
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 17 May 2022
Sec. Psychopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.823826
 Hikaru Hori1*†
Hikaru Hori1*† Norio Yasui-Furukori2†
Norio Yasui-Furukori2† Naomi Hasegawa3
Naomi Hasegawa3 Jun-ichi Iga4
Jun-ichi Iga4 Shinichiro Ochi4
Shinichiro Ochi4 Kayo Ichihashi5
Kayo Ichihashi5 Ryuji Furihata6
Ryuji Furihata6 Yoshitaka Kyo7
Yoshitaka Kyo7 Yoshikazu Takaesu8
Yoshikazu Takaesu8 Takashi Tsuboi9
Takashi Tsuboi9 Fumitoshi Kodaka10
Fumitoshi Kodaka10 Toshiaki Onitsuka11
Toshiaki Onitsuka11 Tsuyoshi Okada12
Tsuyoshi Okada12 Atsunobu Murata3
Atsunobu Murata3 Hiroko Kashiwagi3,13
Hiroko Kashiwagi3,13 Hitoshi Iida1
Hitoshi Iida1 Naoki Hashimoto14
Naoki Hashimoto14 Kazutaka Ohi15
Kazutaka Ohi15 Hisashi Yamada3,16
Hisashi Yamada3,16 Kazuyoshi Ogasawara17
Kazuyoshi Ogasawara17 Yuka Yasuda3,18
Yuka Yasuda3,18 Hiroyuki Muraoka19
Hiroyuki Muraoka19 Masahide Usami20
Masahide Usami20 Shusuke Numata21
Shusuke Numata21 Masahiro Takeshima22
Masahiro Takeshima22 Hirotaka Yamagata23
Hirotaka Yamagata23 Tatsuya Nagasawa24
Tatsuya Nagasawa24 Hiromi Tagata25
Hiromi Tagata25 Manabu Makinodan26
Manabu Makinodan26 Mikio Kido27,28
Mikio Kido27,28 Eiichi Katsumoto29
Eiichi Katsumoto29 Hiroshi Komatsu30
Hiroshi Komatsu30 Junya Matsumoto3
Junya Matsumoto3 Chika Kubota13
Chika Kubota13 Kenichiro Miura3
Kenichiro Miura3 Akitoyo Hishimoto31
Akitoyo Hishimoto31 Koichiro Watanabe9
Koichiro Watanabe9 Ken Inada19
Ken Inada19 Hiroaki Kawasaki1
Hiroaki Kawasaki1 Ryota Hashimoto3
Ryota Hashimoto3In several clinical guidelines for schizophrenia, long-term use of anticholinergic drugs is not recommended. We investigated the characteristics of the use of anticholinergics in patients with schizophrenia by considering psychotropic prescription patterns and differences among hospitals. A cross-sectional, retrospective prescription survey at the time of discharge was conducted on 2027 patients with schizophrenia from 69 Japanese hospitals. We examined the relations among psychotropic drug prescriptions regarding anticholinergic prescription. We divided the hospitals into three groups—low rate group (LG), medium rate group (MG), and high rate group (HG)—according to their anticholinergic prescription rates, and analyzed the relationship between anticholinergic prescription rates and antipsychotic prescription. Anticholinergic drugs were prescribed to 618 patients (30.5%), and the prescription rates were significantly higher for high antipsychotic doses, antipsychotic polypharmacy, and first-generation antipsychotics (FGAs) use. The anticholinergic prescription rate varied considerably among hospitals, ranging from 0 to 66.7%, and it was significantly higher in patients with antipsychotic monotherapy, antipsychotic polypharmacy, and normal and high doses of antipsychotics in HG than in those LG and MG. The anticholinergics prescription rate in patients with second-generation antipsychotic monotherapy in HG was also significantly higher than in those LG and MG; however, the difference was no longer significant in patients with FGA monotherapy. Conclusively, in addition to high antipsychotic doses, antipsychotic polypharmacy, and FGA use, hospital characteristics influence the prescribing of anticholinergic drugs.
Schizophrenia is one of the top 15 causes of health burden in the world (1). The characteristics of its clinical presentation are positive and negative symptoms and cognitive impairment (2). In recent years, the goal of treatment for schizophrenia has been recovery, which requires improvement in the level of functioning, including cognitive dysfunction (3–8). Antipsychotic medication is the mainstay for managing schizophrenia, and psychosocial treatments, including cognitive-behavioral therapy, social skills training, assertive community treatment, supported employment, occupational therapy (9), and teaching illness, are helpful as well.
Antipsychotics induce a blockade of the dopamine D2 receptors, and this antagonism in the limbic system may be responsible for the effect on positive symptoms, whereas the antagonism of dopamine transmission in the basal ganglia causes extrapyramidal symptoms (EPS). In clinical practice, anticholinergics are widely used for treating and preventing EPS induced by antipsychotic use (10). In general, first-generation antipsychotics (FGAs) are prone to cause EPS, whereas this risk decreases in the case of second-generation antipsychotics (SGAs), whereas antipsychotic polypharmacy causes a higher EPS rate than antipsychotic monotherapy (11). Moreover, studies have shown that antipsychotic-induced EPS is mostly dose-dependent (12).
Anticholinergics are also known to be associated with peripheral side effects, such as urinary disturbances, dry mouth, and constipation. Moreover, its main adverse effects are often associated with impaired cognitive functioning in schizophrenia (13–15). Therefore, in current treatment guidelines for schizophrenia, prophylactic use of anticholinergics is not recommended in the long term (13, 16–18). The high use rate of long-term concurrent anticholinergics with antipsychotics has been noted as an important therapeutic issue in some countries. For instance, Xiang et al. (19) found that anticholinergics were administered to more than half of the considered schizophrenia patients in 2009. In addition, a previous Japanese study reported that adjunctive anticholinergics were prescribed to 56–75% of chronic schizophrenia inpatients even though they were taking SGAs (20). A European study performed in six countries found that 30% of patients treated with SGAs were also prescribed anticholinergics (21). Based on these findings, one can conclude that significant discrepancies between the recommended treatment guidelines and real-world clinical practice in schizophrenia exist globally (22, 23).
To disseminate and implement uniform clinical guidelines, the Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE) project was started in 2016 (24–31). The EGUIDE project started with the cooperation of 22 hospitals, and as of 2021, more than 240 hospitals (44 universities) have participated. In brief, the EGUIDE project disseminates the guidelines through lectures and case discussions. Previous reports from the EGUIDE project have shown that there are large interinstitutional differences in prescribing treatment patterns for schizophrenia and major depressive disorders (24, 25, 26). We hypothesized that this would be the case for anticholinergic prescriptions as well, with large variations in prescribing patterns from hospital to hospital, influenced by the underlying antipsychotic prescriptions.
In this study, we analyzed the anticholinergic prescriptions for patients with schizophrenia in Japan using data at the time of discharge, further classified the patients into three groups according to the anticholinergic prescription rates at each hospital, and then compared them in terms of antipsychotic dosage, antipsychotic polypharmacy/monotherapy, and FGA/SGA monotherapy.
Using data from the EGUIDE project, a cross-sectional pharmacological survey was first conducted in 2016. A survey was conducted on all psychotropics on patients with schizophrenia who were discharged from participating hospitals between April and September before the educational program (25, 26, 32).
In total, 83 hospitals participated in this survey. A cross-sectional case record survey was conducted using standardized data collection from each study site, involving a sample of 2,177 patients who had been diagnosed with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria at the time of discharge. As our study aimed to investigate anticholinergics prescription status, 77 patients who were not administered antipsychotics were excluded from the analysis. Hospitals with <10 discharges during the study period were also excluded (14 hospitals, 73 patients). Finally, a total of 69 hospitals (33 university hospitals, 19 national/public hospitals, and 17 private hospitals) were included in this study (Figure 1). In this work, we analyzed data from the first year before joining the EGUIDE program from 2016 to 2018.
