- 1Department of Nursing, Third Xiangya Hospital of Central South University, Changsha, China
- 2Key Laboratory of Medical Information Research, College of Hunan Province, Central South University, Changsha, China
- 3Xiangya School of Nursing, Central South University, Changsha, China
- 4School of Nursing and Healthcare Leadership, University of Washington, Tacoma, WA, United States
- 5Department of Cardiothoracic Vascular Surgery, Affiliated Hospital of Jinggangshan University, Ji’an, China
- 6Department of Child, Family, and Population Health Nursing, School of Nursing, University of Washington, Seattle, WA, United States
Background: Various studies show that sleep quality, life skills, and cortisol are associated with depressive symptoms, separately. However, the relationships between sleep quality, life skills, cortisol, and depressive symptoms remain unclear. Thus, this study aims to examine the mediating or moderating roles of life skills and cortisol in the relationship between sleep quality and depressive symptoms.
Methods: A retrospective cross-sectional study was performed among 212 adolescents with childhood household dysfunction (CHD) from August to October 2020 in China. We used the Pittsburgh Sleep Quality Index, the Secondary School Student Life Skills Rating Scale, and the Center for Epidemiologic Studies Depression Scale to measure sleep quality, life skills, and depressive symptoms, respectively. Additionally, 65 participants provided blood samples to assess their blood cortisol levels. Analyses included correlations, regressions, and structural equation models. Bootstrapping was performed to examine the mediation effect. Multiple regression analysis was performed to examine the moderation effect.
Results: The results showed that sleep quality and life skills were significantly associated with depressive symptoms (p < 0.01). Life skills mediated the relationship between sleep quality and depressive symptoms. Cortisol moderated the relationship between sleep quality and depressive symptoms.
Conclusion: Our findings support potential mediating and moderating roles of life skills and cortisol in the relationship between sleep quality and depressive symptoms, which suggests improving sleep quality, life skills are of great significance in the prevention and intervention of depression in adolescents with CHD, and disseminating knowledge about the high risk of developing increased depressive symptoms among adolescents with CHD with higher cortisol levels is indicated.
Introduction
Childhood household dysfunction (CHD) refers to “children growing up in a family environment that may undermine their safety, stability, and sense of bonding” and includes experiences such as parental divorce/separation, substance abuse, severe diseases, or imprisonment (1). In China, CHD is prevalent among adolescents, with the number reaching over 0.5 million in 2018 (2). Adolescents with CHD often live in low-income areas with limited mental health resources, and they feel more loneliness and less secure compared to those with fewer CHD experiences (3). Adolescents with experiences of CHD are more likely to report depression, anxiety disorders, suicidal ideation, suicide attempt, distress, smoking, and substance abuse (4, 5). Therefore, CHD is a risk factor for various mental health problems.
Among the various negative psychological outcomes, depression is one of the most common and widely reported psychological problems among children and adolescents with CHD (6). As the subclinical stage of depression, depressive symptoms are a series of symptoms characterized by significant depressive mood, mainly including low spirits, decreased interest, physical discomfort, fatigue, and so on (7). The prevalence of depressive symptoms among adolescents is high and is still growing rapidly. The past two decades have seen an increase in elevated depressive symptoms among adolescents, with a point prevalence increasing from 24% between 2001 and 2010 to 37% between 2011 and 2020 (8). Previous studies have consistently shown that, compared with adolescents with no experience of CHD, adolescents with CHD experience depressive symptoms at a higher rate (SB = 0.045) (9). Depressive symptoms are associated with a wide range of unhealthy behaviors and negative health outcomes among adolescents. A large number of studies have shown that adolescents with depressive symptoms are at higher risk for substance abuse behaviors and suicide (10, 11). Depressive symptoms are listed as the second leading cause of death among adolescents aged between 11 and 17 (12). However, the etiology of depressive symptoms is still undefined. Besides hereditary and environmental factors (13), the effect of sleep quality on depression has received increasing attention over a long period.
Sleep quality is critical for health and development, and it is an important marker of psychological health (14). Adolescents’ poor sleep quality is related to various negative mental health outcomes, including depressive symptoms (15, 16). Recent evidence from cross-sectional studies in adolescent students shows that poor sleep quality is associated with increased depressive symptoms throughout adolescence (17). Adolescents with sleep disturbance are 2.47 times more likely to experience depressive symptoms than those without sleep problems (18). There is evidence to support the prospective role of poor sleep quality in the development of depressive symptoms in adolescents. A meta-analysis of 23 longitudinal studies among adolescents and a systematic review found sleep disturbance acts as a precursor to the development of depressive symptoms, which can predict the occurrence and outcome of depression (19, 20). Furthermore, a recent review on the association between depressive symptoms and sleep disturbance has revealed the potential mechanisms, and sleep disturbance could contribute to increased levels of inflammatory markers (e.g., IL−6, TNF, and CRP), a rapid decrease in monoamines [e.g., serotonin (5-HT), norepinephrine (NE), and dopamine], and circadian rhythm disruption (e.g., clock genes dysregulation), which are closely related to the development of depressive symptoms (20). Therefore, poor sleep quality may be a modifiable risk factor for increasing an individual’s susceptibility to depressive symptoms.
Life skills, also known as psychosocial ability, refer to the ability of individuals to adopt adaptive and positive behaviors to effectively deal with various needs and challenges in their daily life. It is considered to be a useful and effective coping ability under various stress conditions (21). WHO (21) holds that the core strategies of life skills mainly include the following: effective communication skills, decision-making, creative thinking, interpersonal relationship skills, problem-solving, critical thinking, self-awareness, empathy, and coping with stress and emotion. Numerous studies have documented the positive effects of life skills on improving mental health outcomes, especially depressive symptoms. For instance, several recent reviews have shown that life skill interventions are effective in preventing mental disorders including depressive symptoms in adolescents, especially among those in vulnerable environments [e.g., (22, 23)]. According to the transactional stress-coping model, the consequence of the interplay between stressful conditions and coping ability determines the onset of adverse mental health outcomes (24). Poor sleep quality is a stressful condition and is characterized by increased vulnerability to stressful stimuli and events, which can weaken ability to cope with stress in everyday life (25). Life skills serve as one of the most effective, useful, and universal coping abilities to help individuals better adapt to stress that may lead to psychological problems such as depressive symptoms (26). Pearlin’s stress process theory emphasizes on the process of stress can be seen as combining three major conceptual domains: the sources of stress, the mediators of stress, and the consequences of stress. In the process of the consequences of stress caused by the sources of stress (i.e., poor sleep quality), the mediators of stress are vital. Pearlin argues that coping (i.e., an individual’s ability to handle and solve the stressful condition) such as life skills plays a significant role in mediating the impact of stressful events such as poor sleep quality on depressive symptoms (27). So, we believe that life skills may mediate the relationship between sleep quality and depressive symptoms. That is, poor sleep quality acts as a chronic stressor that can be disruptive to life skills, which consequently contributes to depressive symptoms. Zhang et al. (28) found that coping style plays a mediating role in the association between sleep quality and depressive symptoms among college nursing students. However, no research has focused on life skills as the mediator between sleep quality and depressive symptoms among adolescents with CHD.
