
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 11 March 2022
Sec. Aging Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.826135
This article is part of the Research Topic Insights in Aging Psychiatry: 2021 View all 12 articles
 Valentina Moschini1†
Valentina Moschini1† Salvatore Mazzeo2,3†
Salvatore Mazzeo2,3† Silvia Bagnoli2
Silvia Bagnoli2 Sonia Padiglioni4,5
Sonia Padiglioni4,5 Filippo Emiliani2
Filippo Emiliani2 Giulia Giacomucci2
Giulia Giacomucci2 Carmen Morinelli1
Carmen Morinelli1 Assunta Ingannato2
Assunta Ingannato2 Tommaso Freni2
Tommaso Freni2 Laura Belloni4,5
Laura Belloni4,5 Camilla Ferrari2
Camilla Ferrari2 Sandro Sorbi2,3
Sandro Sorbi2,3 Benedetta Nacmias2,3*
Benedetta Nacmias2,3* Valentina Bessi2
Valentina Bessi2Objective: HTT is a gene containing a key region of CAG repeats. When expanded beyond 39 repeats, Huntington disease (HD) develops. HTT genes with <35 repeats are not associated with HD. The biological function of CAG repeat expansion below the non-pathological threshold is not well understood. In fact higher number of repeats in HTT confer advantageous changes in brain structure and general intelligence, but several studies focused on establishing the association between CAG expansions and susceptibility to psychiatric disturbances and to other neurodegenerative disease than HD. We hypothesized that HTT CAG repeat length below the pathological threshold might influence mood and personality traits in a longitudinal sample of individuals with Subjective Cognitive Decline.
Methods: We included 54 patients with SCD. All patients underwent an extensive neuropsychological battery at baseline, APOE genotyping and analysis of HTT alleles. We used the Big Five Factors Questionnaire (BFFQ) and Hamilton Depression Rating Scale (HDRS), respectively, to assess personality traits of patients and depression at baseline. Patients who did not progress to Mild Cognitive Impairment (MCI) had at least 5-year follow-up time.
Results: In the whole sample, CAG repeat number in the shorter HTT allele was inversely correlated with conscientiousness (Pearson = −0.364, p = 0.007). There was no correlation between HDRS and CAG repeats. During the follow-up, 14 patients [25.93% (95% C.I. = 14.24–37.61)] progressed to MCI (MCI+) and 40 [74.07% (95% C.I. = 62.39–85.76)] did not (MCI−). When we performed the same analysis in the MCI+ group we found that: CAG repeat length on the shorter allele was inversely correlated with energy (Pearson = 0.639, p = 0.014) and conscientiousness (Pearson = −0.695, p = 0.006). CAG repeat length on the longer allele was inversely correlated with conscientiousness (Pearson = −0.901, p < 0.001) and directly correlated with emotional stability (Pearson = 0.639, p = 0.014). These associations were confirmed also by multivariate analysis. We found no correlations between BFFQ parameters and CAG repeats in the MCI− group.
Discussion: Personality traits and CAG repeat length in the intermediate range have been associated with progression of cognitive decline and neuropathological findings consistent with AD. We showed that CAG repeat lengths in the HTT gene within the non-pathological range influence personality traits.
Huntingtin (HTT) is a gene coding for a soluble peptide which is widely expressed during development, being essential for embryogenesis (1), and plays crucial roles in axonal trafficking, regulation of gene transcription, and cell survival in post developmental life (2). HTT contains a key region of CAG repeats which is translated into a corresponding polyglutamine stretch (3). The expansion of CAG triplet beyond 40 repeats leads to the dysfunction and death of neurons in the striatum and in other brain regions, causing Huntington's disease (HD) (4), a neurodegenerative disorder characterized by cognitive, motor and psychiatric disturbance (5).
Several studies showed that carriers of HTT CAG repeats in the pathological range (HD carriers) had a defined personality profile, characterized by higher conscientiousness, lower emotional stability (6) and lower social cognition (7) as compared to controls.
However, there are only a few studies exploring the effect of CAG repeats in the non-pathological range on personality traits and mood. Killoran et al. showed that the individuals with a number of CAG repeats in the range of the so-called intermediate alleles (IA) (27–35 CAG repeats) were more likely to experience trouble with motivation, to have thoughts of suicide and generally reported more mood and behavior problems than those with <27 repeats (8). Other authors showed that IAs of HTT are associated with higher prevalence of neurodegenerative conditions, such as Alzheimer's disease (AD) (9) and to the non-fluent variant of primary progressive aphasia (10). IA proportion has been reported to be significantly increased in AD patients (8) as compared to healthy controls and HTT levels were increased in neuronal cells in the hippocampus of AD cases (11). Alzheimer-type lesions, in turns, were found more frequently in autopsy of HD patients [from 67 (12) to 80% (13)] as compared to healthy controls. Finally, a recent work by our group found that HTT CAG expansions influence neuropsychological functions in individuals experiencing Subjective Cognitive Decline (SCD) or Mild Cognitive Impairment (MCI) (14).
