- Birmingham and Solihull Mental Health NHS Foundation Trust, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom
Self-other distinction refers to the ability to distinguish between our own and other people's physical and mental states (actions, perceptions, emotions etc.). Both the right temporo-parietal junction and brain areas associated with the human mirror neuron system are likely to critically influence self-other distinction, given their respective contributions to theory of mind and embodied empathy. The degree of appropriate self-other distinction will vary according to the exact social situation, and how helpful it is to feel into, or remain detached from, another person's mental state. Indeed, the emotional resonance that we can share with others affords the gift of empathy, but over-sharing may pose a downside, leading to a range of difficulties from personal distress to paranoia, and perhaps even motor tics and compulsions. The aim of this perspective paper is to consider how evidence from behavioral and neurophysiological studies supports a role for problems with self-other distinction in a range of psychiatric symptoms spanning the emotional, cognitive and motor domains. The various signs and symptoms associated with problematic self-other distinction comprise both maladaptive and adaptive (compensatory) responses to dysfunction within a common underlying neuropsychological mechanism, compelling the adoption of more holistic transdiagnostic therapeutic approaches within Psychiatry.
Introduction
What Is Self-Other Distinction and Why Is It Important?
Humans are innately wired to respond to others' emotional states. Most of us understand what it is to vicariously feel other's pain, and if we are lucky, their happiness. This emotional resonance that we can share with others appears automatic, and its greatest gift is that of affective empathy and our ability to respond sensitively to the needs of others. However, successful navigation of the social world also requires that we can contemplate the contrasting perspectives of self and other, and too much sharing may have a downside.
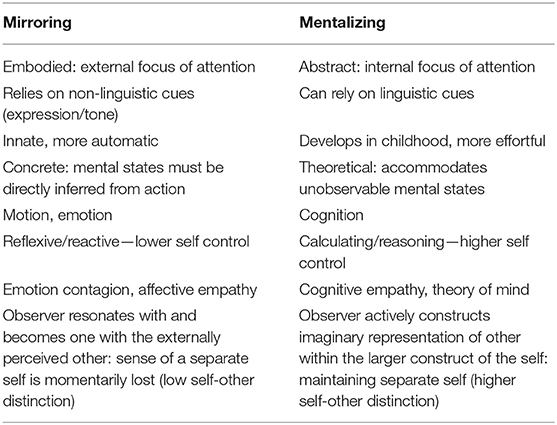
Self-other distinction refers to the ability to distinguish between our own and other people's physical and mental states, including actions, perceptions, emotions etc. Low self-other distinction (or self-other blending/merging) is associated with processes that contribute to the recognition of mental states in others including imitation or mirroring. However, low self-other distinction can be associated with misattributions of mental states when these differ for self and other, a situation when abstract mentalizing that holds in mind these opposing perspectives is important. Mirroring tends to occur in response to visual stimuli and for embodied mental states, whereas mentalizing is critical when visual cues to mental states are potentially misleading or not readily observable (e.g., verbal tasks; understanding beliefs; deception etc.). The appropriate degree of self-other distinction will therefore vary according to the exact social situation e.g., affective empathy may involve low self-other distinction, whereas understanding false belief requires higher self-other distinction (Table 1).
Which Brain Regions Contribute to Self-Other Distinction?
One neural network highly relevant to self-other distinction is the Mirror Neuron System [MNS: (1)] which was first identified in primates (2–4). The MNS is thought to underpin motor simulation of observed actions, providing a basis for imitation, and to draw upon visual cues to support the understanding of action goals [e.g., (5, 6)], in turn facilitating inferences about emotions, and perhaps beliefs and intentions (7, 8), although its exact contribution to empathy continues to be investigated (9). In humans, the MNS includes the inferior frontal gyrus (BA44/45), superior temporal and inferior parietal lobe (10–12). This network develops very early in childhood (13), and may be automatically activated before the second network, the mentalizing system (14, 15), which includes the medial prefrontal cortex, precuneus, and right temporal parietal junction (rTPJ). The mentalizing network supports more conscious reasoning about mental states (15, 16).
