
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 03 June 2022
Sec. Aging Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.833767
This article is part of the Research TopicCommunity Series in Understanding Brain Aging – Volume IIView all 7 articles
 Xue Sun1†
Xue Sun1† Lina Wang1*†
Lina Wang1*† Xinhua Shen2
Xinhua Shen2 Cheng Huang1
Cheng Huang1 Zhuqin Wei1
Zhuqin Wei1 Liming Su1
Liming Su1 Simeng Wang1
Simeng Wang1 Xiaoshen Liu3
Xiaoshen Liu3 Xueting Zhen4
Xueting Zhen4Background: Non-pharmacological interventions are promising for delaying cognitive decline in older adults with mild cognitive impairment (MCI). Although some studies have demonstrated adherence rates and factors influencing participation in single modality non-pharmacological interventions, little is known about the level and correlates of adherence to multimodal non-pharmacological interventions (MNPIs) in older adults with MCI.
Objective: This study aimed to explore the adherence level and the correlates of adherence to MNPIs in older adults with MCI.
Methods: A cross-sectional design was employed. Community-dwelling older adults aged 60 years and over were recruited from senior community centers and healthcare centers in Huzhou from March 2019 to December 2020. Data were collected by a general information questionnaire and the adherence scale of cognitive dysfunction management (AS-CDM) in older adults with MCI. Hierarchical regression analyses were applied to explore the correlates of adherence to MNPIs.
Results: A total of 216 completed questionnaires were finally analyzed. Of these, 68.52% were female, and 45.4% of the participants had no less than 6 years of education. The overall mean score for adherence was 117.58 (SD = 10.51) out of 160, equivalent to 73.49 in the hundred-mark system, indicating a medium-level adherence to MNPIs in older adults with MCI. Of the five dimensions of adherence (AS-CDM), self-efficacy scored the highest, and the lowest was perceived barriers. The univariate analysis showed that the factors associated with the adherence to MNPIs were: regular physical exercise, meat-vegetable balance, absence of multimorbidity, high level of education, living alone, and living in urban (p < 0.05). In the hierarchical regression analysis, the final model explained 18.8% of variance in overall adherence (p < 0.01), which high school (Beta = 0.161, p < 0.05), college and above more (Beta = 0.171, p < 0.05), meat-vegetarian balance (Beta = 0.228, p < 0.05), regular physical exercise (Beta = 0.234, p < 0.05), and presence of multimorbidity (Beta = −0.128, p < 0.05) significantly contributed to adherence. In addition, nearly 80% of older adults with MCI preferred MNPIs.
Conclusion: Early assessment and management of adherence to MNPIs were essential in older adults with MCI. Furthermore, the findings shed light on several critical areas of intervention to improve adherence to MNPIs in older adults with MCI.
Clinical Trial Registration: http://www.chictr.org.cn/showproj.aspx?proj=35363, ChiCTR1900020950 (Registered on January 23, 2019).
Mild cognitive impairment (MCI) is an intermediate stage between normal aging and early dementia, and is mainly manifested by a progressive decline in memory or other cognitive functions (1). The overall prevalence of MCI ranges from 6.7 to 25.2% with age (2). Meanwhile, the annual progression rate from MCI to dementia varies between 8 and 15%, and 50% of people with MCI will progress to dementia within 5 years, meaning that it is an essential condition to identify and treat (3, 4). However, updated guidelines from the American Academy of Neurology (AAN) stated that no high-quality evidence exists to support pharmacological treatments for older adults with MCI (2). Given the effectiveness of non-pharmacological interventions in improving cognitive function, such as physical exercise, cognitive training, and dietary treatments, most studies are more inclined to non-pharmacological interventions (2, 5).
It cannot be denied that long-term adherence plays a crucial role in completing a variety of non-pharmacological interventions. However, older adults with MCI are physically inactive and their adherence to physical exercise is poor (6, 7). In a 1-year exercise management program (7), 41.8% of individuals completed <1/3 of this program, and 10.4% of individuals performed none of the exercise sessions, with the mean adherence of 33.2 ± 25.5%. Low motivation, lack of interest, poor health status, and lower socioeconomic status have been identified as factors influencing the adherence to exercise participation in older adults (7, 8). Although the evidence suggests that the Mediterranean Diet (MD) has a better effect on slowing cognitive decline and decreasing the risk of developing Alzheimer's disease (9), more than half of the participants (52.1%) showed low adherence to the MD in older adults (10). In contrast, higher MD adherence was significantly associated with younger age, female, higher educational level, and better anthropometric parameters (10). In addition, cognitive training provides the best non-pharmacological approach to improving cognitive function in older adults with MCI (11). Training method, duration, fatigue or other health-related limitations, and the difficulty of the cognitive tasks may affect individual adherence to cognitive training (12, 13). Therefore, there is much room for improvement in adherence to cognitive dysfunction management activities for older adults with MCI.
