- 1Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Kowloon, Hong Kong SAR, China
- 2Department of Orthopaedics and Traumatology, The University of Hong Kong, Pokfulam, Hong Kong SAR, China
- 3Department of Orthopedic Surgery, Rush University Medical Center, Chicago, IL, United States
- 4International Spine Research and Innovation Initiative, Rush University Medical Center, Chicago, IL, United States
- 5Medical Research Center Oulu, Oulu University Hospital and University of Oulu, Oulu, Finland
- 6Rehabilitation Services of South Karelia Social and Health Care District, Lappeenranta, Finland
- 7Finnish Institute of Occupational Health, Oulu, Finland
- 8Department of Biomedical Engineering, The Hong Kong Polytechnic University, Kowloon, Hong Kong SAR, China
Introduction: Lumbar multifidus muscle (LMM) dysfunction is thought to be related to pain and/or disability in people with chronic low back pain (CLBP). Although psychosocial factors play a major role in pain/disability, they are seldom considered as confounders in analyzing the association between LMM and CLBP.
Objectives: This study aimed to determine: (1) differences in psychological factors, insomnia, and LMM characteristics between people with and without CLBP; (2) associations between psychological factors, insomnia, or LMM characteristics and low back pain (LBP) intensity or LBP-related disability in people with CLBP; and (3) whether LMM characteristics are related to LBP symptoms in people with CLBP after considering confounders.
Methods: Seventy-eight volunteers with CLBP and 73 without CLBP provided sociodemographic information, filled the 11-point numeric pain rating scale and Roland-Morris disability questionnaire (RMDQ). They completed the Hospital Anxiety and Depression Scale (HADS), Pain Catastrophizing Scale (PCS), Fear Avoidance Belief Questionnaire (FAB), and Insomnia Severity Index Scale (ISI). Resting and contracted thickness of LMM at L4-S1 levels were measured from brightness-mode ultrasound images. Percent thickness changes of LMM at L4-S1 levels during contraction were calculated. Resting LMM stiffness at L4-S1 was measured by shear wave elastography. Associations among LMM, psychosocial or insomnia parameters and clinical outcomes were analyzed by univariate and multivariate analyses.
Results: People with CLBP demonstrated significantly higher LBP-intensity, RMDQ, HADS, FAB, PCS, and ISI scores than asymptomatic controls (p < 0.05). The former also had significantly smaller percent thickness changes of LMM at L4/L5 during contraction. LBP-intensity was positively related to scores of PCS-total, PCS-helplessness, FAB-total, FAB-work, and ISI in people with CLBP (p < 0.05). RMDQ scores were positively associated with the scores of HADS-total, HADS-depression, PCS-total, FAB-total, FAB-physical activity, PCS-helplessness, and ISI in people with CLBP (p < 0.05). FAB-work and ISI scores together explained 24% of LBP-intensity. FAB-total scores alone explained 34% of variance of LBP-related disability in people with CLBP.
Conclusion: More fear-avoidance belief or insomnia is related to greater LBP-intensity and/or LBP-related disability in people with CLBP. Although people with CLBP were thought to have aberrant LMM morphometry/function, no LMM characteristics were related to LBP-intensity or LBP-related disability after considering other confounders.
Introduction
Low back pain (LBP) affects approximately 80% of adults at least once in their lifetime and is one of the leading causes of disability globally (1). LBP is defined as pain or discomfort between the twelfth ribs and buttocks (2). Although most LBP cases recover spontaneously, some people with LBP may experience chronic low back pain (CLBP) lasting for 3 months or more (3). The point prevalence of CLBP in the United States has been documented to be 13.1% (4). CLBP is one of the major causes of exorbitant treatment costs, and indirect costs due to sick leaves in the United States (5).
Morphometric and functional changes in lumbar multifidus muscle (LMM) may be related to CLBP (6–8). Since LMM is a spinal stabilizer that provides approximately two-thirds of spinal stability (9), aberrant changes in morphometry [e.g., muscle atrophy (10, 11) or fatty infiltration (12, 13)] or functional deficits of LMM (e.g., altered muscle activity and/stiffness) (14–16) may be related to the development or maintenance of CLBP. For instance, Danneels et al. reported low levels of surface electromyography activity in LMM among people with CLBP as compared to healthy individuals. Similarly, Masaki et al. (16) reported that the average LMM stiffness of people with CLBP was significantly higher than that of asymptomatic controls. Higher LBP intensity was significantly associated with higher LMM stiffness among people with CLBP (16). However, because prior research investigating the associations between LMM characteristics and CLBP clinical outcomes did not consider the influences of other confounders, it remains unclear whether their associations persist after taking confounders into account.
Multiple confounding factors are known to be related to CLBP. Compared to healthy individuals, people with CLBP are 2.3 to 3.2 folds more likely to have comorbidities (e.g., depression, anxiety and insomnia) (5). Previous research has suggested that various psychological factors [e.g., anxiety, depression, pain catastrophizing, fear-avoidance beliefs (FAB), etc.] are associated with pain intensity and/or disability in people with CLBP (17–22). In addition to mood disturbances, impaired sleep has been reported in people with CLBP (23, 24). Approximately 55% of people with CLBP experience insomnia (25), which is defined as sleep disturbance or difficulty in initiating sleep (26). People with CLBP also demonstrated significantly poorer sleep quality/quantity than asymptomatic individuals (23, 27).
Given the above, it is conceivable that correlations between various characteristics (e.g., resting and contracted LMM thickness, percent thickness changes during contraction, and resting muscle stiffness) of LMM and clinical outcomes in patients with CLBP may be modified after considering various psychological and/or sleep-related factors. A better understanding of these associations can improve the clinical management of these patients. Therefore, the current study aimed to: (1) compare the psychological factors, insomnia, and LMM characteristics between people with and without CLBP; (2) quantify the correlations between various psychological factors, sleep disturbance, or LMM characteristics and clinical outcomes (intensity of LBP and LBP-related disability) in people with CLBP; and (3) determine whether LMM characteristics are related LBP or LBP-related disability in people with CLBP after considering other confounders.
Materials and Methods
Participants and Study Design
This case-control study was conducted in a university laboratory. Individuals aged between 18 and 65 years were eligible for the study. Participants with CLBP (n = 78) were recruited from a public hospital, while asymptomatic participants (n = 73) were recruited from the university campus. People with CLBP were recruited if: (1) they experienced non-specific CLBP [defined as pain not attributable to a specific cause (28)] with or without leg pain that lasted for 3 months or more (3), that required medical consultation; and (2) their LBP intensity was at least 5 out of 10 on an 11-point numeric pain rating scale (NPRS). Age-matched asymptomatic controls should not experience an episode of LBP in the last 24 months. Exclusion criteria for all participants were: history of neurological disease, systemic inflammatory disease, previous spinal surgery, spinal fractures/tumors, metabolic disease, confirmed or suspected pregnancy, and indication for spine surgery.
Data Collection Procedures
Following the provision of informed written consent as suggested by the Human Subjects Ethics Sub-committee of the university (HSEAR20151027007-01), participants were instructed to complete a battery of questionnaires related to their demographics, Pain Intensity, LBP-related disability, fear-avoidance beliefs, pain catastrophizing, anxiety, depression, and insomnia.
Demographic Questionnaire
The questionnaire asked questions related to the participant’s age, gender, body mass index, education level, work status, married status, and smoking and drinking habits.
Standardized Questionnaires
Pain: An 11-point numeric pain rating scale (NPRS) was used to quantify LBP intensity, with “0” representing “no pain at all” and “10” representing “the worst imaginable pain” (29). Participants were asked to choose a number best represented: (1) the current level of pain; as well as (2) the least and (3) worst levels of pain during the past 24 h. The pain level over the past 24 h was estimated using the average of three ratings (30). The pain intensity level was categorized as mild (1–5), moderate (6–8) and severe (9–10) (31). A cut-off score of >4 is considered as the minimal clinically important change in people with CLBP (32). The scale has shown excellent test-retest reliability [intraclass correlation coefficient (ICC) = 0.99] in assessing pain intensity among people with musculoskeletal pain (33).
Low back pain-related disability: participants’ functional disability was assessed by the Hong Kong-Chinese version of the 24-item Roland-Morris Disability Questionnaire (RMDQ) (34). It evaluates the impact of LBP on daily function, with scores ranging from 0 to 24 (0 means no disability; 24 means maximum disability). From the total score, the disability was classified into mild (0–8), moderate (9–16), and high (17–24) severity (34). A cut-off score of >4 indicates people with dysfunctional LBP (35). RMDQ has demonstrated excellent test-retest reliability (ICC = 0.94) in assessing LBP-related disability in people with non-specific CLBP (34).
Mood: The Chinese version of the Hospital Anxiety and Depression Scale (HADS) was used to assess anxiety and depression (36). It consists of two 7-item subscales measuring anxiety (HADS-A) and depression (HADS-D). Each of the 14 items is scored from 0 to 3 (37). Total scores of <7, 8–10, 11–14, and 15–21 in each subscale indicate non-cases, mild, moderate, and severe problems, respectively (38). A cut-off value of >8 is considered as clinically significant scores in each subscale of anxiety or depression (39). For the total score, >13 is considered as clinically significant scores for both anxiety and depression (39). This questionnaire has shown excellent internal consistency (Cronbach’s alpha = 0.84) in evaluating anxiety and depression among Chinese patients with cancer and their family caregivers (40).
Pain catastrophizing: The Chinese version pain catastrophizing scale (PCS) was used to assess pain catastrophizing (41). This 13-item questionnaire consists of 3 subscales: rumination, magnification, and helplessness (41). Total PCS scores of 30 or above signify clinically significant pain catastrophizing in people with chronic pain (42). It has demonstrated excellent internal consistency for the total PCS score (α = 0.9) (41).
