- 1Department of Neurology, Anting Hospital, Shanghai, China
- 2Department of Central Laboratory, Yancheng Third People’s Hospital, The Sixth Affiliated Hospital of Nantong University, Yancheng, China
- 3Department of Neurology, Yancheng Third People’s Hospital, The Sixth Affiliated Hospital of Nantong University, Yancheng, China
Mounting evidence suggests that social cognitive abilities [including theory of mind (ToM) and empathy] are impaired in adult patients with epilepsy. Although the deficits in overall ToM in epilepsy have been documented well, the effects of epilepsy on empathic ability and specific subcomponents of ToM remain unclear. The primary aim of this study was to provide the first meta-analytic integration of ToM and empathy in adult patients with epilepsy, and to decompose these constructs to clearly differentiate their distinct (cognitive ToM and affective empathy) and overlapping (affective ToM/cognitive empathy) components. This meta-analysis included 28 studies. Adult patients with temporal lobe epilepsy (TLE) and frontal lobe epilepsy (FLE) showed impairments in cognitive ToM and affective ToM/cognitive empathy compared to the healthy controls (HCs); no group differences were identified for affective empathy. Besides, cognitive ToM was impaired in adult patients with idiopathic generalized epilepsy (IGE) and focal seizures (caused by epileptogenic foci) outside the temporal and frontal lobes (extra-TLE/FLE) and no group differences were evident for affective ToM/cognitive empathy compared to the HCs. Moreover, relative to the HCs, no group differences were identified for affective empathy in adult patients with IGE. Additionally, no (statistically) significant difference was observed between the magnitude of ToM/empathy impairment in adult patients who underwent and those who did not undergo epilepsy surgery. These quantitative findings suggest differential impairment of the core aspects of social cognitive processing in adult patients with epilepsy, which may contribute to the development of structured cognitive interventions (i.e., social cognitive training) for adult patients with epilepsy.
Introduction
Epilepsy, one of the most common neurological disorders, affects over 50 million people worldwide (1). It is characterized by chronic, unprovoked, and recurrent seizures (2). Epilepsy is usually complicated by numerous neurobiological disorders, cognitive impairment, and psychosocial ramifications, which lead to a severe economic burden and deterioration in the quality of life (1–5).
Cognitive impairment, including memory impairment, language dysfunction, attention deficit, executive dysfunction, and social cognitive impairment, is considered to be a common symptom of epilepsy (6–14). According to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (15), social cognition is a core neurocognitive domain, which is defined as the ability to explain and predict the behavior of others based on their beliefs, feelings and intentions, and interact in complex social environments and relationships (16–20). Social cognition is a multidimensional construct that mainly involves theory of mind (ToM), empathy, social perception and knowledge, and attribution bias (21–23).
ToM is, in turn, a core domain of social cognition, which denotes the ability to understand and act according to the mental states (beliefs, intentions, and desires) of other humans (24–26). ToM is a complex ability that encompasses multiple components, mainly the cognitive and affective domains (27). Cognitive ToM refers to the ability to derive inferences about the thoughts, intentions, beliefs, and motivations of others, while affective ToM is the ability to infer others’ feelings, affective states, and emotions (28, 29).
Empathy, another core aspect of social cognition, refers to the ability to understand and feel another’s emotions and to respond appropriately and with compassion (30–35). Empathy, akin to ToM, is also a complex and multifaceted phenomenon, which mainly comprises the cognitive and affective dimensions (36). Cognitive empathy is the ability to understand the thoughts and emotions of others, while affective empathy confers the ability to feel and share the emotions of another the emotional state of others (37–41).
Notably, although conceptually there are differences between affective ToM and cognitive empathy, these two constructs are difficult to distinguish at the level of purely behavioral assessment (42–50). Furthermore, the overlap between affective ToM and cognitive empathy is frequently noted (42–44, 46, 50). Specifically, they both involve attributions to emotional state of others. Therefore, we use the terms affective ToM and cognitive empathy interchangeably herein.
Although numerous recent studies have assessed ToM and empathy deficits in adult patients with epilepsy, their conclusions have been inconsistent (51–55), which may be attributed to low statistical power, since a majority of these studies enrolled small patient populations (56–59). A quantitative meta-analysis may improve the statistical power, estimate the severity of these deficits, and refine the conclusions drawn from the inconsistent findings of previous studies (20).
To the best of our knowledge, no meta-analysis has investigated empathy deficits in adult patients with epilepsy. Although two meta-analyses examined the differences in ToM between patients with epilepsy and HCs (11, 13), no previous meta-analysis has investigated the differences between cognitive ToM and affective ToM in adult patients with epilepsy. Moreover, the two above-mentioned meta-analyses only included studies that investigated five specific ToM tasks (faux-pas task, false belief tasks, reading the mind in the eyes task, strange stories task, and cartoon ToM task), and some other important ToM tasks were not included (such as the Yoni task and the movie for the assessment of social cognition). Additionally, both previous meta-analysis included patients from different age groups. Furthermore, Bora and Meletti (11) investigated ToM deficits in temporal lobe epilepsy (TLE). Although Stewart et al. (13) investigated ToM impairment in different types of epilepsy [TLE, frontal lobe epilepsy (FLE), idiopathic generalized epilepsy (IGE), and focal seizures outside the temporal and frontal lobes (extra-TLE/FLE)], they included only two studies to investigate ToM impairment in patients with IGE and extra-TLE/FLE, and three studies to investigate ToM impairment in patients with FLE, owing to limitations of the other available studies.
Therefore, the primary aim of this study was to provide the first meta-analytic integration of ToM and empathy in adult patients with epilepsy and investigate the cognitive and affective subcomponents of these two entities. Specific subgroup analyses were conducted to evince a clear differentiation between the separate components (cognitive ToM and affective empathy) and overlapping components (affective ToM/cognitive empathy). Furthermore, subgroup analyses were performed to assess whether the deficits in ToM and empathy were related to the site of the epileptogenic focus (including TLE, FLE, IGE, and extra-TLE/FLE), considering that epileptic seizures are categorized by seizure onset into focal, generalized, combined generalized, and focal, and unknown (1). Moreover, subgroup analyses were conducted in adult patients with TLE who underwent and those who did not undergo epilepsy surgery [pre-surgical studies (TLE-TL-) and post-surgical studies (TLE-TL +)], to investigate whether temporal lobectomy is related to ToM and empathy deficits in adult patients with TLE. Furthermore, we evaluated the effect of potential variables such as mean age, sex (ratio of female patients in the epilepsy group), education level, age of epilepsy onset, disease duration, monthly seizure frequency, number of anti-epileptic drugs (AEDs) administered, and intelligence quotient (IQ) scores on social cognition. We hope that this meta-analysis will provide a more comprehensive and nuanced understanding of the effect of epilepsy on ToM and empathy in adults with epilepsy.
Methods
Study Registration
This meta-analysis protocol was registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (ID: INPLASY 2021120039).
Literature Search Strategy and Data Sources
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (60). Databases including Web of Science, PubMed, and Embase were searched on November 20, 2021 using the following search terms: [“epileps*” or “seizure disorder”] AND [“social cognition” or “theory of mind” or “ToM” or “mentalising” or “mentalizing” or “empath*” or “perspective taking”]. Furthermore, other resources, such as the reference lists of all included studies, were searched to identify studies that were not indexed in these databases.
Inclusion Criteria
The inclusion criteria for this review were as follows: (1) studies published in English in peer-reviewed journals, (2) studies that used measures to assess at least one domain of ToM or empathy performance, (3) studies comparing ToM or empathy performance between adult patients with epilepsy and HCs, and (4) studies that reported adequate data to calculate the effect sizes of ToM or empathy.
Exclusion Criteria
Studies were excluded for the following reasons: (1) absence of comparisons of ToM or empathy between patients with epilepsy and HCs, (2) the study sample overlapped with another study with a larger sample size, (3) studies that grouped patients with different sites of epileptogenic foci together, and (4) studies whose a sample size was less than 10 (26).
Study Quality Assessment
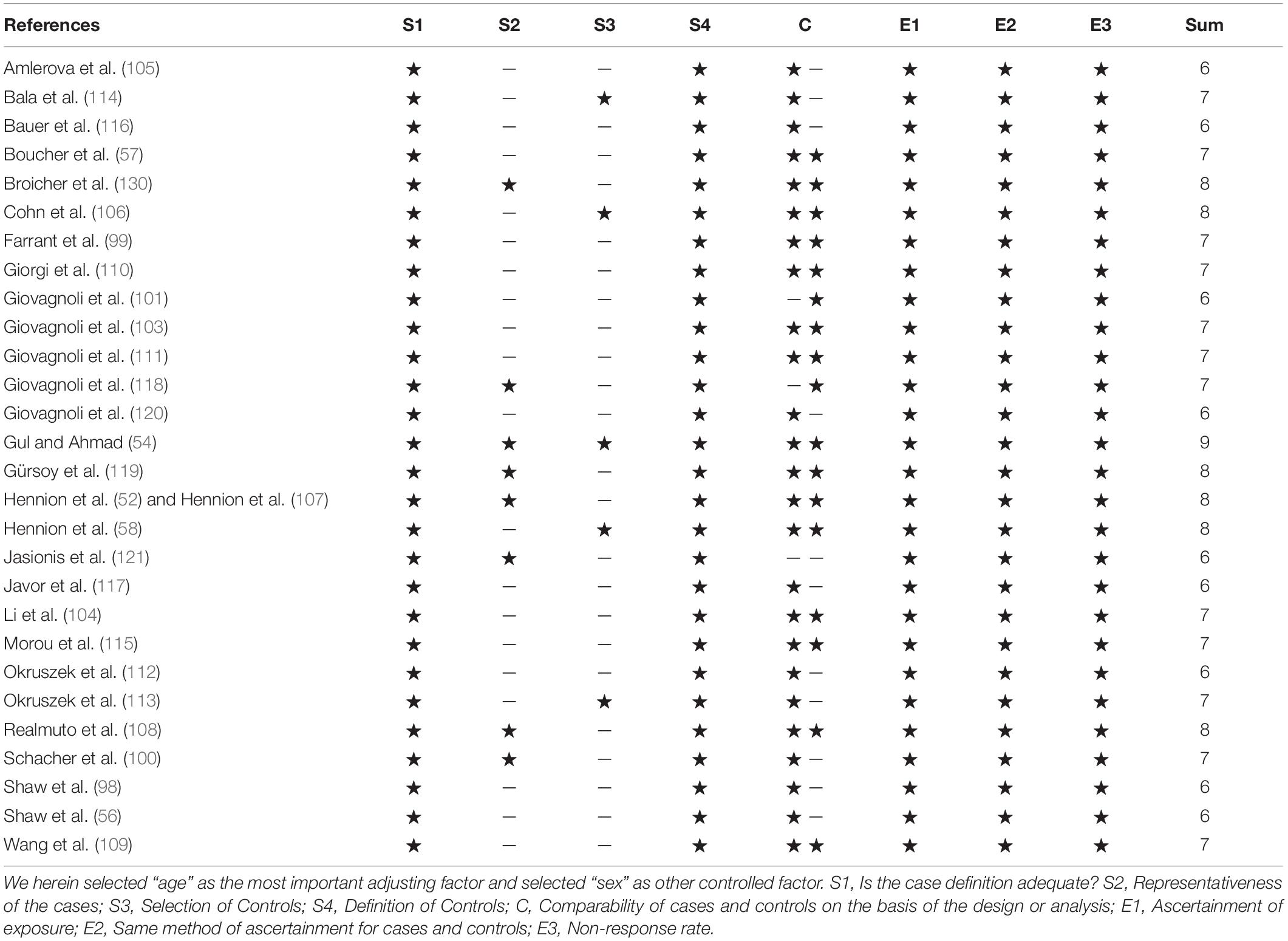
A nine-star protocol was used to assess study quality, based on the Newcastle-Ottawa Scale. The study was considered to be of high quality if the star rating was ≥ 7 (61).