The adherence assessment tools are commonly applied to evaluate individuals' level of adherence at baseline or during the intervention, and indirectly to reflect the feasibility and acceptability of cognitive dysfunction management activities (14). Most previous studies relied on a single indicator/goal attainment to measure adherence to a single non-pharmacological intervention in older adults with MCI (15–18). For exercise intervention, adherence is often evaluated by the number of completions, duration of exercise, and tolerance to exercise intensity (15). A goal/task attainment scale is commonly used in cognitive training to assess adherence (16). As for diet interventions, adherence is always evaluated indirectly by the amount or frequency of food intake (17, 18). In recent years, multimodal non-pharmacological interventions (MNPIs) have become an emerging (19). A current systematic review showed that MNPIs provided more significant improvements in cognitive-motor abilities and cognitive function in older adults with MCI (20). However, the preference to MNPIs in older adults with MCI is poorly understood. Moreover, little is known about the level and correlates of adherence to MNPIs in this population. Identifying correlates of adherence to MNPIs is crucial to developing therapeutic interventions aiming to increase control, prevent adverse effects of treatment, and improve long-term outcomes.
The Health Belief Model (HBM) has been used to explain and predict to adherence with health and medical care recommendations for more than 20 years (21, 22). This model defines the key factors that explain health behaviors, including an individual's perceived threat to disease (perceived susceptibility), the belief of consequence (perceived severity), potential positive benefits of action (perceived benefits), perceived barriers to action, exposure to factors that prompt action (cues to action), and confidence in the ability to succeed (self-efficacy) (21). Researchers have used the HBM as a generalized conceptual framework to explain and predict health behaviors engagement or adherence across a spectrum of medical conditions in various subjects (23–25). In our previous work (26), we have developed the adherence scale of cognitive dysfunction management (AS-CDM) based on HBM for older adults with MCI. AS-CDM involves three kinds of non-pharmacological interventions: physical exercise, cognitive training, and dietary treatment. The AS-CDM can be used to assess the overall level of adherence to MNPIs in which individuals are currently participating or may participate in the future, in terms of both adherence behavior and adherence intentions. Meanwhile, it also used to evaluate the preferred intervention modality of cognitive dysfunction management (single/mixed modality), with good validity and internal consistency reliability (see Supplementary Materials 1). Considering that HBM allows for the inclusion of individual-level variables influences on subsequent action (27), diverse potential individual-level factors, including socio-demographic, biological (medical) and lifestyle and behavior characteristics, may influence the level of adherence to MNPIs in older adults with MCI.
Therefore, our study seeks to answer three primary questions:
(1) Whether older adults with MCI are inclined to adopt the MNPIs strategy.
(2) What is the level of adherence to MNPIs in older adults with MCI?
(3) What are the correlates that may explain the differences in adherence to MNPIs at individual-level in older adults with MCI?
This study was approved by the Medical Ethics Committee of the Third People's Hospital of Huzhou of Zhejiang Province, following the guidelines of the Declaration of Helsinki (reference number 2018-031) and registered at Chictr.org.cn (ChiCTR1900020950). Written informed consents were obtained from each participant. This study complied with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies (28).
This research was a cross-sectional study conducted in Huzhou city, Zhejiang, China. The participants were included: (1) age ≥ 60 years old; (2) diagnosed of MCI; (3) absence of self-reported visual or auditory impairment; and (4) able to make an informed consent. A trained neurologist-psychiatrist made the evaluation based on the diagnostic criterion for MCI (29). The following were adopted as MCI operational criteria at screening: (1) report of a relative decline in cognitive functioning during the past year by the participant or informant; (2) normal general cognitive function, including a Beijing version of Montreal Cognitive Assessment score (15< MoCA score was < 26) (30, 31); (3) intact activities of daily living (ADL score was < 16) (32); (4) absence of dementia, including a Mini-Mental State Examination (MMSE) score of 25-30 (33).
The exclusion criteria of the study were: (1) a history of neurological, psychiatric, and other severe medical issues that may affect brain function; (2) a history of alcohol dependency or other addiction within 10 years; (3) taking any medications in the past 6 months which may cause impaired or improved cognitive performance.
The sample size was calculated by considering the assumptions for the single population proportion. The prevalence of MCI in China of the previous study was 14.7% (34), the overall rate is 14.7% (π = 0.147). The allowable error is 5% (δ = 0.05), and uα/2 is the 1 –α/2 percentile of the standard normal distribution, which is 1.96 when α = 0.05 (α = 0.05, uα/2 = 1.96). Therefore, the following formula: n = ([1 – π])/δ2= (1.962*0.147*0.853)/0.052=192. Meanwhile, considering invalid questionnaires and expanding the sample size by 10%, the total sample size was 211.
We recruited a convenience sample of community-dwelling older adults aged 60 years and over from senior community centers and healthcare centers in Huzhou from March 2019 to December 2020. Our staff distributed recruitment leaflets at community health centers and local senior centers. Local healthcare providers also helped to make referrals to our study. Word of mouth resulted in the recruitment of older adults as well. In addition, our study team conducted free health lectures and screening events on cognitive impairments to attract interested populations. Individuals who showed interest were invited for an in-person interview to screen for eligibility by three trained staff. In the first round of eligibility assessments, a total of 832 potential participants were recruited with complaints of memory impairment. The second round of eligibility assessments included basic demographic data, functional assessment of daily activities, general cognitive function, medical history. A trained neurologist-psychiatrist made the evaluation based on the diagnostic criterion for MCI. Finally, 583 individuals were excluded due to ineligibility, and 249 eligible participants were included in the study.