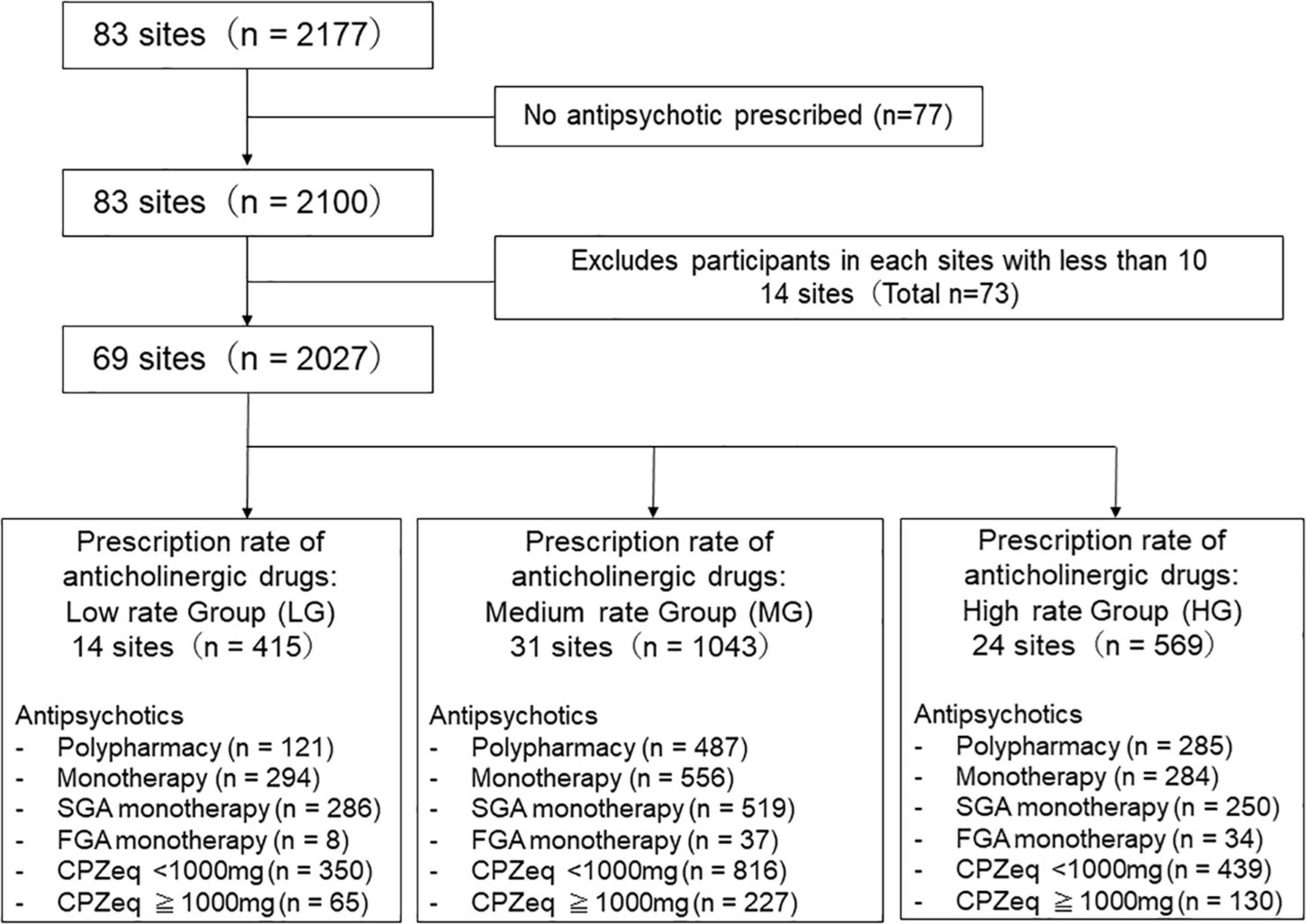
Figure 1. Study flow chart. The institutions were divided into three groups according to the anticholinergics prescription rate.
The EGUIDE project members collected the data. Age, gender, and clinical characteristics of the patients were collected. Information about the types and doses of all antipsychotic drugs was collected from medical records. Doses of antipsychotic drugs were converted into approximate chlorpromazine equivalent milligrams (CPZeq) (33). Based on previous reports (34), we defined the high-dose and normal-dose groups as ≥1,000 mg/day and <1,000 mg/day of CPZeq for antipsychotics, respectively. We also used biperiden equivalent (BPeq). Anticholinergics include amantadine, benztropine, biperiden, mazaticol, procyclidine, promethazine, and trihexyphenidyl.
The hospitals were divided into three groups according to the anticholinergic prescription rate. In the EGUIDE project, we aimed to reduce the prescription rate of anticholinergic drugs to <20%. To see the educational effect, we categorized the prescribing groups by hospital prescribing rate of 20%. We defined and categorized into three groups [low rate group (LG) (0≤% of anticholinergic prescription <20), medium rate group (MG) (20=% of anticholinergic prescription <40), and high rate group (HG) (40<%anticholinergic prescription)]. We compared the use of antipsychotics dosage, antipsychotics monotherapy/polypharmacy, and FGA/SGA monotherapy among these three groups of patients according to the anticholinergics prescription rate.
The ethics committees of the National Center of Neurology and Psychiatry and each participating university/hospital/clinic approved the entire study protocol, and the study was carried out in accordance with the latest version of the Declaration of Helsinki. Patients were able to opt-out of the purpose and procedures of the study and refuse study participation. The study protocol has been registered in the University Hospital Medical Information Network Registry (UMIN000022645).
All statistical analyses were performed using SPSS software for Windows (Version 26.0). The data are presented as mean ± standard deviations. We used the Shapiro–Wilk test to assess the data correspondence to the normal distribution. Demographic characteristics and the results were compared among the groups. The anticholinergic prescription rate was calculated as: schizophrenic patients prescribed anticholinergics at discharge/all discharged schizophrenia patients. Spearman’s correlation analysis was used to determine the correlation of the dosages of antipsychotics (CPZeq) and anticholinergics (BP). The descriptive statistics were tabulated using either the Chi square test or analysis of variance to test for significant differences across the groups with Post-hoc Bonferroni. The level of statistical significance was set at p < 0.05. For multiple comparison test, a p-value of <0.033 (0.05/3) was considered significant.
We enrolled 2,027 patients diagnosed with schizophrenia (924 males and 1,103 females). All baseline demographic data are summarized in Table 1. In this survey, 618 participants (30.5%) were administered with anticholinergics, the most common being biperiden (n = 493), trihexyphenidyl (n = 86), and promethazine (n = 68). A comparison of anticholinergic prescription rates for the Top 3 of these groups, biperiden, trihexyphenidyl, and promethazine, is shown in Supplementary Table 1. Prescribing rates for all three anticholinergics were higher for high antipsychotic doses, antipsychotic polypharmacy, and FGA use.
Among all samples, normal doses of antipsychotics had lower anticholinergic prescription rates than high doses (Antipsychotic high-dose: 43%, antipsychotic normal-dose: 27%, p = 2.0 × 10–10), and antipsychotic monotherapy had lower anticholinergic prescription rates than antipsychotic polypharmacy (antipsychotic monotherapy: 21%, antipsychotic polypharmacy: 43%, p = 2.2 × 10–16). Furthermore, among the monotherapy, FGA monotherapy had a higher anticholinergic prescription rate than SGA monotherapy (FGA monotherapy: 57%, SGA monotherapy 19%, p = 4.6 × 10–15) (Figure 2). A positive correlation was found between the antipsychotic dosage (CPZeq) and anticholinergics dosage (BPeq) [rs = 0.33, p = 6.4 × 10–35 (Figure 3)] and anticholinergic prescription rate in each hospital [rs = 0.34, p = 0.0004 (Figure 4)].
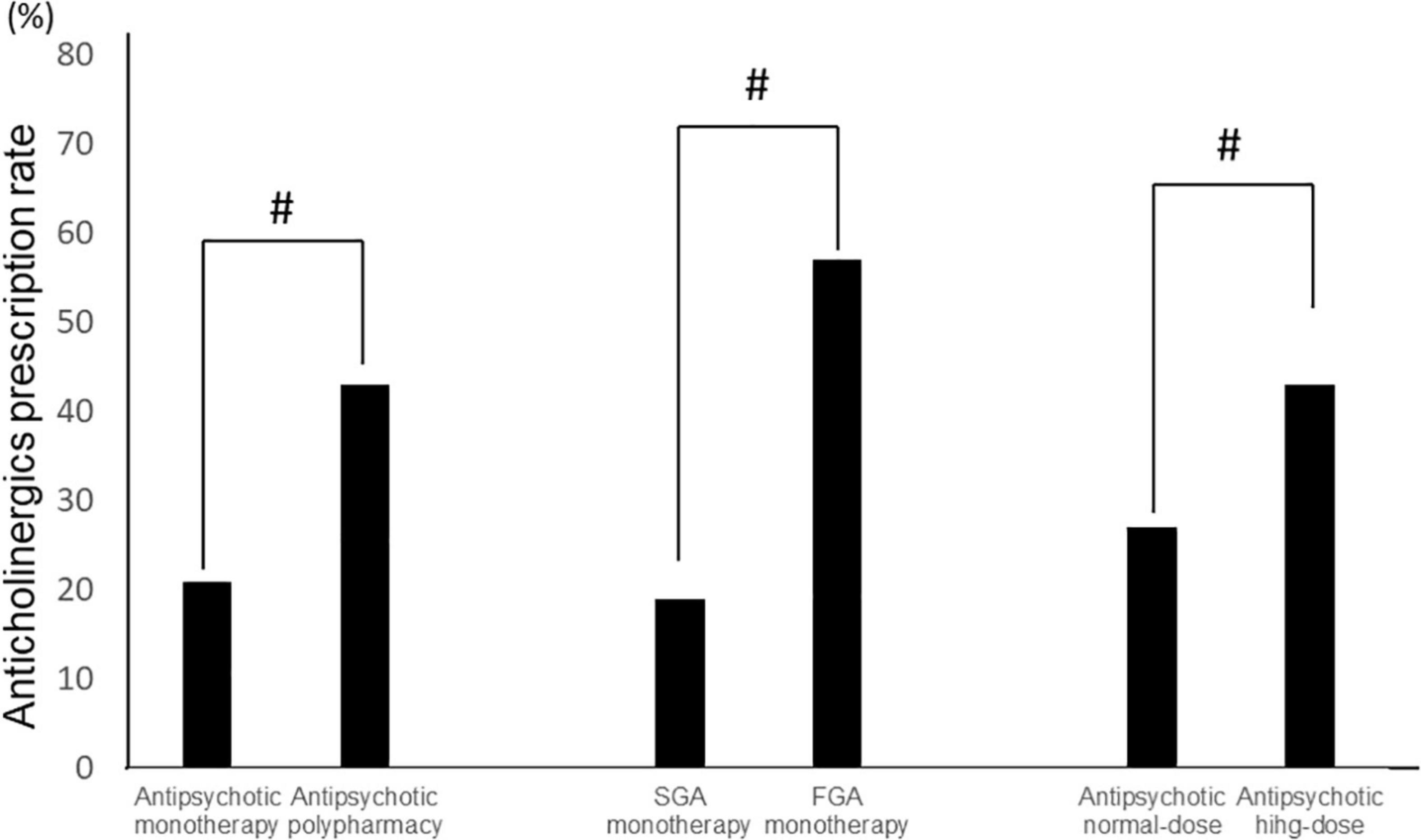
Figure 2. Comparison of anticholinergic prescription rates (antipsychotic monotherapy vs. antipsychotic polypharmacy, SGA monotherapy vs. FGA monotherapy, and antipsychotic normal-dose vs. antipsychotic high-dose). #p < 0.01. FGA, first generation antipsychotic; SGA, second generation antipsychotic.
