
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 16 June 2022
Sec. Schizophrenia
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.867461
This article is part of the Research Topic Prognostic Imaging Biomarkers in Psychotic Disorders View all 7 articles
 Tsutomu Takahashi1,2*
Tsutomu Takahashi1,2* Daiki Sasabayashi1,2
Daiki Sasabayashi1,2 Yoichiro Takayanagi1,3
Yoichiro Takayanagi1,3 Atsushi Furuichi1,2
Atsushi Furuichi1,2 Haruko Kobayashi1,2
Haruko Kobayashi1,2 Kyo Noguchi4
Kyo Noguchi4 Michio Suzuki1,2
Michio Suzuki1,2Deficit syndrome schizophrenia is a characteristic subtype defined by persistent negative symptoms and poor functional outcomes; however, the biological mechanisms underlying this specific subtype have not yet been elucidated in detail. The present magnetic resonance imaging study examined the prevalence of duplicated Heschl’s gyrus (HG), a potential neurodevelopmental marker, in schizophrenia patients with (N = 38) and without (N = 37) the deficit syndrome. The prevalence of the HG duplication pattern bilaterally was higher in the whole schizophrenia group than in 59 matched healthy controls. Furthermore, the prevalence of right HG duplication was significantly higher in the deficit schizophrenia group than in the non-deficit schizophrenia group. The HG pattern in schizophrenia was not associated with clinical variables, including illness duration, medication, and symptom severity, while right HG duplication correlated with higher scores for Proxy for the Deficit Syndrome. The present results suggest that the prominent neurodevelopmental pathology associated with gyral formation of HG may contribute to enduring negative symptomatology in schizophrenia.
Schizophrenia is characterized by substantial clinical and biological heterogeneity, where negative symptoms are a core component that mainly account for long-term disability and poor functional outcomes in patients with the disorder (1, 2). The deficit form of schizophrenia (D-Sz), a well-defined clinical subgroup independent of the DSM (3) or ICD (4) subtype classification, is characterized by primary (i.e., not secondary to other factors, such as positive symptoms, comorbid depression, and extrapyramidal side effects) and enduring negative symptoms (5–7). The DSM/ICD subtypes of schizophrenia based on symptom profiles (e.g., paranoid, disorganized, and undifferentiated) have been eliminated because the subtype classification changes with time and cannot estimate their outcomes (8). On the other hand, the D-Sz defined by established classification tools {i.e., the Schedule for the Deficit Syndrome (9) or the Proxy for the Deficit Syndrome [PDS; (10)]} has a subtype stability over time and rather homogeneous outcome (7). While the etiological factors associated with D-Sz have not yet been identified, neuroimaging evidence of enhanced interregional cortical coupling (11) and altered gross brain morphology (e.g., gyrification patterns) (12, 13) specifically in D-Sz appear to support its prominent neurodevelopmental pathology. Therefore, brain morphological characteristics associated with early neurodevelopmental abnormalities, which likely exist at illness onset (14), may be a prognostic biomarker of worse long-term functioning in schizophrenia.
Recent magnetic resonance imaging (MRI) studies on schizophrenia revealed an increased prevalence of a duplicated Heschl’s gyrus (HG) pattern (15–17), which may reflect the development of an anomalous cytoarchitecture in utero (18, 19). This alteration in the gross brain morphology has been detected irrespective of illness stages [i.e., high-risk status (17), both first-episode (15) and chronic (16) stages] and the medication status (15–17) of schizophrenia. Furthermore, these studies suggested that the duplicated HG pattern was associated with severe prodromal symptomatology (17), but rather mild positive psychotic symptoms after onset (15, 16), as well as prominent verbal fluency deficit (17). These findings likely support the possibility that the HG gyrification pattern may contribute to specific clinical syndrome in schizophrenia. In addition, it has been suggested that the duplicated pattern of the HG, which participates in emotional processing (20), is associated with regional brain hypoactivity (21). It may be thus hypothesized that the patients with D-Sz, who are characterized by persistent negative symptoms, have an increased prevalence of HG duplication, but no MRI studies to date have specifically examined the HG duplication patterns in D-Sz.
Therefore, we herein used MRI to investigate and compare duplicated HG patterns in the D-Sz and non-deficit subtype of schizophrenia (ND-Sz) with those in matched healthy controls. We had previously explored brain characteristics of our D-Sz cohort and found gross morphological changes associated with early neurodevelopment [e.g., small adhesio interthalamica, altered distribution of the orbitofrontal sulcogyral pattern; (12)] and gray matter reduction in the insular cortex (unpublished data). The present study aimed to further expand these studies to HG duplication pattern, a recently identified early neurodevelopmental marker. Based on previous findings showing HG duplication in schizophrenia as a stable neurodevelopmental marker (15–17) and a putative prominent neurodevelopmental pathology in its deficit subtype (11–13), we predicted that the prevalence of HG duplication may be higher in patients with schizophrenia, particularly those with D-Sz. Furthermore, we investigated the relationships between the HG pattern and the clinical characteristics of patients in the different subgroups.