The general information questionnaire was designed by researchers, which had a tripartite structure: (1) socio-demographic information (e.g., sex, age, marital status, education, living conditions, income); (2) lifestyle information, including eating habits, physical exercise, smoking status, alcohol consumption; and (3) medical characteristics, such as family history of Alzheimer's and presence of multimorbidity. Details of the names and value assignments of each variable were described in Supplementary Materials 2.
The adherence scale of cognitive dysfunction management (AS-CDM) was used to assess the overall level of adherence to MNPIs in which individuals are currently participating or may participate in the future, in terms of both adherence behavior and adherence intentions (26). The AS-CDM composes six dimensions: perceived susceptibility to MCI (7 items), perceived severity to MCI (4 items), perceived benefits of cognitive dysfunction management of MNPIs (3 items), perceived barriers to performing the cognitive dysfunction management (7 items), cues to complete the cognitive dysfunction management (3 items), self-efficacy (8 items), 32 items in total with higher scores indicating better adherence of cognitive dysfunction management. The content validity of AS-CDM was 0.92 according to the expert consultation and empirical analysis, and the Cronbach's α coefficient was 0.904.
The Beijing Version of the Montreal Cognitive Assessment Test (MoCA) was administered to assess global cognitive function (30). The MoCA tests includes analysis of visuospatial, executive ability, naming, attention, memory, language, abstraction, and orientation skills, aggregated for a maximum score of 30. Its scores range from 0 to 30, with a higher number indicating better cognitive performance. By using a cut-off score of 26, it gives the optimal sensitivity (92.4%) and specificity (88.4%) in distinguishing between older adults with MCI and those with intact cognitive function (35).
The Chinese version of the Lawton and Brody's Activities of Daily Living Scale was used to assess physical functional status (32). The scale consists of the Physical Self-Maintenance Scale (PSMS) with six questions and the Instrumental Activities of Daily Living Scale (IADL) with eight questions. The scores range from 14 to 56, with a higher score indicating a lower level of ADL functioning. A score of <16 means that older adults with MCI have their activities of daily living intact. The Cronbach's α coefficient was 0.93 and Split-half coefficient was 0.91; the range of intraclass correlation coefficient of the 14 items with total score of the scale was 0.84–0.96 (36).
Data were collected using a structured questionnaire through a face-to-face interview. Trained health professionals facilitated data collection and supervision to ascertain the quality of data. The training was given to data collectors and supervisors for 2 days duration to minimize measurement bias. Furthermore, the older adults with MCI completed the questionnaires about 30 min. We gave a small gift to those who completed this survey as compensation.
Data were analyzed in SPSS 25.0 (SPSS Inc., Chicago, IL, USA). Normally distributed continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were presented as frequency and percentage. Differences in the level of adherence between/among groups by each variable were analyzed by independent samples t-test or ANOVA. Hierarchical regression analyses were further conducted to identify the correlates of adherence to MNPIs for older adults with MCI. In the first model (model A), socio-demographic variables were included; In the second model (model B), the lifestyle information was entered; The disease-related factors were entered in the last model (model C). The level of statistical significance was set at p < 0.05.
The flow diagram (Figure 1) shows 249 participants were included in this study and 33 did not complete the questionnaire. Finally, a total of 216 completed questionnaires were finally analyzed. Of these, 68.52% were female, and 45.4% of the participants had no <6 years of education, and only 6% had more than 12 years of education. Less than 15% of the participants were single (divorced/widowed), and 14.4% lived alone. More than 75% of the participants were engaged in physical labor, and most of the participants have a monthly income of <3,000. The detailed demographic data are summarized in Table 1.
The overall mean score of AS-CDM was 117.58 (SD = 10.51) out of 160. Females had higher AS-CDM compared to males. As shown in Table 2, statistically significant differences in adherence were distributed by education level (p = 0.001), living conditions (p = 0.048), registered residence (p = 0.029), presence of multimorbidity (p = 0.034), eating habits (p = 0.003), and physical exercise (p = 0.003). Participants with the following characteristics had better adherence, including regular physical exercise, meat-vegetable balance, absence of multimorbidity, high education level, living alone, and living in urban.
According to Model 1 in Table 3, socio-demographic factors accounted for 10.2% of the variance of adherence (p < 0.001). Of these, junior school (Beta = 0.159, p < 0.05), high school (Beta = 0.206, p < 0.05), college and above more (Beta = 0.216, p < 0.05), living alone (Beta = 0.133, p < 0.05) had significant effects on the variance of adherence in model 1. After controlling for socio-demographic variates, lifestyle factors further significantly accounted for 17.2% of the variance of adherence in model 2. Physical exercise and eating habits were associated with AS-CDM scores, which explained variance increased by 7% (R2 change = 0.07, p < 0.001). Adding disease-related factors to model 3 accounted for an additional 1.6% of the variance of adherence (p < 0.001). In model 3, high school (Beta = 0.161, p < 0.05), college and above more (Beta = 0.171, p < 0.05), meat-vegetarian balance (Beta = 0.228, p < 0.05), regular physical exercise (Beta = 0.234, p < 0.05), and presence of multimorbidity (Beta = −0.128, p < 0.05) contributed significantly to adherence. The final model explained 18.8% of the total variance of adherence to MNPIs in older adults with MCI.