The rTPJ is of particular importance in self-other distinction, given its role in multi-sensory integration and sense of embodiment (17), and activation during tasks where the differing beliefs of self and other are salient (18, 19). Further evidence comes from the effects of rTPJ stimulation on tasks that require self-other distinction such as imitation inhibition tasks (20–22). Most studies suggest that rTPJ activity is positively associated with self-other distinction (8), such that activations may emphasize incongruence between self and other, or allow for switching between their related representations [e.g., (23)].
What Might Happen If Self-Other Distinction Goes Wrong?
Within the social cognitive domain, indicators of low self-other distinction include motor imitation, and emotion contagion, when we effectively take on the physical and emotional states of others. These acts of mirroring encourage the automatic sacrifice of a sense of separate self as the observer becomes one with the perceived other. This loss of self-other distinction could be less likely to occur in the context of mentalizing, which may involve the conscious and controlled construction of an imaginary other (or alternative self) perhaps subordinate to and easily distinguishable from the one's true self. In sum, sense of oneself as a unique individual entity, and as the originator or controller of perceived internal and external states (e.g., actions and emotions), may be vulnerable to the effects associated with loss of self-other distinction and the mirroring experience. On the other hand, in some cases, too much self-other distinction could be problematic.
The aim of this perspective paper is to synthesize the evidence suggesting that problems with self-other distinction are relevant to the development of numerous psychiatric disorders, building on previous research (8, 17) through the integration of additional evidence in the form of both behavioral and neurophysiological studies within the field of psychiatry. While many factors may influence self-other distinction (e.g., executive dysfunction; self-efficacy; sensory impairments), this opinion piece focuses on processes that are typically associated with mirroring, with reference to the contrast with conscious mentalizing. Key questions were: 1. Is it possible to identify primary (direct) vs. secondary (indirect) signs of problematic self-other distinction? 2. Are there secondary signs with opposing/compensatory effects? I will argue that a range of clinical symptoms across emotional, cognitive and motor domains constitute various manifestations of impaired self-other distinction, resulting from dysfunction within a common underlying neural mechanism, with important implications including in terms of treatment approaches.
Relevance of Self-Other Distinction to Psychiatry
Self-Other Distinction Within the Emotional Domain
The primary cause of loss of self-other distinction within the emotional domain is likely to be high emotion contagion. MNS responses are emotion specific and more sensitive to negative valence (24, 25), therefore excessive resonance with others experiencing negative emotion is likely to result in increased personal distress. High personal distress is common in psychiatric disorders but is not usually accompanied by high empathic concern (Table 2). Perhaps continued experience of personal distress can prove aversive, leading individuals to self-report lower empathic concern as they become more focused on resolving their own internal emotional state. The unpleasantness of excessive emotional resonance could also contribute to social anxiety and social anhedonia (8). Furthermore, the relationship between performance on social cognitive tasks and emotional resonance may fall on an inverted U-curve helping to explain patterns of social cognitive performance (e.g. inconsistent impairment across tasks) in numerous psychiatric disorders (8, 17).
Frequent unregulated emotional contagion may encourage confusion around the source (self/other) of experienced emotional states. Alexithymia (286), or difficulty identifying and expressing emotions, could be one consequence of this confusion stemming from vicarious experience of other's emotions in the absence of a linking situational cause for that emotion in oneself (8). However, alexithymia could indicate reduced attention to internal states which in turn reduces the salience of excessive emotional resonance or personal distress (8, 287). Other forms of emotional blunting (e.g., constricted/flat affect), and perhaps dissociation, could support similar regulatory functions in terms of avoiding exposure to problematic emotions of self and/or other. Such emotional responses may be largely unconscious conditioned responses to the primary problem of loss of self-other distinction within the emotional domain.
