
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 11 May 2022
Sec. Psychopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.828088
This article is part of the Research Topic Novel Antipsychotics Within and Beyond Clinical Trials: The Treatment of Overlapping Psychiatric Disorders with D3-D2 Partial Agonists View all 28 articles
Background: Cariprazine's efficacy and safety have been previously tested in adult patients with acute mania associated with bipolar I disorder, but there is no available data in FEM. The objective of this study is to assess the efficacy and safety of cariprazine in combination with a mood stabilizer in treating FEM as well as to evaluate patients' adherence to the treatment.
Methods: FEM patients were recruited from the acute inpatient unit at Lleida University Hospital Santa Maria, between January and June 2021. Their symptoms were evaluated using the Young Mania Rating Scale (YMRS) and the Clinical Global Impressions–Severity (CGI-S) scale at admission and at discharge. Akathisia was assessed using the Barnes Akathisia Rating Scale. Patient adherence to medication treatment was assessed 30 days after discharge using the Morisky, Green and Levine Medication Adherence Scale. Socio-demographic and clinical information were further collected.
Results: Eleven patients with FEM were involved, seven women and four men. Their mean age was 26.00+/-6.37 years. Mean hospitalization was 17.36+/−4.7 days. Cariprazine was combined with a mood stabilizer: lithium in seven patients and divalproex in four. Mean YMRS change from baseline was −24.55+/−7.5 and the mean CGI-S change from baseline was −2.55+/−0.82. Regarding adverse events, two (18.2%) patients presented with akathisia. At the 30-day treatment-adherence assessment, six (54.5%) patients were adherent and four (36.4%) had moderate adherence.
Conclusion: In this sample, cariprazine in combination with mood stabilizers proved to be safe and effective in the treatment of FEM with more than half the patients being adherent to treatment. Therefore, cariprazine add-on is a good choice for promoting the long-term adherence of patients, thus minimizing the risk of relapse and improving prognosis.
Bipolar disorder (BD) is a chronic and disabling mental disorder, characterized by recurrent mood episodes of depression, mania, hypomania and mixed affective states with periods of full or partial remission (1). It is associated with high burden of disease and psychosocial dysfunction, affecting more than 1% of the general population (2). Mania is the most recognizable phase of the disorder, and its presence is key for diagnosis (2). Its characteristic symptoms include, among others, grandiosity, reduced need for sleep, distractibility, increased flight of ideas, impulsivity, and occasionally, it is further accompanied by psychotic symptoms (3). Mania has been associated with impaired psychosocial functioning and cognition (4), and patients sometimes require hospitalization in order to stabilize their psychopathological condition (5).
Given the recurring nature of the disorder, the emphasis of treatment is not only on the resolution of acute symptoms, but also on the assurance of long-term prophylaxis of mood episodes (6). Therefore, treatment by a multidisciplinary team is recommended, combining psychological and pharmacological treatment options (7). Regarding the treatment of manic episodes, the main objectives are the resolution of acute manic symptoms, behavioural and cognitive symptoms as well as psychotic symptoms, if present (6). Clinical guidelines for the pharmacological treatment of acute mania offer recommendations based on evidence, safety, and tolerability (8). One of the most recently published ones is The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders (6) which recommends oral monotherapy, if possible, with aripiprazole, asenapine, risperidone, quetiapine or cariprazine. If monotherapy is insufficient, second-generation antipsychotics can be combined with a mood-stabilizing agent: lithium or valproate (6). Lithium is considered to be the gold standard for the maintenance treatment of BD; however, its onset of action is slower than that of antipsychotics in the treatment of acute mania (9). Therefore, many clinicians combine lithium or other mood stabilizers with an atypical antipsychotic in order to treat the manic phase of BD. In fact, a combination therapy is recommended as first-line treatment option with greater efficacy than monotherapy with lithium or divalproex alone [Ogawa et al. (10); Pacchiarotti et al. (8)]. This latter treatment approach was applied for the purposes of the present study in first-episode mania (FEM) patients.
