
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Psychiatry, 04 April 2022
Sec. Anxiety and Stress Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.837774
This article is part of the Research TopicCommunity Series in Interplay of Stress, Pain and Psychiatric Diseases - Volume IIView all 6 articles
 Qiu Zhao1,2†
Qiu Zhao1,2† Yuan Han3†
Yuan Han3† Xiao-Yi Hu1,2†
Xiao-Yi Hu1,2† Song Zhang4†
Song Zhang4† Long Zhang5
Long Zhang5 Jun Wang1,2
Jun Wang1,2 Qian-Qian Zhang1,2
Qian-Qian Zhang1,2 Ming-Shu Tao1,2
Ming-Shu Tao1,2 Jia-xing Fang1,2
Jia-xing Fang1,2 Jie Yang1,2
Jie Yang1,2 Rong-Guang Liu1,2
Rong-Guang Liu1,2 Xun Sun1,2
Xun Sun1,2 Jian Zhou1,2
Jian Zhou1,2 Xiang Li1,2
Xiang Li1,2 Mannan-Abdul1,2
Mannan-Abdul1,2 Hongxing Zhang2
Hongxing Zhang2 He Liu6*†
He Liu6*† Jun-Li Cao1,2*
Jun-Li Cao1,2*Objective: This study aimed to explore transcranial electrical stimulation (tES) to relieve peripartum anxiety and depressive symptoms in women undergoing cesarean section with combined spinal–epidural anesthesia.
Methods: This double-blind, randomized, sham-controlled trial was conducted in the Affiliated Hospital of Xuzhou Medical University from March 2021 and May 2021. One hundred and forty-eight full-term parturients giving birth by elective cesarean section were selected, and 126 were included in the intent-to-treat analysis. Parturients were provided standardized anesthesia and randomized to the active-tES (a-tES) group and sham-tES group. Parturients and outcome assessors were blinded to treatment allocation. The primary outcome was the changes in peripartum mental health disorders, including anxiety, assessed by the Pregnancy-Related Anxiety Questionnaire-Revised 2 (PRAQ-R2). Secondary outcomes included peripartum depressive symptoms, assessed by the Edinburgh Postnatal Depression Scale (EPDS), maternal satisfaction, fatigue level, sleep quality index, and pain score during and after operation. Data were collected before entering the operating room (T0), between post-anesthesia and pre-surgery (T1), before leaving the operating room (T2), and at 24 h post-surgery (T3).
Results: One hundred and twenty-six eligible parturients were enrolled in the two groups: a-tES group (N = 62) and sham-tES group (N = 64). Treatment with tES resulted in significantly lower scores of anxiety compared with sham-tES (T2: P < 0.001; T3: P = 0.001). Moreover, the a-tES groups showed a significant reduction in depression scores (T2: P = 0.003; T3: P = 0.032).
Conclusion: In this randomized pilot study, tES treatment is efficacious in alleviating peripartum anxiety and depressive symptoms in women undergoing cesarean section and has been demonstrated to be a novel strategy for improving peripartum mental health disorders.
Clinical Trial Registration: [www.chictr.org.cn], identifier [ChiCTR2000040963].
Peripartum anxiety and/or depression is a common mental health disorder during pregnancy (1, 2). The overall prevalence during pregnancy is 15.2% for any anxiety disorder and 22.9% for anxiety symptoms (3); furthermore, peripartum depression has been nominated as a common complication of pregnancy and affects one in every seven women (4). Sixty percent of women with peripartum depression have preexisting comorbid psychiatric disorders, of which more than 80% are anxiety disorders (5). Peripartum mental health disorders can lead to poor maternal–infant physical health and negative birth outcomes (6–8). Studies have shown that pregnant women with anxiety and/or depression have experienced more nausea and vomiting, show instability in their professional behavior, and visit the obstetrician more frequently during pregnancy compared to pregnant women without anxiety and/or depression (9). Moreover, women with peripartum anxiety are more likely to experience preterm birth, have infants with lower than average birth weight (i.e., <2,500 g), and have infants with increased probability of being admitted to the NICU (10). Meta-analysis from 17 pooled studies showed that peripartum anxiety was significantly associated with preterm birth: 5 pooled studies showed a significant effect on spontaneous preterm birth, and 12 pooled studies showed lower infant birth weight (3). Moreover, if these disorders are being ignored or left untreated, they have adverse effects on women and their children, ranging from increased risk of poor adherence to medical care, exacerbation of medical conditions, loss of interpersonal and financial resources, smoking and drug addiction, suicide, and infanticide (11). Therefore, peripartum mental health disorders, including anxiety and/or depression, are associated with increased risks of maternal and infant mortality and morbidity and are recognized as a significant patient safety issue (12).
Peripartum anxiety and/or depression is often underdiagnosed and inadequately treated (5). The current recommended method for anxiety disorders in the general population is psychotherapy (13); however, its efficacy is not definitive. It has been documented that women who experience symptoms of anxiety and depression are commonly prescribed antidepressants. However, before becoming pregnant, they mostly abandon taking drugs because of the insecurities regarding the potential teratogenicity of the antidepressants (14, 15).
Considering the significant impact of peripartum mental health disorders on both the mother and the newly born child, it is imperative to explore effective therapeutic strategies. Non-pharmacological and non-invasive interventions are innovative approaches that can be a feasible strategy for the treatment of mental health disorders (16, 17). Research shows that psychiatric disorders might result from a maladaptive neuroplasticity of the prefrontal and limbic regions, with hypoactivation of the DLPFC (18–20) or abnormalities in amygdala processing (21, 22). tES is a non-invasive method of applying low-intensity electrical current to the DLPFC for treating neurological conditions and psychiatric disorders (23, 24). tES can be anticipated as a therapy for anxiety, pain, insomnia, depression, headache, fibromyalgia, and numerous affective disorders (25, 26). An early meta-analysis by Klawansky and colleagues (27) identified eight sham-controlled randomized trials for anxiety; the notable result arising from the meta-analysis was that the pooled result for the eight studies analyzing the treatment of anxiety with continuous scales was in favor of tES at a statistically significant level (effect size estimate = −0.5883; 95% confidence interval = −0.9503, −0.2262), and the result in favor of tES remained significant when they dropped the three studies that provided no convincing sensation in their sham protocol.