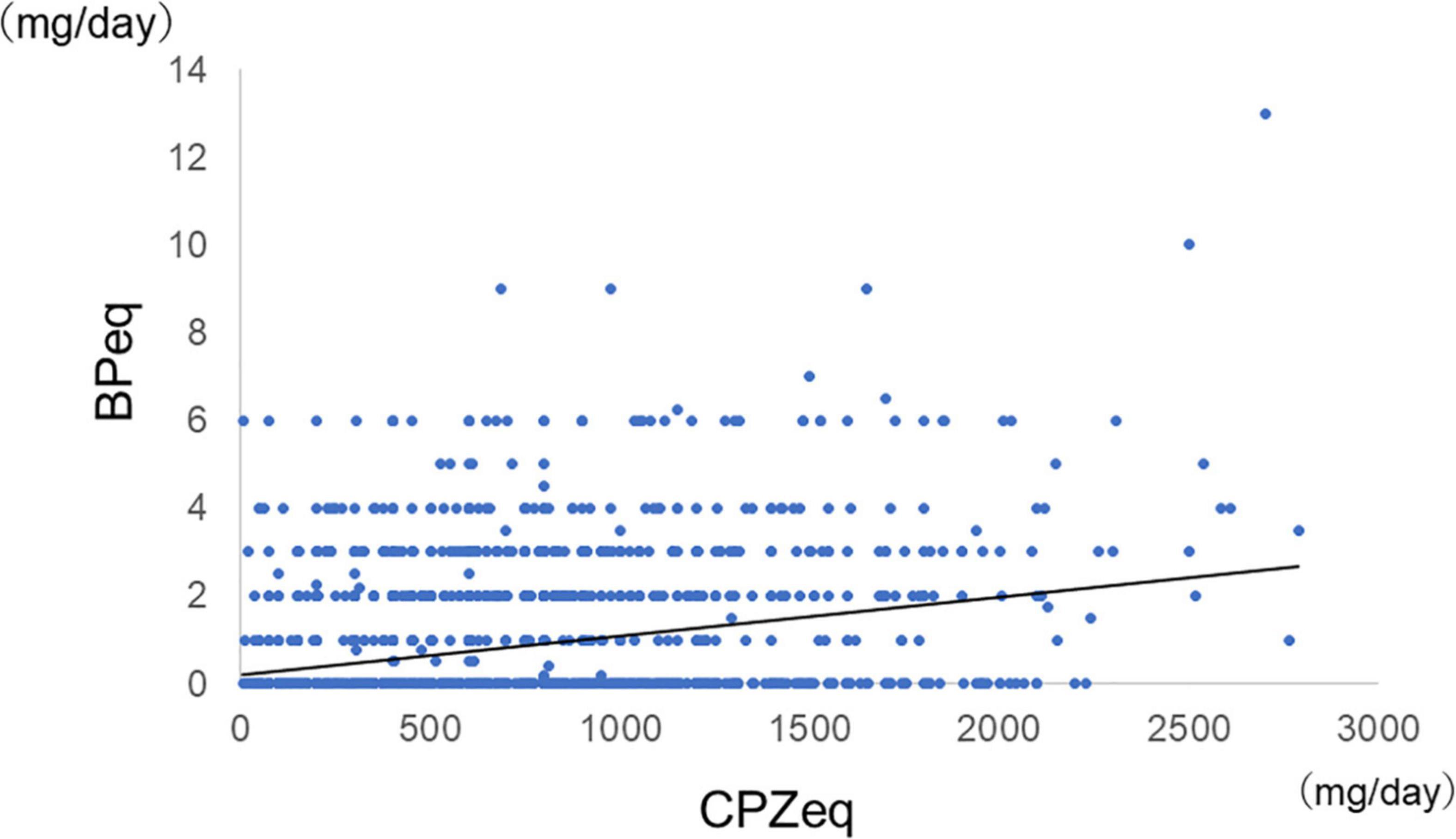
Figure 3. Correlation between antipsychotic dosage and dosage of anticholinergics (n = 2,027). rs = 0.33, p = 6.4 × 10–15. CPZeq, chlorpromazine equivalent; BPeq, biperiden equivalent.
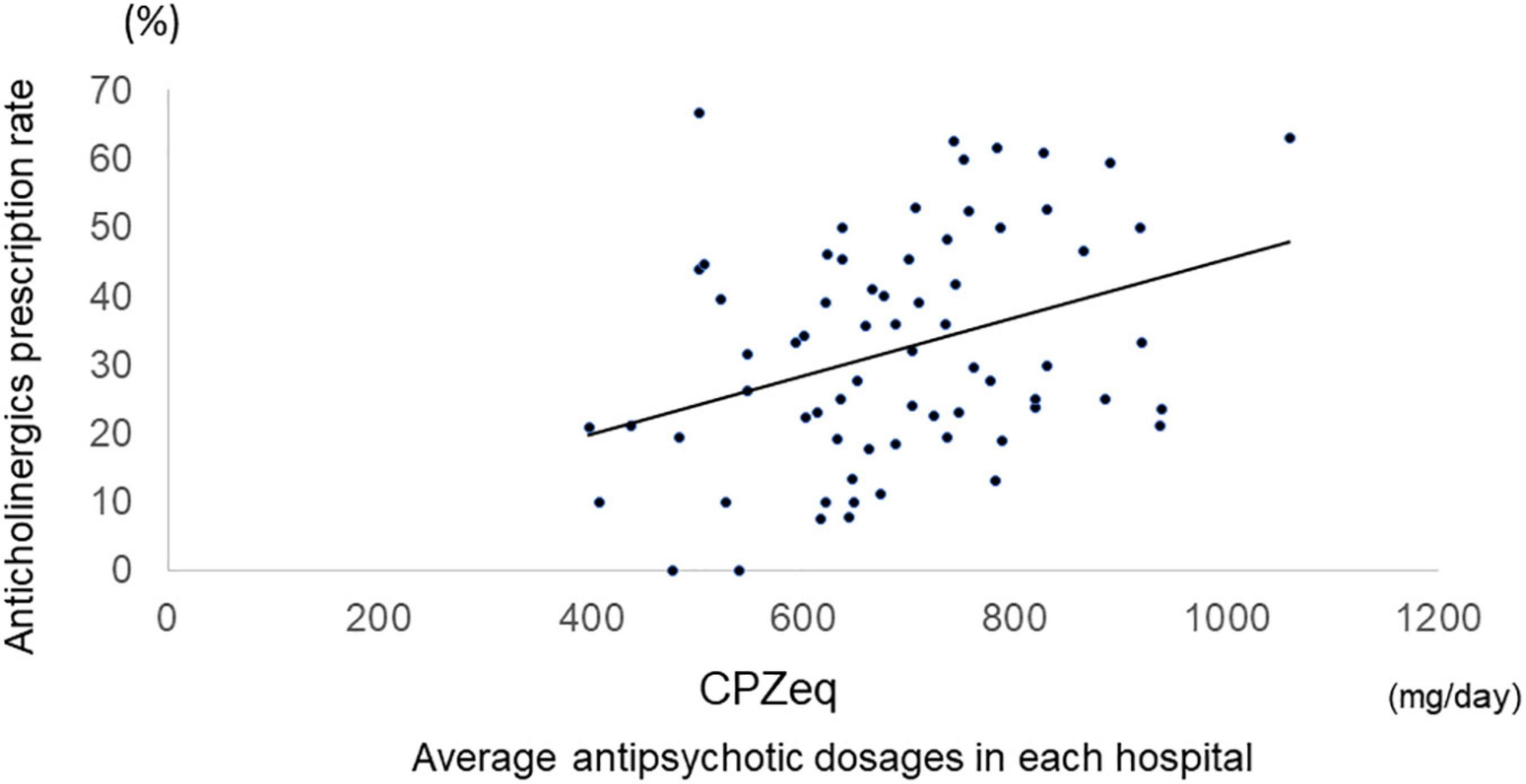
Figure 4. Correlation between antipsychotic average dosages in each hospital and anticholinergic prescription rate. rs = 0.34, p = 0.0004. CPZeq, chlorpromazine equivalent.
There was a significant difference in the average proportion of patients receiving anticholinergics among the hospitals, ranging from 0 to 66.7% (Figure 5). The patients were classified into three groups according to the rate of anticholinergics prescriptions at each hospital. Among the three groups, there were significant differences in age, doses of antipsychotics, antipsychotics monotherapy rate, and prescriptions rate of anxiolytics/hypnotics and mood stabilizers; LG had significantly lower doses of antipsychotics and prescriptions rate of anxiolytics/hypnotics and significantly higher rates of antipsychotic monotherapy than MG and HG (Table 2).