Although sleep quality and life skills are the important factors that affect depressive symptoms, the relationship between sleep quality and depressive symptoms may change because of other factors. Cortisol is a stress hormone widely recognized as an indicator for both physiological and psychological stress that affects the health status of adolescents (29). According to a recent systematic review, cortisol, as an objective and direct measure, especially cortisol in the morning, is more strongly associated with psychological distress and is possibly more important as a predictor of depressive symptoms than other subjective measures (30). Numerous literature has shown that those with higher morning waking cortisol levels have a higher risk for subsequent depression disorders (31, 32). A cross-sectional study on the potential role of cortisol dysregulation (morning plasma cortisol, 24-h urinary free cortisol, and cortisol metabolites) in depressive symptoms found that depressive symptoms in primary care subjects not consulting for their mood were associated with higher morning plasma cortisol, but not urinary cortisol or its metabolites (33). Furthermore, the cortisol awakening response can interact with stressful events (such as acute interpersonal stress) to prospectively predict depressive symptoms, which has been demonstrated in some longitudinal studies of adolescent girls (34, 35). According to the hypothesis of rapid eye movement sleep emotional homeostasis by Goldstein and Walker (36), poor sleep quality as a stressful event can interact with cortisol for the development of major depression. A recent review pointed out that the increase in morning serum cortisol caused by the dysregulation of the hypothalamus–pituitary–adrenal axis plays a positive moderating role in the relationship between poor sleep and mental health problems (37). However, whether cortisol moderates the association between sleep quality and depressive symptoms in adolescents with CHD remains uncovered.
This study aimed to explore the mediating or moderating role of life skills and cortisol in the relationship between sleep quality and depressive symptoms. The main hypotheses of this study were as follows:
Hypothesis 1. Life skills play the mediating roles in the relationship between sleep quality and depressive symptoms among adolescents with CHD.
Hypothesis 2. Cortisol moderates the relationship between sleep quality and depressive symptoms.
Materials and Methods
Participants and Procedures
This cross-sectional study was conducted from August to October 2020 in Hunan Province, South-Central China. Based on the Gross Domestic Product (GDP) ranking of Hunan Provincial Statistics Bureau (38), two cities were selected for the study: Changsha City (the first ranking; lies in the east of Hunan Province, mostly of Han ethnicity with high incomes) and Zhangjiajie City (the last ranking; lies in the west of Hunan Province, mostly of minority ethnicity with low incomes). Then, a random number generator computer program was used to select two counties from each city, leading to a final sampling frame of four counties.
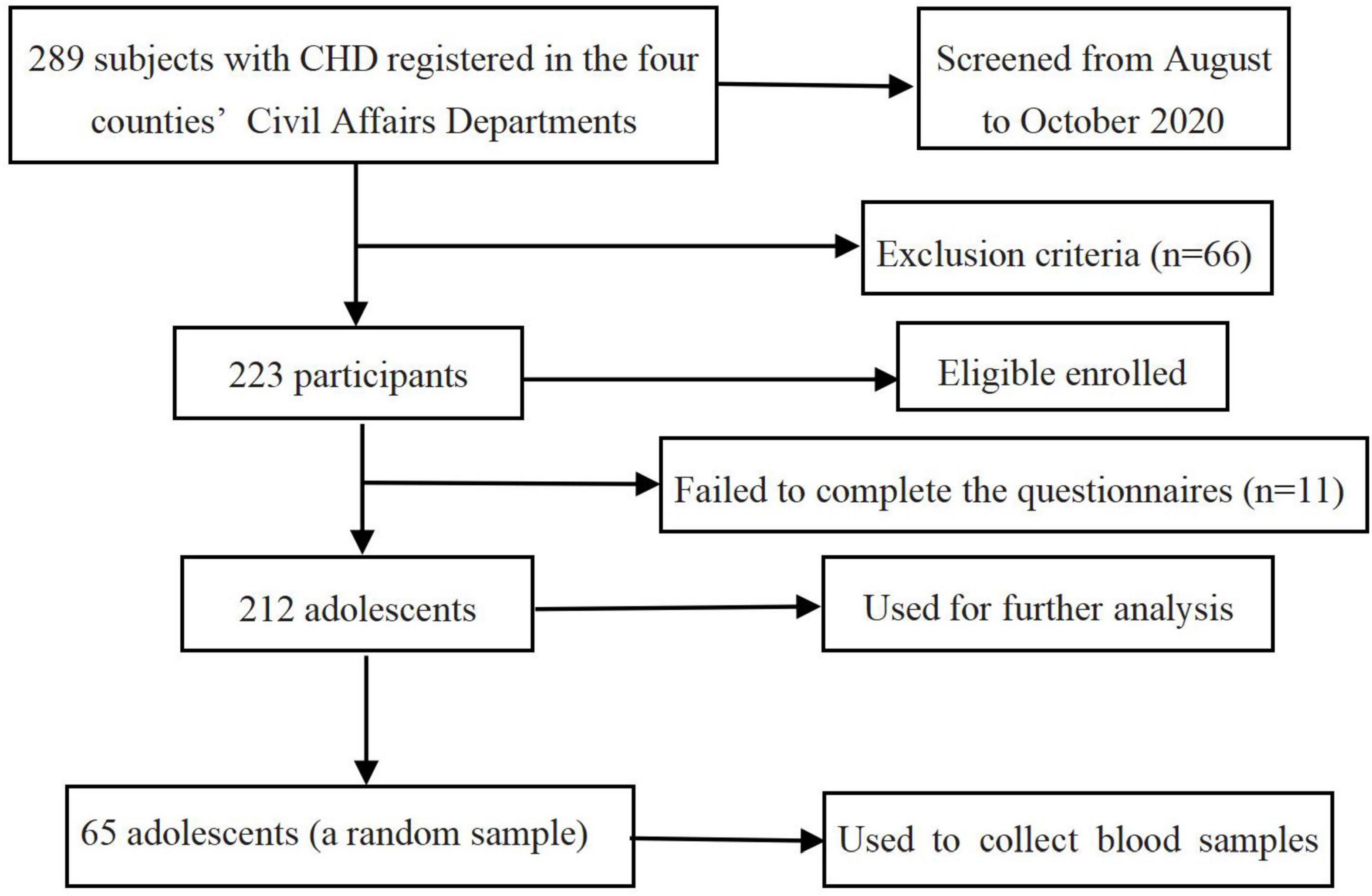
Participants were recruited from the middle or high school students with CHD (N = 289) registered in the four counties’ Civil Affairs Departments. Those with mental illness and unable to communicate normally due to developmental delay were excluded from the study. In total, 223 adolescents completed the survey, among whom 212 (95.1%) were included in the final analysis (11 invalid questionnaires were excluded due to more than 30% missing items of each scale). To measure blood cortisol levels, we collected blood samples from a random sample of 65 students. The flowchart of subject enrollment and screening in this study is shown in Figure 1.