Thirty-eight patients with D-Sz, 37 with ND-Sz, and 59 healthy control subjects were enrolled in the present study (Table 1). We have previously investigated other brain structures (i.e., midline brain structures, orbitofrontal surface morphology) in this cohort (12), but this is the first study that specifically examined the relationship between the HG patterns and D-Sz using our data. However, the majority of the healthy controls and 51/75 schizophrenia patients examined in the present study had been included in our previous studies on HG patterns in the schizophrenia spectrum (16) and the volume-by-gyrification relationship of the HG in first-episode schizophrenia (15).
Briefly, schizophrenia patients meeting the ICD-10 research criteria (4) assessed via a structured clinical interview [the Comprehensive Assessment of Symptoms and History (22)] and chart review were recruited at Toyama University Hospital. Just prior to MRI being performed, clinical symptoms were examined by experienced psychiatrists using the Brief Psychiatric Rating Scale [BPRS (23)] and the Scale for the Assessment of Negative/Positive Symptoms [SANS/SAPS (24)]. As previously described (12, 13), patients were divided into the D and ND subgroups based on their PDS scores [i.e., blunted affect − (anxiety + guilty feelings + depressed mood + hostility items)] according to BPRS (10). Although the PDS score accurately reflects primary and enduring negative symptomatology in patients with schizophrenia (10, 25), we herein classified patients with the top (≥−3) and bottom (≤−8) 25[percentage] of PDS scores in our full schizophrenia dataset into the D and ND subgroups, respectively, to increase the likelihood of a correct classification (11).
Healthy subjects who had been screened using a questionnaire for a personal or family history of psychiatric disease in first-degree relatives (26) were selected from our previous studies (15, 16) based on matching to the patient group for demographic characteristics (e.g., age, sex, height, and parental education; Table 1).
All participants were physically healthy and screened for gross brain abnormalities using MRI. Exclusion criteria for patients and controls were as follows: (1) a lifetime history of serious head injury, seizure, neurological disease, or substance abuse disorder; (2) a history of electroconvulsive therapy; and (3) other medical conditions that may affect mental condition and/or brain functioning (e.g., thyroid dysfunction, steroid use, hypertension, and diabetes). The study protocol was approved by the Committee on Medical Ethics of the University of Toyama (No. I2013006). Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
All participants underwent the same imaging protocol at Toyama University Hospital using a 1.5T Magnetom Vision scanner (Siemens, Erlangen, Germany) to obtain 160–180 contiguous T1-weighted 1-mm-thick sagittal slices via the 3D gradient-echo sequence FLASH. The following imaging parameters were used: TR/TE = 24 ms per 5 ms; flip angle = 40°; FOV = 256 mm; and matrix = 256 × 256 pixels, with a voxel size of 1.0 mm × 1.0 mm × 1.0 mm.
Brain images were reconstructed into 1-mm-thick contiguous coronal images that perpendicularly aligned with the anterior commissure-posterior commissure line using Dr. View software (Infocom, Tokyo, Japan).
One rater (TT) assessed all MR images under blind conditions for subject identities. As previously described (15–17), the HG was classified into the single or duplicated pattern by referring to images in three directions; the duplicated pattern was further divided into partial duplication by the sulcus intermedius [common stem duplication (CSD)] or complete duplication accompanied by a second HG [complete posterior duplication (CPD)] (Figure 1). A second rater with no knowledge of the subjects’ identity (DS) independently assessed the HG patterns in a subset of randomly selected 15 brains. Intra- (TT) and inter-rater (TT and DS) reliabilities for the classification of HG were both higher than 0.83 (tested by Cronbach’s α).

Figure 1. Different Heschl’s gyrus gyrification patterns (colored in blue) in the left hemisphere on MR images in three directions and a schematic diagram on axial directions. A, anterior; CPD, complete posterior duplication; CSD, common stem duplication; FTS, first transverse sulcus; HG, Heschl’s gyrus; HS, Heschl’s sulcus; L, lateral; P, posterior; M, medial; PP, planum polare; PT, planum temporale; sHG, second Heschl’s gyrus; sHS, second Heschl’s sulcus; SI, sulcus intermedius. Note that these patterns were presented also in our previous publications (15–17).