Fear-avoidance beliefs: The level of pain-related fear was evaluated by the Hong Kong-Chinese version of the 16-item Fear-Avoidance Beliefs Questionnaire (FAB). It has demonstrated excellent internal consistency (α = 0.8), reliability, and validity in measuring fear-avoidance beliefs in people with CLBP (17, 43). Each item was graded on a 7-point Likert-type scale (0 means completely disagree; 6 means completely agree). It consists of 2 subscales: (1) beliefs about damage from physical activity (FAB-PA) [4 items (2,3,4,5); score range: 0 to 24]; and (2) beliefs about damage from work-related activities (FAB-W) [7 items (6,7,9,10,11,12,15); score range: 0 to 42]. The remaining five items are excluded from the calculation. The FAB-PA subscale is classified as low (0–14) and high fear levels (15–24). The FAB-W subscale is also classified as low (0–33) and high fear levels (34–42). The overall total score was calculated by adding the score of both subscales (17). The cut-off scores of >13 and >29 for FAB-PA and FAB-W, respectively, have been reported to be predictive of poor clinical outcome (disability) in people with LBP (44). For FAB-Total, cut-off scores of ł48 are considered to predict persistent disability in the future (45).
Insomnia: The severity of insomnia was assessed by the Chinese version of the 7-item Insomnia Severity Index (ISI). Each item is rated on a 5-point Likert scale (e.g., 0 = no insomnia; 4 = very severe insomnia) (46). The total scores were interpreted as no insomnia (0–7), sub-threshold insomnia (8–14), moderate insomnia (15–21), and severe insomnia (22–28) (47). A cut-off value of 10 is considered to be optimal to detect insomnia in the community (48). The ISI has demonstrated good test-retest reliability (α = 0.88) in people with chronic pain (49).
Lumbar Multifidus Muscle Assessments
Lumbar multifidus muscle morphometry and function: Bilateral parasagittal images of LMM at the L4/L5 and L5/S1 levels at rest and during submaximal contraction were captured with separate brightness-mode ultrasound videos on Supersonic Imagine® (Aixplorer Innovative UltraFast™ Ultrasound Imaging, France). This non-invasive ultrasonography technique has been used to estimate muscle activation (50). It has shown good to excellent intra-examiner (ICC = 0.86–0.90) and inter-examiner (ICC = 0.86–0.93) reliability in evaluating resting/contracted thickness and percent thickness change in LMM (51, 52). The participant in the prone position performed contralateral leg lifts three times to touch a bar fixed at 5-cm height in order to elicit submaximal voluntary contraction of LMM (53). The lumbar curve at rest was maintained at around 10°. The resting and contracted LMM thicknesses in the recorded brightness-mode videos were then measured on the ultrasonography device. The thickness was determined from the distance between the posterior tip of the facet joint and the inside edge of the overlying fascia (Figure 1). The average of three measured thickness ratios (thickness contracted - thickness rest/thickness rest x 100%) of each LMM muscle was used for statistical analysis.
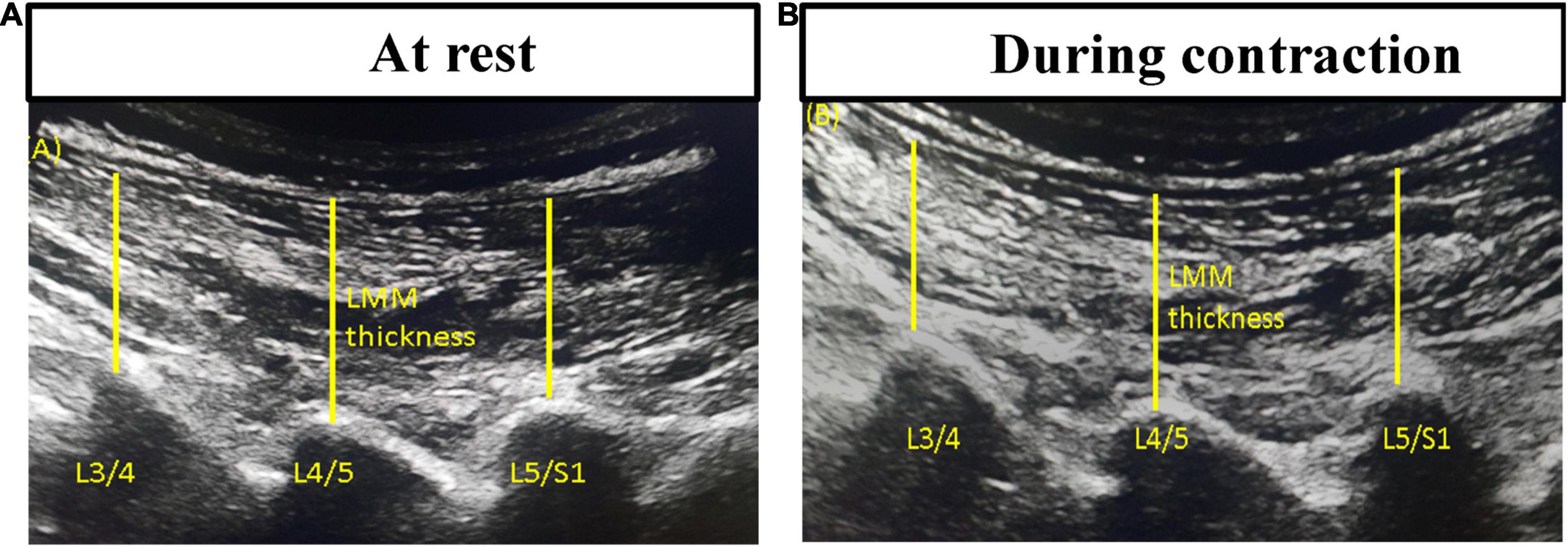
Figure 1. Thickness measurements of lumbar multifidus muscles using bright-mode ultrasound images (A) at rest and (B) during contraction.
The shear modulus (stiffness) of bilateral LMM at the L4/L5 and L5/S1 levels of the participants were assessed at rest by supersonic shear wave imaging (SSI) function of Supersonic Imagine®. The resting LMM stiffness at each muscle level was measured thrice. A curved (1–6 MHz) SSI ultrasound probe was placed parallel to LMM fibers at the target level (54). The probe sent multiple ultrasound push beams focused on various depths to deform and to create shear waves in LMM. The machine detected the shear waves and generated the resulting 2-dimensional shear modulus color maps at 1 sample/second. On each map, two standardized circular regions of interest (ROIs) with 5 mm diameter were placed between 1 and 2 cm depth of the target LMM (Figure 2). The average pixel intensity within the ROIs on each map indicates the LMM shear modulus. The shear modulus (μ) within each ROI was automatically calculated by the software using the formula μ = ρv2, where ρ is the muscle mass density and v is shear wave speed (55). The resting LMM stiffness was estimated by averaging the shear modulus of each LMM muscle at rest.
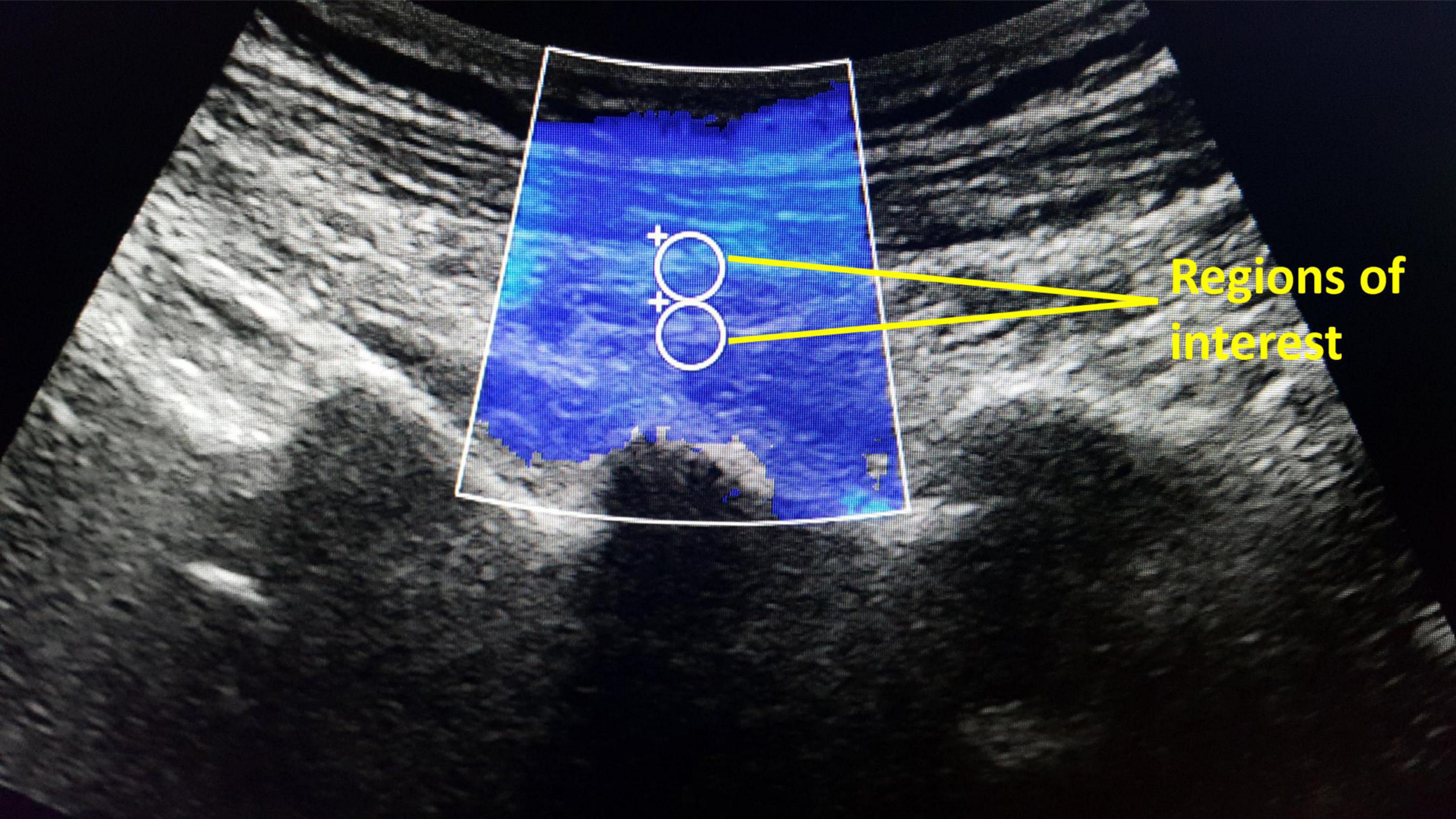
Figure 2. The supersonic shear wave imaging for lumbar multifidus stiffness measurements based on average pixel intensity within two regions of interest (5 mm diameter).
