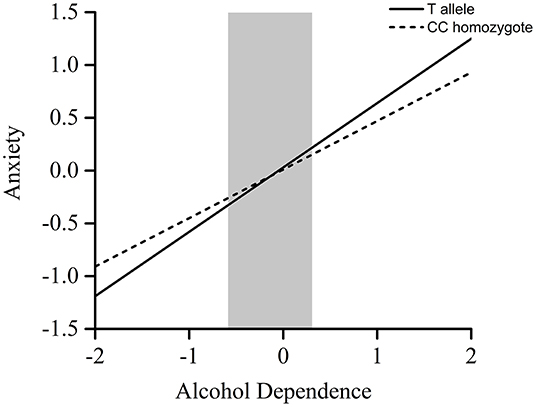
Figure 1. RoS test on anxiety from alcohol dependence in POMC rs2071345 allelic groups. Gray shaded area represents 95% CI of the crossover point C of the interaction on the alcohol dependence axis. Ninety-five percent CI of C ranged from −0.59 to 0.31.
Re-parameterized Regression Analysis
In order to test the specific form of G × E, re-parameterized regression analysis was conducted by using the regression models adapted from Belsky et al. (36). Results involving rs2071345 × environment (severity of alcohol dependence) interaction (see Table 3) showed that the weak differential susceptibility model (model B) had strong fit to data (R2 = 0.23, p < 0.001), in which the slopes for severity of alcohol dependence in CC homozygote group (B1 = 0.34, SE = 0.07, p < 0.001) and T allele group (B2=0.53, SE = 0.05, p < 0.001) were significant. The estimated point and 95% CI of crossover point C both fell within the range of alcohol dependence C = −0.17 (SE = 0.47), 95%CI = [−1.09, 0.75]. Furthermore, the weak differential susceptibility model could explain more variance (ΔR2 = 0.05, p < 0.001) by adding one more parameter than the strong differential susceptibility model, explaining more variance (ΔR2 = 0.13, p < 0.001) by adding two more parameters than the strong diathesis-stress model, explaining more variance (ΔR2 = 0.01, p < 0.05) by adding one more parameter than the weak diathesis-stress model, which demonstrated that CC homozygote were non-plasticity homozygote and T allele was plasticity allele in anxiety.
Discussion
We examined the interactions between POMC rs2071345 polymorphism with alcohol dependence on anxiety symptoms during acute alcohol withdrawal, and further explored the nature of POMC rs2071345 × alcohol dependence by testing two competing models: diathesis-stress vs. differential susceptibility.
First, as expected, significant concurrent associations were found between alcohol dependence severity and anxiety symptoms during acute alcohol withdrawal, and further analysis revealed that the severity of alcohol dependence increased the risk of anxiety symptoms. It is in alignment with previous studies (37–40). Further, we found that the POMC rs2071345 is unexpectedly associated with the severity of anxiety symptoms during acute alcohol withdrawal, which previously has not been reported. Considering the location of the variant in the genomic structure of the POMC gene, rs2071345 may be involved in the regulation of transcription factor binding, which would need to be confirmed by further molecular biological experiments.
Next, in the anxiety model, POMC rs2071345 polymorphism significantly moderates the association between severity of alcohol dependence and anxiety symptoms during acute alcohol withdrawal, confirming the hypothesis we proposed and supporting the diathesis-stress theory. Furthermore, all the indexes in the re-parameterized regressions indicated that the POMC rs2071345 polymorphism × environment (alcohol problem severity) interactions were consistent with the weak diathesis-stress model among male problem drinkers with anxiety symptoms. Specifically, compared to adults with CC homozygote of POMC rs2071345, those with the T allele reported more anxiety symptoms when experiencing more severe alcohol withdrawal as measured by alcohol problem severity. That is, T allele of POMC rs2071345 may be a genetic risk gene, affecting the stability of transcribed mRNA which is one of the main mechanisms of adaptation to stress (41). The findings suggest that CC homozygote of POMC rs2071345 may buffer the effects of alcohol dependence, such that carriers of the CC homozygote of POMC rs2071345 may be better equipped to handle problematic situations and challenges that arise from a higher level of alcohol dependence or other stressors. Alternatively, carriers of the CC homozygote of POMC rs2071345 may not need to rely on lower level of alcohol dependence or be as sensitive to lower level of alcohol dependence as those with T allele of POMC rs2071345, suggesting higher level of alcohol dependence may not confer the same level of risk among carriers of the CC homozygote of POMC rs2071345. These findings together suggest that the stress from different sources may interact with different vulnerability genes, even belonging to the same functional group. As such, our study provides new evidence for the moderating function of the POMC polymorphism in the association between current stress as measured by the severity of alcohol dependence during withdrawal and anxiety symptoms.