Although tES can be beneficial in relieving anxiety (28) and other psychiatric disorders in the general population, as far as we know, there are no randomized controlled trials to evaluate the effects of tES on peripartum mental health disorders. The main objective of this pilot study was to explore the novel strategy, tES, to relieve peripartum mental health disorders, including anxiety and depressive symptoms, in women undergoing cesarean section. The secondary aim was to evaluate the effects of the intervention on postpartum fatigue, maternal satisfaction, and Visual Analogue Scale (VAS) score during and after the operation.
For this pilot randomized clinical trial, ethical approval was obtained from the Ethical Committee of the Affiliated Hospital of Xuzhou Medical University (ethics identifier XYFY2021-KL040-01, Chairperson Prof Tie Xu), Jiangsu, China, on 18 March 2021. The study was registered on the Chinese clinical trial registry1 with the identifier ChiCTR2000040963. All procedures performed in the study involving human participants were following the ethical standards of the institutional and/or national research committee, the 2013 Declaration of Helsinki, and the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline (29). Written informed consent was obtained from all subjects participating, a legal surrogate, or the parents in this trial.
The study adopted a double-blind, a randomized sham-controlled clinical trial. From March 2021 to May 2021, parturients were recruited at the Affiliated Hospital of Xuzhou Medical University.
One hundred and forty-eight full-term parturients giving birth by elective cesarean section were recruited at the Affiliated Hospital of Xuzhou Medical University. Inclusion criteria were (1) elective cesarean section, 38–42 weeks of gestational age, and good fetal heartbeat (120–160 bpm); (2) desire for combined spinal–epidural anesthesia; and (3) ASA class II (30). Exclusion criteria were (1) age younger than 18 years or older than 45 years; (2) ASA classes I, III, and IV; (3) eclampsia during pregnancy or cerebrovascular diseases; (4) experience with tES, forehead skin damage, or allergy; (5) intracorporeal implantation of electronic devices (e.g., pacemakers or other metal devices); (6) preexisting mental illness (but not anxiety disorders and depression) or history of psychotropic substance use within one month; and (7) inability to understand or refusal to sign informed consent.
The principal investigators identified and enrolled patients. Using a permuted block method (31) (with a block size of 4 or 6) and an interactive voice–web response system, patients were randomly (1:1) assigned to receive a-tES or sham-tES. The follower and patients were blinded to treatment assignment. Group allocations were kept in opaque sealed envelopes sequentially numbered and disclosed by a health care practitioner not directly involved in the parturients’ clinical management and data collection. Each code was revealed just as the parturients entered the operating room to determine which stimulation methods would be prepared. All parturients used equipment, but for the sham control group, it would turn off automatically after 30 s, so the patients were blinded for grouping. The equipment was withdrawn immediately after the stimulation, so the follower was not aware of the grouping.
Standardized anesthesia was provided, including fasting for 6–8 h before anesthesia. Upon arrival into the operating room, the women were placed supine with the bed tilted 30° to the left. Standard monitoring was applied, consisting of electrocardiography (ECG), non-invasive blood pressure, and continuous pulse oximetry, and then the nurse would open the upper limb veins. All parturients were then placed in the left lateral position, and an anesthetist with at least 5 years’ experience performed combined spinal–epidural anesthetic using a needle-through-needle set at the estimated L2–L3 or L3–L4 vertebral interspace. Specifically, after skin disinfection and local skin infiltration with 2% lidocaine, an 18G Tuohy epidural needle was used to identify the epidural space, applying the loss of resistance method through the midline approach. A 27G Whitacre spinal needle with a pencil-point tip was passed through the Tuohy needle into the subarachnoid space. Entry into the subarachnoid space was confirmed by free cerebrospinal fluid outflow. Subarachnoid medication consisted of 0.75% bupivacaine hydrochloride 1.0–1.5 ml. The spinal needle was then withdrawn, and an epidural catheter was threaded into the epidural space. The epidural catheter was gently aspirated and checked for the absence of cerebrospinal fluid, and the epidural needle was removed with an epidural catheter inserted 3–5 cm into the epidural space in a cephalad direction.
After the intrathecal injection, parturients were turned supine; after maternal vital signs turned stable and during the time before surgery begins, the study staff again assessed the Pregnancy-Related Anxiety Questionnaire-Revised 2 (PRAQ-R2) and Edinburgh Postnatal Depression Scale (EPDS) scales, which would be completed within 5–10 min. Assessment of sensory block was done in each dermatomal level bilaterally for loss to cold sensation, 5 ml 2% lidocaine would be administrated for supplement of analgesia by epidural catheter if necessary, and the operation was to start only when the sensory block level was not below T6. Then gel electrodes were immediately placed on the frontal skull, and the tES equipment (GY168A) delivered a direct current of 2 mA (2 mA/5 cm × 2 cm = 0.2 mA/cm2; maximum energy output: 2 mA; pulse width: 250 μs; frequency: 120 Hz; duration: 20 min) to parturients in the a-tES group. For the sham-tES group, electrodes were placed on the same place, and sham stimulation (maximum energy output: 2 mA; pulse width: 250 μs; frequency: 120 Hz; duration: 30 s) was given to parturients. Following previously established methods of clinical studies of brain stimulation, the current was turned off automatically after 30 s of stimulation (32, 33). These methods provide the same initial sensory feelings of tES conditions, specifically, itching and tingling feelings on the scalp for the first few seconds of tES (32, 33). Both groups reported experiencing the same sensation during the 30 s period, and no participants described any differences between the conditions. Assessment of sensory block was done in each dermatomal level bilaterally for loss to cold sensation, and the operation was to start only when the sensory block level was not below T6. The anesthesiologists decided whether to add supplemental IV fluids and intraoperative analgesia.
After admission, maternal baseline information (including age, BMI, gestational age, birth order, gravidity, parity, menstrual regularity, education, urban residence, family population and income, health of other children, and Pittsburgh Sleep Quality Index (PSQI) score) was collected, and written informed consent was obtained 1 day before operation. Intraoperative information (total time, anesthesia time, surgery time, lumbar puncture clearance, level of anesthesia, drugs for lumbar anesthesia, VAS for visceral traction pain during operation, fluid intake, bleeding volume, urine volume, 1 and 5 min Apgar score, hypotension, and/or hypertension) was collected on the day of surgery.