For FEM patients, medication adherence is an important aspect to consider, as it impacts on the efficacy of pharmacotherapy and therefore later disease-outcome (11). Thus, treatment should be initiated with cautious use of medications and slow titration, as early experiences of tolerability and side-effects prime later expectations and subsequent adherence, especially in FEM (12).
Cariprazine is a dopamine D2–D3 partial agonist with high affinity to D3 receptors. It is approved for the treatment of schizophrenia and the depressive and manic/mixed episodes associated with bipolar I disorder by the Food and Drug Administration, and it has shown efficacy as adjunctive treatment for major depressive disorder (13). It binds with high affinity to dopamine D2 and D3 receptors and to serotonin 5HT1A and 5HT2B receptors and with moderate affinity to serotonin 5HT2A receptors (14). A distinctive characteristic of cariprazine is that it has the highest affinity for D3 receptors among other antipsychotics; in fact, it is greater than that of dopamine itself (14). This makes cariprazine the only antipsychotic that can occupy the D3 receptors in the presence of dopamine in the living brain (15). Three short-term clinical trials have confirmed the efficacy of cariprazine over placebo (16–18), and a long-term (19) clinical trial confirmed the safety and tolerability of cariprazine in patients with bipolar I mania. The dose range in mania is 3–6 mg/day (20) with treatment-emergent affective switches reported with very low doses (21). Based on cariprazine's good tolerability and safety profile, it could not only treat mania effectively, but also improve treatment-adherence, therefore improving long-term outcomes of patients with FEM (22).
Although clinical trials are the gold standard of clinical research, they have some disadvantages, including that the data is not generalisable, as there are marked differences between patients involved in clinical trials and those seen in real-world settings (23). For instance, patients enrolled in trials are carefully screened using rigorous criteria, and comorbidities and adjunctive medications are highly controlled – all these aspects do not seem feasible in clinical practice (24). Therefore, it is important to supplement the knowledge gained from clinical trials with data gained from real-world evidence, such as electronic health and medical records, electronic devices and applications, case series or observational and naturalistic studies (25).
The objective of this study is to assess cariprazine's efficacy and safety in combination with mood stabilizers in treating FEM as well as patients' adherence to the treatment.
This study is an observational study including patients over the age of 18 with a diagnosis of FEM where the medical decision was taken to initiate cariprazine treatment before the start of the study. Patients were recruited from the acute inpatient unit at Santa Maria University Hospital (Lleida, Spain) between January and June 2021. Diagnosis was based on the clinical assessment of the presentation at first inpatient hospitalization, following the DSM-5 A-D criteria for a manic episode (3).
Patients with a Young Mania Rating Scale (YMRS) (26) [Spanish version (27)] total score ≥ 18 at admission were included. Exclusion criteria included the presence of mixed symptoms, previous manic or psychotic episodes; mental intellectual disability; previous antipsychotic-use; and manic episode attributable to the physiological effects of substances or other medical conditions.
All patients were treated with cariprazine flexible doses (3–6 mg/day) in combination with a mood stabilizer: lithium (800–1,200 mg/day) or divalproex (1,000–1500 mg/day). Following the local recommendations for inpatients with severe mania (8), in addition to the antipsychotic treatment, a mood stabilizer was introduced in all cases. No specific timing of the start of the mood stabilizer is provided by the guidelines, but authors chose to start it on day 2 of cariprazine treatment, as it is the usual practice in their hospital. Treatment with both medications were maintained.
Socio-demographic and clinical information were collected. Patients were evaluated using YMRS and Clinical Global Impressions-Severity of Illness (CGI-S) scales at admission and discharge. Response (≥50% reduction in YMRS score at discharge) and remission (YMRS score ≤ 12 at discharge) were further assessed, using conventional cut-off criteria (28).
Based on previous safety studies of cariprazine (19, 29), the development of akathisia was measured using the Barnes Akathisia Rating Scale (BARS) (30). Further safety assessment included the evaluation of insomnia, headache and suicidality using a clinical interview conducted by the treating psychiatrist. Clinical laboratory tests conducted at baseline and discharge evaluated prolactin and metabolic (total cholesterol, LDL, HDL, triglycerides, and fasting glucose) changes, in line with common clinical practice for therapeutic monitoring (31).