The current study contributes to the existing literature by providing valuable information about the underlying etiology of alcohol dependence and anxiety during acute alcohol withdrawal and has several notable strengths. First, to our knowledge, this study is the first to examine the G × E interactions on this POMC polymorphism, alcohol dependence severity and anxiety during acute alcohol withdrawal, providing preliminary evidence for the distinct G × E interactions on alcohol dependence and anxiety. Further, with the newly developed approach of regions of significance (RoS) analysis, the present study explored whether the G × E interactions would be consistent with the diathesis-stress model or the differential susceptibility model and determined the range of values of the environment where the environment-predicting-outcome regression lines significantly differ from each other (42). Finally, by focusing on the re-parameterized regression analysis, the present study is likely to maximize the statistical power by aligning analyses with hypotheses of interest and can directly compare and evaluate different G × E hypotheses (36).
There are several limitations in the present study. First, only males were investigated. Previous work has demonstrated differences between men and women in regard of OXTR polymorphisms (43–45), which highlights the importance of further studies of sex differences concerning differential diathesis. Second, our data on the associations between genes, alcohol dependence severity, and anxiety were cross-sectional, which did not allow for cross-lagged relationships between alcohol dependence severity and anxiety across different genotypes to be tested. Therefore, future research with longitudinal design will be needed to explore the G × E interaction across different genotypes. Third, an additional uncontrolled factor is the possibility that various withdrawal symptoms may contribute to anxiety, which could be explored in further research. Fourth, the current study only estimated the interactions between the POMC rs2071345 polymorphism with alcohol problem severity on anxiety symptoms, which is another limitation. Previous work demonstrated that β-END differentially affected anxiety and depression (46), highlighting the importance of further studies of the interactions between depression, POMC rs2071345 polymorphisms, and alcohol dependence.
Conclusion
The present study provides preliminary evidence for distinct G × E interactions such that the POMC rs2071345 polymorphism interacted with alcohol dependence on male problem drinkers' anxiety during acute alcohol withdraw. These findings contribute to a more comprehensive view of the complex genetic etiology of problem drinkers' negative emotions during alcohol withdrawal.
With regard to the nature of G × E interactions on anxiety observed in the present study, our findings were in accordance with the diathesis-stress hypothesis. These empirical findings have important implications for interpreting genetic moderation of alcohol problem severity on individual differences of adults' negative emotion during alcohol withdrawal. The findings might also encourage more work at the molecular level on the role of the underlying mechanisms in response to environment and in modulating anxiety, especially in relation to functional studies of neural systems.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Peking University Health Science Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LC, FW, and Y-HC designed the study. LH, LW, YL, and FW contributed to data acquisition. LH, LW, MN, and WW drafted the manuscript. LH, LW, MN, FZ, YZ, and GS participated in data analysis and interpretation. All authors read and approved the final manuscript.
