- 1Department of Otorhinolaryngology–Head and Neck Surgery, First Affiliated Hospital of Nanchang University, Nanchang, China
- 2Department of Anesthesiology, First Affiliated Hospital of Nanchang University, Nanchang, China
Background: Obstructive sleep apnea (OSA) is associated with hypertension; however, the associations between cardinal features of OSA, such as intermittent hypoxia (IH) and sleep fragmentation (SF), and blood pressure remain unclear. We performed this study to address this issue.
Method: We investigated 335 subjects with the polysomnography (PSG) tests. Data, including basic characteristics, PSG parameters, and blood pressure, were collected. We calculated p-values for linear trends of blood pressure across oxygen-desaturation index (ODI)/microarousal index (MAI) quartiles. Logistic regressions were used to determine the risk factors for abnormal blood pressure and to detect the multiplicative interaction between ODI and MAI with blood pressure.
Results: After adjusting for multiple variables, compared with subjects with lower ODI quartiles, those with higher ODI quartiles had significant higher systolic blood pressure (SBP) and diastolic blood pressure (DBP) (p for trend = 0.010 and 0.018, respectively). And compared with subjects with lower ODI quartiles, those with higher ODI quartiles were also more likely to have abnormal DBP and hypertension after adjusting for multiple variables. Similarly, compared with subjects with lower MAI quartiles, those with higher MAI quartiles had significant higher SBP and DBP, and were more likely to have abnormal DBP and hypertension. No significant multiplicative interactions between ODI and MAI with blood pressure were detected.
Conclusion: Subjects with more severe IH/SF had significant higher blood pressure and were more likely to have abnormal DBP and hypertension than those with less severe IH/SF. No interaction between IH and SF on the relationship with blood pressure was shown.
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder and its prevalence increases with age and obesity (1). A recent study reported a 23.4 and 49.7% prevalence of moderate-to-severe OSA in females and males, respectively (2). Multiple studies have demonstrated an association between OSA and cardiovascular diseases, particularly hypertension. The Sleep Heart Health study reported a prevalence of 59, 62, and 67% for hypertension in mild, moderate, and severe OSA, respectively (3). Meanwhile, OSA is particularly common in refractory hypertension (4, 5). Studies have also demonstrated an independent relationship between OSA severity and blood pressure. A large cross-sectional study demonstrated that, after adjusting for age, body mass index (BMI), and gender, OSA severity index was a significant predictor of both systolic (SBP) and diastolic (DBP) blood pressures, and each additional apneic event per hour of sleep increased the odds of hypertension by about 1% (6). A linear increase in SBP and DBP with increasing OSA severity was also demonstrated after adjusting for age, BMI, and gender (7).
Obstructive sleep apnea is characterized by two cardinal pathophysiological features, including intermittent hypoxia (IH) and sleep fragmentation (SF). IH has long been recognized as one of the mechanisms in the development of OSA-related hypertension, and rodent studies have demonstrated a robust association between IH and hypertension (8). It has been suggested that IH may increase the sympathetic nervous system and renin-angiotensin system activity, (9–11) cause endothelial dysfunction, (12, 13) and contribute to inflammation and metabolic disorders (14, 15) to induce hypertension. Recent evidence also implicates SF as a distinct trigger for elevated blood pressure. Rodent studies have demonstrated that SF contributes to sympathetic activation and endothelial dysfunction, which promotes the development of hypertension (16). However, the results of clinical studies have been inconsistent. Some studies have demonstrated a positive association between SF and the risk for hypertension, (17–19) while others have found no effects of SF on blood pressure (20–22). Most of these clinical studies had some limitations, including small sample sizes (19–21) or focusing on specific populations (17, 18). And whether the risk of hypertension increases with the elevating severity of IH/SF is not yet known. In addition, the interaction between IH and SF in the association with blood pressure has not been evaluated.
We performed this study to address these gaps. We aimed to deeply assess the independent associations of the two cardinal features of OSA, IH and SF, with blood pressure, and the effects of the interaction between IH and SF.
Materials and Methods
Subjects
Adult inpatients with suspected OSA who underwent polysomnography (PSG) between January 2014 and January 2021 at the First Affiliated Hospital of Nanchang University were included. The following criteria were used to exclude 110 subjects: known hypertension previously (n = 65); known diabetes mellitus previously (n = 12); previous OSA treatment (n = 15); serious systemic diseases (e.g., heart failure or renal failure) (n = 2); and missing data (n = 16). A total of 335 patients with complete data were included (Figure 1).
Figure 1. Enrollment flow chart for the study population. OSA, obstructive sleep apnea; PSG, polysomnography.
Basic Characteristics
Comprehensive medical histories and blood tests were obtained from the subjects. The medical history included gender, age, hypertension, diabetes, other diseases, and smoking and alcohol consumption. Weights and heights were recorded and BMI was calculated as weight (kg)/height (m2). Blood tests were also performed, including fasting glucose levels.
