- 1Department of Clinical Psychology, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
- 2Department of Medicine II, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
- 3Department of Psychosomatic Medicine, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
Background: Previous studies have shown dysfunctional emotion processing in patients with inflammatory bowel diseases (IBD), characterized by a hypersensitivity to negative emotions and a hyposensitivity to positive emotions. Models of emotion processing emphasize the importance of bodily sensations to the experience of emotions. Since there have been no studies on whether emotion-associated bodily sensations are changed in IBD, we investigated the experience of bodily sensations related to valence and arousal, together with their links to emotional awareness, as one domain of interoceptive sensibility relevant to emotion processing.
Methods: Using a topographical self-report measure, 41 IBD patients in clinical remission and 44 healthy control (HC) participants were asked to indicate where and how intensely in their body they perceive changes when experiencing emotions of positive and negative valence, as well as relaxation and tension. Additionally, we used self-report questionnaires to assess emotional awareness as one domain of an individual’s interoceptive sensibility, gastrointestinal-specific anxiety (GSA), and psychological distress.
Results: Patients with IBD reported higher emotional awareness but lower intensities of perceived changes in their bodily sensations related to valence and arousal of emotional processing. IBD patients reported less intense bodily activation during positive emotions and less intense bodily deactivation during negative emotional states in comparison to HC participants. Higher emotional awareness and psychological distress were linked to stronger experiences of emotion-related bodily sensations in IBD patients.
Conclusion: Inflammatory bowel diseases patients exhibited alterations in how they link bodily sensations to their emotional experience. Such persistent changes can affect a patient’s wellbeing and are related to higher levels of anxiety and depression among IBD patients, even in remission.
Introduction
Patients with inflammatory bowel disease (IBD) report poorer quality of life, including impairments in their physical and mental wellbeing, than healthy individuals (1). IBD is often accompanied by increased attention to and anxiety about gastrointestinal sensations, resulting in greater psychological distress (2). Psychological distress and biased emotion experience have been shown to be subjectively and objectively related to disease activity (3, 4). These relationships are consistent with the biopsychosocial model of illness, according to which the disease course affects and is affected by biological, social, and psychological factors, such as emotions and cognitions (5). The perception of bodily sensations significantly influences the experience of stress and emotions (6, 7). Thus, chronic conditions associated with altered visceral and interoceptive processing, such as gastrointestinal inflammation, might result in impaired emotional functioning, potentially affecting patients’ quality of life and psychological wellbeing (8, 9).
Studies on emotional experience in IBD have pointed toward the presence of emotional dysfunctions, characterized by decreased sensitivity to positive emotional cues (9, 10), increased sensitivity to negative emotions and increased arousal during emotional experience (11). Moreover, neuroimaging findings have indicated a reduced activation within the limbic system in response to positive emotional stimuli in this patient group (10). In IBD, biological and emotional factors interact trough the brain-gut axis, as the limbic system communicates with the intrinsic neural network of the gastrointestinal tract via the sympathetic and parasympathetic nerves (12). Alterations in these brain regions have been associated with impaired regulation of the sympathetic and parasympathetic stress response in IBD patients, promoting the proinflammatory activity (4, 13). Thus, poor emotional functioning is thought to negatively affect the course of the disease and to be linked to greater psychological distress in IBD.
According to dimensional models of emotion, emotions are arranged in a two-dimensional space defined by the dimensions of valence and arousal (14). Valence refers to the hedonic value of an emotion, that is, the subjective feeling of pleasantness or unpleasantness. Arousal refers to the subjective state of feeling relaxation or tension (15) and represents the level of autonomic activation that an emotions elicits. Both dimensions influence peoples’ approach–avoidance behavior: high positive arousal states trigger the appetitive motivational system, while low negative arousal states are experienced as not rewarding and are therefore avoided (16). An individual’s level of emotional arousal is modulated by interoceptive processes (17). Part of an individual’s interoceptive abilities is the ability to link the conscious experience of emotions to the physiological sensations perceived in different regions of the body (18). A study by Nummenmaa et al. (19) showed that the experience of emotions is linked to topographical patterns of activation and deactivation perceived in the body, suggesting their representation in the somatosensory system (19, 20). Emotional awareness constitutes a distinct feature of one’s interoceptive sensibility and describes the ability to attribute specific bodily sensations to physiological manifestations of emotions (21). Although it has been shown that IBD patients exhibit certain alterations in both emotion and interoceptive processing (22), no study has investigated the role of interoceptive sensibility in the embodiment of valence and arousal among patients with IBD.
The present study aims to contribute to the understanding of how emotional valence and arousal are subjectively experienced in the body among patients with IBD and whether emotion-related sensations, possibly altered by increased anxiety about gastrointestinal sensations (23), are linked to patients’ emotional awareness. Using a topographical self-report measure adapted from Nummenmaa et al. (19), we assessed perceived changes in bodily sensations associated with experiences of positive and negative valence, as well as tension and relaxation, among patients with IBD and healthy control participants. We hypothesized that (I.) IBD patients would report stronger bodily sensations for negative emotions and more attenuated sensations for positive emotions than healthy control participants. We also expected that (II.) interoceptive sensibility and gastrointestinal-specific anxiety (GSA) would be related to the severity of alterations in emotion-related bodily sensations in IBD, particularly for negative emotions. To investigate whether these changes show distinct topographical patterns, especially in body areas strongly related to IBD symptoms, we explored whether IBD patients exhibit altered bodily sensations in the abdomen related to the experience of valence and arousal.
Materials and Methods
Participants
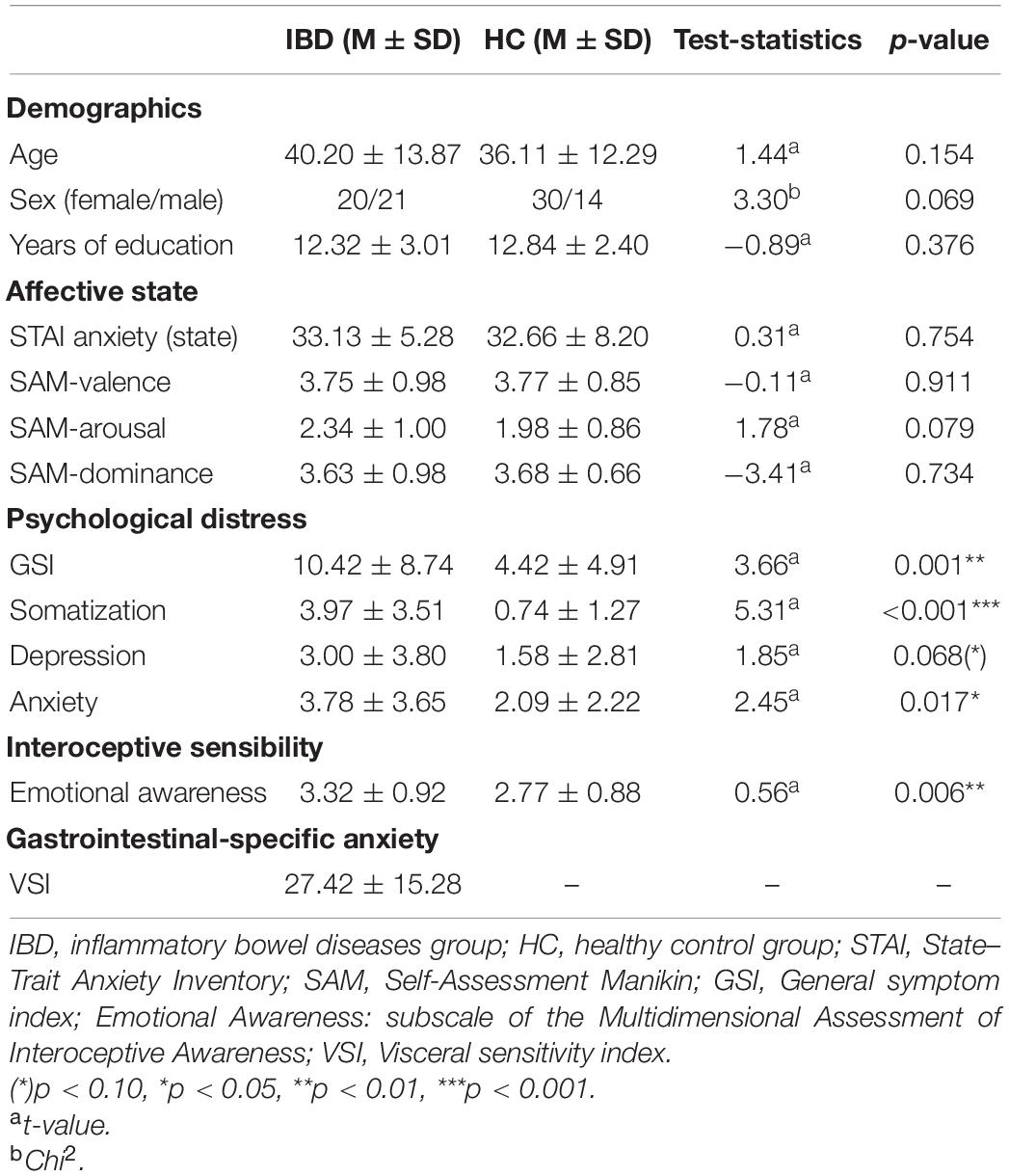
A total of 86 individuals between 18 and 65 years of age participated in the study. Of these, 42 met the diagnostic criteria for IBD, and 44 were healthy control (HC) participants. We excluded one participant from the IBD group because of a lack of understanding of the instructions. Thus, the final sample consisted of 41 IBD patients (20 female, 21 male) and 44 HC participants (30 female, 14 male; Table 1). Outpatients attending the IBD outpatient unit at the University Medical Center Mannheim were recruited during the routinely control visits and invited to participate in the study. Patients, who met the inclusion and exclusion criteria and provided written informed consent, took part in the study after the physical examination. Overall, 29 patients with Crohn’s disease (CD) and 12 patients with ulcerative colitis (UC) in clinical remission were included. We focused on examining IBD patients in clinical remission to avoid potentially confounding effects of active disease symptoms on the measures of emotional experience. Furthermore, we were interested in examining whether alterations in emotion experience could also be observed during clinical remission, potentially affecting a patient’s wellbeing even when acute disease symptoms have subsided. CD patients exhibited a mean Harvey–Bradshaw Index score (24) of 1.76 ± 1.83 (range: 0–16), and UC patients exhibited a mean Partial Mayo Score (25) of 1.58 ± 1.51 (range: 0–7). The average age for disease onset reported by the patients was 25.54 ± 10.24 years. The mean disease duration reported by the patients was 14.65 ± 12.64 years. Diagnostic procedures and gastroenterological examinations were carried out on all patients by fully trained physicians specialized in the care of patients with IBD. Exclusion criteria were biological signs of disease activity (fecal calprotectin level > 200 μg/g, C-reactive protein > 50 mg/L, HBI score > 5, Partial Mayo score > 3), current use of corticosteroids, use of psychotropic medications, and current or past neurological or psychiatric diseases. For further details on sample characteristics, see Table 1. Details on treatment medication, comorbidities, previous surgeries, and extraintestinal manifestations were collected from medical reports and are provided in Supplementary Table 1.