Screening and Data Extraction
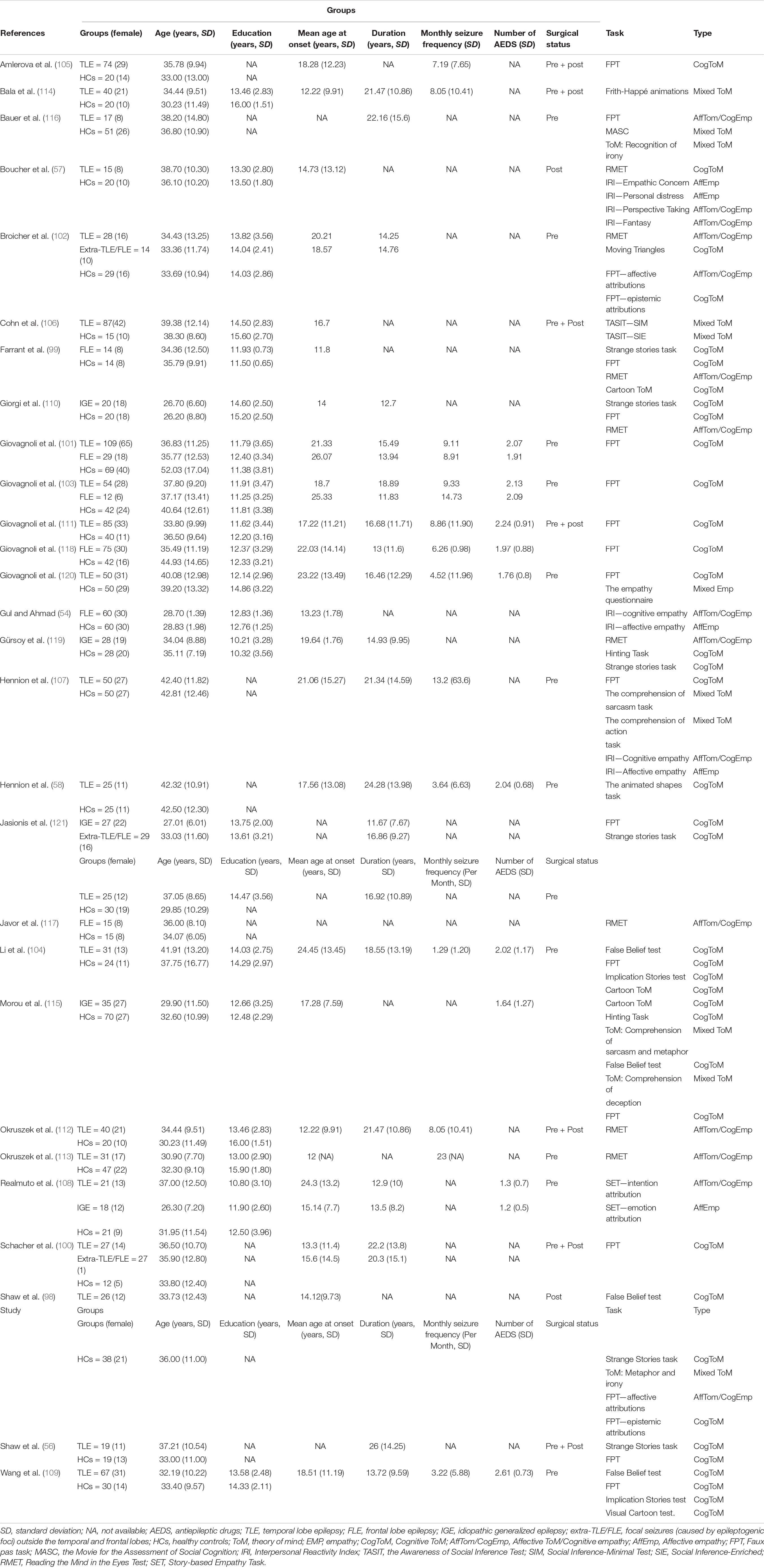
Two investigators independently completed article retrieval, screening, data extraction, and quality evaluation. The following data were extracted: (a) title information, such as the name of the first author, year of publication, and title; (b) sample characteristics, such as the number of participants in the epilepsy and control groups, mean age, sex (female and male patients), epilepsy type, monthly seizure frequency, whether surgery was performed or not, number of AEDs, education level, disease duration, and IQ score; (c) the tasks were divided into the cognitive and affective subcomponents for both ToM and empathy; and (d) the data were used to calculate the mean effect sizes of ToM or empathy.
Statistical Analysis
Data were analyzed using Stata 15.0 with a random-effects model (62). Hedges g and 95% confidence intervals (CIs) were calculated to estimate differences in ToM and empathy between adult patients with epilepsy and HCs (63). The interpretation of Hedges g was similar to that of Cohen d: 0.2 indicated a small effect, 0.5 indicated a medium effect, and 0.8 indicated a large effect (64). Negative effect sizes indicated poorer performance of the adult patients with epilepsy compared to the HCs.
When studies did not provide a total mean score on a particular measure (i.e., overall ToM, overall empathy, cognitive ToM, affective empathy, and affective ToM/cognitive empathy), but reported more than one ToM or empathy task, a pooled effect size was aggregated by computing the mean effect size (and standard error) (65). The heterogeneity of the mean weighted effect sizes across analyses was tested using the I2 test, and the degree of heterogeneity was deemed low, moderate, or large when the value of I2 was equal to or larger than 0, 50, or 75%, respectively (66). Publication bias was assessed using funnel plots and Egger’s test (67). If publication bias was found, the trim-and-fill method was applied to obtain effect sizes adjusted for publication bias (68).
Meta-regression analyses were conducted for age, sex, education level, age at epilepsy onset, disease duration, monthly seizure frequency, number of AEDs, and IQ scores. A minimum of 10 data points was required for each relevant predictor variable and the social cognitive ability under assessment for each of these analyses (69).
Notably, since we used the terms affective ToM and cognitive empathy interchangeably in this paper, we defaulted that the data of affective ToM/cognitive empathy is the same as the data of affective ToM or cognitive empathy.
Results
Study Characteristics
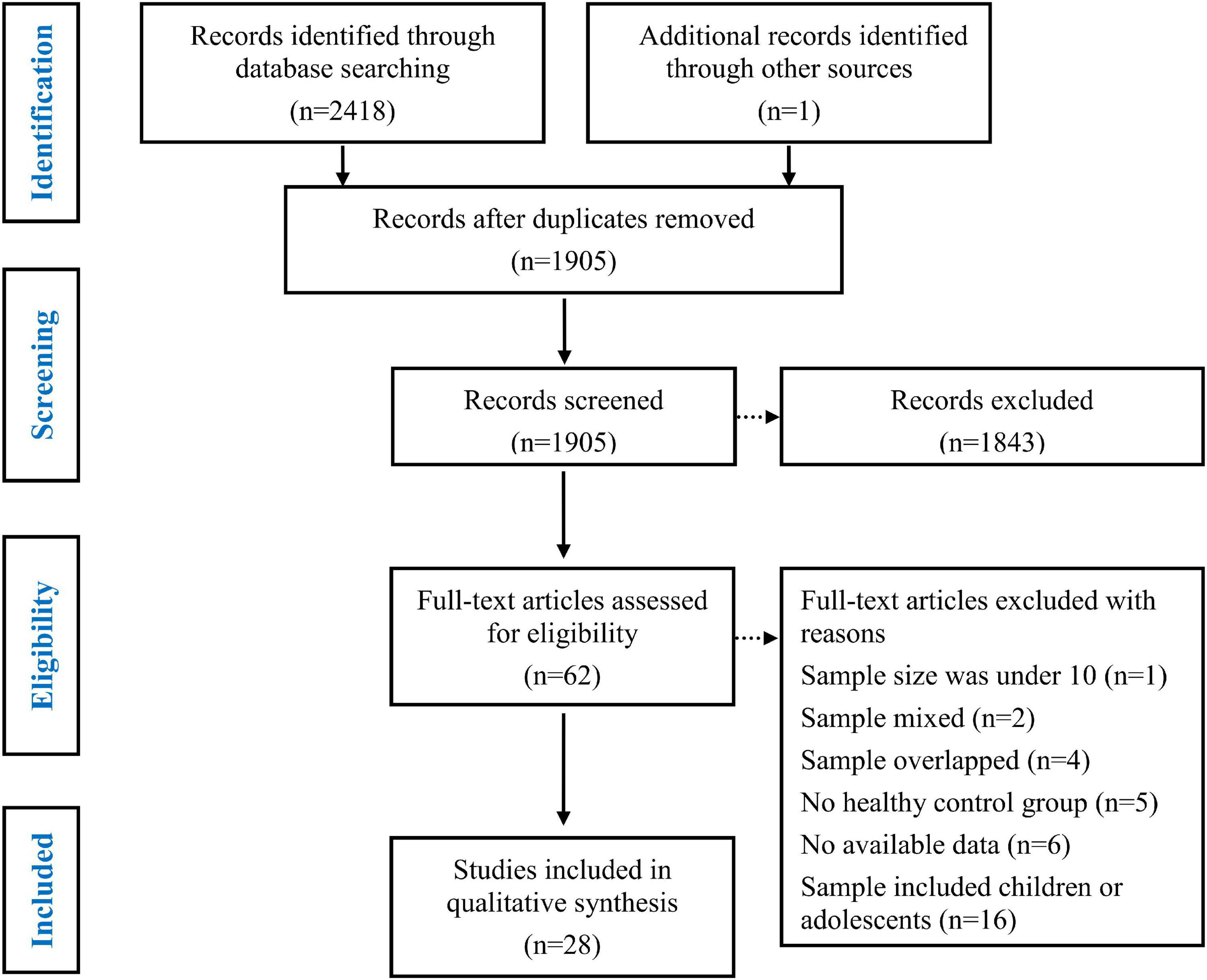
The details of the study selection process are depicted in Figure 1. Initially, 2418 articles were retrieved from three databases (Web of Science, PubMed, and Embase) and one article was retrieved from other sources. After eliminating duplicate studies, 1905 articles remained, which were then subjected to title and abstract screening. Subsequently, 62 full text papers were reviewed. Thirty-four of the 62 studies were excluded for the following reasons: the sample size was under 10 (K = 1) (70), the sample was mixed and included patients with epilepsy and other diseases (K = 1) (71); patients with different sites of the epileptogenic focus were grouped together (K = 1) (25); the samples overlapped with those of other studies (K = 4) (52, 72–74); the study did not include an HC group (K = 5) (75–79); data were insufficient to calculate the effect sizes and standard errors of ToM or empathy (K = 6) (80–85); and the study population included children or adolescents (K = 16) (51, 53, 55, 59, 86–97). Eventually, 28 studies were included in the meta-analysis (Table 1) (54, 56–58, 98–121). The studies included 902 adult patients with TLE (21 studies), 205 adult patients with FLE (6 studies), 128 adult patients with IGE (5 studies), and 70 adult patients with extra-TLE/FLE (3 studies).
The results of the assessment of study quality are presented in Table 2, and the mean score was 6.86 (SD = 0.79). Nineteen of the 28 case-control studies were awarded ≥ 7 stars and considered to be of high quality.
Theory of Mind and Empathy in Adult Patients With Temporal Lobe Epilepsy vs. Healthy Controls
The key results from this meta-analysis are presented in Table 3. Adult patients with TLE exhibited significant impairments in overall ToM [g = −0.91, 95% CI (−1.05, −0.77), K = 21; Figure 2], and moderate impairment in overall empathy [g = −0.71, 95% CI (−0.89, −0.52), K = 11; Figure 2] compared to HCs. The analysis of the overlapping and distinct subcomponents of these constructs revealed an association between adult patients with TLE with significant and severe deficits in cognitive ToM [g = −0.91, 95% CI (−1.10, −0.72), K = 15; Figure 3] and moderate deficits in affective ToM/cognitive empathy [g = −0.76, 95% CI (−0.88, −0.63), K = 11; Figure 3]. However, no group differences were evident for affective empathy [g = −0.16, 95% CI (−0.49, 0.17), K = 3; refer to Figure 3]. There was no heterogeneity across studies for affective ToM/cognitive empathy (I2 = 0), small heterogeneity for affective empathy (I2 = 29%), moderate heterogeneity for overall ToM (I2 = 66%) and overall empathy (I2 = 52%), and significant heterogeneity for studies on cognitive ToM (I2 = 78%). Egger’s test did not reveal a significant publication bias for overall ToM, overall empathy, cognitive ToM, affective ToM/cognitive empathy, or affective empathy.
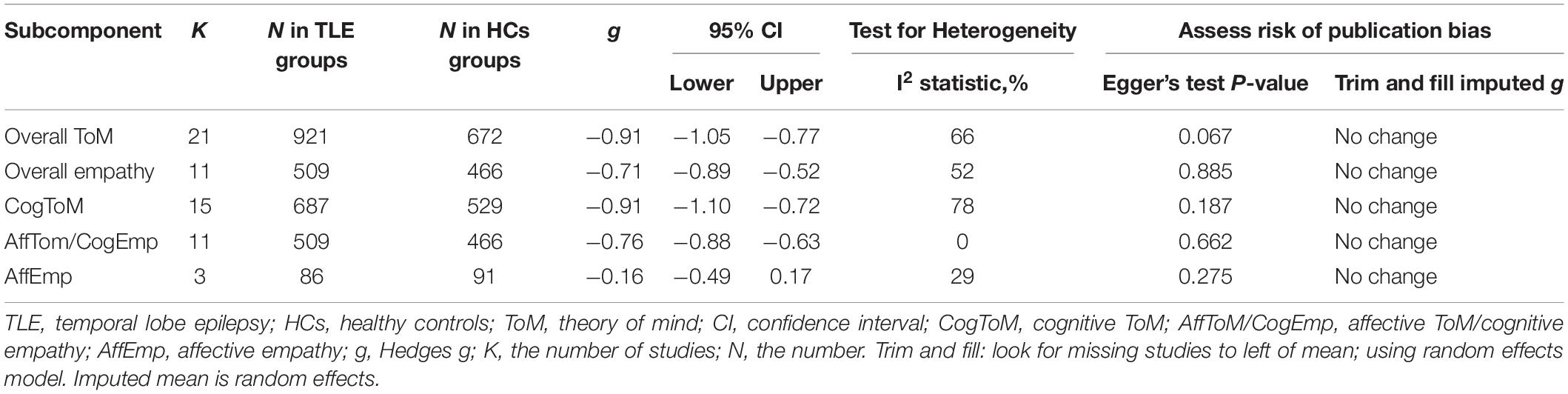
Table 3. Mean effects for ToM and empathy subcomponents comparing participants with TLE against healthy controls and tests for publication bias.
