
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 10 May 2022
Sec. Psychopathology
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.873953
This article is part of the Research TopicCommunity Series in Overeating and Decision Making VulnerabilitiesView all 6 articles
 Xiao-Dong Han1†
Xiao-Dong Han1† Hong-Wei Zhang1†
Hong-Wei Zhang1† Ting Xu1
Ting Xu1 Lin Liu1
Lin Liu1 Hui-Ting Cai2
Hui-Ting Cai2 Zi-Qi Liu2
Zi-Qi Liu2 Qing Li3
Qing Li3 Hui Zheng2*
Hui Zheng2* Tao Xu4,5*
Tao Xu4,5* Ti-Fei Yuan2,6,7*
Ti-Fei Yuan2,6,7*Impulsiveness is a stable personal characteristic that contributes to obesity and may interact with it. Specifically, obesity is caused by unrestrained impulse eating that is not consciously controlled and leads to a hormonal imbalance that also can impair impulse control. However, the mechanism of this relationship is unclear. In our study, 35 obese individuals (body mass index, BMI > 28) were recruited and matched with 31 healthy controls (BMI < 24) in age and education level. All the participants underwent a resting-state fMRI and completed the Barratt Impulsiveness Scale-11. The results showed that patients with obesity had a significantly lower fractional amplitude of low-frequency fluctuations (fALFF) in the bilateral dorsolateral prefrontal cortex (dlPFC) and higher fALFF in the left fusiform cortex. In addition, non-planning impulsiveness was positively correlated with BMI. Importantly, we found that the right dlPFC completely mediated the relationship between non-planning impulsiveness and BMI. Our findings suggest that impulsivity is statistically more likely to precede obesity than to precede impulsivity and contributes to obesity by downregulating spontaneous activity in the dlPFC. This suggests that the dlPFC, which is associated with executive control, may be able a potential target for treating obesity.
Currently, humans can rapidly obtain food and therefore require more effort to control their appetites and eat healthily. The personality trait of impulsiveness is a risk factor for overeating and obesity (1). Beyond mere cosmetic concerns, obesity also poses a significant health risk for individuals, as it can lead to diabetes, cardiovascular disease, and cognitive impairment (2). The prevalence of obesity has increased globally since 1950, as 13% of adults (18 years or older) were obese in 2016 (3). Imbalances in energy consumption lead to obesity if more calories are consumed and/or less energy is expended (4). The link between impulsiveness and obesity is relatively straightforward: children that struggle with delayed gratification and poor impulse control are more likely to eat high-fat foods when they are available (5). One survey showed that patients with obesity are higher in impulsiveness (6), and impulsiveness can also significantly predict the development and treatment of obesity (7). Although there may be more complex associations between impulsiveness and obesity, excess body fat deposits (whether caused by diet or otherwise) have been shown to lead to low-grade inflammation and alter the production of hormones that affect neuronal structure and function related to motivation and impair executive control (8).
Obesity alters subcortical structures (i.e., the basal ganglia) that are related to motivation as well as cortical structures (i.e., the prefrontal cortex [PFC]) that are related to executive functions (9). In classic theory, the most powerful drive toward overeating is the motivation of hunger and cravings for specific food. Motivation-related subcortical structures include the nucleus accumbens (NAcc), which is related to reward, the amygdala, which is related to fear, and the striatum, which is related to habitual behaviors (10). A recent UK Biobank study showed that patients with obesity have lower gray matter volume of subcortical areas (the thalamus, caudate nucleus, putamen, globus pallidus, hippocampus, nucleus accumbens, and amygdala) and aberrant white matter microstructure (11). The behavioral outcome of the personality trait of impulsiveness seems to be a reduction in executive control that can lead to overeating. A brain perfusion scan study showed that in response to drinking glucose, obese adolescents show reduced cerebral perfusion in brain regions involved in executive function (the PFC) and increased perfusion in areas of the brain involved in homeostasis (the hypothalamus) (12). An magnetic resonance spectroscopy study demonstrated blunted brain energy consumption and impaired systematic glucose uptake in obese participants compared with normal-weight participants, indicating neuroenergetic dysregulation in the insula (13). However, it remains unclear which specific brain regions play an important role in impulsiveness and obesity.
The dorsolateral prefrontal cortex (dlPFC) is a key region of executive control, which is also involved in impulsive decision-making (14) and is correlated with impulsiveness (15). The resting-state functional magnetic resonance imaging (rs-fMRI) index seems to reflect personality (16). ALFF is a common resting-state fMRI index that indicates improvement in brain functions and provides a potential target for clinical settings, such as neuromodulation. Low frequency (typically 0.01–0.08 Hz) fluctuation is a stable indicator of spontaneous neural activity (17) that has been used to detect changes in brain state in various diseases, including Alzheimer's disease (18), schizophrenia (19), and obesity (20). In 2018, one study found that the ALFF of the hippocampus in 30 obese patients decreased significantly after bariatric surgery and was negatively correlated with postoperative leptin and anxiety (21). In addition, this study found that 1 month postoperatively, the ALFF was changed and that there was not a group difference between patients with obesity and healthy controls (HCs). This may be because the ALFF is sensitive to physiological noise and not very robust. Previous studies have proposed utilizing the fractional ALFF (fALFF) method, that is, the ratio of the power spectrum of the low-frequency range (0.01–0.08 Hz) to the power spectrum of the entire frequency range, which more accurately reflects the intensity of brain activity (22).