The average total score of the AS-CDM for all participants was 117.58 ± 10.51. Of these, the average score of perceived susceptibility dimension was 3.99 ± 0.38, perceived severity was 3.72 ± 0.76, perceived benefits was 3.40 ± 0.89, perceived barriers was 2.80 ± 0.86, cues to action was 3.96 ± 0.63, and self-efficacy was 4.14 ± 0.55 (see Figure 2A). There was a statistically significant difference in the six dimensions of the AS-CDM (F = 108.4, p < 0.001). The lowest score was for the dimension of perceived barrier. It can be significantly influenced by educational level, monthly income, eating habits, and race (see Figure 2B). In contrast, self-efficacy was the highest scoring of the adherence dimensions, and participants who had regular physical exercise and lived in the urban showing greater self-efficacy on the AS-CDM assessment (see Figure 2C). The influence factors with statistically significant of other dimensions are shown in Figures 2D–F.
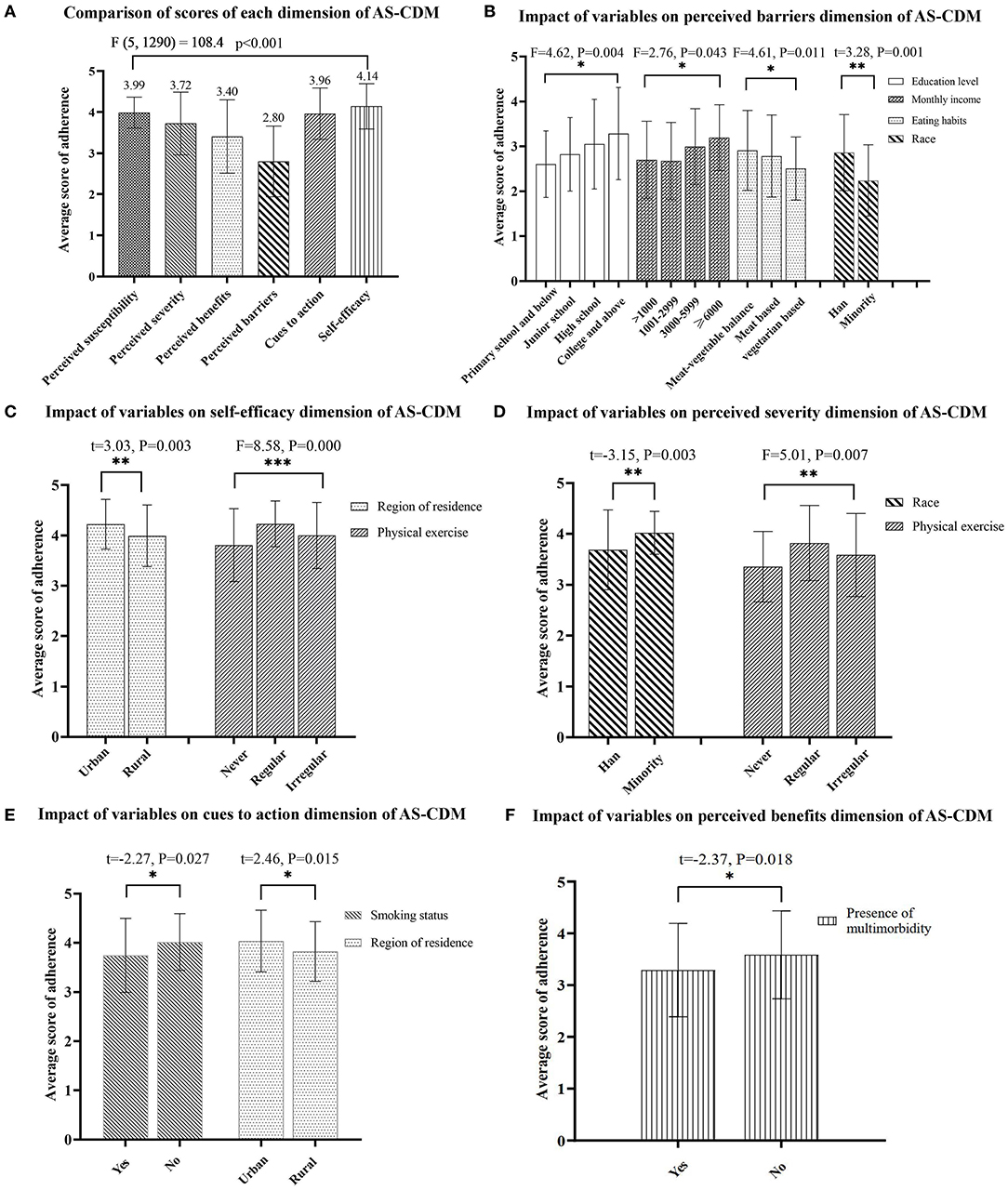
Figure 2. (A–F) Analysis of variables associated with dimensions scores of adherence. *p < 0.05, **p < 0.01, ***p < 0.001.
The dimension of self-efficacy in the AS-CDM was used to evaluate the preferred modality of cognitive dysfunction management in this study. Of these, items 1 and 2 indicate the preference of exercise intervention, items 3 and 4 represent preference for the cognitive training intervention, and items 5–8 for preference on the dietary intervention. Each item was scored on a 5-point Likert scale; a score of ≥4 was satisfied simultaneously for all items of a specific intervention preference, representing a preference for this intervention method. On the contrary, any item score < of 4 points is defined as uncertainty. In this study, the preferred intervention modality can be classified as single modality, mixed modality, and uncertain by scoring of the specific intervention modality. As shown in Table 4, nearly 80% of older adults with MCI preferred a mixed modality of non-pharmacological interventions (i.e., MNPIs).