Overnight Polysomnography Parameters
The subjects received a standard laboratory-based PSG (Alice 4 or 5; Respironics, Pittsburgh, PA, United States). Electroencephalogram (EEG), electrooculogram (EOG), electrocardiogram (ECG), electromyogram (EMG), nasal and oral airflow, thoracic and abdominal respiratory effort, pulse oximetry, posture, and snoring data were obtained. Sleep stages, respiratory events, and microarousals were scored automatically using a computer software and were subsequently checked manually by a skilled technician, following the American Academic Sleep Medicine (AASM) criteria. The apnea–hypopnea index (AHI) was defined as the number of apnea and hypopnea events per hour during sleep. The oxygen-desaturation index (ODI) was defined as the number of times per hour of sleep that the blood oxygen level dropped by ≥ 4% from the baseline. The microarousal index (MAI) was defined as the number of arousals per hour of sleep, and an arousal was defined as an abrupt shift in the EEG frequency, including alpha, theta and/or frequencies > 16Hz (but not spindles) that lasted at least 3 s, with at least 10 s of stable sleep preceding the change. The lowest oxygen saturation value during sleep was referred to as the LSpO2 (23). As AHI and ODI have strong collinearity and both of them can represent IH, we chose one of them (ODI) to perform the analyses. We chose MAI to represent SF.
Blood Pressure
Three daytime blood pressure measurements were recorded after at least 5 min of rest in a sitting position in accordance with the American Society of Hypertension guidelines using a mercury sphygmomanometer, and their mean was calculated. The blood pressure threshold for hypertension was 140/90 mmHg. According to the Guidelines for Prevention and Treatment of Hypertension in China, hypertension was defined as abnormal SBP (≥ 140 mmHg) or DBP (≥ 90 mmHg) or both (24).