Recent evidence has suggested that patients with obesity show increased impulsiveness and that increased impulsiveness also predicts a higher body mass index (BMI) (23–25). It is also known that executive function regions such as the dlPFC play an important role in moderating impulsiveness (26). However, it is extremely challenging to determine exactly how impulsiveness interacts with obesity, especially since the brain modifies this relationship. Evidence on the mediating role of resting-state brain activity in the relationship between impulsiveness and obesity is lacking. In this study, we performed a resting-state analysis to map fALFF patterns and correlated those patterns with BMI. The results of this investigation could be a major step toward understanding the precise relationship between impulsiveness and obesity. We hypothesized that (1) altered spontaneous activity would be observed in the prefrontal region and that (2) this activity would mediate impulsiveness and BMI. This study was preregistered in the open science framework (https://osf.io/rkwn8).
From September 2020 to December 2021, we recruited 37 obese subjects (BMI > 28, age from 19.95 to 49 years old, 7 male) through the Weight Loss Metabolism Clinic in China and 33 healthy volunteer (BMI < 24, age from 21.59 to 56.3 years old, 9 male) matched on demographic factors through local advertisements. According to the data processing flow, the magnetic resonance data of the last 35 obese patients and 31 healthy patients were included in the analysis. For the specific quality control of the subjects, see the data processing flow section. We found that these two groups have significant differences in BMI and non-planing impulsiveness. There was no group difference in Gender, Diabetic comorbidities, age, and years of education. For more information, see Table 1. All participants (1) were at least 18 years old; (2) could read and understand the description of each item in the questionnaire; and (3) signed written consent. There were exclusion criteria in both groups for people with neurologic diseases or other psychiatric disorders. (1) history of head trauma, (2) neurodevelopmental (intellectual disability), (3) neurocognitive (dementia) conditions, 4 addiction or substance use disorder. The ethics committee of Shanghai Sixth People's Hospital approved the study. All procedures were in accordance with the Declaration of Helsinki.
First, all participants were briefed on the goal of the experiment and completed the informed consent form. Then, basic demographic information (age, education level, and gender) was collected, and BMIs (equal to [weight in kilograms divided by height in meters]2) were calculated to determine the degree of obesity. Second, participants completed the Chinese version of the Barratt Impulsiveness Scale-11 (BIS-11) and underwent a 12-min MRI scan. Two participants in the HC group (2/33) didn't finish the main questionnaire, so remove from the following analysis.
The BIS-11 is a widely used 30-item scale that measures trait impulsiveness in adults (27). The Chinese version of the BIS-11 consists of three subscales: non-planning impulsiveness (non-plan imp), motor impulsiveness (motor imp), and cognitive impulsiveness (cog imp). Cognitive impulsiveness reflects impulsive thoughts and anticipation of the results of actions. Motor impulsiveness reflects the impulse to act. Non-planning impulsiveness reflects a lack of future planning (28). Each item was measured on a 5-point Likert score from 1 “never” to 5 “very often.” The score range for each subscale is from 10 to 50 points; the subscale scores and the total score are converted to a range of 0 to 100 points. That is, the score of each subscale = [(Sum of points of each item−10) ÷ 40] × 100,” and the average imp score = the sum of the three subscales/3. Higher scores indicate higher impulsiveness.
MRI data were acquired by a 3.0-Tesla Siemens Prisma scanner with a 64-channel head coil at the Shanghai Sixth People's Hospital (Shanghai, China). The fMRI images were acquired using an echo-planar imaging pulse sequence with the following parameters: total volume = 210, repetition time = 2,000 ms, echo time = 30 ms, flip angle = 90°, number of slices = 33, transverse orientation, field of view = 224 × 224 mm2, matrix size = 64 × 64, slice thickness = 3.5 mm. The structural images were acquired using a magnetization-prepared rapid gradient-echo sequence with the following parameters: repetition time = 2,000 ms, echo time 2.32 ms, inversion time = 900 ms, flip angle = 8°, number of slices = 208, field of view = 230 × 230 mm2, matrix size = 256 × 256, slice thickness = 0.9 mm.
The Data Processing Assistant for Resting-State fMRI v5.2 (http://rfmri.org/dparsf) was used to preprocess and analyse the MRI data. To prevent instability in the data, the first 10 volumes were discarded. After slice timing, reorientation, and realignment to the middle slice, T1 coregistration was applied. A skull-stripped T1 image was segmented using Diffeomorphic Anatomical Registration Through Exponentiated Lie (DARTEL) algebra. Covariances associated with nuisance variables were then regressed out. Motion was controlled with a 24-parameter Friston model (29). We excluded subjects if their head movements or rotation were >2.0° or 2.0 mm, resulting in the exclusion of two patients (2/37) and zero healthy controls (0/31). A combination of cerebrospinal fluid, white matter, and global signals reduced physiological artifacts (29). The DARTEL tool was used to compute transformations from individual native space to Montreal Neurological Institute space of 3 × 3 × 3 mm3. Then, we calculated the fALFF. A fast Fourier transform was used to transform the filtered time series of each voxel into a frequency domain and subsequently into a power spectrum. In each voxel, the square root of the signal was measured over 0.01–0.08 Hz. The fALFF was normalized through transformation to a z score. Finally, a 4-mm full-width, half-maximum Gaussian kernel was used for spatial smoothing.
