
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 28 April 2022
Sec. Child and Adolescent Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.806669
This article is part of the Research TopicLongitudinal Data Analysis in Child and Adolescent Mental HealthView all 10 articles
 Kaori Endo1†
Kaori Endo1† Daniel Stanyon1†
Daniel Stanyon1† Syudo Yamasaki1
Syudo Yamasaki1 Miharu Nakanishi1,2
Miharu Nakanishi1,2 Junko Niimura1
Junko Niimura1 Sho Kanata3
Sho Kanata3 Shinya Fujikawa4
Shinya Fujikawa4 Yuko Morimoto5
Yuko Morimoto5 Mariko Hosozawa6
Mariko Hosozawa6 Kaori Baba7
Kaori Baba7 Nao Oikawa8
Nao Oikawa8 Naomi Nakajima1
Naomi Nakajima1 Kazuhiro Suzuki9
Kazuhiro Suzuki9 Mitsuhiro Miyashita10
Mitsuhiro Miyashita10 Shuntaro Ando1,4
Shuntaro Ando1,4 Mariko Hiraiwa-Hasegawa11
Mariko Hiraiwa-Hasegawa11 Kiyoto Kasai4,12
Kiyoto Kasai4,12 Atsushi Nishida1*
Atsushi Nishida1*Background: Attention-deficit/hyperactivity disorder (ADHD) develops in early childhood and carries lifelong impact, but early identification and intervention ensure optimal clinical outcomes. Prolonged or excessive parenting stress may be a response to infant behavioral differences antecedent to developmental disorders such as ADHD, and therefore represents a potentially valuable inclusion in routine early-life assessment. To investigate the feasibility of using routinely-collected self-reported maternal parenting stress as a risk marker for child ADHD, this study investigated the longitudinal association between maternal parenting stress from 1 to 36 months after childbirth and child ADHD in early adolescence.
Methods: The sample comprised 2,638 children (1,253 girls) from the Tokyo Teen Cohort population-based birth cohort study. Mothers recorded parenting stress five times from 1 to 36 months following childbirth in the Maternal and Child Health Handbook, a tool used for routine early-life assessment in Japan. Nine years later, mothers evaluated their child's ADHD symptoms at 12 y using the hyperactivity/inattention subscale from the Strength and Difficulties Questionnaire.
Results: Approximately 7.5% of parents reported that they had parenting stress at 36 m after childbirth. 6.2% of children were evaluated as above the cut-off for ADHD symptoms at 12 y. Parenting stress at 1 and 3–4 m was not associated with child ADHD symptoms at 12 y. However, child ADHD symptoms at 12 y was significantly associated with parenting stress at 9–10 m (unadjusted OR = 1.42, p =.047, 95% CI [1.00, 2/00]), 18 m (unadjusted OR = 1.57, p =.007, 95% CI [1.13, 2.19]) and 36 m (unadjusted OR = 1.67, p =.002, 95% CI [1.20, 2.31]). These associations remained after adjustment for child's sex, age in months and family income.
Conclusions: We identified associations between parenting stress at 9–10, 18 and 36 m after childbirth and child ADHD symptoms at 12 years old. Self-reported parenting stress data may have utility as an early indicator for ADHD risk. Participation in early-life health checks, assessment of parenting stress, and tailoring support to family needs should be promoted for early identification and intervention for ADHD.
Attention-deficit/hyperactivity disorder (ADHD) is a psychological disorder impacting individuals across the lifespan, and may lead to poorer health outcomes such as depression, psychotic disorder, attempted suicide, and completed suicide (1–3). Approximately 30% of people with ADHD in childhood experience persisting symptoms in adulthood (1). While ADHD is a chronic condition, early identification and intervention have been shown to greatly improve outcomes. For example, interventions for preschool and school-aged children may facilitate social skills and reduce behavioral problems (4, 5). Additionally, parent training has demonstrated benefits for both child, via reduced internalizing and externalizing behaviors, and parent, via increased confidence and decreased parenting stress (6, 7).
