
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry , 22 April 2022
Sec. Molecular Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.828476
This article is part of the Research Topic Neurobiological Underpinnings of Cognitive Impairment and Pharmacological Treatments View all 6 articles
Uric acid is commonly known for its bad reputation. However, it has been shown that uric acid may be actively involved in neurotoxicity and/or neuroprotection. These effects could be caused by oxidative stress or inflammatory processes localized in the central nervous system, but also by other somatic diseases or systemic conditions. Our interest was to summarize and link the current data on the possible role of uric acid in cognitive functioning. We also focused on the two putative molecular mechanisms related to the pathological effects of uric acid—oxidative stress and inflammatory processes. The hippocampus is a prominent anatomic localization included in expressing uric acid's potential impact on cognitive functioning. In neurodegenerative and mental disorders, uric acid could be involved in a variety of ways in etiopathogenesis and clinical presentation. Hyperuricemia is non-specifically observed more frequently in the general population and after various somatic illnesses. There is increasing evidence to support the hypothesis that hyperuricemia may be beneficial for cognitive functioning because of its antioxidant effects but may also be a potential risk factor for cognitive dysfunction, in part because of increased inflammatory activity. In this context, gender specificities must also be considered.
Uric acid (UA) is the final oxidation product of adenine and guanine metabolism (1). It is formed from these exogenous purines and endogenously from damaged, dying and dead cells (2). In humans, an enzyme called urate oxidase loses its functional activity so that further oxidation of UA is no longer possible. Consequently, humans must cope with much higher levels of UA compared to other mammals (3). UA is generally known for its bad reputation. The fact that about 90% of UA filtered in renal glomeruli is reabsorbed and that humans maintain high levels of UA raised the idea that UA should be considered not only as a metabolic waste but also as a molecule with important physiological activity. The beneficial effects of UA were proposed by Kellog and Fridovich (4) and further explored and developed by Ames et al. (5) three decades ago. In vitro experiments showed that UA is a potent scavenger of singlet oxygen, peroxyl radicals (RO), and hydroxyl radicals (5). It also protects the cell from oxidative damage by chelating metal ions (6) and acting as a specific inhibitor of radicals generated by the decomposition of peroxynitrite (ONOO−) (7). Because of these effects, UA is considered a very potent free radical scavenger, accounting for more than half of the antioxidant capacity of plasma (8). On the other hand, many epidemiological and experimental data show the oxidative potential of UA. Various cells, after being exposed to UA, generate reactive oxygen species (9). UA, as a pro-oxidant, can decrease nitric oxide (NO) production, induce lipid peroxidation, and interact with peroxynitrite to generate free radicals (9). In recent years, the mechanisms by which UA mediates inflammation have attracted the interest of scientists. UA has been found to contribute importantly to immune responses even in the absence of microbial stimulation (10).
UA (2,6,8 trioxypurine—C5H4N4O3) is a heterocyclic organic compound that is the end product of the oxidation of two purine nucleic acids, adenine and guanine. The enzymatic pathway for the degradation of purines is complex and involves numerous enzymes. Briefly, adenosine monophosphate (AMP) is converted to inosine, while guanine monophosphate (GMP) is converted to guanosine by nucleotidase. The nucleosides, inosine and guanosine, are further converted by purine nucleoside phosphorylase (PNP) to the purine bases hypoxanthine and guanine, respectively. Hypoxanthine is then oxidized to xanthine by xanthine-oxidase (XO) and guanine is deaminated to form xanthine by guanine deaminase. Xanthine is again oxidized by xanthine oxidase to form the final product, UA (11). Being a weak acid with a high dissociation constant, UA circulates in plasma (pH 7.4) predominantly in the form of urate (98%), a monovalent sodium salt (1). UA is mainly formed in the liver, intestine and vascular endothelium from endogenous (nucleoproteins) and exogenous (dietary proteins) precursor proteins (2). Approximately two-thirds of the UA load (65–75%) is excreted by the kidneys, while the gastrointestinal tract eliminates one-third (25–35%) (12). Most of the serum UA is freely filtered in kidney glomeruli, and about 90% of the filtered UA is reabsorbed, while only 10% is excreted in the urine (1).
The UA serum level is the result of a balance between dietary purine intake, xanthine oxidase activity, and renal UA excretion (13). When the balance is disturbed, hyperuricemia or hypouricemia occurs. Hyperuricemia has been arbitrarily defined as a value >7 mg/dl in men and >6.5 mg/dl in women, while hypouricemia is defined as a serum urate concentration ≤ 2 mg/dl (14). Numerous epidemiological studies showed elevated UA levels in patients with gout (15), chronic kidney disease (16), cardiovascular diseases (17), metabolic syndrome and obesity (18), confirming its role as a risk factor and useful marker for prediction of progression and outcome in these diseases.
Hyperuricemia has been studied as a possible driving force in the development of intelligence in primates (19). The presence of hyperuricemia has been shown to confer an evolutionary advantage through greater stimulation of the cerebral cortex (20), which could be attributed to its structural similarity to the known psychostimulant caffeine (19), but has also led to longer life in hominids due to its antioxidant effects (21). These UA metabolic properties may have allowed humans to develop higher brain mass (in terms of volume), better intellectual performance (22), and possibly evolutionary supremacy (20) compared with other mammals. The relationship between hyperuricemia and intellectual activity was then established in different population samples (23, 24). The importance of this relationship has been confirmed at the level of cortical stimulation and/or facilitation of learning processes (22).
Epidemiological studies showed that hyperuricemia is increasing worldwide (25). All these features of elevated UA levels were no longer beneficial but rather became risk factors in modern humans, suggesting that UA plays an important pathogenic role in “diseases of civilization” (26). A recent confluence of biochemical, epidemiological and clinical data has pointed to the far-reaching neuroprotective potential of this endogenous antioxidant but also highlighted its role in inflammatory processes. Although a relatively simple substance, the implications of UA's complex effects on health and disease must be considered.
Understanding the mechanisms by which high UA levels affect neuroplasticity and cognitive functioning could provide a potential therapeutic approach to counteract diseases associated with hyperuricemia. Considering the complexity of the human organism, none of the metabolites, including UA, can be considered one-sidedly. In this article, we attempt to elucidate the role of UA in cognitive functioning based on its involvement in oxidative stress and inflammatory processes.
This narrative review was performed by an exhaustive electronic search of the PubMed and Web of Science databases using the terms “uric acid” and “cognition”; “uric acid” and “oxidative stress” “uric acid” and “neuroinflammation;” “uric acid” and “neuroprotection;” “uric acid” and “neurotoxicity”. There was no restriction on the year of publication. We searched for studies published in English, but there were no regional restrictions. We did not pre-specify a preferred study methodology, so there was no restriction on a particular study design. Experimental studies, randomized or non-randomized clinical trials, cohort studies, and case-control studies were considered. We did not limit the assessment of cognitive functioning to a particular test or specify the method of serum UA (sUA) measurement. Abstracts of potentially relevant titles were assessed, and the full text of potentially eligible studies was reviewed. We performed a forward and backward search for relevant papers and repeated this process until no new titles were found. Letters, comments, editorials, practice guidelines, conference proceedings, theses, case studies and unpublished data were excluded.
UA acts as a pro-oxidant by increasing free radical production, causing inflammation, and altering the production of NO (27). UA can become a pro-oxidant by forming radicals in reactions with other various oxidants (28), including its relevant interaction with peroxynitrite (27, 29). These radicals predominantly target lipids, low-density lipoprotein (LDL), and membranes rather than other cellular components. At the same time, the hydrophobic environment created by lipids is unfavorable for UA to exert its antioxidative properties. UA cannot scavenge lipophilic radicals and cannot break the radical chain propagation within lipid membranes (30). UA can oxidize LDL in the presence of copper ions (Cu+ and Cu++) and lipid hydroperoxidases (31). UA decreases the bioavailability of NO and inhibits cell migration and proliferation in endothelial cells, mediated in part by C-reactive protein (CRP) expression and oxidative stress (32). It also decreases mitochondrial deoxyribonucleic acid (DNA) contents and intracellular adenosine triphosphate (ATP) concentrations associated with reactive oxygen species (ROS) production (33) (Figure 1A). However, the reaction of UA with peroxynitrite can also generate radicals, consistent with the ability of UA to become pro-oxidant under various circumstances (34).