Imaging differences between obesity and HC were measured using a two-sample t-test and p < 0.001 for voxels, p < 0.05 for clusters, GRF corrected. The statistical analysis and plotting were based on Jamovi v2.2.2 (https://www.jamovi.org/) and GraphPad v 9.0 (https://www.graphpad.com/). The summaries of quantitative variables with a normal distribution are expressed as the means and standard deviations; categorical data are expressed as rates. The group comparisons between obese and healthy controls were performed by independent t-tests (the comparison of the gender distribution in each group was verified by a χ2 test). Then, we used the Pearson correlation analysis to detect the correlation between brain activity and impulsiveness. For an original significance of α = 0.05, we used the Bonferroni correction for multiple testing (α' = α/m, where m is the number of comparisons; in this study, m = 9).
We used a mediation test to explore the mediating effect of spontaneous brain activity on the relationship between impulsiveness personality and obesity. We used WebPower (http://psychstat.org/mediation) to calculate the sample size (path a = −0.5, path b = −0.5, power = 0.8) for this mediation analysis. We used the general linear model mediation model (jamm Advanced Mediation Models v1.0.5) in Jamovi to perform the mediation analysis. A normal bootstrap method was used to construct a 95% confidence interval for significance testing of the mediating effects. The mediation analysis was to test the hypothesis that spontaneous brain activity mediated the relationship between impulsiveness and BMI. We also checked the reverse model, that spontaneous brain activity mediates BMI, which influences non-planning impulsiveness (see Supplementary Material). Following the mediation test, we also performed hierarchical clustering based on the significant brain activity and impulsiveness to explore how the impulsiveness and brain activity will be the intermediate phenotype for obesity. And after that, the χ2 test for association was used to test the association between brain activity and impulsiveness. The number of participants finally included in all data was 35+31, a total of 66.
We found that patients with obesity had a lower fALFF in the bilateral dlPFC (x = −30, y = 45, z = 30, k = 122, max-t = −5.62; x = 30, y = 45, z = 30, max-t = −4.91, k = 95) and a higher fALFF in the left fusiform region (x = −33, y = −51, z = −6, max-t = 5.15, k = 54). More detail is shown in Figure 1.
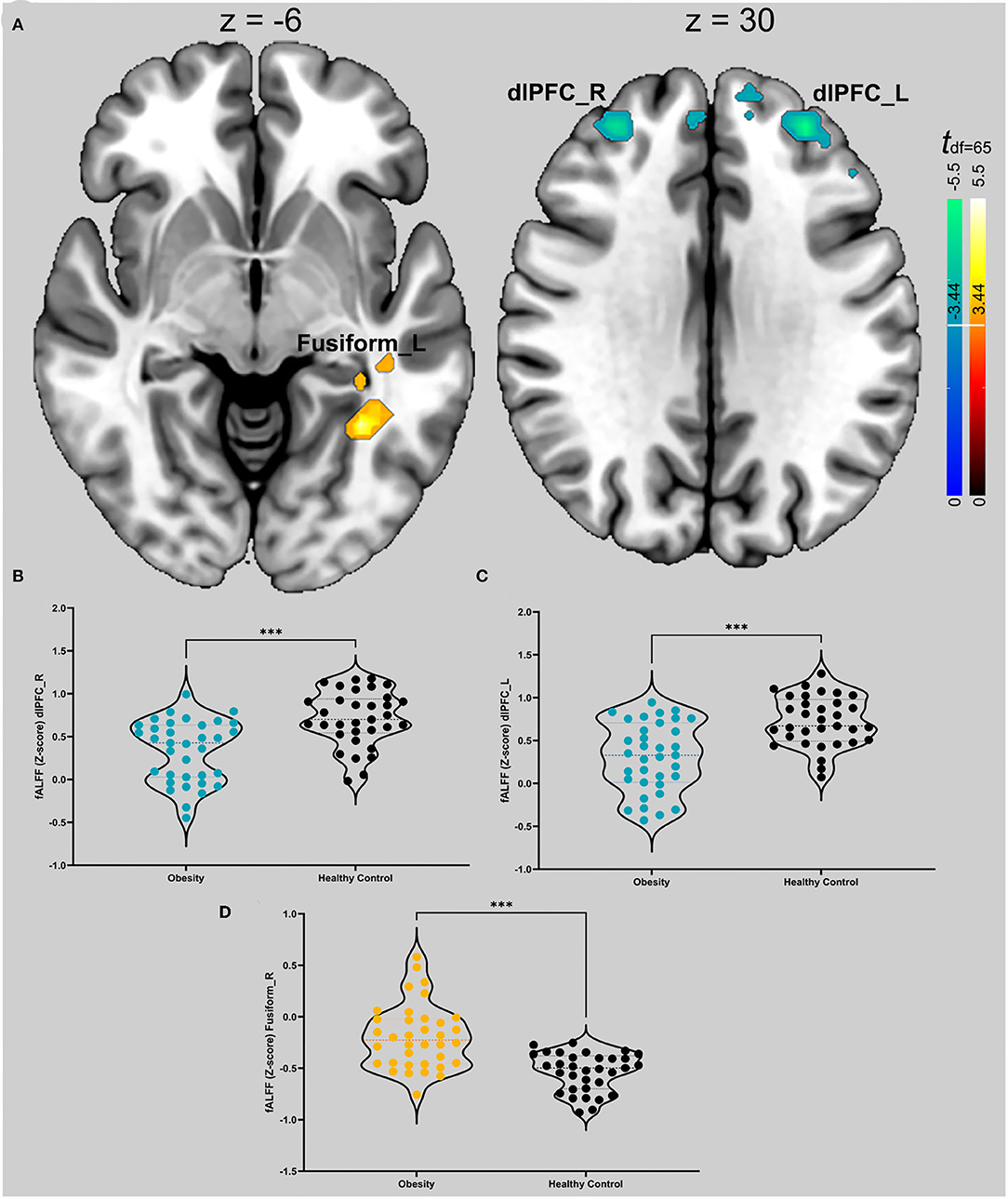
Figure 1. (A) t map of differences in spontaneous activity of the groups. Hot colors (red to bright yellow) indicate that obese patients have higher spontaneous brain activity in those regions; cool colors (blue) indicate that obese patients have lower spontaneous activity. The thresholds for the two comparisons are ±3.44. The slice on the graph is the cross section of the vertical axis with the maximum threshold. (B) Violin diagrams of the intensity values corresponding to the dlPFC of the two groups on the right. The colored dots in the figure are obese patients, and the black dots are healthy controls. The dotted lines represent the first, second, and third quantiles. We used an independent samples t test. *** Indicates p < 0.001 in (A–D). (C) Violin graphs of the intensity values corresponding to the left dlPFC in the two groups. (D) Violin graphs of the intensity values corresponding to the left fusiform gyrus in the two groups.