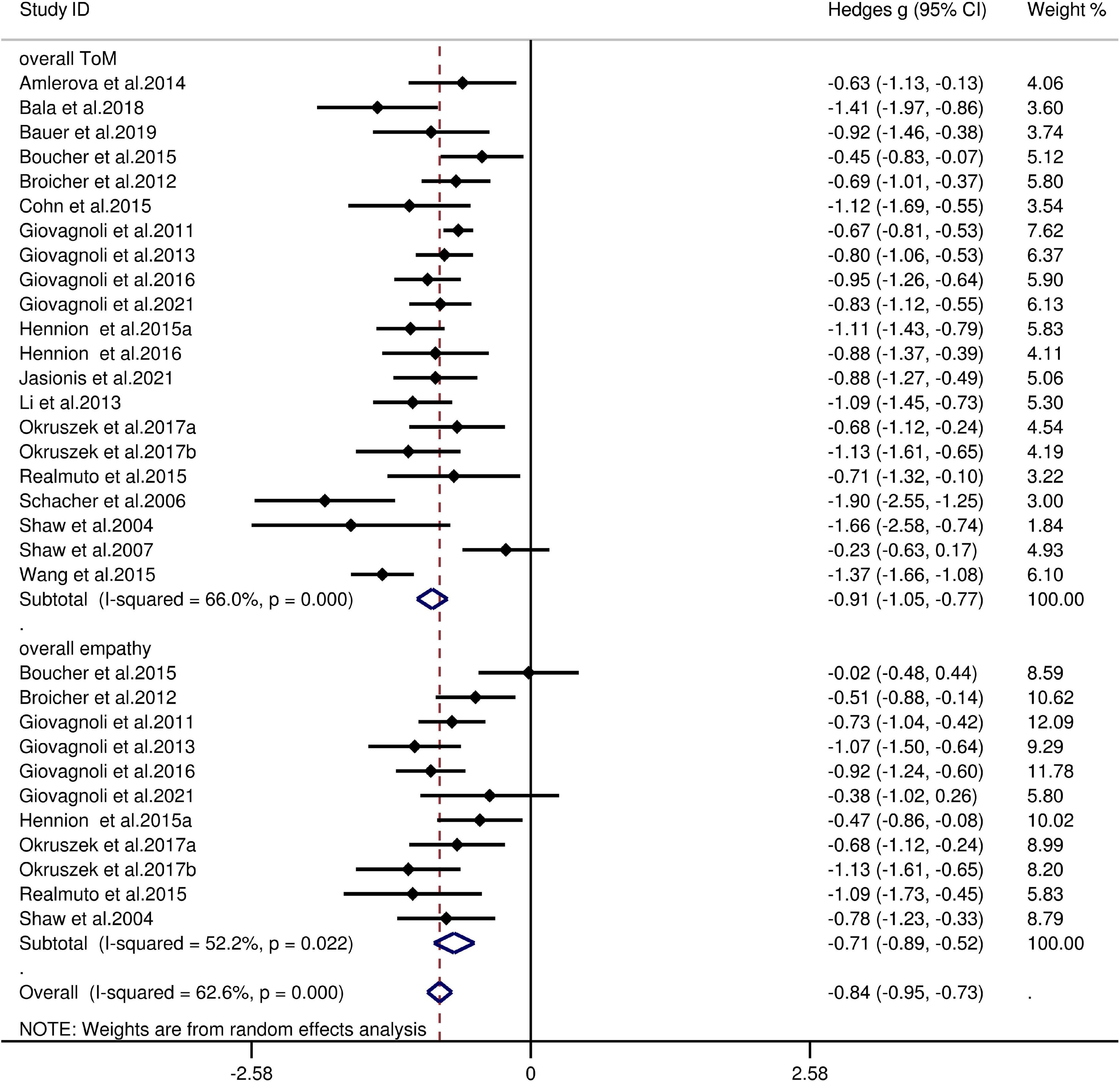
Figure 2. Forest plots showing effect size estimates (Hedges g) for overall ToM and overall empathy differences between TLE and healthy controls. CI, confidence interval; TLE, temporal lobe epilepsy; ToM, theory of mind.
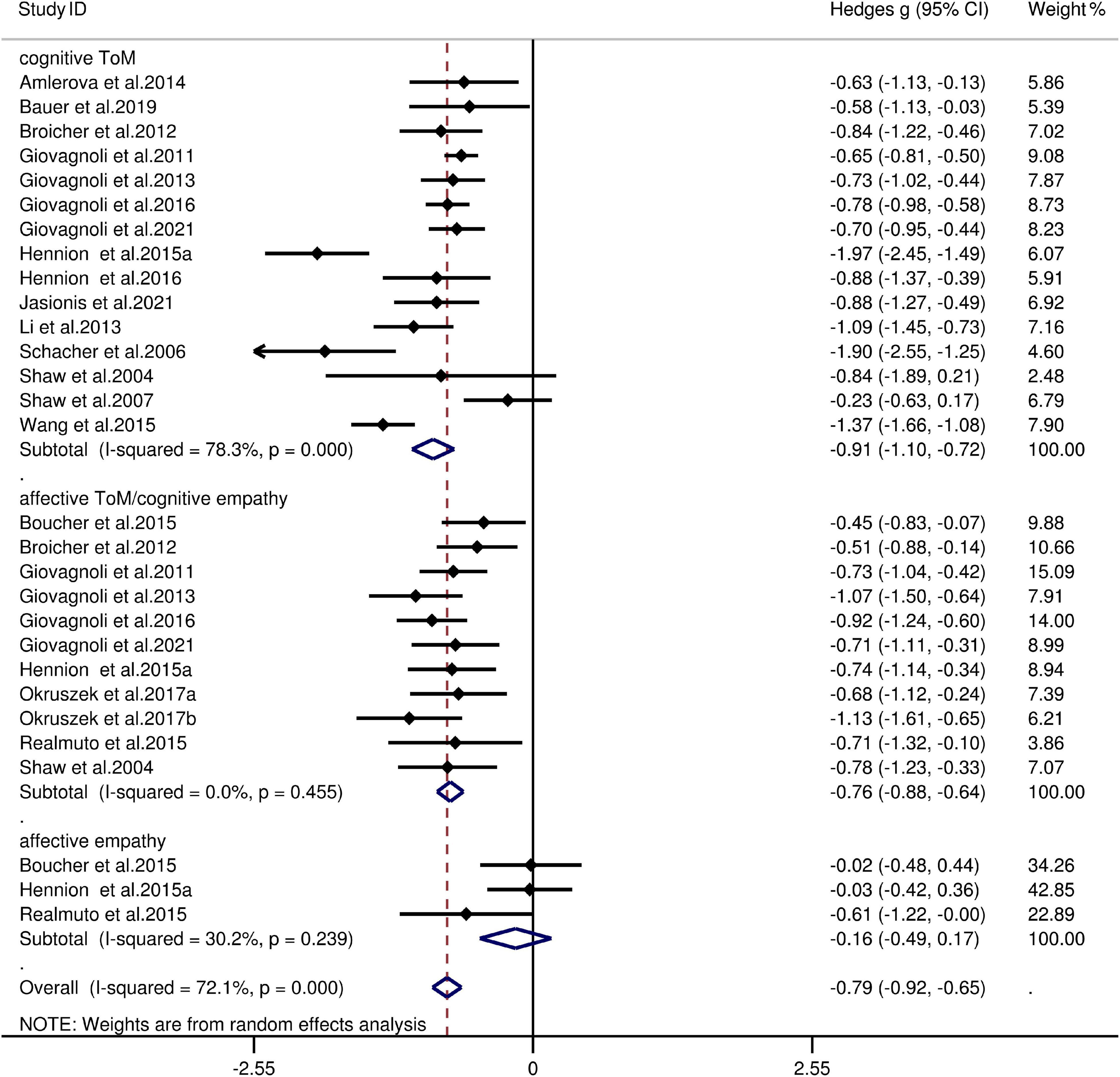
Figure 3. Forest plots showing effect size estimates (Hedges g) for cognitive ToM, affective ToM/cognitive empathy, and affective empathy differences between TLE and healthy controls. CI, confidence interval; TLE, temporal lobe epilepsy; ToM, theory of mind.
Theory of Mind and Empathy in Adult Patients With Frontal Lobe Epilepsy vs. Healthy Controls
The key results from this meta-analysis are presented in Table 4. Adult patients with FLE exhibited significant impairment in overall ToM [g = −0.93, 95% CI (−1.28, −0.57), K = 6; Figure 4] and overall empathy [g = −0.94, 95% CI (-1.36, -0.53), K = 6; Figure 4] compared to the HCs. The examination of the overlapping and distinct subcomponents of these constructs revealed associations between adult patients with FLE and severe deficits in cognitive ToM [g = −1.06, 95% CI (−1.31, −0.80), K = 4; Figure 4] and affective ToM/cognitive empathy [g =−0.96, 95% CI (−1.40, −0.51), K = 6; Figure 4]. However, no group differences were evident for affective empathy [g = −0.31, 95% CI (−0.67, 0.05), K = 1; Figure 4]. There was no heterogeneity across studies for affective empathy (I2 = 0) and moderate heterogeneity for cognitive ToM (I2 = 65%), but a significant variation was observed for overall ToM (I2 = 85%), overall empathy (I2 = 84%), and affective ToM/cognitive empathy (I2 = 77%). Egger’s test did not reveal significant publication bias for overall ToM, overall empathy, cognitive ToM, or affective ToM/cognitive empathy.
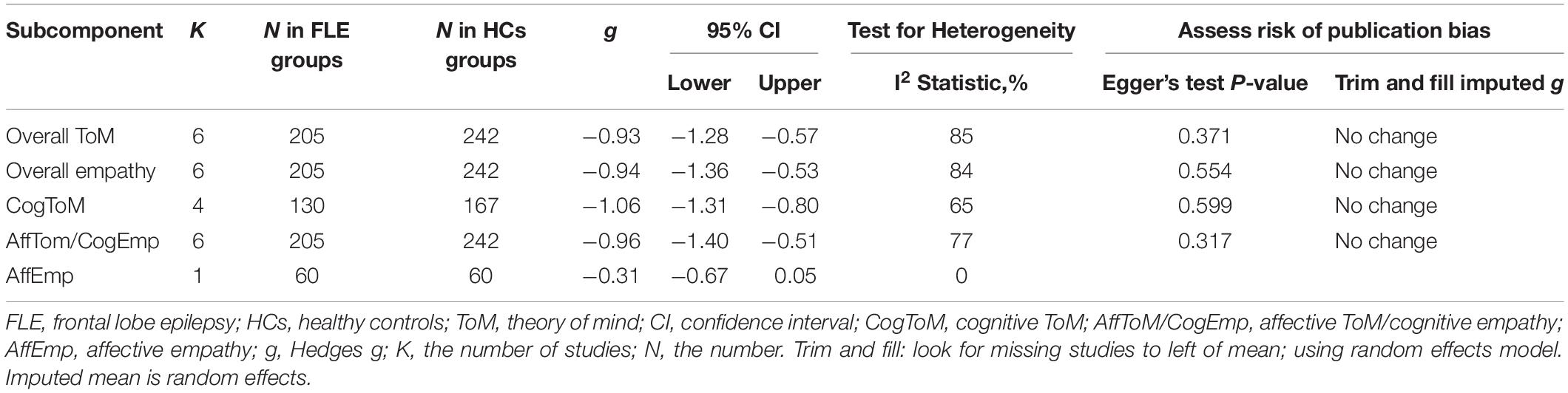
Table 4. Mean effects for ToM and empathy subcomponents comparing participants with FLE against healthy controls and tests for publication bias.