Precise identification of contributors to low adherence will be crucial for improving treatment effectiveness and distinguishing individuals needing additional supervision to decrease the risk of disease or complications (37). To our knowledge, this is the first study that examined the level and correlates of adherence to MNPIs in older adults with MCI. The adherence to MNPIs was medium-level for older adults with MCI in this study. Our findings showed significant differences in the adherence to MNPIs in the education level, physical exercise, eating habits, and multimorbidity status of participants.
Whether single modality or multimodal interventions, treatment adherence might be different across the project categories. In previous RCT studies, most participants could adhere to a specific treatment during the supervised intervention; however, the level of adherence declined over the long term (7, 14). In a study of memory support systems training for older adults with MCI (14), participants in the intervention group had the highest adherence at the end of the training; however, it was consistently declining at the 8-week and 6-month follow-up visit after training. Our results showed a medium-level of adherence to MNPIs, a similar level of adherence reported by Mosca (65.5%) (38) and Lam (79%) (39). Notably, the cross-sectional data analysis in this study may overestimate the adherence of participants. Nevertheless, the present study extends our understanding of the evaluation of adherence to MNPIs in older adults with MCI.
The hierarchical regression analysis in this study mainly confirmed that higher level of education was associated with higher adherence to MNPIs in older adults with MCI. Previous research have also shown that higher level of education significantly predicted higher post-intervention adherence to behavioral intervention in older adults with MCI (40). Individuals with higher education may be more effective in understanding the potential benefits and actively overcoming barriers to adherence to health interventions (40). This reasonable explanation is also confirmed by the results shown in Figure 2B of this study. What's more, higher education attainment may be associated with higher cognitive reserve, which may prevent further cognitive decline and ultimately delay the onset of dementia (41, 42). Therefore, individuals with higher education may have less cognitive impairment and be more able to adhere to interventions (40). Conversely, this finding suggests that those with lower levels of education may require substantially more programmatic support to be successful with interventions (40). In this study, only 6% of participants received more than 12 years of education, which is consistent with the distribution characteristics of the education level of older adults with MCI in China (43), indicating that most older adults with MCI have a lower level of education and poorer adherence to MNPIs. Therefore, community healthcare providers should give more attention to the less educated older adults with MCI. Regular reminders and encouragement are necessary to enforce good adherence and further optimize the benefits of MNPIs.
Physical exercise and meat-vegetable balance were identified to have a significant positive association with adherence in older adults with MCI in model 2. Consistent with previous studies (44, 45), these data suggest that the level of adherence to the MNPIs was higher among participants with regular physical exercise than among those with non-regular physical exercise. It is noteworthy that the scores of both self-efficacy and perceived severity dimensions were significantly different in varying exercise states (shown in Figures 2C,D). By inference, regular exercise may improve the individuals' adherence to MNPIs by boosting their self-efficacy. Many previous studies have demonstrated the cognition-enhancing effects of exercise in older adults with MCI (46, 47). Therefore, it is recommended that primary healthcare providers should facilitate physical exercise recommendations and customize exercise programs for individuals at high risk of developing MCI.
The study results confirm that eating habits were significantly correlated with adherence to MNPIs in older adults with MCI. Participants with a meat-vegetables balance habit showed better adherence than those who ate only meat or a predominantly vegetarian diet. This finding may be attributed to the balance of meat-vegetables as a healthy diet model, similar to the Mediterranean diet composition. The latter has been proved to be an independent protective factor for cognitive function (5). The similarity of these two dietary patterns may promote the scores of diet intervention adherence of MNPIs in older adults with MCI.
In addition, the results of this study identified the status of live-alone as an independent contributor to high adherence to MNPIs. Picorelli et al. also found that living alone was associated with better adherence to exercise programs for older adults (8). This interaction can be interpreted in one of three ways: (1) Those who live alone may not have any other family members to share household responsibilities that require energy expenditure (48). (2) Older adults living alone may have complex health needs and are more likely to be the highest users of health care services (49), including MNPIs. (3) Additionally, interventions that increase social engagement (such as physical exercise, cognitive training, and dietary treatment) may reduce loneliness (50), which led to high adherence to MNPIs among participants living alone in this study. Conversely, older adults with MCI who live with family exhibit poor adherence. Therefore, it is necessary to encourage individuals and their family members to make decisions to engage in meaningful daily activities.
The final regression model illustrated that participants with multimorbidity had lower adherence to MNPIs. Adverse effects of medications may worsen cognitive function and further impede participation in MNPIs in older adults with MCI (51). Additionally, functional limitations caused by somatopathy may also diminish the adherence to MNPIs (8). This reasonable explanation is also supported by the findings shown in Figure 2F of this study. Therefore, healthcare providers need to ensure safe and effective medication administration via health education for older adults. Meanwhile, strategies to guide or reduce the use of potentially inappropriate medications (PIMs) should be implemented to minimize their adverse effects on cognitive/physical function in older adults with MCI (52). Furthermore, physical function assessment and implementation of functionally matched MNPIs are necessary for older adults with MCI suffering from multimorbidity.