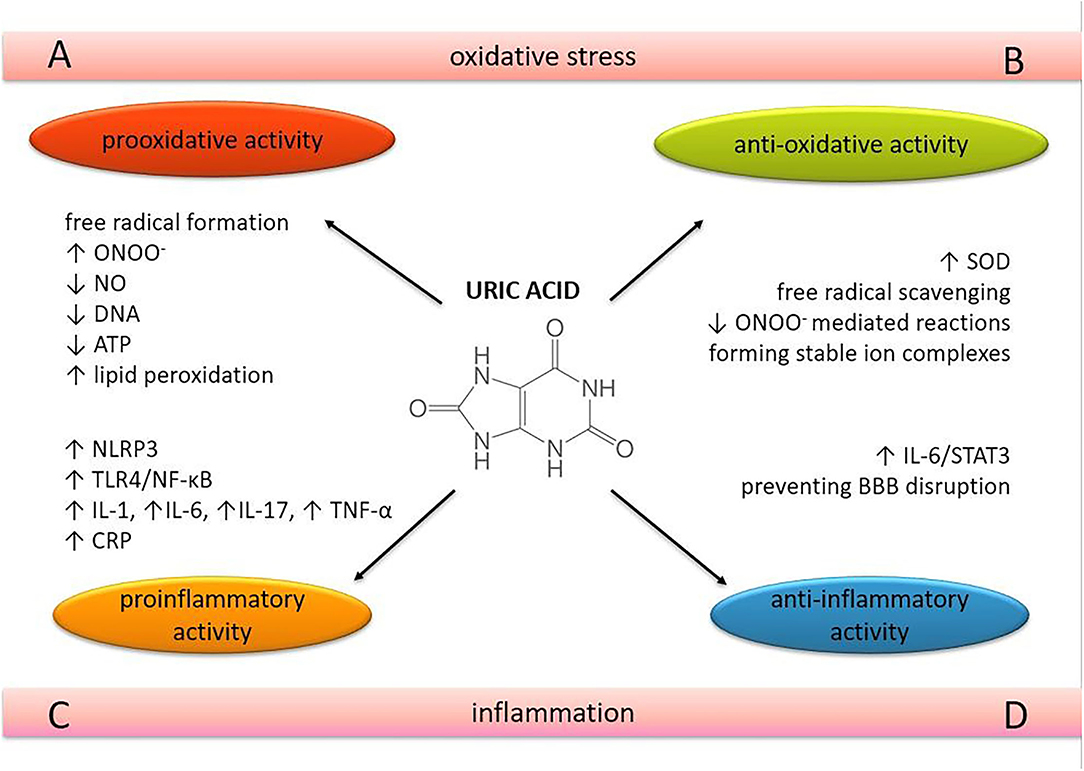
Figure 1. The role of uric acid in oxidative stress and neuroinflammation. The dual nature of uric acid in terms of oxidative and inflammatory processes in brain tissue. ONOO−, peroxynitrite; NO, nitric oxide; SOD, superoxide dismutase; DNA, deoxyibonucleic acid; oxidative (A,B) and inflammatory processes. (C,D) in brain tissue. ATP, adenosine 5'-triphosphate; NLRP3, nucleotide-binding and oligomerization domain-like receptor protein 3; TLR4, Toll-like receptor 4; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL, interleukin; TNF-α, tumor necrosis factor-alpha; CRP, C-reactive protein; STAT 3, signal transducer and activator of transcription 3; BBB, blood-brain barrier.
Oxidative stress has been shown to occur in various cells exposed to UA, such as vascular endothelial cells (32, 33, 35), adipocytes (36), renal tubular cells (37), hepatocytes (38), etc. The high oxygen consumption in neurons leads to the formation of an excessive amount of ROS in the central nervous system (CNS). Compared with other organs, the brain has a lower antioxidant capacity, which makes it particularly vulnerable to oxidative stress (39). The lipid structure of neuronal membranes with unsaturated fatty acids makes neurons extremely sensitive to lipid peroxidation (40). Oxidative damage in the CNS is the result of oxidation and nitration of proteins, lipids, and DNA, leading to necrosis and apoptosis of neuronal cells (41).
UA is a very potent free radical scavenger and is considered one of the most important antioxidants in human plasma (5) (Figure 1B). It has been suggested that UA may exert neuroprotective effects because of its antioxidant properties. The neuroprotective effect of this purine metabolite was demonstrated in cultured rat hippocampal neurons exposed to excitatory and metabolic toxicity. It also resulted in stabilization of calcium homeostasis and preservation of mitochondrial function (42). Serum UA levels have been shown to have a significant positive correlation with total serum antioxidant capacity in healthy human volunteers after acute administration (41, 43) and also in hypoxia-induced conditions (44). Superoxide dismutase (SOD) is an antioxidant enzyme that scavenges superoxide anion (O) by converting this free radical into oxygen (O2) and hydrogen peroxide (H2O2). Hink et al. demonstrated that UA effectively preserves and enhances extracellular SOD activity in mice at concentrations approaching physiological levels in humans (45). Removal of O prevents its reaction with NO, thus blocking the formation of peroxynitrite (ONOO−), a very potent oxidant implicated in the pathogenesis of several CNS diseases. Peroxynitrite can interact with almost all cellular structures, causing severe cellular damage (46, 47). Squadrito et al. have shown that UA cannot scavenge ONOO− directly but acts as a specific inhibitor of radicals such as CO and NO2, which are formed when ONOO− reacts with CO2 (48). The protective effect of UA against ONOO− was confirmed in the experimental autoimmune encephalomyelitis (EAE) model in mice, which is a model of multiple sclerosis (MS) (49). In mice with developed EAE, exogenously administered UA penetrated the already compromised blood-brain barrier (BBB) and blocked peroxynitrite (ONOO−) mediated tyrosine nitration and apoptotic cell death in inflamed areas of the spinal cord tissue.
UA, as a selective inhibitor of certain peroxynitrite-mediated reactions, blocked the toxic effects of peroxynitrite on primary spinal cord neurons in vitro in a dose-dependent manner and also inhibited both the decline in mitochondrial respiration and the enhanced release of lactate dehydrogenase (LDH) (50). In a mouse model of spinal cord injury (SCI), treatment with UA prevented nitrotyrosine formation, lipid peroxidation, and neutrophil infiltration into spinal cord tissue and significantly improved locomotor dysfunction in mice (50). These results support the possibility that elevating UA levels may provide a therapeutic approach for the treatment of SCI as well as other neurological diseases with a peroxynitrite-mediated pathological substrate.
UA is thought to have a pro-inflammatory effect by triggering interleukin (IL)-1β-mediated inflammation via activation of the nucleotide-binding and oligomerization domain (NOD)-like receptor protein (NLRP) 3 inflammasome, a multimolecular complex whose activation appears to play a central role in many pathological inflammatory conditions (51, 52). It also induces the expression of CRP in human vascular cells (32). Epidemiological studies have shown that UA is positively associated with several pro-inflammatory markers such as CRP, white blood cell count, IL-6 and tumor necrosis factor-alpha (TNF-α), and predicts an increase in their levels over a 3-year follow-up (53, 54). A recent randomized, double-blind, placebo-controlled pilot study revealed the positive correlation between serum UA levels and IL-6, IL-17, and TNF-α, suggesting that xanthine oxidase inhibitors reduce serum UA levels but also the levels of these cytokines in patients with gout (55) (Figure 1A). A positive relationship between serum UA and acute-phase reactants such as CRP, fibrinogen, ferritin, and complement C3 was confirmed in a dose-dependent manner, also suggesting that UA induces the pro-inflammatory effect through the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway (56) (Figure 1C).
Elevated UA levels induced by a high-UA diet (HUAD) triggered the expression of pro-inflammatory cytokines, activated the Toll-like receptor 4 (TLR4)/NF-κB pathway, and increased gliosis in the hippocampus (57) and mediobasal hypothalamus of Wistar rats (58). Furthermore, serum UA was able to cross the BBB and act as a potent inflammatory stimulus (57, 58). Some authors found a linear correlation between serum UA levels and UA levels from cerebrospinal fluid (CSF) and confirmed that BBB impairment was associated with higher CSF levels of UA (59). TLR signaling pathways culminate in the activation of the transcription factor NF-κB, which controls the expression of an array of inflammatory cytokine genes (60). In addition, the activation of the TLR4/NF-κB signaling pathway also occurs in other pathological states which are induced by UA, such as pancreatic β-cell death (61) and renal tubules (62). These results indicate that the pathogenic effect of UA may be manifested by inflammation in the hippocampus, suggesting NF-κB activation as an important signaling pathway. Aliena-Valero et al. demonstrated that exogenous administration of UA increases IL-6 levels and plays a neuroprotective role through the activation of the IL-6/signal transducer and activator of transcription 3 (STAT3) signaling pathway, which in turn leads to modulation of relevant mediators of oxidative stress, neuroinflammation, and apoptotic cell death in the brain (63).
The protective role of UA has also been observed in CNS inflammatory processes. In the EAE model, exogenous treatment with UA prevented disruption of BBB integrity and reduced its permeability to inflammatory cells (49) (Figure 1D). Pre-treatment with UA attenuated meningeal inflammation, BBB, and intracranial hypertension in a dose-dependent manner in the adult rat pneumococcal meningitis model. As UA levels increased to approach levels found in humans, the severity of inflammation decreased as a function of UA concentration (64).
Cognition as a higher brain function consists of major cognitive domains: memory, attention, language, executive functions and visuospatial functions (65). All of these domains can be affected and impaired by certain diseases, processes, or toxins, resulting in cognitive dysfunction (65). Cognitive impairment is a chronic neurodegenerative condition characterized by poor learning and memory (66). Cognitive impairment can be a consequence of the physiological aging process (67, 68), but it can also accompany neurodegenerative (69) and neuropsychiatric disorders (70, 71). It is well-established that oxidative stress and inflammation are important pathogenic mechanisms that lie in the background of these conditions (72–77).