Before data collection, the research team explained the purpose of the study to potentially eligible adolescents at the schools. Adolescents who agreed to take part in this study returned home with informed consent forms. After approval was obtained from both the guardians and adolescents, the adolescents then completed the questionnaires in a single session during school hours. Investigators were present at the research sites to answer any questions raised by the participants. The blood samples were collected between 5:30 a.m. and 6:00 a.m. within 30 min of waking up the next morning in the presence of both the teacher and a trained researcher in a private room at their schools.
This study protocol and procedures were reviewed and approved by the Ethical Committee of the Xiangya School of Nursing, Central South University. Participants were provided with information regarding the study, and their informed consent was obtained.
Sample Size Estimation
Since it was a cross-sectional study, the method of calculating the sample size by multivariable correlation study [equation: N = (U1–α/2S/δ)2] was used to estimate the required sample size. The pre-survey of 30 adolescents showed that the Sdepressive symptoms = 0.11, Ssleep quality = 0.14, and Slife skills = 0.13. According to the allowable error δ = 0.02 (39), significance level α = 0.05, the sample size required for each variable is 116, 188, and 161, respectively. Therefore, at least 188 samples were required to be included in this study, and 212 samples were finally included.
Measures
The research measures in this study consist of six parts:
Sociodemographic Characteristics
An investigator-developed questionnaire was used to obtain general demographic information such as age, gender, ethnicity, whether the participants was an only child, region of residence, annual family income, primary caregivers, and caregivers’ educational level.
Childhood Household Dysfunction
Childhood household dysfunction was measured using a checklist from the Chinese Ministry of Civil Affairs (40) and referred to a child whose parents had not fulfilled guardianship and parenting responsibility for more than 6 months for either one of the following: (1) incarceration, (2) severe illness determined by each locality according to the actual situation, (3) substance abuse, (4) mother remarriage, (5) death, or (6) missing. The cumulative variance contribution rate obtained via confirmatory factor analysis was 85.36% in this study.
Sleep Quality
Sleep quality during the previous month was assessed by the 19-item Chinese version of the Pittsburgh Sleep Quality Index (PSQI), which was developed for the Chinese population by Liu et al. (41). The PSQI consists of seven domain scores (subjective sleep quality; sleep latency; sleep duration; sleep efficiency; sleep disturbances; sleep medication use; and daytime dysfunction), each ranging from 0 to 3. The PSQI also yields a global score (sum of all items from the seven domains), with higher scores indicating poorer sleep quality and more severe sleep disturbances/problems. The PSQI has demonstrated good internal consistency among Chinese people (41). In this study, the PSQI also demonstrated good internal consistency with a Cronbach’s alpha of 0.87.
Life Skills
Life skills of adolescents in China were assessed using the Secondary School Student Life Skills Rating Scale (SSS-LSRS) developed by Zhao et al. (42). The scale includes 47 dichotomized items rated as “0” for “no” and “1” for “yes” and yields 8 major components (problem-solving ability, empathy skills, communication skills, self-esteem, sociability skills, interpersonal morality, self-efficacy, stress coping, and emotion regulation). The total score ranges from 0 to 47, with higher total scores representing better life skills. The questionnaire demonstrated good internal consistency in a previous study (43). In this study, the SSS-LSRS also demonstrated good internal consistency with a Cronbach’s alpha of 0.91.
Depressive Symptoms
Depressive symptoms in the past month were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) (44). The scale includes a total of 20 items and yields 4 components (depressive affect, somatic complaints, interpersonal problems, and positive affect). All items are scored on a 4-point Likert-type frequency scale, ranging from 0 (rarely or none of the time) to 3 (most or all of the time). The total score on the CES-D (sum of all items from the four components) ranges from 0 to 60, with higher scores indicating more severe depressive symptoms. According to a previous study among Chinese adolescents (45), the CES-D demonstrated good internal consistency. In this study, the CES-D also demonstrated good internal consistency with a Cronbach’s alpha of 0.94.
Blood Cortisol Level
To measure blood cortisol levels, we randomly selected 65 participants (35 girls and 30 boys) for a blood draw (antecubital venipuncture). A 3 ml of blood sample was collected from each participant in the early morning about 30 min (between 5:30 a.m. and 6:00 a.m.) after they woke up. Blood draws were performed in the schools by clinically experienced registered nurses. Participants were asked to avoid any food, drink, smoking, or sports 30 min prior to blood collection. After collection, all samples were placed in an incubator at 4–8°C and transported to the laboratory. Subsequently, samples were centrifuged and stored at −20°C in the laboratory until further analysis. Blood cortisol levels (nmol/L) were determined by Elecsy and Cobas e 411 Analyser (Roche Diagnostic, Switzerland), which uses the electrochemiluminescence immunoassay (ECLIA). Each sample was analyzed in duplicate and was conducted according to the manufacturer’s instructions. The analytical sensitivity of the assay was 0.05 μg/dL, with intra-assay and inter-assay coefficients of variation of <10%. For cortisol, the inter-assay variation was 2.0% and the intra-assay variation was 3.8%, which meets the EP5-A2 standard (acceptable coefficients are less than 10% for inter and intra) of CLSI (46). This means that the assay has good precision. For our analyses, we used the mean of the duplicates of each sample.
Statistical Analysis
The data were analyzed in SPSS 26.0 and Amos 24.0 software. A p < 0.05 was considered statistically significant. Descriptive statistics were examined including frequencies/proportions and means/standard deviations. Pearson’s correlations were performed to examine correlations among sleep quality, life skills, and depressive symptoms. A structural equation model was used to examine the mediating effect of life skills between sleep quality and depressive symptoms. In the model, latent variables were entered for sleep quality (independent variable) and depressive symptoms (dependent variable). The mediating variable of life skills was entered as an observed variable. The bias-corrected percentile bootstrap CI method was used to calculate 95% confidence intervals (95% CIs) of the coefficients for the total, direct, and indirect effects. Coefficients were considered statistically significant if the 95% CI did not cross 0. Multiple linear regression analysis was performed to examine the moderating effect of cortisol levels between sleep quality and depressive symptoms. The regression coefficient of the interaction item of “cortisol levels” and “sleep quality” was significant, which indicates that the moderating effect of cortisol levels was significant. Age, gender, ethnicity, living region, caregivers’ education level, and the type of CHD were controlled as covariates in all analysis. To illuminate moderating effect when performing a simple slope test, we used the Johnson–Neyman method recommended by Bauer and Curran (47) to analyze the specific situation of the interaction and determine the significant interval of simple slope [i.e., the effects of the independent variable (sleep quality) on the dependent variable (depressive symptoms)] within the whole value range [(Mmin, Mmax)] of moderator (cortisol levels) and show the moderating effect of cortisol levels on the relationship between sleep quality and depressive symptoms when the level of cortisol reaches a certain value. In the moderating effect diagram, the X-axis represents the value of the moderator (centered and logarithmized cortisol levels), the Y-axis represents the slope of the effect of centered sleep quality on depressive symptoms, the solid line in the center represents the changing trend of the effect of centered sleep quality on depressive symptoms with the change of the value of moderator (centered and logarithmized cortisol levels), and the horizontal line perpendicular to the X-axis represents the thresholds of the moderator.