Some psychiatric disorders feature anti-social behaviors which should prompt an emotional reaction in others, such as the compulsive socially inappropriate urges seen in Tourette syndrome (TS). TS is associated with heightened personal distress and increased emotional reactivity to emotional facial expressions (26, 36), and patients who experience urges to make offensive remarks/gestures find them troubling as they don't consciously wish to cause distress (288). On the surface, socially inappropriate actions imply emotional disregard, and emphasize self-other distinction because the patient's transgression is in direct antagonism to the others' emotional needs (8) i.e., the anti-social action (at least momentarily) separates the perpetrator from the victim because the intention and action goals associated with their anti-social act conflict with the desired mental state of the victim. However, in addition to counteracting any feeling of excessive emotional resonance, such actions promote control over the emotional state of others. Therefore, rather than emphasizing self-other distinction, anti-social urges could arise from an unconscious urge to prompt a negative emotional mental state within another that matches the patient's own negative internal state (i.e., reduced self-other distinction). This may provide a better explanation for some emotionally provocative and antagonistic behaviors seen in Borderline Personality Disorder (BPD) and Narcissistic Personality Disorder (NPD).
Self-Other Distinction Within the Cognitive Domain
Excessive emotional resonance with others and arising difficulties with self-other distinction could have a broader effect on conscious experience of cognitive mental states including judgments about the origin of these. Difficulty knowing whether a thought or intention arose from the self explains many symptoms of psychosis [e.g., (155)] including delusions relating to thought transfer and telepathy. Incorrect assumptions that one is aware of the cognitive mental state of another could also reduce mentalizing leading to egocentric errors (289). Projection of negative emotions or intentions onto others, as seen in disorders such as BPD and schizophrenia (including on social cognitive tasks: Table 2), is likely to prompt social anxiety and paranoia. If a projected thought is positive, it could encourage grandiosity. Doubts about whether thoughts are internally generated may also underlie magical thinking as seen in Obsessive-compulsive Disorder (OCD), explaining the association between negative sense of agency and likelihood thought action fusion (287) i.e., the belief that thinking about events makes those events more likely to happen.
In some cases, loss of self-other distinction may weaken the stability of our overall conscious construct of self, as most clearly seen in BPD and schizophrenia. When this occurs, it appears all the more important to develop cognitive strategies that help restore self-other boundaries. Strategies are likely to include conscious avoidance of mentalizing, helping to explain the low self-reported perspective taking that often accompanies high personal distress (Table 2), and perhaps poor performance on social cognitive tasks. In addition, impulsive non-conformity, whereby individuals with schizophrenia express strong opposition to convention and the opinions or expectations of others, even where this would seem harmful or irrational, may enhance cognitive self-other distinction. Similar characteristics can be seen in NPD, where rivalry and entitlement emphasize one's own uniqueness, and deception may be used to maintain differentiation between the cognitive mental states of self and other (152).
Self-Other Distinction Within the Motor Domain
Excessive motor resonance in the form of echophenomena is likely to indicate loss of self-other distinction within the motor domain. Similar more subtle characteristics may be observed during imitation inhibition tasks, through magnetoencephalography, or perhaps when exploring susceptibility to the rubber-hand illusion (Table 2). Given the role of the MNS in emotion contagion there is likely to be a link between motor resonance and neural limbic response [e.g., (290, 291)], and therefore greater motor resonance and a tendency to emotional dysregulation (although MNS activity may not always manifest as observable movement). Difficulties in deciding whether the self is the agent of movements and related sensory events could help to explain the perception of involuntary movements, and perhaps depersonalization, in some psychiatric disorders. Weakened sense of ownership of personal actions could encourage impulsivity, and in more severe cases, delusions of control.
One proposed mechanism thought to influence self-other distinction is based on movement efference and predictive sensory feedback [e.g., (292, 293)], whereby dysfunction impairs determination of self-produced actions and effects, with relevance to conditions such as psychosis (294–296). Disrupted sensory feedback (alike excessive motor resonance) could have a conscious cognitive correlate in the form of altered sense of agency. Indeed, sense of agency appears to consist of both intrinsic (i.e., a more conscious, cognitive experience of agency) and extrinsic (i.e., sensorimotor experience of body ownership) aspects, and differences in integrating or balancing intrinsic and extrinsic self-representation networks could impair self-other distinctions (297).