Most of older adults with MCI were inclined to MNPIs strategy in this study. A minority of participants may select a single modality due to lack of interest, health-related restrictions, and family diet plans. Twelve of these participants had no clear preference for any specific intervention. This may be partly explained by individual differences in the level of healthcare consciousness in the management of cognitive impairment. For this reason, healthcare providers should strengthen cognitive-related health education to facilitate the self-consciousness of cognitive function management and behavior adherence in older adults with MCI.
The most important strength of this study is evaluating the level and correlates of adherence to MNPIs in older adults with MCI. This topic has significant implications in screening at-risk individuals with poor adherence to cognitive dysfunction management and ineffectively promoting their cognitive-health behavior. This study also provides evidence that a high educational level, meat-vegetarian balanced diet habits, regular physical exercise, and absence of multimorbidity are associated with high adherence to MNPIs in older adults with MCI, indicating the potential targets of adherence-promoting interventions. The new findings in this study, although preliminary, could provide more valuable information to formulate guidelines on the operationalization and implementation of MNPIs for older adults with MCI.
The findings in this study should be understood within the context of several limitations. (1) The study location was only in Huzhou city, China, with relatively small sample size, and the generalizability of the findings is limited. Meanwhile, the convenience sampling used in this study makes the results of this study potentially susceptible to sampling bias. A large sample size, random sampling, and multi-center study are needed in the future. (2) This study was a cross-sectional design, which is unsuitable for examining causality, and longitudinal studies on MNPIs intervention for older adults with MCI are needed to determine these findings. (3) The final model in this study demonstrates that the included variables did not account for all the variance of adherence (18.8%). According to the Multidimensional Adherence Model (53), therefore, further exploration of the contribution of variables related to the healthcare system (patient-provider relationship) and other potential variables (the duration of the intervention, fatigue, social network size, social support, depressive symptomatology) is needed to formulate a more comprehensive attribution model of adherence to MNPIs for older adults with MCI.
Positive effects of MNPIs have been reported and are promising for delaying cognitive decline. This study provides the first illustration of the level and correlates of adherence to MNPIs in older adults with MCI. Older adults with MCI included in this study presented medium-level adherence, although most participants were inclined to MNPIs strategy. Education level, eating habits, physical exercise, and multimorbidity status were associated with MNPIs adherence in older adults with MCI. These findings suggest the need for education on MNPIs and the potential directions for health promotion programs for older adults with MCI. Furthermore, the above correlation will help screen high-risk MCI individuals with low adherence to NMPIs, and provide a more proactive and precise intervention program.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
LW: devised research concept, research design, and critical revision of the manuscript for intellectual content. XSu: drafted the first version of the manuscript, submitted the manuscript for publication, and assisted in the development of the research design. XSh: contributed to the design of the study. CH, ZW, and LS: assisted with participant enrolment and consenting and data acquisition plan. SW, XL, and XZ: assisted with statistical analytic planning. All authors contributed to the design and drafting of the manuscript and read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Nos. 72174061 and 71704053), the China Scholarship Council Foundation (No. 201908330251), and the Zhejiang Provincial College Students Scientific and Technological Innovation Activities-Zhejiang Xinmiao Talents Program (No. 2021R431031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.833767/full#supplementary-material
1. Tangalos EG, Petersen RC. Mild cognitive impairment in geriatrics. Clin Geriatr Med. (2018) 34:563–89. doi: 10.1016/j.cger.2018.06.005
2. Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
3. Petersen RCJNEJM. Clinical practice. Mild Cogn Impairment. (2011) 364:2227–34. doi: 10.1056/NEJMcp0910237
4. Petersen RC. Mild cognitive impairment. Continuum. (2016) 22:404–18. doi: 10.1212/CON.0000000000000313