Parenting a child is often stressful for parents in general, but parenting children with developmental difficulties may be especially challenging (8). A previous meta-analysis suggested that parents of children with ADHD symptoms experience significantly more parenting stress compared to parents of children without ADHD symptoms (9). Although early precursors of ADHD may be expressed as early as 3–18 months (10–14), ADHD is usually only considered for diagnosis once a child begins pre-school (15). As a result, parents caring for very young children with early ADHD symptoms may endure increased stress for prolonged periods before detection by support services. Prolonged parenting stress may lead to poorer parent mental health outcomes and harsh parenting strategies, both of which are reciprocally related to child ADHD symptom severity (16–18). Therefore, measuring parenting stress could have utility as both an early-life ADHD risk indicator and a signal to health professionals to provide relevant support for caregivers and their child before this cycle begins.
In the present study, we wished to investigate whether parenting stress could be clinically useful as a broad screening method for childhood ADHD risk. Using data from the Tokyo Teen Cohort (TTC), a prospective population-based birth cohort study in Japan (N = 3171), we investigated longitudinal associations between maternal parenting stress during the first 3 years following childbirth and child ADHD at age 12 within a community sample. To ensure clinical feasibility of potential screening, we used the Maternal and Child Health handbook (MCH), which is an already widely-adopted and routinely-used tool for infant health assessment in Japan. The MCH collects parenting stress data via a single self-report item and thus presents minimal time or response burden.
We used data from the TTC study (19), which is a population-based birth cohort study of child health and development using data from children and their caregivers. For the first wave of this cohort study, 3171 households with a child aged 10 were randomly sampled using the resident register from three municipalities (Setagaya, Chofu, and Mitaka) in Tokyo, Japan. For the second wave (age 12), 3,007 households participated (follow-up rate: 94.8%). At both waves, trained interviewers obtained written informed consent from the child's primary caregivers. As part of TTC's wider data collection procedure, participants were asked to complete a set of questionnaires. The study protocol of TTC was approved by the institutional review boards from the Tokyo Metropolitan Institute of Medical Science (Approval number [12–35]), SOKENDAI (Graduate University for Advanced Studies [2012002]), and the University of Tokyo [10057].
Participants were asked to fill in anonymous self-report questionnaires including questions about ADHD symptoms and sociodemographic characteristics (child's sex, age in months, and family income). Participants were also requested to report the responses recorded in their Maternal and Child Health handbook (MCH) on maternal parenting stress.
The MCH handbook is a booklet distributed to newly pregnant women in Japan to facilitate routine assessment of child development and health by mothers as well as healthcare professionals (20). In 2018, 98% of all pregnant women reported receiving the MCH handbook within the first 20 weeks of pregnancy (21). The MCH handbook is used when mothers and their children attend health check-ups offered by the local health center or pediatric clinics, scheduled at regular intervals coinciding with developmental milestones (1, 3–4, 9–10, 18, and 36 months). In advance of each check-up, mothers answer the following question: “As a result of parenting, are there times when you feel distressed or find it difficult to cope?” Mothers can choose one of the following three responses: “yes,” “no,” or “difficult to say”. In the analysis, these responses were reclassified into dichotomous categories: “no” or “yes/difficult to say,” since we assumed that “difficult to say” may reflect that respondents were experiencing parenting stress but were reluctant to indicate so. The primary caregiver copied the parenting stress information from MCH handbook on the self-report questionnaire at the first wave of TTC (age 10).
Child ADHD symptoms were assessed using the hyperactivity/inattention subscale from the parent-report Strength and Difficulties Questionnaire (SDQ) (22, 23) at the second wave of the TTC study (age 12). The subscale consists of two items for inattention, two items for hyperactivity, and one item for impulsiveness (24). The three possible responses were “not true” [0], “somewhat true” (1), and “certainly true” (2). The responses from these five items were summed to produce a score from 0 to 10, with higher scores reflecting greater ADHD symptom burden. This subscale offers good predictive and discriminant validity across gender and age groups in adolescence (24). A cut-off value of 7 points or more was used for indicating high risk of ADHD, in line with previous studies (25–27).