As a potent antioxidant in the human body, but also as a mediator in inflammatory processes, UA is the subject of increasing research focused on its influence on cognitive functioning (summarized in Table 1). Although the impact of UA on cognitive functions is undoubtedly confirmed, the exact mechanisms by which cognitive changes occur are not fully understood.

Table 1. Correlation between serum uric acid levels (sUA) and cognitive functioning (CogF) in various study populations.
The neuroanatomy of cognition should be considered in more detail in this context. The hippocampus is the brain region that plays a critical role in learning and memory (presented in the center of Figure 2). Hippocampal dysfunction can alter cognitive abilities (113). Inflammation of the hippocampus has been associated with various neurological dysfunctions (114, 115). The inflammatory responses also lead to neuronal death and blockade of neurogenesis, which in turn leads to cognitive impairment (116, 117).
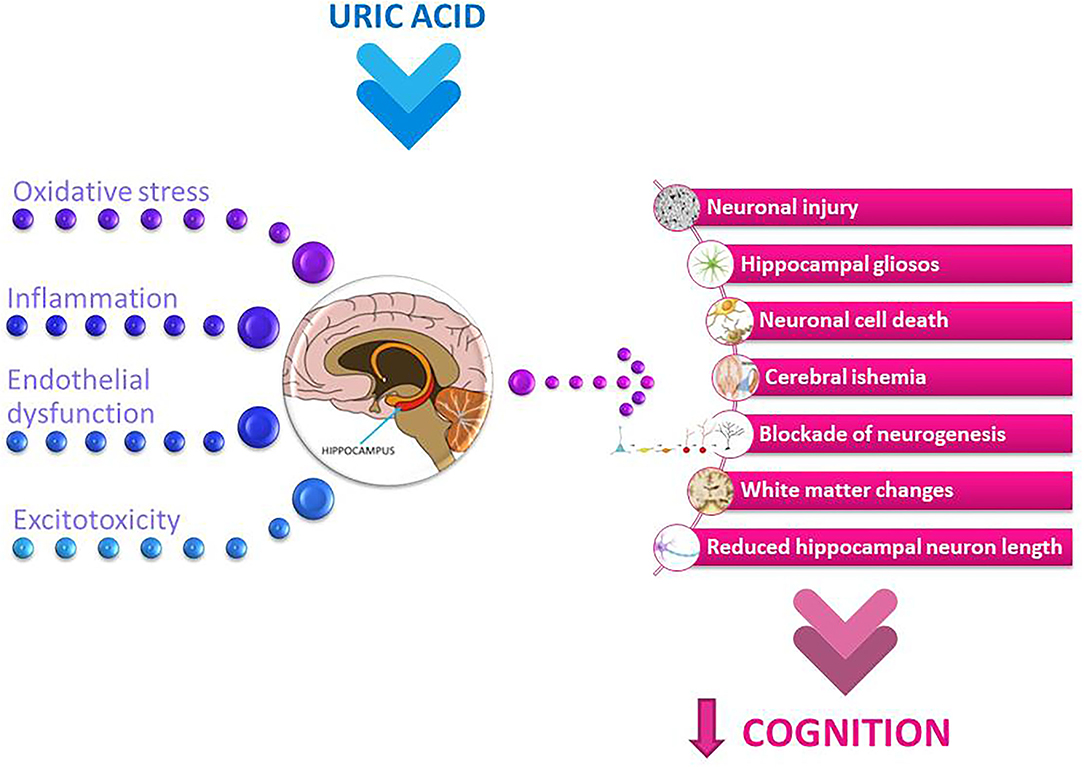
Figure 2. Potential mechanisms involved in uric acid-related cognitive dysfunction. The summary of the main pathological mechanisms of uric acid, such as oxidative stress and neuroinflammation, along with endothelial dysfunction and excitotoxicity, which may collectively affect neuronal and brain function and further implicate uric acid-related cognitive decline.
In physiological settings, UA levels have been measured in the serum and its values were established to be in the range of 3.5 and 7.2 mg/dL in adult males and postmenopausal women and between 2.6 and 6.0 mg/dL in premenopausal women (118). Under physiological conditions, the brain relies relatively little on UA for antioxidant defense because UA molecules cannot leak through an intact BBB (119). The relation between UA serum levels and CSF levels represents a crucial aspect in assessing the influence of UA on brain tissue. In healthy subjects, CSF UA levels are about ten times lower than serum levels (120). Examining CSF metabolite in a healthy population sample, Reavis et al. (121) recently found that men have higher CSF UA levels than women [median (25th−75th) 5,353.71 ng/mL (4,041.17–7,102.65) vs. 9,008.48 ng/mL (7,033.92–11,906.72), p < 0.0001]. BBB destruction is thought to play an important role in neuroinflammation and oxidative stress (122). Previous studies have shown that the concentrations of CSF UA in patients with an impaired BBB depend partly on serum UA concentrations and partly on the balance between production and consumption in the CNS (123). Some authors have proposed the CSF-to-plasma UA ratio as a marker of BBB integrity (124). The urate transporter URAT1, expressed on cilia and the apical surface of ventricular ependymal cells lining the wall of the ventricles that separates CSF and brain tissue, may also represent a novel UA transport mechanism involved in the regulation of UA homeostasis in the brain (125). Desideri et al. provided the evidence that UA could exert detrimental effects on brain structure and function by directly influencing the viability of neuronal cells and their ability to establish synaptic connections in the in vitro model of Alzheimer's dementia (AD), depending on the levels of exposure of cells to UA (126). This effect of UA was observed starting from the dose of 40 mM, while lower UA concentration did not significantly influence cell biology, suggesting a dose-dependent effect of this purine metabolite. The reduction of cells viability under UA exposure was observed starting from a dose that could be achieved in CSF in a condition of mild hyperuricemia (i.e., 400 mM) (118). Intraperitoneal injection of UA was found to elevate both plasma and brain urate levels by 55 and 36.8%, respectively, in rats (127). In recent years, there is increasing evidence of positive correlations between CSF UA and sUA levels in patients with neuroinflammatory and neurodegenerative diseases, supporting the hypothesis of a strong influence of UA on the brain and cognition (59, 123, 128). In the presence of hyperuricemia, the diffusion of UA through the BBB could increase the concentrations of UA in CSF to the levels that might exert detrimental effects on cells biology by promoting the onset and/or progression of neuronal damage, further leading to cognitive impairment. It has been shown that each μmol/L increase in plasma UA was associated with about a 5% increase in CSF UA in patients with mild cognitive impairment (59).
Shao et al. showed that systemic hyperuricemia, induced by HUAD in rats over a 12-week period resulted in cognitive dysfunction manifested by decreased spatial learning and memory (57). TLR4 activation can reduce hippocampal pyramidal neuron dendrite length and impair hippocampal-dependent spatial reference memory in an inflammation-dependent manner (129), thus suggesting the potential for TLR4 activation by UA which lead to cognitive impairment. Decreased SOD activity was also detected in the hippocampal tissue of HUAD rats, suggesting pro-oxidant activity of UA. The concentration-dependent correlation between serum levels of UA and hippocampal gliosis has also been confirmed in humans (57). Hypothesizing that the effects of UA on cognition may be related to its concentration and exposure period, Tian et al. explored the effects of long-term elevated serum UA level on cognitive function and hippocampus. This UA elevation induced by HUAD during 48 weeks was significantly associated with the risk of cognitive impairment (130). Elevated UA levels induced oxidative stress and increased the expression of TNF-α and amyloid beta peptide (Aβ) in the rat's hippocampus, suggesting that both oxidative stress and inflammation could mediate the pathogenesis of cognitive impairment induced by UA (130). This study also suggests that the detrimental effects of higher UA levels on cognitive functioning are likely to become apparent only above a certain serum UA concentration. In this review, we have addressed the problems of the possible different impacts of this molecule on cognitive functioning, especially in the context of oxidative and inflammatory changes, and further tried to enlighten its diverse impact of cognition in various neuropsychiatric disorders and regarding somatic functioning in animal models and human studies.