Patient adherence to medication treatment was assessed 30 days after discharge using the Morisky Green Levine Medication Adherence Scale (MGLS) (32). Patients were categorized as: MGL = 0–1 representing low adherence, MGL = 2–3 representing moderate adherence, and MGL = 4 representing high adherence.
Descriptive statistics were calculated for the demographic and safety data. For evaluating the change on the YMRS and CGI-S measures, the related-samples Wilcoxon Signed Rank Test was conducted.
The study was carried out following the latest version of the Declaration of Helsinki, and the local ethics committee approved the study (CEIC-2341).
For a summary of patient characteristics, refer to Table 1. Seven women and four males with FEM were included in the study (N = 11) with a mean age of 26 +/−6.37 years. Mean hospitalization for the observed episode was 17.36 +/−4.7 days. Mean cariprazine dose was 4.64 mg +/−1.25 mg/day, administered once daily in the morning. Lithium carbonate was given to seven patients with a mean dose of 1085.71 +/−157.36, and divalproex sodium to four patients with a mean dose of 1,125 +/−250 mg/day. Regarding psychiatric comorbid conditions, one patient had attention-deficit hyperactivity disorder, one had post-traumatic stress disorder and four had substance use disorder.
The mean YMRS score was 35.55 +/−7.79 at admission and 11 +/−2.19 at discharge, p = 0.003, change from baseline was −24.55 +/−7.5 (Figure 1). All patients achieved a clinically significant response (≥50% reduction in YMRS score at discharge). Eight (72.7%) patients achieved clinically significant remission (YMRS ≤ 12) and three (27.3%) patients showed minimal symptoms at the end of the hospitalization (YMRS = 13–19). Those with minimal symptoms at discharge showed psychotic symptoms at admission; had larger duration of untreated mania; and received the highest dose of cariprazine (6 mg/day) during the hospitalization. Mean CGI-S decreased from 4.82 +/−0.87 at admission to 2.27 +/−0.65 at discharge, p = 0.003, change from baseline is therefore −2.55 +/−0.82 (Figure 2).
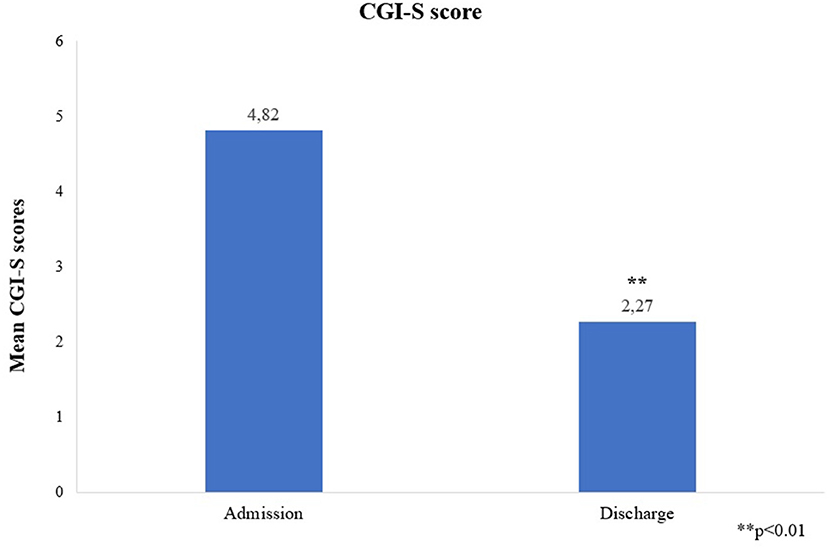
Figure 2. Mean CGI-S scores at admission and discharge. CGI-S, Clinical Global Impression – Severity Scale. **p < 0.01.
For a summary of the safety outcomes, refer to Table 2. Regarding adverse events, two (18.2%) patients developed akathisia (one moderate and one marked), one (9.1%) experienced insomnia and two (18.2%) reported headache. No suicidal behaviour was noted in any of the patients. Mean change in prolactin level from baseline to discharge was −4.97 +/−5.05 ng/mL for females and −3.28 +/−2.7 ng/mL for males. Mean change of metabolic parameters was: 1.90 +/- 8.87 mg/dL for total cholesterol; 1.84 +/−13.49 mg/dL for LDL cholesterol; −2.97 +/−6.06 mg/dL for HDL cholesterol; 16.71 +/−21.09 mg/dL for Triglycerides; and 7.85 +/−13.78 for fasting glucose.