Results
Participant Demographics
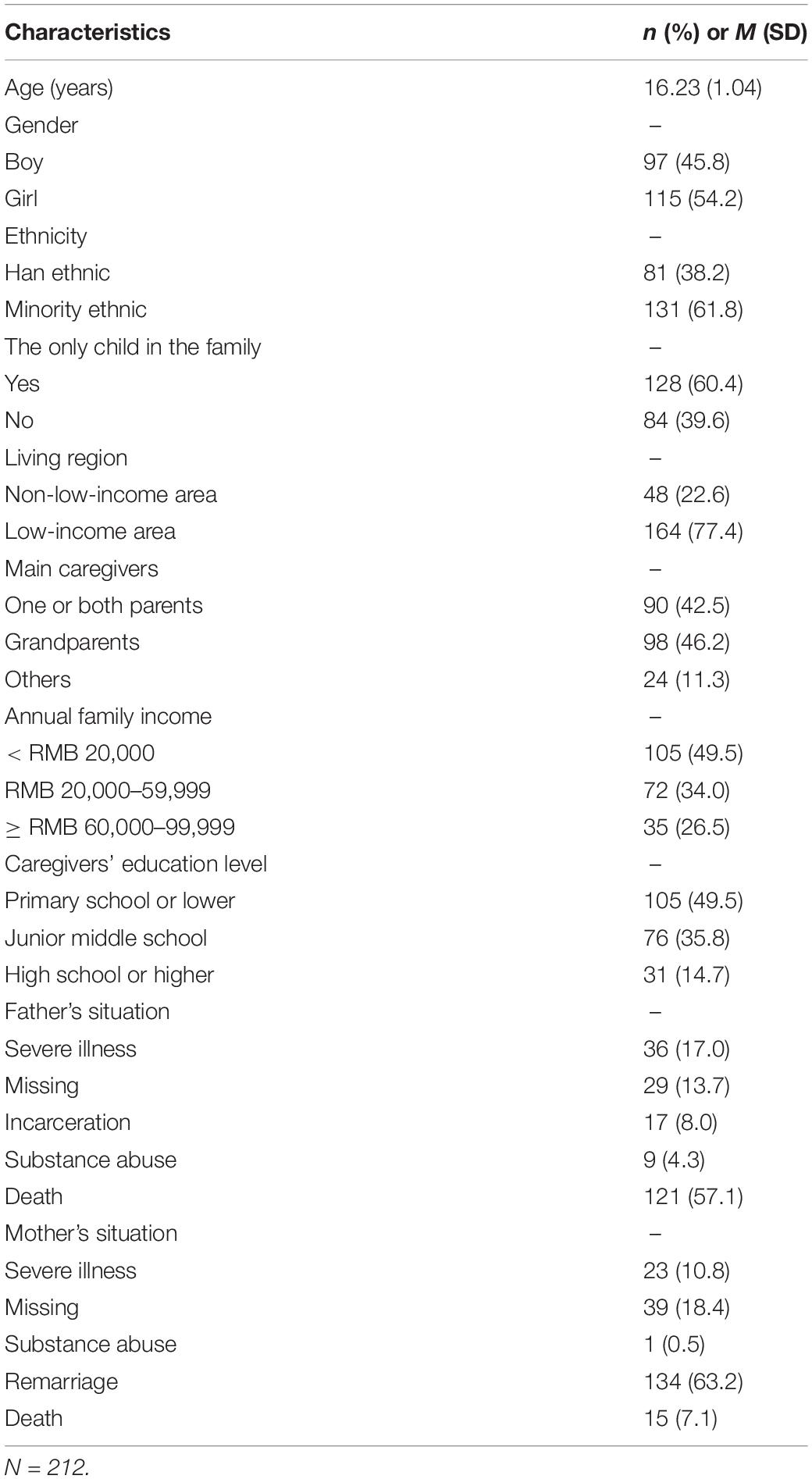
Table 1 shows summarized demographic characteristics for the 212 adolescents with CHD in the analysis sample (mean age = 16.23 years, SD = 1.04). Of the adolescents, 54.2% were girls, 61.8% were of a minority ethnic group, 39.6% were only children, and 77.4% lived in a low-income area. Of the caregivers, 46.2% were grandparents, 42.5% were parents (one or both), and 11.3% had another (“other”) relationship with the adolescent. Regarding household dysfunction, 57.1% of participants’ fathers died and 63.2% of participants’ mothers remarried.
Correlations Among Sleep Quality, Life Skills, and Depressive Symptoms
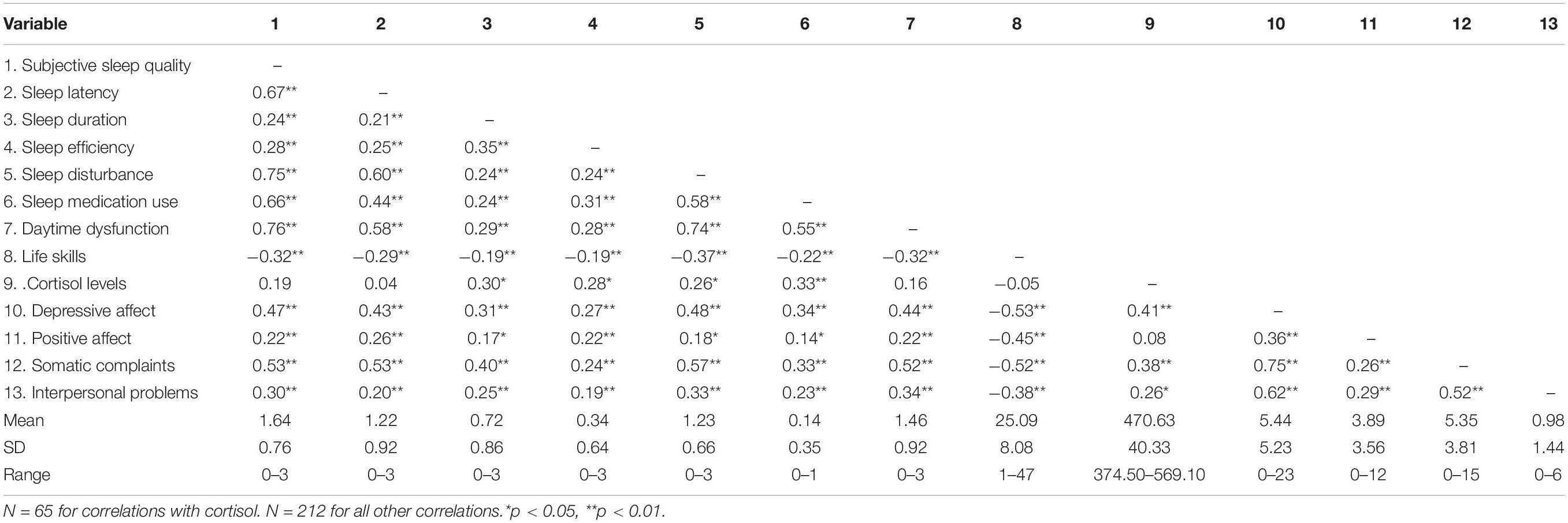
There were significant correlations among sleep quality, life skills, and depressive symptoms, as shown in Table 2. Positive correlations between all dimensions of sleep quality (variables 1–7) and depressive symptoms (variables 10–13) were identified (r’s = 0.14–0.76, p < 0.05). However, life skills (variable 8) were negatively related to all dimensions of depressive symptoms (variables 10–13) (r’s = −0.53 to −0.38, p < 0.01), as well as all dimensions of sleep quality (variables 1–7; r’s = −0.32 to −0.19, p < 0.01). Additionally, cortisol levels (variable 9) were positively related to some dimensions of sleep quality (variables 3−6) (r’s = 0.26–0.33, p < 0.05) and depressive symptoms (variables 10, 12, 13) (r’s = 0.26–0.41, p < 0.05).