The Pearson correlation analysis showed that non-planning impulsiveness was positively correlated with BMI (df = 65, r = 0.34, p = 0.005) and negatively correlated with the fALFF in the right dlPFC (df = 65, r = −0.38, p = 0.002); the fALFF in the right dlPFC was negatively correlated with BMI (df = 65, r = −0.52, p < 0.001) (Figures 2A–C).
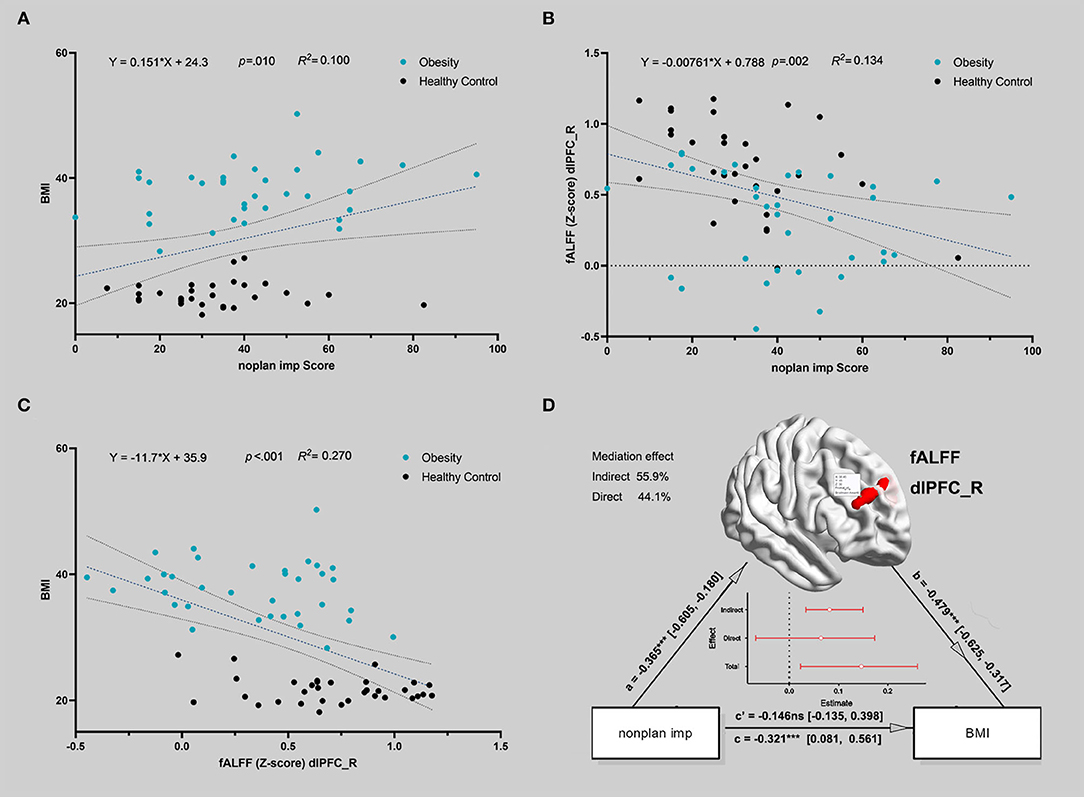
Figure 2. The correlation between the activity of the brain region during delayed gratification and the severity of IGD. (A) Non-planning impulsiveness and body mass index (BMI) were positively correlated in all participants. The blue dots represent patients, and the black dots represent healthy controls. A linear fit was conducted, and the dotted line indicates the 95% confidence interval of the dotted line (in A–C). (B) Non-planning impulsiveness and the fALFF in the right dlPFC were negatively correlated in all participants. (C) The fALFF in the right dlPFC and BMI were negatively correlated in all participants. (D) The indirect path (via lower fALFF in the right dlPFC) completely mediated the relationship between non-planning impulsiveness and BMI for all participants. The red line in the figure represents the standardized estimation coefficient; the y-intercept indicates that spontaneous brain activity mediates the influence of impulsivity on obesity.
Figure 2D shows that the indirect path via a lower fALFF (a*b, beta = 0.175, standard error = 0.065, 95% confidence interval = [0.075, 0.329], Z = 2.697, p = 0.007, mediation = 54.48%) completely mediated the direct path from non-planning impulsiveness to BMI (c, beta = 0.146, standard error = 0.133, 95% confidence interval = [−0.135, 0.398], Z = 1.098, p = 0.272, mediation = 45.52%). Additionally, the reverse model was estimated, and its results did not support the opposite relationship, that spontaneous brain activity mediated BMI and led to more non-planning impulsiveness. This model is discussed in more detail in the Supplementary Material. The χ2 test on the hierarchical clustering (including the fALFF in right dlPFC and non-planning impulsiveness score) also showed a higher rate of Cluster 1 in the obese group than in the HC group (χ2 value = 15.241, p < 0.001) (Figure 3).