Tics and compulsions can be associated with sensorimotor abnormalities (298, 299) and alterations in sense of agency for action (Table 2). While tics are reported as feeling at the most semi-voluntary, and tend not to appear goal directed, one effect of these internally generated fragments of motor activity is to interrupt motor resonance with external others, helping to support self-other differentiation, and perhaps developing into a habit conditioned to the experience of internal emotional stress. That is, the sensory fulfillment associated with tics and motor compulsions may arise through the acting out of a self-initiated action which helps to confirm (perhaps subconsciously) internal control over movement and related neural motor activity, counterintuitively helping to re-establish sense of agency. Given that both emotion and sense of self are relevant to self-harm (300), compulsive self-harm may be another symptom through which a self-initiated motor act enables a sense of self-control or internal agency over a perceived emotional or sensory state.
Self-Other Distinction Within the Brain
Excessive resonance with others is perhaps most likely to be reflected in atypical activity within the MNS, as seen in disorders including TS and schizophrenia (26, 249). More generally, inferior parietal and inferior frontal activations have been shown to be atypical during social cognitive tasks in TS, ASD and BPD; unusual resting state activity has been revealed in schizophrenia; and structural changes have been associated with symptoms of OCD and NPD (Table 2). Problems with self-other distinction may also manifest as atypical activity within the mentalizing system, perhaps as hypo-activation of rTPJ when mentalizing is cued or hyper-activation when it is not [e.g., 29, 46]. Many studies have revealed that the right TPJ in particular, may demonstrate atypical activity during social cognitive tasks in patient populations with symptoms linked to problems with self-other distinction.
Perhaps the best evidence links brain dysfunction directly to behavioral signs of self-other distinction problems or related symptoms. For example, in TS, global measures of echophenomena and urges to tic have been associated with rTPJ activity during two different social cognitive tasks (26, 184). In schizophrenia, psychosis has been linked to negative symptoms (249) and excessive activity within the MNS (83), while reduced neural synchrony involving rTPJ has been implicated in impaired social communication in autism (283). Overall however, few studies have attempted to explore specific associations.
Discussion
Primary Effects, Secondary Symptoms and Coping Strategies
Many neuropsychiatric disorders feature emotional, cognitive and/or motor features that are likely to indicate problems with self-other distinction. Within each of these domains, we may identify both signs of low self-other distinction, and characteristics or behaviors that could constitute secondary effects or coping strategies which serve to increase self-other distinction. For example, frequent emotion contagion may lead to emotional dysregulation, and detachment from emotional experiences may combat personal distress. Cognitive features associated with poor self-other distinction may manifest as paranoia or projection, and potential coping strategies include avoidance of perspective taking or buffering sense of self through grandiosity or impulsive non-conformity. Excessive motor resonance with others (e.g., poor imitation inhibition) may reduce sense of physical agency and encourage the development of tics and compulsions that may help to restore this.
A novel contribution of the hypotheses presented herein is that they can account for a range of seemingly contradictory behaviors and self-defeating symptoms. There is irony in that many of the symptoms that arise through difficulties with self-other distinction, and reflect greater resonance with others' mental states, could appear to suggest hypo-mentalizing or antagonism toward others. This highlights the importance of considering both ability and application. Where over-application occurs, resulting difficulties may be as great as in cases of under-application.
While the concept of self-other distinction can be applied to cognition, emotion or movement, it's also important to consider automaticity, or implicit vs. explicit processes and skills, where possible. For example, processes that reduce self-other distinction and involve the motor and limbic system (e.g., emotion contagion) appear fairly implicit or automatic (301, 302), although some individuals may be more susceptible to the cues that initiate this. In contrast, complex higher level mentalizing may be to some extent more explicit or controllable (16, 186, 303). An over-responsive MNS leading to frequent limbic dysregulation may initiate confusion around sense of agency, which then becomes more generalized to thought and action. In general, as we cannot directly observe another person's thought, it makes sense for cognitive signs to occur further downstream. For example, while excessive automatic emotion contagion is often a primary sign, secondary effects such as reduced perspective taking or conscious attention to other's emotions, may help to compensate for the primary problem (i.e., low self-other distinction). Other indirect signs (e.g., tics and motor compulsions) may seem less conscious, although differentiating between conscious strategies and automatic compulsive responses can be challenging. Furthermore, regulatory or compensatory effects may occur across domains, supported by the finding that both cognitive (thought action fusion; sense of agency) and emotional (personal distress) factors mediate the relationship between emotion contagion and alexithymia (287).