Exclusion criteria for the HC group were chronic medical conditions, chronic medication intake, use of psychotropic medication, and current or past neurological or psychiatric diseases, as well as general gastrointestinal complaints during the 4 weeks prior the experiment. The study was approved by the Ethics Committee of the Medical Faculty Mannheim at Heidelberg University, and all participants gave their written informed consent before participating in the study. Please note that a subsample of participants from the IBD and the healthy control group in this study was also included in another manuscript on interoception and emotion perception published by our group [see Atanasova et al. (22)].
All of the participants completed a battery of psychometric questionnaires assessing psychological distress, interoceptive sensibility, and gastrointestinal-specific anxiety. The participants then performed a computer-based task, and changes in the participants’ bodily sensations when experiencing valence and arousal were assessed.
Questionnaires
Brief Symptom Inventory
To characterize the participants, we assessed psychological distress using the Brief Symptom Inventory (BSI-18) (26). BSI-18 is a self-report measure of somatization, depression, and anxiety. Scores on three subscales with six items each are combined into a global symptom severity index (GSI). GSI scores range from 0 to 72 points, and scores on each of the three subscales range from 0 to 24 points, with higher scores indicating higher symptom severity. Cronbach’s alphas for the 18 items were 0.86 for the IBD group and 0.81 for the HC group.
Affective State
Prior to testing, the current affective states of the participants were evaluated using the state version of the State–Trait Anxiety Inventory [STAI; Spielberger et al. (27)]. The STAI is a 20-item scale that measures subjective feelings of apprehension, tension, nervousness, worry, and activation of the autonomic nervous system on a four-point Likert scale. Item scores are added to obtain the scale total score (which ranges from 20 to 80), with higher scores indicating higher anxiety. Internal consistency was good, with Cronbach’s α = 0.76 for the IBD group and 0.90 for the HC group. Arousal, valence, and dominance levels were assessed using the Self-Assessment Manikin [SAM; Bradley and Lang (28)]. The SAM is a non-verbal pictorial assessment technique that measures valence/pleasure, perceived arousal, and perceptions of dominance on a nine-point Likert scale. Higher scores indicate more positive valence, higher arousal, and higher perceived dominance.
Visceral Sensitivity Index
Gastrointestinal-specific anxiety was measured using the Visceral sensitivity index [VSI; Labus et al. (29)]. The 15-item scale assesses worry, fear, vigilance, sensitivity, and avoidance, as well as gastrointestinal-related cognitions and behaviors. Items are scored on a reversed six-point scale. The overall VSI score ranges from 0 to 75, with higher scores indicating more severe GSA. VSI was developed specifically for patients with functional gastrointestinal disorders and is not suitable for assessment of healthy individuals. For that reason, we utilized this self-report measure for the IBD group only. The internal consistency of this scale for the current IBD sample was good, with Cronbach’s α = 0.89.
Multidimensional Assessment of Interoceptive Awareness
We assessed Emotional Awareness as the feature of interoceptive sensibility that is most strongly related to the experience of emotions in the body, as it comprises an individual’s awareness that certain physical sensations are the sensory aspects of emotional states. For this purpose, we used the Multidimensional Assessment of Interoceptive Awareness [MAIA; Mehling et al. (21)] and its Emotional Awareness subscale, which comprises five items assessed on a six-point Likert scale. The subscale score is calculated as the mean item score, with higher scores indicating higher emotional awareness. Emotional Awareness exhibited acceptable internal consistency, with Cronbach’s α = 0.76 for the IBD group and 0.86 for the HC group. For further details on the MAIA and its subscales, see Supplementary Material.
Experimental Procedure
Participants were asked to indicate changes in their bodily sensations when they experienced emotional states of different valence (positive/negative) and arousal (relaxation/tension) during everyday life, using a topographical self-report method adapted from Nummenmaa et al. (19). Initially, participants were familiarized with the task and instructed to evaluate which bodily regions they typically felt as being activated or deactivated when experiencing a particular emotional state. Thus, the task did not involve inducing actual emotions by experimental manipulation. To indicate these changes, participants were asked to color the body areas where they perceived sensations of activation and deactivation by selecting a color from a nine-point color bar ranging from blue (−4 = “very strong deactivation”) to red (+4 = “very strong activation”) (see Supplementary Figure 1). Participants indicated their responses by painting the bodily regions using successive strokes on the presented body templates. If they did not perceive any changes in some parts of the body, participants were instructed to leave these uncolored. The four instructions (positive emotions, negative emotions, relaxation, and tension) were presented in a pseudorandomized order. The task was presented on a 14″ computer screen. The front and back body templates comprised 366 × 195 pixels, and the diameter of the painting tool was 13 pixels. The task was programmed using the Presentation® software (Version 20.1, Neurobehavioral Systems, Inc., Berkeley, CA, United States).1
Dependent Variables
For each participant and trial, color code values (ranging from -4 to + 4) were stored in a numeric array for each pixel of the body templates and spatially smoothed by averaging the color code value of each pixel and its surrounding pixels (±3 pixels).
To assess changes in bodily sensations, we calculated (a) a change score representing the average of the absolute values of the pixels and (b) a change score across those pixels that indicated either a deactivation or an activation. This procedure was conducted for all pixels of the body templates to derive a global whole-body score, as well as for different regions of interest (ROIs). The ROIs were predefined based on the findings of Nummenmaa et al. (19) and included the head, chest, arms, abdomen, legs, and back as separate body regions (see Supplementary Figure 2). Whole-body and ROI analyses were performed using custom pipelines run with MATLAB 2020a (The MathWorks, Inc., Natick, MA, United States).
Data Analysis
The dependent variables were analyzed separately for the experience of valence and arousal using mixed-effects analysis-of-variance designs (mixed-effects ANOVAs). Testing for normality of residuals (Kolmogorov–Smirnov test, visual inspection of Q–Q plots) revealed that the normality assumptions underlying parametric analyses were violated. Therefore, a non-parametric approach was employed, using a rank-aligned ANOVA (30, 31). Global change scores were analyzed in 2 × 2 designs with the between-subjects factor “group” (IBD/HC) and an additional experimental factor, i.e., “valence” (positive/negative) in one case and “arousal” (relaxation/tension) in another. To analyze more specifically the changes in activation and deactivation, we extended these designs by an additional within-subject “type of change” (activation/deactivation) factor, resulting in two 2 × 2 × 2-mixed ANOVA designs. To further characterize the effects in the ANOVA designs, post-hoc comparisons were conducted using ANOVA sub-designs and pairwise comparisons. Due to an unequal distribution of participants’ sex in the groups, exploratory analyses including the additional factor “sex” (female/male) were computed to examine the influence of this variable on the reported bodily sensations related to valence and arousal.
To investigate the links between participants’ emotion-related bodily sensations, gastrointestinal-specific anxiety, and emotional awareness, Spearman’s rank correlation coefficients were calculated. In addition to our preregistered analyses (see below), ROI scores for the experience of valence and arousal in distinct parts of the body were analyzed using exploratory 2 × 2 × 2 rank-aligned ANOVA designs with the same factors as those used in the whole-body analyses. The statistical analyses were carried out using SPSS v.27.0 (IBM Corp., United States) and the ARTool package implemented in RStudio v.1.2.1335 (RStudio, PBC, Boston, MA, United States).2 The significance level was set to p < 0.05. To control for multiple testing in the correlation analyses, we applied a false-discovery rate (FDR) correction, as recommended by Benjamini and Hochberg (32), and we reported the corresponding p-values, denoted as pFDR. For all analyses of an exploratory nature, uncorrected p-values are reported.
Preregistration
The hypotheses, sample size, methods, exclusion criteria, and planned analyses were preregistered before data collection and can be accessed at https://aspredicted.org/blind.php?x=hu4n7k. According to our preregistration, a sample size of 120 participants was planned. However, because of the COVID-19 outbreak and pandemic-related restrictions, the number of participants was reduced. Furthermore, in contrast to our preregistered recruitment procedure, the HC group comprised more women and less men compared to the IBD group, resulting in two samples not fully matched as initially planned. Mean age and education level of all participants have been balanced and both groups were comparable with respect to these variables. All remaining aspects of the study were carried out in accordance with the pre-registered protocol unless stated otherwise. Note that because of length restrictions, only data on emotional experience of valence and arousal are reported in this manuscript. Data on body image perception and the experience of specific emotions, as described in the same preregistration, are reported in separate articles.