Regarding treatment-adherence, six (54.5%) patients displayed high adherence, four (36.4%) moderate adherence and one (9.1%) patient had low adherence to the medication, as shown by the MGLS score at day 30 after discharge.
This was the first study to specifically investigate cariprazine's efficacy in combination with a mood stabilizer in FEM. In this sample, mean YMRS scores and CGI-S scores showed a great reduction from admission to discharge with all patients achieving clinically significant response. Furthermore, 72% patients achieved clinically significant remission.
These findings are in line with those of clinical trials. The short-term efficacy and safety/tolerability of cariprazine was confirmed in three 3-week placebo-controlled studies (16–18) in adult patients with acute manic or mixed episodes associated with bipolar I disorder. Flexible-dose cariprazine 3–12 mg/day was used in two studies (16, 17) and a fixed/flexible dose scheme (3–6 mg/day or 6–12 mg/day) was used in the third (18). In each trial, improvement from baseline to week 3 in YMRS score, CGI-S scores as well as rates of response were significantly greater for cariprazine- than for placebo-treated patients. Remission rates also showed statistical significance in favour of cariprazine over placebo (33). This aspect is of high significance, as the persistence of symptoms after the acute treatment of mania was shown to be associated with worse illness-outcome and an increased risk of relapse (33). Therefore, the fact that cariprazine patients achieved response and remission has clinical significance in the improvement of prognosis (33).
In general, the first stages of most diseases require a simpler approach and treatment response is usually more favourable, obtaining a greater benefit with less risk (34). After the first episode, multiple relapses and a progressive worsening of psychosocial functioning and cognition often occur. However, it is thought that first episodes represent a window of intervention to improve clinical results and patient's quality of life (5). Despite that, guidelines focusing on the treatment of FEM are scarce (35). Treatment in FEM yields complete remission of the manic syndrome in most cases, but it may take longer for males, younger patients, or those with psychotic features or a longer duration of untreated mania (11).
Furthermore, BD is associated with more frequent relapses than other psychiatric diseases (36) with non-adherence to pharmacological treatment (37) and residual symptoms after an acute manic episode being the best predictors of relapse (33). There are several factors influencing adherence: individual-specific sociodemographic factors, insight, cognition as well as illness-specific factors, like illness-severity or comorbidities (38). Of note, there are medication-specific factors as well, like the complexity of the medication regimen and side-effects (39). Regarding cariprazine, it is given orally once daily; can be taken with or without food; can be taken at any time of the day; and neither age, gender nor smoking influence dose administration (40), making the medication regimen easy to comply with.
Regarding side-effects, akathisia incidence in our sample (18.2%) was similar to those reported in acute double-blind studies (pooled data of the three short-term studies: 19.8% for the 3–6 mg/day dose-range) (29). Prevalence of insomnia and headache, (9.1 and 18.2%, respectively) were also similar to the outcomes of the pooled analysis (8.7, 13.7% in the same dose-range) (29).
Regarding the metabolic parameters, there was a slight increase observed in triglycerides, fasting glucose and cholesterol levels (except for HDL cholesterol) in our sample. These findings are generally similar to those observed in clinical trials (29). In addition, mean metabolic variations were inferior to 5% for total cholesterol and LDL cholesterol but not for HDL cholesterol; mean triglyceride variations were inferior to 20–30% and mean fasting glucose increase was inferior to 10 mg/dL, which are within normal ranges (31, 41).
Mean change in prolactin level from baseline to discharge in this sample was similar to those reported in post-hoc analyses conducted on pooled data of the three short-term studies Patel et al. (42). Decrease in prolactin level was seen as a consequence of the D2 partial agonism, especially in females.