Therefore, in the present study we hypothesized that HTT CAG repeat length below the pathological threshold might influence mood and personality traits in a longitudinal sample of individuals with SCD.
We included 54 consecutive patients who complained memory disorder and self-referred to the Center for Alzheimer's disease and Adult Cognitive Disorders of Careggi Hospital in Florence. Inclusion criteria were: (1) complaining of cognitive decline with a duration of ≥ 6 months; (2) normal functioning on the Activities of Daily Living and the Instrumental Activities of Daily Living scales (15); (3) unsatisfied criteria for dementia or MCI at baseline (16, 17). Exclusion criteria were: (1) history of head injury, current neurological and/or systemic disease, symptoms of psychosis, major depression, alcoholism or other substance abuse. No patient had family history of Huntington disease.
The local ethics committee approved the protocol of the study. All participants gave written informed consent. All procedures involving experiments on humans have been done in accordance with the ethical standards of the Committee on Human Experimentation of the institution in which the experiments were done or in accordance with the Helsinki Declaration of 1975. Specific national laws have been observed.
At baseline, all participants underwent: (1) comprehensive family and clinical history, general and neurological examination; (2) extensive neuropsychological battery including assessment of cognitive complaints, depressive symptoms, and premorbid intelligence; (3) brain MRI or CT scan; (4) peripheral blood collection to analyze Apolipoprotein E (APOE) and HTT genotypes. A positive family history of dementia was defined as one or more first-degree relatives with documented cognitive decline. Disease duration was defined as the time from the onset of symptoms to the first neurological or neuropsychological evaluation.
All patients underwent clinical and neuropsychological follow-up every 12 or 24 months. Progression to MCI and conversion to AD were defined according to the National Institute on Aging-Alzheimer's Association (16, 17).
All patients underwent an extensive neuropsychological assessment, consisting of: global measurements (Mini-Mental State Examination), tasks exploring verbal and spatial working memory (Digit Span; Corsi Tapping Test), verbal long-term memory (Five Words and Paired Words Acquisition; Recall after 10 min; Recall after 24 h; 15 Words of Rey Babcock Short Story Immediate and Delayed Recall), language [Token Test; Category Fluency Task, Phonemic Fluency Test (15)],visual-spatial abilities (Rey-Osterrieth Complex Figure copy), visual-spatial long-term memory [Rey-Osterrieth Complex Figure test (18)], attention/executive function [Dual Task (19), and Trail Making Test (20)], everyday memory (Rivermead Behavioral Memory Test) (18). All raw test scores were adjusted for age, education and gender according to the correction factor reported in validation studies for the Italian population (15, 18–22).
Cognitive complaints were explored using a survey based on the Memory Assessment Clinics-Questionnaire (MAC-Q) (23).The presence and severity of depressive symptoms was evaluated by the 22-item Hamilton Depression Rating Scale (HRSD) (24). Premorbid intelligence, as a cognitive reserve proxy, was assessed at by Test di Intelligenza Breve (TIB, i.e., Brief Intelligence Test) (25), an Italian version of the National Adult Reading Test (NART) (26).
Personality traits were assessed by the Big Five Factors Questionnaire (BFFQ), a self-administered inventory following a widely accepted five-traits personality model (27). The five dimensions of the BFFQ are: (1) emotional stability: the resilience to unpleasant emotions like anger, anxiety, depression, self-pity and worry; (2) energy: being active, assertive, energetic, enthusiastic, outgoing, and talkative; (3) conscientiousness: the degree of organization, persistence, and motivation in goal-directed behavior; (4) agreeableness: the quality of interpersonal orientation to compassion, including adjectives like appreciative, forgiving, generous, kind, sympathetic, and trusting; (5) openness to culture and experience: active imaginations, aesthetic sensitivity, intellectual curiosity, wide variety of interests. Each of the five dimensions of personality consists of two sub-dimensions defined in turn by 24 items. Subjects rated their level of agreement on a five-point scale ranging from “strongly agree” to “strongly disagree”.
Subjects' DNA was isolated from peripheral blood using a standard automated method (QIAcube, QIAGEN). APOE genotypes were investigated by High Resolution melting Analyses (HRMA) (28). Two sets of PCR primers were designed to amplify APOE regions encompassing rs7412 [NC_000019.9:g.45412079C>T] and rs429358 (NC_000019.9:g.45411941T>C). Patients who were carriers of the ε4 allele (one or two APOEε4 alleles) were classified as APOEε4+, while patients who were not carriers of ε4 allele (no APOEε4 alleles) were classified as APOEε4−.