Results
Valence: Positive and Negative Emotions
Whole-Body Sensations
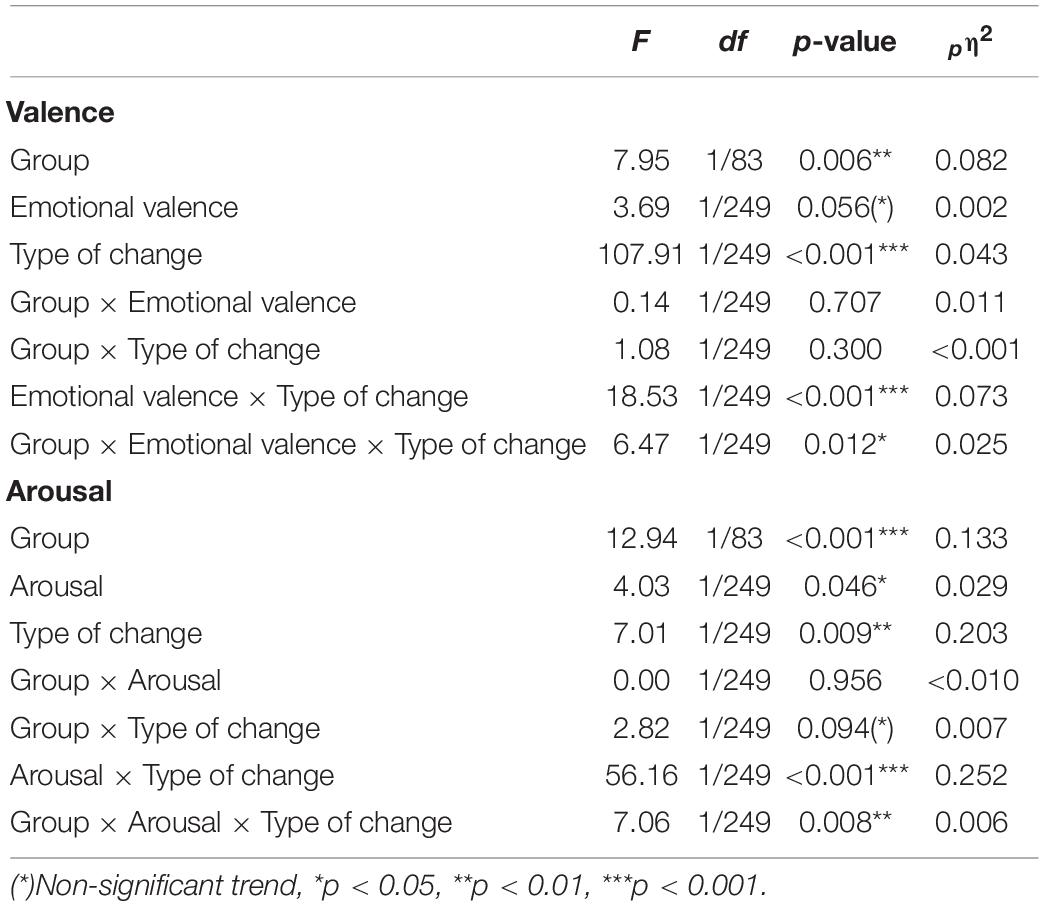
IBD patients reported significantly less perceived change in their bodily sensations compared to HC (main effect “group”: F1, 83 = 6.90, p = 0.010, pη2 = 0.077), without a difference between positive and negative emotions (main effect “valence”: F1, 83 = 0.23, p = 0.635, pη2 = 0.003; group × valence: F1, 83 < 0.01, p = 0.988, pη2 < 0.0001; Figure 1). To investigate whether participants’ sex influenced these effects, we repeated the analyses with “sex” as an additional between-subjects factor. These revealed no influence of the factor “sex.” For further details see Supplementary Table 6.
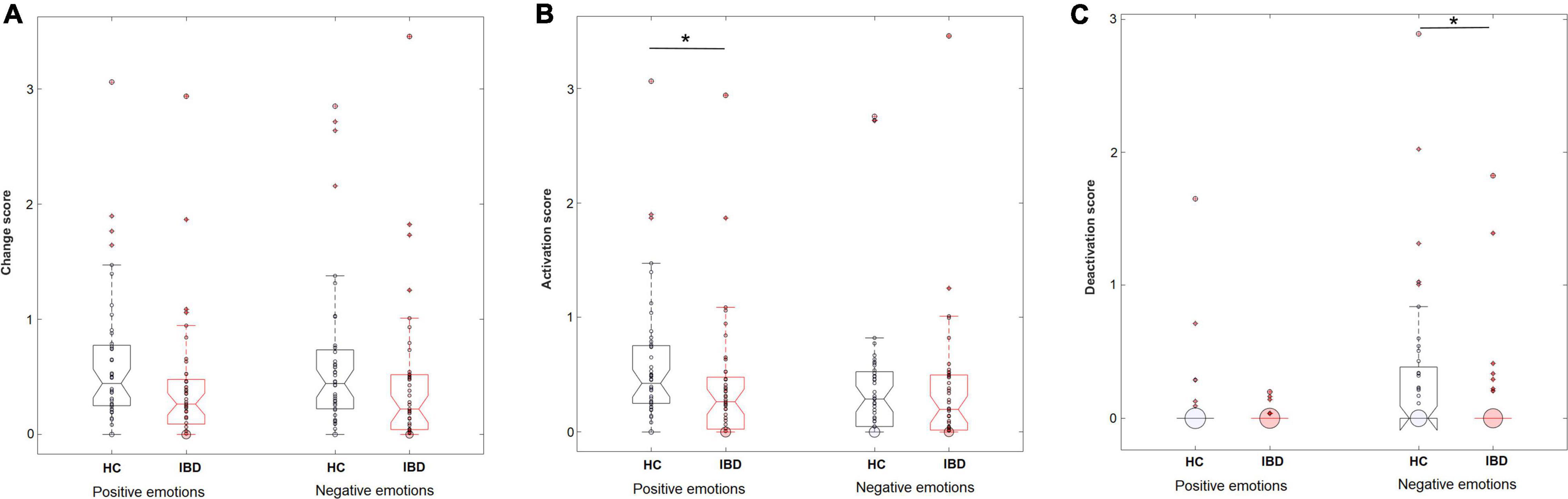
Figure 1. Perceived levels of overall changes in the body (A), sensations of activation (B), and sensations of deactivation (C) in the body during the experience of positive and negative emotions in HC (n = 44) and IBD (n = 41). *p < 0.05.
Separating the changes in bodily activation and deactivation revealed that differences between the groups depended on whether the participants judged activation or deactivation for positive or negative emotions (group × valence × type of change: F1, 249 = 6.47, p = 0.012, pη2 = 0.025). Decomposing the design into 2 × 2 ANOVA sub-designs revealed a significant group difference for bodily deactivation, influenced by the emotional valence (group × valence: F1, 83 = 9.56, p = 0.003, pη2 = 0.103): Compared with HC, IBD patients reported a smaller difference in the perceived deactivation between positive and negative emotions (p = 0.005), caused by a lower deactivation associated with negative emotions (p = 0.015, positive emotions: p = 0.746; see Figure 1). For bodily activation, the groups differed depending on the emotional valence at a trend-level significance level (2 × 2 ANOVA sub-design for activation: main effect “group”: F1, 83 = 4.84, p = 0.031, pη2 = 0.055; group × valence: F1, 83 = 3.81, p = 0.054, pη2 = 0.044). Compared with HC, the IBD group reported a smaller difference between positive and negative emotions (p = 0.018). This effect was caused by a significantly lower bodily activation during positive emotions (p = 0.015) but not during negative emotions in the IBD group (p = 0.587; see Figure 1). All main and interaction effects are reported in Table 2 but are not further described because of their restricted interpretability caused by higher-order interaction effects.
Extending the analysis by the additional factor “sex” revealed that the group differences depending on the factors “valence” and “type of change” were not influenced by the participant’s sex (group × sex × valence × type of change: F1, 243 = 0.05, p = 0.826, pη2 < 0.001; group × valence × type of change: F1, 243 = 6.02, p = 0.015, pη2 = 0.024). However, independently of the assessed emotional valence, sex influenced the group differences in the reported bodily activation and deactivation (group × sex × type of change: F 1, 243 = 5.51, p = 0.020, pη2 = 0.022). Decomposing the design into two separate 2 × 2 × 2 ANOVA sub-designs indicated that this effect was caused by lower perceived deactivation reported by male patients with IBD compared to male healthy control participants (p = 0.041; Supplementary Figure 5) while there was no difference between female patients with IBD and female HC. For further details see Supplementary Table 7.
Exploratory Regions of Interest Analyses
Statistical analyses of the ROI scores revealed that the groups differed only in the overall change in their bodily sensations experienced in the back (main effect “group”: F1, 83 = 7.75, p = 0.007, pη2 = 0.085), with IBD patients reporting less change of sensation in this area compared to HC (see Supplementary Table 2).
Inflammatory bowel diseases and HC participants differed in their differentiation between bodily activation and deactivation depending on whether they evaluated positive or negative emotions. This pattern was observed for the chest, legs, and back, as well as at trend-level significance levels for the head, arms, and abdomen (group × valence × type of change; see Table 3). 2 × 2 ANOVA sub-designs revealed that compared to HC, IBD patients reported smaller differences in their bodily deactivation between positive and negative emotions for the head, chest, arms, back, and abdomen (all ps < 0.03), resulting from a lower perceived deactivation during negative emotions (all ps ≤ 0.044) but not during positive emotions (all ps ≥ 0.890; see Figure 2). For body activation, the two groups differed significantly, with IBD patients reporting less perceived activation in the back and legs, independent of the emotional valence (2 × 2 ANOVA sub-design for activation: main effects “group”: all ps ≤ 0.014; group × valence: ps = 0.081). For further details, see Supplementary Figure 3.