Before entering the operating room, parturients were arranged to the preoperative preparation room for checking parturient information, peripheral venous cannulation and so on, at which they were evaluated with the PRAQ-R2 and EPDS scales (T0). After the anesthesia was administered, when the maternal vital signs were stable, and before surgery begins, the study staff again assessed the PRAQ-R2 and EPDS scales, which would be completed within 5–10 min (T1). After cesarean section, parturients were evaluated before leaving the operating room, and we evaluate their PRAQ-R2 and EPDS for the third time (T2). At 24 h after cesarean section, parturients were evaluated in the maternity ward, including PRAQ-R2 and EPDS, postpartum childbearing fatigue, maternal satisfaction, VAS after operation, and postoperative complications such as nausea and vomiting, chill, dyspnea, and dizziness (T3).
Pregnancy-Related Anxiety Questionnaire-Revised 2 (34) was the primary outcome measure used to measure anxiety level. The PRAQ-R2 contains 10 items with its response score ranging from 1 to 5, with higher scores indicating higher levels of pregnancy-related anxiety. Primiparous women with PRAQ-R scores of ≥26 and parous women with PRAQ-R scores of ≥21 are considered to be suffering from anxiety (35).
Edinburgh Postnatal Depression Scale (36) is a self-report questionnaire consisting of 10 items, with a 4-point Likert scale (0–3), designed to assess postpartum depression. This scale addresses the intensity of depressive symptoms within the previous 7 days and has been used in several studies both with pregnant and postpartum women, namely, in Portugal (37). The threshold for postpartum depression was defined as a total score of ≥13. The higher the score, the more severe the depressive symptoms.
This questionnaire consists of 22 items specifically designed to assess maternal satisfaction with neuraxial anesthesia for elective cesarean section. Satisfaction with four elements is assessed: the anesthetics, insertion of the needle into the back, the side effects, and the atmosphere in the theater. Each item is scored on a 7-point scale, and the scores are added to give a total score (minimum score 22 and maximum score 154), with a higher score representing higher satisfaction (38).
In the Fatigue Identification Form, the mother chooses from several symptom-related adjectives. Scores range from a low of 30 to a high of 120 (39).
A 100 mm VAS is by far the most frequently used assessment instrument to evaluate analgesic effects of various therapies and detect minute pain changes during analgesic administration. Participants were asked to make a hatch mark on a 100 mm line that represents their average pain intensity: 0–3 points for mild pain, 4–6 points for moderate pain, and 7–10 points for severe pain (40).
PSQI is a widely used and well-validated 19-item self-administered survey designed for the subjective evaluation of sleep quality and disturbances in clinical populations. It provides a global score ranging from no sleep difficulty to severe difficulties. The 19 items are categorized into seven clinically derived components including sleep duration, disturbances during sleep, sleep latency, dysfunction during the day due to sleepiness, efficiency of sleep, overall sleep quality, and need medication to sleep. Each component score is weighted equally from 0 to 3, and PSQI score is calculated by adding the scores for each question to obtain a global score (0–21). Higher global PSQI scores indicate poorer sleep. PSQI was used to understand maternal postnatal sleep (41).
Additional outcomes included nausea and vomiting, chill, dyspnea, and dizziness.
The sample size estimate of this study was predetermined and posted on a publicly accessible server.2 The principle of data analysis and the statistical plan were decided before the initiation of the study. This experiment used the principle of intentionality analysis and interpolated the data using the random forest method.
The sample size was determined a priori using PASS15. Our preliminary data showed that after stimulation, the mean anxiety score of the intervention group was 14.65, and the standard deviation was 3.82, while that of the control group was 16.83. We chose a study power of 0.80 and a significance level of 0.05 and used a two-sided significance level to find significant difference in mean scores; we then derived that 49 patients per group were required. Considering 20% loss of follow-up, the sample size was increased to 63 per group. Thus, 126 patients were recruited and randomized into two groups. All subjects completed the intervention period and follow-up, and there were no dropouts.
Normally distributed data are represented by mean ± SD. Non-normally distributed data are represented by median (interquartile range). Count data are expressed by number (percentage). The Shapiro–Wilk and Levine’s test were applied to assess the normality of the distribution and homogeneity of variance of the data, respectively. The repeated measurement data, such as anxiety score and depression score at various time points of T0, T1, T2, and T3, were compared using a linear mixed-effects model. The linear mixed-effects model was performed using the lmerTest package in the R software (R version 3.6.1). The group, time (modeled as a categorical variable), and group-and-time interaction were fixed effects, and the random effect was a random intercept for subjects. Secondary comparisons were made using t-test for parametric, continuous data; Mann–Whitney U test for non-parametric, continuous data; and Fisher’s exact tests for binomial data. Finally, dividing them into anxiety and non-anxiety groups based on the anxiety score before leaving the operation room (T2). The glm function in R software is used to perform a single-factor logistic regression analysis to explore the risk factors that may affect anxiety. Then the variables with P < 0.1 in the single-factor logistic regression analysis are included in the multi-factor logistic regression analysis. P < 0.05 was considered statistically significant, and the R software for Windows was used for all statistical analyses.
One hundred and forty-eight parturients presenting for elective cesarean section were approached for participation in the study between March 2021 and May 2021. Among them, 126 eligible parturients were enrolled in the a-tES group (N = 62) and sham-tES group (N = 64). During the study period, no complications were observed among patients who completed the treatment protocols and who tolerated the tES treatments well. Data from all parturients were analyzed according to their assigned group. The flow chart of the study with parturient enrollment, allocation, follow-up, and analysis is shown in Figure 1. Experimental design and timeline of the two experimental sessions (active-tES and sham-tES) are shown in Figure 2. There were no significant differences in sociodemographic and clinical variables at baseline between the groups (Table 1).