Statistical Analysis
Continuous data with a normal distribution were presented as means ± standard deviation, while those with skewed distribution were presented as medians (with interquartile range). Categorical data were presented as numbers (%). The p-values for trends of basic characteristic, PSG parameters and blood pressure across ODI quartiles or MAI quartiles were calculated using the polynomial linear trend test for continuous variables and the linear-by-linear association test for dichotomous variables. Binary logistic regression analyses were used to determine the risk factors for abnormal SBP, abnormal DBP, and hypertension, along with the linear trends of the risk of abnormal blood pressure across ODI quartiles or MAI quartiles. Multiplicative interactions were detected with logistic regression. All statistical analyses were performed using SPSS Statistics 21.0 software (IBM Corp., Armonk, NY, United States). P < 0.05 was taken to indicate statistical significance.
Results
Basic Characteristics
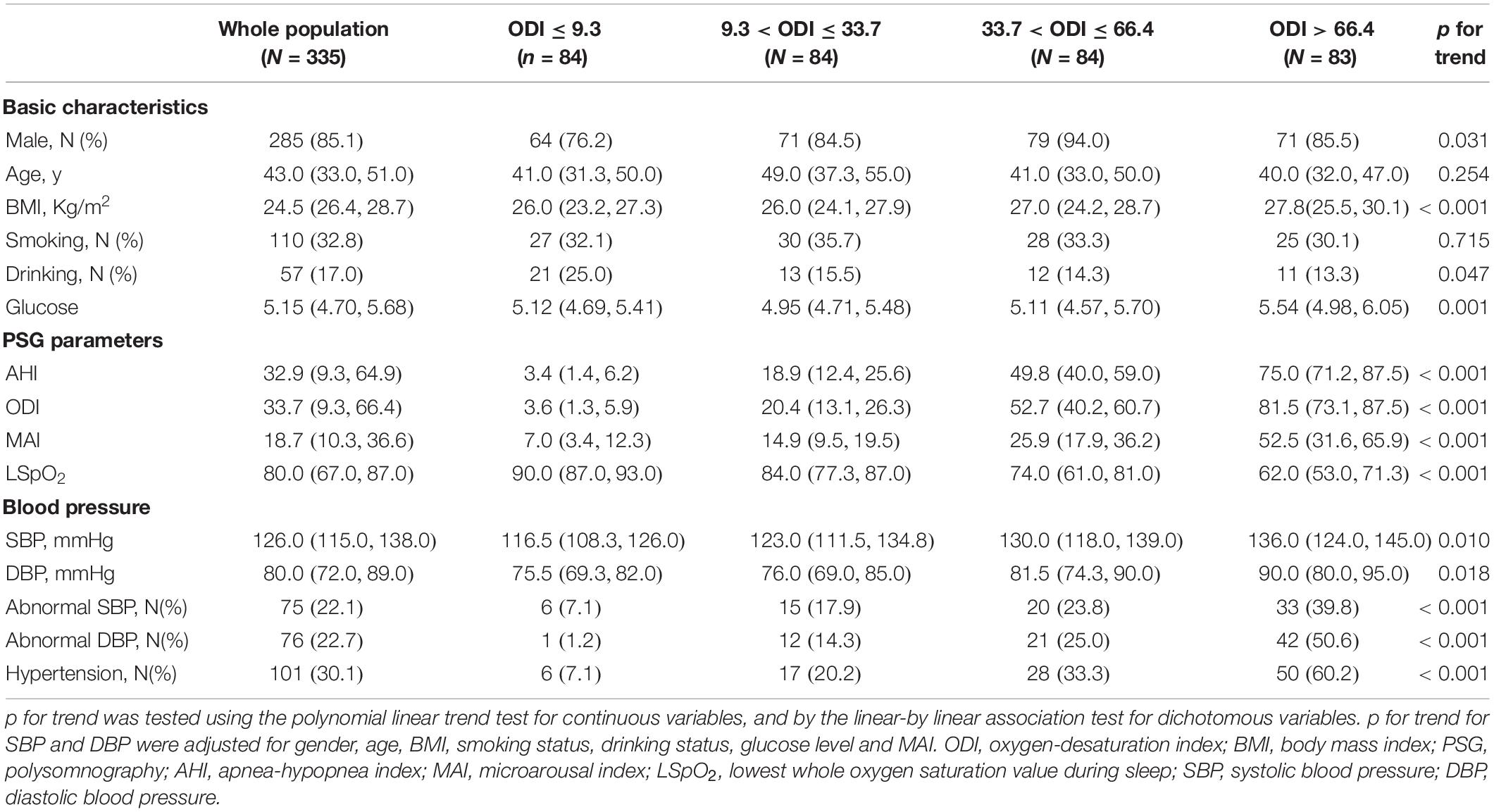
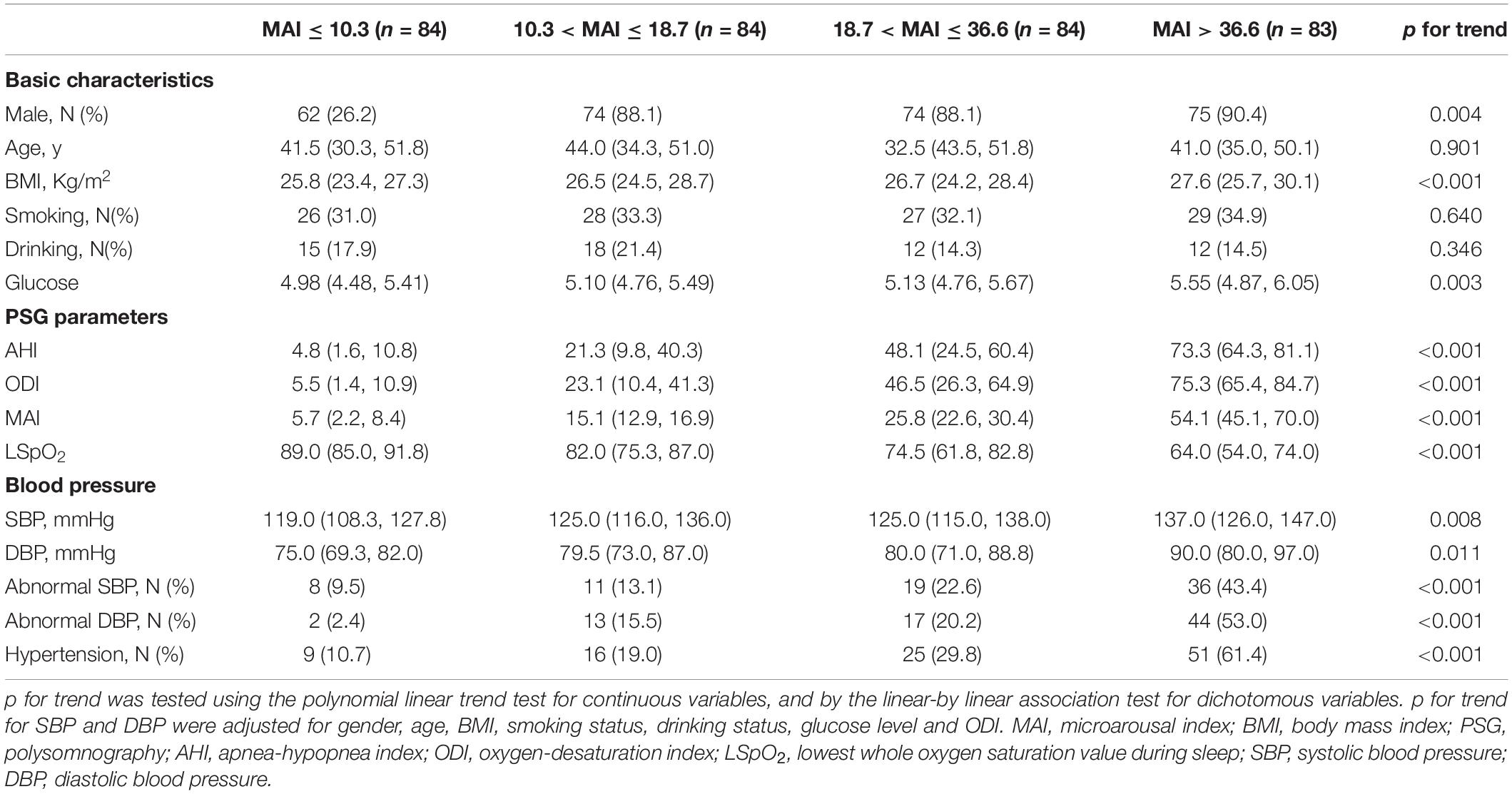
The 335 subjects included in this study were categorized in the basis of ODI (≤ 9.3, 9.3–33.7, 33.7–66.4, and > 66.4) and MAI (≤ 10.3, 10.3–18.7, 18.7–36.6, and > 36.6) quartiles. Most subjects with a high ODI were obese males, and this group had a higher percentage of drinkers, higher glucose levels, and more severe OSA (demonstrated by higher AHI, ODI, and MAI levels, and lower LSpO2 levels) (all p-values < 0.05) (Table 1). The percentages of subjects with abnormal SBP, abnormal DBP, and hypertension increased from 7.1 