The results of epidemiological studies on the relationship between UA and cognition are conflicting. The study examining the cognitive decline in the population of healthy older women showed that elevated UA levels were associated with poorer working memory and slower manual speed but not with global cognitive functioning, learning/memory, verbal fluency or visuo-constructional functions (84). In the study of elderly adults with mildly elevated UA levels, poorer working and verbal memory were observed compared with those with low-intermediate UA concentrations (80). It has also been shown that even mildly elevated UA levels can lead to both structural and functional brain changes. The results of the Invecchiare in Chianti (InCHIANTI) cross-sectional study suggest a positive association between high circulating levels of UA and the presence of dementia syndrome (82). A positive correlation between circulating UA levels and cognitive decline was demonstrated in a cohort of pharmacologically untreated young elderly subjects (79). In the Rotterdam Scan Study, hyperuricemic patients exhibited white matter atrophy compared to normouremic subjects. This structural change was followed by a deterioration in cognitive abilities, as evidenced by poorer information-processing speed and decreased executive functionality (81). Beydoun et al. showed that in older men, a significant increase in serum UA levels was associated with faster cognitive decline over time in a visual memory/visuo-construction ability test (83). Elevated serum UA levels were associated with changes in spontaneous brain activities and also followed by lower learning/memory and attention/executive functions (78). Lower neuropsychological assessment scores were notably detected in word fluency tests and number connection tests and were observed in males with pre-hyperuricemia and hyperuricemia (78). Because the cognitive changes occurred before hyperuricemia, this finding may be relevant to the clinical management of patients with pre-hyperuricemia and hyperuricemia. These results also suggest that the changes in cognitive functions affected by the different serum UA levels are gender-specific. This study demonstrated that the changes in spontaneous brain activity occurred mainly in the pallidum and putamen, which were correlated with scores of verbal fluency tests and number connection tests. The pallidum and putamen are the structures that make up the basal ganglia, which are involved in motor control and learning and in the selection and activation of cognitive, executive, and emotional programs (131). The gender-related effect has also been observed in some other studies. A study of 1,451 cognitively healthy adults found that elevated baseline sUA was related to decreased attention and visuospatial abilities in males. There were no noticeable findings in females (90). In a large cohort of 1,598 healthy older people, mean age 72 years, with a follow-up of 12 years, Latourte et al. found an increased risk of developing dementia in those with high sUA levels, even after multiple adjustment (91). A strong association was found with vascular or mixed dementia and no significant association with AD. The authors found no significant association between sUA levels and magnetic resonance imaging markers of cerebrovascular disease or hippocampal volume (91). Elevated UA levels could contribute to endothelial dysfunction (132) and subsequent white matter lesions (85) by reducing the availability of NO in the brain, which in turn leads to poorer cognitive performance. In addition, UA could also contribute to endothelial dysfunction through its pro-oxidant properties (133) (see in Figure 2).
In contrary to previous observations, a large prospective population-based cohort study of 4,618 participants aged 55 years and older showed that elevated UA levels were associated with a decreased risk of dementia. Participants without dementia who were followed up later in life and developed hyperuricemia also had better cognitive performance across all cognitive domains assessed in the study but after adjustment for several cardiovascular risk factors (97). Elevated serum UA levels adversely affected subjects with normal cognition, whereas a protective trend was observed in individuals with cognitive impairment. Interestingly, higher sUA levels were associated with a slower decline in cognitive scores and brain metabolism in females with mild cognitive impairment (MCI), and this effect was found in apolipoprotein E4 carriers but not in non-carriers (99). The cohort study of very old people (age 90–108 years) showed that higher sUA levels were associated with a lower risk of cognitive impairment, but only in men (98). This gender-dependent effect was also suggested in a cross-sectional analysis from the Brazilian Longitudinal Study of Adult Health (ELSA-Brazil) cohort (100). Similar results were obtained from studies among Chinese older adults examining the association between blood UA levels and risk for MCI, suggesting a protective role of high blood UA levels. The findings highlight the potential of managing UA in daily life for preserving cognitive abilities in later life (101, 102). High circulating UA levels correlated positively with improved muscle function and cognitive performance in elderly subjects (96). In a prospective study, elevated baseline UA levels were associated with subsequently enhanced cognitive performance, even in the specific cognitive domain (95).
Both vascular pathology and oxidative stress have been associated with increased risk of dementia and cognitive impairment (134, 135). The results of a recent study by Sun et al., demonstrated that serum UA levels were significantly higher in the PSCI group than in the non-PSCI group, suggesting that serum UA levels may serve as a predictive factor for PSCI (93). In the analysis of data from the Impairment of Cognition and Sleep (ICONS) study, both low and high sUA levels were associated with an elevated incidence of PSCI in males but not in females (94). Although UA has potent antioxidant properties, it can accelerate the oxidative stress reaction under certain pathological conditions, such as ischaemia (136, 137).
Several recent systematic reviews have carefully examined the data on sUA and the relationship with dementia/cognition and provided a more comprehensive synthesis of the evidence. The systematic review by Khan and colleagues showed that in 31 studies using mostly case-control data, sUA was lower in dementia patients compared with control subjects without dementia (138). This review concluded that the relationship between sUA and dementia/cognitive impairment was not consistent across dementia groups, with an apparent association for AD and Parkinson's-disease-related dementia (PDD), but not in cases of mixed dementia or Vascular Dementia (VaD). There was no correlation between scores on Mini-Mental State Examination (MMSE) and sUA level, except in patients with PDD. Similar results were provided by the meta-analysis of cohort studies by Pan et al. (139). Another systematic review assessed the association between sUA and AD (140). Based on 11 case-control studies with 2,708 participants, the sUA levels were not significantly different between patients with AD and healthy controls. Thus, on the basis of these systematic reviews, there is no convincing evidence to date that higher sUA levels are associated with a lower risk of dementia, except possibly in PDD.
Elevated UA levels are an important risk factor for chronic kidney disease (CKD), and numerous studies have shown that these patients are at higher risk for cognitive impairment (86–88). It is also well-established that cerebrovascular lesions are an important risk factor for the development of cognitive decline in CKD patients (141, 142) and that UA plays an important role in these lesions (143, 144). Moreover, direct neuronal injury by uremic toxins can significantly alter cognitive functions in patients at all stages of CKD (87, 145, 146) (summarized in Figure 2). The negative correlation between sUA and MMSE scores was also found in elderly patients receiving maintenance haemodialysis. This correlation was independent of demographic and clinical characteristics (92).
The association between UA and subsequent cognitive performance in patients that carry a high vascular burden showed that low UA levels were associated with poorer cognitive performance, manifested by lower global cognitive scores, memory scores, executive scores, and visuospatial scores (110). A stronger UA effect on cognitive performance was found in older patients (>65 years old), with a significant age interaction for global cognitive, executive, and attention scores. The main finding of this study is that among men with long-lasting cardiovascular diseases and a high vascular burden, lower UA levels were associated with poorer cognitive functions assessed a decade later (110). Higher serum UA levels were independently associated with poorer cognitive performance in chronic heart failure patients (89). Furthermore, these UA effects were manifested in men but not in women.
The potential contribution of UA to cognitive reserve could be attributed to its potent antioxidant properties. Euser et al. examined the association between serum UA and lower dementia risk and better cognitive function later in life in a large prospective population-based cohort study over an 11-year follow-up period (97). Higher levels of UA were associated with lower dementia risk and better cognitive function in later life. UA has revealed neuroprotective effects after experimental cerebral ischemia (42). These findings support the central role of oxyradicals in excitotoxic and ischemic neuronal injury and suggest a potential therapeutic use of UA in ischemic stroke (refer to Figure 2). Neurological impairment at stroke onset and final infarction size at follow-up were inversely related to the concentration of UA (147). Recent studies on the recovery of cognitive function after stroke by the use of UA may also indicate its possible role in the formation of a cognitive reserve (97).
There is growing evidence that UA may exert neuroprotective properties by suppressing neuroinflammation and inhibiting oxidative stress in neurodegenerative disorders (148, 149). In the experimental model of MS, exogenous treatment with UA prevented disruption of BBB integrity, reduced its permeability to inflammatory cells, decreased oxidative stress, and promoted the survival rate (49). Clinical trials consistently suggest that higher serum UA levels are related to a slower progression of Parkinson's disease (PD) (103, 104). PD patients with cognitive dysfunction also have lower serum levels of UA compared to those without cognitive dysfunction (111). The controlled longitudinal study that examined the evolution of cognitive changes and the prognostic value of the UA levels on cognition in the PD-patient cohort demonstrated that the level of both plasma and sUA remained stable over the 3-year period with subtle cognitive changes (150). Given that UA may have a neuroprotective effect in PD, maintaining or even increasing the sUA levels would be beneficial for PD-patients. This study also suggests that it is important to keep body weight and diet stable to avoid fluctuations in UA levels. Recent epidemiological studies showed decreased levels of UA in amyotrophic lateral sclerosis (ALS) patients compared to matched controls and subsequently linked higher UA baseline levels with slower progression and prolonged survival (105–107). In Huntington's disease, functional decline was negatively correlated with UA levels (108). The inverse association between serum UA and AD risk was confirmed in the meta-analysis by Du et al. (109). Patients with depression seem to have significantly lower serum UA levels compared to patients with delirium, dementia, amnesia, and other cognitive disorders (112). The decrease in serum UA levels was related to the antimanic, anticonvulsant, and antiagressive effects of lithium and allopurinol (151). UA levels were also decreased in subjects with first-episode psychosis (152), and further reduced plasma UA levels suggest a defect in the antioxidant defense system in schizophrenia (153). Numerous studies have shown that serum UA levels were lower or tended to decrease in patients with neurodegenerative and mental disorders. Increased serum UA levels could reduce the risk of onset and slow the progression of cognitive decline, thus confirming the hypothesis of a protective role of UA in these disorders (Figure 1B). Opposite to these results, recent research by Borovcanin et al. pointed to the correlation of sUA levels with negative symptoms in patients with schizophrenia after acute treatment, especially important when considering that negative symptoms and cognitive deficits in schizophrenia share many features (154).