HTT CAG repeat expansion was determined by a polymerase chain reaction amplification assay, using fluorescently labeled primers (29). The size of the fragment was determined by capillary electrophoresis using SeqStudio Genetic Analyzer (ThermoFisher) and the GeneMapper version 4.0 software (Applied Biosystems). A set of HTT CAG alleles, whose lengths were confirmed by DNA sequencing, was used to provide size standards. Patients who were carriers of the intermediate allele [at least one HTT allele with CAG-repeat sizes of 27–35 repeats (5)] were classified as IA+, while patients who were not carriers were classified as IA−.
We tested for normality by the Shapiro-Wilk test. Patient groups were characterized by using means and standard deviations, median and interquartile range (IQR), frequencies or percentages and 95% confidence interval (95%C.I.) for continuous distributed variables, continuous non-normally distributed variables and categorical variables, respectively. Depending on the distribution of our data, we used t-test or non-parametric Mann-Whitney-U Tests for between-groups comparisons, Pearson's correlation coefficient or non-parametric Spearman's ρ (rho) to evaluate correlations between groups' numeric measures, and chi-square tests to compare categorical data. We calculated the size effect by Cohen's d for normally distributed numeric measures, η2 for Mann-Whitney-U Test and Cramer's V for categorical data. We used backward linear regressions as multivariate analyses. Bonferroni correction was applied to correct for multiple comparisons dividing 0.05 by the numbers of variables included in each analysis (adjusted statistical significance levels are reported in the caption of each table). All statistical analyses were performed with SPSS software v.25 (SPSS Inc., Chicago, USA) and the computing environment R 4.0.3 (R Foundation for Statistical Computing, Vienna, 2013).
Our sample included 37 male and 13 females. Mean age at baseline was 60.80 (± 7.33). All the patients were Caucasian. Median CAG repeats lengths were 18.00 (IQR 4.00, range: 12–29) in the shorter allele and 21.00 (4.00, range: 16–31) in the longer allele. The most common HTT alleles had 18 (shorter alleles) and 18, 21 and 22 (longer alleles) CAG-repeats (Figure 1). Six out of 54 patients [11.11% (95% CI 2.73–19.49)] were carriers of intermediate alleles of HTT gene (IAs+). Among these, one patient was homozygous for IA (29 and 31 CAG-repeats). There was no significant difference in disease duration, family history of AD, sex, years of education, TIB, MMSE, HDRS and BFFQ score between IAs− and IAs+. There was no difference in any neuropsychological test score between IAs− and IAs+.
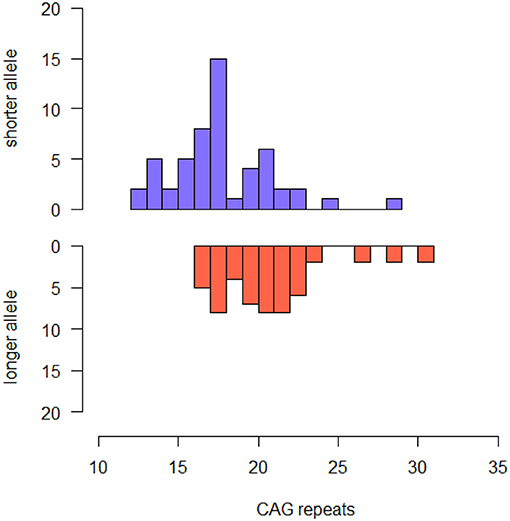
Figure 1. Histogram describing the frequency of CAG repeat lengths of shorter and longer alleles in the whole sample.
Patients were followed-up for a median time of 13 years. During the follow-up, 14 patients [25.93% (95% C.I. = 14.24–37.61)] progressed to MCI (MCI+) and 40 patients [74.07% (95% C.I. = 62.39–85.76)] did not progress to MCI (MCI−) with a mean follow-up time of 15.29 (IQR 4.44). Follow-up time of MCI−was significantly longer than mean progression time of MCI+ [8.35 (IQR 6.33), p < 0.001, η2 = 0.27] years. There was no difference in proportion of IA between MCI−and MCI+ (p = 0.661).
At baseline no significant differences were found with respect to disease duration, family history of AD, sex, years of education, TIB, MMSE, HDRS and BFFQ score between MCI−and MCI+ (Table 1).
In the whole sample, energy was directly correlated with conscientiousness (Pearson = 0.318, p = 0.019) and openness (Pearson = 0.614, p < 0.001). Emotional stability was directly correlated with conscientiousness (Pearson = 0.357, p = 0.008) and inversely correlated with HDRS (Pearson = −0.30, p = 0.028). Openness was significantly higher in man than in women (51.47 ± 10.51 vs. 4481 ± 9.80, p = 0.028, Cohen's d = 0.66). BFFQ scores were not associated with demographic features, neuropsychological scores and APOE genotype.
CAG repeat number in the shorter allele was inversely correlated with conscientiousness (Pearson = −0.364, p = 0.007). There was no correlation between CAG repeat numbers and HDRS scores.