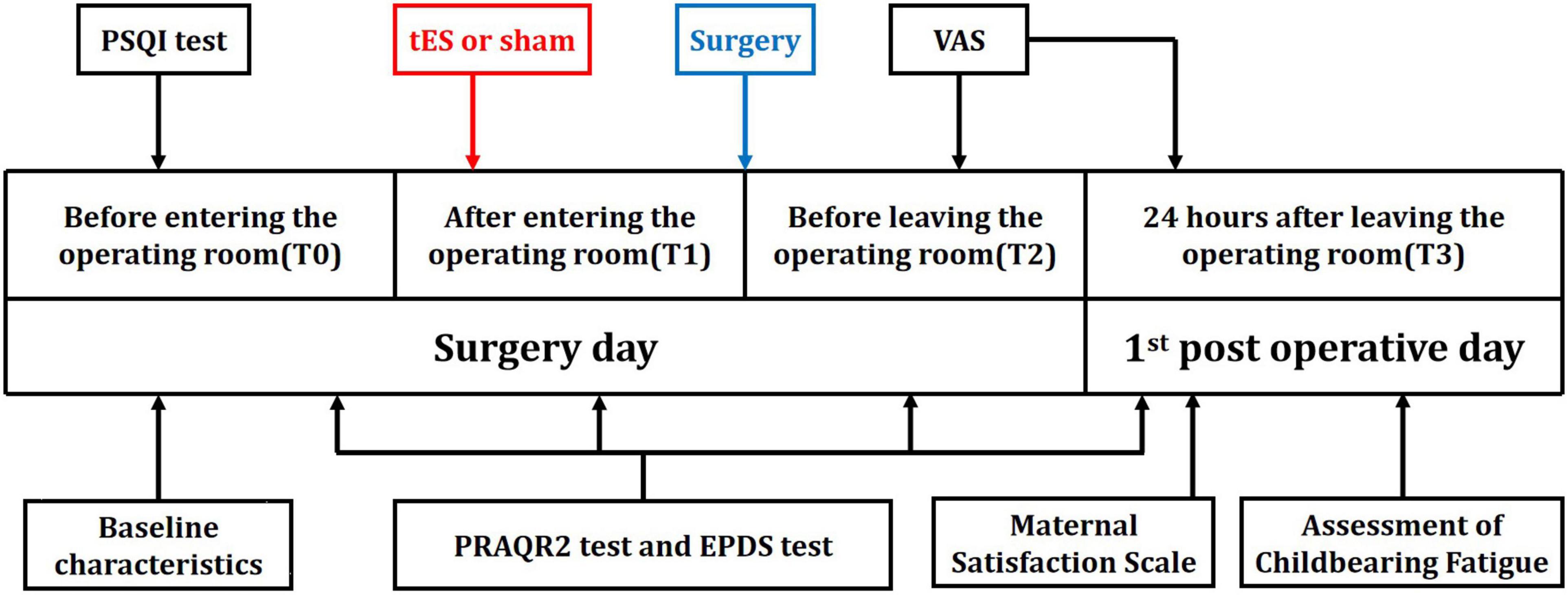
Figure 2. Timeline of experimental design. Experimental design and timeline of the two experimental sessions (active-tES and sham-tES).
Throughout the trial, for all parturients, a decrease in anxiety levels was observed in the two treatment groups. Intragroup analyses showed a significant difference between T0, T1, T2, and T3. Using a mixed-effects linear model in lmerTest package of R software, we found no significant difference in scores between the two groups before stimulation, but after stimulation, anxiety scores in the a-tES group were significantly lower than that in the sham-tES group (T2: P < 0.001; T3: P = 0.001, Table 2 and Figure 3A). There were significant differences between groups and time comparisons (P = 0.002; P < 0.001, Table 2).
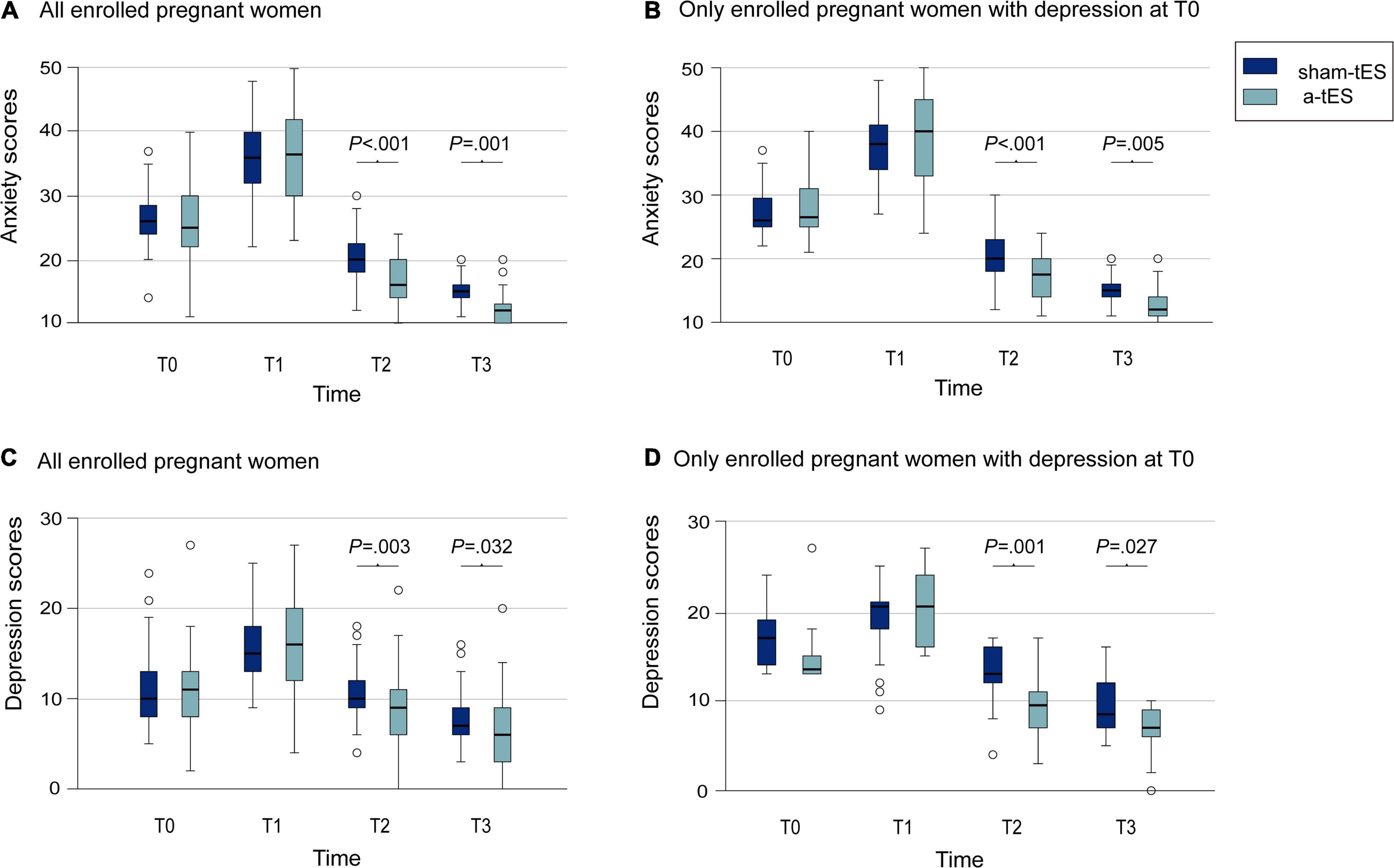
Figure 3. Comparison of anxiety and depression scores of pregnant women between the a-tES group and sham-tES group. (A) Changes in anxiety scores in all enrolled pregnant women. (B) Changes in anxiety scores in only enrolled pregnant women with anxiety at T0. (C) Changes in depression scores for all enrolled pregnant women. (D) Changes in depression scores for only enrolled pregnant women with depression at T0.