Based on the results of experimental and clinical studies, UA seems to play a dual role as a pro- and antioxidant. The balance between the two effects reflects a very complex interplay of factors that include the concentration of UA, the nature and concentration of free radicals, the presence and concentration of other antioxidant molecules, and the various cascades involved (155). However, recent studies suggest that the antioxidant properties of UA are not solely responsible for its beneficial effects in the CNS. UA has been recognized as an important metabolite in protecting spinal cord neurons from glutamate-induced toxicity (156). UA is also thought to have beneficial effects within the normal range, whereas detrimental effects are more likely to occur in hyperuricemia.
Recently, however, there has been a growing body of evidence from clinical and basic research supporting the hypothesis that hyperuricemia, in part through increased inflammatory activity, may be a potential risk factor for cognitive dysfunction. Taking these lines of evidence together, UA appears to exert a protective effect on brain tissue and neurons during the initial stage of elevated UA levels, primarily through its potent antioxidant activity, but long-term elevation appears to trigger an inflammatory response that leads to brain tissue damage. Thus, UA metabolism may be a so-called double-edged sword in terms of the inflammatory and/or oxidative responses it induces in brain tissue, although, its harmful effects appear to outweigh the benefits of UA in most cases.
Although numerous factors contributing to cognitive impairment have been identified to date, UA appears to be an important participant in the onset and/or progression of cognitive decline in various disorders. There is still conflicting evidence about UA's pathophysiological role and its clinical significance in influencing cognitive dysfunction. This may be partly explained by UA's dual nature and different properties, but also by a variety of distinct pathologies that can lead to many constellations of cognitive domain's dysfunctions.
The UA impact on cognitive abilities may have a long evolution course, suggesting that the effects of UA on cognition should be explored in the terms of long, chronic exposure. The more informative study design would be a prospective long-term follow-up cohort study with a relatively large sample of older adults and measurement of sUA fluctuations concurrently with cognitive testing. Cognitive functions should be assessed with a wider range of domain-specific neurological tests. This would allow us to understand global patterns of cognitive fluctuation over time. In this context, it would also be important to investigate accompanying neuroanatomical and neurophysiological changes. Identification of modifiable risk factors is also important, as this will provide greater insight into pathophysiology, risk stratification and potential interventions. Collection of data on dietary habits, medication and comorbidity is necessary to fully exclude the influence of confounding factors. Further research on these complex topics is needed to help design and implement interventions to preserve cognitive capacities in health and various diseases.
MB presented the idea and initial structuration of this review article. NM and KV drew a figure and a table. All authors have searched the literature and given some new insights into specific fields of their competencies, read, discussed, and accepted responsibility for the entire content of this submitted manuscript and approved its submission.
This work was supported by the Ministry of Science and Technological Development of the Republic of Serbia, No. 175069 and the Faculty of Medical Sciences, University of Kragujevac, No. JP 03/16.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Bojana Mircetic for language editing.
1. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. (2016) 213:8–14. doi: 10.1016/j.ijcard.2015.08.109
2. El Ridi R, Tallima H. Physiological functions and pathogenic potential of uric acid: a review. J Adv Res. (2017) 8:487–93. doi: 10.1016/j.jare.2017.03.003
3. Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. (2002) 19:640–53. doi: 10.1093/oxfordjournals.molbev.a004123
4. Kellogg EW, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. (1977) 252:6721–8. doi: 10.1016/S0021-9258(17)39909-X
5. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. (1981) 78:6858–62. doi: 10.1073/pnas.78.11.6858
6. Davies KJA, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. (1986) 235:747–54. doi: 10.1042/bj2350747
7. Whiteman M, Ketsawatsakul U, Halliwell B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann N Y Acad Sci. (2002) 962:242–59. doi: 10.1111/j.1749-6632.2002.tb04072.x
8. Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med. (1993) 14:615–31. doi: 10.1016/0891-5849(93)90143-I
9. Kang D-H, Ha S-K. Uric acid puzzle: dual role as anti-oxidantand pro-oxidant. Electrolytes Blood Press. (2014) 12:1. doi: 10.5049/EBP.2014.12.1.1
10. Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep. (2011) 13:160–6. doi: 10.1007/s11926-011-0162-1
11. Chaudhary K, Malhotra K, Sowers J, Aroor A. Uric acid - key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med. (2013) 3:208–20. doi: 10.1159/000355405
12. Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. (2015) 77:323–45. doi: 10.1146/annurev-physiol-021113-170343
13. Tana C, Busetto L, Di Vincenzo A, Ricci F, Ticinesi A, Lauretani F, et al. Management of hyperuricemia and gout in obese patients undergoing bariatric surgery. Postgrad Med. (2018) 130:523–35. doi: 10.1080/00325481.2018.1485444
14. Esparza Martín N, García Nieto V. Hypouricemia and tubular transport of uric acid. Nefrologia. (2011) 31:44–50. doi: 10.3265/Nefrologia.pre2010.Oct.10588
15. Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK. Serum uric acid and the risk of incident and recurrent gout: a systematic review. J Rheumatol. (2017) 44:388–96. doi: 10.3899/jrheum.160452
16. Bellomo G. Uric acid and chronic kidney disease: a time to act? World J Nephrol. (2013) 2:7. doi: 10.5527/wjn.v2.i2.17
17. Viazzi F, Garneri D, Leoncini G, Gonnella A, Muiesan ML, Ambrosioni E, et al. Serum uric acid and its relationship with metabolic syndrome and cardiovascular risk profile in patients with hypertension: insights from the I-DEMAND study. Nutr Metab Cardiovasc Dis. (2014) 24:921–7. doi: 10.1016/j.numecd.2014.01.018
18. King C, Lanaspa MA, Jensen T, Tolan DR, Sánchez-Lozada LG, Johnson RJ. Uric acid as a cause of the metabolic syndrome. Contrib Nephrol. (2018) 192:88–102. doi: 10.1159/000484283
20. Sofaer JA, Emery AE. Genes for super-intelligence? J Med Genet. (1981) 18:410–3. doi: 10.1136/jmg.18.6.410
21. Watanabe S, Kang D-H, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. (2002) 40:355–60. doi: 10.1161/01.HYP.0000028589.66335.AA
22. Stetten D, Hearon JZ. Intellectual level measured by army classification battery and serum uric acid concentration. Science. (1959) 129:1737. doi: 10.1126/science.129.3365.1737
23. Tovchiga O V., Shtrygol' SY. Uric acid and central nervous system functioning (a literature review). Biol Bull Rev. (2014) 4:210–21. doi: 10.1134/S2079086414030086
24. Kennett KF, Cropley AJ. Uric acid and divergent thinking: a possible relationship. Br J Psychol. (1975) 66:175–80. doi: 10.1111/j.2044-8295.1975.tb01453.x
25. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the national health and nutrition examination survey 2007-2008. Arthritis Rheum. (2011) 63:3136–41. doi: 10.1002/art.30520