A one-way analysis of variance (ANOVA) or the χ2 test (or Fisher’s exact test when more than 20[percentage] of cells had expected counts <5) was employed for group comparisons of demographic and clinical data.
We used the χ2 test to evaluate group differences (controls vs. whole schizophrenia, D- vs. ND-Sz) in HG gyrification pattern distributions in each hemisphere. Because the HG duplication was more prevalent in the patients especially for D-Sz, the odds ratio was calculated to estimate the relationship between HG duplication and relative risk of schizophrenia and its subtype. The relationships between HG patterns and clinical variables [onset age, illness duration, medication (dose, duration), total SANS/SAPS and BPRS scores, and PDS scores] were assessed by an analysis of covariance (ANCOVA) with age as a covariate, followed by Duncan’s test. p < 0.05 was considered to indicate a significant difference.
The score for the blunted affect subscale was significantly higher in the D-Sz group than in the ND-Sz group, while delusions, hallucinations, and attention deficits were more severe in the ND group (Table 1). On the other hand, no significant differences were observed in the age of onset, duration of illness, or medication status between the subgroups. In accordance with the literature (27), the proportion of males was higher in the D-Sz group than in the ND-Sz group.
Comparisons between all patients with schizophrenia (N = 75) and healthy controls (N = 59) revealed significant differences for both the left (χ2 = 6.69, p = 0.035) and right (χ2 = 10.86, p = 0.004) hemispheres (Table 2). The prevalence of bilateral HG duplication patterns (CSD or CPD) was higher in patients than in the controls [left, χ2 = 5.90, p = 0.015, odds ratio = 2.37 (95[percentage] CI, 1.18–4.77); right, χ2 = 10.22, p = 0.001, odds ratio = 3.33 (95[percentage] CI, 1.59–7.03)] (Figure 2). We then examined right-handed subjects only because handedness distribution differed between the control and whole patient groups (Fisher’s exact test, p = 0.034); the results obtained remained essentially the same (Supplementary Material).
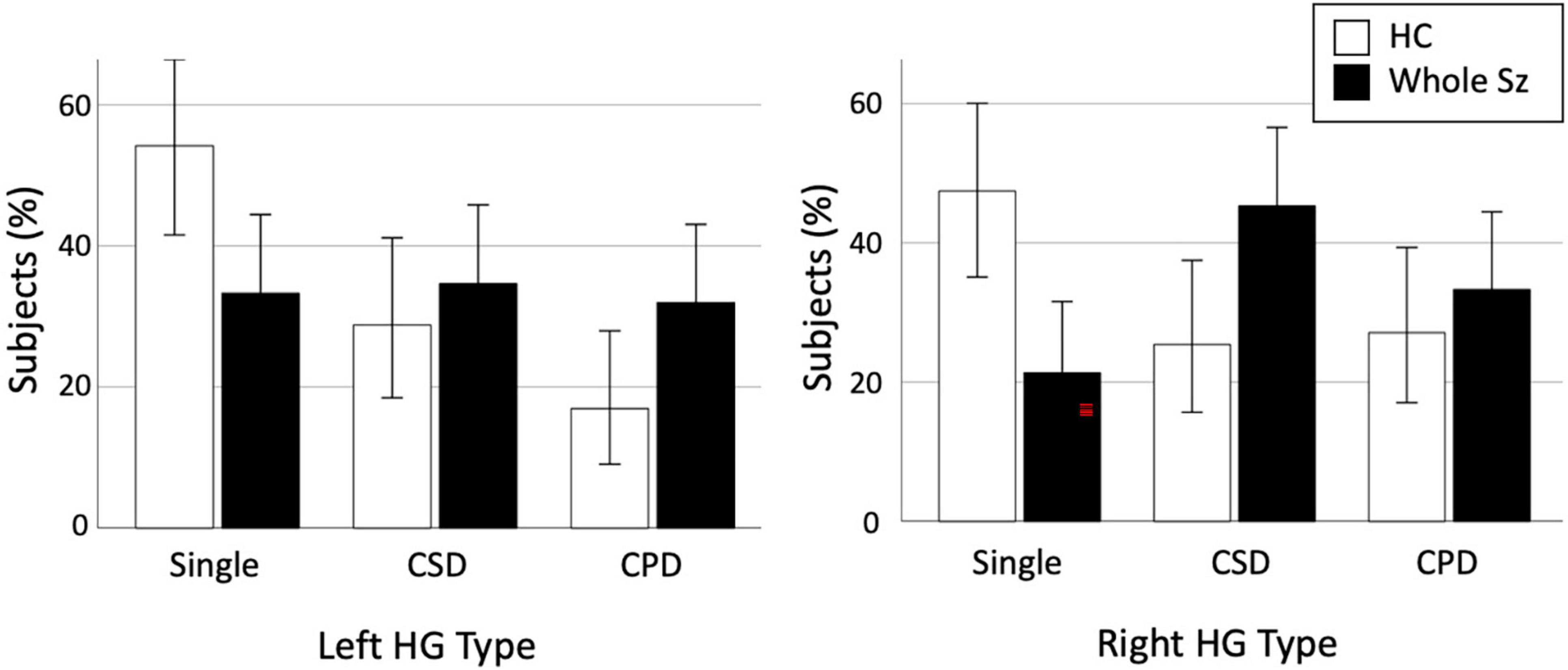
Figure 2. Prevalence of each Heschl’s gyrus (HG) gyrification pattern in healthy controls (HC) and whole schizophrenia (Sz). Error bars indicate 95[percentage] confidence intervals. CPD, complete posterior duplication; CSD, common stem duplication.