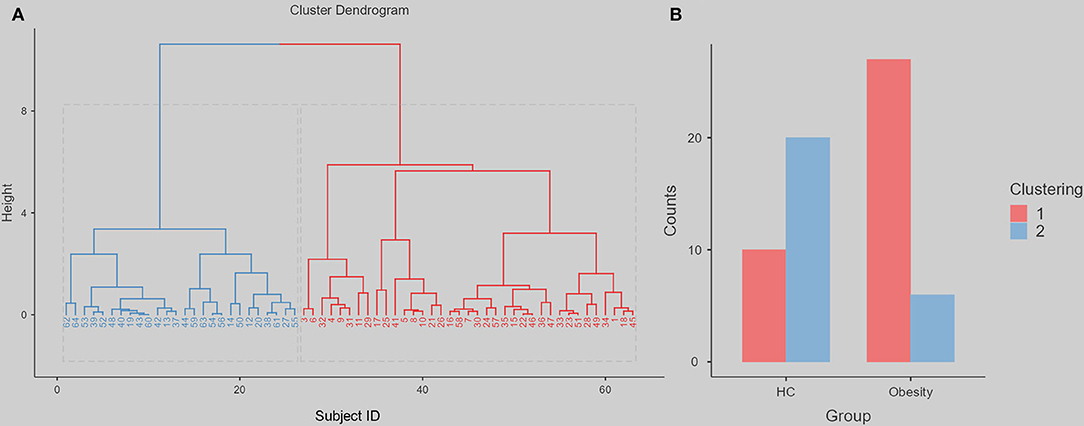
Figure 3. The hierarchical clustering dendrogram and following difference between obesity group and HC. We use the height 8, can divide all the participants into two clustering. The red represents clustering 1, the blue represents clustering 2. (A) The Cluster Dendrogram. (B) The Group Counts for each clustering.
This study aimed to explore whether brain activity can mediate the relationship between individual impulsiveness and obesity. Our analysis yielded two main results. This study was the first to find that obese patients have significantly lower spontaneous brain activity in the dlPFC, which supports our first hypothesis. Second, we found that the fALFF in the right dlPFC completely mediated the relationship between non-planning impulsiveness and obesity. These results indicate that spontaneous resting brain activity is a potential biomarker for obesity-related impulsiveness and regulates obesity outcomes.
Resting-state fMRI seems to be more strongly correlated with impulsive personality traits than obesity. First, not all the trait impulsiveness subscales correlate with obesity (30). In our study, we found that patients with obesity had increased non-planning impulsiveness than that of HCs. The results we obtained are not entirely consistent with previous results. The majority of previous surveys of obese patients showed higher impulsivity to exercise or attention than healthy individuals (25). Only a few studies related to food addiction have reported that unplanned impulsivity predicts BMI (31), which may indicate a significant number of potential food addicts in obese people. Further unplanned impulses also modulate the efficacy of transcranial direct current stimulation as an appetite intervention in obese patients (32). The relationship between non-planning impulsiveness and loss of control during a Binge maybe indicate the underlaying brain function altered in obesity (33). We found a significant decrease in the fALFF in the bilateral dlPFC in patients with obesity, which may indicate that the fALFF in the dlPFC is a relatively stable potential biomarker for obesity. Previous studies have reported many changes in brain regions in obese patients, such as decreased ALFF values in the hippocampus and putamen after bariatric surgery (20). Our findings contribute new evidence about cortical activity in obese patients. A previous meta-analysis showed that changes in the dlPFC are common in patients with obesity and patients with substance use disorders (34). This may be because obese patients undergo outpatient bariatric and metabolic surgery and therefore have more difficulty reducing food cravings, similar to a food addiction (35).
The dlPFC results may provide new insights into the treatment of obesity. This region is involved in important cognitive functions such as attention, memory, and executive control (36). The dlPFC is an emerging target area for non-invasive brain stimulation psychiatric interventions, such as treating major depression disorder, obsessive-compulsive disorder, and addiction (37). The dlPFC participates in several functional-based brain networks (38), such as the salience network (SN) and executive control network (ECN), which are related to impulsiveness (39). The SN is the key network for labeling relevant information. ECN controls reward-based decision-making. As previous studies have pointed out, coordinated alterations in the SN, ECN, and default mode network (DMN) facilitate the diagnosis and treatment of obesity (40). The clustering analysis based on fALFF in right dlPFC and non-planning impulsiveness score showed a higher rate of Cluster 1 in the obesity group than in the HC group. Non-planning impulsivity in the obesity population is more of an intermediate phenotype, and spontaneous neural activity in the right dlPFC may be a potential biomarker.