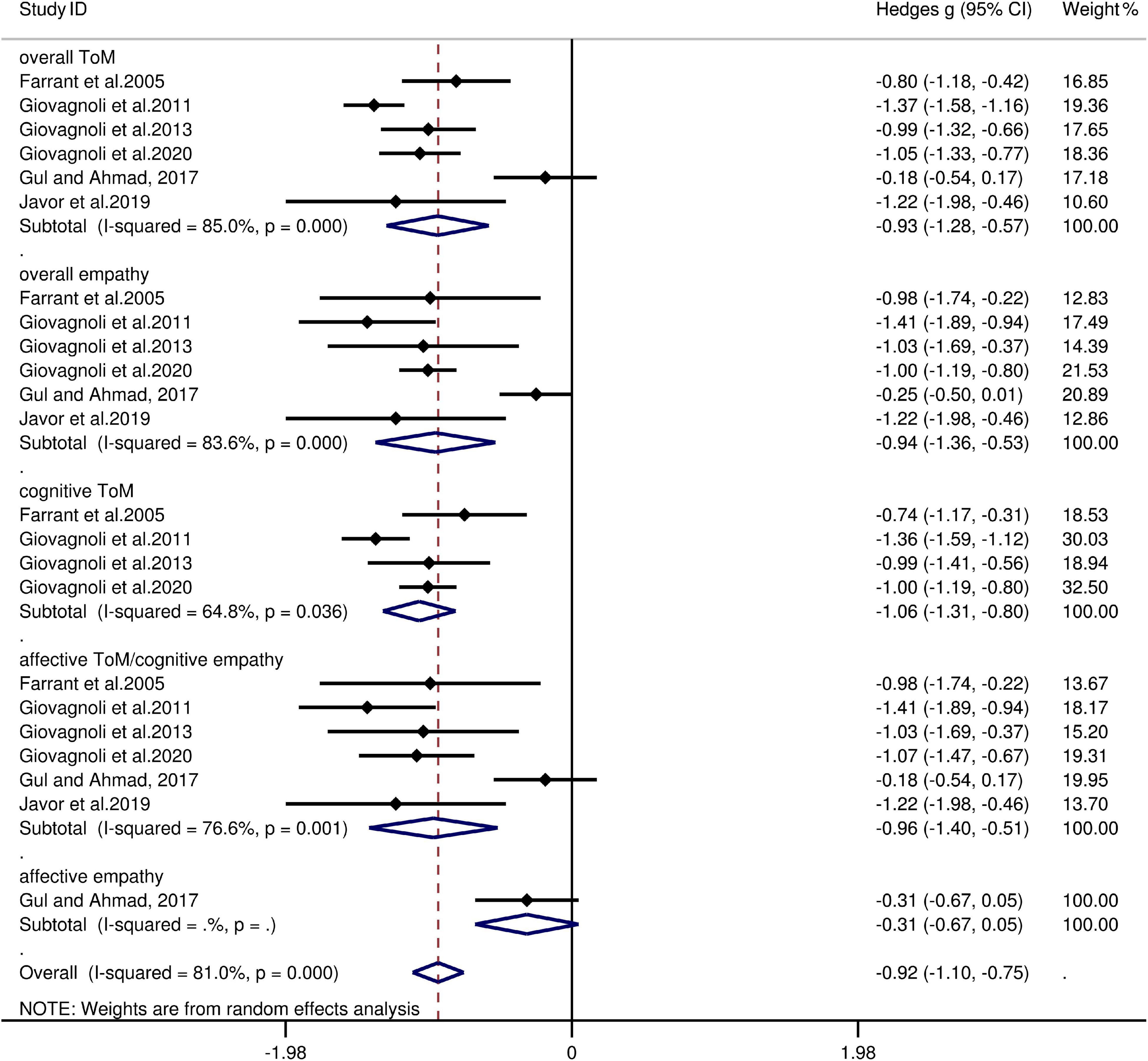
Figure 4. Forest plots showing effect size estimates (Hedges g) for overall ToM, overall empathy, cognitive ToM, affective ToM/cognitive empathy, and affective empathy differences between FLE and healthy controls. CI, confidence interval; FLE, frontal lobe epilepsy; ToM, theory of mind.
Theory of Mind and Empathy in Adult Patients With Idiopathic Generalized Epilepsy vs. Healthy Controls
The key results from this meta-analysis are shown in Table 5. Adult patients with IGE exhibited mild deficits in overall ToM [g = −0.42, 95% CI (−0.58, −0.27), K = 5; Figure 5] and cognitive ToM [g = −0.498, 95% CI (−0.77, −0.23), K = 4; Figure 5] compared to the HCs. However, no group differences were evident for overall empathy [g = −0.36, 95% CI (−0.74, 0.02), K = 4; Figure 5], affective ToM/cognitive empathy [g = −0.33, 95% CI (−0.69, 0.04), K = 4; Figure 5], and affective empathy [g = −0.24, 95% CI (−0.86, 0.38), K = 1; Figure 5]. There was no heterogeneity across studies for affective empathy (I2 = 0), small heterogeneity for overall ToM (I2 = 16%), cognitive ToM (I2 = 48%), affective ToM/cognitive empathy (I2 = 46%), and moderate heterogeneity for overall empathy (I2 = 51%). Egger’s test did not reveal significant publication bias for overall ToM, overall empathy, cognitive ToM, or affective ToM/cognitive empathy.
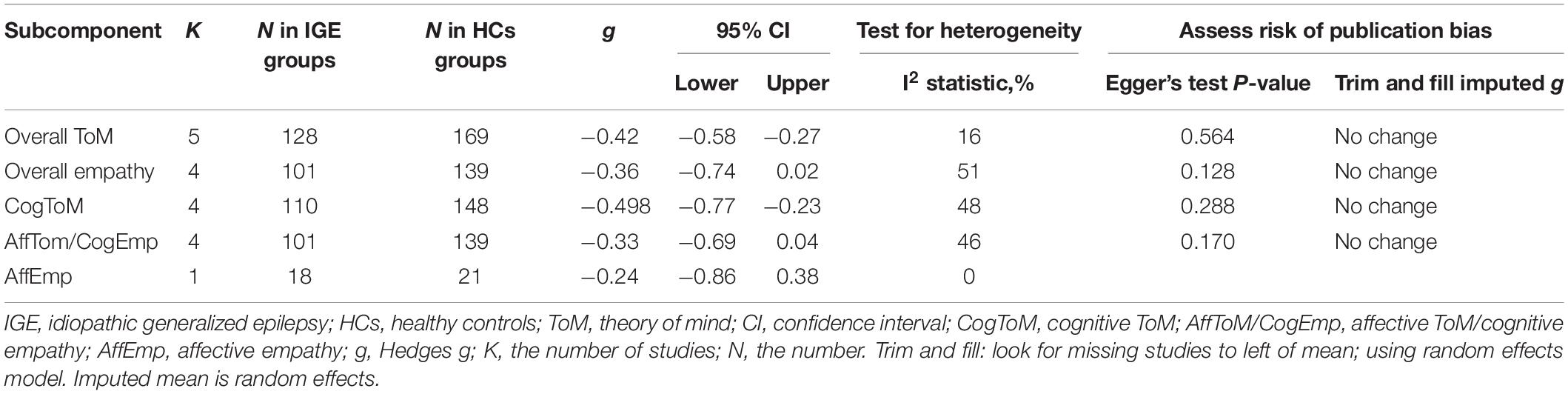
Table 5. Mean effects for ToM and empathy subcomponents comparing participants with IGE against healthy controls and tests for publication bias.
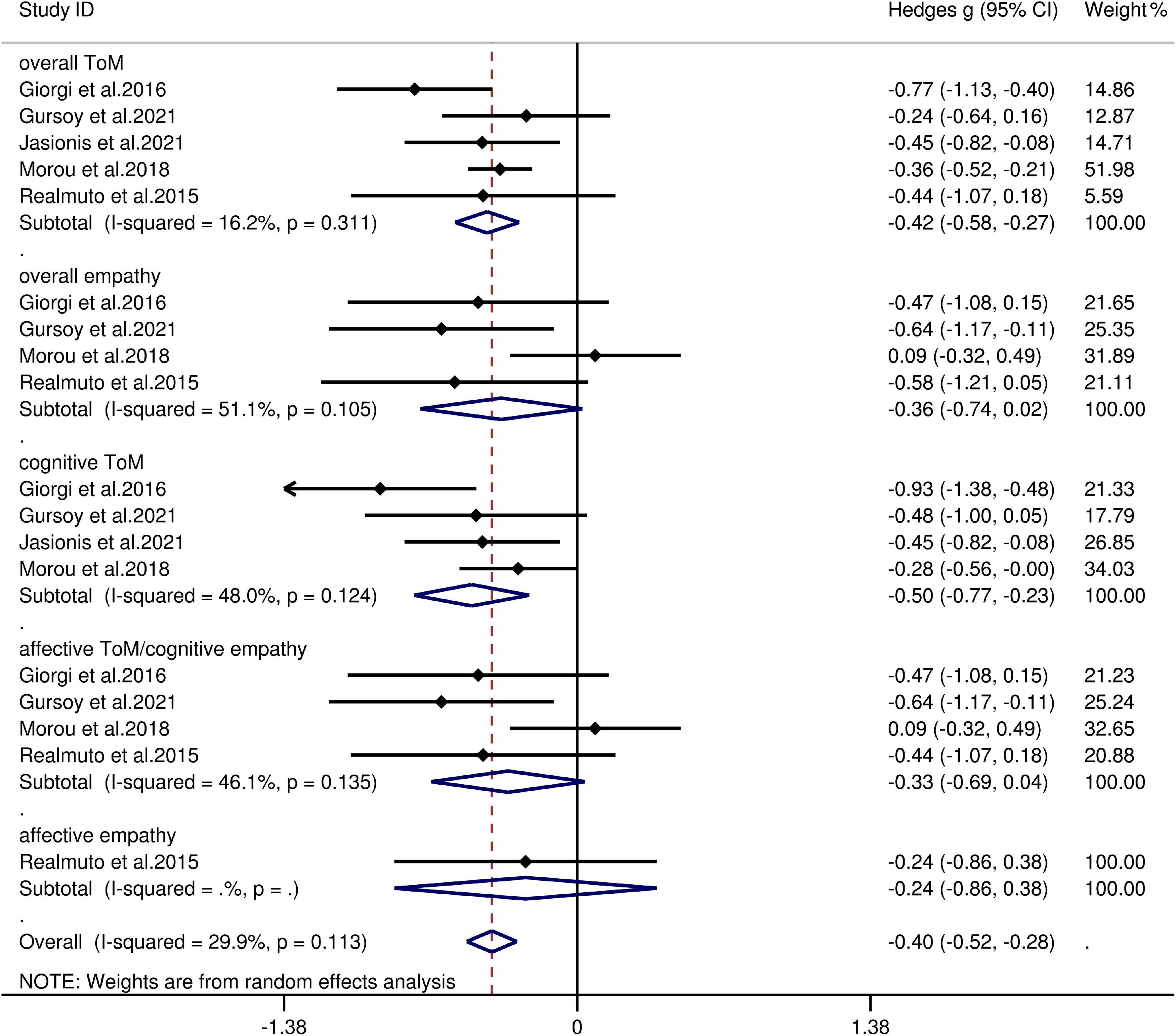
Figure 5. Forest plots showing effect size estimates (Hedges g) for overall ToM, overall empathy, cognitive ToM, affective ToM/cognitive empathy, and affective empathy differences between IGE and healthy controls. CI, confidence interval; IGE, idiopathic generalized epilepsy; ToM, theory of mind.
Theory of Mind and Empathy in Adult Patients With Extra-Temporal Lobe Epilepsy/Frontal Lobe Epilepsy vs. Healthy Controls
The key results obtained from this meta-analysis are depicted in Table 6. Adult patients with extra-TLE/FLE showed mild deficits in overall ToM [g = −0.48, 95% CI (−0.85, −0.12), K = 3; Figure 6] and cognitive ToM [g = −0.499, 95% CI (−0.88, −0.12), K = 3; Figure 6] compared to HCs. However, no group differences were evident for overall empathy [g = −0.26, 95% CI (−0.71, 0.18), K = 1; Figure 6] and affective ToM/cognitive empathy [g = −0.26, 95% CI (−0.71, 0.18), K = 1; Figure 6]. There was no heterogeneity across studies for overall empathy (I2 = 0), affective ToM/cognitive empathy (I2 = 0), and little heterogeneity for overall ToM (I2 = 48%) and cognitive ToM (I2 = 44%). Egger’s test was not significant for overall or cognitive ToM.
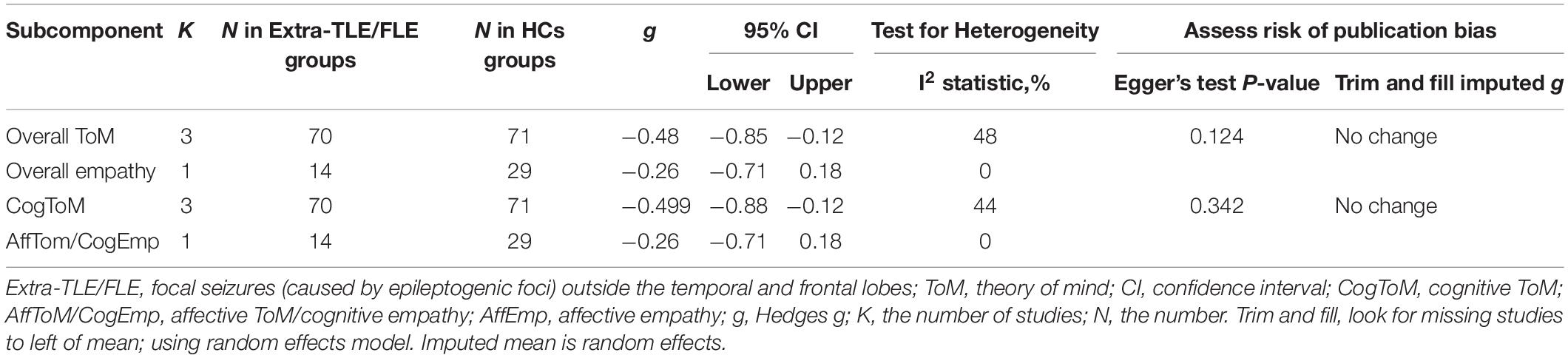
Table 6. Mean effects for ToM and empathy subcomponents comparing participants with Extra-TLE/FLE against HCs and tests for publication bias.