Child age in months (calculated from birth date and survey response date), sex and socio-economic status (measured via family income) were adjusted for in analyses as potential confounders. Family income was categorized into 0–5, 5–10, and 10+ million yen per year, representing the lower, middle and upper thirds of household income distribution in Tokyo.
Bivariate binomial logistic regression analysis was used to examine the association between the presence of parenting stress at 1, 3–4, 9–10, 18, and 36 m, and child ADHD symptoms at age 12. Multiple binomial logistic regression analysis adjusting for sex, age in months, and annual household income followed for the above analysis. A full information maximum likelihood (FIML) estimation procedure was adopted to handle missing data (28) under the assumption of missing at random (MAR). All analyses were performed in Mplus 8.4.
Of the 3,007 households that participated the second wave (age 12) survey of the TTC study, 353 were excluded for the following reasons: did not own an MCH handbook, could not confirm whether they possessed an MCH handbook, had not responded to any items in the MCH handbook regarding parenting stress, had not reported child ADHD symptoms at age 12. Thus, the final analysis included 2,654 households (88.2%). Among the 2,654 cases, about half (52.5%) of children were boys. The mean age in months at the second survey wave was 145.9 months. 64.5% of participating households had annual household incomes below 10 million yen. Among 3,007 participants, more than 70% recorded parenting stress at all timepoints (73.4% at 1, 86.4% at 3–4, 85.3% at 9–10, 85.5% at 18, and 85.4% at 36 months).
As shown in Table 1, the prevalence of mothers reporting parenting stress was highest at 1 m (41.4%) and lowest at 9–10 m (26.5%). 6.1% of children had SDQ attention/hyperactivity scores indicating high risk of ADHD symptoms at age 12.
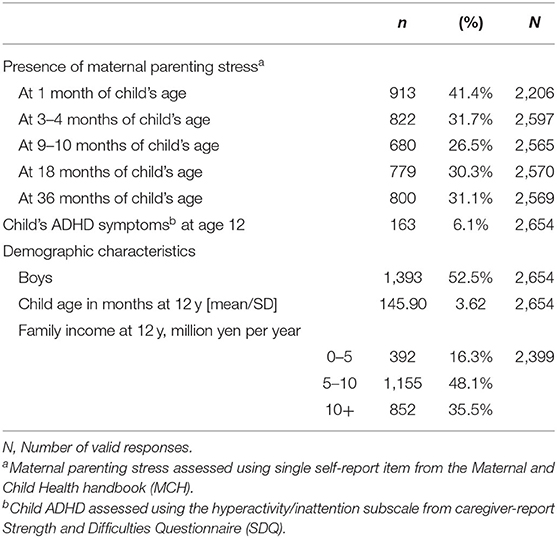
Table 1. Prevalence of maternal parenting stress during the first 3 years following childbirth, child ADHD at age 12, and demographic characteristics at age 12.
Table 2 shows the result of binomial logistic regression analysis of ADHD symptoms at age 12 from maternal parenting stress during the first 3 years after childbirth. At 1 m and 3–4 m, associations between parenting stress and ADHD symptoms at age 12 were not significant. However, ADHD symptoms at age 12 was significantly associated by parenting stress at 9–10 m (OR = 1.42, p = 0.047, 95% CI [1.00, 2.00]), 18 m (OR = 1.57, p = 0.007, 95% CI [1.13, 2.19]) and 36 m (OR = 1.67, p = 0.002, 95% CI [1.20, 2.31]) with increasing strength at each time point. After adjustment for child's sex, age in months, and annual household income, ADHD symptoms at age 12 remained significantly associated by parenting stress at 9–10 m (OR = 1.43, p = 0.048, 95% CI [1.00, 2.05]), 18 m (OR = 1.57, p = 0.010, 95% CI [1.11, 2.21]) and 36 m (OR = 1.64, p = 0.005, 95% CI [1.16, 2.30]) while associations between parenting stress at 1 and 3–4 m and ADHD symptoms at age 12 remained insignificant.