In subgroup comparisons between the D- and ND-Sz groups, a significant difference was only observed for the right hemisphere (left, χ2 = 0.50, p = 0.779; right, χ2 = 7.23, p = 0.027). The prevalence of right HG duplication was significantly higher in the D-Sz subgroup than in the ND-Sz subgroup [χ2 = 5.36, p = 0.021, odds ratio = 4.08 (95[percentage] CI, 1.22–13.42)] (Figure 3). When only patients with HG duplication patterns (i.e., CSD vs. CPD) were examined, a subgroup difference was not observed (left, χ2 = 0.07, p = 0.786; right, χ2 = 1.91, p = 0.167).
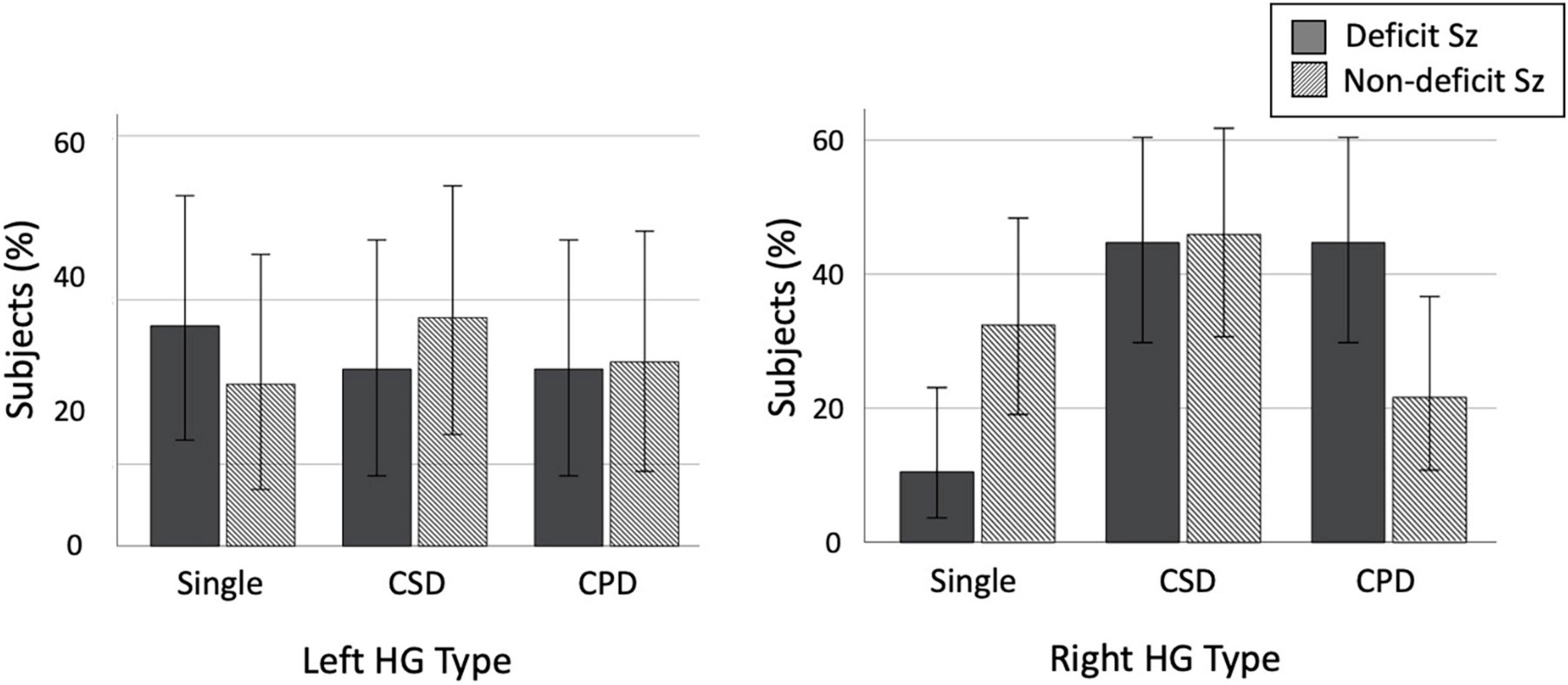
Figure 3. Prevalence of each Heschl’s gyrus (HG) gyrification pattern in patients with deficit and non-deficit schizophrenia (Sz). Error bars indicate 95[percentage] confidence intervals. CPD, complete posterior duplication; CSD, common stem duplication.