Then we compared the changes in the number and proportion of anxious mothers at each time point and found that the a-tES group was significantly lower than the sham-tES group at T2 (P = 0.002, Table 2). But the number of both groups was 0 at T3; this requires us to carry out further experiments to verify and explore.
Further sensitivity analysis showed that for parturients who were anxious at T0, we also found that the anxiety scores after stimulation was significantly lower than those in the control group (T2: P < 0.001; T3: P = 0.005, Table 2 and Figure 3B), consistently with before. Time differences in anxiety scores were also statistically significant (P < 0.001).
Meanwhile, we find that depression scores of all parturients were lower in the a-tES group after stimulation (T2: P = 0.003; T3: P = 0.032; Table 2 and Figure 3C). Time differences were equally significant (P < 0.001).
There were no significant differences in the change in numbers and proportion of mothers who have depressive symptoms (T2: P = 0.23, T3: P = 0.49, Table 2).
Exploring even further, for parturients who have depressive symptoms at T0, we carried out sensitivity analysis and also found lower depression scores in the a-tES group after stimulation (T2: P = 0.001; T3: P = 0.027; Table 2 and Figure 3D). The difference in scores between the two groups changed significantly over time (P < 0.001).
tES had no effect on intraoperative nausea and vomiting, chills, and dyspnea but reduced the incidence of dizziness in the intervention group (P = 0.018) (Table 3). VAS score during operation was the maximum value of visceral traction pain from delivery of the fetus to completion of suturing. VAS scores during and after operation were lower in the tES group (P = 0.040; P = 0.012, Table 3). There was no difference in maternal satisfaction score, postpartum fatigue scores score, and PSQI score between groups (Table 3).
Specifically, according to parity, patients with an anxiety score less than 21 points were evaluated as non-anxious, and patients with a score greater than or equal to 21 points were evaluated as anxious. For patients with a parity more than 1, those with an anxiety score of less than 26 were mediated as non-anxious, and those with an anxiety score greater than or equal to 26 were considered to have anxiety. A two-category logistic regression analysis was carried out to determine whether there is anxiety as a two-category outcome indicator.
Single factor logistic regression analysis shows that the P-values of groups gravidity, parity (time), occupation, education, and lumbar puncture interspace are less than 0.1 (Table 4). Incorporating these factors into the multivariate logistic regression analysis, we found that only the group showed significant statistical differences. There was no difference in other indicators, further indicating that the two groups were balanced and comparable, and the intervention group was less likely to have anxiety than the control group (Table 5).
Peripartum mental health disorders, including anxiety and depressive pregnancy, are common, and there is an increased risk of depression in pregnant women who have anxiety (42). Peripartum mental health disorder can lead to poor maternal infant physical health and negative birth outcomes. To avoid these hazards, people often use pharmacological and non-pharmacological treatments. It is well known that benzodiazepines and benzodiazepine-related drugs are generally prescribed for the treatment of anxiety disorders (43). These drugs have anxiolytic, hypnotic, and anticonvulsant properties and may relieve symptoms in the short term. There is no doubt about that benzodiazepines are effective in non-pregnant populations. However, when used during pregnancy, benzodiazepine-related drugs pass readily through the placenta, with a greater placental transfer in late pregnancy, compared to early pregnancy (44). Some work has found that neonates exposed to benzodiazepines in utero are more likely to have respiratory difficulties, particularly if exposure is late in gestation (45). The US Food and Drug Administration has categorized various drugs according to their risk during pregnancy and lactation (46). Most drugs, such as lorazepam, oxazepam, and diazepam, are categorized as D, indicating that there is evidence of human fetal risk (47). In view of the above considerations, we prefer to explore non-pharmacological physical therapy methods for the relief of anxiety and depression in women during pregnancy, which are non-invasive and safe and do not pose risks to the mother and fetus (48). Therefore, we discovered tES, a typical low-intensity transcranial electric stimulation, which has been proven to be effective for psychological disorders such as generalized anxiety, with a high degree of safety and almost no adverse effects on the fetus and the mother (49).
Guleyupoglu et al. reported that tES evolved from the concept of “electro-sleep” and was first investigated at the beginning of the 20th century (25). It was hypothesized that tES treatment did not in fact induce sleep, but rather the sleep was a side effect of the relaxing induced by the current stimulation, resulting in changing the name from “electro-sleep” to “transcranial electrical stimulation” (25, 50). Several studies have proposed the therapeutic efficacy of non-invasive brain stimulation for treating neurological conditions and psychiatric disorders, such as generalized anxiety disorder (GAD), depression, addiction, stroke, and pain (20, 22, 26). The authors performed the cathode positioned over the right DLPFC and the anode over the contralateral deltoid with a current of 2 mA for 30 min, and it was found that there was a significant improvement of anxiety and a discrete improvement in depression during the treatment (51).
The DLPFC has a potential role in top-down control of processes involved in mood disturbances, including the orbitofrontal cortex and medial prefrontal cortex. Since the DLPFC tonically inhibits the amygdala, neuromodulation of these nuclei could improve this inhibition (18, 52). This is essential for balancing the stress response because anxiety and depression are associated with prefrontal cortex hypoactivity and lack of inhibitory neural mechanisms (52). tES is not only employed to modulate cortical excitability in a target region but also induces changes in the interconnected areas and cortico-subcortical circuits (24, 53, 54). A meta-analysis, which included 61 single-session, sham-controlled, crossover DLPFC tDCS studies, concluded that overall participants across trials and analyses revealed a small, significant effect of a-tDCS on improving RTs and accuracy in cognitive tasks (55). They also find gender differences (i.e., stronger increase in accuracy following a-tDCS in females). Women take a more “top-down” cognitive strategy than men, relying more heavily on higher-order frontal regions, which is enhanced by DLPFC tDCS (56).