26. Mertz DP. Gout hazard as the price for development of intelligence? Dtsch Med Wochenschr. (1974) 99:24–6.
27. Glantzounis G, Tsimoyiannis E, Kappas A, Galaris D. Uric acid and oxidative stress. Curr Pharm Des. (2005) 11:4145–51. doi: 10.2174/138161205774913255
28. Maples KR, Mason RP. Free radical metabolite of uric acid. J Biol Chem. (1988) 263:1709–12. doi: 10.1016/S0021-9258(19)77933-2
29. Vásquez-Vivar J, Santos AM, Junqueira VBC, Augusto O. Peroxynitrite-mediated formation of free radicals in human plasma: EPR detection of ascorbyl, albumin-thiyl and uric acid-derived free radicals. Biochem J. (1996) 314:869–76. doi: 10.1042/bj3140869
30. Muraoka S, Miura T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol Toxicol. (2003) 93:284–9. doi: 10.1111/j.1600-0773.2003.pto930606.x
31. Bagnati M, Perugini C, Cau C, Bordone R, Albano E, Bellomo G. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: a study using uric acid. Biochem J. (1999) 340:143–52. doi: 10.1042/bj3400143
32. Kang D-H, Park S-K, Lee I-K, Johnson RJ. Uric acid–induced c-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. (2005) 16:3553–62. doi: 10.1681/ASN.2005050572
33. Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. (2012) 121:e71–8. doi: 10.1159/000345509
34. Santos CXC, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. (1999) 372:285–94. doi: 10.1006/abbi.1999.1491
35. Yu M-A, Sánchez-Lozada LG, Johnson RJ, Kang D-H. Oxidative stress with an activation of the renin–angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. (2010) 28:1234–42. doi: 10.1097/HJH.0b013e328337da1d
36. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Physiol. (2007) 293:C584–96. doi: 10.1152/ajpcell.00600.2006
37. Ryu E-S, Kim MJ, Shin H-S, Jang Y-H, Choi HS, Jo I, et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Physiol. (2013) 304:F471–80. doi: 10.1152/ajprenal.00560.2012
38. Lanaspa MA, Sanchez-Lozada LG, Choi Y-J, Cicerchi C, Kanbay M, Roncal-Jimenez CA, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress. J Biol Chem. (2012) 287:40732–44. doi: 10.1074/jbc.M112.399899
39. Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. (2017) 360:201–5. doi: 10.1124/jpet.116.237503
40. Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. (2007) 87:1175–213. doi: 10.1152/physrev.00047.2006
41. Markesbery WR, Lovell MA. Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch Neurol. (2007) 64:954. doi: 10.1001/archneur.64.7.954
42. Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. (1998) 53:613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1
43. Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. (2001) 38:365–71. doi: 10.1097/00005344-200109000-00005
44. Sinha S, Singh SN, Ray US. Total antioxidant status at high altitude in lowlanders and native highlanders: role of uric acid. High Alt Med Biol. (2009) 10:269–74. doi: 10.1089/ham.2008.1082
45. Hink HU, Santanam N, Dikalov S, McCann L, Nguyen AD, Parthasarathy S, et al. Peroxidase properties of extracellular superoxide dismutase role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol. (2002) 22:1402–8. doi: 10.1161/01.ATV.0000027524.86752.02
46. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. (2007) 87:315–424. doi: 10.1152/physrev.00029.2006
47. Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Cell Mol Physiol. (2004) 287:L262–8. doi: 10.1152/ajplung.00295.2003
48. Squadrito GL, Cueto R, Splenser AE, Valavanidis A, Zhang H, Uppu RM, et al. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys. (2000) 376:333–7. doi: 10.1006/abbi.2000.1721
49. Hooper DC, Scott GS, Zborek A, Mikheeva T, Kean RB, Koprowski H, et al. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood–CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. (2000) 14:691–8. doi: 10.1096/fasebj.14.5.691
50. Scott GS, Cuzzocrea S, Genovese T, Koprowski H, Hooper DC. Uric acid protects against secondary damage after spinal cord injury. Proc Natl Acad Sci USA. (2005) 102:3483–8. doi: 10.1073/pnas.0500307102
51. Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. (2006) 116:2262–71. doi: 10.1172/JCI28075
52. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. (2006) 440:237–41. doi: 10.1038/nature04516
53. Ruggiero C, Cherubini A, Ble A, Bos AJG, Maggio M, Dixit VD, et al. Uric acid and inflammatory markers. Eur Heart J. (2006) 27:1174–81. doi: 10.1093/eurheartj/ehi879
54. Ruggiero C, Cherubini A, Miller E, Maggio M, Najjar SS, Lauretani F, et al. Usefulness of uric acid to predict changes in c-reactive protein and interleukin-6 in 3-year period in italians aged 21 to 98 years. Am J Cardiol. (2007) 100:115–21. doi: 10.1016/j.amjcard.2007.02.065
55. Huang YY, Ye Z, Gu SW, Jiang ZY, Zhao L. The efficacy and tolerability of febuxostat treatment in a cohort of Chinese Han population with history of gout. J Int Med Res. (2020) 48:300060520902950. doi: 10.1177/0300060520902950
56. Spiga R, Marini MA, Mancuso E, Di Fatta C, Fuoco A, Perticone F, et al. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB signaling pathway in HepG2 cells. Arterioscler Thromb Vasc Biol. (2017) 37:1241–9. doi: 10.1161/ATVBAHA.117.309128
57. Shao X, Lu W, Gao F, Li D, Hu J, Li Y, et al. Uric acid induces cognitive dysfunction through hippocampal inflammation in rodents and humans. J Neurosci. (2016) 36:10990–1005. doi: 10.1523/JNEUROSCI.1480-16.2016
58. Lu W, Xu Y, Shao XX, Gao F, Li Y, Hu J, et al. Uric acid produces an inflammatory response through activation of NF-κB in the hypothalamus: implications for the pathogenesis of metabolic disorders. Sci Rep. (2015) 5:12144. doi: 10.1038/srep12144
59. Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF. Uric acid as a CNS antioxidant. J Alzheimers Dis. (2010) 19:1331–6. doi: 10.3233/JAD-2010-1330
60. Kawai T, Akira S. Signaling to NF-κB by toll-like receptors. Trends Mol Med. (2007) 13:460–9. doi: 10.1016/j.molmed.2007.09.002
61. Jia L, Xing J, Ding Y, Shen Y, Shi X, Ren W, et al. Hyperuricemia causes pancreatic β-cell death and dysfunction through nf-κb signaling pathway. PLoS ONE. (2013) 8:e78284. doi: 10.1371/journal.pone.0078284
62. Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, et al. Uric acid induces renal inflammation via activating tubular NF-κB Signaling pathway. PLoS ONE. (2012) 7:e39738. doi: 10.1371/journal.pone.0039738
63. Aliena-Valero A, Rius-Pérez S, Baixauli-Martín J, Torregrosa G, Chamorro Á, Pérez S, et al. Uric acid neuroprotection associated to IL-6/STAT3 signaling pathway activation in rat ischemic stroke. Mol Neurobiol. (2021) 58:408–23. doi: 10.1007/s12035-020-02115-w
64. Kastenbauer S, Koedel U, Becker BF, Pfister HW. Experimental meningitis in the rat: protection by uric acid at human physiological blood concentrations. Eur J Pharmacol. (2001) 425:149–52. doi: 10.1016/S0014-2999(01)01137-2
65. Franco Á de O, Starosta RT, Roriz-Cruz M. The specific impact of uremic toxins upon cognitive domains: a review. Brazilian J Nephrol. (2019) 41:103–11. doi: 10.1590/2175-8239-jbn-2018-0033
66. Chang K-W, Zong H-F, Rizvi MY, Ma K-G, Zhai W, Wang M, et al. Modulation of the MAPKs pathways affects Aβ-induced cognitive deficits in Alzheimer's disease via activation of α7nAChR. Neurobiol Learn Mem. (2020) 168:107154. doi: 10.1016/j.nlm.2019.107154
67. Popa-Wagner A, Buga A-M, Popescu B, Muresanu D. Vascular cognitive impairment, dementia, aging and energy demand. A vicious cycle. J Neural Transm. (2015) 122:47–54. doi: 10.1007/s00702-013-1129-3
68. Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer's disease. Neurologia. (2000) 15:93–101.