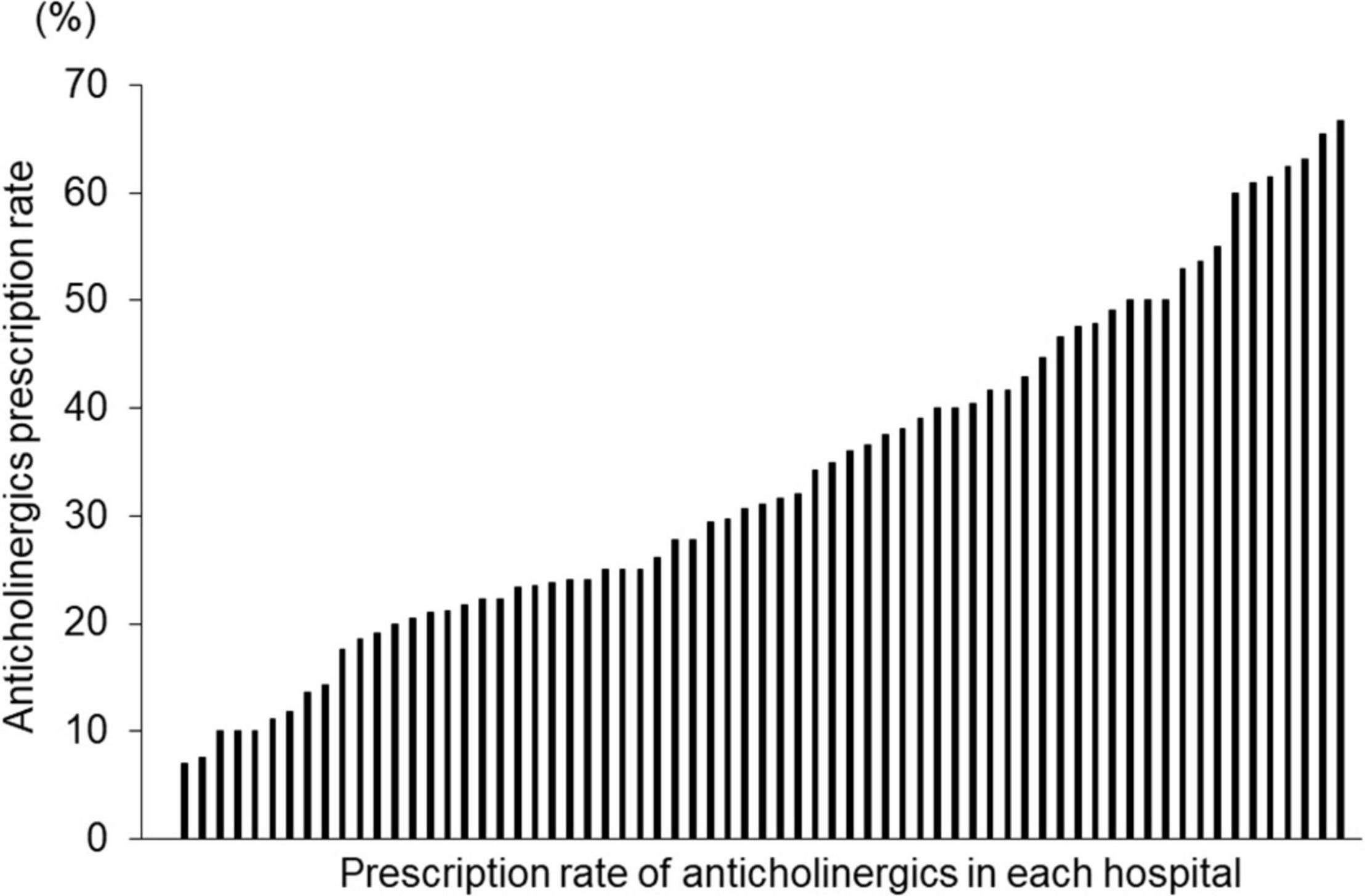
Figure 5. Proportion of patients with schizophrenia receiving anticholinergics in 69 hospitals. Vertical axis: Proportion of receiving anticholinergics in each hospital. Horizontal axis: Each hospital (N = 69).
We divided the patients into two groups according to the antipsychotic dose: high-dose (≥1,000 mg/day CPZeq) and normal-dose (<1,000 mg CPZeq). The Chi-square test showed a significant difference (p < 0.01). The rate of anticholinergic prescriptions was significantly higher in the high-dose group for all LG, MG, and HG groups (LG: 9.4, 20.0%; MG: 24.8, 33.9%; and HG: 45.1, 70.8% for normal and high doses, respectively). When comparing the rate of anticholinergic prescriptions in these three groups, a significant difference (p = 2.2 × 10–16 and 1.4 × 10–14 for normal and high doses, respectively) was observed. In the normal-dose group, HG had a significantly higher anticholinergic prescription rate than LG and MG, and MG had a significantly higher anticholinergic prescription rate than LG. In the high-dose group, HG had a significantly higher anticholinergic prescription rate than LG and MG (Figure 6). Moreover, LG in the normal-dose group had a significantly lower anticholinergic prescription rate than MG (p = 8.5 × 10–12) and HG (p < 2.2 × 10–16) in the high-dose group and LG in the higher dose group had significantly lower anticholinergic prescription rate than HG (p = 0.003) in the normal-dose group. HG in the higher anticholinergic prescription rate than MG (p < 2.2 × 10–16) in the normal-dose group. HG in the normal dose group had significantly lower prescription rate in the HG in the higher-dose group (p = 2.2 × 10–16).
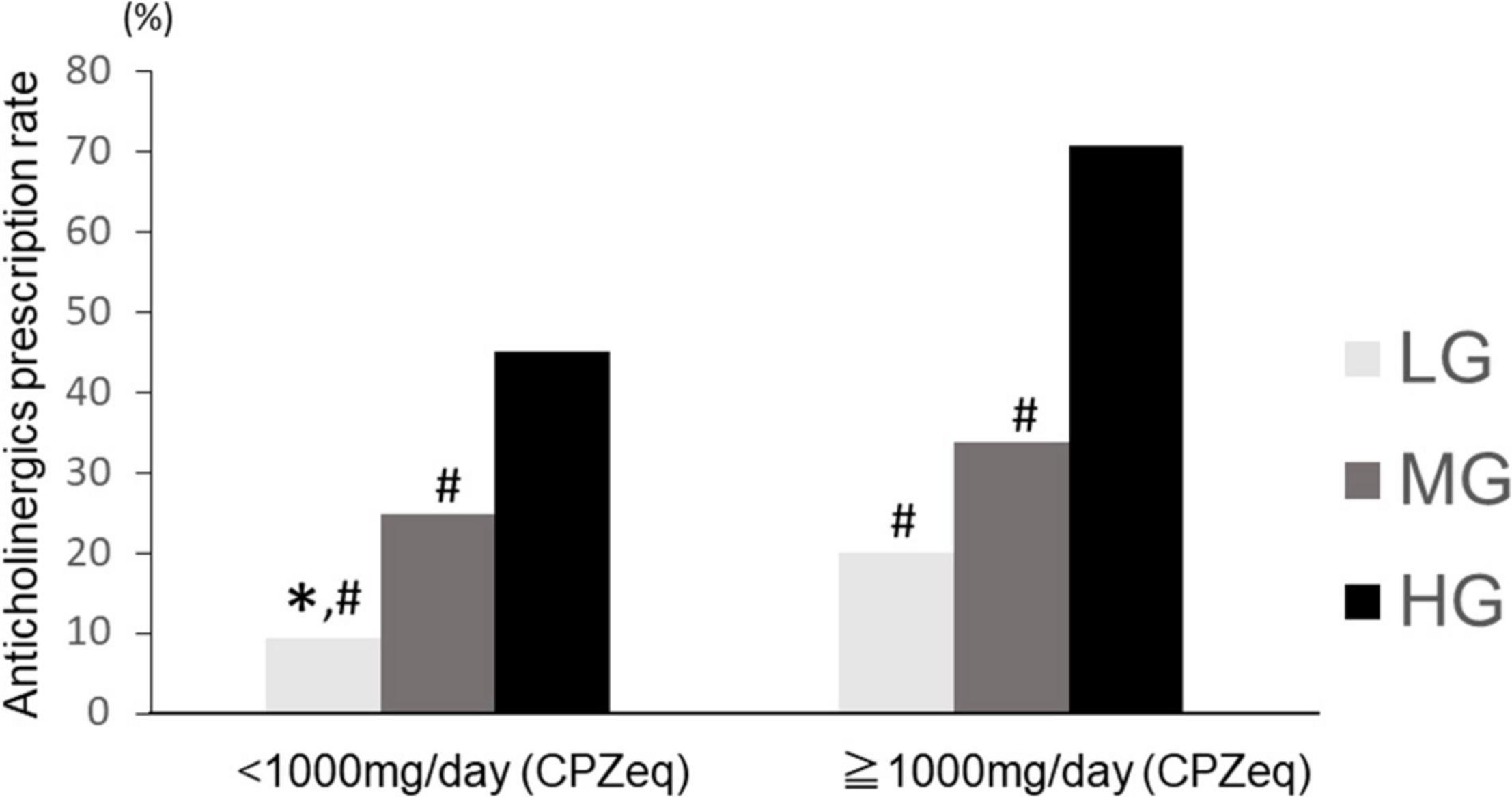
Figure 6. Anticholinergics prescription rate at high and normal doses of antipsychotics in the three groups. LG, low rate group; MG, medium rate group; HG, high rate group. Normal dose (CPZeq < 1,000 mg): LG (n = 350), MG (n = 816), HG (n = 439). High dose (CPZeq = 1,000 mg): LG (n = 65), MG (n = 227), HG (n = 130). ∗p < 0.033 vs. MG. #p < 0.033 vs. HG.