Psychotic features are common in bipolar mania, some studies estimating it to be around 68% (43) which is similar to our sample, where 72.7% of the patients experienced psychotic symptoms. The presence of psychotic symptoms leads to an earlier age of onset and more severe mood episodes, requiring more frequent hospitalizations – making it crucial to find an effective treatment for this patient population (44). A study explored the pharmacological treatments and found that having bipolar mania with psychotic features is associated with receiving a combination therapy of an antipsychotic and an anticonvulsant agent (44). However, there is no evidence of superiority of any first-line antipsychotic (8). In our study, cariprazine in combination with lithium or divalproex sufficiently addressed psychotic symptoms in FEM.
Overall, cariprazine has an easy medication regimen and a favourable safety profile. Cariprazine's long-term tolerability was demonstrated by Ketter and colleagues (19) in a 16-week open-label cariprazine 3–12 mg/day study, with akathisia being a common adverse event. They showed low rates of sedation or weight gain and although akathisia occurred in one-third of the patients, it yielded low rates of discontinuation as it was managed effectively. Therefore, cariprazine could be a good choice of pharmacotherapy to ensure adherence from the first stages of the disease. In terms of 30-day adherence, in our study, six (54.5%) patients displayed high adherence, four (36.4%) moderate adherence and one (9.1%) patient had low adherence to the medication. This is an encouraging data for adherence, but studies are needed to examine this further.
One of the limitations of the data presented here is the small sample size. In addition, conclusions regarding the efficacy and risk/benefit profile of cariprazine are difficult to be drawn on due to the lack of an active comparator; multiple doses; and the concomitant medication. Also, many of the patients in this sample had a psychiatric comorbid condition which is known to negatively influence many aspects of the disorder – including less favourable treatment response, especially to lithium – making it complicated to draw accurate conclusions from these findings. Furthermore, patients were followed up for a short period only, warranting the need for longer observations to conclude long-term effects.
In this sample, cariprazine in combination with a mood stabilizer (lithium or divalproex) was effective in resolving the acute manic episode of FEM patients and it proved to be safe and well-tolerated with a low rate of adverse effects. Since the BD internationals guidelines recommend choosing treatment based on not only efficacy, but also short-term and long-term safety and tolerability, cariprazine is a good choice of pharmacotherapy. Given cariprazine's gentle safety profile and ease of administration, it likely improves patient's adherence to treatment and therefore helps minimizing the risk of relapse and improves prognosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Comitè d'Ètica d'Investigació amb medicaments de Lleida (CEIC-2341). The patients/participants provided their written informed consent to participate in this study.
RP-G and VL-B were responsible for conception and design as well as initial drafting of the manuscript. GA-H and EA were responsible for revising the manuscript critically for important intellectual content of the version of the manuscript to be published. All authors read and approved the final manuscript.
Gedeon Richter provided funds for the open access publication fees.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vieta E, Durgam S, Lu K, Ruth A, Debelle M, Zukin S. Effect of cariprazine across the symptoms of mania in bipolar I disorder: analyses of pooled data from phase II/III trials. Euro Neuropsychopharmacol. (2015) 25:1882–91. doi: 10.1016/j.euroneuro.2015.08.020
2. Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, alabrese JR, et al. Bipolar disorders. Nat Rev Dis Prim. (2018) 4:1–16. Available online at: https://www.nature.com/articles/nrdp20188 (accessed February 24, 2022).
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM−5) [Internet]. (2013). Available online at: https://www.psychiatry.org/psychiatrists/practice/dsm (accessed February 24, 2022).
4. Vieta E, Sanchez-Moreno J. Acute and long-term treatment of mania. Dialog Clin Neurosci. (2008) 10:165. Available online at: /pmc/articles/PMC3181868/ (accessed February 24, 2022).
5. Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. (2016) 387:1561–72. Available online at: http://www.thelancet.com/article/S014067361500241X/fulltext (accessed February 24, 2022).
6. Malhi GS, Bell E, Boyce P, Bassett D, Berk M, Bryant R, et al. The 2020 royal australian and new zealand college of psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. (2020) 22:805–21. Available online at: https://onlinelibrary.wiley.com/doi/full/10.1111/bdi.13036 (accessed February 24, 2022).
7. Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian network for mood and anxiety treatments (CANMAT) and international society for bipolar disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. (2018) 20:97–170. Available online at: https://onlinelibrary.wiley.com/doi/full/10.1111/bdi.12609 (accessed February 24, 2022).
8. Pacchiarotti I, Anmella G, Colomer L, Vieta E. How to treat mania. Acta Psychiatrica Scandinavica. (2020) 142:173–92. Available online at: https://onlinelibrary.wiley.com/doi/full/10.1111/acps.13209 (accessed February 24, 2022).
9. Licht RW. Lithium: still a major option in the management of bipolar disorder. CNS Neurosci Therapeutics. (2012) 18:219–26. Available online at: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1755-5949.2011.00260.x (accessed February 24, 2022).
10. Ogawa Y, Tajika A, Takeshima N, Hayasaka Y, Furukawa TA. Mood stabilizers and antipsychotics for acute mania: a systematic review and meta-analysis of combination/augmentation therapy vs. monotherapy. CNS Drugs. (2014) 28:989–1003. Available online at: https://link.springer.com/article/10.1007/s40263-014-0197-8 (accessed February 24, 2022).
11. Ramain J, Conus P, Golay P. A narrative review of intervention in first-episode affective psychoses. J Psych Res. (2021) 143:123–37. Available online at: https://pubmed.ncbi.nlm.nih.gov/34487989/ (accessed February 24, 2022).
12. McIntyre RS, Berk M, Brietzke E, Goldstein BI, López-Jaramillo C, Kessing LV, et al. Bipolar disorders. Lancet. (2020) 396:1841–56. Available online at: http://www.thelancet.com/article/S0140673620315440/fulltext (accessed February 24, 2022).
13. Durgam S, Earley W, Guo H, Li D, Németh G, Laszlovszky I, et al. Efficacy and safety of adjunctive cariprazine in inadequate responders to antidepressants: a randomized, double-blind, placebo-controlled study in adult patients with major depressive disorder. J Clin Psych. (2016) 77:371–8. Available online at: https://pubmed.ncbi.nlm.nih.gov/27046309/ (accessed February 24, 2022).
14. Kiss B, Horváth A, Némethy Z, Schmidt É, Laszlovszky I, Bugovics G, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Experim Therap. (2010) 333:328–40. Available online at: https://pubmed.ncbi.nlm.nih.gov/20093397/ (accessed February 24, 2022).
15. Stahl SM. Mechanism of action of cariprazine. CNS Spectrums. (2016) 21:123–7. Available online at: https://pubmed.ncbi.nlm.nih.gov/26956157/ (accessed February 24, 2022).
16. Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. (2015) 17:63–75. Available oonline at: https://pubmed.ncbi.nlm.nih.gov/25056368/ (accessed February 24, 2022).
17. Sachs GS, Greenberg WM, Starace A, Lu K, Ruth A, Laszlovszky I, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Aff Disord. (2015) 174:296–302. Available online at: https://pubmed.ncbi.nlm.nih.gov/25532076/ (accessed February 24, 2022).
18. Calabrese JR, Keck PE, Starace A, Lu K, Ruth A, Laszlovszky I, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psych. (2015) 76:284–92. Available online at: https://pubmed.ncbi.nlm.nih.gov/25562205/ (accessed February 24, 2022).
19. Ketter TA, Sachs GS, Durgam S, Lu K, Starace A, Laszlovszky I, et al. The safety and tolerability of cariprazine in patients with manic or mixed episodes associated with bipolar I disorder: a 16-week open-label study. J Aff Disord. (2018) 225:350–6. Available online at: https://pubmed.ncbi.nlm.nih.gov/28843918/ (accessed February 24, 2022).
20. Gedeon Richter Plc. Vraylar (cariprazine) [package insert]. (2015) Available online at: www.fda.gov/medwatch (accessed February 24, 2022).
21. Pons-Cabrera MT, Palacios-Garrán R, Tardón-Senabre L, Fernández-Plaza T, Marco-Estrada O, Madero S, et al. Cariprazine-induced mania: a case series report. Bipolar Disord. (2021) Available online at: https://pubmed.ncbi.nlm.nih.gov/34797609/ (accessed February 24, 2022).