We performed this analysis in the MCI− and MCI+ groups separately. In the MCI+ group CAG-repeat length on the shorter allele was inversely correlated with conscientiousness (Pearson = −0.695, p = 0.006, Figure 2A) and energy (Pearson = −0.694 p = 0.006, Figure 2B); CAG repeat length on the longer allele was inversely correlated with conscientiousness (Pearson = −0.901, p < 0.001, Figure 2C) and directly correlated with emotional stability (Pearson = 0.639, p = 0.014, Figure 2D). We found no correlations between BFFQ parameters and CAG repeats in the MCI− group. There was no association between HDRS score and CAG repeat length neither in MCI− nor in MCI+ group.
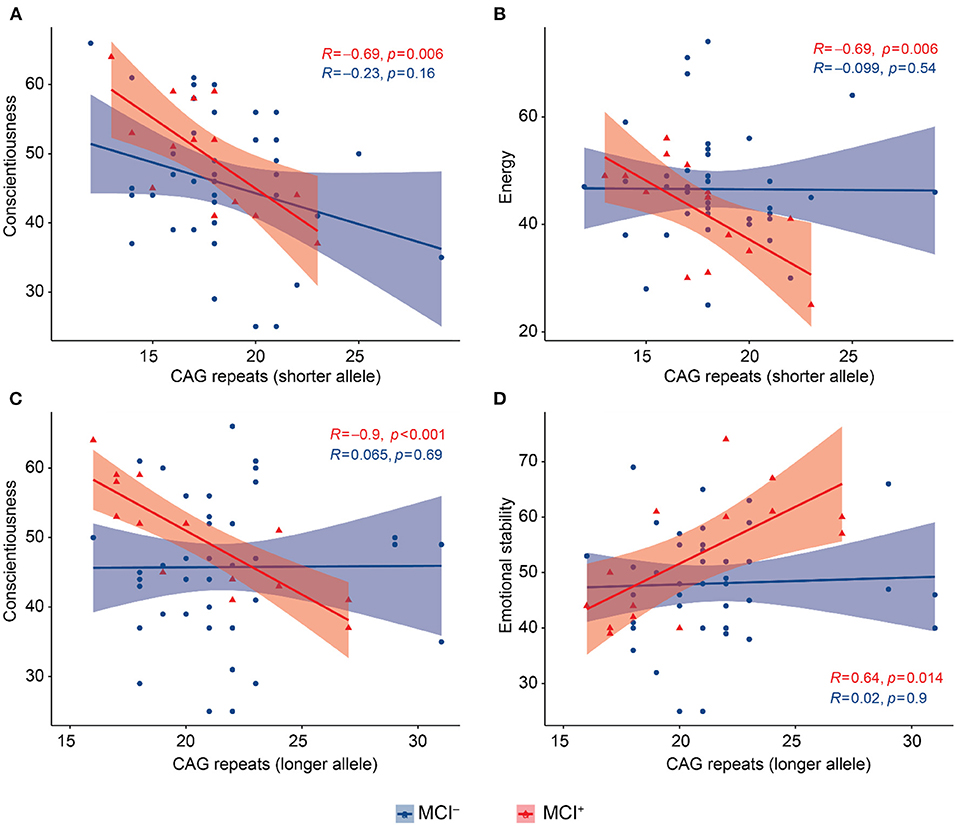
Figure 2. Scatter plots with lines of best fit (95% C.I.) showing the relationship between CAG repeat lengths and BFFQ dimensions in MCI− and MCI+. Pearson correlation coefficient (R) and level of significance (p) are reported (statistical significance at the p < 0.05). (A) CAG repeat length on the shorter allele was inversely correlated with conscientiousness in MCI+. (B) CAG repeat length on the shorter allele was inversely correlated with energy in MCI+. (C) CAG repeat length on the longer allele was inversely correlated with conscientiousness in MCI+. (D) CAG repeat length on the longer allele was directly correlated with emotional stability in MCI+.
To ascertain that the associations between personality traits and CAG repeat lengths were independent from confounding factors, we performed multiple regression analyses in the MCI+ group. Energy, emotional stability and conscientiousness were considered as dependent variables one at a time. CAG repeat numbers in the shorter and longer allele were both considered as independent variables. Variables that have been found to be associated with personality traits have been considered as covariates. There was no evidence of multicollinearity among covariates, as assessed by tolerance values >0.10. Energy remains significantly associated with CAG repeat length on the HTT shorter allele [B = −2.33 (95%CI = −4.02:−6.4), p = 0.011]; emotional stability remains significantly associated with CAG repeats on the HTT longer allele [B = 2.30 (95%CI = 1.26:3.34), p = 0.001] and with HDRS [B = −1.49 (95%CI = −2.24:−0.73), p = 0.001]; conscientiousness remains significantly associated with HTT longer allele [B = −1.46 (95%CI = −2.26:−0.66), p = 0.002] (Table 2).