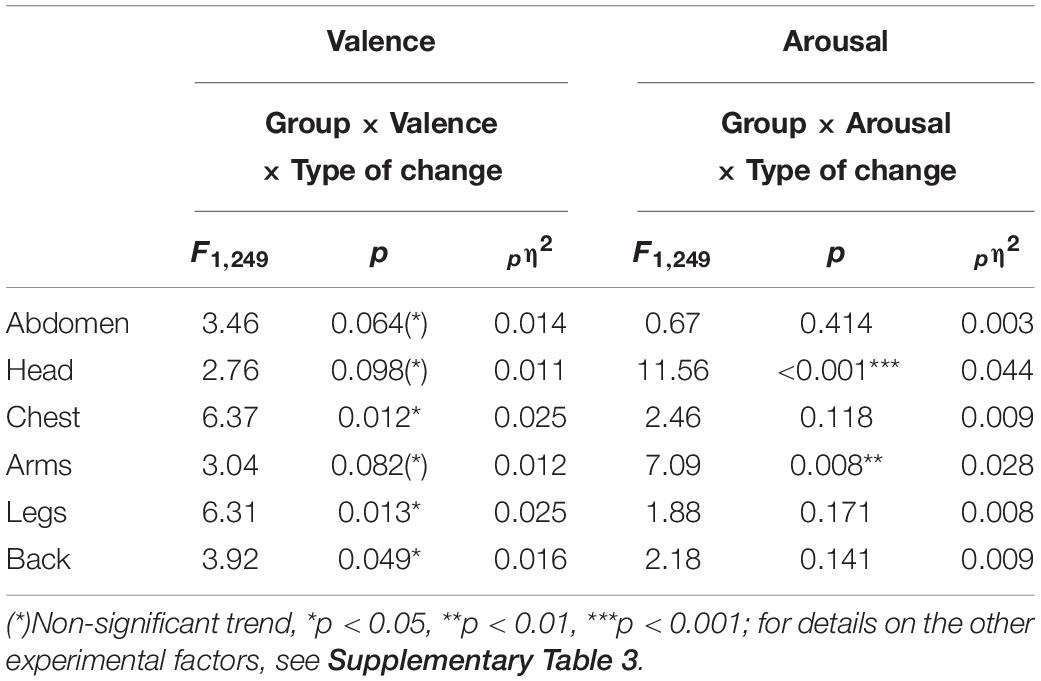
Table 3. Interaction effects between the factors “Group” (IBD/HC), “Valence” (positive/negative), “Arousal” (relaxed/tensed), and “Type of change” (activation/deactivation) for all predefined ROI.
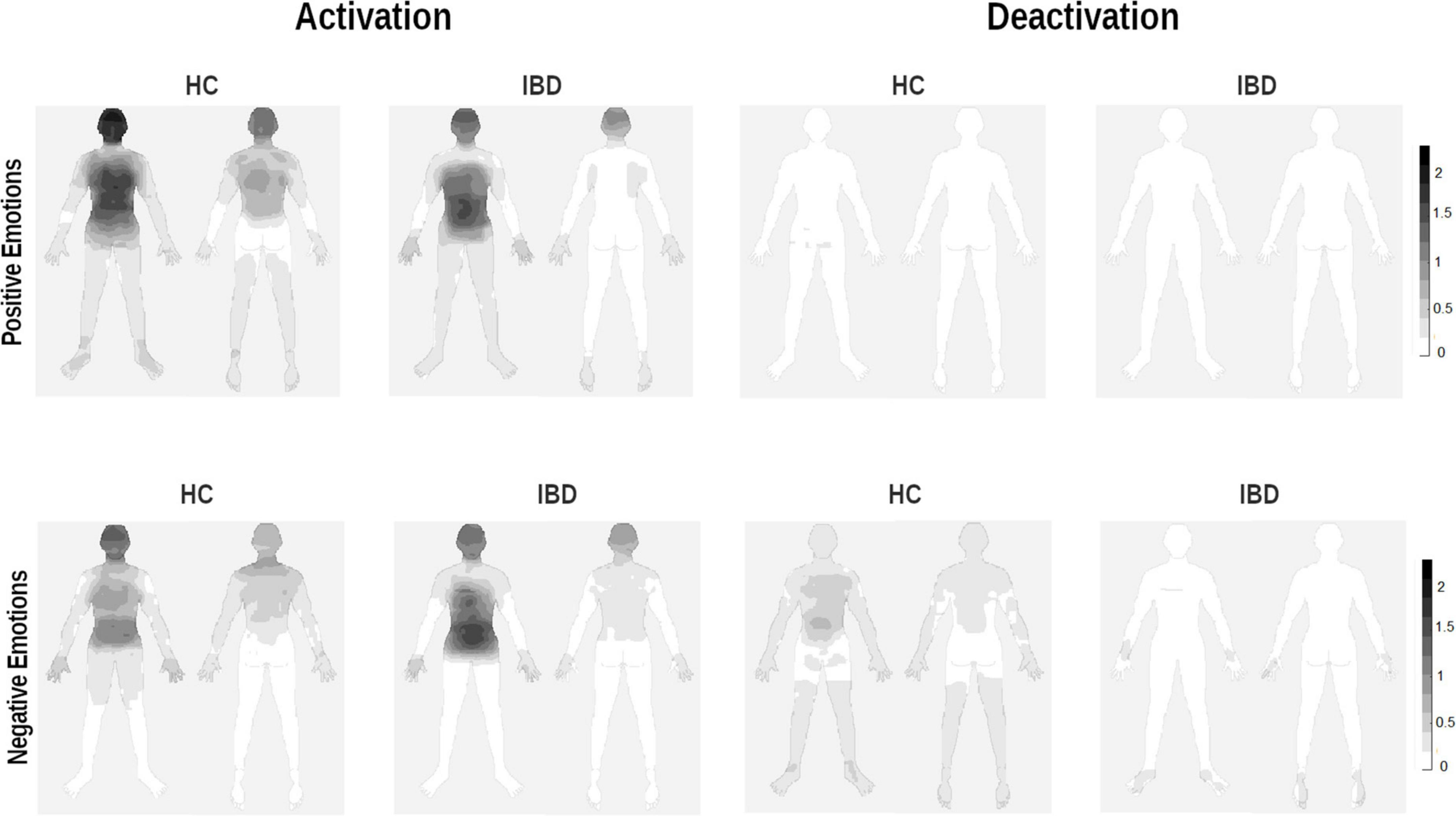
Figure 2. Whole-body topographies visualizing the perceived activation and deactivation for the experience of positive and negative emotions in HC participants and IBD patients (0 = “no activation/deactivation”; 4 = “very strong activation/deactivation”).
Arousal: Relaxation and Tension
Whole-Body Sensations
IBD patients reported less perceived change in their bodily sensations related to the experience of arousal (main effect “group”: F1, 83 = 10.20, p = 0.002, pη2 = 0.109) without a difference between the experiences of relaxation and tension (group × arousal: F1, 83 = 0.52, p = 0.474, pη2 = 0.006; main effect “arousal”: F1, 83 = 0.52, p = 0.474, pη2 = 0.006). The medians and data ranges for these conditions are shown in Figure 3. Extending the ANOVA design by the additional between-subjects factor “sex” revealed no influence of the participants’ sex on the evaluation of bodily sensation related to arousal. For further details see Supplementary Table 6.
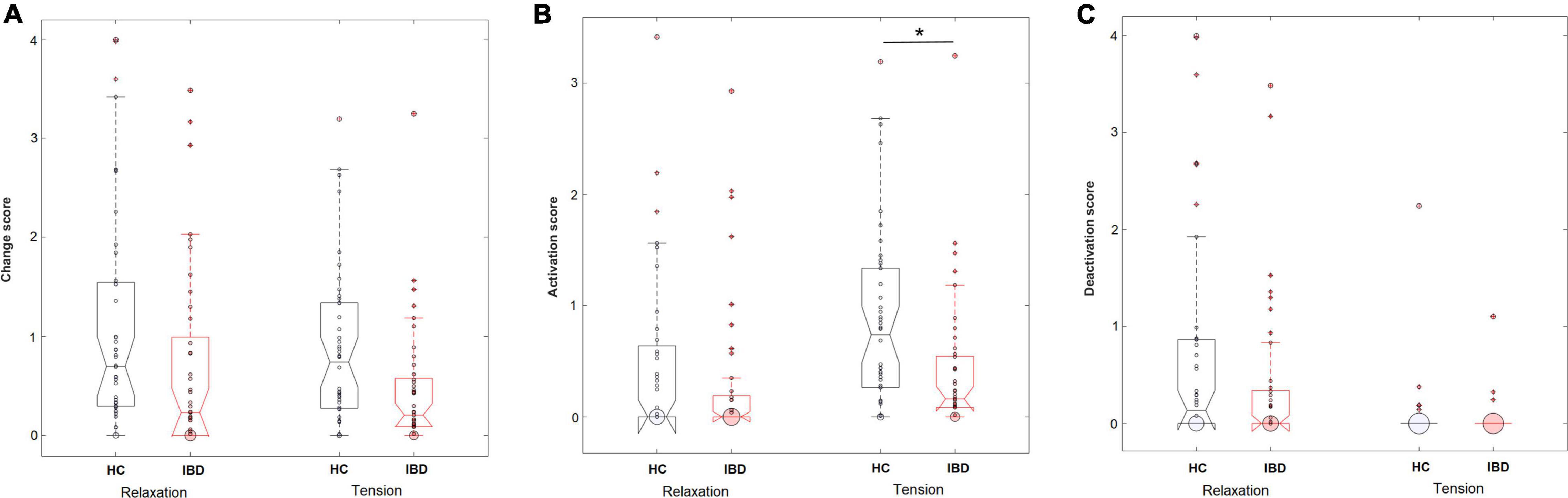
Figure 3. Perceived levels of overall changes in the body (A), sensations of activation (B), and sensations of deactivation (C) in the body during the experience of relaxation and tension in HC (n = 44) and IBD (n = 41). *p < 0.05.