22. Stahl SM, Laredo S, Morrissette DA. Cariprazine as a treatment across the bipolar I spectrum from depression to mania: mechanism of action and review of clinical data. Therap Adv Psychopharmacol. (2020) 10:2045125320905752. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/32110377 (accessed February 24, 2022).
23. Corrigan-Curay J, Sacks L, Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. (2018) 320:867–8. Available online at: https://jamanetwork.com/journals/jama/fullarticle/2697359 (accessed February 24, 2022).
24. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence - what is it and what can it tell us? N Eng J Med. (2016) 375:2293–7. Available online at: https://pubmed.ncbi.nlm.nih.gov/27959688/ (accessed February 24, 2022).
25. Radawski CA, Hammad TA, Colilla S, Coplan P, Hornbuckle K, Freeman E, et al. The utility of real-world evidence for benefit-risk assessment, communication, and evaluation of pharmaceuticals: case studies. Pharmacoepidemiol Drug Safety. (2020) 29:1532–9. Available online ar: https://pubmed.ncbi.nlm.nih.gov/33146901/ (accessed February 24, 2022).
26. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry : J Mental Sci. (1978) 133:429–35. Available online at: https://pubmed.ncbi.nlm.nih.gov/728692/ (accessed February 24, 2022).
27. Colom F, Vieta E, Martínez-Arán A, Garcia-Garcia M, Reinares M, Torrent C, et al. Spanish version of a scale for the assessment of mania: validity and reliability of the young mania rating scale. Medicina Clinica. (2002) 119:366–71. Available online at: https://pubmed.ncbi.nlm.nih.gov/12372167/ (accessed February 24, 2022).
28. Bourin M, Thibaut F. How to assess drugs in the treatment of acute bipolar mania? Front Pharmacol. (2013) 4. Available online at: https://pmc/articles/PMC3557457/ (accessed February 24, 2022).
29. Earley W, Durgam S, Lu K, Debelle M, Laszlovszky I, Vieta E, et al. Tolerability of cariprazine in the treatment of acute bipolar I mania: a pooled post-hoc analysis of 3 phase II/III studies. J Aff Disord. (2017) 215:205–12. Available online at: https://pubmed.ncbi.nlm.nih.gov/28343051/ (accessed February 24, 2022).
30. Barnes TRE. A rating scale for drug-induced akathisia. Br J Psych : J Mental Sci. (1989) 154:672–6. Available online at: https://pubmed.ncbi.nlm.nih.gov/2574607/ (accessed February 24, 2022).
31. de Hert M, Dekker JM, Wood D, Kahl KG, Holt RIG, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European psychiatric association (EPA), supported by the European association for the study of diabetes (EASD) and the European society of cardiology (ESC). Eur Psych. (2009) 24:412–24. doi: 10.1016/j.eurpsy.2009.01.005
32. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. (1986) 24:67–74. Available online at: https://pubmed.ncbi.nlm.nih.gov/3945130 (accessed February 24, 2022).
33. Earley W, Durgam S, Lu K, Ruth A, Németh G, Laszlovszky I, et al. Clinically relevant response and remission outcomes in cariprazine-treated patients with bipolar I disorder. J Affect Disord. (2018) 226:239–44. doi: 10.1016/j.jad.2017.09.040
34. Salagre E, Dodd S, Aedo A, Rosa A, Amoretti S, Pinzon J, et al. Toward precision psychiatry in bipolar disorder: staging 20. Front Psychiatry. (2018) 9:641. doi: 10.3389/fpsyt.2018.00641
35. Power P. Intervening early in bipolar disorder in young people: a review of the clinical staging model. Irish J Psychol Med. (2015) 32:31–43. Available online at: https://pubmed.ncbi.nlm.nih.gov/30185268/ (accessed February 24, 2022).
36. Angst J, Gamma A, Sellaro R, Lavori PW, Zhang H. Recurrence of bipolar disorders and major depression. A life-long perspective. Eur Arc Psychiatry Clin Neurosci. (2003) 253:236–40. Available online at: https://pubmed.ncbi.nlm.nih.gov/14504992/ (accessed February 24, 2022).