Funding
This study was supported by the Technology Support Project of Xinjiang (2017E0267, FW), Natural Science Foundation of Xinjiang Uyghur Autonomous Region (2018D01C228, FW), Tianshan Youth Project–Outstanding Youth Science and Technology Talents of Xinjiang (2017Q007, FW), Beijing Natural Science Foundation (7152074, FW), and the Opening Project of Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences (YL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Durazzo TC, Meyerhoff DJ. Psychiatric, demographic, and brain morphological predictors of relapse after treatment for an alcohol use disorder. Alcohol Clin Exp Res. (2017) 41:107–16. doi: 10.1111/acer.13267
2. Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2018) 392:1015–35. doi: 10.1016/S0140-6736(18)31310-2
3. Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. (2015) 72:757–66. doi: 10.1001/jamapsychiatry.2015.0584
4. Walker LC. A balancing act: the role of pro-and anti-stress peptides within the central amygdala in anxiety and alcohol use disorders. J Neurochem. (2021) 157:1615–43. doi: 10.1111/jnc.15301
5. King M, Semlyen J, Tai SS, Killaspy H, Osborn D, Popelyuk D, et al. A systematic review of mental disorder, suicide, and deliberate self harm in lesbian, gay and bisexual people. BMC Psychiatry. (2008) 8:70. doi: 10.1186/1471-244X-8-70
6. Singhal A, Ross J, Seminog O, Hawton K, Goldacre MJ. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. (2014) 107:194–204. doi: 10.1177/0141076814522033
7. Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. (2001) 158:1568–78. doi: 10.1176/appi.ajp.158.10.1568
8. de Castro-Catala M, Peña E, Kwapil TR, Papiol S, Sheinbaum T, Cristóbal-Narváez P, et al. Interaction between FKBP5 gene and childhood trauma on psychosis, depression and anxiety symptoms in a non-clinical sample. Psychoneuroendocrinology. (2017) 85:200–9. doi: 10.1016/j.psyneuen.2017.08.024
9. Chubar V, Van Leeuwen K, Bijttebier P, Van Assche E, Bosmans G, Van den Noortgate W, et al. Gene–environment interaction: new insights into perceived parenting and social anxiety among adolescents. Eur Psychiatry. (2020) 63:e64. doi: 10.1192/j.eurpsy.2020.62
10. Malhi GS, Das P, Outhred T, Dobson-Stone C, Irwin L, Gessler D, et al. Effect of stress gene-by-environment interactions on hippocampal volumes and cortisol secretion in adolescent girls. Aust N Z J Psychiatry. (2019) 53:316–25. doi: 10.1177/0004867419827649
11. Rehan W, Antfolk J, Johansson A, Aminoff M, Sandnabba NK, Westberg L, et al. Gene-environment correlation between the dopamine transporter gene (DAT1) polymorphism and childhood experiences of abuse. J Interpers Violence. (2018) 33:2059–72. doi: 10.1177/0886260515622299
12. Wall TL, Luczak SE, Hiller-Sturmhöfel S. Biology, genetics, and environment: underlying factors influencing alcohol metabolism. Alcohol Res. (2016) 38:59–68.
13. Zhang Y, Ming QS, Yi JY, Wang X, Chai QL, Yao SQ. Gene-gene-environment interactions of serotonin transporter, monoamine oxidase a and childhood maltreatment predict aggressive behavior in Chinese adolescents. Front Behav Neurosci. (2017) 11:17. doi: 10.3389/fnbeh.2017.00017
14. Fraley GS, Ritter S. Immunolesion of norepinephrine and epinephrine afferents to medial hypothalamus alters basal and 2-deoxy-D-glucose-induced neuropeptide Y and agouti gene-related protein messenger ribonucleic acid expression in the arcuate nucleus. Endocrinology. (2003) 144:75–83. doi: 10.1210/en.2002-220659
15. Wagner CG, McMahon CD, Marks DL, Daniel JA, Steele B, Sartin JL. A role for agouti-related protein in appetite regulation in a species with continuous nutrient delivery. Neuroendocrinology. (2004) 80:210–8. doi: 10.1159/000082735
16. Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. (2007) 148:5531–40. doi: 10.1210/en.2007-0745