The Mediation of Life Skills in the Relationship Between Sleep Quality and Depressive Symptoms
We constructed a structural equation model for the relationships of sleep quality and life skills with depressive symptoms. The model was a good fit to the data, χ2 = 137.05, df = 52, χ2/df = 2.64, GFI = 0.91, IFI = 0.93, TLI = 0.91, CFI = 0.93, RMSEA = 0.079, and there were significant differences in all paths, as shown in Figure 2. The results of the bootstrap analyses further indicated that sleep quality was significantly associated with life skills (direct effect β = −0.39, 95% CI [−0.51, −0.25], p < 0.001) and that life skills were significantly associated with depressive symptoms (direct effect β = −0.43, 95% CI [−0.56, −0.28], p < 0.001). Sleep quality was significantly associated with depressive symptoms (total effect: β = 0.66, 95% CI [0.54, 0.75], p < 0.001; direct effect: β = 0.49, 95% CI [0.36, 0.61], p < 0.001). All these results suggested that life skills (β = 0.17, 95% CI [0.09, 0.25]) acted as a mediator in the relationship between sleep quality and depressive symptoms, and the mediating effect of life skills accounts for 25.8% of the total effect, as shown in Table 3.

Figure 2. Structural model of life skills as mediator of sleep quality and depressive symptoms. SQ, Pittsburgh Sleep Quality Index; CES-D, Center for epidemiological studies depression scale.
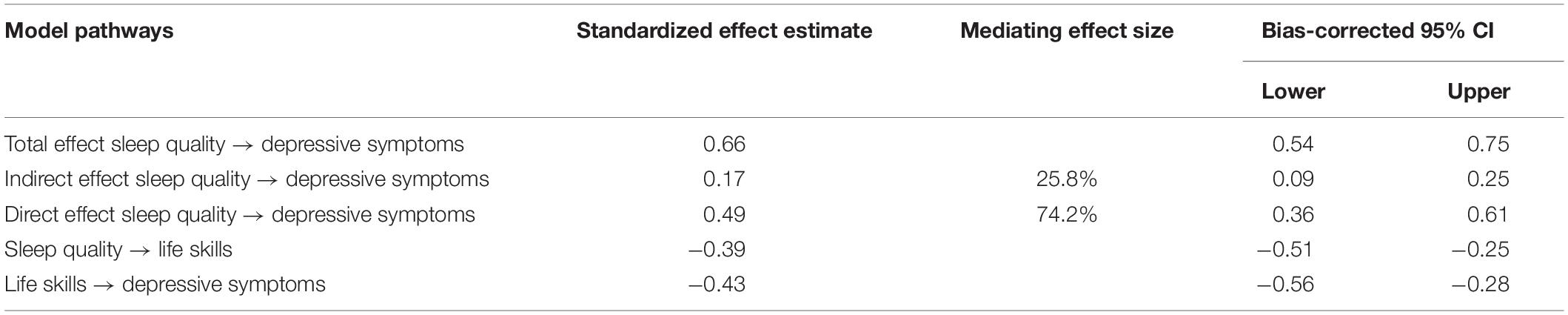
Table 3. The total, indirect, and direct effects of sleep quality on depressive symptoms with life skills as a mediator.
The Moderation of Cortisol Levels in the Relationship Between Sleep Quality and Depressive Symptoms
For moderation analyses with the dependent variable CES-Dtotal, a significant interaction was found for sleep quality and cortisol levels (ΔR2 = 0.07; ΔF1,61 = 8.23; p = 0.006; b = 0.27; t = 2.87; p = 0.006), suggesting that the positive relationship between sleep quality and depressive symptoms was intensified by higher cortisol levels. However, when the centered and logarithmized cortisol values are less than the threshold of −0.55, the moderating effect of cortisol levels on the relationship between sleep quality and depressive symptoms is not significant. An overview of all moderation analyses is shown in Table 4. The interaction effects are presented in Figure 3.
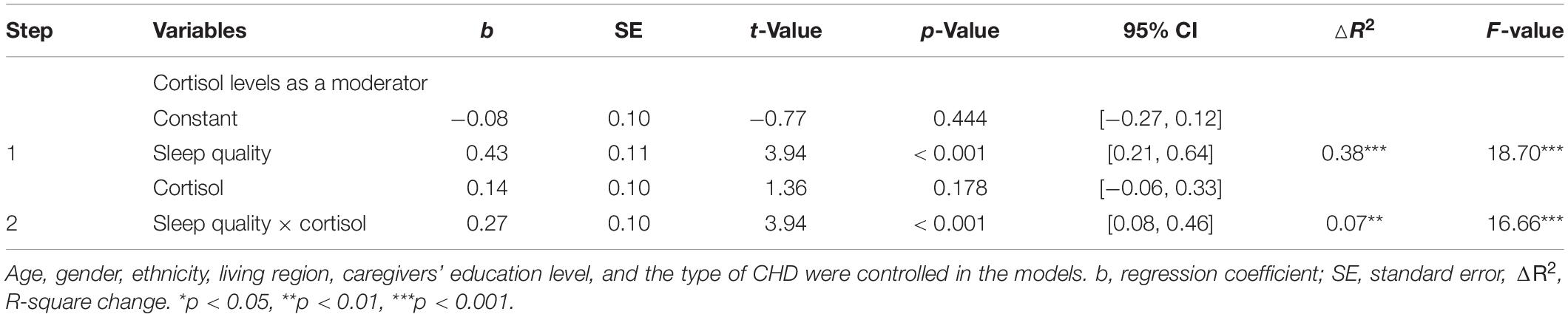
Table 4. Overview of significant moderation model (including confounders) with sleep quality (SQ) as independent variables, depressive symptoms (CES-Dtotal) as dependent variables, and cortisol levels as moderators.