Data Analyses
Statistical analyses were performed using SPSS software (Version 26, IBM Corp., Armonk, NY, United States). Since Shapiro-Wilk tests indicated that our data was not normally distributed, non-parametric tests were used for data analyses. Descriptive statistics were conducted to summarize demographic characteristics (median and interquartile range) pain intensity, and RMDQ scores, HADS scores, FAB scores, PCS scores, ISI scores, while the mean and standard deviation were used to report LMM parameters in people with and without CLBP. Mann-Whitney U tests were used to compare between-group differences in psychological and insomnia scores. Linear mixed model analysis, which is robust for non-parametric data, was used for between-group comparisons of LMM characteristics after adjusting for age, gender and body mass index (BMI) (56). LMM characteristics. Spearman’s rank correlation coefficients were used to evaluate the relationships among demographic characteristics, pain intensity, RMDQ scores, HADS scores, FAB scores, PCS scores, ISI scores, and LMM stiffness and LMM thickness ratios. The strength of the correlation was classified as very weak (0.00–0.19), weak (0.20–0.39), moderate (0.40–0.59), strong (0.60–0.79), and very strong (0.80–1.0) (57). Partial correlation analyses between pain intensity and LMM parameters were performed by adjusting for psychological variables that were significantly related to pain intensity. Likewise, partial correlation analyses between LBP-related disability and LMM parameters were conducted by adjusting for psychological variables that significantly related to LBP-related disability. Psychological, insomnia, and LMM variables that demonstrated significant correlations with the 11-point NPRS or RMDQ score were then entered into two separate multiple linear regression models using a stepwise approach (p < 0.05 for entry, p > 0.10 for removal) to evaluate the relation between LMM characteristics and pain intensity or LBP-related disability in people with CLBP after accounting for various confounders. The significance level was set at p < 0.05 for all tests.
Results
Demographic Data
Demographic data of 78 participants with CLBP and 73 asymptomatic participants are shown in Table 1. There were no significant differences in age, body mass index, percentage of male, occupation, smoking status, and alcohol use, except for education levels and marital status between groups.
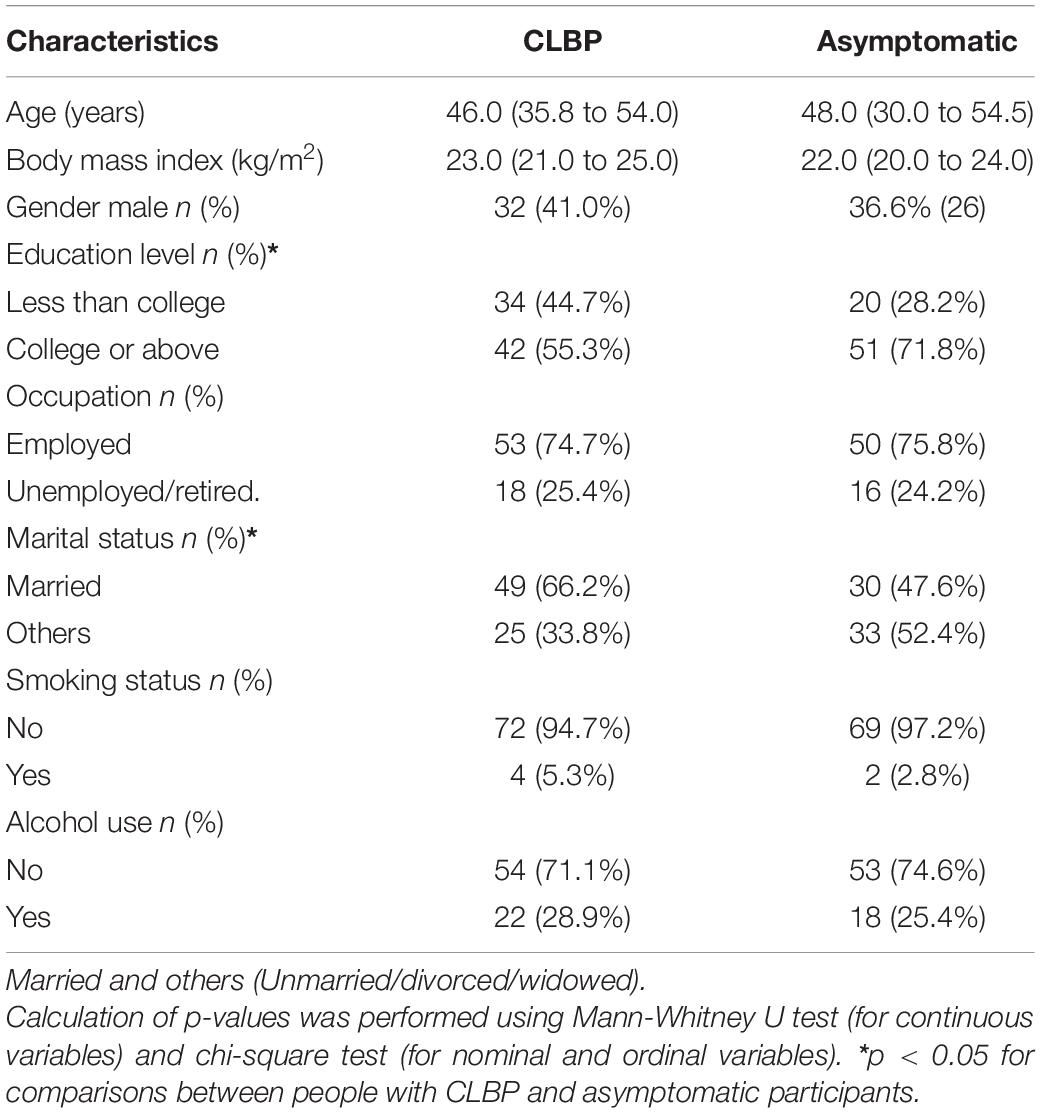
Table 1. Characteristics of participants with chronic low back pain (CLBP) and asymptomatic individuals [Median (interquartile range)].
Psychological and Sleep Parameters
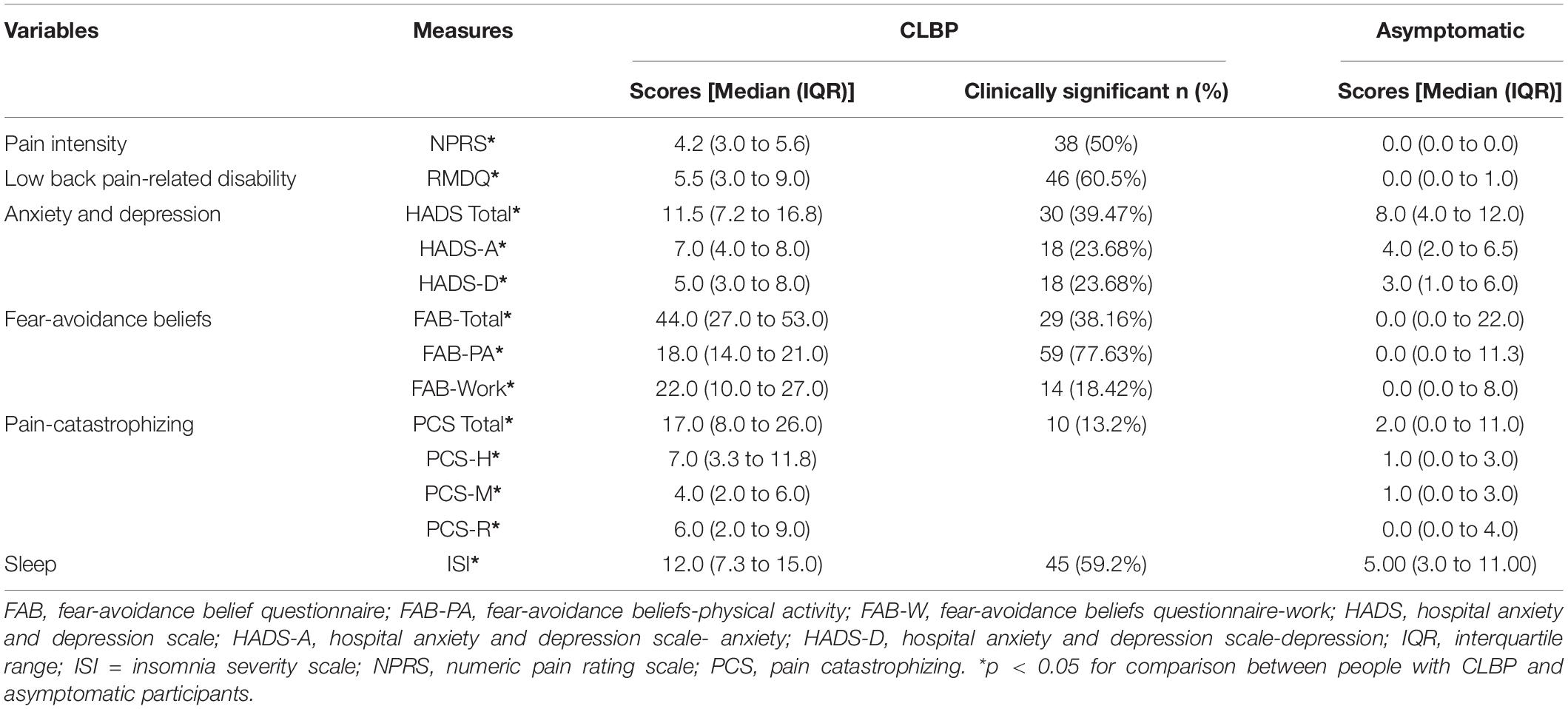
People with CLBP demonstrated significantly higher pain intensity, disability, HADS, FAB, PCS, and ISI scores than asymptomatic participants (p < 0.05). Fifty percent and 61% of people with CLBP had clinically significant pain and disability, respectively, while 40 and 38% had clinically significant mood and fear-avoidance beliefs problems, respectively. Ten percent and 59% had clinically significant pain-catastrophizing and insomnia, respectively (Table 2).