In the present study we explored the association between CAG repeat lengths in the HTT gene with personality traits and mood in a sample of SCD patients followed-up for a median time of 13 years. As the main result we found that the length of CAG repeats in the HTT gene within the non-pathological range are associated with personality traits in SCD patients who progressed to MCI but not in patients who remained stable. In particular, lower level of conscientiousness and energy and higher level of emotional stability were correlated with higher number of CAG repeats. These associations were independent from possible confounding variables and were not influenced by depressive symptoms measured by HDRS. An association between HTT variants and personality traits was shown by Larsen et al. (6). Interestingly, these authors found opposite associations: HD carriers and healthy first-degree relatives (individuals at risk for HD) had greater conscientiousness and lower emotional stability as compared to controls. However, they did not find any difference between HD carriers and non-carriers at risk for HD, concluding that there is no direct effect of the HTT gene on personality traits. The discrepancies with our results could be due to the different populations considered. Larsen et al. enrolled both HD carriers and HD non-carriers with family history for HD while none of the individuals included in our sample had family history for HD. It is known that HD symptoms and onset are influenced also by modifier genes other than HTT (30, 31). Therefore, we could speculate the opposite effect of HTT in individuals at risk and not at risk for HD might follow a polygenic effect.
Moreover, Larsen et al. did not report CAG repeat numbers of the control group. So, we do not know if the effect on personality traits is due to the higher number of CAG repeats or to the family history for HD. We aim to test these hypotheses in future studies on larger samples.
To the best of our knowledge there are no previous reports showing an effect of HTT CAG repeat length on personality traits in non-HD patients. Even more importantly, our results showed that his effect may depend on the pathological background of the SCD.
SCD is an heterogeneous condition including normal aging, psychiatric conditions, neurologic and medical disorders, substance use, and medication (32). Longitudinal data allowed us to isolate patients whose memory symptoms were more probably due to a neurodegenerative condition underlying SCD. In other words, it is probable that the SCD of patients who progressed to MCI was due to a degenerative disease already active at the SCD stage.
We already showed that CAG repeat lengths in HTT may differently influence neuropsychological performances according to cognitive status of subjects (14). In more detail, we showed that a higher number of GAC repeats in the non-pathological range was associated with higher scores in tasks assessing executive function, memory, visual–spatial ability and language in SCD patients but to lower scores in the same cognitive domain in MCIs. Therefore, we might speculate that the effect found in this study of HTT variants both on cognitive function and on personality traits may be evident only when a pathological process occurs. In other words, as mutant HTT leads to a defined personality profile in HD patients, the number of CAG repeats in HTT influence personality traits and neuropsychological function also in patients with a probable neurodegenerative process underlying a subjective or objective cognitive decline.
The biological substrate of this effect might lie in the interaction of HTT protein with a number of proteins with a role in microtubule-based axon trafficking (33, 34). In particular, wild-type Hintingtin protein specifically enhances the vesicular transport of Brain Derived Natriuretic Factor (BDNF) (35), a neurotrophic factor involved in synaptic connections (36), neural growth (37), synaptic plasticity (38), and essential for long-term potentiation underlying hippocampus-related memory (14, 39). PolyQ tracts in Huntingtin protein stabilize interactions (40), according to a non-linear relation with the best function reached at an intermediate number of CAG repeats and then showing a progressive decrease (41). We might speculate that the interaction between Huntingtin and BDNF or the effect of this interaction on axonal trafficking may depend not only on the length of PolyQ tracts, but also on a possible pathological process underlying the progression from SCD to MCI. We think that our results could represent first clinical evidence to further explore this mechanism.
It would be interesting to know if this effect changes over time, for instance, collecting BFFQ data also at the end of the follow-up and in patients without SCD.
The relationships of personality traits with SCD and risk of progression of cognitive decline have been already reported by previous works with different results. Lower conscientiousness has been associated with higher complaints about their memory in SCD (42). Prospective studies indicate that individuals who score higher on conscientiousness have a slower rate of cognitive decline and reduced risk of developing dementia, even in the presence of AD neuropathology (43, 44). Conscientiousness is even associated with less amyloid deposition in cognitively normal aging (45). Low emotional stability has been reported as a risk factor for clinical AD and memory deficit has been associated with a faster rate of cognitive decline (46), while higher emotional stability seems to moderate the effect of the APOEε4 on cognitive function and risk of dementia (47). On the contrary, in a previous study we showed that higher emotional stability was linked to higher risk of progression from SCD to MCI.
We are not aware of studies showing association of energy with SCD and progression to objective cognitive impairment. Nevertheless, it is well recognized that patients with AD dementia showed a reduction of social life, tendency to social isolation, loss of motivation or reduced initiative (48).
Interestingly, recent studies showed that carriers of IAs had higher β-amyloid burden and developed MCI more frequently than non-carriers of IAs (49).