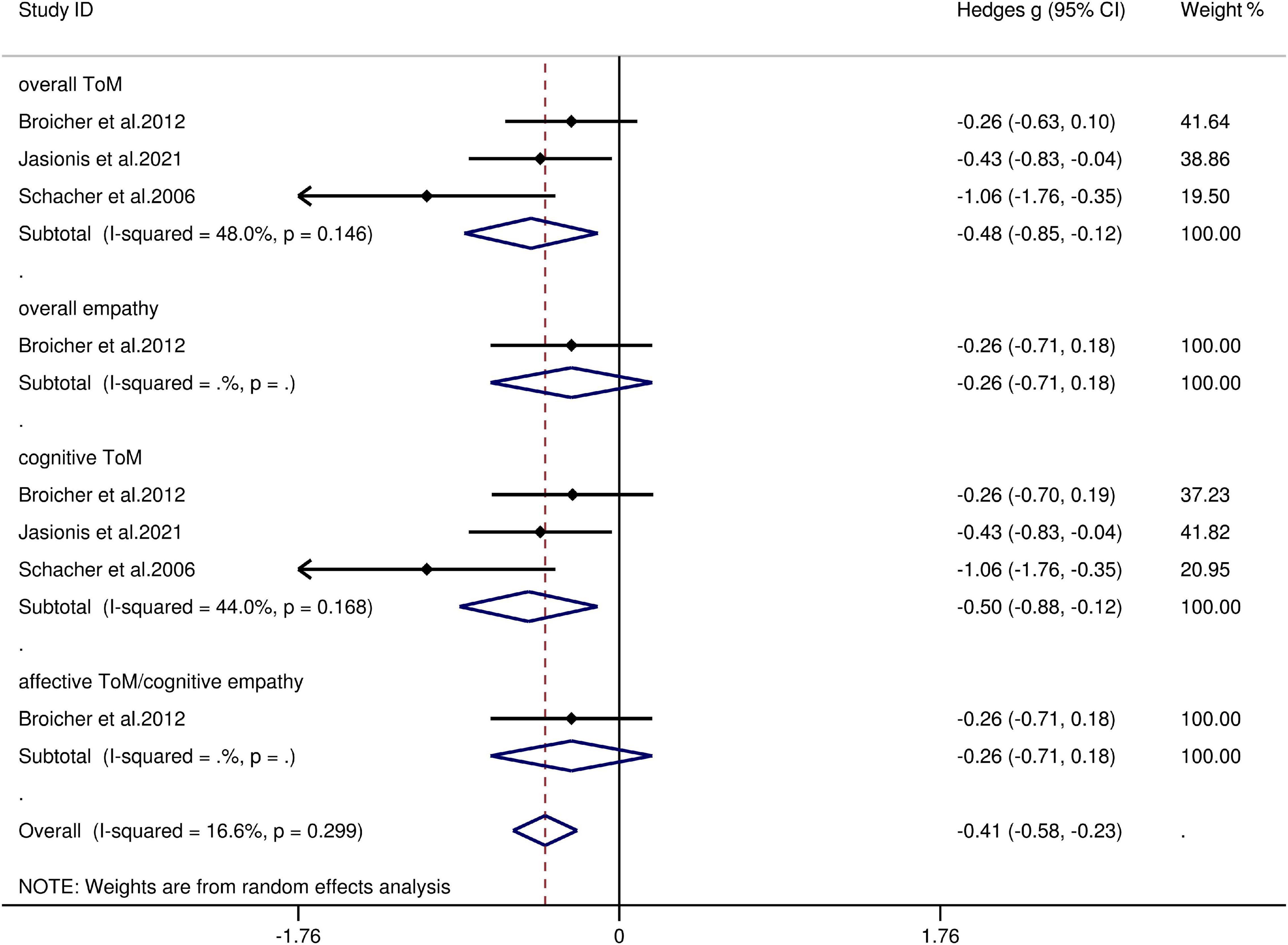
Figure 6. Forest plots showing effect size estimates (Hedges g) for overall ToM, overall empathy, cognitive ToM, and affective ToM/cognitive empathy differences between Extra-TLE/FLE and healthy controls. CI confidence interval, Extra-TLE/FLE focal seizures (caused by epileptogenic foci) outside the temporal and frontal lobes, ToM theory of mind.
Empathy and Theory of Mind in Adult Patients With Temporal Lobe Epilepsy With and Without Epilepsy Surgery vs. Healthy Controls
Table 7 depicts the key results obtained from this meta-analysis. The performance of adult patients with TLE-TL- and TLE-TL + with respect to overall ToM (g = −0.89 and g = −0.92), overall empathy (g = −0.77 and g = −0.57), cognitive ToM (g = −0.89 and g = −0.77), and affective ToM/cognitive empathy (g = −0.79 and g = −0.65) was inferior to that of the HCs. However, no group differences were evident for overall empathy (g = −0.27 and g = −0.02). The effect sizes of the TLE-TL- and TLE-TL + groups were comparable for overall ToM (Q = 0.03, df = 1, p = 0.863), overall empathy (Q = 0.82, df = 1, p = 0.366), cognitive ToM (Q = 0.34, df = 1, p = 0.56), affective ToM/cognitive empathy (Q = 1.01, df = 1, p = 0.314), and affective empathy (Q = 0.46, df = 1, p = 0.498).
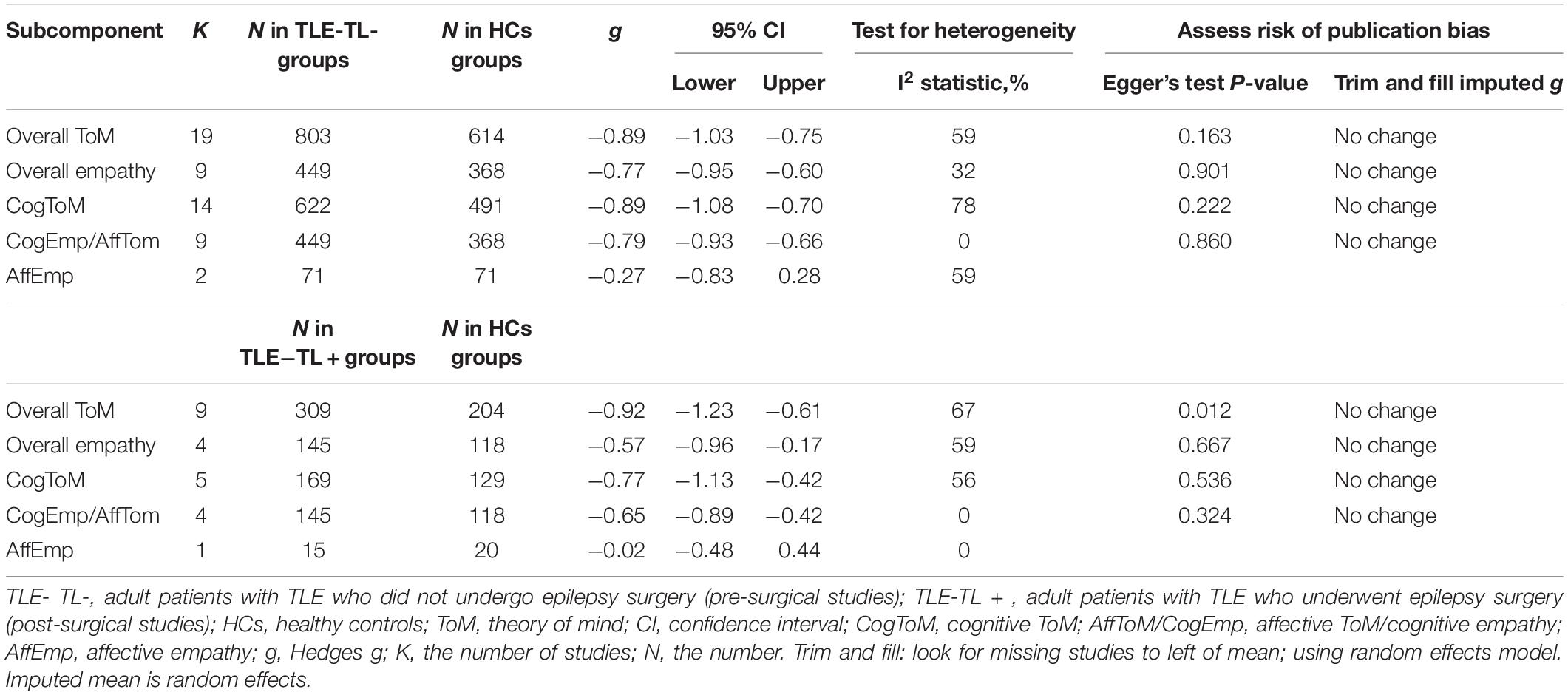
Table 7. Mean effects for ToM and empathy subcomponents comparing participants with TLE-TL- and TLE-TL + against healthy controls and tests for publication bias.
Egger’s test was not significant, except for overall ToM in adult patients with TLE-TL +. However, a trim-and-fill analysis did not result in imputation of any studies, and the effect size remained the same.
Meta-Regression Analyses
Meta-regressions were not conducted for the effect of potential variables (age, sex, education level, age at epilepsy onset, disease duration, monthly seizure frequency, number of AEDs, and IQ score) on the severity of ToM/empathy in adult patients with FLE, IGE, or extra-TLE/FLE, as less than 10 studies contributed to the data for this subcomponent.
The variables (age, sex, education level, age at epilepsy onset, disease duration, and monthly seizure frequency) associated with adult patients with TLE did not account for any significant variance in overall ToM (p = 0.871, 0.218, 0.582, 0.996, 0.712, and 0.318, respectively), overall empathy (p = 0.871, 0.218, 0.582, 0.996, 0.712, and 0.318, respectively), cognitive ToM (p = 0.871, 0.218, 0.582, 0.996, 0.712, and 0.318, respectively), and affective ToM/cognitive empathy (p = 0.871, 0.218, 0.582, 0.996, 0.712, and 0.318, respectively). No meta-regressions were conducted for the variables associated with affective empathy, or for those (number of AEDs and IQ score) associated with overall ToM, overall empathy, cognitive ToM, and affective ToM/cognitive empathy, as less than 10 studies provided data for this subcomponent.
Discussion
To the best our knowledge, this meta-analysis is the first to investigate the patterns of ToM and empathy function in adult patients with epilepsy. The meta-analysis included 28 studies, and combined samples of 902 adult patients with TLE (21 studies), 205 adult patients with FLE (6 studies), 128 adult patients with IGE (5 studies), and 70 adult patients with extra-TLE/FLE (3 studies). Adult patients with TLE and FLE exhibited impairments in overall ToM, overall empathy, cognitive ToM, and affective ToM/cognitive empathy, but no significant differences were observed for affective empathy, compared to the HCs. Overall and cognitive ToM were both impaired in adult patients with IGE and extra-TLE/FLE, but no group differences were evident for overall empathy or affective ToM/cognitive empathy, compared to the HCs. Moreover, relative to the HCs, no group differences were identified for affective empathy in adult patients with IGE. The subgroup analysis found no statistically significant difference in the degree of ToM/empathy impairment between adult patients with TLE-TL- and TLE-TL +. The meta-regression analysis indicated that there was no significant relationship between the variables (age, sex, education level, age at epilepsy onset, disease duration, and monthly seizure frequency) and the magnitude of the effect sizes in adult patients with TLE.
A large effect size was observed for overall ToM (g = −0.91, K = 21 and g = −0.93, K = 6) between adult patients with TLE/FLE and the HCs, which was consistent with the findings of Stewart et al. (13) (g = −0.92, K = 9 and g = −1.03, K = 3, respectively). Subsequently, we analyzed the sub-components of ToM and found that adult patients with TLE/FLE exhibited impairment in cognitive ToM (g = −0.91 and g = −1.06) and affective ToM (g = −0.76 and g = −0.96). The results may be consistent with the neuropathological progression of patients with TLE/FLE, as both the cognitive and affective ToM networks are composed of a core network, including the anterior dorsal medial prefrontal cortex and temporoparietal junction. Moreover, the cognitive ToM network involves the dorsal striatum and dorsal anterior cingulate cortex, while the affective ToM network involves the ventromedial and orbitofrontal cortices, ventral striatum, ventral anterior cingulate cortex, and amygdala (122, 123). Coincidentally, there is a clear overlap between the neural networks involved in patients with TLE/FLE and the cognitive and affective ToM networks (58, 124–132).