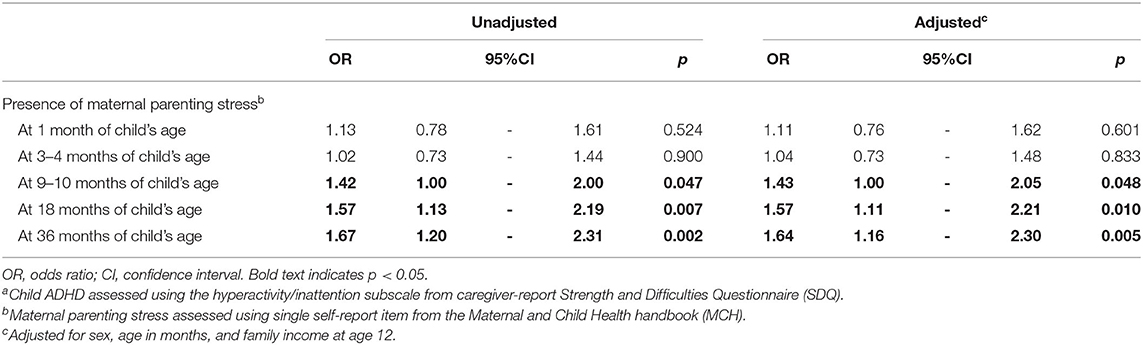
Table 2. Logistic regressions ofchild ADHD at age 12a from maternal parenting stress during first 3 years following childbirth.
To our knowledge, this is the first study to investigate the longitudinal association between maternal parenting stress at multiple intervals from 1 to 36 months post-childbirth and child ADHD symptoms at 12 years old in a population-based birth cohort sample. Our analyses found that parenting stress at 9–10, 18 and 36 months was associated (with subsequent increasing strength) with child ADHD symptoms at 12 years old, though parenting stress at earlier time points showed no such association. This finding suggests that self-reported parenting stress from 9 months may be a useful measure for early identification of children at risk of ADHD.
Our finding that parenting stress at 9–10 months associated ADHD symptoms in adolescence, with increasing strength at 18 and 36 months, raises the question of what differences emerge from 9 to 36 months in children who later develop ADHD that underlie this increase in parenting stress. Sleep disturbances between 0 and 5 years are associated with ADHD in adolescence (29, 30), and are associated with poorer parent mental health (31). Recent studies have also found heightened emotional reactivity at 6 and 9 months to be associated with ADHD, suggesting temperamental differences may emerge around this period (11, 12). The increasing strength of the relationship at 18 and 36 months may reflect that as motor development progresses and the child becomes more mobile, behavior manifesting core ADHD symptoms (e.g., hyperactivity and attentional difficulties) begins to emerge and impact more strongly on parents' wellbeing. Further research with young children with ADHD is necessary to determine how and what developmental differences emerge at this early age.
Parenting stress is a contributor to the increased likelihood of parents of children with ADHD using harsh or negative parenting strategies (16, 32), which may in turn exacerbate externalizing behaviors and other deleterious outcomes associated with ADHD (16, 33), highlighting the pressing need for early intervention. Several meta-analyses have demonstrated that behavioral parent training (PT) may reduce parenting stress, lead to positive parenting, and improve long-term outcomes for both the child and the parent (34–37). PT may confer reduced benefits when the parent themselves has ADHD (38, 39), though a more recent study found limited impact of parental ADHD symptoms on treatment outcomes (40). In either case, since ADHD is a highly heritable condition (41), health practitioners should ensure parental treatment needs (if any) are addressed alongside those of the child to ensure optimal outcomes for both.
This study shows the utility of the MCH handbook (and by extension, single-item self-report measures of parenting stress) for early identification of ADHD risk. This builds on a recent study examining the possibility of early identification of autism via developmental delay recorded in the MCH handbook (42) to encourage utilization of MCH data for early screening of mental health problems. We recommend that health professionals working with families aim to routinely capture parenting stress information, and specifically target parenting stress reduction when building family-specific support programmes.