5. Wu L, Sun DJR. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. (2017) 7:41317. doi: 10.1038/srep41317
6. Tak EC, van Uffelen JG, Paw MJ, van Mechelen W, Hopman-Rock M. Adherence to exercise programs and determinants of maintenance in older adults with mild cognitive impairment. J Aging Phys Act. (2012) 20:32–46. doi: 10.1123/japa.20.1.32
7. Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. (2007) 55:158–65. doi: 10.1111/j.1532-5415.2007.01035.x
8. Picorelli AM, Pereira LS, Pereira DS, Felício D, Sherrington C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: a systematic review. J Physiother. (2014) 60:151–6. doi: 10.1016/j.jphys.2014.06.012
9. Klimova B, Novotny M, Schlegel P, Valis M. The effect of mediterranean diet on cognitive functions in the elderly population. Nutrients. (2021) 13:2067. doi: 10.3390/nu13062067
10. Mantzorou M, Vadikolias K, Pavlidou E, Tryfonos C, Vasios G, Serdari A, et al. Mediterranean diet adherence is associated with better cognitive status and less depressive symptoms in a Greek elderly population. Aging Clin Exp Res. (2021) 33:1033–40. doi: 10.1007/s40520-020-01608-x
11. Zhao X, Wang L, Ge C, Liu X, Chen M, Zhang C. Effect of process-based multi-task cognitive training program on executive function in older adults with mild cognitive impairment: study rationale and protocol design for a randomized controlled trial. Front Psychiatry. (2020) 11:655. doi: 10.3389/fpsyt.2020.00655
12. Forman EM, Goldstein SP, Flack D, Evans BC, Manasse SM, Dochat C. Promising technological innovations in cognitive training to treat eating-related behavior. Appetite. (2018) 124:68–77. doi: 10.1016/j.appet.2017.04.011
13. Shatil E, Metzer A, Horvitz O, Miller A. Home-based personalized cognitive training in MS patients: a study of adherence and cognitive performance. NeuroRehabilitation. (2010) 26:143–53. doi: 10.3233/NRE-2010-0546
14. Greenaway MC, Duncan NL, Smith GE. The memory support system for mild cognitive impairment: randomized trial of a cognitive rehabilitation intervention. Int J Geriatr Psychiatry. (2013) 28:402–9. doi: 10.1002/gps.3838
15. Hawley-Hague H, Horne M, Skelton DA, Todd C. Review of how we should define (and measure) adherence in studies examining older adults' participation in exercise classes. BMJ Open. (2016) 6:e011560. doi: 10.1136/bmjopen-2016-011560
16. Wilz G, Schinkthe D, Soellner RJGtJoG, Psychiatry G. Goal attainment and treatment compliance in a cognitive-behavioral telephone intervention for family caregivers of persons with dementia. GeroPsych. (2011) 24:115–25. doi: 10.1024/1662-9647/a000043
17. Gardener S, Gu Y, Rainey-Smith SR, Keogh JB, Clifton PM, Mathieson SL, et al. Adherence to a Mediterranean diet and Alzheimer's disease risk in an Australian population. Transl Psychiatry. (2012) 2:e164. doi: 10.1038/tp.2012.91
18. van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. (2015) 6:154–68. doi: 10.3945/an.114.007617
19. Yu F, Lin FV, Salisbury DL, Shah KN, Chow L, Vock D, et al. Efficacy and mechanisms of combined aerobic exercise and cognitive training in mild cognitive impairment: study protocol of the ACT trial. Trials. (2018) 19:700. doi: 10.1186/s13063-018-3054-0
20. Yorozuya K, Kubo Y, Tomiyama N, Yamane S, Hanaoka H. A systematic review of multimodal non-pharmacological interventions for cognitive function in older people with dementia in nursing homes. Dement Geriatr Cogn Disord. (2019) 48:1–16. doi: 10.1159/000503445
21. Cerkoney KA, Hart LK. The relationship between the health belief model and compliance of persons with diabetes mellitus. Diabetes Care. (1980) 3:594–8. doi: 10.2337/diacare.3.5.594
22. Alogna M. Perception of severity of disease and health locus of control in compliant and noncompliant diabetic patients. Diabetes Care. (1980) 3:533–4. doi: 10.2337/diacare.3.4.533
23. Barker, Gordon T, Mansberger, Steven L. Psychometric properties of the reduced version of the Glaucoma Treatment Compliance Assessment Tool (GTCAT). Ophthalmic Epidemiol. (2019) 26 (1):55–62. doi: 10.1080/09286586.2018.1516785
24. Kawakami, Aki, Tanaka, Makoto, Nishigaki, Masakazu, et al. A screening instrument to identify ulcerative colitis patients with the high possibility of current non-adherence to aminosalicylate medication based on the Health Belief Model: a cross-sectional study. BMC Gastroenterol. (2014) 14:220. doi: 10.1186/s12876-014-0220-z
25. Karimy M, Azarpira H, Araban M. Using health belief model constructs to examine differences in adherence to pap test recommendations among Iranian women. Asian Pac J Cancer Prev. (2017) 18:1389–94. doi: 10.22034/APJCP.2017.18.5.1389
26. Xt Z. Development and relia bility and validitv test of compliance prediction scale in cognitive function management for older adults with mild cognitive impairment (dissertation/master's thesis). Huzhou University, Zhejiang (2020).