Moderate (g = −0.71, K = 11) and large effect sizes (g = −0.94, K = 6) were found for the comparison of overall empathy between adult patients with TLE/FLE and the HCs, respectively. Subsequently, we analyzed the subcomponents of empathy and found that adult patients with TLE/FLE exhibited impairment in cognitive empathy, but no group differences were evident for affective empathy. These quantitative findings are consistent with the findings of broader research, which indicates that cognitive empathy and affective empathy are separate domains that differ in their requirements for effortful processing (27, 48, 133–135). Specifically, cognitive empathy is a slow and laborious process that requires the individual’s attention and time, while affective empathy is an automatic and spontaneous response that operates at a minimal level of consciousness (48, 136). Thus, these two components of empathy may pose different challenges for adult patients with TLE/FLE. Since the cognitive requirements for affective empathy are low, it could be expected that this ability remains relatively conserved in adult patients with TLE/FLE. Moreover, considering the limited number of included studies that contributed to the effect size of affective empathy in adult patients with TLE/FLE (K = 3 and K = 1, respectively), the findings should be interpreted with caution.
Group differences were not observed for overall empathy in adult patients with IGE and the effect size for overall ToM impairment was small (g = −0.42, K = 5), which differed from the findings of Stewart et al. (13), who conducted a meta-analysis of two studies and reported moderate impairment in the overall ToM (g = −0.59) in patients with IGE compared to the HCs. The results of our quantitative analyses indicated that the deficits in ToM among adult patients with IGE were subtle. This may be related to the structural abnormalities in areas recognized as ToM hubs, including the temporoparietal neocortices and mesial prefrontal (137–141). Our study also focused on the sub-components of ToM. The results of previous qualitative studies suggested that the cognitive and affective ToM domains are impaired in patients with IGE (119, 141). However, these findings are inconsistent with those of the current quantitative meta-analysis, which showed that adult patients with IGE had mild impairments in cognitive ToM, but no difference was found in affective ToM. Nevertheless, these findings should be interpreted with caution, considering the paucity of studies contributing to the effect size of affective ToM in adult patients with IGE (K = 4).
Currently, anterior temporal lobectomy (ALT) is the most common type of epilepsy surgery for adults with TLE. ALT typically entails resection of the anterior parts of temporal lobe (including the hippocampus, amygdala, anterior part of the fusiform gyrus, and adjacent neocortical temporal tissue) (114, 142), which are usually activated in ToM or empathy tasks (143). Thus, it could be hypothesized that ALT may result in the risk of a decline in ToM or empathy in patients with TLE (114, 142). However, the current quantitative findings showed no significant differences in the degree of ToM/empathy impairment between adult patients with TLE-TL- and TLE-TL +. This may be because this type of surgical treatment is commonly performed only in patients with drug resistant epilepsy; thus, most of patients with TLE-TL + have experienced symptoms of epilepsy for many years. Such prolonged and uncontrolled seizures may cause alterations in brain tissue of epileptic zone and consequently its functions (11, 114). In addition, some of patients with TLE-TL + suffer from epilepsy since birth or early childhood, and early-onset epilepsy may also trigger early brain reorganization, resulting in a functional compensation after surgical treatment (106). Therefore, temporal lobectomy may not significantly worsen patient’s performance in ToM or empathy tasks. However, any individual improvements or decline may be masked by group comparisons, and the results should consider methodological heterogeneity among studies (144).
Limitations
This meta-analysis had several limitations. First, we only included cross-sectional studies, while more longitudinal studies are required to investigate the dynamic changes in the ToM and empathy functions in adult patients with epilepsy. Second, although 28 studies were included in this meta-analysis, few studies contributed to the mean effect size for affective empathy between adult patients with TLE or FLE or IGE, and HCs (K = 3, K = 1, and K = 1, respectively). Additionally, only three studies provided data on the comparison between adult patients with IGE and HCs; therefore, further research in this area is warranted in the future. Third, although we investigated some demographic and clinical variables (i.e., age, sex, education level, age at epilepsy onset, disease duration, and monthly seizure frequency) that may affect ToM and empathy in adults with TLE, other factors (such as number of AEDs and IQ score) were not examined, owing to the paucity of data available in the original studies. Similarly, potential variables associated with the severity of ToM or empathy were not examined in adult patients with FLE, IGE, or extra-TLE/FLE. Further studies are needed to fully clarify the potential effects of these factors on ToM- and empathy-associated features in adult patients with epilepsy.
Conclusion
In conclusion, our data provide important clarifications on how the two interrelated social cognitive abilities, ToM and empathy, are affected in adults with epilepsy. The results of this meta-analysis suggest that adult patients with TLE and FLE showed impairments in cognitive ToM and affective ToM/cognitive empathy, but no significant differences were found in affective empathy. Besides, cognitive ToM was impaired in adult patients with IGE and extra-TLE/FLE, but no group differences were evident for affective ToM/cognitive empathy. Moreover, relative to the HCs, no group differences were identified for affective empathy in adult patients with IGE. Additionally, the degree of ToM/empathy impairment did not differ significantly between adult patients with TLE-TL- and TLE-TL +. These quantitative results suggest a differential impairment in the core aspects of social cognitive processing (including ToM and empathy) in adult patients with epilepsy, which may contribute to the development of structured cognitive interventions (i.e., social cognitive training) for this patient population.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZY and GW: study design and critical revision of the manuscript. HW, PZ, JZha, and PP: analysis and interpretation of data. HW and PZ: drafting of the manuscript. All authors approval of the final version for submission.
Funding
This work was supported by the Jiangsu Commission of Health (LGY20180390).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all authors of the studies included. This protocol was prospectively registered at the International Platform of Registered Systematic Review and Meta-analysis Protocols (ID: INPLASY 2021120039).
References
1. Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. (2019) 393:689–701. doi: 10.1016/s0140-673632596-0
2. Adamczyk B, Węgrzyn K, Wilczyński T, Maciarz J, Morawiec N, Adamczyk-Sowa M. The most common lesions detected by neuroimaging as causes of epilepsy. Medicina (Kaunas). (2021) 57:294. doi: 10.3390/medicina57030294
3. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:459–80. doi: 10.1016/s1474-442230499-x
4. Janson MT, Bainbridge JL. Continuing burden of refractory epilepsy. Ann Pharmacother. (2021) 55:406–8. doi: 10.1177/1060028020948056
5. Tombini M, Assenza G, Quintiliani L, Ricci L, Lanzone J, Di Lazzaro V. Epilepsy and quality of life: what does really matter? Neurol Sci. (2021) 42:3757–65. doi: 10.1007/s10072-020-04990-6
6. Martin RC, Griffith HR, Faught E, Gilliam F, Mackey M, Vogtle L. Cognitive functioning in community dwelling older adults with chronic partial epilepsy. Epilepsia. (2005) 46:298–303. doi: 10.1111/j.0013-9580.2005.02104.x
7. Griffith HR, Martin RC, Bambara JK, Marson DC, Faught E. Older adults with epilepsy demonstrate cognitive impairments compared with patients with amnestic mild cognitive impairment. Epilepsy Behav. (2006) 8:161–8. doi: 10.1016/j.yebeh.2005.09.004
8. Piazzini A, Canevini MP, Turner K, Chifari R, Canger R. Elderly people and epilepsy: cognitive function. Epilepsia. (2006) 47(Suppl. 5):82–4. doi: 10.1111/j.1528-1167.2006.00884.x
9. Griffith HR, Martin RC, Bambara JK, Faught E, Vogtle LK, Marson DC. Cognitive functioning over 3 years in community dwelling older adults with chronic partial epilepsy. Epilepsy Res. (2007) 74:91–6. doi: 10.1016/j.eplepsyres.2007.01.002
10. Witt JA, Werhahn KJ, Krämer G, Ruckes C, Trinka E, Helmstaedter C. Cognitive-behavioral screening in elderly patients with new-onset epilepsy before treatment. Acta Neurol Scand. (2014) 130:172–7. doi: 10.1111/ane.12260
11. Bora E, Meletti S. Social cognition in temporal lobe epilepsy: a systematic review and meta-analysis. Epilepsy Behav. (2016) 60:50–7. doi: 10.1016/j.yebeh.2016.04.024
12. Miller LA, Galioto R, Tremont G, Davis J, Bryant K, Roth J, et al. Cognitive impairment in older adults with epilepsy: characterization and risk factor analysis. Epilepsy Behav. (2016) 56:113–7. doi: 10.1016/j.yebeh.2016.01.011
13. Stewart E, Catroppa C, Lah S. Theory of mind in patients with epilepsy: a systematic review and meta-analysis. Neuropsychol Rev. (2016) 26:3–24. doi: 10.1007/s11065-015-9313-x
14. Sen A, Capelli V, Husain M. Cognition and dementia in older patients with epilepsy. Brain. (2018) 141:1592–608. doi: 10.1093/brain/awy022
15. American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association (2013).
16. Baron-Cohen S. Is asperger syndrome/high-functioning autism necessarily a disability? Dev Psychopathol. (2000) 12:489–500. doi: 10.1017/s0954579400003126
17. Brüne M, Brüne-Cohrs U. Theory of mind–evolution, ontogeny, brain mechanisms and psychopathology. Neurosci Biobehav Rev. (2006) 30:437–55. doi: 10.1016/j.neubiorev.2005.08.001
18. Call J, Tomasello M. Does the chimpanzee have a theory of mind? 30 years later. Trends Cogn Sci. (2008) 12:187–92. doi: 10.1016/j.tics.2008.02.010
19. Henry A, Tourbah A, Chaunu MP, Rumbach L, Montreuil M, Bakchine S. Social cognition impairments in relapsing-remitting multiple sclerosis. J Int Neuropsychol Soc. (2011) 17:1122–31. doi: 10.1017/s1355617711001147
20. Lin X, Zhang X, Liu Q, Zhao P, Zhong J, Pan P, et al. Social cognition in multiple sclerosis and its subtypes: a meta-analysis. Mult Scler Relat Disord. (2021) 52:102973. doi: 10.1016/j.msard.2021.102973
21. Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. (2008) 34:1211–20. doi: 10.1093/schbul/sbm145
22. Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. (2015) 16:620–31. doi: 10.1038/nrn4005
23. Lin X, Zhang X, Liu Q, Zhao P, Zhang H, Wang H, et al. Theory of mind in adults with traumatic brain injury: a meta-analysis. Neurosci Biobehav Rev. (2021) 121:106–18. doi: 10.1016/j.neubiorev.2020.12.010
24. McDonald S, Flanagan S. Social perception deficits after traumatic brain injury: interaction between emotion recognition, mentalizing ability, and social communication. Neuropsychology. (2004) 18:572–9. doi: 10.1037/0894-4105.18.3.572
25. Bujarski KA, Flashman L, Li Z, Tosteson TD, Jobst BC, Thadani VM, et al. Investigating social cognition in epilepsy using a naturalistic task. Epilepsia. (2016) 57:1515–20. doi: 10.1111/epi.13477
26. Leppanen J, Sedgewick F, Treasure J, Tchanturia K. Differences in the theory of mind profiles of patients with anorexia nervosa and individuals on the autism spectrum: a meta-analytic review. Neurosci Biobehav Rev. (2018) 90:146–63. doi: 10.1016/j.neubiorev.2018.04.009
27. Lin X, Zhang X, Liu Q, Zhao P, Zhong J, Pan P, et al. Empathy and theory of mind in multiple sclerosis: a meta-analysis. Front Psychiatry. (2021) 12:628110. doi: 10.3389/fpsyt.2021.628110
28. Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. (2004) 3:71–100. doi: 10.1177/1534582304267187
29. Heitz C, Noblet V, Phillipps C, Cretin B, Vogt N, Philippi N, et al. Cognitive and affective theory of mind in dementia with Lewy bodies and Alzheimer’s disease. Alzheimers Res Ther. (2016) 8:10. doi: 10.1186/s13195-016-0179-9
30. Lawrence EJ, Shaw P, Baker D, Baron-Cohen S, David AS. Measuring empathy: reliability and validity of the empathy quotient. Psychol Med. (2004) 34:911–9. doi: 10.1017/s0033291703001624
31. Leiberg S, Anders S. The multiple facets of empathy: a survey of theory and evidence. Prog Brain Res. (2006) 156:419–40. doi: 10.1016/s0079-612356023-6
32. Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. (2011) 54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014
33. Henry JD, von Hippel W, Molenberghs P, Lee T, Sachdev PS. Clinical assessment of social cognitive function in neurological disorders. Nat Rev Neurol. (2016) 12:28–39. doi: 10.1038/nrneurol.2015.229
34. de Waal FBM, Preston SD. Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci. (2017) 18:498–509. doi: 10.1038/nrn.2017.72
35. Timmers I, Park AL, Fischer MD, Kronman CA, Heathcote LC, Hernandez JM, et al. Is empathy for pain unique in its neural correlates? A meta-analysis of neuroimaging studies of empathy. Front Behav Neurosci. (2018) 12:289. doi: 10.3389/fnbeh.2018.00289
36. Baars BJ, Gage NM. Fundamentals of Cognitive Neuroscience. Cambridge, MA: Academic Press (2010).
37. Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. (2011) 17:18–24. doi: 10.1177/1073858410379268
38. Wondra JD, Ellsworth PC. An appraisal theory of empathy and other vicarious emotional experiences. Psychol Rev. (2015) 122:411–28. doi: 10.1037/a0039252
39. van Zonneveld L, Platje E, de Sonneville L, van Goozen S, Swaab H. Affective empathy, cognitive empathy and social attention in children at high risk of criminal behaviour. J Child Psychol Psychiatry. (2017) 58:913–21. doi: 10.1111/jcpp.12724
40. Bartochowski Z, Gatla S, Khoury R, Al-Dahhak R, Grossberg GT. Empathy changes in neurocognitive disorders: a review. Ann Clin Psychiatry. (2018) 30:220–32.