to 39.8%, from 1.2 to 50.6%, and from 7.1 to 60.2%, respectively, across the ODI quartiles (all p-values < 0.001) (Table 1). Similarly, most of the subjects with a high MAI were obese males, and this group had higher glucose levels and more severe OSA (all p-values < 0.05) (Table 2). The percentages of subjects with abnormal SBP, abnormal DBP, and hypertension increased from 9.5 to 43.4%, from 2.4 to 53.0%, and from 10.7 to 61.4%, respectively, across MAI quartiles (all p-values < 0.001) (Table 2).
Oxygen-Desaturation Index and Blood Pressure
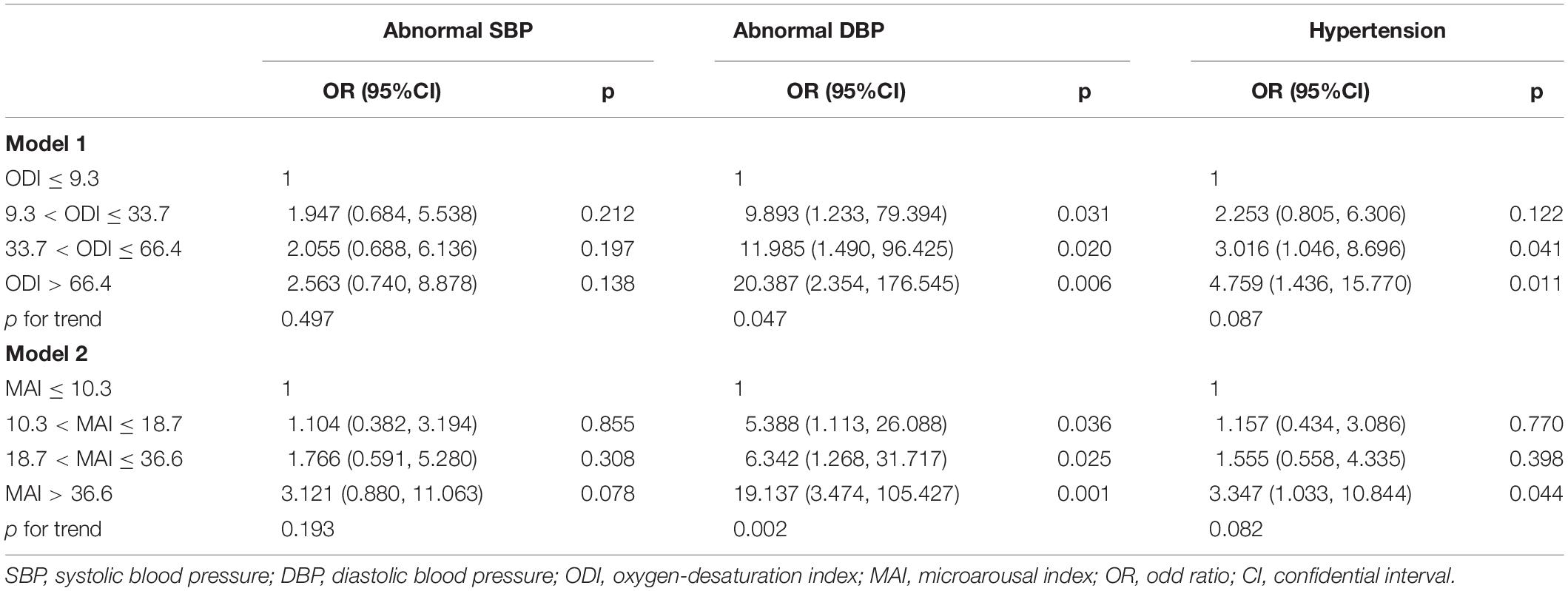
After adjusting for gender, age, BMI, smoking status, drinking status, glucose levels, and MAI, compared with subjects with lower ODI quartiles, those with higher ODI quartiles had significant higher SBP and DBP (p for trend = 0.010 and 0.018, respectively) (Table 1 and Figures 2A,B). Logistic regression analyses showed that compared with subjects with lower ODI quartiles, those with higher ODI quartiles were more likely to have abnormal DBP, and a significant linear trend for the risk of abnormal DBP and increasing ODI quartiles was seen (Table 3 and Model 1), with odds ratios (ORs) [95% confidential interval (CI)] of 1 (reference), 9.893 (1.233, 79.394), 11.985 (1.490, 96.425), and 20.387 (2.354, 176.545), respectively (p for trend = 0.047). Although no significant linear trend for hypertension with increasing ODI quartiles was seen (p for trend = 0.087), compared with subjects with the lowest ODI quartile, those with higher ODI quartiles were more likely to have hypertension [33.7 < ODI ≤ 66.4 vs. ODI ≤ 9.3, OR (95%CI) = 3.016 (1.046, 8.696), p = 0.041; and ODI > 66.4 vs. ODI ≤ 9.3, OR (95%CI) = 4.759 (1.436, 15.770), p = 0.011] (Table 3 and Model 1).