Lumbar Multifidus Muscle Parameters
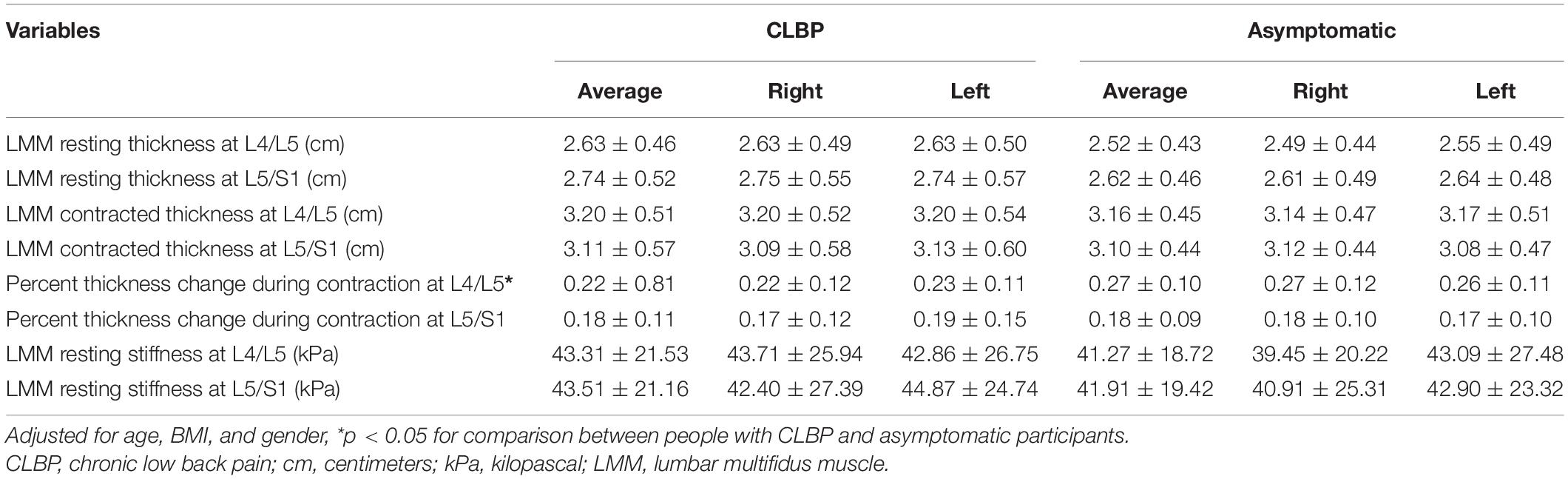
Between-group comparisons of LMM characteristics at L4/L5 and L5/S1 are reported in Table 3. After adjusting for age, gender and BMI, the percent thickness change of LMM during contraction at L4/L5 was significantly greater in asymptomatic participants than that in people with CLBP (p < 0.05). There were no significant differences in LMM resting thickness, contracted thickness, or LMM resting stiffness at both levels between people with and without CLBP. Likewise, the percent thickness change of LMM at L5/S1 during contraction was not statistically different between groups.
Correlations Between Pain Intensity and Demographic, Psychological, or Lumbar Multifidus Muscle Parameters
None of the demographic variables were associated with pain intensity. Table 4 shows the interrelation among various psychological and sleep variables, LMM variables, LBP intensity, and LBP-related disability. Spearman’s correlation analyses showed that pain intensity was significantly but weakly correlated with PCS-Total scores (ρ = 0.29, p < 0.05), and was moderately correlated with the scores of PCS-H (ρ = 0.34, p < 0.05), FAB-Total (ρ = 0.30, p < 0.05), FAB-W (ρ = 0.39, p < 0.05), and ISI (ρ = 0.44, p < 0.05) in people with CLBP. Partial correlation analysis revealed no significant association between any LMM parameters and LBP intensity.

Table 4. The interrelations among various psychological and insomnia variables, lumbar multifidus muscle (LMM) variables, low back pain (LBP) intensity, and LBP-related disability in people with chronic LBP.
Correlations Between Low Back Pain-Related Disability and Demographic, Psychological, or Lumbar Multifidus Muscle Parameters
Spearman’s correlation analyses showed that RMDQ scores were significantly, but weakly correlated with age (ρ = 0.26, p < 0.05), HADS-total (ρ = 0.26, p < 0.05), HADS-D (ρ = 0.28, p < 0.05), PCS-Total scores (ρ = 0.29, p < 0.05). RMDQ scores were also moderately correlated with the education level (ρ = −0.36, p < 0.05), FAB-Total scores (ρ = 0.34, p < 0.05), FAB-PA scores (ρ = 0.24, p < 0.05), FAB-W scores (ρ = 0.24, p < 0.05), PCS-H scores (ρ = 0.33, p < 0.05), ISI scores (ρ = 0.24, p < 0.05) in people with CLBP. No significant correlation was noted between RMDQ scores and any LMM parameters. Partial correlation analysis found that LMM parameters were not significantly related to LBP-related disability.
Factors Explaining Low Back Pain-Intensity
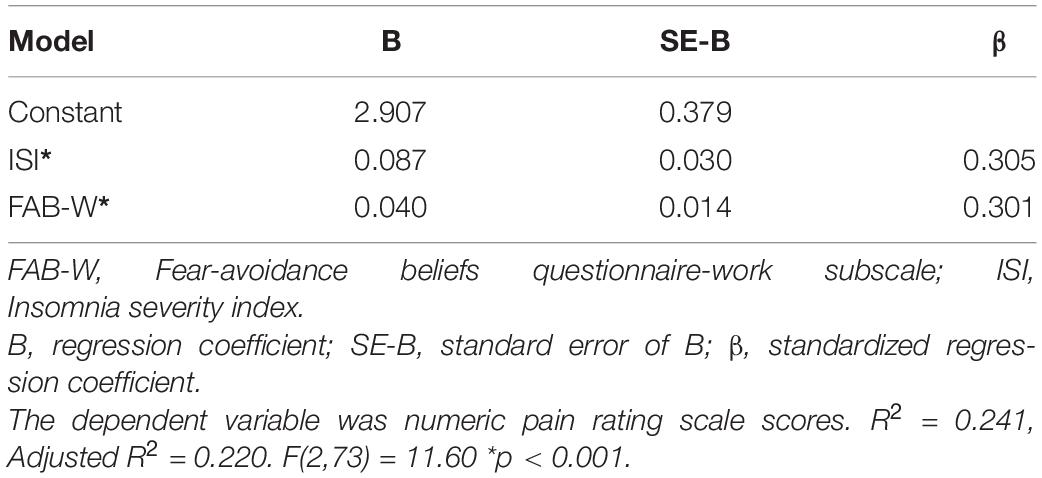
Since no significant associations were noted between LMM parameters and LBP intensity or disability, only those psychological and sleep parameters were included in the regression models. Three independent variables were eligible for the entry to the regression model for predicting LBP intensity (FAB-W, PCS-H, and ISI scores). The final model accounted for approximately 24% of the variance of pain intensity (R2 = 0.241; adjusted R2 = 0.220). Specifically, high ISI scores and FAB-W scores were associated with higher pain intensity in people with CLBP (Table 5). The unique variance explained by each of the two independent variables indexed by the squared semi-partial correlations was relatively low (insomnia and fear-avoidance beliefs about work each only accounted for approximately 8% of the variance of pain intensity).
Factors Explaining Low Back Pain-Related Disability
A two-stage hierarchical linear regression analysis was used to predict the level of disability reported by people with CLBP. In the first block, age and education levels were entered as a covariate; in the second block, HADS-D, FAB-T, PCS-H, and ISI scores were entered simultaneously as the primary variables of interest. Results of the hierarchical regression analysis are shown in Table 6. Only education level, entered on the first block, was a significant covariate, F(2, 73) = 4.035, p = 0.022. For the final block, the model was statistically significant F(6,69) = 5.926, p < 0.001, R2 = 0.340, Adjusted R2 = 0.283 and the FAB-T score accounted for 34% of the variance in RMDQ scores.