Furthermore, there was no significant effect of sex on HG duplication in the controls, the schizophrenia group as a whole, or each schizophrenia subgroup.
The HG pattern did not affect the onset age, duration of illness, medications, or symptom ratings (total SANS, SAPS, and BPRS scores) in the D- and ND-Sz subgroups. As predicted by significant contribution of right HG duplication to the D-Sz (described above), in the schizophrenia group as a whole, patients with the right duplicated pattern had a significantly higher PDS score (i.e., higher characteristic tendency for the deficit subtype) than those with right single HG [F(1,72) = 4.66, p = 0.034; post hoc test, p = 0.034]. This difference in the PDS score was especially evident between patients with CPD (mean = −4.24, SD = 3.87) and those with the single pattern (mean = −8.00, SD = 3.90) [F(2,71) = 3.74, p = 0.029; post hoc test, p = 0.007]. On the other hand, the left HG pattern in the schizophrenia group as a whole did not affect these clinical variables.
While its preliminary nature with a small number of subjects, we demonstrated for the first time that prevalence of right HG duplication was higher in patients with D-Sz than in those with ND-Sz. This difference was not explained by demographic or clinical differences in these subgroups, except for the PDS score, which reflects the trait characteristics of deficit syndrome. These results suggest that prominent early neurodevelopmental abnormalities associated with sulcal formation during gestation may contribute to the characteristic clinical manifestations of D-Sz, such as enduring negative symptomatology and poor functional outcomes.
The present study demonstrated that the prevalence of the right HG duplication patterns was significantly higher in patients with schizophrenia, particularly those with the deficit subtype, than in healthy controls, while the HG duplication itself has been reported in approximately 30–50[percentage] of healthy adults (28–30). The functional significance of this anatomical variation currently remains unclear, but may reflect inter-individual differences in the cytoarchitectonic development of the primary auditory cortex in utero (18, 19); duplicated HG has been implicated in the development of learning disabilities (31, 32) and the inhibition of HG activity in auditory processes (21) after birth, even in non-clinical populations. HG is a primary brain region of auditory processing, but also participates in emotional processing (20) and social communication (33). Therefore, the core clinical features of D-Sz, such as persistent blunted affect and prominent cognitive deficits, particularly in social and verbal domains (7), may be partly attributed to neurodevelopmental abnormalities associated with HG sulcus formation. Indeed, our previous study on the early stages of psychosis (17) revealed a correlation between the HG duplication pattern and verbal fluency deficits, although it was evident between different duplication patterns (i.e., CSD vs. CPD) in the left hemisphere. Since brain gyrification may reflect regional neural connectivity (34), our hypothesis needs to be tested in a cohort with more detailed clinical assessments using the multimodal neuroimaging of brain connectivity/function. Potential clinical significance of different HG duplication patterns (CSD, CPD) should be also tested in such future studies.
The present results on HG duplication patterns were not influenced by illness chronicity or medication, supporting previous neuroimaging findings suggesting a prominent early neurodevelopmental pathology in D-Sz [e.g., small adhesio interthalamica (12) and gyrification pattern changes (12, 13)]. We failed to replicate our previous findings in larger and less confounded (i.e., first-episode) schizophrenia groups to show that the right CPD pattern specifically contributed to less severe positive symptomatology (15, 16); however, the right CPD pattern in the present cohort correlated with the trait characteristics of deficit syndrome (i.e., PDS score). In addition, the right lateralized group difference in the HG pattern in the present study may support right hemisphere dominance for emotion processing, particularly for negative emotional information (35), as well as D-Sz having severe and prolonged neurodevelopmental abnormalities. Right HG generally develops 1–2 weeks earlier than left HG during mid-to-late gestation (18) and is more complex (18, 29); therefore, gyral formation of HG in D-Sz may be more affected on the right hemisphere. Since a meta-analysis of gray and white matter volumes across various brain regions found no significant differences between D- and ND-Sz (36) and these volumetric data are affected by various confounding factors (e.g., illness chronicity and medication), a better predictive biomarker of the clinical subtype and course of schizophrenia may be gross brain morphology, which is strongly related to early neurodevelopment.