As far as we aware, this is the first randomized sham-controlled trial to assess the short-term effects of tES for peripartum mental health disorders in parturients undergoing cesarean section with combined spinal–epidural anesthesia. We found that tES performed over the DLPFC (2 mA for 20 min) ameliorated peripartum anxiety and depression score, whether for parturients who were anxious or depressed at T0, or for all mothers. There were also significant differences in the number and proportion change of anxious mothers (T2: P = 0.002). In the a-tES group, VAS scores during the operation and after operation were decreased (P = 0.040; P = 0.012), and the incidence of dizziness during surgery was also reduced (P = 0.018). However, there were no significant differences in other peripartum complications, maternal satisfaction score, postpartum fatigue score, and PSQI score between the groups.
In this trial, the intervention group outperformed the control group before leaving the operating room, both in terms of change in scores and number of anxious women only and in terms of changes in general anxiety scores. For a variety of reasons, mothers may briefly experience sudden increases in anxiety and depression levels and decreases in mental health during the perioperative period; these may be quickly relieved by the progression of surgery and anesthesia and the birth of the fetus, so that a proportion of women who are anxious before they enter the operating theater fall back to normal levels of anxiety both before they leave the theater and at the postoperative follow-up. However, it is easy to see from our data that tES did reduce maternal anxiety scores before leaving the operating theater compared to the control group scores, reducing the number and proportion of anxious people, with a statistically significant difference, and similarly, maternal depression scores were significantly reduced. More conclusions need to be verified by further research. In other words, tES improves maternal perioperative mental health. tES reduced pain scores at intraoperative and postoperative follow-up. In terms of intraoperative complications, tES did not increase the incidence of perioperative complications such as nausea and vomiting and chills in either group but reduced the incidence of dizziness, which may be related to the neurosensory effects it brings (57).
Future studies with larger sample sizes and more extended follow-up periods are needed to clarify the effectiveness of non-invasive brain stimulation for peripartum anxiety and depression that is refractory to conventional treatments. Research on its long-term effects may provide additional and more valid evidence for the role of tES in relieving peripartum mental health disorders of women undergoing cesarean section.
There are limitations of the pilot study that should be addressed. First, the study performed only one session of a-tES. Over the last decade, the single-session approach has been used to investigate a wide array of cognitive functions, including perception, verbal fluency, visual search, attention, etc. Despite that, studies have shown significant reductions in anxiety levels in patients with generalized anxiety disorders using multiple sessions of stimulation (58). Therefore, multiple sessions during the a-tES protocol for peripartum anxiety are recommended for future investigations to potentially increase the therapeutic efficacy. Secondly, we had a short follow-up period, and there was loss of data for long-term effects of a-tES. Thirdly, we did not follow up the newborns after the Apgar score determined in the operating room and missed the effects of a-tES on the newborns in the maternity ward. So, in a further study, we will extend the follow-up period and add more attention to the newborn.
In summary, the main results of this pilot randomized study show that a-tES can improve the peripartum mental health disorders, including anxiety and depressive symptoms, of women undergoing cesarean section with combined spinal–epidural anesthesia.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of the Affiliated Hospital of Xuzhou Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
J-LC designed the study, gave critical review of the manuscript, approved the final version, and was accountable for the work. HL and YH designed the study, prepared the manuscript, gave critical review of the manuscript, approved the final version, and were accountable for the work. M-ST, Q-QZ, and JZ helped conduct the study and collect the data. XL, J-XF, JY, R-GL, JW, and XS analyzed and interpreted the data. QZ, X-YH, and SZ conducted the study, collected the data, prepared the manuscript, helped test samples, and analyzed the data. LZ, Mannan-Abdul, and HZ helped prepare the manuscript and gave a critical review of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported in part by grants from the National Natural Science Foundation of China (NSFC81720108013, NSFC31771161, and NSFC81230025 to J-LC; NSFC81300957 and NSFC82171227 to HL; NSFC81771453 and NSFC31970937 to HZ), Jiangsu Provincial Special Program of Medical Science (BL2014029 to J-LC), Basic and Clinical Research Center in Anesthesiology of Jiangsu Provincial “Science and Education for Health” Project (J-LC), Jiangsu Provincial Natural Science Foundation (BK20190047 to HZ), Zhejiang Provincial Natural Science Foundation (LY22H090019 to HL), the Priority Academic Program Development of Jiangsu Higher Education Institutions (19KJA610005 to HZ), Distinguished Professor Program of Jiangsu (HZ), Jiangsu Province Innovative and Entrepreneurial Talent Program and Jiangsu Province Innovative and Entrepreneurial Team Program (HZ), Xuzhou Medical University start-up grant for excellent scientist (D2018010 and D2019025D to HZ), the Natural Science Foundation of Shanghai (21ZR1411300 to YH), and Shenkang Clinical Study Foundation of Shanghai (SHDC2020CR4061 to YH).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Dipesh Chaudhury of New York University Abu Dhabi (NYUAD), Abu Dhabi, United Arab Emirates (UAE), for his great help in language polishing, considerable comments, and sound suggestions. We would also like to thank all of the participants of this study.