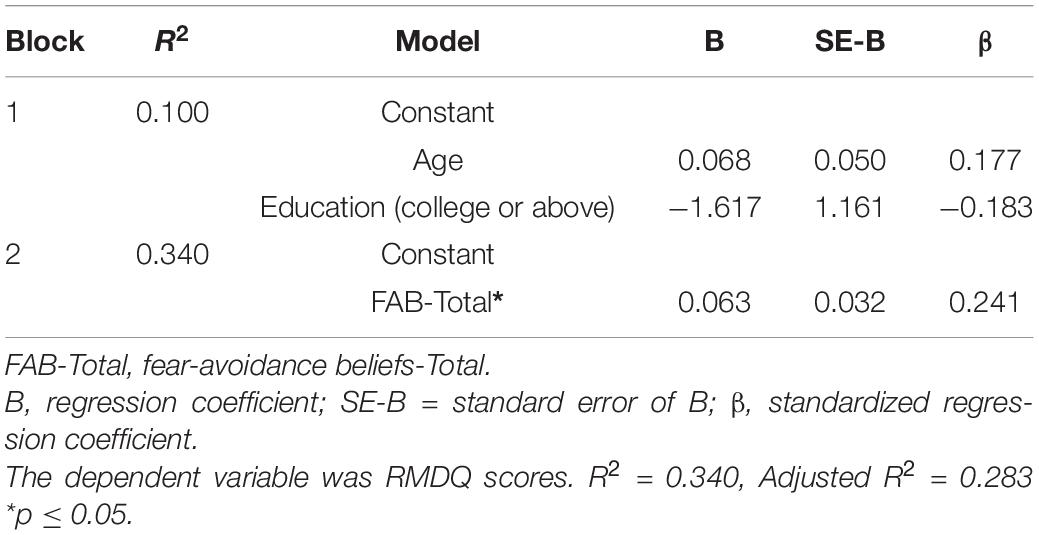
Table 6. Summary of hierarchical regression model predicting of Roland Morris Disability Questionnaire scores.
Discussion
Although individuals with CLBP had significantly smaller percent thickness change of LMM at the L4/L5 level during submaximal contraction than asymptomatic controls, no LMM parameters were significantly related to LBP-intensity or LBP-related disability in people with CLBP. Conversely, multiple psychological factors (e.g., pain catastrophizing and fear-avoidance beliefs) and insomnia were significantly related to LBP-intensity or LBP-related disability in individuals with CLBP. After considering various factors, FAB-W and ISI scores together explained 24% of the variance of pain intensity in individuals with CLBP. Similarly, FAB-Total scores explained 34% of the variance of LBP-related disability in people with CLBP. Taken together, our results lend support to the idea that the influence of psychological factors is more significant than the effect of LMM parameters on LBP intensity and LBP-related disability.
Percent Thickness Change During Contraction
The average percent thickness change at L4/L5 during submaximal contraction in people with CLBP was less than that of asymptomatic participants accords with previous research by Kiesel et al. (15). They found significant differences in percent thickness change at L4/L5 between patients with CLBP and healthy individuals (15). However, our other LMM measurements showed no significant differences in resting or contracted LMM thickness at L4/L5 and L5/S1 levels, or no significant difference in resting LMM stiffness at L4/L5 and L5/S1 levels between people with and without CLBP. These findings concur with prior research. Sweeney et al. revealed no significant difference in resting thickness at L4/L5 and L5/S1 levels between patients with CLBP and healthy individuals (58). Wong et al. (51) demonstrated that the contracted thickness of LMM at L3/L4 and L4/L5 levels in patients with CLBP did not differ from that of asymptomatic individuals. Likewise, previous research found no significant difference in LMM stiffness at L4/L5 level between people with and without CLBP in different postures (59). Koppenhaver et al. also found that LMM resting stiffness at L4/L5 in patients with CLBP (n = 60) was comparable to that of healthy people (n = 60) (60). Although consistent non-significant findings may be attributed to the great variability in LMM thickness or stiffness among people with and without CLBP, it may also imply that certain pain related LMM changes only occur in some patient subgroups, or other LMM measurements (e.g., electromyography, functional cross-sectional area on magnetic resonance images) may be more sensitive to detect subtle differences in LMM parameters between people with and without CLBP.
Our non-significant correlations between the percent thickness change of LMM at the L4/L5 or L5/S1 level during contraction and pain or LBP-related disability (even after controlling for psychological factors) concurred with previous research (61). Zielinski et al. (61) reported no significant correlation between percent thickness change of LMM at L3/L4 and LBP or LBP-related disability in patients with CLBP at baseline. Interestingly, although their patients reported a significant reduction in disability after performing stabilization exercises, post-treatment improvements in Oswestry Low Back Pain Disability Questionnaire scores in these patients were not significantly related to the corresponding alteration in percent thickness change at the L3/L4 level. Similarly, two systematic reviews found that post-treatment changes in resting thickness, cross-sectional area or endurance of LMM were unrelated to the improvements in LBP or LBP-related disability in people with LBP (62, 63). Similar to our findings, a cross-sectional study found that neither LMM cross-sectional area nor thickness at the L4/L5 or L5/S1 level was significantly correlated to RMDQ scores among 45 people with CLBP (64). Another systematic review also found inconsistent evidence regarding the association between baseline percent thickness change of LMM during contraction and ensuing clinical outcomes after various non-surgical treatments (65). Given that the negative results may be ascribed to the suboptimal sensitivity of Brightness-mode ultrasonography in detecting selective impairments in the activation of deep LMM fibers among patients with CLBP (66), future studies should adopt other advanced technologies (e.g., intramuscular electromyography, ultrafast ultrasonography, multivoxel magnetic resonance spectroscopy, or diffusion magnetic resonance imaging) (67) to evaluate the potential relationship between LMM morphometry or function and clinical outcomes in patients with CLBP.
Pain Catastrophizing
Similar to previous research, the current study found that pain catastrophizing was correlated with disability in people with CLBP (68, 69), but it did not predict LBP-related disability when it was concurrently evaluated together with other cognitive factors (70). Depression is one of the most common mental health conditions affecting people with chronic pain (71). Our study revealed that HAD-total scores and its depression subscale had weak positive correlations with LBP-related disability. These findings agreed with previous research. Hung et al. reported that the depression subscale was correlated with Oswestry Disability Index in people with CLBP (n = 225; r = 0.46) (72). Further, negative thoughts, low self-esteem, and decreased motivation for activity are symptoms of depression, which can negatively affect daily functioning and may contribute to disability (73).
Fear-Avoidance Beliefs
Fear-avoidance beliefs are known to be related to pain intensity and LBP-related disability in patients with LBP (74–76). Mannion et al. (77) reported that reduced FAB total scores were significantly correlated with decreases in the disability scores. Numerous reasons may lead to the presence of fear-avoidance beliefs in patients. People experiencing pain may reduce their physical activity level because they fear that any movement may aggravate their pain intensity, which in turn becomes a vicious cycle leading to disability (78, 79). Fear may also disturb the neural control pathway for automaticity, resulting in deficits in trunk motor control and increased trunk variability during walking in uncontrolled daily-living environments (80), which may heighten the risk of LBP. Further, some people with CLBP believe that any painful movements may damage their spine or may intensify their suffering (81). Additionally, healthcare professionals’ fear-avoidance beliefs regarding LBP may inadvertently influence their patients’ beliefs (82). Therefore, healthcare professionals should evaluate and minimize their patients’ fear-avoidance behaviors. Given that psychological interventions (e.g., cognitive-behavior therapy) are significantly better than routine treatment (83), back-care advice (84) or exercises (85, 86) in reducing fear-avoidance beliefs in patients with LBP, healthcare professionals should be either trained to deliver behavioral psychological interventions (87) or refer indicated patients to psychologists for proper management.
Insomnia
Almost 60% of our participants with CLBP reported clinically significant insomnia. Our findings also suggest that insomnia is one of the significant predictors of pain intensity in people with CLBP, which concurs with previous research that higher ISI scores were associated with higher pain intensity in people with CLBP (88). Similarly, a recent systematic review revealed low- to moderate-quality evidence that improved sleep quantity/quality is significantly related to improved LBP-related disability or reduced LBP in patients with CLBP (89). However, sleep disturbances and pain may affect each other reciprocally to form a vicious cycle because some brain regions (e.g., mesencephalic periaqueductal gray, thalamus, and raphe magnus) responsible for the initiation and maintenance of sleep are also involved in pain modulation (90).
Other factors may also explain the relation between sleep disturbances and pain. Different patients with chronic pain may have different circadian pain rhythms (91) and chronotypes (92). Some may have the highest pain intensity at wake-up that decreases during the day, while others may experience similarly high pain intensity in the morning that gradually decreases until it increases again from afternoon to night. Conversely, some may have the lowest pain intensity at waking and pain gradually increases over time (91). It has been postulated that those with high pain intensity in the morning may have suboptimal melatonin secretion at night, which may contribute to chronic sleep disturbances and increased pain perception in these patients (93). Interestingly, people with chronotype E (i.e., most active in the evening) experience a higher degree of musculoskeletal pain compared to those with chronotype M (i.e., most active in the morning). Collectively, circadian pain rhythms and chronotypes may have influence on pain (92).
In addition to the circadian pain rhythms, sleeplessness may affect pain sensitivity (94, 95). Insomnia is a known risk factor for developing back pain in asymptomatic individuals (96). Studies have found that sleep disturbance may affect the descending inhibitory pain pathways causing increased pain sensitivity (23, 97). Impaired sleep may also increase inflammatory cytokines that increase pain sensitivity (98, 99). A meta-analysis found that impaired sleep was significantly associated with higher levels of pro-inflammatory cytokines [e.g., interleukin (IL)-6] and biomarkers (e.g., C-reactive protein in the blood) (100) which might be related to more disability (101). Although the mechanisms underlying cytokines and disability remain to be determined, it is plausible that cytokines (e.g., IL-6, IL-1 and tumor necrosis factor-α) directly cause sarcopenia and functional impairments (102–105). Sleep-related changes in pain modulation may also limit functional abilities or activities of daily living in people with CLBP (106, 107). Regardless of the mechanisms, a large-scale prospective study involving 6,200 people with CLBP revealed that those with frequent sleeplessness at baseline had a lower probability of LBP recovery 11 years later (108). Therefore, preventing/reducing sleep-related problems in people with CLBP may improve their long-term prognosis. Future studies are warranted to evaluate the effects of sleep or pain interventions in modifying sleep, pain, and disability in people with CLBP.