We compared the anticholinergic prescription rates of the patients treated with antipsychotic monotherapy and polypharmacy. The Chi square test showed a significant difference (p < 0.01). In all previously considered groups, the rate of anticholinergics was significantly lower for patients with antipsychotic monotherapy than for those with antipsychotic polypharmacy (LG: 7.1, 20.7%, MG: 21.2, 35.5%, HG: 36.6, 65.3% for antipsychotic monotherapy and polypharmacy, respectively) (Figure 7). In the antipsychotic polypharmacy, LG had a significantly lower anticholinergics prescription rate than HG (p = 7.0 × 10–15), and HG had a significantly higher anticholinergic prescription rate than MG (p = 3.6 × 10–14). In the antipsychotic monotherapy group, LG had a significantly lower anticholinergics prescription rate than MG (p = 3.3 × 10–6) and HG (p = 2.7 × 10–16) (Figure 7). Moreover, LG in the antipsychotic polypharmacy had a significantly higher antipsychotic prescription rate than LG (p = 0.002) in the antipsychotic monotherapy. LG in the monotherapy had a significantly lower anticholinergic prescription rate than MG (p < 2.2 × 10–16) and HG (p < 2.2 × 10–16) in the antipsychotic polypharmacy. In this way, the characteristics of the proportion of anticholinergics prescriptions rate across the hospitals were maintained, regardless of the patients being on antipsychotic polypharmacy or monotherapy.
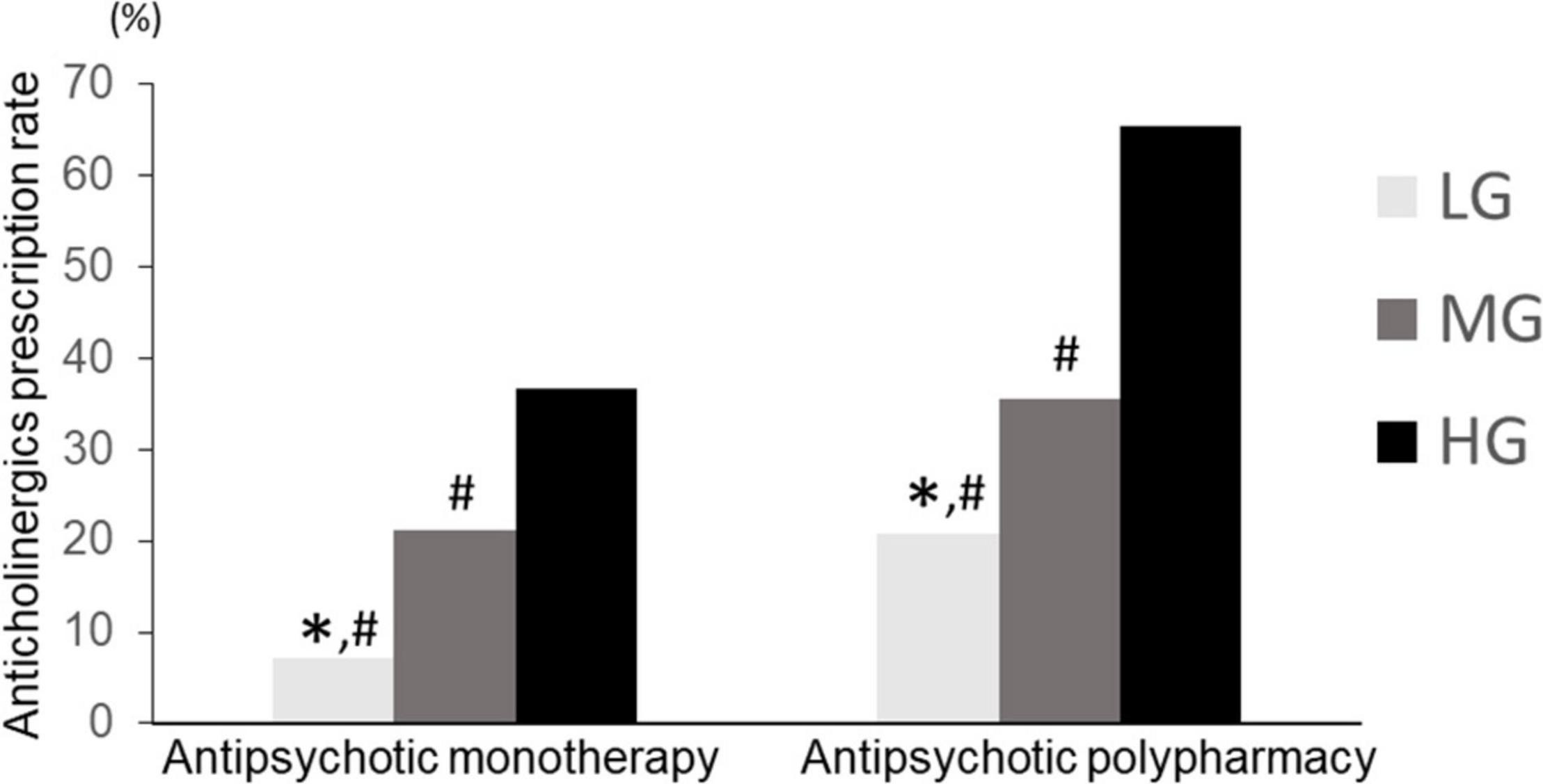
Figure 7. Anticholinergic prescription rate of LG, MG, and HG (antipsychotic monotherapy vs. antipsychotic polypharmacy). LG, low rate group. MG, medium rate group; HG, high rate group. Monotherapy, LG (n = 294), MG (n = 556), HG (n = 284). Polypharmacy: LG (n = 121), MG (n = 487), HG (n = 285). ∗p < 0.033 vs. MG. #p < 0.033 vs. HG.
Anticholinergics prescription rates were compared by dividing the antipsychotic monotherapy group into FGA monotherapy and SGA monotherapy. The Chi-square test showed a significant difference (p < 0.01). In the SGA monotherapy group, the anticholinergics prescriptions rate was significantly different among LG, MG, and HG groups (LG: 5.9%, MG: 19.5%, HG: 32.0%; p = 1.0 × 10–14). In a post-hoc analysis, LG and MG had significantly lower anticholinergic prescription rates than HG (p = 2.0 × 10–7, 5.4 × 10–15 for LG vs. HG and MG vs. HG, respectively) and MG had significantly lower anticholinergics prescription rates than HG (p = 5.6 × 10–4). Further, in the FGA monotherapy group, there was no significant difference in the rate of anticholinergics prescriptions among the three groups (LG: 50.0%, MG: 45.9%, HG: 70.6%; p = 0.104) (Figure 8). Moreover, LG in the SGA monotherapy had a significantly lower anticholinergic prescription rate than LG (p = 6.9 × 10–4), MG (p = 5.5 × 10–6), and HG (p = 2.0 × 10–13) in the FGA monotherapy. MG in the FGA monotherapy had a significantly higher anticholinergic prescription rate than LG (p = 1.1 × 10–11) and MG (p = 0.005) in the SGA monotherapy. HG in the SGA monotherapy had a significantly lower anticholinergic prescription rate than HG (p = 4.1 × 10–14) in the FGA monotherapy. Alternatively, treatment with SGA maintained the characteristics of the anticholinergics prescription rate among hospitals, whereas the FGA therapy did not follow any characteristics of anticholinergics prescription rate among hospitals.
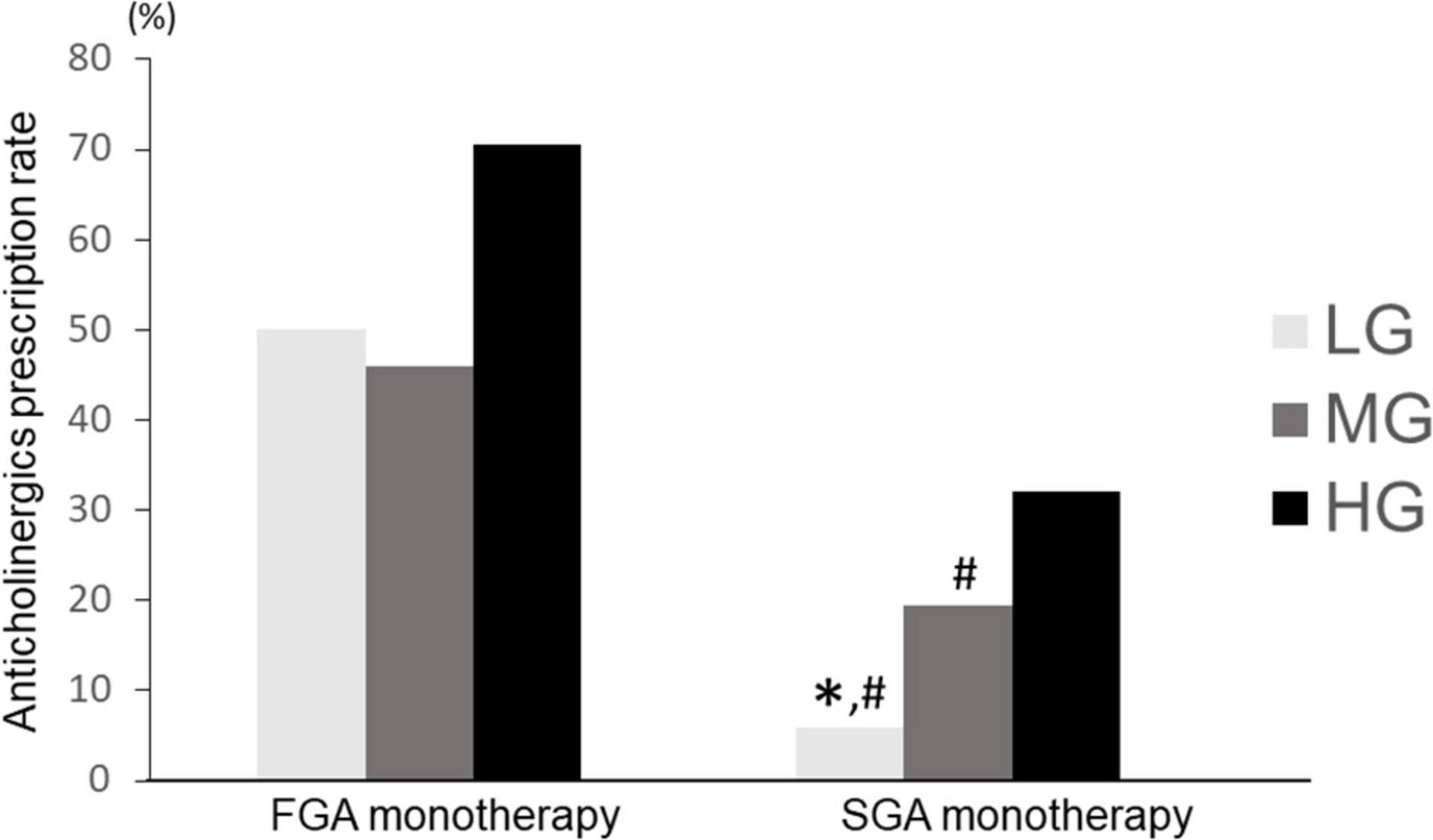
Figure 8. Comparison of anticholinergic prescription rates among LG, MG, and HG (SGA vs. FGA). LG, low rate group; MG, medium rate group; HG, high rate group; SGA, second generation antipsychotic; FGA, first generation antipsychotic. FGA monotherapy: LG (n = 8), MG (n = 37), HG (n = 34); SGA monotherapy: LG (n = 286), MG (n = 519), HG (n = 250); ∗p < 0.033 vs. MG. #p < 0.033 vs. HG.