69. Chen P-H, Cheng S-J, Lin H-C, Lee C-Y, Chou C-H. Risk factors for the progression of mild cognitive impairment in different types of neurodegenerative disorders. Behav Neurol. (2018) 2018:1–8. doi: 10.1155/2018/6929732
70. Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. (2012) 11:141–68. doi: 10.1038/nrd3628
71. Menon V. Brain networks and cognitive impairment in psychiatric disorders. World Psychiatry. (2020) 19:309–10. doi: 10.1002/wps.20799
72. Van Raamsdonk JM, Vega IE, Brundin P. Oxidative stress in neurodegenerative disease: causation or association? Oncotarget. (2017) 8:10777–8. doi: 10.18632/oncotarget.14650
73. Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev. (2015) 2015:1–18. doi: 10.1155/2015/610813
74. Salim S. Oxidative stress and psychological disorders. Curr Neuropharmacol. (2014) 12:140–7. doi: 10.2174/1570159X11666131120230309
75. Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, et al. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. (2019) 45:742–51. doi: 10.1093/schbul/sby125
76. Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci. (2019) 1437:57–67. doi: 10.1111/nyas.13712
77. Katarina V, Gordana T, Svetlana MD, Milica B. Oxidative stress and neuroinflammation should be both considered in the occurrence of fatigue and depression in multiple sclerosis. Acta Neurol Belg. (2020) 120:853–61. doi: 10.1007/s13760-018-1015-8
78. Lin L, Zheng LJ, Joseph Schoepf U, Varga-Szemes A, Savage RH, Wang YF, et al. Uric acid has different effects on spontaneous brain activities of males and females: a cross-sectional resting-state functional MR imaging study. Front Neurosci. (2019) 13:763. doi: 10.3389/fnins.2019.00763
79. Cicero AFG, Desideri G, Grossi G, Urso R, Rosticci M, D'Addato S, et al. Serum uric acid and impaired cognitive function in a cohort of healthy young elderly: data from the brisighella study. Intern Emerg Med. (2015) 10:25–31. doi: 10.1007/s11739-014-1098-z
80. Schretlen DJ, Inscore AB, Jinnah HA, Rao V, Gordon B, Pearlson GD. Serum uric acid and cognitive function in community-dwelling older adults. Neuropsychology. (2007) 21:136–40. doi: 10.1037/0894-4105.21.1.136
81. Verhaaren BFJ, Vernooij MW, Dehghan A, Vrooman HA, De Boer R, Hofman A, et al. The relation of uric acid to brain atrophy and cognition: the rotterdam scan study. Neuroepidemiology. (2013) 41:29–34. doi: 10.1159/000346606
82. Ruggiero C, Cherubini A, Lauretani F, Bandinelli S, Maggio M, Di Iorio A, et al. Uric acid and dementia in community-dwelling older persons. Dement Geriatr Cogn Disord. (2009) 27:382–9. doi: 10.1159/000210040
83. Beydoun MA, Canas JA, Dore GA, Beydoun HA, Rostant OS, Fanelli-Kuczmarski MT, et al. Serum uric acid and its association with longitudinal cognitive change among urban adults. J Alzheimers Dis. (2016) 52:1415–30. doi: 10.3233/JAD-160028
84. Vannorsdall TD, Kueider AM, Carlson MC, Schretlen DJ. Higher baseline serum uric acid is associated with poorer cognition but not rates of cognitive decline in women. Exp Gerontol. (2014) 60:136–9. doi: 10.1016/j.exger.2014.10.013
85. Hoth KF, Tate DF, Poppas A, Forman DE, Gunstad J, Moser DJ, et al. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. (2007) 38:308–12. doi: 10.1161/01.STR.0000254517.04275.3f
86. Madan P, Kalra OP, Agarwal S, Tandon OP. Cognitive impairment in chronic kidney disease. Nephrol Dial Transplant. (2006) 22:440–4. doi: 10.1093/ndt/gfl572
87. Etgen T, Sander D, Chonchol M, Briesenick C, Poppert H, Forstl H, et al. Chronic kidney disease is associated with incident cognitive impairment in the elderly: the INVADE study. Nephrol Dial Transplant. (2009) 24:3144–50. doi: 10.1093/ndt/gfp230
88. McQuillan R, Jassal SV. Neuropsychiatric complications of chronic kidney disease. Nat Rev Nephrol. (2010) 6:471–9. doi: 10.1038/nrneph.2010.83
89. Niu W, Yang H, Lu C. The relationship between serum uric acid and cognitive function in patients with chronic heart failure. BMC Cardiovasc Disord. (2020) 20:381. doi: 10.1186/s12872-020-01666-z
90. Kueider AM, An Y, Tanaka T, Kitner-Triolo MH, Studenski S, Ferrucci L, et al. Sex-Dependent associations of serum uric acid with brain function during aging. J Alzheimers Dis. (2017) 60:699–706. doi: 10.3233/JAD-170287
91. Latourte A, Soumaré A, Bardin T, Perez-Ruiz F, Debette S, Richette P. Uric acid and incident dementia over 12 years of follow-up: a population-based cohort study. Ann Rheum Dis. (2018) 77:328–35. doi: 10.1136/annrheumdis-2016-210767
92. Zhang J, Tang L, Hu J, Wang Y, Xu Y. Uric acid is associated with cognitive impairment in the elderly patients receiving maintenance hemodialysis—A two-center study. Brain Behav. (2020) 10:e01542. doi: 10.1002/brb3.1542
93. Sun J, Lv X, Gao X, Chen Z, Wei D, Ling Y, et al. The association between serum uric acid level and the risk of cognitive impairment after ischemic stroke. Neurosci Lett. (2020) 734:135098. doi: 10.1016/j.neulet.2020.135098
94. Liu Q, Liao X, Pan Y, Jin A, Zhang Y. Association between serum uric acid levels and cognitive function in patients with ischemic stroke and transient ischemic attack (TIA): a 3-month follow-up study. Neuropsychiatr Dis Treat. (2021) 17:991–9. doi: 10.2147/NDT.S300893
95. Wang T, Wu Y, Sun Y, Zhai L, Zhang D. A prospective study on the association between uric acid and cognitive function among middle-aged and older Chinese. J Alzheimers Dis. (2017) 58:79–86. doi: 10.3233/JAD-161243
96. Wu Y, Zhang D, Pang Z, Jiang W, Wang S, Tan Q. Association of serum uric acid level with muscle strength and cognitive function among Chinese aged 50-74 years. Geriatr Gerontol Int. (2013) 13:672–7. doi: 10.1111/j.1447-0594.2012.00962.x
97. Euser SM, Hofman A, Westendorp RGJ, Breteler MMB. Serum uric acid and cognitive function and dementia. Brain. (2008) 132:377–82. doi: 10.1093/brain/awn316
98. Li J, Dong BR, Lin P, Zhang J, Liu GJ. Association of cognitive function with serum uric acid level among Chinese nonagenarians and centenarians. Exp Gerontol. (2010) 45:331–5. doi: 10.1016/j.exger.2010.01.005
99. Lee Y, Park M, Jeong SH, Kang SW, Baik K, Jung JH, et al. Effects of baseline serum uric acid and apolipoprotein E4 on longitudinal cognition and cerebral metabolism. Neurobiol Aging. (2021) 106:223–31. doi: 10.1016/j.neurobiolaging.2021.05.003
100. Baena CP, Suemoto CK, Barreto SM, Lotufo PA, Benseñor I. Serum uric acid is associated with better executive function in men but not in women: baseline assessment of the ELSA-Brasil study. Exp Gerontol. (2017) 92:82–6. doi: 10.1016/j.exger.2017.03.010
101. Chen C, Lyu YB, Li CC, Cai JF, Zhang XC, Liu YC, et al. Association of blood uric acid and cognitive impairment in oldest-old aged 80 years and older in 9 longevity areas of China. Zhonghua Yu Fang Yi Xue Za Zhi. (2021) 55:39–44.
102. Chen C, Li X, Lv Y, Yin Z, Zhao F, Liu Y, et al. High blood uric acid is associated with reduced risks of mild cognitive impairment among older adults in china: a 9-year prospective cohort study. Front Aging Neurosci. (2021) 13:747686. doi: 10.3389/fnagi.2021.747686
103. Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. (2009) 66:1460–8. doi: 10.1001/archneurol.2009.247
104. Schwarzschild MA, Schwid SR, Marek K, Watts A, Lang AE, Oakes D, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. (2008) 65:716–23. doi: 10.1001/archneur.2008.65.6.nct70003
105. Keizman D, Ish-Shalom M, Berliner S, Maimon N, Vered Y, Artamonov I, et al. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci. (2009) 285:95–9. doi: 10.1016/j.jns.2009.06.002
106. Paganoni S, Zhang M, Zárate AQ, Jaffa M, Yu H, Cudkowicz ME, et al. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J Neurol. (2012) 259:1923–8. doi: 10.1007/s00415-012-6440-7
107. Kataoka H, Kiriyama T, Kobayashi Y, Horikawa H, Ueno S. Clinical outcomes and serum uric acid levels in elderly patients with amyotrophic lateral sclerosis aged ≥ 70 years. Am J Neurodegener Dis. (2013) 2:140–4.
108. Auinger P, Kieburtz K, Mcdermott MP. The relationship between uric acid levels and Huntington's disease progression. Mov Disord. (2010) 25:224–8. doi: 10.1002/mds.22907
109. Du N, Xu D, Hou X, Song X, Liu C, Chen Y, et al. Inverse association between serum uric acid levels and Alzheimer's disease risk. Mol Neurobiol. (2016) 53:2594–9. doi: 10.1007/s12035-015-9271-6
110. Molshatzki N, Weinstein G, Streifler JY, Goldbourt U, Tanne D. Serum uric acid and subsequent cognitive performance in patients with pre-existing cardiovascular disease. PLoS ONE. (2015) 10:e0120862. doi: 10.1371/journal.pone.0120862
111. Wang XJ, Luo WF, Wang LJ, Mao CJ, Wang L, Liu CF. Study on uric acid and the related factors associated with cognition in the patients with Parkinson's disease. Natl Med J China. (2009) 89:1633–5.
112. Wen S, Cheng M, Wang HH, Yue J, Wang HH, Li G, et al. Serum uric acid levels and the clinical characteristics of depression. Clin Biochem. (2012) 45:49–53. doi: 10.1016/j.clinbiochem.2011.10.010
113. Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. (2014) 508:88–92. doi: 10.1038/nature13028
114. Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S, et al. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. (2014) 40:9–17. doi: 10.1016/j.bbi.2014.03.005
115. Giovanello KS, Schnyer D, Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. (2009) 19:111–7. doi: 10.1002/hipo.20491
116. Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. (2016) 353:777–83. doi: 10.1126/science.aag2590
117. Heneka MT, Carson MJ, Khoury J El, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. (2015) 14:388–405. doi: 10.1016/S1474-4422(15)70016-5
118. Desideri G, Castaldo G, Lombardi A, Mussap M, Testa A, Pontremoli R, et al. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci. (2014) 18:1295–306.