Most intriguingly, spontaneous brain activity can explain the impact of impulsivity on obesity outcomes, and its effect is very strong. Previous studies suggest that the impaired impulse control associated with obesity is caused by imbalanced dynamic cross-network interactions (41). There are many treatments for obesity, but the high levels of impulsiveness, anxiety, binge eating, food addiction, and other mental illness symptoms associated with obesity have made it unrealistic to develop treatments for weight loss (42). Even the most effective bariatric surgery is still associated with the risk of regaining weight, and psychotic symptoms are an important predictor of this weight gain. Furthermore, hierarchical clustering supports the use of fALFF to study obesity, and the involvement of unplanned impulses suggests that it is no longer appropriate to treat obesity as a mere metabolic disorder problem (43). There is an urgent need to develop effective, safe, and easy-to-use obesity treatment options such as non-invasive brain stimulation. However, the mediation effect is simply a statistical result; thus, we must be careful when concluding, such as theories that interventions addressing food-related impulsivity are more effective than other interventions (44). Several studies have investigated the interventional effect of non-inclination brain stimulation in obese patients. Early rTMS intervention in the left dlPFC of 29 obese patients effectively reduced fat mass and appetite (45). Recently, deep coils were used to stimulate the bilateral insula and prefrontal cortex of 9 patients, which was found to effectively reduce body weight and found an increase in prefrontal functional connectivity (46). This may suggest that non-invasive brain stimulation may be an effective treatment option for obesity, a multifactorial confounding disease. In addition, the relatively weaker but more portable transcranial direct current stimulation may have wider applicability. For example, a combined hypocaloric diet may have been effective in reducing sweets cravings in overweight individuals (47), and changes in neural activity may themselves have potential roles as predictive biomarkers for tDCS and cognitive training (CT) in the dlPFC The resulting prefrontal EEG asymmetry predicts a reduction in BMI (48). To improve treatment outcomes, more studies need to determine pre-treatment biomarkers of treatment outcomes to determine which patients may respond to specific treatments.
This study has several limitations. First, our patients were recruited mainly from a population in good health and with high socioeconomic status. This may introduce bias in our research because the sample may be more concerned with the quality of life and have a higher level of social support than other samples. Follow-up research should use more random sampling methods. Second, cross-sectional comparisons cannot make proper causal inferences. This study did not include anxiety and depressive mood in our analysis, and we believe that this comorbidity factor would weaken the overall statistical power if controlled for as a covariate. Especially when anxiety and depression themselves are highly correlated with obesity (49), it is a very dangerous move to try to eliminate the major factors, which may be the abnormal phenotypes shared by anxiety and depression with obesity. As our previous study explored (50). Therefore, future exploratory research should use longitudinal methods. Third, our participants were mainly female; thus, gender differences in impulsivity and obesity must be considered (51). Obesity is the last observable result, resulting from excess consumption and intake of calories. We hypothesize that obese people are less physically active than healthy people under modern living conditions (52). Due to the lack of monitoring of subject exercise levels in this study, we were not able to discuss obesity outcomes within a unified dynamic energy balance framework (53). As part of future studies, we will also include a self-reported International Physical Activity Questionnaire or exercise monitoring wristbands for control measurements. Add more evidence regarding “healthier” community building designs as a means of reducing obesity (54).
In conclusion, these results indicate that the dlPFC can serve as a biomarker of obesity and that its activity mediates the path from increased non-planning impulsiveness to higher BMI. Our results may facilitate the development of novel treatment strategies, such as non-invasive brain stimulation, and advance the personalized treatment of obesity.
The original contributions presented in the study are included in the article/Supplementary Material further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the human research review board of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
X-DH, Z-QL, and H-WZ: writing-original draft. TX, QL, and LL: resources. H-TC: writing-review and editing. HZ: project administration, methodology, and software. TX: resources and conceptualization. T-FY: conceptualization and supervision. All authors contributed to the article and approved the submitted version.
This work was supported by clinical retrospective study of Shanghai Jiao Tong University Affiliated Sixth People's Hospital (YNHG201912 to H-WZ), the Natural Science Foundation of China (82171486), Natural Science Foundation of Shanghai (21ZR1448400 to TX), the Interdisciplinary Program of Shanghai Jiao Tong University (YG2021ZD23 to TX), General Science Foundation of Shanghai Sixth People's Hospital (YNMS202114 to TX).
QL was employed in Siemens Healthcare Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to acknowledge Yin Liu, Hang-bin Zhang, Tong Wu, Pang Tang, Yi Zhang, and Yan-Bin Zheng for their help with data collection and advice on writing the article.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.873953/full#supplementary-material
1. Meule A, Platte P. Facets of impulsivity interactively predict body fat and binge eating in young women. Appetite. (2015) 87:352–7. doi: 10.1016/j.appet.2015.01.003