A comparison of anticholinergic doses for each group in the categories described in “Antipsychotic Dosage and Anticholinergic Prescription in Each Group, Antipsychotic Prescription Pattern (Polypharmacy vs. Monotherapy) and Anticholinergic Prescription in Each Group, and Antipsychotic Monotherapy (First Generation Antipsychotic vs. Second Generation Antipsychotic) and Anticholinergic Prescription in Each Group” is presented in Supplementary Table 2. As with anticholinergic prescribing rates, dosages were higher for antipsychotics high dose, antipsychotic polypharmacy, and FGA use, as well as in hospitals that prescribed more anticholinergics (e.g., HG).
The results of this study show that anticholinergics were prescribed in approximately 30% of the considered patients with schizophrenia, which is similar to previous studies (35–38). In Japan, when anticholinergic drugs are used for schizophrenia patients, biperiden is often prescribed. To our knowledge, there is no clear evidence showing that biperiden is more effective or has fewer side effects than other anticholinergics. Biperiden was also the drug of choice in a previous study of the effects of anticholinergic drug reduction on cognitive function in Japanese patients with schizophrenia (39). Therefore, it appears that biperiden is often used in Japan for the treatment and prevention of extrapyramidal symptoms. In this study, we found that anticholinergic prescriptions varied by the hospital. Hospitals with high anticholinergic prescribing (e.g., HG) had high anticholinergic prescription rates for all prescribing patterns: dosage (high or normal), antipsychotic polypharmacy, and SGA monotherapy (Figures 6–8). In contrast, for FGA monotherapy, anticholinergic prescribing rates were high even in hospitals with fewer anticholinergic prescriptions (e.g., LG) (Figure 8). These results suggest that anticholinergic drugs may be prescribed even though they are not recommended in treatment guidelines, and the EGUIDE project is expected to optimize prescribing.
The purposes of the EGUIDE project are to educate psychiatrists about the treatment guidelines and to see if the quality of their clinical practice changes. In Asian studies (19, 37), the prescription rate for anticholinergic drugs is approximately 30%, similar to this study. Contrarily, studies in Europe have shown that the rate is <20% (40). This study showed that the prescription rate of anticholinergic drugs varied greatly from hospital to hospital. Particularly in hospitals classified as MG and HG groups, further education on anticholinergic prescription is needed. In addition, hospitals that were in the LG group will need to continue this status with education on the guidelines.
In general, antipsychotics have a dopamine D2 receptor blocking effect, and schizophrenia with EPS has a higher rate of D2 receptor blockade (41). A recent review reported that D2 receptor occupancy in the striatum greater than 78% increases the risk of EPS (42). In this study, we have also revealed a positive correlation between the antipsychotic dose and the anticholinergic use (Figure 3).
In this study, we divided the doses into high and normal doses and compared them across hospitals. The results of this study showed that hospitals with a higher frequency of anticholinergic usage had higher rates of anticholinergic prescription for both high and normal doses. Alternatively, rather than being the effect of antipsychotic dosage, the output may be more due to the prescribing habits of each hospital.
HG and MG also had a higher rate of antipsychotic polypharmacy. Consistent with the results of previous studies (43), the use of antipsychotic polypharmacy may be more likely to elicit EPS, resulting in increased use of anticholinergics. An interesting result of the present study is the observation that the anticholinergic prescribing rate is influenced by hospital characteristics, even if it is limited to antipsychotic monotherapy. This may be a consequence of the fact that hospitals that prescribe more anticholinergics (e.g., HG) either administer these prophylactically before the development of EPS or prescribe them without switching antipsychotics when EPS develops. In the current study, the monotherapy group was further divided into FGA monotherapy and SGA monotherapy for analysis, and in the SGA monotherapy group, anticholinergics were prescribed more frequently in hospitals with a higher prescribing rate, similar to the other findings of this study. Interestingly, there was no significant difference in anticholinergic prescription rates among the three groups in FGA monotherapy. This may be due to the pharmacological characteristics of FGA, i.e., when FGA is used as monotherapy, it must be prescribed even in hospitals that do not normally administer anticholinergics frequently (e.g., LG).
This study also revealed the proportion of concomitant anticholinergics use for each antipsychotic (Figure 9). Anticholinergics were used more frequently in FGA polypharmacy, followed by SGA polypharmacy, FGA monotherapy, and SGA monotherapy. Interestingly, there was the tendency of low rate of prescription of anticholinergics in patients with long-acting injection (LAI), which is in line with several previous studies suggesting that LAI has lower usage as antiparkinsonian drug than oral agents (44–46). Based on these results, it is advisable to plan prescriptions with SGA monotherapy. Regarding antipsychotics selection, it may be advisable to choose drugs that are less likely to produce extrapyramidal symptoms or to use LAI during the maintenance phase.
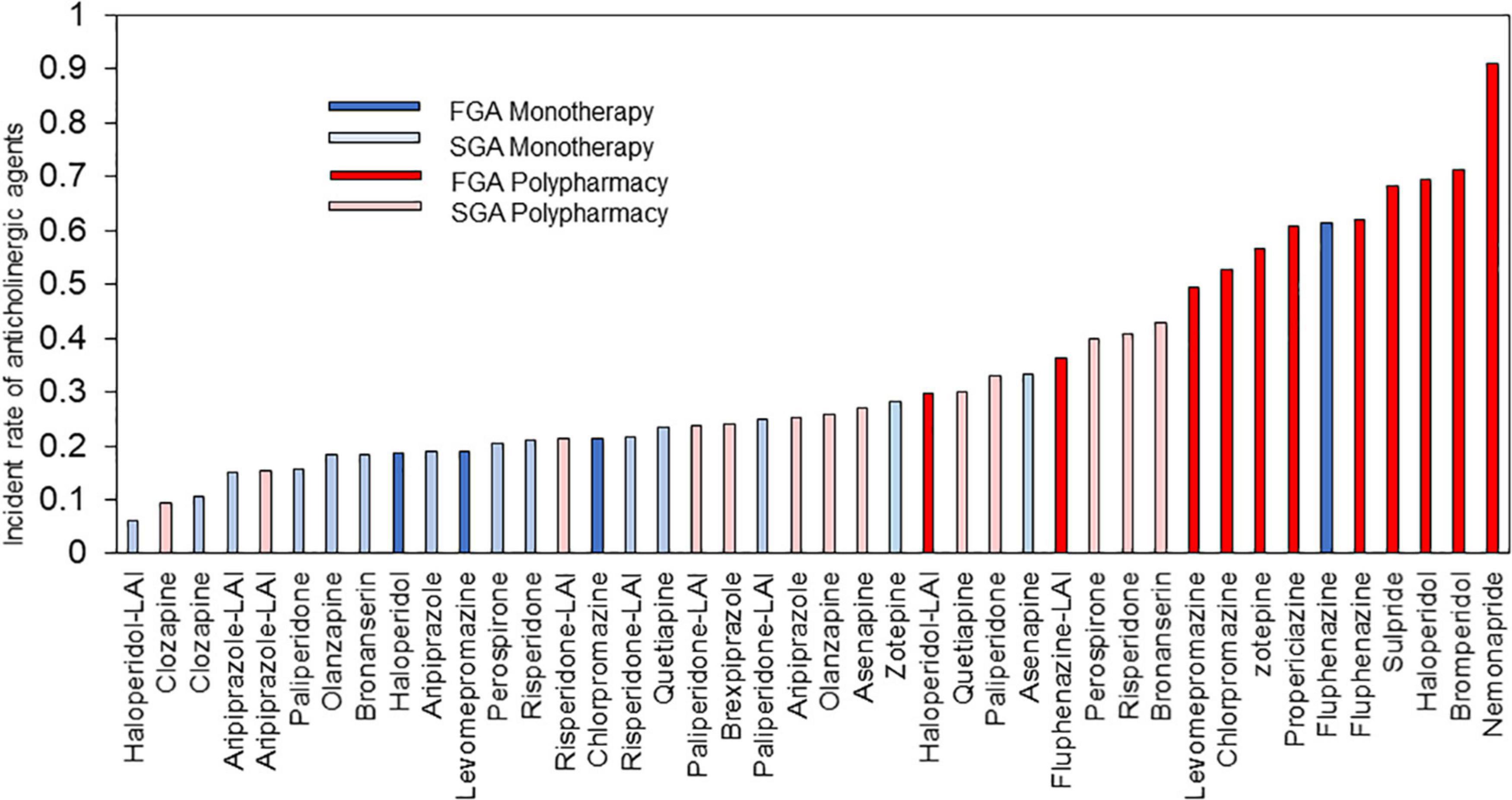
Figure 9. Anticholinergic drugs prescription rate of each antipsychotic. LAI, long-acting injection; FGA, first generation antipsychotic; SGA, second generation antipsychotic.