1. Liu Y, Guo N, Li T, Zhuang W, Jiang H. Prevalence and associated factors of postpartum anxiety and depression symptoms among women in Shanghai, China. J Affect Disord. (2020) 274:848–56. doi: 10.1016/j.jad.2020.05.028
2. Zemestani M, Fazeli Nikoo Z. Effectiveness of mindfulness-based cognitive therapy for comorbid depression and anxiety in pregnancy: a randomized controlled trial. Arch Womens Ment Health. (2020) 23:207–14. doi: 10.1007/s00737-019-00962-8
3. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. (2017) 210:315–23. doi: 10.1192/bjp.bp.116.187179
4. Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). (2005) 119:1–8. doi: 10.1037/e439372005-001
5. Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. (2013) 70:490–8. doi: 10.1001/jamapsychiatry.2013.87
6. Muzik M, McGinnis EW, Bocknek E, Morelen D, Rosenblum KL, Liberzon I, et al. Ptsd symptoms across pregnancy and early postpartum among women with lifetime Ptsd diagnosis. Depress Anxiety. (2016) 33:584–91. doi: 10.1002/da.22465
7. Hoffman C, Dunn DM, Njoroge WFM. Impact of postpartum mental illness upon infant development. Curr Psychiatry Rep. (2017) 19:100. doi: 10.1007/s11920-017-0857-8
8. Dubber S, Reck C, Muller M, Gawlik S. Postpartum bonding: the role of perinatal depression, anxiety and maternal-fetal bonding during pregnancy. Arch Womens Ment Health. (2015) 18:187–95. doi: 10.1007/s00737-014-0445-4
9. Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med. (2007) 20:189–209. doi: 10.1080/14767050701209560
10. Yonkers KA, Smith MV, Forray A, Epperson CN, Costello D, Lin H, et al. Pregnant women with posttraumatic stress disorder and risk of preterm birth. JAMA Psychiatry. (2014) 71:897–904. doi: 10.1001/jamapsychiatry.2014.558
11. Madigan S, Oatley H, Racine N, Fearon RMP, Schumacher L, Akbari E, et al. A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. J Am Acad Child Adolesc Psychiatry. (2018) 57:645–57.e8. doi: 10.1016/j.jaac.2018.06.012
12. Kendig S, Keats JP, Hoffman MC, Kay LB, Miller ES, Moore Simas TA, et al. Consensus bundle on maternal mental health: perinatal depression and anxiety. Obstet Gynecol. (2017) 129:422–30. doi: 10.1097/AOG.0000000000001902
13. Berard A, Zhao JP, Sheehy O. Antidepressant use during pregnancy and the risk of major congenital malformations in a cohort of depressed pregnant women: an updated analysis of the Quebec pregnancy cohort. BMJ Open. (2017) 7:e013372. doi: 10.1136/bmjopen-2016-013372
14. Lemon E, Vanderkruik R, Arch JJ, Dimidjian SA. Treating anxiety during pregnancy: patient concerns about pharmaceutical treatment. Matern Child Health J. (2020) 24:439–46. doi: 10.1007/s10995-019-02873-7
15. Walton GD, Ross LE, Stewart DE, Grigoriadis S, Dennis CL, Vigod S. Decisional conflict among women considering antidepressant medication use in pregnancy. Arch Womens Ment Health. (2014) 17:493–501. doi: 10.1007/s00737-014-0448-1
16. Kar SK, Sarkar S. Neuro-stimulation techniques for the management of anxiety disorders: an update. Clin Psychopharmacol Neurosci. (2016) 14:330–7. doi: 10.9758/cpn.2016.14.4.330
17. Abraha I, Rimland JM, Trotta FM, Dell’Aquila G, Cruz-Jentoft A, Petrovic M, et al. Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series. BMJ Open. (2017) 7:e012759. doi: 10.1136/bmjopen-2016-012759
18. Ironside M, Browning M, Ansari TL, Harvey CJ, Sekyi-Djan MN, Bishop SJ, et al. Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: a randomized clinical trial. JAMA Psychiatry. (2019) 76:71–8. doi: 10.1001/jamapsychiatry.2018.2172
19. Ong WY, Stohler CS, Herr DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol. (2019) 56:1137–66. doi: 10.1007/s12035-018-1130-9
20. Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. (2016) 127:1031–48. doi: 10.1016/j.clinph.2015.11.012
21. Carnevali L, Mancini M, Koenig J, Makovac E, Watson DR, Meeten F, et al. Cortical morphometric predictors of autonomic dysfunction in generalized anxiety disorder. Auton Neurosci. (2019) 217:41–8. doi: 10.1016/j.autneu.2019.01.001
22. Vicario CM, Salehinejad MA, Felmingham K, Martino G, Nitsche MA. A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neurosci Biobehav Rev. (2019) 96:219–31. doi: 10.1016/j.neubiorev.2018.12.012
23. Sagliano L, Atripaldi D, De Vita D, D’Olimpio F, Trojano L. Non-invasive brain stimulation in generalized anxiety disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 93:31–8. doi: 10.1016/j.pnpbp.2019.03.002
24. Todder D, Gershi A, Perry Z, Kaplan Z, Levine J, Avirame K. Immediate effects of transcranial direct current stimulation on obsession-induced anxiety in refractory obsessive-compulsive disorder: a pilot study. J ECT. (2018) 34:e51–7. doi: 10.1097/YCT.0000000000000473
25. Guleyupoglu B, Schestatsky P, Edwards D, Fregni F, Bikson M. Classification of methods in transcranial electrical stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J Neurosci Methods. (2013) 219:297–311. doi: 10.1016/j.jneumeth.2013.07.016
26. Kuo MF, Chen PS, Nitsche MA. The application of tDCS for the treatment of psychiatric diseases. Int Rev Psychiatry. (2017) 29:146–67. doi: 10.1080/09540261.2017.1286299
27. Klawansky S, Yeung A, Berkey C, Shah N, Phan H, Chalmers TC. Meta-analysis of randomized controlled trials of cranial electrostimulation. Efficacy in treating selected psychological and physiological conditions. J Nerv Ment Dis. (1995) 183:478–84. doi: 10.1097/00005053-199507000-00010
28. de Lima AL, Braga FMA, da Costa RMM, Gomes EP, Brunoni AR, Pegado R. Transcranial direct current stimulation for the treatment of generalized anxiety disorder: a randomized clinical trial. J Affect Disord. (2019) 259:31–7. doi: 10.1016/j.jad.2019.08.020
29. Schulz KF, Altman DG, Moher D, Consort G. [CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials (Chinese version)]. Zhong Xi Yi Jie He Xue Bao. (2010) 8:604–12. doi: 10.3736/jcim20100702
30. Doyle DJ, Goyal A, Bansal P, Garmon EH. American Society of Anesthesiologists Classification. Treasure Island, FL: StatPearls (2021).