Therapeutic Implications, Limitations and Remaining Questions
The struggle to achieve a healthy and functional balance of self-other distinction may manifest in a range of forms, from tics in TS, to repetitive cycles of affiliation followed by antagonism in BPD. The theory presented suggests while those with neurodevelopmental, anxiety and personality disorders express differing constellations of internalizing and externalizing symptoms, overlapping difficulties with self-other distinction imply shared dysfunction within a common underlying neuropsychological mechanism. Therefore the potential therapeutic benefit of addressing difficulties with self-other distinction should be extensive, once the specific associations between self-other distinction and the suggested related symptoms and coping mechanisms have been established. Psychological interventions have begun to consider factors which overlap with the self-other distinction theme (e.g., self-awareness; emotion regulation; mentalizing), including metacognitive approaches for psychosis [e.g., (304, 305)], and personality disorders (306, 307). Other emerging interventions combine non-invasive brain stimulation with social cognitive (308) or sensori-motor (309) related training. Future related research should seek to first fully define and operationalise the construct of self-other distinction, before identifying reliable measures (e.g., self-other overlap index) that can be used in assessment and evaluation. Ultimately we should seek to harness what we can from behaviors that appear to counteract a problem with self-other distinction in order to inform therapeutic strategies.
The proposed hypotheses prompt further unanswered questions. For example, longitudinal studies are necessary to test whether suggested primary signs of low self-other distinction (e.g., emotion contagion; echophenomena) precede the development of other symptoms such as alexithymia, blunted affect, paranoia, antagonistic behaviors. This would identify risk factors and targets for early intervention. While there should be common overlap in the underlying mechanisms, individual differences in neural organization or stage of development of self-other distinction difficulties or compensatory responses, would help to explain the predominance of features within a given domain e.g., motor in TS vs. cognitive in schizophrenia. Diagnostic and therapeutic approaches would also be informed by a better understanding of the specific neural networks and structures involved, as well as factors such as the relationship between self-other distinction and executive dysfunction (e.g., cognitive flexibility). Can most of the symptoms described be linked to dysfunction of rTPJ, and is this synonymous with over-activation of the MNS or altered functional connectivity between the mirroring and mentalizing networks? Recent studies have revealed rTPJ activation in relation to forward predictions in both highly social (310) and less social (311) contexts, so further related clinical research using carefully selected experimental tasks is needed.
Many psychiatric symptoms appear likely to stem from low self-other distinction. However, some behavioral problems may reflect excessive self-other distinction as a primary effect. For example, the data on autism seems to suggest a mixed pattern, which could be linked to motor and/or MNS dysfunction [(312–314); but see (315)]. Social cognition is frequently impaired in movement disorder (316) and an impaired motor system will likely impair self-other distinction through loss of feedback between motor resonance and emotional processes (317). In relation to primary and secondary effects, primary psychopathy is thought to involve a fundamental deficit in affective empathy and therefore high self-other distinction, whereas secondary psychopathy may involve indirect symptoms or those arising through a coping strategy (318). It is possible that some of the signs and symptoms presented here that are suggestive of high self-other distinction constitute primary rather than secondary effects. Furthermore, some behaviors could reflect either high or low self-other distinction [e.g., hypo-imitation: (319)] and whether an individual may fluctuate between polarized high or low self-other distinction (e.g., due to rTPJ dysfunction) remains to be explored. Other more general limitations include the challenges in reviewing the literature and drawing comparisons across different studies and disorders, because of variations in terms used, co-morbidities, reliability of self-report and unknown impact of medications.