119. Becker BF, Kastenbauer S, Ködel U, Kiesl D, Pfister HW. Urate oxidation in CSF and blood of patients with inflammatory disorders of the nervous system. Nucleosides Nucleotides Nucleic Acids. (2004) 23:1201–4. doi: 10.1081/NCN-200027469
120. Láhoda F, Athen D. Typing of uric acid level in cerebrospinal fluid in neurological and psychiatric diseases. Adv Exp Med Biol. (1977) 76 B:256–8. doi: 10.1007/978-1-4684-3285-5_37
121. Reavis ZW, Mirjankar N, Sarangi S, Boyle SH, Kuhn CM, Matson WR, et al. Sex and race differences of cerebrospinal fluid metabolites in healthy individuals. Metabolomics. (2021) 17:13. doi: 10.1007/s11306-020-01757-0
122. Zlokovic B V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. (2008) 57:178–201. doi: 10.1016/j.neuron.2008.01.003
123. Dujmovic I, Pekmezovic T, Obrenovic R, Nikolić A, Spasic M, Stojkovic MM, et al. Cerebrospinal fluid and serum uric acid levels in patients with multiple sclerosis. Clin Chem Lab Med. (2009) 47:848–53. doi: 10.1515/CCLM.2009.192
124. Niklasson F, Hetta J, Degrell I. Hypoxanthine, xanthine, urate and creatinine concentration gradients in cerebrospinal fluid. Ups J Med Sci. (1988) 93:225–32. doi: 10.3109/03009738809178548
125. Tomioka NH, Nakamura M, Doshi M, Deguchi Y, Ichida K, Morisaki T, et al. Ependymal cells of the mouse brain express urate transporter 1 (URAT1). Fluids Barriers CNS. (2013) 10:31. doi: 10.1186/2045-8118-10-31
126. Desideri G, Gentile R, Antonosante A, Benedetti E, Grassi D, Cristiano L, et al. Uric acid amplifies aβ amyloid effects involved in the cognitive dysfunction/dementia: evidences from an experimental model in vitro. J Cell Physiol. (2017) 232:1069–1078. doi: 10.1002/jcp.25509
127. Gong L, Zhang Q-L, Zhang N, Hua W-Y, Huang Y-X, Di P-W, et al. Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson's disease: linking to Akt/GSK3β signaling pathway. J Neurochem. (2012) 123:876–85. doi: 10.1111/jnc.12038
128. Shu Y, Li H, Zhang L, Wang Y, Long Y, Li R, et al. Elevated cerebrospinal fluid uric acid during relapse of neuromyelitis optica spectrum disorders. Brain Behav. (2017) 7:e00584. doi: 10.1002/brb3.584
129. Okun E, Barak B, Saada-Madar R, Rothman SM, Griffioen KJ, Roberts N, et al. Evidence for a developmental role for TLR4 in learning and memory. PLoS ONE. (2012) 7:e47522. doi: 10.1371/journal.pone.0047522
130. Tian T, Liu X run, Li T ting, Nie Z chao, Li S jing, Tang Y, et al. Detrimental effects of long-term elevated serum uric acid on cognitive function in rats. Sci Rep. (2021) 11:6732. doi: 10.1038/s41598-021-86279-y
131. Ring HA, Serra-Mestres J. Neuropsychiatry of the basal ganglia. J Neurol Neurosurg Psychiatry. (2002) 72:12–21. doi: 10.1136/jnnp.72.1.12
132. Kato M, Hisatome I, Tomikura Y, Kotani K, Kinugawa T, Ogino K, et al. Status of endothelial dependent vasodilation in patients with hyperuricemia. Am J Cardiol. (2005) 96:1576–8. doi: 10.1016/j.amjcard.2005.07.068
133. Patterson RA, Horsley ETM, Leake DS. Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: important role of uric acid. J Lipid Res. (2003) 44:512–21. doi: 10.1194/jlr.M200407-JLR200
134. Erkinjuntti T, Gauthier S. The concept of vascular cognitive impairment. Dement Clin Pract. (2009) 24:79–85. doi: 10.1159/000197886
135. Nunomura A, Moreira PI, Castellani RJ, Lee H, Zhu X, Smith MA, et al. Oxidative damage to RNA in aging and neurodegenerative disorders. Neurotox Res. (2012) 22:231–48. doi: 10.1007/s12640-012-9331-x
136. Zhu A, Zou T, Xiong G, Zhang J. Association of uric acid with traditional inflammatory factors in stroke. Int J Neurosci. (2016) 126:335–41. doi: 10.3109/00207454.2015.1015723
137. Hayden MR, Tyagi SC. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr Metab. (2004) 1:10. doi: 10.1186/1743-7075-1-10
138. Khan AA, Quinn TJ, Hewitt J, Fan Y, Dawson J. Serum uric acid level and association with cognitive impairment and dementia: systematic review and meta-analysis. Age. (2016) 38:16. doi: 10.1007/s11357-016-9871-8
139. Pan S-Y, Cheng R-J, Xia Z-J, Zhang Q-P, Liu Y. Risk of dementia in gout and hyperuricaemia: a meta-analysis of cohort studies. BMJ Open. (2021) 11:e041680. doi: 10.1136/bmjopen-2020-041680
140. Chen X, Guo X, Huang R, Chen Y, Zheng Z, Shang H. Serum uric acid levels in patients with Alzheimer's disease: a meta-analysis. PLoS ONE. (2014) 9:e94084. doi: 10.1371/journal.pone.0094084
141. Miglinas M, Cesniene U, Janusaite MM, Vinikovas A. Cerebrovascular disease and cognition in chronic kidney disease patients. Front Cardiovasc Med. (2020) 7:96. doi: 10.3389/fcvm.2020.00096
142. Drew DA, Weiner DE. Cognitive impairment in chronic kidney disease: keep vascular disease in mind. Kidney Int. (2014) 85:505–7. doi: 10.1038/ki.2013.437
143. Kanellis J, Kang D-H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. (2005) 25:39–42. doi: 10.1016/j.semnephrol.2004.09.007
144. Talebi A, Amirabadizadeh A, Nakhaee S, Ahmadi Z, Mousavi-Mirzaei SM. Cerebrovascular disease: how serum phosphorus, vitamin D, and uric acid levels contribute to the ischemic stroke. BMC Neurol. (2020) 20:116. doi: 10.1186/s12883-020-01686-4
145. Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. (2009) 73:920–7. doi: 10.1212/WNL.0b013e3181b72629
146. Hailpern SM, Melamed ML, Cohen HW, Hostetter TH. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: third national health and nutrition examination survey (NHANES III). J Am Soc Nephrol. (2007) 18:2205–13. doi: 10.1681/ASN.2006101165
147. Chamorro Á, Obach V, Cervera Á, Revilla M, Deulofeu R, Aponte JH. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke. (2002) 33:1048–52. doi: 10.1161/hs0402.105927
148. Wu SH, Shu XO, Milne G, Xiang YB, Zhang X, Cai Q, et al. Uric acid correlates to oxidation and inflammation in opposite directions in women. Biomarkers. (2015) 20:225–31. doi: 10.3109/1354750X.2015.1068852
149. Huang T-T, Hao D-L, Wu B-N, Mao L-L, Zhang J. Uric acid demonstrates neuroprotective effect on Parkinson's disease mice through Nrf2-ARE signaling pathway. Biochem Biophys Res Commun. (2017) 493:1443–9. doi: 10.1016/j.bbrc.2017.10.004
150. Annanmaki T, Pohja M, Parviainen T, Hakkinen P, Murros K. Uric acid and cognition in Parkinson's disease: a follow-up study. Parkinsonism Relat Disord. (2011) 17:333–7. doi: 10.1016/j.parkreldis.2011.01.013
151. Machado-Vieira R, Lara DR, Souza DO, Kapczinski F. Purinergic dysfunction in mania: an integrative model. Med Hypotheses. (2002) 58:297–304. doi: 10.1054/mehy.2001.1543
152. He Q, You Y, Yu L, Yao L, Lu H, Zhou X, et al. Uric acid levels in subjects with schizophrenia: a systematic review and meta-analysis. Psychiatry Res. (2020) 292:113305. doi: 10.1016/j.psychres.2020.113305
153. Yao JK, Reddy R, Van Kammen DP. Abnormal age-related changes of plasma antioxidant proteins in schizophrenia. Psychiatry Res. (2000) 97:137–51. doi: 10.1016/S0165-1781(00)00230-4
154. Borovcanin MM, Janicijevic SM, Mijailovic NR, Jovanovic IP, Arsenijevic NN, Vesic K. Uric acid potential role in systemic inflammation and negative symptoms after acute antipsychotic treatment in schizophrenia. Front Psychiatry. (2022) 12:822579. doi: 10.3389/fpsyt.2021.822579
155. Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. (2008) 27:608–19. doi: 10.1080/15257770802138558
Keywords: uric acid, cognition, neuroprotection, neurotoxicity, oxidative stress, inflammation
Citation: Mijailovic NR, Vesic K and Borovcanin MM (2022) The Influence of Serum Uric Acid on the Brain and Cognitive Dysfunction. Front. Psychiatry 13:828476. doi: 10.3389/fpsyt.2022.828476
Received: 03 December 2021; Accepted: 17 March 2022;
Published: 22 April 2022.
Edited by:
Chuang Wang, Ningbo University, ChinaReviewed by:
Rajib Paul, Pandit Deendayal Upadhayaya Adarsha Mahavidyalaya, IndiaCopyright © 2022 Mijailovic, Vesic and Borovcanin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milica M. Borovcanin, bWlsaWNhYm9yb3ZjYW5pbkB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.