When separating the effects of bodily activation and deactivation, analyses showed a significant effect of the type of change on the group difference for relaxation and tension (group × arousal × type of change: F1, 249 = 7.06, p = 0.008, pη2 = 0.028). 2 × 2 ANOVA sub-designs revealed a significant group difference for the perceived bodily deactivation, depending on whether participants evaluated relaxation or tension (group × arousal: F1, 83 = 6.05, p = 0.016, pη2 = 0.068). Compared to HC, IBD patients reported a smaller difference in the experienced bodily deactivation during relaxation and tension (p = 0.001). For bodily activation, the two groups differed significantly depending on whether relaxation or tension was evaluated (2 × 2 ANOVA sub-design for activation: group × arousal: F1, 83 = 9.39, p = 0.003, pη2 = 0.102): Compared to HC, the IBD group exhibited a smaller difference in their experienced bodily activation for relaxation and tension, resulting from a significantly lower perceived activation during tension (p = 0.002; Figure 4) but not during relaxation (p = 0.152). For further details, see Table 2.
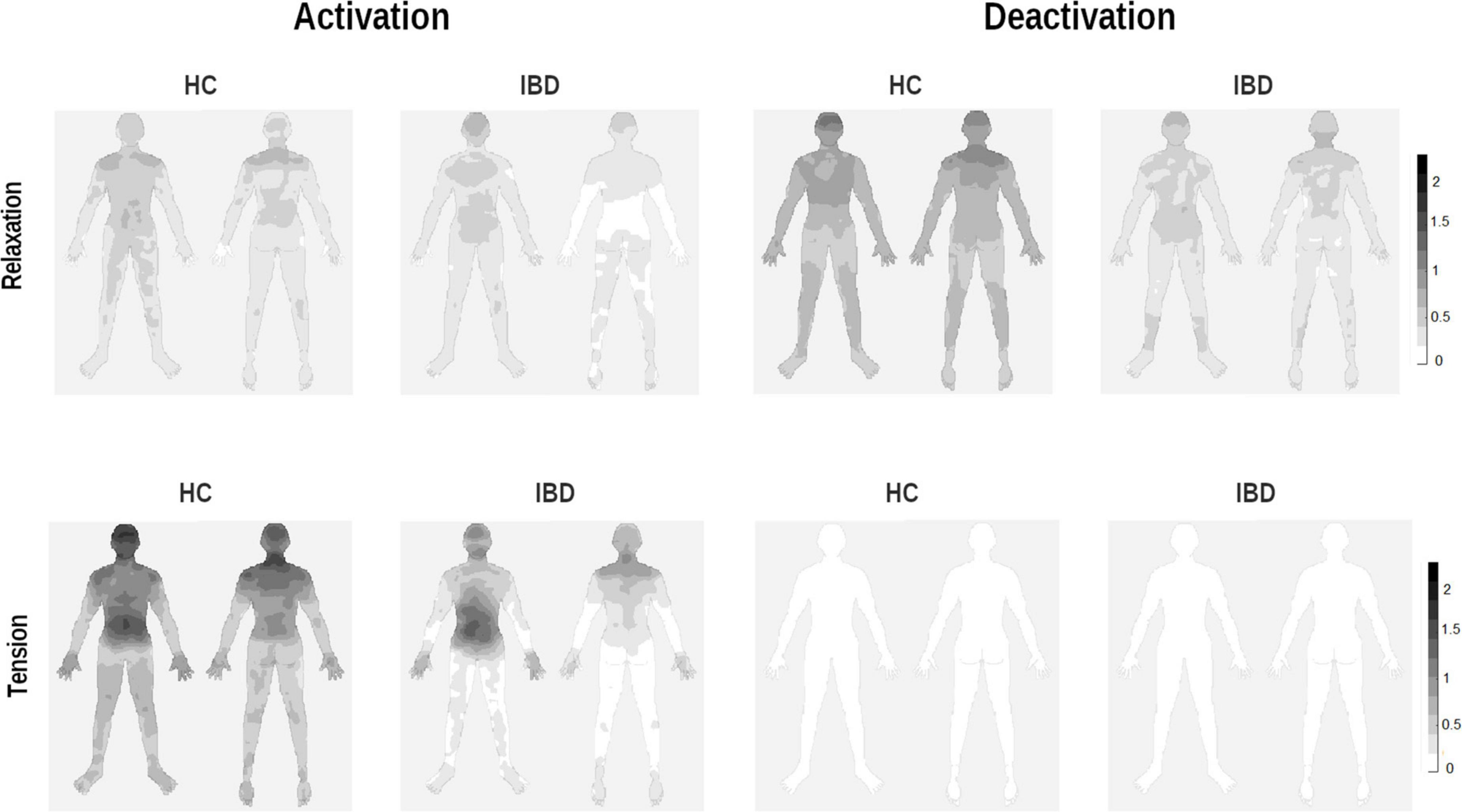
Figure 4. Whole-body topographies visualizing perceived activation and deactivation for the experience of relaxation and tension in HC participants and IBD patients (0 = “no activation/deactivation”; 4 = “very strong activation/deactivation”).
Exploratory analyses including the additional factor “sex” revealed that differences between the groups depending on arousal and the type of change were influenced by the participant’s sex (group × sex × arousal × type of change interaction effect: F1, 243 = 12.41, p < 0.001, pη2 = 0.049). Decomposing the design into two separate 2 × 2 × 2 ANOVA sub-designs for activation and deactivation scores revealed significant 3-way interactions in both analyses (group × sex × arousal: bodily activation: F1, 81 = 6.08, p = 0.016, pη2 = 0.069; bodily deactivation: F1, 81 = 12.37, p < 0.001, pη2 = 0.133) which, however, suggest a differential pattern of influence of participant’s sex on the reported bodily activation and deactivation scores. For further details see Supplementary Table 7.
Differences in the reported bodily activation were caused by lower scores for the experience of tension among male IBD patients compared to male healthy control participants (p = 0.002; Supplementary Figure 6), without differences for females with and without IBD (p = 0.713). For bodily deactivation, male patients with IBD reported significantly lower deactivation scores for the experience of relaxation compared to healthy male participants (p = 0.046; Supplementary Figure 7), while no differences were found for the experience of tension (p = 0.408). Females did not differ in the reported deactivation scores for relaxation or tension (ps > 0.812).
Exploratory Regions of Interest Analyses
Inflammatory bowel diseases patients reported less perceived change in their bodily sensations across all ROIs (all main effects “group”: ps < 0.044), except for the abdomen (p = 0.987; see Supplementary Table 2).
For the head and the arms, the statistical analysis results showed that the differences between the two groups were influenced by whether the participants judged changes to be linked to relaxation or tension and activation or deactivation (group × arousal × type of change, see Table 3). 2 × 2 ANOVA sub-designs showed that the group difference in bodily deactivation and activation was influenced by whether relaxation or tension was evaluated (group × arousal for deactivation: ps ≤ 0.003; activation: ps ≤ 0.011). Compared to HC, IBD patients exhibited a significantly smaller difference in their perceived bodily deactivation during relaxation and tension (all ps ≤ 0.005), caused by less perceived deactivation for relaxation in the head and arms in IBD (ps ≤ 0.097; see Figure 4). For bodily activation, the difference between relaxation and tension was significantly smaller in the IBD group than in the HC group (ps ≤ 0.001): IBD patients reported significantly lower levels of activation during tension than those in the HC group (ps ≤ 0.009). For further details, see Supplementary Figure 4.
Correlations Between Emotion-Associated Bodily Sensations and Interoceptive Sensibility or Gastrointestinal-Specific Anxiety
Emotional Awareness
While changes in bodily sensations for valence and arousal were stronger in participants reporting higher levels of emotional awareness, there were no differences between the groups in these relationships. For further details, see Supplementary Table 4.
Valence
In the differentiation between bodily activation and deactivation, higher emotional awareness was linked to greater bodily activation for the experience of positive emotions only in the IBD group (IBD: rs = 0.462, p = 0.002, pFDR = 0.004; HC: rs = 0.116, p = 0.454, pFDR = 0.454, comparison between groups: Z = 1.702, p = 0.044). In contrast, higher emotional awareness was linked to stronger bodily activations during negative emotions to a comparable extent in both groups (IBD: rs = 0.381, p = 0.014, pFDR = 0.028; HC: rs = 0.304, p = 0.045, pFDR = 0.090, comparison between groups Z = 0.388, p = 0.349).
Arousal
Higher emotional awareness was associated with higher levels of bodily activation during tension in the IBD group at trend-level significance. The two groups did not differ in the strength of this association (IBD: rs = 0.339, p = 0.030, pFDR = 0.060; HC: rs = 0.284, p = 0.062, pFDR = 0.124; Z = 0.271, p = 0.393). No significant associations were found for relaxation (IBD: rs = 0.188, p = 0.238, pFDR = 0.238; HC: rs = 0.271, p = 0.076, pFDR = 0.152; comparison between groups: Z = −0.389, p = 0.348).
There were no significant associations between emotional awareness and perceived bodily deactivation for either valence or arousal for either group (all pFDR ≥ 0.426).
Gastrointestinal-Specific Anxiety
For the IBD group, the correlation analysis results revealed no significant associations between GSA and patients’ perceived bodily sensations related to the experience of valence and arousal (all pFDR ≥ 0.252).
Anxiety and Depression Symptoms
Although all IBD patients met the criteria for clinical remission, they reported higher levels of anxiety and—at least on a descriptive level—depression than the HC participants (see Table 1). Therefore, we performed additional correlation analyses to explore whether symptoms of anxiety and depression are related to the experience of valence and arousal in the body. In the IBD group, greater anxiety and depression were associated with higher bodily activation during negative emotions (all ps < 0.001) and tension (all ps ≤ 0.011) and higher levels of deactivation when feeling relaxed (all ps ≤ 0.019). For further details, see Supplementary Table 5.