17. Femenia T, Perez-Rial S, Uriguen L, Manzanares J. Prodynorphin gene deletion increased anxiety-like behaviours, impaired the anxiolytic effect of bromazepam and altered GABAA receptor subunits gene expression in the amygdala. J Psychopharmacol. (2011) 25:87–96. doi: 10.1177/0269881110367724
18. Grisel JE, Bartels JL, Allen SA, Turgeon VL. Influence of β-endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology. (2008) 200:105–15. doi: 10.1007/s00213-008-1161-4
19. Kiefer F, Jahn H, Schick M, Wiedemann K. Alcohol intake, tumour necrosis factor-α, leptin and craving: factors of a possibly vicious circle? Alcohol Alcohol. (2002) 37:401–4. doi: 10.1093/alcalc/37.4.401
20. Xu Y-Y, Ge J-F, Chen J, Liang J, Pang L-J, Gao W-F, et al. Evidence of a relationship between plasma leptin, not Nesfatin-1, and craving in male alcohol-dependent patients after abstinence. Front Endocrinol. (2020) 11:159. doi: 10.3389/fendo.2020.00159
21. de Lima RMS, Dos Santos Bento LV, di Marcello Valladao Lugon M, Barauna VG, Bittencourt AS, Dalmaz C, et al. Early life stress and the programming of eating behavior and anxiety: sex-specific relationships with serotonergic activity and hypothalamic neuropeptides. Behav Brain Res. (2020) 379:112399. doi: 10.1016/j.bbr.2019.112399
22. Zhang H, Kranzler HR, Weiss RD, Luo X, Brady KT, Anton RF, et al. Pro-opiomelanocortin gene variation related to alcohol or drug dependence: evidence and replications across family-and population-based studies. Biol Psychiatry. (2009) 66:128–36. doi: 10.1016/j.biopsych.2008.12.021
23. Chang HS, Won ES, Lee HY, Ham BJ, Kim YG, Lee MS. The association of proopiomelanocortin polymorphisms with the risk of major depressive disorder and the response to antidepressants via interactions with stressful life events. J Neural Transm. (2015) 122:59–68. doi: 10.1007/s00702-014-1333-9
24. Babineau V, Green CG, Jolicoeur-Martineau A, Bouvette-Turcot AA, Minde K, Sassi R, et al. Prenatal depression and 5-HTTLPR interact to predict dysregulation from 3 to 36 months–a differential susceptibility model. J Child Psychol Psychiatry. (2015) 56:21–9. doi: 10.1111/jcpp.12246
26. Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. (2009) 135:885–908. doi: 10.1037/a0017376
27. Van Batenburg-Eddes T, Brion MJ, Henrichs J, Jaddoe VW, Hofman A, Verhulst FC, et al. Parental depressive and anxiety symptoms during pregnancy and attention problems in children: a cross-cohort consistency study. J Child Psychol Psychiatry. (2013) 54:591–600. doi: 10.1111/jcpp.12023
28. Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. (1971) 127:1653–8. doi: 10.1176/ajp.127.12.1653
29. Skinner HA, Sheu W-J. Reliability of alcohol use indices. The lifetime drinking history and the MAST. J Stud Alcohol. (1982) 43:1157–70. doi: 10.15288/jsa.1982.43.1157
30. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12:371–9. doi: 10.1016/S0033-3182(71)71479-0
31. Tanaka-Matsumi J, Kameoka VA. Reliabilities and concurrent validities of popular self-report measures of depression, anxiety, and social desirability. J Consult Clin Psychol. (1986) 54:328–33. doi: 10.1037/0022-006X.54.3.328
32. Nielsen DM, Ehm MG, Weir BS. Detecting marker-disease association by testing for Hardy-Weinberg disequilibrium at a marker locus. Am J Hum Genet. (1998) 63:1531–40. doi: 10.1086/302114
33. Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis-stress: recommendations for evaluating interaction effects. Dev Psychopathol. (2012) 24:389–409. doi: 10.1017/S0954579412000065
34. Widaman KF, Helm JL, Castro-Schilo L, Pluess M, Stallings MC, Belsky J. Distinguishing ordinal and disordinal interactions. Psychol Methods. (2012) 17:615–22. doi: 10.1037/a0030003
35. Zhang X, Sun H, Wang F, Niculescu M, Shen G, Zhou S, et al. The interaction between genetic variant ZNF804A rs1344706 and alcohol withdrawal on impulsivity: evidence for the diathesis-stress model. Front Psychiatry. (2022) 12:761237. doi: 10.3389/fpsyt.2021.761237