3. Obesity and overweight. Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed January 16, 2022).
4. Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. (2011) 378:826–37. doi: 10.1016/S0140-6736(11)60812-X
5. Kelly NR, Jaramillo M, Ramirez S, Altman DR, Rubin SG, Yang SB, et al. Executive functioning and disinhibited eating in children and adolescents. Pediatr Obes. (2020) 15:e12614. doi: 10.1111/ijpo.12614
6. Thamotharan S, Lange K, Zale EL, Huffhines L, Fields S. The role of impulsivity in pediatric obesity and weight status: a meta-analytic review. Clin Psychol Rev. (2013) 33:253–62. doi: 10.1016/j.cpr.2012.12.001
7. Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behav Res Ther. (2007) 45:1071–5. doi: 10.1016/j.brat.2006.05.009
8. Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. (2014) 42:10–21. doi: 10.1016/j.bbi.2014.04.001
9. Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. (2012) 13:279–86. doi: 10.1038/nrn3212
10. Hong T, He C, Gu Z-K, Xie J-J, Lu Q, Li Y-Q, et al. Psychometric investigation of the Chinese version of the habit, reward and fear scale (HRFS). Brain Behav. (2021) 11:e2364. doi: 10.1002/brb3.2364
11. Dekkers IA, Jansen PR, Lamb HJ. Obesity, Brain volume, and white matter microstructure at MRI: a cross-sectional UK biobank study. Radiology. (2019) 292:270. doi: 10.1148/radiol.2019194010
12. Jastreboff AM, Sinha R, Arora J, Giannini C, Kubat J, Malik S, et al. Altered brain response to drinking glucose and fructose in obese adolescents. Diabetes. (2016) 65:1929–39. doi: 10.2337/db15-1216
13. Jauch-Chara K, Binkofski F, Loebig M, Reetz K, Jahn G, Melchert UH, et al. Blunted brain energy consumption relates to insula atrophy and impaired glucose tolerance in obesity. Diabetes. (2015) 64:2082–91. doi: 10.2337/db14-0421
14. Zhang W, Li G, Manza P, Hu Y, Wang J, Lv G, et al. Functional abnormality of the executive control network in individuals with obesity during delay discounting. Cereb Cortex. (2021) 21:333. doi: 10.1093/cercor/bhab333
15. Volkow ND, Baler RD. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. (2015) 38:345–52. doi: 10.1016/j.tins.2015.04.002
16. Reuter M, Stark R, Hennig J, Walter B, Kirsch P, Schienle A, et al. Personality and emotion: test of Gray's personality theory by means of an fMRI study. Behav Neurosci. (2004) 118:462–9. doi: 10.1037/0735-7044.118.3.462
17. Zang Y-F, He Y, Zhu C-Z, Cao Q-J, Sui M-Q, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. (2007) 29:83–91. doi: 10.1016/j.braindev.2006.07.002
18. Zhou F, Huang S, Zhuang Y, Gao L, Gong H. Frequency-dependent changes in local intrinsic oscillations in chronic primary insomnia: A study of the amplitude of low-frequency fluctuations in the resting state. Neuroimage Clin. (2017) 15:458–65. doi: 10.1016/j.nicl.2016.05.011
19. Fryer SL, Roach BJ, Ford JM, Turner JA, van Erp TGM, Voyvodic J, et al. Relating Intrinsic Low-Frequency BOLD Cortical Oscillations to Cognition in Schizophrenia. Neuropsychopharmacology. (2015) 40:2705–14. doi: 10.1038/npp.2015.119
20. Chao S-H, Liao Y-T, Chen VC-H, Li C-J, McIntyre RS, Lee Y, et al. Correlation between brain circuit segregation and obesity. Behav Brain Res. (2018) 337:218–27. doi: 10.1016/j.bbr.2017.09.017
21. Zhang Y, Ji G, Li G, Hu Y, Liu L, Jin Q, et al. Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. Int J Obes. (2019) 43:842–51. doi: 10.1038/s41366-018-0126-x
22. Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. (2008) 172:137–41. doi: 10.1016/j.jneumeth.2008.04.012
23. Kakoschke N, Lorenzetti V, Caeyenberghs K, Verdejo-García A. Impulsivity and body fat accumulation are linked to cortical and subcortical brain volumes among adolescents and adults. Sci Rep. (2019) 9:2580. doi: 10.1038/s41598-019-38846-7
24. Bénard M, Camilleri GM, Etilé F, Méjean C, Bellisle F, Reach G, Hercberg S, Péneau S. Association between Impulsivity and Weight Status in a General Population. Nutrients. (2017) 9:217. doi: 10.3390/nu9030217
25. Meule A, Blechert J. Trait impulsivity and body mass index: a cross-sectional investigation in 3073 individuals reveals positive, but very small relationships. Health Psychol Open. (2016) 3:2055102916659164. doi: 10.1177/2055102916659164
26. Friedman NP, Robbins TW. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology. (2022) 47:72–89. doi: 10.1038/s41386-021-01132-0
27. Raiha S, Yang G, Wang L, Dai W, Wu H, Meng G, et al. Altered reward processing system in internet gaming disorder. Front Psychiatry. (2020) 11:599141. doi: 10.3389/fpsyt.2020.599141
28. Li X-Y, Phillips Mr Xu D, Zhang Y-L, Yang S-J, Tong Y-S, Wang Z-Q, et al. Reliability and validity of an adapted Chinese version of Barratt Impulsiveness Scale. Chinese Mental Health J. (2011) 25:610–5. https://dx.doi.org/10.3390%2Fijerph15030504
29. Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, et al. comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. (2013) 76:183–201. doi: 10.1016/j.neuroimage.2013.03.004
30. Meule A, Blechert J. Indirect effects of trait impulsivity on body mass. Eat Behav. (2017) 26:66–9. doi: 10.1016/j.eatbeh.2017.01.012
31. Raymond K-L, Lovell GP. Food addiction symptomology, impulsivity, mood, and body mass index in people with type two diabetes. Appetite. (2015) 95:383–9. doi: 10.1016/j.appet.2015.07.030
32. Georgii C, Goldhofer P, Meule A, Richard A, Blechert J. Food craving, food choice and consumption: The role of impulsivity and sham-controlled tDCS stimulation of the right dlPFC. Physiol Behav. (2017) 177:20–6. doi: 10.1016/j.physbeh.2017.04.004
33. Nasser JA, Gluck ME, Geliebter A. Impulsivity and test meal intake in obese binge eating women. Appetite. (2004) 43:303–7. doi: 10.1016/j.appet.2004.04.006
34. von Deneen KM, Liu Y. Obesity as an addiction: Why do the obese eat more? Maturitas. (2011) 68:342–5. doi: 10.1016/j.maturitas.2011.01.018
35. Gluck ME, Viswanath P, Stinson EJ. Obesity, appetite, and the prefrontal cortex. Curr Obes Rep. (2017) 6:380–8. doi: 10.1007/s13679-017-0289-0
36. Gazzaniga M, Ivry RB, Mangun GR. Ph.D. Cognitive Neuroscience: The Biology of the Mind (Fifth Edition). Fifth. New York: W. W. Norton & Company (2018).