Figure 2. Blood pressure across ODI quartiles. SBP, systolic blood pressure; DBP, diastolic blood pressure; ODI, oxygen-desaturation index.
Microarousal Index and Blood Pressure
After adjusting for multiple variables, including ODI, compared with subjects with lower MAI quartiles, those with higher MAI quartiles had significant higher SBP and DBP (p = for trend 0.008 and 0.011, respectively) (Table 2 and Figures 3A,B). Logistic regression analyses showed that compared with subjects with lower MAI quartiles, those with higher MAI quartiles were more likely to have abnormal DBP, and a significant linear trend for the risk of abnormal DBP and increasing MAI quartiles was seen (Table 3 and Model 2), with ORs (95% CI) of 1 (reference), 5.388 (1.113, 26.088), 6.342 (1.268, 31.717), and 19.137 (3.474, 105.427) (p for trend = 0.002). Although no significant linear trend for hypertension with increasing MAI quartiles was seen (p for trend = 0.082), compared with subjects with the lowest MAI quartile, those with the highest MAI quartile were more likely to have hypertension [MAI > 36.6 vs. MAI ≤ 10.3, OR (95%CI) = 3.347 (1.033, 10.844), p = 0.044] (Table 3 and Model 2).
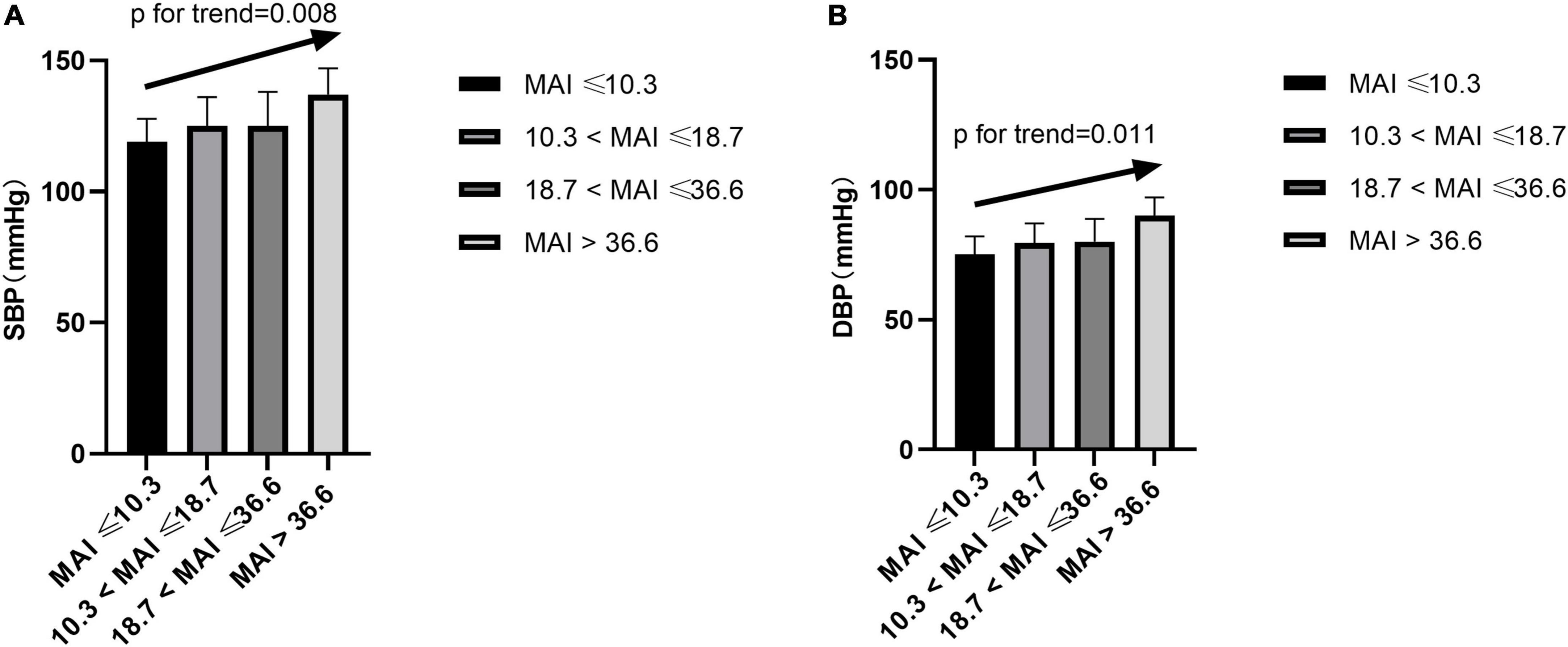
Figure 3. Blood pressure across MAI quartiles. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAI, microarousal index.