Taking this evidence together, we could speculate that CAG repeat number in HTT gene might be one of the genetic factors mediating the effect of conscientiousness, emotional stability and energy on neurodegeneration and progression of cognitive decline. We aim to better explore this hypothesis in further studies, first of all expanding our sample size. Indeed, the small sample size is the first limitation of the present work, in particular when we classified patients according to the progression to MCI (only 14 patients were MCI+). Moreover, only six patients were carriers of the IA. A larger sample would allow us to assess for personality trait differences between IA− and IA+. Another limitation is the lack of a control group. Finally, as it is a single-center study, there may be biases regarding assessment and diagnosis procedures and inclusion of only Caucasian participants.
However, this study has some remarkable strengths: this is the first study assessing the correlation of CAG repeat lengths with personality traits in patients without family history of HD. This may lead to the exclusion of a polygenic effect of modifier genes which are involved in HD symptoms and onset in individuals with family history for HD. The second strength is the very long, median follow-up time. In fact, follow-up time in the MCI−is much longer than the time of conversion of MCI+. This information allows us to minimize the possible underestimation of progression to MCI.
We showed that length of CAG repeats in the HTT gene within the non-pathological range might influence personality traits in SCD patients who will progress to MCI. Low level of conscientiousness, low level of energy and high level of emotional stability are associated with a higher number of CAG repeats. Both personality traits and CAG repeat length in the intermediate range have been associated with progression of cognitive decline and neuropathological findings consistent with AD. If confirmed by further studies on larger samples, our results may be the key to reveal the missing link among HTT gene, personality traits and neurodegenerative process.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana. The patients/participants provided their written informed consent to participate in this study.
SS, BN,VB, and SM: conceptualization. VM, SM, SB, SP, AI, VB, and LB: methodology. SM: statistical analysis. VM, VB, SM, FE, CM, GG, and SP: investigation. VB, SM, SB, and CF: resources. SM, GG, CM, and TF: data curation. VM, SM, and VB: writing—original draft preparation. VM, SM, BN, and VB: writing—review and editing. VB, BN, and SS: supervision and project administration. VB and BN: funding acquisition. All authors have read and agreed to the published version of the manuscript.
This research project was funded by Tuscany Region (Grant No 20RSVB—PREVIEW: PRedicting the EVolution of SubjectIvE Cognitive Decline to Alzheimer's Disease With machine learning) and by RICATEN21 (Ateneo Università di Firenze, fondi Ateneo 2021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Smith R, Brundin P, Li J-Y. Synaptic dysfunction in Huntington's disease: a new perspective. Cell Mol Life Sci. (2005) 62:1901–12. doi: 10.1007/s00018-005-5084-5
2. Schulte J, Littleton JT. The biological function of the Huntingtin protein and its relevance to Huntington's disease pathology. Curr Trends Neurol. (2011) 5:65–78.
3. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's disease collaborative research group. Cell. (1993) 72:971–83. doi: 10.1016/0092-8674(93)90585-E
4. Reiner A, Albin RL, Anderson KD, D'Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci U S A. (1988) 85:5733–7. doi: 10.1073/pnas.85.15.5733
5. ACMG/ASHG statement. Laboratory guidelines for Huntington disease genetic testing. The american college of medical genetics/american society of human genetics Huntington disease genetic testing working group. Am J Hum Genet. (1998) 62:1243–7. doi: 10.1086/301846
6. Larsen IU, Mortensen EL, Vinther-Jensen T, Nielsen JE, Knudsen GM, Vogel A. Personality traits in Huntington's disease: an exploratory study of gene expansion carriers and non-carriers. Am J Med Genet B Neuropsychiatr Genet. (2016) 171:1153–60. doi: 10.1002/ajmg.b.32501
7. Eddy CM, Rickards HE. Theory of mind can be impaired prior to motor onset in Huntington's disease. Neuropsychology. (2015) 29:792–8. doi: 10.1037/neu0000190