Discussion
How bodily sensations are perceived and evaluated in the context of emotional experiences is essential to the way people perceive emotions in everyday life and—in the case of IBD—ultimately how these influence patient’s psychological wellbeing. Since studies on emotional experience in IBD are sparse, the main objective of this study was to investigate the potential alterations in emotion-related bodily sensations and their link to interoceptive sensibility and gastrointestinal-specific anxiety (GSA) in remitted IBD patients compared with healthy individuals. Based on the dimensional model of emotion (14), we investigated how the two dimensions of valence and arousal are experienced in an embodied way by examining perceived bodily activation and deactivation during positive and negative emotions, relaxation, and tension.
Compared to healthy individuals, patients with IBD reported less intense bodily changes associated with the experience of valence and arousal. Moreover, IBD patients varied differently in their perceived sensations of the opposite poles of these dimensions, that is, positive and negative emotions for valence and relaxation and tension for arousal, respectively. Our findings support the need for fine-grained analysis to capture the complex changes in emotion-related bodily sensations, since group differences depend on whether participants evaluate perceived activation or deactivation in different areas of the body. For IBD patients, the patterns of bodily activation and deactivation reported for positive and negative emotions, as well as for relaxation and tension, were more similar than these observed among healthy individuals. The reduced bodily activation during negative and highly arousing emotional states reported for the IBD group might suggest less intense and thereby less distressing emotional experience. However, lower levels of activation linked to positive emotions and decreased deactivation during relaxation might point to detrimental alterations in these domains of emotional experience, which might be of particular importance for patients’ wellbeing. Our results of diminished emotion-related bodily sensations in IBD indicate a more detached experience of emotions, suggesting an emotional avoidance mechanism in this patient group. While patients might perceive this strategy as helpful to avoid a confrontation with overwhelming feelings and stress (33), emotional avoidance may contribute to the maintenance of anxiety and depressive symptoms in the long term (34–36). Finally, exploratory analyses suggest that differences between patients with IBD and healthy control participants differ between men and women, particularly when evaluating bodily sensations related to the experience of arousal with stronger effects in men compared to women.
Experience of Valence in the Body
Overall, IBD patients exhibited significantly less perceived change in their emotion-related bodily sensations. Despite the higher levels of subjectively perceived emotional awareness observed in the IBD group, when asked to identify changes in their bodies, patients reported less intense experiences of bodily activation and deactivation compared to healthy individuals. This finding may indicate that patients with IBD are less able to differentiate between the bodily sensations linked to specific emotion experiences. In line with this interpretation, several studies have demonstrated higher levels of alexithymia in IBD, that is the difficulty to name and differentiate between different emotions (37). Moreover, recent evidence have indicated impairments in patients’ ability to recognize their own feelings and those of others (38). Taken together, these findings suggest that IBD symptoms detrimentally affect patients’ capacity to discriminate between different emotions based on their bodily sensations.
We observed decreased body deactivation during negative emotional states in IBD for several body areas, including the one most affected by IBD symptoms, that is, the abdomen, as well as other less directly disease-related body regions. Thus, altered experience of negative emotions in the body is not limited only to body regions associated with aberrant physiological feedback in IBD, such as gastric sensations, for example, as initially expected. In contrast to Vianna et al. (11), who suggested that emotional dysfunctions occur only for acute IBD patients, we found differences in how emotions are experienced among remitted IBD patients. More precisely, our findings of less activation during positive emotional states suggest decreased sensitivity to positive emotional content among patients with IBD. Previous studies have suggested functional alterations in brain regions associated with the processing of affective information (10) and visceral signals in IBD patients (39, 40). Therefore, impaired integration of affective and visceral signals in IBD patients could result in less intense experience of positive emotions, leading to more depressive symptoms (41), which can in turn affect the course of the disease (9). As these findings indicate persistent alterations in the experience of positive emotions, even in the remitted stage of the disorder, one might speculate whether it might be useful to coach patients to notice sensations of activation in the body specifically related to positive emotions. Mind-body interventions, such as mindfulness-based stress reduction (MBSR) (42), emphasize the focus on bodily sensations and the appraisal of those sensations as crucial to an individual’s coping with stress and emotions (23, 43, 44). Such interventions are believed to enhance an individual’s sensitivity to body-related emotional information and to encourage the implementation of skillful strategies for coping with difficult emotions. In a segment of IBD patients, that is, those who exhibit difficulties in coping with emotional stress, short interoceptively focused interventions might be useful by coaching patients to localize changes in their visceral sensations and learn to perceive emotion-specific changes in their bodily sensations, focusing on particular body areas (45, 46). By learning to accept emotions and the corresponding physiological reactions of the body, patients could learn to observe these sensations without immediately feeling overwhelmed and trying to avoid these using distraction strategies [for a review of mind-body interventions in IBD see: (47, 48)]. By modifying how patients attend to the interoceptive signals elicited by emotional events and providing them with adaptive emotion regulation skills, such interventions can improve patients’ flexibility in emotional experience and prevent an overgeneralization of avoidance strategies of disease related bodily sensations to those associated with emotions (49, 50). Given the bidirectional relationship between emotion perception and psychopathological symptoms, it should be noted that as our results are only of correlational nature, these clinical implications are only speculative and need further investigation. Finally, exploratory analyses indicated that male patients with IBD reported less perceived bodily deactivation compared to male healthy individuals independently whether they judged bodily sensations associated with positive or negative valence. While this differential effect did not restrict the findings observed for the sensations of positive and negative valence between individuals with and without IBD, it emphasizes the need to take the participant’s sex into account when studying alterations in bodily sensations in IBD.
Experience of Arousal in the Body
With respect to arousal, IBD patients perceived less bodily activation during tension than HC, while noticing smaller differences in deactivation experienced during relaxation and tension. In contrast to valence, this pattern was reported only for body areas not directly related to IBD symptomatology. Despite patients’ lower levels of bodily activation when feeling tense, our results indicate that patients with IBD perceive less bodily deactivation during relaxation and therefore less successfully reduce perceived stress and tension (51, 52). Hence, IBD patients may benefit from practicing relaxation techniques, such as autogenic training or progressive muscle relaxation, in order to better differentiate between bodily sensations caused by tension and relaxation (51, 53). Previous studies have shown the positive effects of such interventions on stress reduction in psychosomatic disorders, suggesting potential benefits for IBD patients’ mental and physical wellbeing (54).
Our findings of significantly less intense perceived bodily sensations associated with valence and arousal may indicate the use of emotional avoidance as an emotion regulation strategy among IBD patients. A more detached bodily experience of emotions may allow patients to avoid confronting overly intense physiological sensations and thereby reduce psychological distress. Consistent with this interpretation, a recent study by Banovic et al. (55) found stronger emotional avoidance and emotional suppression among Crohn’s disease patients than among healthy controls (55). Moreover, previous studies on attachment and mentalizing in IBD revealed greater avoidance tendencies in this clinical population (38, 56). Yet, an avoidant attachment style has been associated with cognitive distancing from emotions and our findings suggest a detachment from the corresponding emotion-related bodily sensations as well. These defensive strategies, however, might further impede patients’ mentalization abilities, leading to interpersonal problems (38). However, another explanation for our results could be the use of distraction as a regulatory strategy. Contemporary findings point to the benefits of different emotion regulation mechanisms depending on the intensity of the emotional state (57). While cognitive reappraisal is thought to be more adaptive in the case of low negative emotional intensities, distraction is preferable when individuals face emotions of high intensity (58). As high emotional intensity usually results in greater activation of the autonomic nervous system, it is conceivable that individuals who are more sensitive to these physiological changes may prefer to use distraction to cope with highly arousing emotions (49). Future studies should investigate whether altered processing of bodily sensations is linked to different emotion regulation strategies and whether these are differentially advantageous for coping with disease-related psychological distress and IBD symptoms. Such research might improve our understanding of the mechanisms underlying the interplay between emotion regulation and the experience of emotions in the body and thereby facilitate the development of personalized psychotherapeutic interventions for IBD. Finally, the patterns related to the experience of arousal differed between female and male participants. While females with and without IBD did not differ significantly, compared to healthy male participants, males with an IBD diagnosis exhibited decreased sensations of bodily deactivation for the experience of relaxation. On the other hand, male patients with IBD reported lower levels of bodily activation during tension, which suggests that patients might show a stronger detachment from their bodily sensations during stress, while experiencing greater difficulties to relax and thus, in coping with sustained stress-related sensations. While recent findings suggest that there are no clear sex differences in the subjective experience of emotions in the body (59) but rather only in the expression of those (60), our findings indicate that men with IBD show attenuated emotion-related sensations, suggesting a stronger emotional avoidance.
Correlations With Interoceptive Sensibility, Gastrointestinal-Specific Anxiety, and Psychological Distress
Inflammatory bowel diseases patients reporting higher levels of emotional awareness, that is, a feature of one’s interoceptive sensibility, exhibit increased bodily sensations during positive and negative emotions, as well as tension (61). The effects of interoception on emotional experience have been discussed repeatedly in the literature, suggesting that interoceptively aware individuals report higher emotional intensity and arousal (62, 63). IBD patients exhibiting higher emotional awareness also report higher levels of bodily activation during positive emotions. Although positive valence is associated with less perceived bodily activation in IBD patients, greater awareness of the connection between physiological sensations and emotions can positively affect the experience of positive emotions. One possible explanation for this effect might be that body- and emotion perception share common neural underpinnings (64), playing crucial roles in the integration of interoceptive and emotional cues (65, 66). Critchley et al. (67) demonstrated that representations of internal bodily sensations in the insula are crucial for the conscious experience of emotions. This suggests that somato-visceral information influences emotion perception in a bottom-up way, indicating that individuals who are more aware of their bodily signals exhibit higher emotional awareness and thus perceive more intense emotions. IBD has been associated with altered activation of the insula, characterized by hyperactivation during visceral pain and stress perception (68, 69). This enhanced insular reactivity might constitute the neural correlate of an increased perception of visceral sensations in IBD and a stronger experience of emotion-related sensations, particularly in those patients who report to be more aware of their body signals.