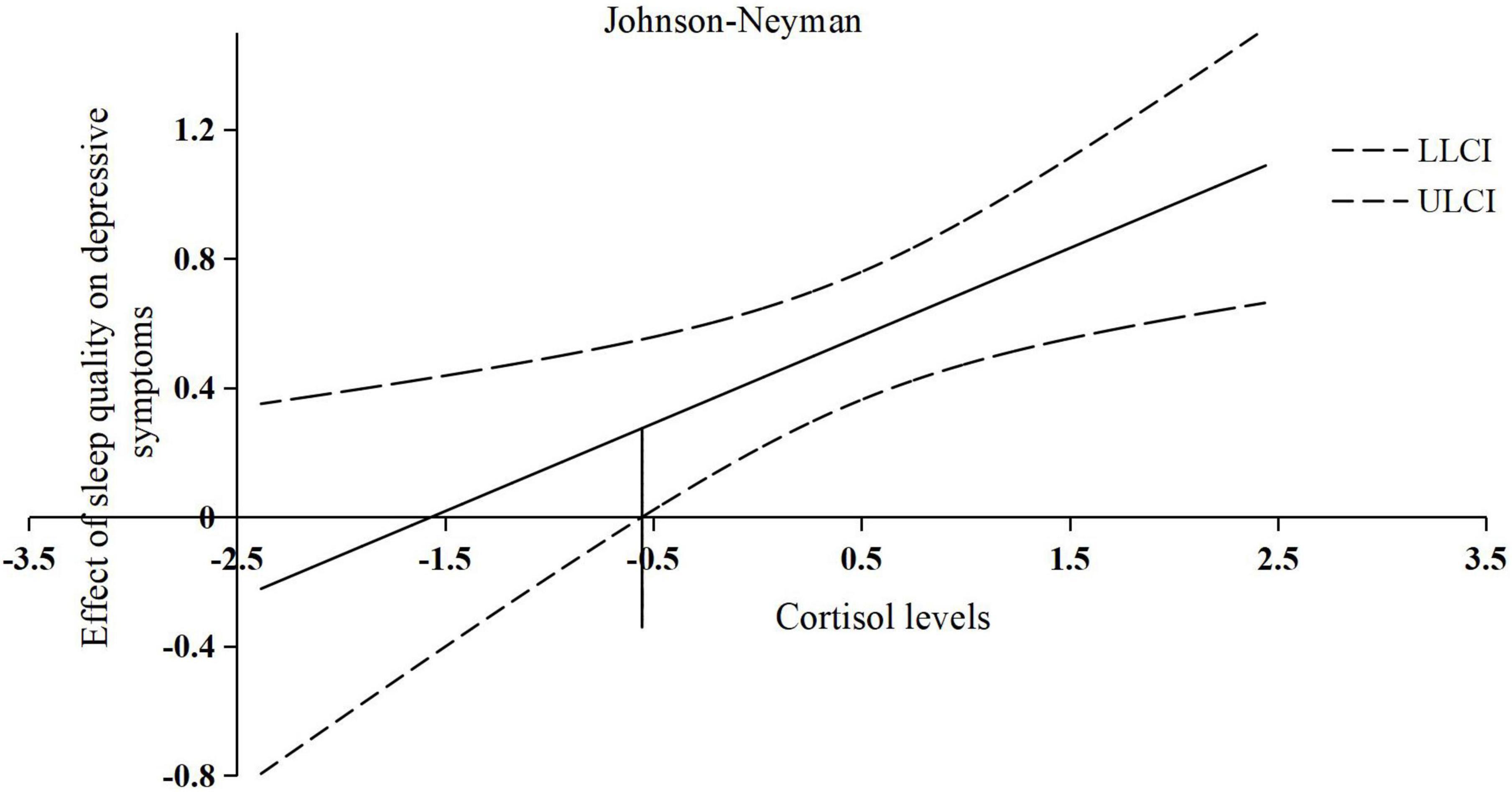
Figure 3. The conditional effect of centered sleep quality on depressive symptoms across the range of centered and logarithmized cortisol levels. X-axis: represents the value of centered and logarithmized cortisol levels; Y-axis: represents the slope of the effect of centered sleep quality on depressive symptoms; the solid line in the center: represents the changing trend of the effect of centered sleep quality on depressive symptoms with the change of the value of centered and logarithmized cortisol levels; the horizontal line at −0.55: represents a threshold of centered and logarithmized cortisol levels; LLCI, lower 95% CI; ULCI, upper 95% CI.
Discussion
To the best of our knowledge, this is the first study to examine the role of life skills and cortisol in the relationship between sleep quality and depressive symptoms among adolescents with CHD. Our results align with prior research showing associations between sleep quality and depressive symptoms (18, 19). Our results also support the theoretical idea that life skills might mediate the association between sleep quality and depressive symptoms. Moreover, cortisol levels might moderate the relationship between sleep quality and depressive symptoms.
The finding that poor sleep quality significantly correlated with greater depressive symptoms is consistent with previous studies showing poor sleep quality as one of the most widely studied risk factors for depressive symptoms (17). Low sleep quality results in poor emotional responses such as heightened reactivity to negative emotional experiences, which further augments an individual’s susceptibility to depressive symptoms (15, 48). Furthermore, the finding that life skills are negatively associated with depressive symptoms also corroborates previous research (23). For instance, Singla et al.’s study showed that the life skill program was effective in preventing depressive symptoms among adolescents by improving emotion regulation and cognitive reappraisal (23).
In this study, life skills played a mediating role in the relationship between sleep quality and depressive symptoms. Poor sleep quality may act as a stressful event that disrupts life skills, and individuals with poor life skills may be less able to adapt to stress and adversity, which could consequently lead to depressive symptoms (26). The mediating effect of life skills in the relationship between sleep quality and depressive symptoms may be explained by Lazarus’s transactional stress-coping model (24) and Pearlin’s stress process theory (27). The transactional stress-coping model holds that cognitive appraisals serve as an essential element in an individuals’ coping process with stressful life events (24), which is closely related to sleep disturbances/problems. A systematic review has revealed that adolescents with sleep disturbances/problems (e.g., insomnia; nightmares; hypersomnia; short sleep duration; and difficulty falling asleep) might adopt negative cognitive appraisals (49). Such negative appraisals (e.g., negative situational and self-appraisals; defeat; and entrapment) may lead to negative coping such as poor life skills and finally contribute to mental health problems including depressive symptoms (27).
Furthermore, our results showed a moderating effect of cortisol levels on the association between sleep quality and depressive symptoms. In detail, cortisol levels might enhance the negative effect of sleep quality on depressive symptoms. In our study, adolescents with low sleep quality and high cortisol levels reported the highest depressive symptoms. This finding is in contrast to a previous longitudinal moderation analysis showing no significant interaction effect between sleep quality and cortisol levels (50). One explanation may be the longer periods contained in the longitudinal study design, making it difficult to observe the maintaining effects of cortisol levels in the association between sleep quality and depressive symptoms that may be affected by other time-evolving environmental factors. Further research is needed to investigate and compare the moderating effect of cortisol on the association between sleep quality and depressive symptoms.