8. Killoran A, Biglan KM, Jankovic J, Eberly S, Kayson E, Oakes D, et al. Characterization of the Huntington intermediate CAG repeat expansion phenotype in PHAROS. Neurology. (2013) 80:2022–7. doi: 10.1212/WNL.0b013e318294b304
9. Menéndez-González M, Clarimón J, Rosas-Allende I, Blázquez M, San Martín ES, García-Fernández C, et al. HTT gene intermediate alleles in neurodegeneration: evidence for association with Alzheimer's disease. Neurobiol Aging. (2019) 76:215.e9–14. doi: 10.1016/j.neurobiolaging.2018.11.014
10. Rosas I, Martínez C, Clarimón J, Lleó A, Illán-Gala I, Dols-Icardo O, et al. Role for ATXN1, ATXN2, and HTT intermediate repeats in frontotemporal dementia and Alzheimer's disease. Neurobiol Aging. (2020) 87:139.e1–e7. doi: 10.1016/j.neurobiolaging.2019.10.017
11. Michael A, Bengt W, Lars OT, Sophia SW. Huntingtin levels are elevated in hippocampal post-mortem samples of Alzheimer's disease brain. Curr Alzheimer Res. (2020) 17:858–67. doi: 10.2174/1567205017666201203125622
12. Jellinger KA. Alzheimer-type lesions in Huntington's disease. J Neural Transm. (1998) 105:787–99. doi: 10.1007/s007020050095
13. Davis MY, Keene CD, Jayadev S, Bird T. The co-occurrence of Alzheimer's disease and Huntington's disease: a neuropathological study of 15 elderly Huntington's disease subjects. J Huntingtons Dis. (2014) 3:209–17. doi: 10.3233/JHD-140111
14. Bessi V, Mazzeo S, Bagnoli S, Giacomucci G, Ingannato A, Ferrari C, et al. The effect of CAG repeats within the non-pathological range in the HTT gene on cognitive functions in patients with subjective cognitive decline and mild cognitive impairment. Diagnostics. (2021) 11:1051. doi: 10.3390/diagnostics11061051
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. (1969) 9:179–86. doi: 10.1093/geront/9.3_Part_1.179
16. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the national institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc. (2011) 7:263–9. doi: 10.1016/j.jalz.2011.03.005
17. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. AlzheimersDement. (2011) 7:270–9. doi: 10.1016/j.jalz.2011.03.008
18. Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A. Rey-Osterrieth complex figure: normative values in an Italianpopulation sample. Neurol Sci. (2002) 22:443–7. doi: 10.1007/s100720200003
19. Baddeley A, Della Sala S, Papagno C, Spinnler H. Dual-task performance in dysexecutive and nondysexecutive patients with a frontal lesion. Neuropsychology. (1997) 11:187–94. doi: 10.1037/0894-4105.11.2.187
20. Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. (1996) 17:305–9. doi: 10.1007/BF01997792
21. Brazzelli M, Della Sala S, Laiacona M. Calibration of the Italian version of the rivermeadbehavioural memory test: a test for the ecological evaluation of memory. Bollettino di Psicologia Applicata. (1993) 206:33–42.
22. Bracco L, Amaducci L, Pedone D, Bino G, Lazzaro MP, Carella F, et al. ItalianMulticentre Study on Dementia (SMID): a neuropsychological test battery for assessingAlzheimer'sdisease. J Psychiatr Res. (1990) 24:213–26. doi: 10.1016/0022-3956(90)90011-E
23. Crook TH, Feher EP, Larrabee GJ. Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatr. (1992) 4:165–76. doi: 10.1017/S1041610292000991
24. Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
25. Colombo L, Sartori G, Brivio C. Stima del quoziente intellettivo tramite l'applicazione del TIB (Test Breve di Intelligenza). Giornale Italiano Psicol. (2002) 3:613–38. doi: 10.1421/1256
26. Nelson H. National Adult Reading Test (NART): For the Assessment of Premorbid Intelligence in Patients with Dementia: Test Manual, NFER-Nelson. Windsor, ON: NFER-Nelson (1982).
27. Costa PT Jr., McCrae RR. The revised NEO personality inventory (NEO-PI-R). In: Boyle GJ, Matthew G, Saklofske DH, editors. The SAGE Handbook of Personality Theory and Assessment: Volume 2 — Personality Measurement and Testing. London: SAGE Publications Ltd. p. 179–98.
28. Sorbi S, Nacmias B, Forleo P, Latorraca S, Gobbini I, Bracco L, et al. APOE allele frequencies in Italian sporadic and familial Alzheimer's disease. Neurosci Lett. (1994) 177:100–2. doi: 10.1016/0304-3940(94)90054-X
29. Jama M, Millson A, Miller CE, Lyon E. Triplet repeat primed PCR simplifies testing for Huntington disease. J Mol Diagn. (2013) 15:255–62. doi: 10.1016/j.jmoldx.2012.09.005
30. Moss DJH, Pardiñas AF, Langbehn D, Lo K, Leavitt BR, Roos R, et al. Identification of genetic variants associated with Huntington's disease progression: a genome-wide association study. The Lancet Neurology. (2017) 16:701–11.