Our study has a number of strengths. First, we used a large, representative population-based birth cohort, giving substantial ecological validity to our findings and allowing us to control for common sociodemographic confounders. Using the MCH handbook gave access to parenting stress data at frequent intervals over the first 36 months. In addition, since MCH handbook data was collected concurrently with infant health check-ups, our retrospective study design avoids the problem of recall bias. However, this study also had some limitations. First, we did not have parenting stress data beyond 36 months, as the MCH handbook does not record parenting stress after this time point. As such, we were unable to determine if this trend of increasing strength of parenting stress on adolescent ADHD symptoms persists as the child ages; this would be a potentially beneficial target for future research. Second, we used parent-reported child ADHD symptoms, rather than researcher-conducted interviews, teacher ratings, or clinical diagnosis. However, the SDQ attention/hyperactivity subscale has robust psychometrics for detecting ADHD (24, 43, 44), which may substantially offset this limitation. Lastly, the presence of parenting stress was captured by a binary-response question used in the MCH handbook, and since there is high social pressure on parenting success there may be a social desirability bias in response patterns. To get a more detailed picture of how parenting stress relates to child ADHD symptoms, future studies may consider using scales such as the Parenting Stress Index (45). This is particularly prescient as many developmental differences in temperament and behavior associated with ADHD may not be disorder-specific; for example, many are also associated with autism (13, 46). Future studies seeking to understand mechanisms or specificity of associations with early parenting stress may therefore benefit from including measures of autistic traits or other developmental differences in their analyses, either as control variables or additional outcomes of interest.
In conclusion, our study found that maternal parenting stress from 9 months post-childbirth was associated with child ADHD symptoms at age 12, with increasing strength at 18 months and 36 months. Maternal parenting stress from 9 months may be an indicator of later child ADHD. This time period may be extremely valuable for early intervention to ensure optimal outcomes in families caring for a child with ADHD.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Tokyo Metropolitan Institute of Medical Science. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
AN, KE, and DS conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript, being supervised by KK, MH-H, SA, and MN. JN, SK, SF, YM, MH, KB, NO, NN, KS, and MM critically reviewed the manuscript for important intellectual content and contributed to the discussion. SY supervised the statistical analysis. All authors contributed to and have approved the final manuscript.
This work was supported by Grant-in-Aid for Transformative Research Areas (21A101) and Grant-in-Aid for Scientific Research on Innovative Areas (JP23118002 and JP16H01689; Adolescent Mind & Self-Regulation) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was also supported in part by JSPS KAKENHI (Grant Nos. JP16H06395, JP16H06398, JP16H06399, JP16K15566, JP16K21720, JP17H05931, JP19K17055, JP19H04877, JP20H01777, JP20H03951, JP21H05171, JP21H05173, and JP21H05174), JST-Mirai Program (Grant No. JPMJMI21J3); AMED (Grant No. JP17ek0109262); the UTokyo Center for Integrative Science of Human Behavior (CiSHuB); and the International Research Center for Neurointelligence (WPI-IRCN) at The University of Tokyo Institutes for Advanced Study (UTIAS). The funding source had no role in the preparation, review, or approval of the manuscript, and the decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to gratefully acknowledge the collaboration of all participants in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.806669/full#supplementary-material
1. Barbaresi WJ, Colligan RC, Weaver AL, Voigt RG, Killian JM, Katusic SK. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. (2013) 131:637–44. doi: 10.1542/peds.2012-2354
2. Nourredine M, Gering A, Fourneret P, Rolland B, Falissard B, Cucherat M, et al. Association of attention-deficit/hyperactivity disorder in childhood and adolescence with the risk of subsequent psychotic disorder. JAMA Psychiatry. (2021) 78:519. doi: 10.1001/jamapsychiatry.2020.4799