Several potential confounding factors in the present study need to be addressed. First, the present study was partly limited by its small sample size for each schizophrenia subgroup. Due to its reliability and stability, the PDS score has been widely used to classify patients into the D and ND subgroups in biological (11, 37–39) and clinical (40–42) studies (10, 25, 41, 43); however, a cross-sectional PDS rating does not have the capacity to directly assess enduring deficit-like features. Therefore, we excluded the ambiguous middle group for the PDS score (−8 to −3) from the current schizophrenia sample in an attempt to reduce potential false classifications, as in previous imaging studies (11–13), which further decreased the number of schizophrenic patients examined. Therefore, the present results need to be confirmed in a well-defined cohort of a large number of patients with D-Sz with clinical follow-up data and/or a semi-structured interview [e.g., the Schedule for the Deficit Syndrome (9)]. Second, the sex ratio significantly differed between the D- and ND-Sz subgroups in the present group, potentially reflecting the general characteristics of D-Sz [more males than females (27)]. However, this difference did not appear to significantly affect the results obtained because there was no significant sex effect on HG patterns. Third, we did not systematically assess the cognitive function of our cohort. Since HG duplication patterns have been implicated in prominent cognitive impairments in D-Sz, as described above, future studies on the gyrification–cognition relationship may provide a more detailed understanding of the pathophysiology of this specific schizophrenia subtype. Finally, although the present HG pattern classification by manual delineation on 1.5T MRI data was reliable (Cronbach’s α > 0.83), our results should be replicated using unbiased automatic analysis on higher resolution images.
In summary, this preliminary MRI study demonstrated that the prevalence of HG duplication was higher in patients with schizophrenia, particularly those with the deficit syndrome subtype who typically exhibit enduring negative symptomatology and poor functional outcomes. Although the HG duplication itself is observed in healthy subjects and there is considerable overlap on the HG pattern distribution between D- and ND-Sz subgroups, it is possible that, in combination with other brain characteristics, the HG pattern in the early stages of schizophrenia may have potential as a prognostic biomarker of worse long-term functioning.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Committee on Medical Ethics of the University of Toyama. The patients/participants provided their written informed consent to participate in this study.
MS and TT conceived the idea and methodology of this study. TT conducted the statistical analyses and wrote the manuscript. DS and HK recruited the participants and were involved in clinical and diagnostic assessments. TT and DS analyzed the MRI data. KN provided the technical support for MRI scanning and data processing. AF managed the MRI and clinical data. MS and YT contributed to the writing and editing of the manuscript. All authors contributed to and approved the final manuscript.
This work was supported by the JSPS KAKENHI Grant Numbers JP18K07550 to TT, JP20H03598 to MS, and JP18K15509 to DS. This work was also partly supported by the Health and Labour Sciences Research Grants for Comprehensive Research on Persons with Disabilities from the Japan Agency for Medical Research and Development (AMED) Grant Number 20dk0307094s0201 to MS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.867461/full#supplementary-material
1. Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. (2020) 16:519–34. doi: 10.2147/NDT.S225643
2. Mosolov SN, Yaltonskaya PA. Primary and secondary negative symptoms in schizophrenia. Front Psychiatry. (2022) 12:766692. doi: 10.3389/fpsyt.2021.766692
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association Press (1994).
4. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization (1993).