31. Genetic Modifiers of Huntington's Disease (GeM-HD) Consortium. Identification of genetic factors that modify clinical onset of Huntington's disease. Cell. (2015) 162:516–26. doi: 10.1016/j.cell.2015.07.003
32. Margolis SA, Kelly DA, Daiello LA, Davis J, Tremont G, Pillemer S, et al. Anticholinergic/sedative drug burden and subjective cognitive decline in older adults at risk of Alzheimer's disease. The Journals of Gerontology: Series A. (2020) doi: 10.1093/gerona/glaa222
33. Engelender S, Sharp AH, Colomer V, et al. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet. (1997) 6:2205–12. doi: 10.1093/hmg/6.13.2205
34. McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem. (2006) 281:3552–9. doi: 10.1074/jbc.M509806200
35. Gauthier LR, Charrin BC, Borrell-Pagès M, Dompierre JP, Rangone H, Cordelières FP, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. (2004) 118:127–38. doi: 10.1016/j.cell.2004.06.018
36. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. (2001) 24:677–736. doi: 10.1146/annurev.neuro.24.1.677
37. Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth factors. (2004) 22:123–31. doi: 10.1080/08977190410001723308
38. McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. (1995) 15:791–803. doi: 10.1016/0896-6273(95)90171-X
39. Bekinschtein P, Cammarota M, Katche C, et al. BDNF is essential to promote persistence of long-term memory storage. Proc Nat Acad Sci. (2008) 105:2711–6. doi: 10.1073/pnas.0711863105
40. Schaefer MH, Wanker EE, Andrade-Navarro MA. Evolution and function of CAG/polyglutamine repeats in protein–protein interaction networks. Nucleic Acids Res. (2012) 40:4273–87. doi: 10.1093/nar/gks011
41. Lee JK, Ding Y, Conrad AL, et al. Sex-specific effects of the Huntington gene on normal neurodevelopment. J Neurosci Res. (2017) 95:398–408. doi: 10.1002/jnr.23980
42. Steinberg SI, Negash S, Sammel MD, Bogner H, Harel BT, Livney MG, et al. Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. Am J Alzheimers Dis Other Demen. (2013) 28:776–83. doi: 10.1177/1533317513504817
43. Terracciano A, Iacono D, O'Brien RJ, Troncoso JC, An Y, Sutin AR, et al. Personality and resilience to Alzheimer's disease neuropathology: a prospective autopsy study. Neurobiol Aging. (2013) 34:1045–50. doi: 10.1016/j.neurobiolaging.2012.08.008
44. Duberstein PR, Chapman BP, Tindle HA, Sink KM, Bamonti P, Robbins J, et al. Personality and risk for Alzheimer's disease in adults 72 years of age and older: a 6-year follow-up. Psychol Aging. (2011) 26:351–62. doi: 10.1037/a0021377
45. Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry. (2007) 64:1204–12. doi: 10.1001/archpsyc.64.10.1204
46. Johansson L, Guo X, Duberstein PR, Hällström T, Waern M, Ostling S, et al. Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology. (2014) 83:1538–44. doi: 10.1212/WNL.0000000000000907
47. Dar-Nimrod I, Chapman BP, Franks P, Robbins J, Porsteinsson A, Mapstone M, et al. Personality factors moderate the associations between apolipoprotein genotype and cognitive function as well as late onset Alzheimer disease. Am J Geriatr Psychiatry. (2012) 20:1026–35. doi: 10.1097/JGP.0b013e318267016b
48. Hsiao Y-H, Chang C-H, Gean P-W. Impact of social relationships on Alzheimer's memory impairment: mechanistic studies. J Biomed Sci. (2018) 25:3. doi: 10.1186/s12929-018-0404-x
49. Mazzeo S, Emiliani F, Bagnoli S, Padiglioni S, Conti V, Ingannato A, et al. Huntingtin gene intermediate alleles influence the progression from subjective cognitive decline to mild cognitive Impairment: a 14-year follow-up study. Eur J Neurol. (2022). doi: 10.1111/ene.15291 [Epub ahead of print].
Keywords: subjective cognitive decline (SCD), mild cognitive impairment (MCI), Huntington (disease), Alzheimer's disease, HTT CAG repeat, personality, intermediate alleles, big five questionnaire
Citation: Moschini V, Mazzeo S, Bagnoli S, Padiglioni S, Emiliani F, Giacomucci G, Morinelli C, Ingannato A, Freni T, Belloni L, Ferrari C, Sorbi S, Nacmias B and Bessi V (2022) CAG Repeats Within the Non-pathological Range in the HTT Gene Influence Personality Traits in Patients With Subjective Cognitive Decline: A 13-Year Follow-Up Study. Front. Psychiatry 13:826135. doi: 10.3389/fpsyt.2022.826135
Received: 30 November 2021; Accepted: 11 February 2022;
Published: 11 March 2022.
Edited by:
Gianfranco Spalletta, Santa Lucia Foundation (IRCCS), ItalyReviewed by:
Yijuang Chern, National Research Program for Biopharmaceuticals, TaiwanCopyright © 2022 Moschini, Mazzeo, Bagnoli, Padiglioni, Emiliani, Giacomucci, Morinelli, Ingannato, Freni, Belloni, Ferrari, Sorbi, Nacmias and Bessi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benedetta Nacmias, YmVuZWRldHRhLm5hY21pYXNAdW5pZmkuaXQ=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.