Limitations
There are several limitations in the current study. First, the cross-sectional study design cannot determine the causal relationship between various LMM, psychological, or sleep parameters and LBP or LBP-related disability in people with CLBP. Future longitudinal studies should determine whether the presence of one or more psychological factors are related to pain intensity or LBP-related disability at future follow-ups. Second, the duration of CLBP was not evaluated in the current study because many participants could not recall their durations of CLBP accurately, which could affect the relations between various factors and CLBP intensity and LBP-related disability. Third, data were collected from self-reported questionnaires, which may lead to social desirability bias and/or recall bias (109). That said, because all the self-reported questionnaires were validated screening tools for various psychological problems in patients with chronic pain (17, 40, 41, 49), they should be suitable for clinical practice and research. Fourth, since the current study only investigated the morphometric changes of LMM in patients with CLBP, the potential associations between aberrant changes in motor control or proprioception of LMM and pain among people CLBP (110, 111) remain uncertain. Future studies should evaluate the correlations between deficits in motor control, proprioception, and/or clinical spinal instability and LBP/LBP-related disability after controlling for psychological and sleep factors. Fifth, FAB, depression and anxiety has been reported to be positively correlated with neuroticism, which is one of the personality traits in people with CLBP (112). It was not within the context of the study to explore personality traits in people with CLBP. Future studies should investigate the influence of personality traits, psychological factors and LMM dysfunctions on LBP-related disability in people with CLBP.
Strengths
This is the first study to evaluate the associations between various LMM parameters and clinical outcomes in patients with CLBP after adjusting for various psychological factors, insomnia, and demographic factors. Our findings highlight the necessity of assessing fear-avoidance beliefs and sleep disturbances in the routine clinical assessments of patients with CLBP, which may better manage these patients.
Conclusion
Since aberrant LMM morphometry or stiffness may only occur in some, but not all, people with CLBP, the current study revealed no significant difference in LMM characteristics between people with and without CLBP (except greater percent thickness change of LMM at L4/L5 level during contraction in asymptomatic individuals). It may also explain why there were no significant associations between any LMM characteristics and LBP-intensity/LBP-related disability in people with CLBP. Conversely, fear-avoidance beliefs or insomnia closely related to pain intensity or disability in people with CLBP. As such, it is important for clinicians to use validated tools to screen for maladaptive fear and sleep disturbances in patients with CLBP so that timely treatments can be given.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Subjects Ethics Sub-committee of the University (HSEAR20151027007-01). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SP and AW: conceptualization and design of the study, recruitment of participants, investigation, and data collection, and writing the original manuscript. AW: project administration. SP, AW, MP, JC, DS, and JK: analysis and interpretation of data. All authors critically revised the draft and approved the final version.
Funding
The current project was funded by the Early Career Scheme (251018/17M).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Kenney KL Lau, Matthew CH Chung, and Charlotte CK Yung for assisting with the data collection. We also thank Raymond Cheung for statistical advice.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.809891/full#supplementary-material
References
1. Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
2. Koes B, Van Tulder M, Thomas S. Diagnosis and treatment of low back pain. BMJ. (2006) 332:1430–4.
3. Rozenberg S. Chronic low back pain: definition and treatment. La Revue Du Praticien. (2008) 58:265–72.
4. Shmagel A, Foley R, Ibrahim H. Epidemiology of chronic low back pain in US adults: data from the 2009-2010 national health and nutrition examination survey. Arthritis Care Res. (2016) 68:1688–94. doi: 10.1002/acr.22890
5. Gore M, Sadosky A, Stacey BR, Tai K-S, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine. (2012) 37:E668–77. doi: 10.1097/BRS.0b013e318241e5de
6. Freeman MD, Woodham MA, Woodham AW. The role of the lumbar multifidus in chronic low back pain: a review. PMR. (2010) 2:142–6. doi: 10.1016/j.pmrj.2009.11.006
7. Hodges PW, Danneels L. Changes in structure and function of the back muscles in low back pain: different time points, observations, and mechanisms. J Orthop Sports Phys Therapy. (2019) 49:464–76. doi: 10.2519/jospt.2019.8827
8. Hofste A, Soer R, Groen GJ, van der Palen J, Geerdink FJ, Oosterveld FG, et al. Functional and morphological lumbar multifidus characteristics in subgroups with low back pain in primary care. Musculoskel Sci Pract. (2021) 55:102429. doi: 10.1016/j.msksp.2021.102429
9. Wilke H-J, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study. Spine. (1995) 20:192–8. doi: 10.1097/00007632-199501150-00011
10. Kamaz M, Kiresi D, Oguz H, Emlik D, Levendoglu F. CT measurement of trunk muscle areas in patients with chronic low back pain. Diagnos Intervent Radiol. (2007) 13:144.
11. Zhang S, Xu Y, Han X, Wu W, Tang Y, Wang C. Functional and morphological changes in the deep lumbar multifidus using electromyography and ultrasound. Sci Rep. (2018) 8:6539. doi: 10.1038/s41598-018-24550-5
12. Mengiardi B, Schmid MR, Boos N, Pfirrmann CW, Brunner F, Elfering A, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology. (2006) 240:786–92. doi: 10.1148/radiol.2403050820
13. Paalanne N, Niinimaki J, Karppinen J, Taimela S, Mutanen P, Takatalo J, et al. Assessment of association between low back pain and paraspinal muscle atrophy using opposed-phase magnetic resonance imaging: a population-based study among young adults. Spine. (2011) 36:1961–8. doi: 10.1097/BRS.0b013e3181fef890
14. Danneels L, Coorevits P, Cools A, Vanderstraeten G, Cambier D, Witvrouw E, et al. Differences in electromyographic activity in the multifidus muscle and the iliocostalis lumborum between healthy subjects and patients with sub-acute and chronic low back pain. Eur Spine J. (2002) 11:13–9. doi: 10.1007/s005860100314
15. Kiesel KB, Underwood FB, Mattacola CG, Nitz AJ, Malone TR. A comparison of select trunk muscle thickness change between subjects with low back pain classified in the treatment-based classification system and asymptomatic controls. J Orthop Sports Phys Therapy. (2007) 37:596–607. doi: 10.2519/jospt.2007.2574
16. Masaki M, Aoyama T, Murakami T, Yanase K, Ji X, Tateuchi H, et al. Association of low back pain with muscle stiffness and muscle mass of the lumbar back muscles, and sagittal spinal alignment in young and middle-aged medical workers. Clin Biomech. (2017) 49:128–33. doi: 10.1016/j.clinbiomech.2017.09.008
17. Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. (1993) 52:157–68. doi: 10.1016/0304-3959(93)90127-B
18. Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. (1999) 80:329–39. doi: 10.1016/s0304-3959(98)00229-2
19. Meyer K, Tschopp A, Sprott H, Mannion AF. Association between catastrophizing and self-rated pain and disability in patients with chronic low back pain. J Rehabil Med. (2009) 41:620–5. doi: 10.2340/16501977-0395
20. Guclu DG, Guclu O, Ozaner A, Senormanci O, Konkan R. The relationship between disability, quality of life and fear-avoidance beliefs in patients with chronic low back pain. Turk Neurosurg. (2012) 22:724–31. doi: 10.5137/1019-5149.JTN.6156-12.1
21. Tsuji T, Matsudaira K, Sato H, Vietri J. The impact of depression among chronic low back pain patients in Japan. BMC Musculoskeletal Disord. (2016) 17:447. doi: 10.1186/s12891-016-1304-4
22. Nava-Bringas TI, Macías-Hernández SI, Vásquez-Ríos JR, Coronado-Zarco R, Miranda-Duarte A, Cruz-Medina E, et al. Fear-avoidance beliefs increase perception of pain and disability in Mexicans with chronic low back pain. Rev Brasil Reumatol. (2017) 57:306–10. doi: 10.1016/j.rbre.2016.11.003
23. Kelly GA, Blake C, Power CK, O’keeffe D, Fullen BM. The association between chronic low back pain and sleep: a systematic review. Clin J Pain. (2011) 27:169–81. doi: 10.1097/AJP.0b013e3181f3bdd5
24. Wang HY, Fu TS, Hsu SC, Hung CI. Association of depression with sleep quality might be greater than that of pain intensity among outpatients with chronic low back pain. Neuropsychiatr Dis Treat. (2016) 12:1993–8. doi: 10.2147/ndt.S110162
25. Bahouq H, Allali F, Rkain H, Hmamouchi I, Hajjaj-Hassouni N. Prevalence and severity of insomnia in chronic low back pain patients. Rheumatol Int. (2013) 33:1277–81. doi: 10.1007/s00296-012-2550-x
26. Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. (2007) 3:S7–10.
27. Marty M, Rozenberg S, Duplan B, Thomas P, Duquesnoy B, Allaert F. Quality of sleep in patients with chronic low back pain: a case-control study. Eur Spine J. (2008) 17:839–44. doi: 10.1007/s00586-008-0660-7
29. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. (2011) 152:2399–404. doi: 10.1016/j.pain.2011.07.005
30. McCaffery M, Beebe A. The numeric pain rating scale instructions. In: McCaffery M, Beebe A, Latham J editors. Pain: Clinic Manual for Nursing Practice. St. Louis: Mosby (1989).