41. Chen J. Empathy for distress in humans and rodents. Neurosci Bull. (2018) 34:216–36. doi: 10.1007/s12264-017-0135-0
42. Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. (2006) 30:855–63. doi: 10.1016/j.neubiorev.2006.06.011
43. Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. (2009) 132:617–27. doi: 10.1093/brain/awn279
44. Dvash J, Shamay-Tsoory S. Theory of mind and empathy as multidimensional constructs. Top Lang Disord. (2014) 34:282–95. doi: 10.1097/tld.0000000000000040
45. Happé F, Cook JL, Bird G. The structure of social cognition: in(ter)dependence of sociocognitive processes. Annu Rev Psychol. (2017) 68:243–67. doi: 10.1146/annurev-psych-010416-044046
46. Preckel K, Kanske P, Singer T. On the interaction of social affect and cognition: empathy, compassion and theory of mind. Curr Opin Behav Sci. (2018) 19:1–6. doi: 10.1016/j.cobeha.2017.07.010
47. Coundouris SP, Adams AG, Henry JD. Empathy and theory of mind in Parkinson’s disease: a meta-analysis. Neurosci Biobehav Rev. (2020) 109:92–102. doi: 10.1016/j.neubiorev.2019.12.030
48. Demichelis OP, Coundouris SP, Grainger SA, Henry JD. Empathy and theory of mind in Alzheimer’s disease: a meta-analysis. J Int Neuropsychol Soc. (2020) 26:963–77. doi: 10.1017/s1355617720000478
49. Shaw DJ, Czekóová K, Pennington CR, Qureshi AW, Špiláková B, Salazar M, et al. You≠me: individual differences in the structure of social cognition. Psychol Res. (2020) 84:1139–56. doi: 10.1007/s00426-018-1107-3
50. Schurz M, Radua J, Tholen MG, Maliske L, Margulies DS, Mars RB, et al. Toward a hierarchical model of social cognition: a neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol Bull. (2021) 147:293–327. doi: 10.1037/bul0000303
51. Jiang Y, Hu Y, Wang Y, Zhou N, Zhu L, Wang K. Empathy and emotion recognition in patients with idiopathic generalized epilepsy. Epilepsy Behav. (2014) 37:139–44. doi: 10.1016/j.yebeh.2014.06.005
52. Hennion S, Szurhaj W, Duhamel A, Lopes R, Tyvaert L, Derambure P, et al. Characterization and prediction of the recognition of emotional faces and emotional bursts in temporal lobe epilepsy. J Clin Exp Neuropsychol. (2015) 37:931–45. doi: 10.1080/13803395.2015.1068280
53. Hu Y, Jiang Y, Hu P, Ma H, Wang K. Impaired social cognition in patients with interictal epileptiform discharges in the frontal lobe. Epilepsy Behav. (2016) 57:46–54. doi: 10.1016/j.yebeh.2016.01.027
54. Gul A, Ahmad H. The relationship between dispositional empathy and prefrontal cortical functioning in patients with frontal lobe epilepsy. Pak J Med Sci. (2017) 33:200–4. doi: 10.12669/pjms.331.11742
55. Jiang Y, Zhu M, Yu F, Wang K. Impaired empathy in patients with idiopathic generalized epilepsy: an event-related potentials study. Epilepsy Behav. (2020) 111:107274. doi: 10.1016/j.yebeh.2020.107274
56. Shaw P, Lawrence E, Bramham J, Brierley B, Radbourne C, David AS. A prospective study of the effects of anterior temporal lobectomy on emotion recognition and theory of mind. Neuropsychologia. (2007) 45:2783–90. doi: 10.1016/j.neuropsychologia.2007.04.020
57. Boucher O, Rouleau I, Lassonde M, Lepore F, Bouthillier A, Nguyen DK. Social information processing following resection of the insular cortex. Neuropsychologia. (2015) 71:1–10. doi: 10.1016/j.neuropsychologia.2015.03.008
58. Hennion S, Delbeuck X, Koelkebeck K, Brion M, Tyvaert L, Plomhause L, et al. A functional magnetic resonance imaging investigation of theory of mind impairments in patients with temporal lobe epilepsy. Neuropsychologia. (2016) 93:271–9. doi: 10.1016/j.neuropsychologia.2016.11.007
59. Braams OB, Meekes J, van Nieuwenhuizen O, Schappin R, van Rijen PC, Blijd-Hoogewys EMA, et al. Epilepsy surgery in children: no further threat to theory of mind. Epileptic Disord. (2019) 21:166–76. doi: 10.1684/epd.2019.1053
60. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
61. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
62. Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. (2015) 20:440–6. doi: 10.1038/mp.2014.59
63. Hedges LV. Distribution theory for glass’s estimator of effect size and related estimators. J Educ Stat. (1981) 6:107–28. doi: 10.2307/1164588
64. Cohen J, Cohen J, Cohen JW, Cohen J, Cohen J, Cohen J, et al. Statistical power analysis for the behavioral science. Technometrics. (1988) 31:499–500.
65. Scammacca N, Roberts G, Stuebing KK. Meta-analysis with complex research designs: dealing with dependence from multiple measures and multiple group comparisons. Rev Educ Res. (2014) 84:328–64. doi: 10.3102/0034654313500826
66. Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
67. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
68. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2015) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
69. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley-Blackwell (2008).
70. Kliemann D, Adolphs R, Paul LK, Tyszka JM, Tranel D. Reorganization of the social brain in individuals with only one intact cerebral hemisphere. Brain Sci. (2021) 11:965. doi: 10.3390/brainsci11080965
71. Shaw P, Bramham J, Lawrence EJ, Morris R, Baron-Cohen S, David AS. Differential effects of lesions of the amygdala and prefrontal cortex on recognizing facial expressions of complex emotions. J Cogn Neurosci. (2005) 17:1410–9. doi: 10.1162/0898929054985491
72. Hu Y, Jiang Y, Hu P, Wang K. Dissociation between affective and cognitive empathy in patients with idiopathic generalized epilepsy. Chin J Neurol. (2014) 47:528–33. doi: 10.3760/cma.j.issn.1006-7876.2014.08.004
73. Toller G, Adhimoolam B, Rankin KP, Huppertz HJ, Kurthen M, Jokeit H. Right fronto-limbic atrophy is associated with reduced empathy in refractory unilateral mesial temporal lobe epilepsy. Neuropsychologia. (2015) 78:80–7. doi: 10.1016/j.neuropsychologia.2015.09.010
74. Operto FF, Pastorino GMG, Mazza R, Di Bonaventura C, Marotta R, Pastorino N, et al. Social cognition and executive functions in children and adolescents with focal epilepsy. Eur J Paediatr Neurol. (2020) 28:167–75. doi: 10.1016/j.ejpn.2020.06.019
75. Lomlomdjian C, Múnera CP, Low DM, Terpiluk V, Solís P, Abusamra V, et al. The right hemisphere’s contribution to discourse processing: a study in temporal lobe epilepsy. Brain Lang. (2017) 171:31–41. doi: 10.1016/j.bandl.2017.04.001
76. Sung C, Connor A. Social-cognitive predictors of vocational outcomes in transition youth with epilepsy: application of social cognitive career theory. Rehabil Psychol. (2017) 62:276–89. doi: 10.1037/rep0000161
77. Abraira L, Sanabria A, Ortega G, Quintana M, Santamarina E, Salas-Puig J, et al. [Social cognition and cognitive functions in patients with epilepsy treated with eslicarbazepine acetate]. Rev Neurol. (2018) 66:361–7.
78. İnanç L, Ünal Y, Semiz ÜB, Kutlu G. Do mentalization skills affect the perception of stigma in patients with epilepsy? Epilepsy Behav. (2018) 88:49–53. doi: 10.1016/j.yebeh.2018.08.022
79. Grewe P, Schulz R, Woermann FG, Brandt C, Doll A, Hoppe M, et al. Very long-term outcome in resected and non-resected patients with temporal lobe epilepsy with medial temporal lobe sclerosis: a multiple case-study. Seizure. (2019) 67:30–7. doi: 10.1016/j.seizure.2019.02.015
80. Giovagnoli AR, Canafoglia L, Reati F, Raviglione F, Franceschetti S. The neuropsychological pattern of Unverricht-Lundborg disease. Epilepsy Res. (2009) 84:217–23. doi: 10.1016/j.eplepsyres.2009.02.004
81. Toller G, Adhimoolam B, Grunwald T, Huppertz HJ, Kurthen M, Rankin KP, et al. Right mesial temporal lobe epilepsy impairs empathy-related brain responses to dynamic fearful faces. J Neurol. (2015) 262:729–41. doi: 10.1007/s00415-014-7622-2
82. Kuchukhidze G, Höfler J, Kronbichler M, Schmid E, Kirschner M, Rainer L, et al. Emotion recognition and social cognition in juvenile myoclonic epilepsy. Z Epileptol. (2019) 32:177–82. doi: 10.1007/s10309-019-0261-y
83. Lunn J, Lewis C, Gannon E. Parent-child mentalizing in pediatric epilepsy. Epilepsy Behav. (2019) 96:6–12. doi: 10.1016/j.yebeh.2019.03.052
84. Shiota MN, Simpson ML, Kirsch HE, Levenson RW. Emotion recognition in objects in patients with neurological disease. Neuropsychology. (2019) 33:1163–73. doi: 10.1037/neu0000587
85. Operto FF, Pastorino GMG, Padovano C, Scuoppo C, Vivenzio V, Coppola G. Social cognition in neuropsychiatric disorders in pediatric age. Brain. (2020) 11:81–8. doi: 10.18662/brain/11.3Sup1/124
86. Genizi J, Shamay-Tsoory SG, Shahar E, Yaniv S, Aharon-Perez J. Impaired social behavior in children with benign childhood epilepsy with centrotemporal spikes. J Child Neurol. (2012) 27:156–61. doi: 10.1177/0883073811414420
87. Lew AR, Lewis C, Lunn J, Tomlin P, Basu H, Roach J, et al. Social cognition in children with epilepsy in mainstream education. Dev Med Child Neurol. (2015) 57:53–9. doi: 10.1111/dmcn.12613
88. Lunn J, Lewis C, Sherlock C. Impaired performance on advanced theory of Mind tasks in children with epilepsy is related to poor communication and increased attention problems. Epilepsy Behav. (2015) 43:109–16. doi: 10.1016/j.yebeh.2014.11.010
89. Raud T, Kaldoja ML, Kolk A. Relationship between social competence and neurocognitive performance in children with epilepsy. Epilepsy Behav. (2015) 52:93–101. doi: 10.1016/j.yebeh.2015.08.028
90. Zilli T, Zanini S, Conte S, Borgatti R, Urgesi C. Neuropsychological assessment of children with epilepsy and average intelligence using NEPSY II. J Clin Exp Neuropsychol. (2015) 37:1036–51. doi: 10.1080/13803395.2015.1076380
91. Stewart E, Catroppa C, Gill D, Webster R, Lawson J, Mandalis A, et al. Theory of mind and social competence in children and adolescents with genetic generalised epilepsy (GGE): relationships to epilepsy severity and anti-epileptic drugs. Seizure. (2018) 60:96–104. doi: 10.1016/j.seizure.2018.06.015
92. Zhang T, Chen L, Wang Y, Zhang M, Wang L, Xu X, et al. Impaired theory of mind in Chinese children and adolescents with idiopathic generalized epilepsy: association with behavioral manifestations of executive dysfunction. Epilepsy Behav. (2018) 79:205–12. doi: 10.1016/j.yebeh.2017.12.006
93. Stewart E, Catroppa C, Gonzalez L, Gill D, Webster R, Lawson J, et al. Theory of mind and social competence in children and adolescents with temporal lobe epilepsy. Neuropsychology. (2019) 33:986–95. doi: 10.1037/neu0000543
94. Bailey K, Im-Bolter N. Theory of mind and language in childhood epilepsy. Mind Brain Educ. (2020) 14:146–54. doi: 10.1111/mbe.12230
95. Lima EM, Rzezak P, Montenegro MA, Guerreiro MM, Valente KDR. Social cognition in childhood epilepsy with centrotemporal spikes. Seizure. (2020) 78:102–8. doi: 10.1016/j.seizure.2020.03.014
96. Pastorino GMG, Operto FF, Padovano C, Vivenzio V, Scuoppo C, Pastorino N, et al. Social cognition in neurodevelopmental disorders and epilepsy. Front Neurol. (2021) 12:658823. doi: 10.3389/fneur.2021.658823