Several limitations should be considered when interpreting the results. First, the cross-sectional design of this study cannot establish causal relationships. However, our study was based on solid theory and past research and still provides useful information on these relationships. Future longitudinal research is needed to further validate our findings. Second, the sample of this study was recruited from Hunan Province, which may not represent adolescents in other parts of China. Further research may consider using a large sample from different areas to test our study hypotheses. Third, this study only examined the relationships between sleep quality, life skills, cortisol level, and depressive symptoms among adolescents with CHD in China. Therefore, it is not clear whether the study’s findings apply to the whole adolescent population. Future studies should evaluate whether similar results could be found when evaluating different adolescent populations living in China. Finally, in our study, we only examined blood cortisol levels once in the early morning, whereas most of the studies measure cortisol levels at three different times (morning, afternoon, and night) of a day [e.g., (51)]. Cortisol releasing has a circadian rhythm (usually, the level of cortisol is highest during 6 a.m. and 8 a.m., and lowest from 10 p.m. to 2 a.m.). Compared with high levels of cortisol in the morning (such as cortisol arousal response), the increase in total cortisol production throughout the day can predict the occurrence of depressive symptoms, which may be more convincing [e.g., (52)]. However, we only collected the subjects’ serum cortisol in the morning, not multiple times a day or total cortisol throughout the day, so the analysis of the results in this study should be reviewed cautiously. Future studies may consider measuring cortisol levels multiple times a day and measuring total cortisol throughout the day to verify the accuracy of the results.
In general, this study was the first to explore the relationships among sleep quality, life skills, cortisol levels, and depressive symptoms. The results showed that sleep quality was significantly associated with depressive symptoms in adolescents. Additionally, the results supported the theoretical idea that life skills may mediate the association between sleep quality and depressive symptoms. Furthermore, the results showed that cortisol levels may moderate the relationship between sleep quality and depressive symptoms. These findings provide new evidence and potential suggestions for the prevention and intervention of adolescent depression. Families, schools, and communities should pay more attention to adolescents with poor sleep quality, low life skills, and high cortisol levels. In addition, improving life skills and sleep quality may also contribute to the improvement in adolescents’ depressive symptoms, which may have lasting beneficial effects into adulthood.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of the Xiangya School of Nursing, Central South University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
PM: conceptualization, formal analysis, writing – review and editing, and visualization. LP: visualization, data curation, formal analysis, and writing – review and editing. WY: visualization, formal analysis, and writing – review and editing. DL, FY, and YC: writing – review and editing. YL: conceptualization, formal analysis, writing – review and editing, and supervision. JH: conceptualization, visualization, formal analysis, and writing – review and editing. All authors contributed to the article and approved the final draft for submission.
Funding
This work was supported by the Hunan Science and Technology Innovation Platform and Talent Plan of China (grant number: 2017TP1004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We warmly thank all the participants of the study for their time and efforts.
References
1. CDC. Preventing Adverse Childhood Experiences. (2021). Available online at: https://www.cdc.gov/violenceprevention/aces/fastfact.html (accessed October 28, 2021).
2. People’s Network.Children Can’t Wait! Half a Million de Facto Unattended Children Will be Guaranteed. New Delhi: People’s Network (2018).
3. Nie QQ, Tian L. l, Scott Huebner E. Relations among family dysfunction, loneliness and life satisfaction in Chinese children: a longitudinal mediation model. Child Indic Res. (2020) 13:839–62.
5. Murray J, Farrington DP, Sekol I. Children’s antisocial behavior, mental health, drug use, and educational performance after parental incarceration: a systematic review and meta-analysis. Psychol Bull. (2012) 138:175–210. doi: 10.1037/a0026407
6. Jane-Costello E, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression? J Child Psychol Psychiatry. (2006) 47:1263–71.
7. Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. (2010) 6:285–312.
8. Shorey S, Ng ED, Wong CHJ. The global prevalence of depression and elevated depressive symptoms among adolescents: a systematic review and meta-analysis. Br J Clin Psychol. (2022) 61:287–305.
9. Obeid S, Al Karaki G, Haddad C, Sacre H, Soufia M, Hallit R, et al. Association between parental divorce and mental health outcomes among Lebanese adolescents: results of a national study. BMC Pediatrics. (2021) 21:455. doi: 10.1186/s12887-021-02926-3
10. Pontes NMH, Ayres C, Wunnenberg M, Pontes MCF. Gender differences in the relationship between prescription opioid misuse and depressive symptoms and suicidality among US high school students. Nurs Outlook. (2021) 69:641–51. doi: 10.1016/j.outlook.2021.01.006
11. Zhang X, Huang R, Ho SY, Lo WS, Lam TH, Wang MP. Alcohol drinking, especially light drinking, and depressive symptoms in adolescents. Drug Alcohol Depend. (2021) 227:108932.
12. Ribeiro JD, Huang X, Fox KR, Franklin JC. Depression and hopelessness as risk factors for suicide ideation, attempts, and death: a meta-analysis of longitudinal studies. Br J Psychiatry. (2018) 212:279–86. doi: 10.1192/bjp.2018.27
13. Zheng Y, Rijsdijk F, Pingault JB, McMahon RJ, Unger JB. Developmental changes in genetic and environmental influences on Chinese child and adolescent anxiety and depression. Psychol Med. (2016) 46:1829–38. doi: 10.1017/S0033291716000313
14. Cho KO. Sleep duration and self-rated health are independently associated with physical activity levels in the Korean population. Iran J Public Health. (2014) 43:590–600.
15. Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. (2017) 31:6–16.
16. O’Callaghan VS, Couvy-Duchesne B, Strike LT, McMahon KL, Byrne EM, Wright MJ. A meta-analysis of the relationship between subjective sleep and depressive symptoms in adolescence. Sleep Med. (2021) 79:134–44.
17. Raniti MB, Allen NB, Schwartz O, Waloszek JM, Byrne ML, Woods MJ. Sleep duration and sleep quality: associations with depressive symptoms across adolescence. Behav Sleep Med. (2017) 15:198–215.
18. Guo L, Deng J, He Y, Deng X, Huang J, Huang G, et al. Prevalence and correlates of sleep disturbance and depressive symptoms among Chinese adolescents: a cross-sectional survey study. BMJ Open. (2014) 4:e005517.
19. Lovato N, Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med Rev. (2014) 18:521–9. doi: 10.1016/j.smrv.2014.03.006
20. Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: a review on bidirectional relationship, mechanisms and treatment. J Cell Mol Med. (2019) 23:2324–32.
21. WHO.Life Skills Education for Children and Adolescents in Schools: Introduction and Guidelines to Facilitate the Development and Implementation of Life Skills Programs. Geneva: WHO (1997).