31. Zelman DC, Hoffman DL, Seifeldin R, Dukes EM. Development of a metric for a day of manageable pain control: derivation of pain severity cut-points for low back pain and osteoarthritis. Pain. (2003) 106:35–42. doi: 10.1016/s0304-3959(03)00274-4
32. Maughan EF, Lewis JS. Outcome measures in chronic low back pain. Eur Spine J. (2010) 19:1484–94. doi: 10.1007/s00586-010-1353-6
33. Gallasch CH, Alexandre NMC. The measurement of musculoskeletal pain intensity: a comparison of four methods. Rev Gaúcha Enfermagem. (2007) 28:260. doi: 10.1097/aog.0b013e318195bd6c
34. Tsang RC. Measurement properties of the Hong Kong Chinese version of the Roland-Morris disability questionnaire. Hong Kong Physiother J. (2004) 22:40–9. doi: 10.1016/s1013-7025(09)70049-1
35. Stratford PW, Riddle DL. A roland morris disability questionnaire target value to distinguish between functional and dysfunctional states in people with low back pain. Physiother Can. (2016) 68:29–35. doi: 10.3138/ptc.2014-85
36. Leung C, Ho S, Kan C, Hung C, Chen C. Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale: a cross-cultural perspective. Int J Psychosom. (1993) 40:29–34.
37. Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. (2003) 1:29. doi: 10.1186/1477-7525-1-29
39. Hinz A, Brähler E. Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J Psychosom Res. (2011) 71:74–8. doi: 10.1016/j.jpsychores.2011.01.005
40. Li Q, Lin Y, Hu C, Xu Y, Zhou H, Yang L, et al. The chinese version of hospital anxiety and depression scale: psychometric properties in chinese cancer patients and their family caregivers. Eur J Oncol Nurs. (2016) 25:16–23. doi: 10.1016/j.ejon.2016.09.004
41. Yap JC, Lau J, Chen PP, Gin T, Wong T, Chan I, et al. Validation of the Chinese Pain Catastrophizing Scale (HK-PCS) in patients with chronic pain. Pain Med. (2008) 9:186–95. doi: 10.1111/j.1526-4637.2007.00307.x
42. Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7:524. doi: 10.1037/1040-3590.7.4.524
43. Cheung PWH, Wong CKH, Cheung JPY. Psychometric validation of the cross-culturally adapted traditional Chinese version of the Back Beliefs Questionnaire (BBQ) and Fear-Avoidance Beliefs Questionnaire (FABQ). Eur Spine J. (2018) 27:1724–33. doi: 10.1007/s00586-018-5576-2
44. Cleland JA, Fritz JM, Brennan GP. Predictive validity of initial fear avoidance beliefs in patients with low back pain receiving physical therapy: is the FABQ a useful screening tool for identifying patients at risk for a poor recovery? Eur Spine J. (2008) 17:70–9. doi: 10.1007/s00586-007-0511-y
45. Landers MR, Creger RV, Baker CV, Stutelberg KS. The use of fear-avoidance beliefs and nonorganic signs in predicting prolonged disability in patients with neck pain. Manual Therapy. (2008) 13:239–48. doi: 10.1016/j.math.2007.01.010
46. Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/s1389-9457(00)00065-4
47. Morin CM. Insomnia: Psychological assessment and management. New York, NY: Guilford press (1993).
48. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. (2011) 34:601–8. doi: 10.1093/sleep/34.5.601
49. Yu DS. Insomnia Severity Index: psychometric properties with Chinese community-dwelling older people. J Adv Nurs. (2010) 66:2350–9. doi: 10.1111/j.1365-2648.2010.05394.x
50. Kiesel KB, Uhl TL, Underwood FB, Rodd DW, Nitz AJ. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man Ther. (2007) 12:161–6. doi: 10.1016/j.math.2006.06.011
51. Wong AY, Parent E, Kawchuk G. Reliability of 2 ultrasonic imaging analysis methods in quantifying lumbar multifidus thickness. J Orthop Sports Phys Therapy. (2013) 43:251–62. doi: 10.2519/jospt.2013.4478
52. Sions JM, Velasco TO, Teyhen DS, Hicks GE. Ultrasound imaging: intraexaminer and interexaminer reliability for multifidus muscle thickness assessment in adults aged 60 to 85 years versus younger adults. J Orthop Sports Phys Ther. (2014) 44:425–34. doi: 10.2519/jospt.2014.4584
53. Hu Y, Wong YL, Lu WW, Kawchuk GN. Creation of an asymmetrical gradient of back muscle activity and spinal stiffness during asymmetrical hip extension. Clin Biomech. (2009) 24:799–806. doi: 10.1016/j.clinbiomech.2009.07.013
54. Teyhen D, Koppenhaver S. Rehabilitative ultrasound imaging. J Physiother. (2011) 57:3. doi: 10.1016/s1836-9553(11)70044-3
55. Gennisson J-L, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagnos Intervent Imaging. (2013) 94:487–95. doi: 10.1016/j.diii.2013.01.022
56. Arnau J, Bono R, Blanca MJ, Bendayan R. Using the linear mixed model to analyze nonnormal data distributions in longitudinal designs. Behav Res Methods. (2012) 44:1224–38. doi: 10.3758/s13428-012-0196-y
57. Evans JD. Straightforward statistics for the behavioral sciences. Stamford: Thomson Brooks (1996).
58. Sweeney N, O’Sullivan C, Kelly G. Multifidus muscle size and percentage thickness changes among patients with unilateral chronic low back pain (CLBP) and healthy controls in prone and standing. Manual Therapy. (2014) 19:433–9. doi: 10.1016/j.math.2014.04.009
59. Chan ST, Fung PK, Ng NY, Ngan TL, Chong MY, Tang CN, et al. Dynamic changes of elasticity, cross-sectional area, and fat infiltration of multifidus at different postures in men with chronic low back pain. Spine J. (2012) 12:381–8. doi: 10.1016/j.spinee.2011.12.004
60. Koppenhaver S, Gaffney E, Oates A, Eberle L, Young B, Hebert J, et al. Lumbar muscle stiffness is different in individuals with low back pain than asymptomatic controls and is associated with pain and disability, but not common physical examination findings. Musculoskelet Sci Pract. (2020) 45:102078. doi: 10.1016/j.msksp.2019.102078
61. Zielinski KA, Henry SM, Ouellette-Morton RH, DeSarno MJ. Lumbar multifidus muscle thickness does not predict patients with low back pain who improve with trunk stabilization exercises. Arch Phys Med Rehabil. (2013) 94:1132–8. doi: 10.1016/j.apmr.2012.12.001
62. Wong AY, Parent EC, Funabashi M, Kawchuk GN. Do changes in transversus abdominis and lumbar multifidus during conservative treatment explain changes in clinical outcomes related to nonspecific low back pain? A systematic review. J Pain. (2014) 377:e371–335. doi: 10.1016/j.jpain.2013.10.008
63. Pinto SM, Boghra SB, Macedo LG, Zheng Y-P, Pang MYC, Cheung JPY, et al. Does motor control exercise restore normal morphology of lumbar multifidus muscle in people with low back pain? A systematic review. J Pain Res. (2021) 14:2543–62. doi: 10.2147/JPR.S314971
64. Rezazadeh F, Taheri N, Okhravi SM, Hosseini SM. The relationship between cross-sectional area of multifidus muscle and disability index in patients with chronic non-specific low back pain. Musculoskeletal Sci Pract. (2019) 42:1–5. doi: 10.1016/j.msksp.2019.03.005
65. Wong AY, Parent EC, Funabashi M, Stanton TR, Kawchuk GN. Do various baseline characteristics of transversus abdominis and lumbar multifidus predict clinical outcomes in nonspecific low back pain? A systematic review. Pain. (2013) 154:2589–602. doi: 10.1016/j.pain.2013.07.010
66. Murillo C, Falla D, Rushton A, Sanderson A, Heneghan NR. Shear wave elastography investigation of multifidus stiffness in individuals with low back pain. J Electromyogr Kinesiol. (2019) 47:19–24. doi: 10.1016/j.jelekin.2019.05.004
67. Kalia V, Leung DG, Sneag DB, Grande FD, Carrino JA. Advanced MRI Techniques for Muscle Imaging. Semin Musculoskelet Radiol. (2017) 21:459–69. doi: 10.1055/s-0037-1604007
68. Van Den Hout JH, Vlaeyen JW, Heuts PH, Sillen WJ, Willen AJ. Functional disability in nonspecific low back pain: the role of pain-related fear and problem-solving skills. Int J Behav Med. (2001) 8:134–48. doi: 10.1207/s15327558ijbm0802_04
69. Thomas E-N, Pers Y-M, Mercier G, Cambiere J-P, Frasson N, Ster F, et al. The importance of fear, beliefs, catastrophizing and kinesiophobia in chronic low back pain rehabilitation. Ann Phys Rehabil Med. (2010) 53:3–14. doi: 10.1016/j.rehab.2009.11.002
70. Woby SR, Roach NK, Urmston M, Watson PJ. The relation between cognitive factors and levels of pain and disability in chronic low back pain patients presenting for physiotherapy. Eur J Pain. (2007) 11:869–77. doi: 10.1016/j.ejpain.2007.01.005
71. Ericsson M II, Linder J, Taylor JE, Haddock CK, Foreyt JP. Depression predicts disability in long-term chronic pain patients. Disabil Rehabil. (2002) 24:334–40. doi: 10.1080/09638280110096241
72. Hung C-I, Liu C-Y, Fu T-S. Depression: an important factor associated with disability among patients with chronic low back pain. Int J Psychiat Med. (2015) 49:187–98. doi: 10.1177/0091217415573937
73. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Therapy. (1995) 33:335–43. doi: 10.1016/0005-7967(94)00075-u
74. Camacho-Soto A, Sowa GA, Perera S, Weiner DK. Fear avoidance beliefs predict disability in older adults with chronic low back pain. PMR. (2012) 4:493–7. doi: 10.1016/j.pmrj.2012.01.017
75. Ferrari S, Chiarotto A, Pellizzer M, Vanti C, Monticone M. Pain self-efficacy and fear of movement are similarly associated with pain intensity and disability in italian patients with chronic low back pain. Pain Pract. (2016) 16:1040–7. doi: 10.1111/papr.12397
76. Buragadda S, Aleisa ES, Melam GR. Fear avoidance beliefs and disability among women with low back pain. Neuropsychiatry. (2018) 8:73–9.