5. Carpenter WT Jr., Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. (1988) 145:578–83. doi: 10.1176/ajp.145.5.578
6. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. (2001) 58:165–71. doi: 10.1001/archpsyc.58.2.165
7. Kirkpatrick B, Galderisi S. Deficit schizophrenia: an update. World Psychiatry. (2008) 7:143–7. doi: 10.1002/j.2051-5545.2008.tb00181.x
8. Mattila T, Koeter M, Wohlfarth T, Storosum J, van den Brink W, de Haan L, et al. Impact of DSM-5 changes on the diagnosis and acute treatment of schizophrenia. Schizophr Bull. (2015) 41:637–43. doi: 10.1093/schbul/sbu172
9. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr. The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. (1989) 30:119–23. doi: 10.1016/0165-1781(89)90153-4
10. Kirkpatrick B, Buchanan RW, Breier A, Carpenter WT Jr. Case identification and stability of the deficit syndrome of schizophrenia. Psychiatry Res. (1993) 47:47–56. doi: 10.1016/0165-1781(93)90054-k
11. Wheeler AL, Wessa M, Szeszko PR, Foussias G, Chakravarty MM, Lerch JP, et al. Further neuroimaging evidence for the deficit subtype of schizophrenia: a cortical connectomics analysis. JAMA Psychiatry. (2015) 72:446–55. doi: 10.1001/jamapsychiatry.2014.3020
12. Takahashi T, Takayanagi Y, Nishikawa Y, Nakamura M, Komori Y, Furuichi A, et al. Brain neurodevelopmental markers related to the deficit subtype of schizophrenia. Psychiatry Res Neuroimaging. (2017) 266:10–8. doi: 10.1016/j.pscychresns.2017.05.007
13. Takayanagi Y, Sasabayashi D, Takahashi T, Komori Y, Furuichi A, Kido M, et al. Altered brain gyrification in deficit and non-deficit schizophrenia. Psychol Med. (2019) 49:573–80. doi: 10.1017/S0033291718001228
14. Takahashi T, Suzuki M. Brain morphologic changes in early stages of psychosis: implications for clinical application and early intervention. Psychiatry Clin Neurosci. (2018) 72:556–71. doi: 10.1111/pcn.12670
15. Takahashi T, Sasabayashi D, Takayanagi Y, Furuichi A, Kido M, Nakamura M, et al. Altered Heschl’s gyrus duplication pattern in first-episode schizophrenia. Schizophr Res. (2021) 237:174–81. doi: 10.1016/j.schres.2021.09.011
16. Takahashi T, Sasabayashi D, Takayanagi Y, Furuichi A, Kido M, Pham TV, et al. Increased Heschl’s gyrus duplication in schizophrenia spectrum disorders: a cross-sectional MRI study. J Pers Med. (2021) 11:40. doi: 10.3390/jpm11010040
17. Takahashi T, Sasabayashi D, Takayanagi Y, Higuchi Y, Mizukami Y, Nishiyama S, et al. Heschl’s gyrus duplication pattern in individuals at risk of developing psychosis and patients with schizophrenia. Front Behav Neurosci. (2021) 15:647069. doi: 10.3389/fnbeh.2021.647069
18. Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. (1977) 1:86–93. doi: 10.1002/ana.410010109
19. Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. (1995) 5:56–63. doi: 10.1093/cercor/5.1.56
20. Concina G, Renna A, Grosso A, Sacchetti B. The auditory cortex and the emotional valence of sounds. Neurosci Biobehav Rev. (2019) 98:256–64. doi: 10.1016/j.neubiorev.2019.01.018
21. Tzourio-Mazoyer N, Marie D, Zago L, Jobard G, Perchey G, Leroux G, et al. Heschl’s gyrification pattern is related to speech-listening hemispheric lateralization: fMRI investigation in 281 healthy volunteers. Brain Struct Funct (2015) 220:1585–99. doi: 10.1007/s00429-014-0746-4
22. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. (1992) 49:615–23. doi: 10.1001/archpsyc.1992.01820080023004
23. Rhoades HM, Overall JE. The semistructured BPRS interview and rating guide. Psychopharmacol Bull. (1988) 24:101–4.
24. Andreasen NC. Scale for the Assessment of Negative Symptoms/Scale for the Assessment of Positive Symptoms. Iowa, AS: The University of Iowa (1984).