27. Olsen, S, Smith S, Oei T. Health belief model predicts adherence to CPAP before experience with CPAP. Eur Respir J. (2008) 32:710–7. doi: 10.1183/09031936.00127507
28. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
29. Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, Scheltens P, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry. (2006) 77:714–8. doi: 10.1136/jnnp.2005.085332
30. Yu J, Li J, Huang X. The beijing version of the montreal cognitive assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. (2012) 12:156. doi: 10.1186/1471-244X-12-156
31. Bosco A, Spano G, Caffò AO, Lopez A, Grattagliano I, Saracino G, et al. Italians do it worse. Montreal Cognitive Assessment (MoCA) optimal cut-off scores for people with probable Alzheimer's disease and with probable cognitive impairment. Aging Clin Exp Res. (2017) 29:1113–120. doi: 10.1007/s40520-017-0727-6
32. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. (1969) 9:179–86. doi: 10.1093/geront/9.3_Part_1.179
33. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
34. Xue J, Li J, Liang J, Chen S. The prevalence of mild cognitive impairment in China: a systematic review. Aging Dis. (2018) 9:706–15. doi: 10.14336/AD.2017.0928
35. Wen HB, Zhang ZX, Niu FS, Li L. The application of montreal cognitive assessment in urban Chinese residents of Beijing. Zhonghua nei ke za zhi. (2008) 47:36–9. doi: 10.3321/j.issn:0578-1426.2008.01.012
36. Qian Shixing, Xiao Shifu, Zhang Xinkai, Li Xia, Zhu Agile, Wang Tao, et al. Reliability and validity of daily living scale for Alzheimer's disease patients and its application. Shanghai Arch Psychiatry. (2010) 22:22–5. doi: 10.3969/j.issn.1002-0829.2010.01.006
37. Coley N, Ngandu T, Lehtisalo J, Soininen H, Vellas B, Richard E, et al. Adherence to multidomain interventions for dementia prevention: data from the FINGER and MAPT trials. Alzheimer's Dementia. (2019) 15:729–41. doi: 10.1016/j.jalz.2019.03.005
38. Mosca IE, Salvadori E, Gerli F, Fabbri L, Pancani S, Lucidi G, et al. Analysis of feasibility, adherence, and appreciation of a newly developed tele-rehabilitation program for people with MCI and VCI. Front Neurol. (2020) 11:583368. doi: 10.3389/fneur.2020.583368
39. Lam LC, Chau RC, Wong BM, Fung AW, Tam CW, Leung GT, et al. A 1-year randomized controlled trial comparing mind body exercise (Tai Chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J Am Med Dir Assoc. (2012) 13:568.e15-20. doi: 10.1016/j.jamda.2012.03.008
40. Sr PAA, DeFeis B, De Wit L, O'Shea D, Mejia A, Chandler M, et al. Functional ability is associated with higher adherence to behavioral interventions in mild cognitive impairment. Clin Neuropsychol. (2020) 34:937–55. doi: 10.1080/13854046.2019.1672792
41. Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's Dementia. (2020) 16:1305–11. doi: 10.1016/j.jalz.2018.07.219
42. O'Shea DM, Langer K, Woods AJ, Porges EC, Williamson JB, O'Shea A, et al. Educational attainment moderates the association between hippocampal volumes and memory performances in healthy older adults. Front Aging Neurosci. (2018) 10:361. doi: 10.3389/fnagi.2018.00361
43. Song M, Wang YM, Wang R, et al. Prevalence and risks of mild cognitive impairment of Chinese community-dwelling women aged above 60 years: a cross-sectional study. Arch Womens Ment Health. (2021) 12, c24:903–911. doi: 10.1007/s00737-021-01137-0
44. Findorff MJ, Wyman JF, Gross CR. Predictors of long-term exercise adherence in a community-based sample of older women. J Women's Health. (2009) 18:1769–76. doi: 10.1089/jwh.2008.1265
45. White JL, Ransdell LB, Vener J, Flohr JA. Factors related to physical activity adherence in women: review and suggestions for future research. Women Health. (2005) 41:123–48. doi: 10.1300/J013v41n04_07
46. Wang L, Wu B, Tao H, Chai N, Zhao X, Zhen X, et al. Effects and mediating mechanisms of a structured limbs-exercise program on general cognitive function in older adults with mild cognitive impairment: a randomized controlled trial. Int J Nurs Stud. (2020) 110:103706. doi: 10.1016/j.ijnurstu.2020.103706
47. Song D, Yu DSF. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: a randomised controlled trial. Int J Nurs Stud. (2019) 93:97–105. doi: 10.1016/j.ijnurstu.2019.02.019
48. Wilcox S, Bopp M, Oberrecht L, Kammermann SK, McElmurray CT. Psychosocial and perceived environmental correlates of physical activity in rural and older african american and white women. J Gerontol Series B Psychol Sci Soc Sci. (2003) 58:P329–37. doi: 10.1093/geronb/58.6.P329
49. Dreyer K, Steventon A, Fisher R, Deeny SR. The association between living alone and health care utilisation in older adults: a retrospective cohort study of electronic health records from a London general practice. BMC Geriatr. (2018) 18:269. doi: 10.1186/s12877-018-0939-4
50. Masi CM, Chen HY, Hawkley LC, Cacioppo JT. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. (2011) 15:219–66. doi: 10.1177/1088868310377394
51. Koyanagi A, Lara E, Stubbs B, Carvalho AF, Oh H, Stickley A, et al. Chronic physical conditions, multimorbidity, and mild cognitive impairment in low- and middle-income countries. J Am Geriatr Soc. (2018) 66:721–7. doi: 10.1111/jgs.15288
52. Bonfiglio V, Umegaki H, Kuzuya M. Potentially inappropriate medications and polypharmacy: a study of older people with mild cognitive impairment and mild dementia. J Alzheimer's Dis. (2019) 71:889–97. doi: 10.3233/JAD-190284
Keywords: mild cognitive impairment, adherence, multimodal interventions, non-pharmacological interventions, cognitive function
Citation: Sun X, Wang L, Shen X, Huang C, Wei Z, Su L, Wang S, Liu X and Zhen X (2022) Correlates of Adherence of Multimodal Non-pharmacological Interventions in Older Adults With Mild Cognitive Impairment: A Cross-Sectional Study. Front. Psychiatry 13:833767. doi: 10.3389/fpsyt.2022.833767
Received: 12 December 2021; Accepted: 12 May 2022;
Published: 03 June 2022.
Edited by:
Gabriela Cohen, Emory University, United StatesReviewed by:
Sanjeev Kumar, University of Toronto, CanadaCopyright © 2022 Sun, Wang, Shen, Huang, Wei, Su, Wang, Liu and Zhen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lina Wang, YXJpbmcyMDAwQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.