22. Sancassiani F, Pintus E, Holte A, Paulus P, Moro MF, Cossu G, et al. Enhancing the emotional and social skills of the youth to promote their wellbeing and positive development: a systematic review of universal school-based randomized controlled trials. Clin Pract Epidemiol Ment Health. (2015) 11(Suppl. 1 M2):21–40. doi: 10.2174/1745017901511010021
23. Singla DR, Waqas A, Hamdani SU, Suleman N, Zafar SW, Saeed K, et al. Implementation and effectiveness of adolescent life skills programs in low- and middle-income countries: a critical review and meta-analysis. Behav Res Ther. (2020) 130:103402. doi: 10.1016/j.brat.2019.04.010
25. Vandekerckhove M, Wang YL. Emotion, emotion regulation and sleep: an intimate relationship. AIMS Neurosci. (2017) 5:1–17.
26. Nasheeda A, Abdullah HB, Krauss SE, Ahmed NB. A narrative systematic review of life skills education: effectiveness, research gaps, and priorities. Int J Adolesc Youth. (2019) 24:362–79.
27. Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. (1981) 22:337–56.
28. Zhang Y, Peters A, Bradstreet J. Relationships among sleep quality, coping styles, and depressive symptoms among college nursing students: a multiple mediator model. J Prof Nurs. (2018) 34:320–5. doi: 10.1016/j.profnurs.2017.12.004
29. Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with a focus on developmental stress, HPA axis function, and hippocampal structure in humans. Neurobiol Dis. (2013) 52:24–37. doi: 10.1016/j.nbd.2012.03.012
30. Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. (2020) 25:321–38.
31. Harris TO, Borsanyi S, Messari S, Stanford K, Cleary SE, Shiers HM. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br J Psychiatry. (2000) 177:505–10.
32. Goodyer IM, Croudace T, Dudbridge F, Ban M, Herbert J. Polymorphisms in BDNF (Val66Met) and 5-HTTLPR, morning cortisol and subsequent depression in at-risk adolescents. Br J Psychiatry. (2010) 197:365–71. doi: 10.1192/bjp.bp.110.077750
33. Capponi V, Carrasco C, Macchiavello S, Undurraga J, Campino C, Carvajal C, et al. Depressive symptoms are associated with higher morning plasma cortisol in primary care subjects. Neuro Endocrinol Lett. (2018) 39:288–93.
34. Stroud CB, Vrshek-Shallhorn S, Norkett EM, Doane LD. The cortisol awakening response (CAR) interacts with acute interpersonal stress to prospectively predict depressive symptoms among early adolescent girls. Psychoneuroendocrinology. (2019) 107:9–18. doi: 10.1016/j.psyneuen.2019.04.017
35. Schuler KL, Ruggero CJ, Goldstein BL, Perlman G, Klein DN, Kotov R. Diurnal cortisol interacts with stressful events to prospectively predict depressive symptoms in adolescent girls. J Adolesc Health. (2017) 61:767–72.
36. Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. (2014) 10:679–708.
37. Kamphuis J, Meerlo P, Koolhaas JM, Lancel M. Poor sleep as a potential causal factor in aggression and violence. Sleep Med. (2012) 13:327–34.
38. Hunan Provincial Statistics Bureau. 2017 Hunan Statistical Year Book. (2018). Available online at: http://222.240.193.190/17tjnj/zk/notepyrightch.htm (accessed July 26, 2021).
39. Xia W, Zhong X, Tao H, Chen Q, Lai M, Tian K. Study on the appropriateness of parameters in sample size estimation method of reference range. Chin J Health Stat. (2019) 36:644–8.
40. Government of the People’s Republic of China. 12 Departments Jointly Issued the “Opinions on Further Strengthening the Protection of De Fac Unattended Children”. Ministry of Civil Affairs of China (2020). Available online at: http://www.gov.cn/xinwen/2020-06/10/content-5407970.htm (accessed January 18, 2022).
41. Liu XC, Tang MQ, Hu L, Wang AZ, Wu HX, Zhao GF, et al. Reliability and validity of the Pittsburgh sleep quality index. Chin J Psychiatry. (1996) 29:103–7.
42. Zhao X, Ma Y, Lv X, Zhuang L. Development of secondary school students’ life skills scale. Chin J Sch Health. (2012) 33:515–20. doi: 10.16835/j.cnki.1000-9817.2012.05.004
43. Zhuang LL, Ma YH, Zhao H, Tian Y, Song J. Reliability and validity of secondary school students’ life skills scale. Chin J Sch Health. (2012) 33:1192–4.
44. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur. (1977) 1:385–401.
45. Zhou M, Zhang G, Rozelle S, Kenny K, Xue H. Depressive symptoms of Chinese children: prevalence and correlated factors among subgroups. Int J Environ Res Public Health. (2018) 15:283.
46. CLSI.EP5-A2 Evaluation of Precision Performance of Quantative Measurement Methods, Approved Guideline. 2nd ed. Wayne, PA: CLSI (2004).
47. Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: inferential and graphical techniques. Multivar Behav Res. (2005) 40:373–400. doi: 10.1207/s15327906mbr4003_5
48. Kahn M, Sheppes G, Sadeh A. Sleep and emotions: bidirectional links and underlying mechanisms. Int J Psychophysiol. (2013) 89:218–28.
49. Littlewood D, Kyle SD, Pratt D, Peters S, Gooding P. Examining the role of psychological factors in the relationship between sleep problems and suicide. Clin Psychol Rev. (2017) 54:1–16.
50. Rothe N, Vogel S, Schmelzer K, Kirschbaum S, Penz M, Wekenborg MK, et al. The moderating effect of cortisol and dehydroepiandrosterone on the relation between sleep and depression or burnout. Compr Psychoneuroendocrinol. (2021) 7:100051.
51. Tonon AC, Carissimi A, Schimitt RL, de Lima LS, Hidalgo MP. How do stress, sleep quality, and chronotype associate with clinically significant depressive symptoms? A study of young male military recruits in compulsory service. Braz J Psychiatry. (2020) 42:54–62. doi: 10.1590/1516-4446-2018-0286
Keywords: depressive symptoms, sleep quality, life skills, cortisol, adolescents, childhood household dysfunction
Citation: Mao P, Peng L, Yuwen W, Liu D, Yan F, Chen Y, Long Y and Hash J (2022) The Mediating and Moderating Roles of Life Skills and Cortisol in the Relationship Between Sleep Quality and Depressive Symptoms Among Chinese Adolescents With Childhood Household Dysfunction. Front. Psychiatry 13:870349. doi: 10.3389/fpsyt.2022.870349
Received: 06 February 2022; Accepted: 07 June 2022;
Published: 12 July 2022.
Edited by:
Li Yang, Peking University Sixth Hospital, ChinaReviewed by:
Jacky Ho Yin Wan, Open Sky Psychology, AustraliaPeng Xiong, Jinan University, China
Joseph D. Wellman, University of Mississippi, United States
Copyright © 2022 Mao, Peng, Yuwen, Liu, Yan, Chen, Long and Hash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yixiang Long, NjAwMTEwQGNzdS5lZHUuY24=
 Ping Mao1,2
Ping Mao1,2 Lianhua Peng
Lianhua Peng Weichao Yuwen
Weichao Yuwen