31. Hilgers RD, Manolov M, Heussen N, Rosenberger WF. Design and analysis of stratified clinical trials in the presence of bias. Stat Methods Med Res. (2020) 29:1715–27. doi: 10.1177/0962280219846146
32. Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. (2006) 117:845–50. doi: 10.1016/j.clinph.2005.12.003
33. Lefaucheur JP. A comprehensive database of published tDCS clinical trials (2005-2016). Neurophysiol Clin. (2016) 46:319–98. doi: 10.1016/j.neucli.2016.10.002
34. Huizink AC, Delforterie MJ, Scheinin NM, Tolvanen M, Karlsson L, Karlsson H. Adaption of pregnancy anxiety questionnaire-revised for all pregnant women regardless of parity: PRAQ-R2. Arch Womens Ment Health. (2016) 19:125–32. doi: 10.1007/s00737-015-0531-2
35. Witteveen AB, De Cock P, Huizink AC, De Jonge A, Klomp T, Westerneng M, et al. Pregnancy related anxiety and general anxious or depressed mood and the choice for birth setting: a secondary data-analysis of the DELIVER study. BMC Pregnancy Childbirth. (2016) 16:363. doi: 10.1186/s12884-016-1158-7
36. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. (1987) 150:782–6. doi: 10.1192/bjp.150.6.782
37. Tendais I, Costa R, Conde A, Figueiredo B. Screening for depression and anxiety disorders from pregnancy to postpartum with the EPDS and STAI. Span J Psychol. (2014) 17:E7. doi: 10.1017/sjp.2014.7
38. Eley VA, Searles T, Donovan K, Walters E. Effect of an anaesthesia information video on preoperative maternal anxiety and postoperative satisfaction in elective caesarean section: a prospective randomised trial. Anaesth Intensive Care. (2013) 41:774–81. doi: 10.1177/0310057X1304100613
39. Pugh LC, Milligan R, Parks PL, Lenz ER, Kitzman H. Clinical approaches in the assessment of childbearing fatigue. J Obstet Gynecol Neonatal Nurs. (1999) 28:74–80. doi: 10.1111/j.1552-6909.1999.tb01967.x
40. Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. (2011) 41:1073–93. doi: 10.1016/j.jpainsymman.2010.08.016
41. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
42. Schofield CA, Battle CL, Howard M, Ortiz-Hernandez S. Symptoms of the anxiety disorders in a perinatal psychiatric sample: a chart review. J Nerv Ment Dis. (2014) 202:154–60. doi: 10.1097/NMD.0000000000000086
43. Shyken JM, Babbar S, Babbar S, Forinash A. Benzodiazepines in pregnancy. Clin Obstet Gynecol. (2019) 62:156–67. doi: 10.1097/GRF.0000000000000417
44. Mcelhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. (1994) 8:461–75. doi: 10.1016/0890-6238(94)90029-9
45. Wikner BN, Stiller CO, Bergman U, Asker C, Kallen B. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. (2007) 16:1203–10. doi: 10.1002/pds.1457
46. Howland RH. Categorizing the safety of medications during pregnancy and lactation. J Psychosoc Nurs Ment Health Serv. (2009) 47:17–20. doi: 10.3928/02793695-20090401-06
47. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. (2015) 212:428–41. doi: 10.1016/j.ajog.2014.10.1106
48. Cancer A, Santi F, Antonietti A. tES to rehabilitate neurodevelopmental disorders: a study on clinical practitioners’ attitudes. Prog Brain Res. (2021) 264:343–61. doi: 10.1016/bs.pbr.2021.01.018
49. Antal A, Alekseichuk I, Bikson M, Brockmoller J, Brunoni AR, Chen R, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. (2017) 128:1774–809. doi: 10.1016/j.clinph.2017.06.001
50. Francis J, Dingley J. Electroanaesthesia–from torpedo fish to TENS. Anaesthesia. (2015) 70:93–103. doi: 10.1111/anae.12887
51. Shiozawa P, Leiva AP, Castro CD, da Silva ME, Cordeiro Q, Fregni F, et al. Transcranial direct current stimulation for generalized anxiety disorder: a case study. Biol Psychiatry. (2014) 75:e17–8. doi: 10.1016/j.biopsych.2013.07.014
52. Sgoifo A, Carnevali L, Alfonso Mde L, Amore M. Autonomic dysfunction and heart rate variability in depression. Stress. (2015) 18:343–52. doi: 10.3109/10253890.2015.1045868
53. Liu A, Voroslakos M, Kronberg G, Henin S, Krause MR, Huang Y, et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. (2018) 9:5092. doi: 10.1038/s41467-018-07233-7
54. Balderston NL, Flook E, Hsiung A, Liu J, Thongarong A, Stahl S, et al. Patients with anxiety disorders rely on bilateral dlPFC activation during verbal working memory. Soc Cogn Affect Neurosci. (2020) 15:1288–98. doi: 10.1093/scan/nsaa146
55. Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt MA. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: influence of stimulation parameters. Brain Stimul. (2016) 9:501–17. doi: 10.1016/j.brs.2016.04.006
56. Butler T, Pan H, Imperato-McGinley J, Voyer D, Cunningham-Bussel AC, Cordero JJ, et al. A network approach to fMRI condition-dependent cognitive activation studies as applied to understanding sex differences. Clin Neurosci Res. (2007) 6:391–8. doi: 10.1016/j.cnr.2007.05.005
57. Raco V, Bauer R, Olenik M, Brkic D, Gharabaghi A. Neurosensory effects of transcranial alternating current stimulation. Brain Stimul. (2014) 7:823–31. doi: 10.1016/j.brs.2014.08.005
58. Nasiri F, Mashhadi A, Bigdeli I, Chamanabad AG, Ellard KK. Augmenting the unified protocol for transdiagnostic treatment of emotional disorders with transcranial direct current stimulation in individuals with generalized anxiety disorder and comorbid depression: a randomized controlled trial. J Affect Disord. (2020) 262:405–13. doi: 10.1016/j.jad.2019.11.064
Keywords: transcranial electrical stimulation, mental health disorders, cesarean section, peripartum anxiety, peripartum depression
Citation: Zhao Q, Han Y, Hu X-Y, Zhang S, Zhang L, Wang J, Zhang Q-Q, Tao M-S, Fang J-x, Yang J, Liu R-G, Sun X, Zhou J, Li X, Mannan-Abdul, Zhang H, Liu H and Cao J-L (2022) Transcranial Electrical Stimulation for Relief of Peripartum Mental Health Disorders in Women Undergoing Cesarean Section With Combined Spinal–Epidural Anesthesia: A Pilot Randomized Clinical Trial. Front. Psychiatry 13:837774. doi: 10.3389/fpsyt.2022.837774
Received: 17 December 2021; Accepted: 14 February 2022;
Published: 04 April 2022.
Edited by:
Chun Yang, Nanjing Medical University, ChinaReviewed by:
Hailong Dong, Fourth Military Medical University, ChinaCopyright © 2022 Zhao, Han, Hu, Zhang, Zhang, Wang, Zhang, Tao, Fang, Yang, Liu, Sun, Zhou, Li, Mannan-Abdul, Zhang, Liu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: He Liu, bGgxMjEwNjFAMTYzLmNvbQ==; Jun-Li Cao, Y2FvamwwMzEwQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.