The current study presents some methodological strengths and limitations as follows. First, the absence of an evaluation of EPS rated by usual scales [e.g., Abnormal Inventory Movement Scale (AIMS) (47), UKU Side Effect Rating Scale (UKU) (48) or DIEPSS (49)] and the cross-sectional design did not allow us to evaluate the successful treatment of EPS with anticholinergics, and in how many patients, anticholinergics are further administered despite the absence of any EPS during long-term treatment, although the use of anticholinergics is connected to the presence of EPS in recent meta-analysis for antipsychotics (50). The EGUIDE project data also do not address side effects associated with anticholinergic drugs. Second, when evaluating the influence of antipsychotic treatment on anticholinergics users, we did not notice any specific effects of low-potency FGAs vs. high-potency FGAs, respectively, due to their different EPS liability. Third, other factors influencing anticholinergics prescription, such as the antipsychotic treatment duration, prescribing attitudes of the psychiatrists, and duration of anticholinergics use, were not surveyed. Fourth, the EGUIDE project focused on inpatients in several Japanese regions and sites; hence, the findings cannot apply to all patients, including outpatients, with schizophrenia in Japan. Further studies are required to overcome the drawbacks mentioned above. Despite the above limitations, the strengths of the study include all cases of patients with schizophrenia at discharge at a significant number of hospitals with >10 cases during the considered period; hence, the observed associations are unlikely to be due to chance.
Conclusively, institutional and biological (pharmacological) factors contribute to the prescription of anticholinergics. Institutional factors influence anticholinergic prescription in antipsychotic monotherapy, high dose of antipsychotics, and SGA monotherapy, whereas biological factors influence anticholinergics prescription in FGA monotherapy.
The datasets presented in this article are not readily available because, the data are not publicly available due to privacy and ethical restrictions (i.e., we did not obtain informed consent on the public availability of raw data). Requests to access the datasets should be directed to RH, cnlvdGFoYXNoaW1vdG81NUBuY25wLmdvLmpw.
The studies involving human participants were reviewed and approved by the ethics committees of the National Center of Neurology and Psychiatry and each participating university/hospital/clinic. The patients/participants provided their written informed consent to participate in this study.
HH, NY-F, and NHase were involved in data collection and data analysis and wrote the first draft of the manuscript. J-II, SO, KIc, RF, YK, YT, TT, FK, ToO, TsO, AM, and HKas were involved in the data analysis and contributed to the interpretation of the data and writing of the manuscript. HI, NHash, KOh, HisY, KOg, YY, HM, MU, SN, MT, HirY, TN, HT, MM, MK, EK, HKo, JM, CK, KM, and AH contributed to the interpretation of the data and data collection. KW and KIn were involved in the study design and contributed to the interpretation of the data. HKaw provided guidance and supported the research. RH supervised the entire project, collected the data, and was involved in the design, analysis, and interpretation of the data. All authors contributed to and approved the final article.
This study was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP 18dk0307060, the AMED under Grant Number JP 19dk0307083, the Health and Labor Science Research Grants (H29-Seishin-Ippan-001, 19GC1201), the Japanese Society of Neuropsychopharmacology, and the Japanese Society of Mood Disorders. The funders had no role in the study design, data collection and analyses, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We appreciate the cooperation of all the individuals who participated in this study. We thank the following psychiatrists belonging EGUIDE project members.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.823826/full#supplementary-material
Supplementary Table 1 | Differences in Top 3 anticholinergic prescribing rates in each group. LG, low rate group; MG, medium rate group; HG, high rate group; FGA, first generation antipsychotic; SGA, second generation antipsychotic.
Supplementary Table 2 | Comparison of anticholinergic doses in each dosing pattern in the three groups. LG, low rate group; MG, medium rate group; HG, high rate group; FGA, first generation antipsychotic; SGA, second generation antipsychotic.
AIMS, abnormal inventory movement scale; BPeq, biperiden equivalent; CPZeq, chlorpromazine equivalent; DIEPSS, drug-induced extrapyramidal symptoms scale; EGUIDE, effectiveness of guidelines for dissemination and education in psychiatric treatment; EPS, extrapyramidal symptoms; FGA, first generation antipsychotic; LAI, long-acting injection; SGA, second generation antipsychotic; UKU, UKU side effect rating scale.
1. Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the global burden of disease study 2010. Lancet. (2012) 380:2129–43. doi: 10.1016/s0140-6736(12)61680-8
2. Joyce EM, Roiser JP. Cognitive heterogeneity in schizophrenia. Curr Opin Psychiatry. (2007) 20:268–72. doi: 10.1097/YCO.0b013e3280ba4975
3. Hori H, Yamada K, Kamada D, Shibata Y, Katsuki A, Yoshimura R, et al. Effect of blonanserin on cognitive and social function in acute phase Japanese schizophrenia compared with risperidone. Neuropsychiatr Dis Treat. (2014) 10:527–33. doi: 10.2147/ndt.s59861
4. Hori H, Yoshimura R, Katsuki A, Sugita AI, Atake K, Nakamura J. Switching to antipsychotic monotherapy can improve attention and processing speed, and social activity in chronic schizophrenia patients. J Psychiatr Res. (2013) 47:1843–8. doi: 10.1016/j.jpsychires.2013.08.024
5. Nemoto T, Uchino T, Aikawa S, Saito J, Matsumoto H, Funatogawa T, et al. Social anxiety and negative symptoms as the characteristics of patients with schizophrenia who show competence-performance discrepancy in social functioning. Psychiatry Clin Neurosci. (2019) 73:394–9. doi: 10.1111/pcn.12848
6. Sampedro A, Peña J, Ibarretxe-Bilbao N, Sánchez P, Iriarte-Yoller N, Ledesma-González S, et al. Mediating role of cognition and social cognition on creativity among patients with schizophrenia and healthy controls: revisiting the shared vulnerability model. Psychiatry Clin Neurosci. (2020) 74:149–55. doi: 10.1111/pcn.12954
7. Shiga T, Horikoshi S, Kanno K, Kanno-Nozaki K, Hikita M, Itagaki S, et al. Plasma levels of dopamine metabolite correlate with mismatch negativity in patients with schizophrenia. Psychiatry Clin Neurosci. (2020) 74:289–93. doi: 10.1111/pcn.12984
8. Onitsuka T, Hirano Y, Nemoto K, Hashimoto N, Kushima I, Koshiyama D, et al. Trends in big data analyses by multicenter collaborative translational research in psychiatry. Psychiatry Clin Neurosci. (2021) 76:1–14. doi: 10.1111/pcn.13311
9. Shimada T, Ohori M, Inagaki Y, Shimooka Y, Ishihara I, Sugimura N, et al. Effect of adding individualized occupational therapy to standard care on rehospitalization of patients with schizophrenia: a 2-year prospective cohort study. Psychiatry Clin Neurosci. (2019) 73:476–85. doi: 10.1111/pcn.12858
10. Bezchlibnyk-Butler KZ, Remington GJ. Antiparkinsonian drugs in the treatment of neuroleptic-induced extrapyramidal symptoms. Can J Psychiatry. (1994) 39:74–84. doi: 10.1177/070674379403900203
11. Kendall T. The rise and fall of the atypical antipsychotics. Br J Psychiatry. (2011) 199:266–8. doi: 10.1192/bjp.bp.110.083766
12. Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. BioMed Res Int. (2014) 2014:656370. doi: 10.1155/2014/656370
13. Barnes TR, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the british association for psychopharmacology. J Psychopharmacol. (2020) 34:3–78. doi: 10.1177/0269881119889296
14. Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. (2004) 161:116–24. doi: 10.1176/appi.ajp.161.1.116
15. Ogino S, Miyamoto S, Miyake N, Yamaguchi N. Benefits and limits of anticholinergic use in schizophrenia: focusing on its effect on cognitive function. Psy Clin Neurosci. (2014) 68:37–49. doi: 10.1111/pcn.12088
16. Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. (2012) 13:318–78. doi: 10.3109/15622975.2012.696143
17. Jablensky A, Castle DJ, Dark F, Humberstone V, Killackey E, Kulkarni J, et al. The 2016 RANZCP guidelines for the management of schizophrenia and related disorders – what’s next? Australas Psychiatry. (2017) 25:600–2. doi: 10.1177/1039856217726691
18. Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, et al. The American psychiatric association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. (2020) 177:868–72. doi: 10.1176/appi.ajp.2020.177901
19. Xiang YT, Wang CY, Si TM, Lee EH, He YL, Ungvari GS, et al. Use of anticholinergic drugs in patients with schizophrenia in Asia from 2001 to 2009. Pharmacopsychiatry. (2011) 44:114–8. doi: 10.1055/s-0031-1275658
20. Yoshio T, Inada T, Uno J, Miwa T, Kitagawa K, Miyahara Y, et al. Prescription profiles for pharmacological treatment of Japanese inpatients with schizophrenia: comparison between 2007 and 2009. Hum Psychopharmacol. (2012) 27:70–5. doi: 10.1002/hup.1272
21. De Hert M, Wampers M, van Winkel R, Peuskens J. Anticholinergic use in hospitalised schizophrenic patients in Belgium. Psychiatry Res. (2007) 152:165–72. doi: 10.1016/j.psychres.2006.07.012
22. Baandrup L. Polypharmacy in schizophrenia. Basic Clin Pharmacol Toxicol. (2020) 126:183–92. doi: 10.1111/bcpt.13384
23. Neta G, Johnson KE. Informing real-world practice with real-world evidence: the value of PRECIS-2. BMC Med. (2018) 16:76. doi: 10.1186/s12916-018-1071-1
24. Furihata R, Otsuki R, Hasegawa N, Tsuboi T, Numata S, Yasui-Furukori N, et al. Hypnotic medication use among inpatients with schizophrenia and major depressive disorder: results of a nationwide study. Sleep Med. (2021) 22:23–30. doi: 10.1016/j.sleep.2021.11.005
25. Ichihashi K, Hori H, Hasegawa N, Yasuda Y, Yamamoto T, Tsuboi T, et al. Prescription patterns in patients with schizophrenia in Japan: first-quality indicator data from the survey of “effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)” project. Neuropsychopharmacol Rep. (2020) 40:281–6. doi: 10.1002/npr2.12122
26. Iida H, Iga J, Hasegawa N, Yasuda Y, Yamamoto T, Miura K, et al. Unmet needs of patients with major depressive disorder – findings from the ‘effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)’ project: a nationwide dissemination, education, and evaluation study. Psychiatry Clinical Neurosci. (2020) 74:667–9. doi: 10.1111/pcn.13143
27. Numata S, Nakataki M, Hasegawa N, Takaesu Y, Takeshima M, Onitsuka T, et al. Improvements in the degree of understanding the treatment guidelines for schizophrenia and major depressive disorder in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep. (2021) 41:199–206. doi: 10.1002/npr2.12173
28. Ogasawara K, Numata S, Hasegawa N, Nakatani M, Makinodan M, Ohi K, et al. Subjective assessment of participants in education programs on clinical practice guidelines in the field of psychiatry. Neuropsychopharmacol Rep. (2022). doi: 10.1002/npr2.12245 [Epub ahead of print].