Oxygen-Desaturation Index and Microarousal Index Interaction
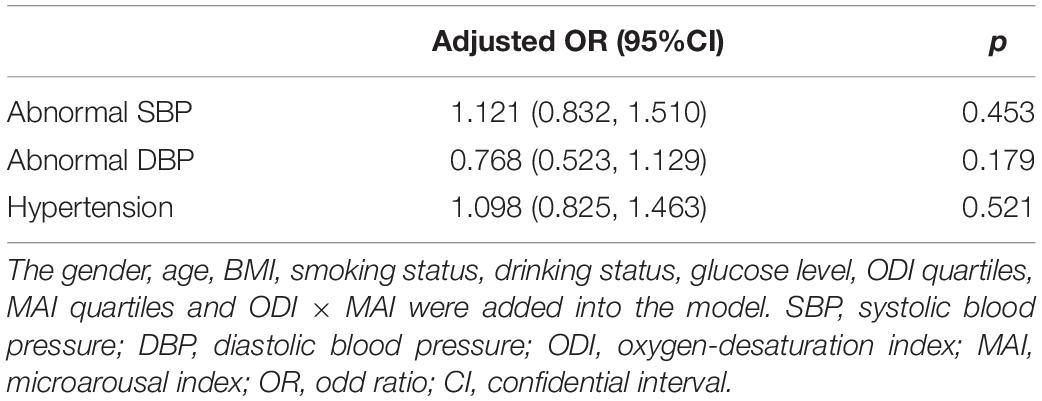
We investigated the effect of a multiplicative interaction between MAI and ODI on blood pressure. After adjusting for multiple variables, there were no statistically significant interactions, with ORs (95%CI) of 1.121 (0.832, 1.510), 0.768 (0.523, 1.129), and 1.098 (0.825, 1.463) for abnormal SBP, abnormal DBP, and hypertension, respectively (Table 4).
Discussion
We found that after adjusting for multiple variables, compared with subjects with lower ODI quartiles, those with higher ODI quartiles had significant higher SBP and DBP. Logistic regression analyses showed that compared with subjects with lower ODI quartiles, those with higher ODI quartiles were more likely to have abnormal DBP and hypertension after adjusting for multiple variables. Similarly, compared with subjects with lower MAI quartiles, those with higher MAI quartiles had significant higher SBP and DBP, and were more likely to have abnormal DBP and hypertension. Furthermore, no significant multiplicative interaction of ODI and MAI on blood pressure was detected.
In this study, we found that those with more severe IH (represented by ODI) had significant higher SBP and DBP, and were more likely to have abnormal DBP and hypertension than those with less severe IH. An increasing number of studies have demonstrated that IH can induce hypertension. In human experimental models, hypoxia has been shown to lead to measurable elevations in blood pressure and sympathetic activation (25–28). IH can stimulate the chemoreceptors and carotid body, and attenuate the activation of carotid baroreceptor, resulting in increased sympathetic activation (29, 30). Persistent sympathetic activation not only elevates blood pressure by increasing vascular resistance and vascular remodeling but also causes sodium retention, impaired natriuresis, and blood pressure elevation by activating the renin-angiotensin system. Meanwhile, IH can promote endothelial dysfunction, systematic inflammation, and metabolic dysfunction, including dyslipidemia and glucose metabolism disorder, which contributes to the development of hypertension (31).