25. Goetz RR, Corcoran C, Yale S, Stanford AD, Kimhy D, Amador X, et al. Validity of a ‘proxy’ for the deficit syndrome derived from the Positive and Negative Syndrome Scale (PANSS). Schizophr Res. (2007) 93:169–77. doi: 10.1016/j.schres.2007.02.018
26. Takahashi T, Suzuki M, Tsunoda M, Kawamura Y, Takahashi N, Maeno N, et al. The association of genotypic combination of the DRD3 and BDNF polymorphisms on the adhesio interthalamica and medial temporal lobe structures. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:1236–42. doi: 10.1016/j.pnpbp.2008.03.014
27. Roy MA, Maziade M, Labbé A, Mérette C. Male gender is associated with deficit schizophrenia: a meta-analysis. Schizophr Res. (2001) 47:141–7. doi: 10.1016/s0920-9964(99)00231-5
28. Leonard CM, Puranik C, Kuldau JM, Lombardino LJ. Normal variation in the frequency and location of human auditory cortex landmarks. Heschl’s gyrus: where is it? Cereb Cortex. (1998) 8:397–406. doi: 10.1093/cercor/8.5.397
29. Abdul-Kareem IA, Sluming V. Heschl gyrus and its included primary auditory cortex: structural MRI studies in healthy and diseased subjects. J Magn Reson Imaging. (2008) 28:287–99. doi: 10.1002/jmri.21445
30. Marie D, Jobard G, Crivello F, Perchey G, Petit L, Mellet E, et al. Descriptive anatomy of Heschl’s gyri in 430 healthy volunteers, including 198 left-handers. Brain Struct Funct. (2015) 220:729–43. doi: 10.1007/s00429-013-0680-x
31. Leonard CM, Voeller KK, Lombardino LJ, Morris MK, Hynd GW, Alexander AW, et al. Anomalous cerebral structure in dyslexia revealed with magnetic resonance imaging. Arch Neurol. (1993) 50:461–9. doi: 10.1001/archneur.1993.00540050013008
32. Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzler J, Mohr CM, et al. Anatomical risk factors for phonological dyslexia. Cereb Cortex. (2001) 11:148–57. doi: 10.1093/cercor/11.2.148
33. Neophytou D, Oviedo HV. Using neural circuit interrogation in rodents to unravel human speech decoding. Front Neural Circuits. (2020) 14:2. doi: 10.3389/fncir.2020.00002
34. Sasabayashi D, Takahashi T, Takayanagi Y, Suzuki M. Anomalous brain gyrification patterns in major psychiatric disorders: a systematic review and transdiagnostic integration. Transl Psychiatry. (2021) 11:176. doi: 10.1038/s41398-021-01297-8
35. Smith SD, Bulman-Fleming MB. An examination of the right-hemisphere hypothesis of the lateralization of emotion. Brain Cogn. (2005) 57:210–3. doi: 10.1016/j.bandc.2004.08.046
36. Chee TT, Chua L, Morrin H, Lim MF, Fam J, Ho R. Neuroanatomy of patients with deficit schizophrenia: an exploratory quantitative meta-analysis of structural neuroimaging studies. Int J Environ Res Public Health. (2020) 17:6227. doi: 10.3390/ijerph17176227
37. Kirkpatrick B, Fernandez-Egea E, Garcia-Rizo C, Bernardo M. Differences in glucose tolerance between deficit and nondeficit schizophrenia. Schizophr Res. (2009) 107:122–7. doi: 10.1016/j.schres.2008.09.023
38. Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, et al. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. (2013) 70:472–80. doi: 10.1001/jamapsychiatry.2013.786
39. Valiente-Gómez A, Amann BL, Mármol F, Oliveira C, Messeguer A, Lafuente A, et al. Comparison of serum BDNF levels in deficit and nondeficit chronic schizophrenia and healthy controls. Psychiatry Res. (2014) 220:197–200. doi: 10.1016/j.psychres.2014.08.039
40. Messias E, Kirkpatrick B, Bromet E, Ross D, Buchanan RW, Carpenter WT Jr., et al. Summer birth and deficit schizophrenia: a pooled analysis from 6 countries. Arch Gen Psychiatry. (2004) 61:985–9.
41. Strauss GP, Harrow M, Grossman LS, Rosen C. Periods of recovery in deficit syndrome schizophrenia: a 20-year multi-follow-up longitudinal study. Schizophr Bull. (2010) 36:788–99. doi: 10.1093/schbul/sbn167
42. Kalisz A, Cechnicki A. The stability of negative syndrome, persistent negative syndrome and deficit syndrome in a twenty-year follow-up study of schizophrenia patients. Psychiatry Res. (2016) 238:236–41. doi: 10.1016/j.psychres.2016.02.012
Keywords: Heschl’s gyrus, schizophrenia, negative symptoms, deficit subtype, early neurodevelopment
Citation: Takahashi T, Sasabayashi D, Takayanagi Y, Furuichi A, Kobayashi H, Noguchi K and Suzuki M (2022) Different Heschl’s Gyrus Duplication Patterns in Deficit and Non-deficit Subtypes of Schizophrenia. Front. Psychiatry 13:867461. doi: 10.3389/fpsyt.2022.867461
Received: 01 February 2022; Accepted: 31 May 2022;
Published: 16 June 2022.
Edited by:
Ingrid Melle, University of Oslo, NorwayReviewed by:
Kazutaka Ohi, Gifu University, JapanCopyright © 2022 Takahashi, Sasabayashi, Takayanagi, Furuichi, Kobayashi, Noguchi and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tsutomu Takahashi, dHN1dG9tdUBtZWQudS10b3lhbWEuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.