97. Wu L, Yang X, Zhang K, Wang X, Yang B. Impairment of eye emotion discrimination in benign childhood epilepsy with centrotemporal spikes: a neuropsychological study. Brain Behav. (2021) 11:e02154. doi: 10.1002/brb3.2154
98. Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS. The impact of early and late damage to the human amygdala on ‘theory of mind’ reasoning. Brain. (2004) 127:1535–48. doi: 10.1093/brain/awh168
99. Farrant A, Morris RG, Russell T, Elwes R, Akanuma N, Alarcón G, et al. Social cognition in frontal lobe epilepsy. Epilepsy Behav. (2005) 7:506–16. doi: 10.1016/j.yebeh.2005.07.018
100. Schacher M, Winkler R, Grunwald T, Kraemer G, Kurthen M, Reed V, et al. Mesial temporal lobe epilepsy impairs advanced social cognition. Epilepsia. (2006) 47:2141–6. doi: 10.1111/j.1528-1167.2006.00857.x
101. Giovagnoli AR, Franceschetti S, Reati F, Parente A, MacCagnano C, Villani F, et al. Theory of mind in frontal and temporal lobe epilepsy: cognitive and neural aspects. Epilepsia. (2011) 52:1995–2002. doi: 10.1111/j.1528-1167.2011.03215.x
102. Broicher SD, Kuchukhidze G, Grunwald T, Krämer G, Kurthen M, Jokeit H. “Tell me how do I feel”–emotion recognition and theory of mind in symptomatic mesial temporal lobe epilepsy. Neuropsychologia. (2012) 50:118–28. doi: 10.1016/j.neuropsychologia.2011.11.005
103. Giovagnoli AR, Parente A, Villani F, Franceschetti S, Spreafico R. Theory of mind and epilepsy: what clinical implications? Epilepsia. (2013) 54:1639–46. doi: 10.1111/epi.12255
104. Li YH, Chiu MJ, Yeh ZT, Liou HH, Cheng TW, Hua MS. Theory of mind in patients with temporal lobe epilepsy. J Int Neuropsychol Soc. (2013) 19:594–600. doi: 10.1017/S1355617713000143
105. Amlerova J, Cavanna AE, Bradac O, Javurkova A, Raudenska J, Marusic P. Emotion recognition and social cognition in temporal lobe epilepsy and the effect of epilepsy surgery. Epilepsy Behav. (2014) 36:86–9. doi: 10.1016/j.yebeh.2014.05.001
106. Cohn M, St-Laurent M, Barnett A, McAndrews MP. Social inference deficits in temporal lobe epilepsy and lobectomy: risk factors and neural substrates. Soc Cogn Affect Neurosci. (2015) 10:636–44. doi: 10.1093/scan/nsu101
107. Hennion S, Delbeuck X, Duhamel A, Lopes R, Semah F, Tyvaert L, et al. Characterization and prediction of theory of mind disorders in temporal lobe epilepsy. Neuropsychology. (2015) 29:485–92. doi: 10.1037/neu0000126
108. Realmuto S, Zummo L, Cerami C, Agrò L, Dodich A, Canessa N, et al. Social cognition dysfunctions in patients with epilepsy: evidence from patients with temporal lobe and idiopathic generalized epilepsies. Epilepsy Behav. (2015) 47:98–103. doi: 10.1016/j.yebeh.2015.04.048
109. Wang WH, Shih YH, Yu HY, Yen DJ, Lin YY, Kwan SY, et al. Theory of mind and social functioning in patients with temporal lobe epilepsy. Epilepsia. (2015) 56:1117–23. doi: 10.1111/epi.13023
110. Giorgi FS, Guida M, Caciagli L, Pagni C, Pizzanelli C, Bonanni E, et al. Social cognition in juvenile myoclonic epilepsy. Epilepsy Res. (2016) 128:61–7. doi: 10.1016/j.eplepsyres.2016.10.017
111. Giovagnoli AR, Parente A, Didato G, Deleo F, Villani F. Expanding the spectrum of cognitive outcomes after temporal lobe epilepsy surgery: a prospective study of theory of mind. Epilepsia. (2016) 57:920–30. doi: 10.1111/epi.13384
112. Okruszek Ł, Bala A, Dziekan M, Szantroch M, Rysz A, Marchel A, et al. Gaze matters! The effect of gaze direction on emotional enhancement of memory for faces in patients with mesial temporal lobe epilepsy. Epilepsy Behav. (2017) 72:35–8. doi: 10.1016/j.yebeh.2017.04.016
113. Okruszek Ł, Bala A, Wordecha M, Jarkiewicz M, Wysokiński A, Szczepocka E, et al. Social cognition in neuropsychiatric populations: a comparison of theory of mind in schizophrenia and mesial temporal lobe epilepsy. Sci Rep. (2017) 7:484. doi: 10.1038/s41598-017-00565-2
114. Bala A, Okruszek Ł, Piejka A, Głêbicka A, Szewczyk E, Bosak K, et al. Social perception in mesial temporal lobe epilepsy: interpreting social information from moving shapes and biological motion. J Neuropsychiatry Clin Neurosci. (2018) 30:228–35. doi: 10.1176/appi.neuropsych.17080153
115. Morou N, Papaliagkas V, Markouli E, Karagianni M, Nazlidou E, Spilioti M, et al. Theory of mind impairment in focal versus generalized epilepsy. Epilepsy Behav. (2018) 88:244–50. doi: 10.1016/j.yebeh.2018.09.026
116. Bauer J, Kegel LC, Steiger BK, Jokeit H. Assessment tools for social cognition in epilepsy. Z Epileptol. (2019) 32:183–6. doi: 10.1007/s10309-019-0260-z
117. Javor A, Ciumas C, Ibarrola D, Ryvlin P, Rheims S. Social cognition, behaviour and therapy adherence in frontal lobe epilepsy: a study combining neuroeconomic and neuropsychological methods. R Soc Open Sci. (2019) 6:180850. doi: 10.1098/rsos.180850
118. Giovagnoli AR, Tallarita GM, Parente A, Pastori C, de Curtis M. The understanding of mental states and the cognitive phenotype of frontal lobe epilepsy. Epilepsia. (2020) 61:747–57. doi: 10.1111/epi.16457
119. Gürsoy SC, Ergün S, Midi Ý, Topçuoðlu V. Theory of mind and its relationship with alexithymia and quality of life in patients with psychogenic nonepileptic seizures: comparisons with generalised epilepsy and healthy controls. Seizure. (2021) 91:251–7. doi: 10.1016/j.seizure.2021.06.032
120. Giovagnoli AR, Parente A, Ciuffini R, Tallarita GM, Turner K, Maialetti A, et al. Diversified social cognition in temporal lobe epilepsy. Acta Neurol Scand. (2021) 143:396–406. doi: 10.1111/ane.13386
121. Jasionis A, Puteikis K, Mameniškienė R. The impact of social cognition on the real-life of people with epilepsy. Brain Sci. (2021) 11:877. doi: 10.3390/brainsci11070877
122. Abu-Akel A, Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia. (2011) 49:2971–84. doi: 10.1016/j.neuropsychologia.2011.07.012
123. Molenberghs P, Johnson H, Henry JD, Mattingley JB. Understanding the minds of others: a neuroimaging meta-analysis. Neurosci Biobehav Rev. (2016) 65:276–91. doi: 10.1016/j.neubiorev.2016.03.020
124. Bartlett PA, Symms MR, Free SL, Duncan JS. T2 relaxometry of the hippocampus at 3T. AJNR Am J Neuroradiol. (2007) 28:1095–8. doi: 10.3174/ajnr.A0505
125. Riederer F, Lanzenberger R, Kaya M, Prayer D, Serles W, Baumgartner C. Network atrophy in temporal lobe epilepsy: a voxel-based morphometry study. Neurology. (2008) 71:419–25. doi: 10.1212/01.wnl.0000324264.96100.e0
126. Frings L, Schulze-Bonhage A, Spreer J, Wagner K. Remote effects of hippocampal damage on default network connectivity in the human brain. J Neurol. (2009) 256:2021–9. doi: 10.1007/s00415-009-5233-0
127. Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. (2009) 34:418–32.
128. Kakeda S, Korogi Y. The efficacy of a voxel-based morphometry on the analysis of imaging in schizophrenia, temporal lobe epilepsy, and Alzheimer’s disease/mild cognitive impairment: a review. Neuroradiology. (2010) 52:711–21. doi: 10.1007/s00234-010-0717-2
129. Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. (2010) 5:e8525. doi: 10.1371/journal.pone.0008525
130. Broicher SD, Frings L, Huppertz HJ, Grunwald T, Kurthen M, Krämer G, et al. Alterations in functional connectivity of the amygdala in unilateral mesial temporal lobe epilepsy. J Neurol. (2012) 259:2546–54. doi: 10.1007/s00415-012-6533-3
131. Ives-Deliperi VL, Jokeit H. Impaired social cognition in epilepsy: a review of what we have learnt from neuroimaging studies. Front Neurol. (2019) 10:940. doi: 10.3389/fneur.2019.00940
132. Strikwerda-Brown C, Ramanan S, Irish M. Neurocognitive mechanisms of theory of mind impairment in neurodegeneration: a transdiagnostic approach. Neuropsychiatr Dis Treat. (2019) 15:557–73. doi: 10.2147/ndt.s158996
133. Gleichgerrcht E, Tomashitis B, Sinay V. The relationship between alexithymia, empathy and moral judgment in patients with multiple sclerosis. Eur J Neurol. (2015) 22:1295–303. doi: 10.1111/ene.12745
134. Patil I, Young L, Sinay V, Gleichgerrcht E. Elevated moral condemnation of third-party violations in multiple sclerosis patients. Soc Neurosci. (2017) 12:308–29. doi: 10.1080/17470919.2016.1175380
135. Golde S, Heine J, Pöttgen J, Mantwill M, Lau S, Wingenfeld K, et al. Distinct functional connectivity signatures of impaired social cognition in multiple sclerosis. Front Neurol. (2020) 11:507. doi: 10.3389/fneur.2020.00507
136. Yu CL, Chou TL. A dual route model of empathy: a neurobiological prospective. Front Psychol. (2018) 9:2212. doi: 10.3389/fpsyg.2018.02212
137. Bernhardt BC, Rozen DA, Worsley KJ, Evans AC, Bernasconi N, Bernasconi A. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. Neuroimage. (2009) 46:373–81. doi: 10.1016/j.neuroimage.2009.01.055
138. O’Muircheartaigh J, Vollmar C, Barker GJ, Kumari V, Symms MR, Thompson P, et al. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology. (2011) 76:34–40. doi: 10.1212/WNL.0b013e318203e93d
139. Ronan L, Alhusaini S, Scanlon C, Doherty CP, Delanty N, Fitzsimons M. Widespread cortical morphologic changes in juvenile myoclonic epilepsy: evidence from structural MRI. Epilepsia. (2012) 53:651–8. doi: 10.1111/j.1528-1167.2012.03413.x
140. Tondelli M, Vaudano AE, Ruggieri A, Meletti S. Cortical and subcortical brain alterations in juvenile absence epilepsy. Neuroimage Clin. (2016) 12:306–11. doi: 10.1016/j.nicl.2016.07.007
141. Guida M, Caciagli L, Cosottini M, Bonuccelli U, Fornai F, Giorgi FS. Social cognition in idiopathic generalized epilepsies and potential neuroanatomical correlates. Epilepsy Behav. (2019) 100:106118. doi: 10.1016/j.yebeh.2019.01.003
142. Kuang Y, Yang T, Gu J, Kong B, Cheng L. Comparison of therapeutic effects between selective amygdalohippocampectomy and anterior temporal lobectomy for the treatment of temporal lobe epilepsy: a meta-analysis. Br J Neurosurg. (2014) 28:374–7. doi: 10.3109/02688697.2013.841854
143. Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci. (2013) 8:123–33. doi: 10.1093/scan/nss119
Keywords: theory of mind, empathy, epilepsy, meta-analysis, cognitive, affective
Citation: Wang H, Zhao P, Zhao J, Zhong J, Pan P, Wang G and Yi Z (2022) Theory of Mind and Empathy in Adults With Epilepsy: A Meta-Analysis. Front. Psychiatry 13:877957. doi: 10.3389/fpsyt.2022.877957
Received: 17 February 2022; Accepted: 04 April 2022;
Published: 27 April 2022.
Edited by:
Ulrike M. Krämer, University of Lübeck, GermanyReviewed by:
Kai Wang, Anhui Medical University, ChinaDaniel Joel Shaw, Aston University, United Kingdom
Copyright © 2022 Wang, Zhao, Zhao, Zhong, Pan, Wang and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: ZhongQuan Yi, eWl6aG9uZ3F1YW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
 HongZhou Wang1
HongZhou Wang1 PanWen Zhao
PanWen Zhao PingLei Pan
PingLei Pan ZhongQuan Yi
ZhongQuan Yi