Results from previous clinical studies about the relationship between SF and blood pressure have been controversial. The PROOF-SYNAPSE study of 780 healthy older subjects found that repetitive sympathetic arousals during sleep were associated with elevated SBP and a higher risk of hypertension (17). (18) found that arousal index was an independent risk factor for DBP in male patients (18). (19) demonstrated that arousal index independently predicted a small percentage of the variance in nocturnal reduction in DBP (19). However, other studies have failed to demonstrate an independent relationship between SF and blood pressure. A study of 1021 subjects showed no independent association between SF and awake blood pressure after controlling for the influence of AHI in subjects with AHI ≥ 1. Two other studies also demonstrated that SF was not independently associated with blood pressure (20, 21). In our study, we found that those with more severe SF (represented by MAI) had significant higher SBP and DBP, and were more likely to have abnormal DBP and hypertension than those with less severe SF. A rodent study has demonstrated that long-term SF induces vascular endothelial dysfunction and mild blood pressure increase, (16) and leads to morphologic vessel changes characterized by elastic fiber disruption and disorganization, increased recruitment of inflammatory cells, and altered expression of senescence markers. This suggests a role for SF in the cardiovascular morbidity related to OSA, including hypertension (16).
In addition to the independent relationships between IH/SF and blood pressure, we also explored the multiplicative effects of IH and SF on blood pressure, and found no evidence of an interaction. This suggests that these two factors contribute independently to abnormal blood pressure.
Meanwhile, we explored the relationships between BMI and hypertension. We found those with higher BMI quartiles were more likely to have abnormal SBP and hypertension than those with lower BMI quartiles, which indicated the important role of obesity in hypertension (Supplementary Table 1). We also detected the interactions between BMI and ODI on abnormal blood pressure, and found no significant interactions (Supplementary Table 2). Furthermore, we divided the subjects into two categories according to the median BMI, and found that only in BMI > 26.37 category, those with higher MAI quartiles were more likely to have abnormal DBP, which needed to be further studied in future (Supplementary Table 3).
This study had some limitations. First, it was a retrospective study, so causality cannot be inferred. Second, the statistical power was relatively low due to the small sample size. Third, although we adjusted for several common confounding factors, other more complex factors, such as lifestyle and exercise, were not taken into consideration. Despite these limitations, the sleep data based on standard PSG and objective measurements increased the credibility of our results.
In conclusion, we found that subjects with more severe IH or SF had significant higher SBP and DBP, and were more likely to have abnormal DBP and hypertension than those with less severe IH or SF. There was no multiplicative interaction between IH and SF regarding the effects on blood pressure. Therefore, it is important to consider the roles of both SF and IH while treating hypertensive patients with OSA.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Internal Review Board of the Institutional Ethics Committee of the First Affiliated Hospital of Nanchang University (Approval No. 2020-12-139), and the study was conducted in accordance with all relevant tenets of the Declaration of Helsinki. This was a retrospective study. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
YYX and YPX designed the study and wrote and manuscript. KY and YYX collected the data. KY performed the analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Jiangxi Provincial Natural Science Foundation Project (Grant No. 20202BABL216028) and the National Natural Science Foundation of China (Grant No. 82060187).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.846275/full#supplementary-material
References
1. Li C, Ford ES, Zhao G, Croft JB, Mokdad AH. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: national health and nutrition examination survey, 2005-2006. Prev Med. (2010) 51:18–23. doi: 10.1016/j.ypmed.2010.03.016
2. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. (2015) 3:310–8. doi: 10.1016/S2213-2600(15)00043-0
3. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep heart health study. JAMA. (2000) 283:1829–36. doi: 10.1001/jama.283.14.1829
4. Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, Paula LD, Amaro A, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. (2011) 58:811–7. doi: 10.1161/HYPERTENSIONAHA.111.179788
5. Martínez-García M, Navarro-Soriano C, Torres G, Barbé F, Caballero-Eraso C, Lloberes P, et al. Beyond resistant hypertension: relationship between refractory hypertension and obstructive sleep apnea. Hypertension. (2018) 72:618–24. doi: 10.1161/HYPERTENSIONAHA.118.11170
6. Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. (2000) 320:479–82. doi: 10.1136/bmj.320.7233.479
7. Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, et al. Population based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. (1997) 157:1746–52. doi: 10.1001/archinte.157.15.1746