3. Chronis-Tuscano A, Molina BSG, Pelham WE, Applegate B, Dahlke A, Overmyer M, et al. Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. (2010) 67:1044–51. doi: 10.1001/archgenpsychiatry.2010.127
4. Jones K, Daley D, Hutchings J, Bywater T, Eames C. Efficacy of the incredible years basic parent training programme as an early intervention for children with conduct problems and ADHD. Child Care Health Dev. (2007) 33:749–56. doi: 10.1111/j.1365-2214.2007.00747.x
5. Shuai L, Daley D, Wang YF, Zhang JS, Kong YT, Tan X, et al. Executive function training for children with attention deficit hyperactivity disorder. Chin Med J (Engl). (2017) 130:549–58. doi: 10.4103/0366-6999.200541
6. Danforth JS, Harvey E, Ulaszek WR, McKee TE. The outcome of group parent training for families of children with attention-deficit hyperactivity disorder and defiant/aggressive behavior. J Behav Ther Exp Psychiatry. (2006) 37:188–205. doi: 10.1016/j.jbtep.2005.05.009
7. Zwi M, Jones H, Thorgaard C, York A, Dennis JA. Parent training interventions for attention deficit hyperactivity disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst Rev. (2011) 2011:CD003018. doi: 10.1002/14651858.CD003018.pub3
8. Deater-Deckard K. Parenting stress and child adjustment: some old hypotheses and new questions. Clin Psychol Sci Pract. (1998) 5:314–32. doi: 10.1111/j.1468-2850.1998.tb00152.x
9. Theule J, Wiener J, Tannock R, Jenkins JM. Parenting stress in families of children with ADHD: a meta-analysis. J Emot Behav Disord. (2013) 21:3–17. doi: 10.1177/1063426610387433
10. Friedman AH, Watamura SE, Robertson SS. Movement-attention coupling in infancy and attention problems in childhood. Dev Med Child Neurol. (2005) 47:660–5. doi: 10.1017/S0012162205001350
11. Sullivan EL, Holton KF, Nousen EK, Barling AN, Sullivan CA, Propper CB, et al. Early identification of ADHD risk via infant temperament and emotion regulation: a pilot study. J Child Psychol Psychiatry. (2015) 56:949–57. doi: 10.1111/jcpp.12426
12. Miller N V, Hane AA, Degnan KA, Fox NA, Chronis-Tuscano A. Investigation of a developmental pathway from infant anger reactivity to childhood inhibitory control and ADHD symptoms: interactive effects of early maternal caregiving. J Child Psychol Psychiatry. (2019) 60:762–72. doi: 10.1111/jcpp.13047
13. Johnson MH, Gliga T, Jones E, Charman T. Annual research review: infant development, autism, and ADHD – early pathways to emerging disorders. J Child Psychol Psychiatry. (2015) 56:228–47. doi: 10.1111/jcpp.12328
14. Gurevitz M, Geva R, Varon M, Leitner Y. Early markers in infants and toddlers for development of ADHD. J Atten Disord. (2014) 18:14–22. doi: 10.1177/1087054712447858
15. Posner J, Polanczyk G V, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. (2020) 395:450–62. doi: 10.1016/S0140-6736(19)33004-1
16. Bhide S, Sciberras E, Anderson V, Hazell P, Nicholson JM. Association between parenting style and socio-emotional and academic functioning in children with and without ADHD: a community-based study. J Atten Disord. (2019) 23:463–74. doi: 10.1177/1087054716661420
17. Mulraney M, Giallo R, Efron D, Brown S, Nicholson JM, Sciberras E. Maternal postnatal mental health and offspring symptoms of ADHD at 8–9 years: pathways via parenting behavior. Eur Child Adolesc Psychiatry. (2019) 28:923–32. doi: 10.1007/s00787-018-1254-5
18. Harold GT, Leve LD, Barrett D, Elam K, Neiderhiser JM, Natsuaki MN, et al. Biological and rearing mother influences on child ADHD symptoms: revisiting the developmental interface between nature and nurture. J Child Psychol Psychiatry. (2013) 54:1038–46. doi: 10.1111/jcpp.12100
19. Ando S, Nishida A, Yamasaki S, Koike S, Morimoto Y, Hoshino A, et al. Cohort profile: The tokyo teen cohort study (TTC). Int J Epidemiol. (2019) 48:1414–414g. doi: 10.1093/ije/dyz033
20. Ichikawa K, Fujiwara T, Nakayama T. Effectiveness of home visits in pregnancy as a public health measure to improve birth outcomes. PLoS ONE. (2015) 10:e0137307. doi: 10.1371/journal.pone.0137307
21. Ministry Ministry of Health Labour Labour Welfare G of J. Report on Regional Public Health Services and Health Promotion Services (2018). Available online at: https://www.mhlw.go.jp/toukei/saikin/hw/c-hoken/18/dl/kekka1.pdf (accessed April 12, 2022).
22. Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. (1997) 38:581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x
23. Matsuishi T, Nagano M, Araki Y, Tanaka Y, Iwasaki M, Yamashita Y, et al. Scale properties of the Japanese version of the Strengths and Difficulties Questionnaire (SDQ): A study of infant and school children in community samples. Brain Dev. (2008) 30:410–5. doi: 10.1016/j.braindev.2007.12.003
24. Algorta GP, Dodd AL, Stringaris A, Youngstrom EA. Diagnostic efficiency of the SDQ for parents to identify ADHD in the UK: a ROC analysis. Eur Child Adolesc Psychiatry. (2016) 25:949–57. doi: 10.1007/s00787-015-0815-0
25. Okumura Y, Yamasaki S, Ando S, Usami M, Endo K, Hiraiwa-Hasegawa M, et al. Psychosocial burden of undiagnosed persistent ADHD symptoms in 12-year-old children: a population-based birth cohort study. J Atten Disord. (2019) 25:108705471983774. doi: 10.1177/1087054719837746
26. Riglin L, Collishaw S, Thapar AK, Dalsgaard S, Langley K, Smith GD, et al. Association of genetic risk variants with attention-deficit/hyperactivity disorder trajectories in the general population. JAMA psychiatry. (2016) 73:1285–92. doi: 10.1001/jamapsychiatry.2016.2817
27. St.Pourcain B, Mandy WP, Heron J, Golding J, Davey Smith G, Skuse DH. Links Between Co-occurring Social-Communication and Hyperactive-Inattentive Trait Trajectories. J Am Acad Child Adolesc Psychiatry. (2011) 50:892–902.e5. doi: 10.1016/j.jaac.2011.05.015
28. Cham H, Reshetnyak E, Rosenfeld B, Breitbart W. Full information maximum likelihood estimation for latent variable interactions with incomplete indicators. Multivariate Behav Res. (2017) 52:12–30. doi: 10.1080/00273171.2016.1245600
29. Carpena MX, Matijasevich A, Loret de Mola C, Santos IS, Munhoz TN, Tovo-Rodrigues L. The effects of persistent sleep disturbances during early childhood over adolescent ADHD, and the mediating effect of attention-related executive functions: Data from the 2004 Pelotas Birth Cohort. J Affect Disord. (2022) 296:175–82. doi: 10.1016/j.jad.2021.09.053
30. Shephard E, Zuccolo PF, Idrees I, Godoy PBG, Salomone E, Ferrante C, et al. Systematic Review and Meta-analysis: The Science of Early-Life Precursors and Interventions for Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. (2021) 61:187–226. doi: 10.1016/j.jaac.2021.03.016
31. Shang CY, Gau SSF, Soong WT. Association between childhood sleep problems and perinatal factors, parental mental distress and behavioral problems. J Sleep Res. (2006) 15:63–73. doi: 10.1111/j.1365-2869.2006.00492.x
32. Modesto-Lowe V, Danforth JS, Brooks D. ADHD does parenting style matter? Clin Pediatr (Phila). (2008) 47:865–72. doi: 10.1177/0009922808319963
33. Roy A, Hechtman L, Arnold LE, Swanson JM, Molina BSG, Sibley MH, et al. Childhood predictors of adult functional outcomes in the multimodal treatment study of attention-deficit/hyperactivity disorder (MTA). J Am Acad Child Adolesc Psychiatry. (2017) 56:687–695.e7. doi: 10.1016/j.jaac.2017.05.020
34. Mulqueen JM, Bartley CA, Bloch MH. Meta-Analysis: Parental Interventions for Preschool ADHD. J Atten Disord. (2015) 19:118–24. doi: 10.1177/1087054713504135
35. Coates J, Taylor JA, Sayal K. Parenting Interventions for ADHD: A Systematic Literature Review and Meta-Analysis. J Atten Disord. (2015) 19:831–43. doi: 10.1177/1087054714535952