36. Belsky J, Pluess M, Widaman KF. Confirmatory and competitive evaluation of alternative gene-environment interaction hypotheses. J Child Psychol Psychiatry. (2013) 54:1135–43. doi: 10.1111/jcpp.12075
37. Anker JJ, Kushner MG. Co-occurring alcohol use disorder and anxiety: bridging psychiatric, psychological, and neurobiological perspectives. Alcohol Res. (2019) 40:arcr.v40.1.03. doi: 10.35946/arcr.v40.1.03
38. Dos Santos DT, Nazário FP, Freitas RA, Henriques VM, de Paiva IS. Alcohol abuse and dependence among Brazilian medical students: association to sociodemographic variables, anxiety and depression. J Subst Use. (2019) 24:285–92. doi: 10.1080/14659891.2018.1562574
39. Lucas Mendes Oliveira Mariane Bagatin Bermudez. Comorbid social anxiety disorder in patients with alcohol use disorder: a systematic review. Journal of Psychiatric Research. (2018) 106:8–14. doi: 10.1016/j.jpsychires.2018.09.008
40. Serrano A, Pavon FJ, Buczynski MW, Schlosburg J, Natividad LA, Polis IY, et al. Deficient endocannabinoid signaling in the central amygdala contributes to alcohol dependence-related anxiety-like behavior and excessive alcohol intake. Neuropsychopharmacology. (2018) 43:1840–50. doi: 10.1038/s41386-018-0055-3
41. Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. (2001) 265:11–23. doi: 10.1016/S0378-1119(01)00350-X
42. Kochanska G, Kim S, Barry RA, Philibert RA. Children's genotypes interact with maternal responsive care in predicting children's competence: diathesis–stress or differential susceptibility? Development and psychopathology. (2011) 23:605–16. doi: 10.1017/S0954579411000071
43. Cicchetti D, Rogosch FA, Hecht KF, Crick NR, Hetzel S. Moderation of maltreatment effects on childhood borderline personality symptoms by gender and oxytocin receptor and FK506 binding protein 5 genes. Dev Psychopathol. (2014) 26:831–49. doi: 10.1017/S095457941400042X
44. Flasbeck V, Moser D, Kumsta R, Brune M. The OXTR single-nucleotide polymorphism rs53576 moderates the impact of childhood maltreatment on empathy for social pain in female participants: evidence for differential susceptibility. Front Psychiatry. (2018) 9:359. doi: 10.3389/fpsyt.2018.00359
45. Weisman O, Pelphrey KA, Leckman JF, Feldman R, Lu Y, Chong A, et al. The association between 2D: 4D ratio and cognitive empathy is contingent on a common polymorphism in the oxytocin receptor gene (OXTR rs53576). Psychoneuroendocrinology. (2015) 58:23–32. doi: 10.1016/j.psyneuen.2015.04.007
Keywords: alcohol dependence, POMC gene polymorphism, rs2071345, anxiety, diathesis-stress model
Citation: Hong L, Wen L, Niculescu M, Zhou F, Zou Y, Shen G, Wang W, Liu Y, Chen Y-H, Wang F and Chen L (2022) The Interaction Between POMC rs2071345 Polymorphism and Alcohol Dependence in Anxiety Symptoms Among Chinese Male Problem Drinkers. Front. Psychiatry 13:878960. doi: 10.3389/fpsyt.2022.878960
Received: 18 February 2022; Accepted: 08 April 2022;
Published: 03 May 2022.
Edited by:
Wenhao Jiang, Southeast University, ChinaReviewed by:
Xingguang Luo, Yale University, United StatesLi Xia, Jiangxi Science and Technology Normal University, China
Minglong Shao, Second Affiliated Hospital of Xinxiang Medical University, China
Copyright © 2022 Hong, Wen, Niculescu, Zhou, Zou, Shen, Wang, Liu, Chen, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Chen, cHN5Y2hvbG9neWNoZW5saUAxNjMuY29t; Fan Wang, RmFuV2FuZ0Biam11LmVkdS5jbg==; Yu-Hsin Chen, eXVoY2hlbkBrZWFuLmVkdQ==
†These authors have contributed equally to this work
 Liuzhi Hong
Liuzhi Hong Lutong Wen
Lutong Wen Michelle Niculescu
Michelle Niculescu Fan Zhou
Fan Zhou Yang Zou
Yang Zou Guanghui Shen
Guanghui Shen Wei Wang
Wei Wang Yanlong Liu
Yanlong Liu Yu-Hsin Chen
Yu-Hsin Chen Fan Wang
Fan Wang Li Chen
Li Chen