8. Fletcher EC. Invited review: physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. (2001) 90:1600–5. doi: 10.1152/jappl.2001.90.4.1600
9. Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Somers VK. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. (2017) 69:841–58. doi: 10.1016/j.jacc.2016.11.069
10. Fletcher EC. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep. (2003) 26:15–9. doi: 10.1093/sleep/26.1.15
11. Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanism. Hypertension. (2010) 56:369–77. doi: 10.1161/HYPERTENSIONAHA.110.152108
12. Abdelnaby K, Zhang C, Khalyfa AA, Foster GE, Beaudin AE, Jorge A, et al. Effect on intermittent hypoxia on plasma exosomal micro RNA signature and endothelial function in healthy adults. Sleep. (2016) 39:2077. doi: 10.5665/sleep.6302
13. Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. (2004) 169:354–60. doi: 10.1164/rccm.200306-756OC
14. Seicean S, Kirchner HL, Gottlieb DJ, Punjabi NM, Resnick H, Sanders M, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the sleep heart health study. Diabetes Care. (2008) 31:1001. doi: 10.2337/dc07-2003
15. Nadeem R, Singh M, Nida M, Waheed I, Khan A, Ahmed S, et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. (2014) 10:475–89. doi: 10.5664/jcsm.3690
16. Alba C, Zhang SX, Eduard P, Qiao Z, Alex GH, Li RC, et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep. (2014) 37:1817–24. doi: 10.5665/sleep.4178
17. Chouchou F, Pichot V, Pépin J, Tamisier R, Celle S, Maudoux D, et al. Sympathetic overactivity due to sleep fragmentation is associated with elevated diurnal systolic blood pressure in healthy elderly subjects: the PROOF-SYNAPSE study. Eur Heart J. (2013) 34:2122. doi: 10.1093/eurheartj/eht208
18. Hu W, Jin X, Chen C, Zhang P, Li D, Su Q, et al. Diastolic blood pressure rises with the exacerbation of obstructive sleep apnea in males. Obesity. (2017) 25:1980–7. doi: 10.1002/oby.21960
19. Loredo JS, Sonia AI, Dimsdale JE. Sleep quality and blood pressure dipping in obstructive sleep apnea. Am J Hypertens. (2001) 14:887–92. doi: 10.1016/s0895-7061(01)02143-4
20. Mitsuo K, Naoko T, Toshikazu S, Kazuomi K. Polysomnography−derived sleep parameters as a determinant of nocturnal blood pressure profile in patients with obstructive sleep apnea. J Clin Hypertens. (2018) 20:1039–48. doi: 10.1111/jch.13308
21. Almeneessier AS, Alshahrani M, Aleissi S, Hammad OS, Bahammam AS. Comparison between blood pressure during obstructive respiratory events in REM and NREM sleep using pulse transit time. Sci Rep. (2020) 10:3342. doi: 10.1038/s41598-020-60281-2
22. Morrell MJ, Finn L, Kim H, Peppard PE, Badr MS, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Crit Care Med. (2000) 162:2091–6. doi: 10.1164/ajrccm.162.6.9904008
23. Iber C, Ancoliisrael S, Chesson A, Quan S. The American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester: American Academy of Sleep Medicine (2007).
24. Writing group of 2018 Chinese Guideline for the Management for Hypertension, Chinese Hypertension League, Chinese Society of Cardiology, Chinese Medical Doctor Association Hypertension Committee, Hypertension Branch of China International Exchange and Promotive Association for Medical and Health care, Hypertension Branch of Chinese Geriatric Medical Association. 2018 Chinese guidelines for the management of hypertension. Chin J Cardiovasc Med. (2019) 24:24–56. doi: 10.3969/j.issn.1007-5410.2019.01.002
25. Foster GE, Brugniaux JV, Pialoux V, Duggan C, Hanly PJ, Ahmed SB, et al. Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans. J Physiol. (2010) 587:3287–99. doi: 10.1113/jphysiol.2009.171553
26. Gianfranco P, Miriam R, Andrea G, Andrea F, Grzegorz B, Francesca G, et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J. (2013) 34:759–66. doi: 10.1093/eurheartj/ehs140
27. Hedner J, Ejnell H, Sellgren J, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl. (1988) 6:S529. doi: 10.1097/00004872-198812040-00166
28. Khoshkish S, Hohl M, Linz B, Arzt M, Linz D. The association between different features of sleep-disordered breathing and blood pressure: a cross-sectional study. J Clin Hypertens. (2018) 20:575–81. doi: 10.1111/jch.13202
29. Rey S, Rio RD, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. (2004) 560:577–86. doi: 10.1113/jphysiol.2004.072033
30. Peng YJ, Nanduri J, Zhang X, Wang N, Raghuraman G, Seagard J, et al. Endothelin-1 mediates attenuated carotid baroreceptor activity by intermittent hypoxia. J Appl Physiol. (2012) 112:187–96. doi: 10.1152/japplphysiol.00529.2011
Keywords: obstructive sleep apnea, blood pressure, intermittent hypoxia, sleep fragmentation, interaction
Citation: Xia Y, You K and Xiong Y (2022) Relationships Between Cardinal Features of Obstructive Sleep Apnea and Blood Pressure: A Retrospective Study. Front. Psychiatry 13:846275. doi: 10.3389/fpsyt.2022.846275
Received: 31 December 2021; Accepted: 02 March 2022;
Published: 08 April 2022.
Edited by:
Melissa Lipford, Mayo Clinic, United StatesReviewed by:
Niels Wessel, Humboldt University of Berlin, GermanyMathias Baumert, University of Adelaide, Australia
Copyright © 2022 Xia, You and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanping Xiong, eGlvbmd5cEBuY3UuZWR1LmNu; Kai You, MTM3MDg2NDQzMEBxcS5jb20=
 Yunyan Xia
Yunyan Xia Kai You
Kai You Yuanping Xiong
Yuanping Xiong