37. Song S, Zilverstand A, Gui W, Li H-J, Zhou X. Effects of single-session vs. multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimulat. (2019) 12:606–18. doi: 10.1016/j.brs.2018.12.975
38. Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. (2011) 106:1125–65. doi: 10.1152/jn.00338.2011
39. Figley CR, Asem JSA, Levenbaum EL, Courtney SM. Effects of body mass index and body fat percent on default mode, executive control, and salience network structure and function. Front Neurosci. (2016) 10:234. doi: 10.3389/fnins.2016.00234
40. Donofry SD, Stillman CM, Erickson KI. A review of the relationship between eating behavior, obesity and functional brain network organization. Soc Cogn Affect Neurosci. (2020) 15:1157–81. doi: 10.1093/scan/nsz085
41. Syan SK, McIntyre-Wood C, Minuzzi L, Hall G, McCabe RE, MacKillop J. Dysregulated resting state functional connectivity and obesity: A systematic review. Neurosci Biobehav Rev. (2021) 131:270–92. doi: 10.1016/j.neubiorev.2021.08.019
42. Suglia SF, Duarte CS, Chambers EC, Boynton-Jarrett R. Social and behavioral risk factors for obesity in early childhood. J Dev Behav Pediatr. (2013) 34:549–56. doi: 10.1097/DBP.0b013e3182a509c0
43. Stanhope KL. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit Rev Clin Lab Sci. (2016) 53:52–67. doi: 10.3109/10408363.2015.1084990
44. Ince B, Schlatter J, Max S, Plewnia C, Zipfel S, Giel KE, et al. Can we change binge eating behaviour by interventions addressing food-related impulsivity? a systematic review. J Eat Disord. (2021) 9:38. doi: 10.1186/s40337-021-00384-x
45. Kim S-H, Chung J-H, Kim T-H, Lim SH, Kim Y, Lee Y-A, et al. The effects of repetitive transcranial magnetic stimulation on eating behaviors and body weight in obesity: A randomized controlled study. Brain Stimulat. (2018) 11:528–35. doi: 10.1016/j.brs.2017.11.020
46. Devoto F, Ferrulli A, Zapparoli L, Massarini S, Banfi G, Paulesu E, et al. Repetitive deep TMS for the reduction of body weight: Bimodal effect on the functional brain connectivity in “diabesity.” Nutr Metab Cardiovasc Dis. (2021) 31:1860–70. doi: 10.1016/j.numecd.2021.02.015
47. de Araujo C, Fitz RC, Natividade GR, Osório AF, Merello PN, Schöffel AC, et al. The effect of transcranial direct current stimulation along with a hypocaloric diet on weight loss in excessive weight people: A pilot randomized clinical trial. Clin Nutr ESPEN. (2020) 40:68–76. doi: 10.1016/j.clnesp.2020.10.005
48. Forcano L, Castellano M, Cuenca-Royo A, Goday-Arno A, Pastor A, Langohr K, et al. Prefrontal Cortex Neuromodulation Enhances Frontal Asymmetry and Reduces Caloric Intake in Patients with Morbid Obesity. Obesity (Silver Spring). (2020) 28:696–705. doi: 10.1002/oby.22745
49. Fulton S, Décarie-Spain L, Fioramonti X, Guiard B, Nakajima S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol Metab. (2022) 33:18–35. doi: 10.1016/j.tem.2021.10.005
50. Zhang H, Liu Z, Zheng H, Xu T, Liu L, Xu T, Yuan T-F, Han X. The influence of childhood emotional abuse on adult obesity. Res Sq. (2021) 21:83. doi: 10.21203/rs.3.rs-1043883/v1
51. Mühlberg C, Mathar D, Villringer A, Horstmann A, Neumann J. Stopping at the sight of food - How gender and obesity impact on response inhibition. Appetite. (2016) 107:663–76. doi: 10.1016/j.appet.2016.08.121
52. Ko GTC. Both obesity and lack of physical activity are associated with a less favorable health-related quality of life in Hong Kong Chinese. Am J Health Promot. (2006) 21:49–52. doi: 10.1177/089011710602100109
53. Manore MM, Larson-Meyer DE, Lindsay AR, Hongu N, Houtkooper L. Dynamic energy balance: an integrated framework for discussing diet and physical activity in obesity prevention-is it more than eating less and exercising more? Nutrients. (2017) 9:905. doi: 10.3390/nu9080905
Keywords: obesity, impulsiveness, resting-state fMRI, mediation effect, dlPFC
Citation: Han X-D, Zhang H-W, Xu T, Liu L, Cai H-T, Liu Z-Q, Li Q, Zheng H, Xu T and Yuan T-F (2022) How Impulsiveness Influences Obesity: The Mediating Effect of Resting-State Brain Activity in the dlPFC. Front. Psychiatry 13:873953. doi: 10.3389/fpsyt.2022.873953
Received: 11 February 2022; Accepted: 11 April 2022;
Published: 10 May 2022.
Edited by:
Qinghua He, Southwest University, ChinaReviewed by:
Liang Gong, Chengdu Second People's Hospital, ChinaCopyright © 2022 Han, Zhang, Xu, Liu, Cai, Liu, Li, Zheng, Xu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Zheng, emguZG10ckBnbWFpbC5jb20=; 0000-0001-9735-5657; Tao Xu, YmFsb3JAc2p0dS5lZHUuY24=; Ti-Fei Yuan, eXRmMDcwN0AxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.