Our results did not reveal any associations between gastrointestinal-specific anxiety (GSA) and emotion experience in the body. In contrast to GSA, which solely reflects patients’ disease-related cognitions, more pronounced general anxiety and depression symptoms were linked to greater bodily activation during positive and negative emotions. This finding is in line with previous evidence that suggests that highly anxious individuals experience emotions in a more intense and threatening way (70). As acute disease activity is normally linked to higher levels of anxiety and depression, this result might indicate that among acute IBD patients, increased emotion-related bodily sensations linked to psychological distress might result in exaggerated emotion perception having detrimental effects on patients’ stress levels, at least for those patients with psychiatric co-morbidities (71).
Given these new findings regarding the role of bodily sensations related to the experience of valence and arousal in IBD, the implementation of interoceptively focused techniques to improve patients’ emotion experience through greater bodily awareness as a supplementary part of patients’ treatment might be of particular importance. Our results raise the possibility that psychological interventions aimed at the improvement of emotion perception in IBD could provide patients with useful skills for engaging with their emotions. As impairments in emotion perception have been shown to be part of the link between disease severity and depression, such supplementary interventions could improve patients’ abilities to cope with their disease symptoms and thus, potentially having positive effects on their psychological wellbeing and quality of life.
Limitations
Some limitations of the present study have to be addressed. First, in contrast to our previously preregistered matching procedure, more female than male healthy individuals were included in the HC group, resulting in an unequal sex distribution between both groups. Second, as we did not include a clinical control group (e.g., irritable bowel syndrome, IBS) and did not collect data on IBS symptoms in the IBD group, we cannot conclude that our findings are limited only to IBD or that they may represent a transdiagnostic feature across several gastrointestinal disorders or even across somatic and mental disorders. Further, we did not directly induce any emotional states but rather asked participants to indicate changes in their bodily sensations based on what they typically perceive when experiencing the presented emotional state. Thus, the participants’ judgments resulted from their recollections of bodily sensations during emotional states in the past. This recall process might be biased by one’s current mood or by recalling only recent situations. Therefore, future studies need to control for these effects by asking participants to indicate their bodily sensations during direct emotion induction. Furthermore, interoception-related features were assessed only via self-report measures, which often do not correspond to participant’s actual interoceptive abilities (72, 73). Moreover, no data on perceived stress and quality of life was collected and thus, future studies are needed to examine the complex interplay of emotion and stress perception as well as their links to quality of life in IBD. Finally, we cannot draw conclusions regarding the direction of causality of any of the observed associations because of the cross-sectional design of the study.
Conclusion
The results of this study indicate that IBD is associated with emotional alterations, characterized by attenuated experiences of emotion-related bodily sensations. The observed stronger detachment from one’s bodily sensations might be a regulatory mechanism emerging from the individual’s attempt to avoid distressing bodily sensations, associated with IBD symptoms and pain, which affects the perception and evaluation of emotion-related bodily sensations due to overgeneralization. The links between emotion-related bodily sensations and anxiety and depressive symptoms suggest that altered emotion perception might contribute to the development and maintenance of psychopathological symptoms during the disease course. However, further studies are needed to investigate whether there is indeed a causal relationship between these domains of functioning and how these affect patients’ quality of life.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Medical Faculty Mannheim, Heidelberg University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KA, WR, and SL designed the study and wrote the protocol. KA, TL, and WR recruited the sample. KA performed the data collection, conducted all statistical analyses, and wrote the first draft of the manuscript. SL, WR, TL, RB-B, and NK provided substantive and conceptual feedback on all drafts. All authors contributed to and have approved the final manuscript.
Funding
This study was supported and funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—GRK2350/1–324164820.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Franziska Unterseher and Paulina Watermann for their help during the recruitment of the patients and data collection. We also want to thank all patients and healthy controls who took part in the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.833423/full#supplementary-material
Footnotes
References
1. Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses—part I. Inflamm Bowel Dis. (2018) 24:742–51. doi: 10.1093/ibd/izx100
2. Bannaga AS, Selinger CP. Inflammatory bowel disease and anxiety: links, risks, and challenges faced. Clin Exp Gastroenterol. (2015) 8:111–7. doi: 10.2147/CEG.S57982
3. Araki M, Shinzaki S, Yamada T, Arimitsu S, Komori M, Shibukawa N, et al. Psychologic stress and disease activity in patients with inflammatory bowel disease: a multicenter cross-sectional study. PLoS One. (2020) 15:e0233365. doi: 10.1371/journal.pone.0233365
4. Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. (2005) 54:1481–91. doi: 10.1136/gut.2005.064261
5. Bevers K, Watts L, Kishino ND, Gatchel RJ. The biopsychosocial model of the assessment, prevention, and treatment of chronic pain. US Neurol. (2016) 12:98–104.
6. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. (2002) 3:655–66. doi: 10.1038/nrn894
7. Chen WG, Schloesser D, Arensdorf AM, Simmons JM, Cui C, Valentino R, et al. The emerging science of interoception: sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. (2021) 44:3–16. doi: 10.1016/j.tins.2020.10.007
8. Kano M, Muratsubaki T, Yagihashi M, Morishita J, Mugikura S, Dupont P, et al. Insula activity to visceral stimulation and endocrine stress responses as associated with alexithymia in patients with irritable bowel syndrome. Psychosom Med. (2020) 82:29–38. doi: 10.1097/PSY.0000000000000729
9. Wilkinson B, Trick L, Knight A, Valton V, Goodhand J, Kennedy NA, et al. Factors associated with depression in people with inflammatory bowel disease: the relationship between active disease and biases in neurocognitive processing. Neurogastroenterol Motil. (2019) 31:e13647. doi: 10.1111/nmo.13647
10. Agostini A, Filippini N, Cevolani D, Agati R, Leoni C, Tambasco R, et al. Brain functional changes in patients with ulcerative colitis: a functional magnetic resonance imaging study on emotional processing. Inflamm Bowel Dis. (2011) 17:1769–77. doi: 10.1002/ibd.21549
11. Vianna EP, Weinstock J, Elliott D, Summers R, Tranel D. Increased feelings with increased body signals. Soc Cogn Affect Neurosci. (2006) 1:37–48. doi: 10.1093/scan/nsl005
12. Mayer EA, Naliboff BD, Craig AB. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. (2006) 131:1925–42. doi: 10.1053/j.gastro.2006.10.026
13. Agostini A, Filippini N, Benuzzi F, Bertani A, Scarcelli A, Leoni C, et al. Functional magnetic resonance imaging study reveals differences in the habituation to psychological stress in patients with crohn’s disease versus healthy controls. J Behav Med. (2013) 36:477–87. doi: 10.1007/s10865-012-9441-1
14. Russell JA. A circumplex model of affect. J Pers Soc Psychol. (1980) 39:1161. doi: 10.1037/h0077714
15. Barrett L. Discrete emotions or dimensions? The role of valence focus and arousal focus. Cogn Emot. (1998) 12:579–99. doi: 10.1080/026999398379574
16. Lang PJ, Bradley MM. Appetitive and defensive motivation: goal-directed or goal-determined? Emot Rev. (2013) 5:230–4. doi: 10.1177/1754073913477511
17. Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Hum Brain Mapp. (2007) 28:9–18. doi: 10.1002/hbm.20258
18. Wiens S. Interoception in emotional experience. Curr Opin Neurol. (2005) 18:442–7. doi: 10.1097/01.wco.0000168079.92106.99
19. Nummenmaa L, Glerean E, Hari R, Hietanen JK. Bodily maps of emotions. Proc Natl Acad Sci USA. (2014) 111:646–51.
20. Ruben J, Schwiemann J, Deuchert M, Meyer R, Krause T, Curio G, et al. Somatotopic organization of human secondary somatosensory cortex. Cereb Cortex. (2001) 11:463–73. doi: 10.1093/cercor/11.5.463
21. Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The multidimensional assessment of interoceptive awareness (MAIA). PLoS One. (2012) 7:e48230. doi: 10.1371/journal.pone.0048230
22. Atanasova K, Lotter T, Reindl W, Lis S. Multidimensional assessment of interoceptive abilities, emotion processing and the role of early life stress in inflammatory bowel diseases. Front Psychiatry. (2021) 12:680878. doi: 10.3389/fpsyt.2021.680878
23. Garland EL, Gaylord SA, Palsson O, Faurot K, Douglas Mann J, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med. (2012) 35:591–602. doi: 10.1007/s10865-011-9391-z
25. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N Engl J Med. (1987) 317:1625–9. doi: 10.1056/NEJM198712243172603
26. Franke GH, Ankerhold A, Haase M, Jäger S, Tögel C, Ulrich C, et al. [The usefulness of the brief symptom inventory 18 (BSI-18) in psychotherapeutic patients]. Psychother Psychosom Med Psychol. (2011) 61:82–6. doi: 10.1055/s-0030-1270518
27. Spielberger C, Goruch R, Lushene R, Vagg P, Jacobs G. Manual for the State-Trait Inventory STAI (form Y). Palo Alto, CA: Mind Garden (1983).
28. Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. (1994) 25:49–59. doi: 10.1016/0005-7916(94)90063-9
29. Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, et al. The visceral sensitivity index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. (2004) 20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x
30. Wobbrock JO, Findlater L, Gergle D, Higgins JJ. The aligned rank transform for nonparametric factorial analyses using only anova procedures. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems: Association for Computing Machinery. Vancouver, BC: (2011). p. 143–6.