29. Takaesu Y, Watanabe K, Numata S, Iwata M, Kudo N, Oishi S, et al. Improvement of psychiatrists’ clinical knowledge of the treatment guidelines for schizophrenia and major depressive disorders using the ‘effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)’ project: a nationwide dissemination, education, and evaluation study. Psychiatry Clin Neurosci. (2019) 73:642–8. doi: 10.1111/pcn.12911
30. Yamada H, Motoyama M, Hasegawa N, Miura K, Matsumoto J, Ohi K, et al. Improvement of psychiatrists’ clinical behaviors in accordance with the treatment guidelines for schizophrenia and major depressive disorders using the ‘effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)’ project: nationwide dissemination, education, and evaluation. Psychiatry Clin Neurosci. (2019) 73:642–8.
31. Yasui-Furukori N, Muraoka H, Hasegawa N, Ochi S, Numata S, Hori H, et al. Association between the examination rate of treatment-resistant schizophrenia and the clozapine prescription rate in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep. (2021) 42:3–9. doi: 10.1002/npr2.12218
32. Hashimoto N, Yasui-Furukori N, Hasegawa N, Ishikawa S, Numata S, Hori H, et al. Characteristics of discharge prescriptions for patients with schizophrenia or major depressive disorder: real-world evidence from the effectiveness of guidelines for dissemination and education (EGUIDE) psychiatric treatment project. Asian J Psychiatr. (2021) 63:102744. doi: 10.1016/j.ajp.2021.102744
33. Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. (2015) 69:440–7. doi: 10.1111/pcn.12275
34. Hori H, Noguchi H, Hashimoto R, Nakabayashi T, Omori M, Takahashi S, et al. Antipsychotic medication and cognitive function in schizophrenia. Schizophr Res. (2006) 86:138–46. doi: 10.1016/j.schres.2006.05.004
35. Broekema WJ, de Groot IW, van Harten PN. Simultaneous prescribing of atypical antipsychotics, conventional antipsychotics and anticholinergics-a European study. Pharm World Sci. (2007) 29:126–30. doi: 10.1007/s11096-006-9063-1
36. Desmarais JE, Beauclair L, Margolese HC. Anticholinergics in the era of atypical antipsychotics: short-term or long-term treatment? J Psychopharmacol. (2012) 26:1167–74. doi: 10.1177/0269881112447988
37. Dong M, Zeng LN, Zhang Q, Yang SY, Chen LY, Najoan E, et al. Prescription of antipsychotic and concomitant medications for adult Asian schizophrenia patients: findings of the 2016 research on Asian psychotropic prescription patterns (REAP) survey. Asian J Psychiatr. (2019) 45:74–80. doi: 10.1016/j.ajp.2019.08.010
38. Gjerden P, Slørdal L, Bramness JG. The use of antipsychotic and anticholinergic antiparkinson drugs in Norway after the withdrawal of orphenadrine. Br J Clin Pharmacol. (2009) 68:238–42. doi: 10.1111/j.1365-2125.2009.03446.x
39. Ogino S, Miyamoto S, Tenjin T, Kitajima R, Ojima K, Miyake N, et al. Effects of discontinuation of long-term biperiden use on cognitive function and quality of life in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:78–83. doi: 10.1016/j.pnpbp.2010.08.030
40. Pristed SG, Correll CU, Nielsen J. Frequency and correlates of anticholinergic use among patients with schizophrenia in Denmark: a nation-wide pharmacoepidemiological study. Psychiatry Res. (2017) 255:198–203. doi: 10.1016/j.psychres.2017.05.033
41. Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. (1992) 49:538–44. doi: 10.1001/archpsyc.1992.01820070032005
42. Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Dopamine D2 receptor occupancy and clinical effects: a systematic review and pooled analysis. Journal Clin Psychopharmacol. (2011) 31:497–502. doi: 10.1097/JCP.0b013e3182214aad
43. Carnahan RM, Lund BC, Perry PJ, Chrischilles EA. Increased risk of extrapyramidal side-effect treatment associated with atypical antipsychotic polytherapy. Acta Psychiatr Scand. (2006) 113:135–41.
44. Bai YM, Ting Chen T, Chen JY, Chang WH, Wu B, Hung CH, et al. Equivalent switching dose from oral risperidone to risperidone long-acting injection: a 48-week randomized, prospective, single-blind pharmacokinetic study. J Clin Psychiatry. (2007) 68:1218–25. doi: 10.4088/jcp.v68n0808
45. Gopal S, Liu Y, Alphs L, Savitz A, Nuamah I, Hough D. Incidence and time course of extrapyramidal symptoms with oral and long-acting injectable paliperidone: a posthoc pooled analysis of seven randomized controlled studies. Neuropsychiatr Dis Treat. (2013) 9:1381–92. doi: 10.2147/ndt.s49944
46. Lasser RA, Bossie CA, Gharabawi GM, Baldessarini RJ. Clinical improvement in 336 stable chronically psychotic patients changed from oral to long-acting risperidone: a 12-month open trial. Int J Neuropsychopharmacol. (2005) 8:427–38. doi: 10.1017/s1461145705005225
47. Guy WA. Abnormal Involuntary Movement Scale (AIMS). Washington, DC: U.S.: Department of Health Education and Welfare (1976). p. 534–7.
48. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand. (1987) 334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x
49. Inada T. Evaluation and Diagnosis of Drug-Induced Extrapyramidal Symptoms. Tokyo: Commentary on the DIEPSS and Guide to Its Usage (1996).
50. Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. (2019) 394:939–51. doi: 10.1016/s0140-6736(19)31135-3
Keywords: schizophrenia, anticholinergic drugs, antipsychotic polypharmacy, antipsychotic monotherapy, first-generation antipsychotics, second-generation antipsychotic (SGA)
Citation: Hori H, Yasui-Furukori N, Hasegawa N, Iga J-i, Ochi S, Ichihashi K, Furihata R, Kyo Y, Takaesu Y, Tsuboi T, Kodaka F, Onitsuka T, Okada T, Murata A, Kashiwagi H, Iida H, Hashimoto N, Ohi K, Yamada H, Ogasawara K, Yasuda Y, Muraoka H, Usami M, Numata S, Takeshima M, Yamagata H, Nagasawa T, Tagata H, Makinodan M, Kido M, Katsumoto E, Komatsu H, Matsumoto J, Kubota C, Miura K, Hishimoto A, Watanabe K, Inada K, Kawasaki H and Hashimoto R (2022) Prescription of Anticholinergic Drugs in Patients With Schizophrenia: Analysis of Antipsychotic Prescription Patterns and Hospital Characteristics. Front. Psychiatry 13:823826. doi: 10.3389/fpsyt.2022.823826
Received: 28 November 2021; Accepted: 04 April 2022;
Published: 17 May 2022.
Edited by:
Ji-chun Zhang, Jinan University, ChinaCopyright © 2022 Hori, Yasui-Furukori, Hasegawa, Iga, Ochi, Ichihashi, Furihata, Kyo, Takaesu, Tsuboi, Kodaka, Onitsuka, Okada, Murata, Kashiwagi, Iida, Hashimoto, Ohi, Yamada, Ogasawara, Yasuda, Muraoka, Usami, Numata, Takeshima, Yamagata, Nagasawa, Tagata, Makinodan, Kido, Katsumoto, Komatsu, Matsumoto, Kubota, Miura, Hishimoto, Watanabe, Inada, Kawasaki and Hashimoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hikaru Hori, aC5ob3JpLmxqQGFkbS5mdWt1b2thLXUuYWMuanA=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.