77. Mannion AF, Junge A, Taimela S, Müntener M, Lorenzo K, Dvorak J. Active therapy for chronic low back pain: part 3. Factors influencing self-rated disability and its change following therapy. Spine. (2001) 26:920–9. doi: 10.1097/00007632-200104150-00015
78. Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. (2012) 28:475–83. doi: 10.1097/AJP.0b013e3182385392
80. Nishi Y, Shigetoh H, Fujii R, Osumi M, Morioka S. Changes in trunk variability and stability of gait in patients with chronic low back pain: impact of laboratory versus daily-living environments. J Pain Res. (2021) 14:1675. doi: 10.2147/JPR.S310775
81. Bunzli S, Smith A, Schütze R, O’Sullivan P. Beliefs underlying pain-related fear and how they evolve: a qualitative investigation in people with chronic back pain and high pain-related fear. BMJ Open. (2015) 5:e008847. doi: 10.1136/bmjopen-2015-008847
82. Darlow B, Fullen BM, Dean S, Hurley DA, Baxter GD, Dowell A. The association between health care professional attitudes and beliefs and the attitudes and beliefs, clinical management, and outcomes of patients with low back pain: a systematic review. Eur J Pain. (2012) 16:3–17. doi: 10.1016/j.ejpain.2011.06.006
83. Linden M, Scherbe S, Cicholas B. Randomized controlled trial on the effectiveness of cognitive behavior group therapy in chronic back pain patients. J Back Musculoskelet Rehabil. (2014) 27:563–8. doi: 10.3233/BMR-140518
84. Lamb SE, Hansen Z, Lall R, Castelnuovo E, Withers EJ, Nichols V, et al. Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. Lancet. (2010) 375:916–23. doi: 10.1016/S0140-6736(09)62164-4
85. Vibe Fersum K, O’Sullivan P, Skouen J, Smith A, Kvåle A. Efficacy of classification-based cognitive functional therapy in patients with non-specific chronic low back pain: a randomized controlled trial. Eur J Pain. (2013) 17:916–28. doi: 10.1002/j.1532-2149.2012.00252.x
86. Vibe Fersum K, Smith A, Kvåle A, Skouen JS, O’Sullivan P. Cognitive functional therapy in patients with non-specific chronic low back pain—a randomized controlled trial 3-year follow-up. Eur J Pain. (2019) 23:1416–24. doi: 10.1002/ejp.1399
87. Zhang Q, Jiang S, Young L, Li F. The effectiveness of group-based physiotherapy-led behavioral psychological interventions on adults with chronic low back pain: a systematic review and meta-analysis. Am J Phys Med Rehabil. (2019) 98:215–25. doi: 10.1097/PHM.0000000000001053
88. Nartea R, Mitoiu B, Nica AS, Constantinovici M, Clantau D. Insomnia in patient with chronic low back pain. Ann Phys Rehabil Med. (2017) 60:e20. doi: 10.1016/j.rehab.2017.07.146
89. Chang R, Wang X, Lin G, Samartzis D, Pinto SM, Wong AY. Are changes in sleep quality/quantity or baseline sleep parameters related to changes in clinical outcomes in patients with non-specific chronic low back pain? a systematic review. Clin J Pain. (2021) 38:292–307. doi: 10.1097/ajp.0000000000001008
90. Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. (2004) 8:119–32. doi: 10.1016/S1087-0792(03)00044-3
91. Tanaka Y, Shigetoh H, Sato G, Fujii R, Imai R, Osumi M, et al. Classification of circadian pain rhythms and pain characteristics in chronic pain patients: an observational study. Medicine. (2021) 100:e26500. doi: 10.1097/MD.0000000000026500
92. Heikkala E, Paananen M, Merikanto I, Karppinen J, Oura P. Eveningness intensifies the association between musculoskeletal pain and health-related quality of life: a Northern Finland Birth Cohort Study 1966. Pain. (2022). Epub ahead of print. doi: 10.1097/j.pain.0000000000002609
93. Fatima G, Sharma V, Verma N. Circadian variations in melatonin and cortisol in patients with cervical spinal cord injury. Spinal Cord. (2016) 54:364–7. doi: 10.1038/sc.2015.176
94. Brennan MJ, Lieberman JA III. Sleep disturbances in patients with chronic pain: effectively managing opioid analgesia to improve outcomes. Curr Med Res Opin. (2009) 25:1045–55. doi: 10.1185/03007990902797790
95. Miller MA, Wright H, Hough J, Cappuccio FP. Sleep and cognition. In: Idzikowski C editor. Sleep and its disorders affect society. London: IntechOpen (2014).
96. Agmon M, Armon G. Increased insomnia symptoms predict the onset of back pain among employed adults. PLoS One. (2014) 9:e103591. doi: 10.1371/journal.pone.0103591
97. Kundermann B, Krieg J-C, Schreiber W, Lautenbacher S. The effects of sleep deprivation on pain. Pain Res Manage. (2004) 9:25–32. doi: 10.1155/2004/949187
98. Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. (2007) 30:1145–52. doi: 10.1093/sleep/30.9.1145
99. Heffner KL, France CR, Trost Z, Ng HM, Pigeon WR. Chronic low back pain, sleep disturbance, and interleukin-6. Clin J Pain. (2011) 27:35. doi: 10.1097/ajp.0b013e3181eef761
100. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiat. (2016) 80:40–52. doi: 10.1016/j.biopsych.2015.05.014
101. Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS. Nonspecific low back pain: inflammatory profiles of patients with acute and chronic pain. Clin J Pain. (2019) 35:818. doi: 10.1097/AJP.0000000000000745
102. Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutrit Metab Care. (2003) 6:295–9. doi: 10.1097/01.mco.0000068965.34812.62
103. Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. (2004) 39:687–99. doi: 10.1016/j.exger.2004.01.009
104. Roubenoff R. Physical activity, inflammation, and muscle loss. Nutrit Rev. (2007) 65:S208–12. doi: 10.1111/j.1753-4887.2007.tb00364.x
105. Santos MLADS, Gomes WF, Queiroz BZ, Rosa NMDB, Pereira DS, Dias JMD, et al. Muscle performance, pain, stiffness, and functionality in elderly women with knee osteoarthritis. Acta Ortopédica Brasileira. (2011) 19:193–7.
106. Di Iorio A, Abate M, Guralnik JM, Bandinelli S, Cecchi F, Cherubini A, et al. From chronic low back pain to disability, a multifactorial mediated pathway: the InCHIANTI study. Spine. (2007) 32:E809. doi: 10.1097/BRS.0b013e31815cd422
107. Naughton F, Ashworth P, Skevington SM. Does sleep quality predict pain-related disability in chronic pain patients? The mediating roles of depression and pain severity. Pain. (2007) 127:243–52. doi: 10.1016/j.pain.2006.08.019
108. Skarpsno ES, Mork PJ, Nilsen TIL, Nordstoga AL. Influence of sleep problems and co-occurring musculoskeletal pain on long-term prognosis of chronic low back pain: the HUNT Study. J Epidemiol Community Health. (2020) 74:283–9. doi: 10.1136/jech-2019-212734
109. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscipl Healthc. (2016) 9:211. doi: 10.2147/JMDH.S104807
110. Meier ML, Vrana A, Schweinhardt P. Low back pain: the potential contribution of supraspinal motor control and proprioception. Neuroscientist. (2019) 25:583–96. doi: 10.1177/1073858418809074
111. Pinto SM, Cheung JP, Samartzis D, Karppinen J, Zheng Y-P, Pang MY, et al. Differences in proprioception between young and middle-aged adults with and without chronic low back pain. Front Neurol. (2020) 11:605787. doi: 10.3389/fneur.2020.605787
Keywords: chronic low back pain, fear-avoidance beliefs, sleep disturbance, lumbar multifidus muscle, CLBP
Citation: Pinto SM, Cheung JPY, Samartzis D, Karppinen J, Zheng Y-p, Pang MYC and Wong AYL (2022) Are Morphometric and Biomechanical Characteristics of Lumbar Multifidus Related to Pain Intensity or Disability in People With Chronic Low Back Pain After Considering Psychological Factors or Insomnia? Front. Psychiatry 13:809891. doi: 10.3389/fpsyt.2022.809891
Received: 08 November 2021; Accepted: 14 March 2022;
Published: 15 April 2022.
Edited by:
Domenico De Berardis, Mental Health Center (CSM) and Psychiatric Service of Diagnosis and Treatment (SPDC), ItalyReviewed by:
Kai Wang, Anhui Medical University, ChinaCarmine Tomasetti, SPDC di Giulianova, Italy
Shu Morioka, Kio University, Japan
Copyright © 2022 Pinto, Cheung, Samartzis, Karppinen, Zheng, Pang and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arnold Y. L. Wong, YXJub2xkLndvbmdAcG9seXUuZWR1Lmhr
 Sabina M. Pinto
Sabina M. Pinto Jason P. Y. Cheung2
Jason P. Y. Cheung2 Jaro Karppinen
Jaro Karppinen Arnold Y. L. Wong
Arnold Y. L. Wong