37. Tohen M, Goldberg JF, Hassoun Y, Sureddi S. Identifying profiles of patients with bipolar i disorder who would benefit from maintenance therapy with a long-acting injectable antipsychotic. J Clin Psych. (2020) 81. Available online at: https://pubmed.ncbi.nlm.nih.gov/32558403/ (accessed February 24, 2022).
38. Sajatovic M, Ignacio R v, West JA, Cassidy KA, Safavi R, Kilbourne AM, et al. Predictors of non-adherence among individuals with bipolar disorder receiving treatment in a community mental health clinic. Compreh Psych. (2009) 50:100–7. doi: 10.1016/j.comppsych.2008.06.008
39. Jawad I, Watson S, Haddad PM, Talbot PS, McAllister-Williams RH. Medication non-adherence in bipolar disorder: a narrative review. Therap Adv Psychopharmacol. (2018) 8:349–63. Available online at: https://pubmed.ncbi.nlm.nih.gov/30524703 (accessed February 24, 2022).
40. European Medicines Agency. EPAR for Reagila. (2017). Available online at: https://www.ema.europa.eu/en/documents/overview/reagila-epar-summary-public_en.pdf (accessed February 24, 2022).
41. Meeusen J, Ueda M, Nordestgaard B, Remaley A. Lipids and Lipoproteins. In: Rifai N, editor. Tietz Textbook of Laboratory Medicine [Internet]. 7th Edition. Saunders (2023). p. 407–8. Available online at: https://books.google.dk/books?id=r6RcEAAAQBAJ&pg=PA407&lpg=PA407&dq=normal+percentage+variation+lipids&source=bl&ots=BQwio-GMZa&sig=ACfU3U04tiDsVXRBB1ropu7-OzIHj2dQ0w&hl=en&sa=X&ved=2ahUKEwii1prqtaL2AhUFR_EDHfJ1Dv8Q6AF6BAgqEAM#v=onepage&q=normal%20percentage%20variation%20lipids&f=false (accessed February 24, 2022).
42. Patel M, Culpepper L, Vieta E, Kelly DL, Moreira T, Earley W. Effects of cariprazine on prolactin levels in patients with bipolar I disorder conclusions background. In: 32nd Annual Psych Congress. (2019). Available online at: https://www.hmpgloballearningnetwork.com/site/pcn/posters/effects-cariprazine-prolactin-levels-patients-bipolar-i-disorder (accessed February 24, 2022).
43. Canuso CM, Bossie CA, Zhu Y, Youssef E, Dunner DL. Psychotic symptoms in patients with bipolar mania. J Aff Disord. (2008) 11):164–9. Available online at: https://pubmed.ncbi.nlm.nih.gov/18378001/ (accessed February 24, 2022).
44. Bjørklund LB, Horsdal HT, Mors O, Gasse C, Østergaard SD. Psychopharmacological treatment of psychotic mania and psychotic bipolar depression compared to non-psychotic mania and non-psychotic bipolar depression. Bipolar Disord. (2017) 19:505–12. Available online at: https://onlinelibrary.wiley.com/doi/full/10.1111/bdi.12504 (accessed February 24, 2022).
Keywords: first episode mania, cariprazine, bipolar disoder, acute mania, presicion medicine, treatment adherence
Citation: Palacios-Garrán R, Llorca-Bofí V, Arteaga-Henriquez G and del Agua E (2022) Cariprazine Use in Combination With a Mood Stabilizer in First Episode Mania. Front. Psychiatry 13:828088. doi: 10.3389/fpsyt.2022.828088
Received: 02 December 2021; Accepted: 15 April 2022;
Published: 11 May 2022.
Edited by:
Peter Falkai, LMU Munich University Hospital, GermanyReviewed by:
Georgios Demetrios Kotzalidis, Sapienza University of Rome, ItalyCopyright © 2022 Palacios-Garrán, Llorca-Bofí, Arteaga-Henriquez and del Agua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Palacios-Garrán, cGFsYWNpb3MuZ2FycmFuQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.