36. Rimestad ML, Lambek R, Zacher Christiansen H, Hougaard E. Short- and long-term effects of parent training for preschool children with or at risk of ADHD: a systematic review and meta-analysis. J Atten Disord. (2019) 23:423–34. doi: 10.1177/1087054716648775
37. Charach A, Carson P, Fox S, Ali MU, Beckett J, Lim CG. Interventions for preschool children at high risk for ADHD: a comparative effectiveness review. Pediatrics. (2013) 131:e1584–604. doi: 10.1542/peds.2012-0974
38. Sonuga-Barke EJS, Daley D, Thompson M. Does maternal ADHD reduce the effectiveness of parent training for preschool children's ADHD? J Am Acad Child Adolesc Psychiatry. (2002) 41:696–702. doi: 10.1097/00004583-200206000-00009
39. Wang CH, Mazursky-Horowitz H, Chronis-Tuscano A. Delivering evidence-based treatments for child attention-deficit/hyperactivity disorder (ADHD) in the context of parental ADHD. Curr Psychiatry Rep. (2014) 16:474. doi: 10.1007/s11920-014-0474-8
40. Forehand R, Parent J, Peisch VD, Sonuga-Barke E, Long N, Breslend NL, et al. Do parental ADHD symptoms reduce the efficacy of parent training for preschool ADHD? A secondary analysis of a randomized controlled trial. Behav Res Ther. (2017) 97:163–9. doi: 10.1016/j.brat.2017.08.002
41. Thapar A, Cooper M, Jefferies R, Stergiakouli E. What causes attention deficit hyperactivity disorder? Arch Dis Child. (2012) 97:260–5. doi: 10.1136/archdischild-2011-300482
42. Hirota T, Bishop S, Adachi M, Shui A, Takahashi M, Mori H, et al. Utilization of the maternal and child health handbook in early identification of autism spectrum disorder and other neurodevelopmental disorders. Autism Res. (2020) 14:1–9. doi: 10.1002/aur.2442
43. Hall CL, Guo B, Valentine AZ, Groom MJ, Daley D, Sayal K, et al. The validity of the strengths and difficulties questionnaire (SDQ) for children with ADHD symptoms. PLoS ONE. (2019) 14:e0218518. doi: 10.1371/journal.pone.0218518
44. Øvergaard KR, Oerbeck B, Friis S, Pripp AH, Biele G, Aase H, et al. Attention-deficit/hyperactivity disorder in preschoolers: The accuracy of a short screener. J Am Acad Child Adolesc Psychiatry. (2018) 57:428–35. doi: 10.1016/j.jaac.2018.03.008
45. Abidin RR. Parenting Stress Index Manual. Charlottesville, VA: Pediatric Psychology Press (1983).
Keywords: maternal parenting stress, ADHD, birth cohort, Maternal and Child Health handbook, adolescent
Citation: Endo K, Stanyon D, Yamasaki S, Nakanishi M, Niimura J, Kanata S, Fujikawa S, Morimoto Y, Hosozawa M, Baba K, Oikawa N, Nakajima N, Suzuki K, Miyashita M, Ando S, Hiraiwa-Hasegawa M, Kasai K and Nishida A (2022) Self-Reported Maternal Parenting Stress From 9 m Is Longitudinally Associated With Child ADHD Symptoms at Age 12: Findings From a Population-Based Birth Cohort Study. Front. Psychiatry 13:806669. doi: 10.3389/fpsyt.2022.806669
Received: 01 November 2021; Accepted: 06 April 2022;
Published: 28 April 2022.
Edited by:
Joseph M. Boden, University of Otago, New ZealandReviewed by:
Tomoya Hirota, University of California, San Francisco, United StatesCopyright © 2022 Endo, Stanyon, Yamasaki, Nakanishi, Niimura, Kanata, Fujikawa, Morimoto, Hosozawa, Baba, Oikawa, Nakajima, Suzuki, Miyashita, Ando, Hiraiwa-Hasegawa, Kasai and Nishida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atsushi Nishida, bmlzaGlkYS1hdEBpZ2FrdWtlbi5vci5qcA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.