31. Kay M, Wobbrock J. ARTool: R package for the Aligned Rank Transform for Nonparametric Factorial ANOVAs. (2019).
32. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B (Methodological). (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
33. Sheppes G, Scheibe S, Suri G, Gross JJ. Emotion-regulation choice. Psychol Sci. (2011) 22:1391–6.
34. Bardeen JR, Tull MT, Stevens EN, Gratz KL. Exploring the relationship between positive and negative emotional avoidance and anxiety symptom severity: the moderating role of attentional control. J Behav Ther Exp Psychiatry. (2014) 45:415–20. doi: 10.1016/j.jbtep.2014.04.006
35. Kelly MM, Forsyth JP. Associations between emotional avoidance, anxiety sensitivity, and reactions to an observational fear challenge procedure. Behav Res Ther. (2009) 47:331–8. doi: 10.1016/j.brat.2009.01.008
36. Hayes SC, Wilson KG, Gifford EV, Follette VM, Strosahl K. Experiential avoidance and behavioral disorders: a functional dimensional approach to diagnosis and treatment. J Consult Clin Psychol. (1996) 64:1152. doi: 10.1037/0022-006x.64.6.1152
37. Martino G, Caputo A, Schwarz P, Bellone F, Fries W, Quattropani MC, et al. Alexithymia and inflammatory bowel disease: a systematic review. Front Psychol. (2020) 11:1763. doi: 10.3389/fpsyg.2020.01763
38. Agostini A, Scaioli E, Belluzzi A, Campieri M. Attachment and mentalizing abilities in patients with inflammatory bowel disease. Gastroenterol Res Pract. (2019) 2019:7847123. doi: 10.1155/2019/7847123
39. Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. (2005) 115:398–409. doi: 10.1016/j.pain.2005.03.023
40. Fan Y, Bao C, Wei Y, Wu J, Zhao Y, Zeng X, et al. Altered functional connectivity of the amygdala in crohn’s disease. Brain Imaging Behav. (2020) 14:2097–106. doi: 10.1007/s11682-019-00159-8
41. Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. (2006) 19:34–9. doi: 10.1097/01.yco.0000191500.46411.00
43. Lutz J, Herwig U, Opialla S, Hittmeyer A, Jäncke L, Rufer M, et al. Mindfulness and emotion regulation—an fMRI study. Social Cognitive and Affective Neuroscience. (2013) 9:776–85. doi: 10.1093/scan/nst043
44. de Jong M, Lazar SW, Hug K, Mehling WE, Hölzel BK, Sack AT, et al. Effects of mindfulness-based cognitive therapy on body awareness in patients with chronic pain and comorbid depression. Front Psychol. (2016) 7:967. doi: 10.3389/fpsyg.2016.00967
45. Davey S, Bell E, Halberstadt J, Collings S. Where is an emotion? Using targeted visceroception as a method of improving emotion regulation in healthy participants to inform suicide prevention initiatives: a randomised controlled trial. Trials. (2020) 21:642. doi: 10.1186/s13063-020-04479-9
46. Fischer D, Messner M, Pollatos O. Improvement of interoceptive processes after an 8-week body scan intervention. Front Hum Neurosci. (2017) 11:452. doi: 10.3389/fnhum.2017.00452
47. Ewais T, Begun J, Kenny M, Rickett K, Hay K, Ajilchi B, et al. A systematic review and meta-analysis of mindfulness based interventions and yoga in inflammatory bowel disease. J Psychosom Res. (2019) 116:44–53. doi: 10.1016/j.jpsychores.2018.11.010
48. Wilke E, Reindl W, Thomann PA, Ebert MP, Wuestenberg T, Thomann AK. Effects of yoga in inflammatory bowel diseases and on frequent IBD-associated extraintestinal symptoms like fatigue and depression. Complement Ther Clin Pract. (2021) 45:101465. doi: 10.1016/j.ctcp.2021.101465
49. Ardi Z, Golland Y, Shafir R, Sheppes G, Levit-Binnun N. The effects of mindfulness-based stress reduction on the association between autonomic interoceptive signals and emotion regulation selection. Psychosom Med. (2021) 83:852–62. doi: 10.1097/PSY.0000000000000994
50. Wynne B, McHugh L, Gao W, Keegan D, Byrne K, Rowan C, et al. Acceptance and commitment therapy reduces psychological stress in patients with inflammatory bowel diseases. Gastroenterology. (2019) 156:935.e–45.e.
51. Jacobson E. Progressive Relaxation: A Physiological and Clinical Investigation of Muscular States and Their Significance in Psychology and Medical Practice. Chicago: University of Chicago Press (1938).
52. Pawlow LA, Jones GE. The impact of abbreviated progressive muscle relaxation on salivary cortisol. Biol Psychol. (2002) 60:1–16. doi: 10.1016/s0301-0511(02)00010-8
53. Schultz JH, Luthe W. Autogenic Training: A Psychophysiologic Approach to Psychotherapy. New York, NY: Grune & Stratton (1959).
54. Shah K, Ramos-Garcia M, Bhavsar J, Lehrer P. Mind-body treatments of irritable bowel syndrome symptoms: an updated meta-analysis. Behav Res Ther. (2020) 128:103462. doi: 10.1016/j.brat.2019.103462
55. Banovic I, Montreuil L, Derrey-Bunel M, Scrima F, Savoye G, Beaugerie L, et al. Toward further understanding of crohn’s disease-related fatigue: the role of depression and emotional processing. Front Psychol (2020) 11:703. doi: 10.3389/fpsyg.2020.00703
56. Agostini A, Rizzello F, Ravegnani G, Gionchetti P, Tambasco R, Straforini G, et al. Adult attachment and early parental experiences in patients with crohn’s disease. Psychosomatics. (2010) 51:208–15. doi: 10.1176/appi.psy.51.3.208
57. Sheppes G, Scheibe S, Suri G, Radu P, Blechert J, Gross JJ. Emotion regulation choice: a conceptual framework and supporting evidence. J Exp Psychol Gen. (2014) 143:163. doi: 10.1037/a0030831
58. Sheppes G. Chapter Four - transcending the “good & bad” and “here & now” in emotion regulation: costs and benefits of strategies across regulatory stages. In: B Gawronski editor. Advances in Experimental Social Psychology. (Vol. 61), Cambridge: Academic Press (2020). p. 185–236.
59. Volynets S, Glerean E, Hietanen JK, Hari R, Nummenmaa L. Bodily maps of emotions are culturally universal. Emotion. (2020) 20:1127. doi: 10.1037/emo0000624
60. Kret ME, De Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. (2012) 50:1211–21. doi: 10.1016/j.neuropsychologia.2011.12.022
61. Schuette SA, Zucker NL, Smoski MJ. Do interoceptive accuracy and interoceptive sensibility predict emotion regulation? Psychol Res. (2020) 85:1894–08. doi: 10.1007/s00426-020-01369-2
62. Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. Interoceptive sensitivity and self-reports of emotional experience. J Pers Soc Psychol. (2004) 87:684–97. doi: 10.1037/0022-3514.87.5.684
63. Herbert BM, Pollatos O, Schandry R. Interoceptive sensitivity and emotion processing: an EEG study. Int J Psychophysiol. (2007) 65:214–27. doi: 10.1016/j.ijpsycho.2007.04.007
64. Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. (2012) 62:493–9. doi: 10.1016/j.neuroimage.2012.05.012
65. Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. (2013) 77:624–38. doi: 10.1016/j.neuron.2013.02.008
66. Berntson GG, Khalsa SS. Neural circuits of interoception. Trends Neurosci. (2021) 44:17–28. doi: 10.1016/j.tins.2020.09.011
67. Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. (2004) 7:189–195. doi: 10.1038/nn1176
68. Rubio A, Pellissier S, Van Oudenhove L, Ly HG, Dupont P, Tack J, et al. Brain responses to uncertainty about upcoming rectal discomfort in quiescent crohn’s disease–a fMRI study. Neurogastroenterol Motil. (2016) 28:1419–32. doi: 10.1111/nmo.12844
69. Agostini A, Ballotta D, Righi S, Moretti M, Bertani A, Scarcelli A, et al. Stress and brain functional changes in patients with crohn’s disease: a functional magnetic resonance imaging study. Neurogastroenterol Motil. (2017) 29:1–10. doi: 10.1111/nmo.13108
70. Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. (2010) 214:451–63. doi: 10.1007/s00429-010-0258-9
71. Hu S, Chen Y, Chen Y, Wang C. Depression and anxiety disorders in patients with inflammatory bowel disease. Front Psychiatry. (2021) 12:714057. doi: 10.3389/fpsyt.2021.714057
72. Murphy J, Brewer R, Plans D, Khalsa SS, Catmur C, Bird G. Testing the independence of self-reported interoceptive accuracy and attention. Quart J Exp Psychol. (2020) 73:115–33. doi: 10.1177/1747021819879826
Keywords: inflammatory bowel disease, emotional awareness, bodily sensations, arousal, valence, emotion perception
Citation: Atanasova K, Lotter T, Bekrater-Bodmann R, Kleindienst N, Reindl W and Lis S (2022) Is It a Gut Feeling? Bodily Sensations Associated With the Experience of Valence and Arousal in Patients With Inflammatory Bowel Disease. Front. Psychiatry 13:833423. doi: 10.3389/fpsyt.2022.833423
Received: 11 December 2021; Accepted: 29 March 2022;
Published: 22 April 2022.
Edited by:
Christoph Pieh, Danube University Krems, AustriaReviewed by:
Alessandro Agostini, University of Bologna, ItalyRichard Gevirtz, Alliant International University, United States
Copyright © 2022 Atanasova, Lotter, Bekrater-Bodmann, Kleindienst, Reindl and Lis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantina Atanasova, S29uc3RhbnRpbmEuQXRhbmFzb3ZhQHppLW1hbm5oZWltLmRl
†These authors have contributed equally to this work
 Konstantina Atanasova
Konstantina Atanasova Tobias Lotter
Tobias Lotter Robin Bekrater-Bodmann
Robin Bekrater-Bodmann Nikolaus Kleindienst
Nikolaus Kleindienst Wolfgang Reindl
Wolfgang Reindl Stefanie Lis
Stefanie Lis