Conclusion
In conclusion, impaired self-other distinction, potentially underpinned by excessive mirroring, and/or hypoactivation of rTPJ, appears to lead to a disturbed sense of agency and the manifestation of a range of psychiatric symptoms across emotional, motor and cognitive domains. These symptoms variously reflect, or attempt to redress, the problematic level of self-other distinction. Understanding the hidden relationship between self-other distinction and symptoms as diverse as paranoia, self-harm, tics and narcissism, and considering the potential compensatory value of compulsive and antagonistic behaviors that are typically viewed as dysfunctional, will enhance our global understanding of mental health and expedite the development of more effective and innovative interventions.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. (2006) 7:942–51. doi: 10.1038/nrn2024
2. Cattaneo L, Rizzolatti G. The mirror neuron system. Arch Neurol. (2009) 66:557–60. doi: 10.1001/archneurol.2009.41
3. Kaplan JT, Iacoboni M. Getting a grip on other minds: mirror neurons, intention understanding, and cognitive empathy. Soc Neurosci. (2006) 1:175–83. doi: 10.1080/17470910600985605
4. Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. (2005) 3:e79. doi: 10.1371/journal.pbio.0030079
5. Umiltà MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, et al. I know what you are doing. Neurophysiol Study Neuron. (2001) 31:155–65. doi: 10.1016/S0896-6273(01)00337-3
6. Keysers C, Paracampo R. Gazzola V. What neuromodulation and lesion studies tell us about the function of the mirror neuron system and embodied cognition. Curr Opin Psychol. (2018) 24:35–40. doi: 10.1016/j.copsyc.2018.04.001
7. Gallese V. Before and below 'theory of mind': embodied simulation and the neural correlates of social cognition. Philos Trans R Soc Lond B Biol Sci. (2007) 362:659–69. doi: 10.1098/rstb.2006.2002
8. Eddy CM. Social cognition and self-other distinctions in neuropsychiatry: Insights from schizophrenia and Tourette syndrome. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 82:69–85. doi: 10.1016/j.pnpbp.2017.11.026
9. Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J Cogn Neurosci. (2009) 21:1229–43. doi: 10.1162/jocn.2009.21189
10. de la Rosa S, Schillinger FL, Bülthoff HH, Schultz J, Uludag K. fMRI adaptation between action observation and action execution reveals cortical areas with mirror neuron properties in human BA 44/45. Front Hum Neurosci. (2016) 10:78. doi: 10.3389/fnhum.2016.00078
11. Binder E, Dovern A, Hesse MD, Ebke M, Karbe H, Saliger J, et al. Lesion evidence for a human mirror neuron system. Cortex. (2017) 90:125–37. doi: 10.1016/j.cortex.2017.02.008
12. Ferrari PF, Gerbella M, Coudé G, Rozzi S. Two different mirror neuron networks: The sensorimotor (hand) and limbic (face) pathways. Neurosci. (2017) 358:300–15. doi: 10.1016/j.neuroscience.2017.06.052
13. Meltzoff AN, Moore MK. Explaining facial imitation: a theoretical model. Early Dev Parent. (1997) 6:179–92. doi: 10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R
14. Frith CD, Frith U. The neural basis of mentalizing. Neuron. (2006) 50:531–4. doi: 10.1016/j.neuron.2006.05.001
15. Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. (2009) 48:564–84. doi: 10.1016/j.neuroimage.2009.06.009
16. Spunt RP, Lieberman MD. The busy social brain: evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychol Sci. (2013) 24:80–6. doi: 10.1177/0956797612450884
17. Eddy CM. The junction between self and other? Temporo-parietal dysfunction in neuropsychiatry. Neuropsychologia. (2016) 89:465–77. doi: 10.1016/j.neuropsychologia.2016.07.030
18. Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. (2003) 19:1835–42. doi: 10.1016/S1053-8119(03)00230-1
19. Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. (2005) 43:1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013
20. Santiesteban I, Banissy MJ, Catmur C, Bird G. Enhancing social ability by stimulating right temporoparietal junction. Curr Biol. (2012) 22:2274–7. doi: 10.1016/j.cub.2012.10.018
21. Giardina A, Caltagirone C, Oliveri M. The temporoparietal junction modulates self-other motor representations during online and offline social motor conflict: an rTMS study. Neuroreport. (2015) 26:1–5. doi: 10.1097/WNR.0000000000000282
22. Hogeveen J, Chartrand TL, Obhi SS. Social mimicry enhances mu-suppression during action observation. Cereb Cortex. (2015) 25:2076–82. doi: 10.1093/cercor/bhu016
23. Sowden S, Catmur C. The role of the right temporoparietal junction in the control of imitation. Cereb Cortex. (2015) 25:1107–13. doi: 10.1093/cercor/bht306
24. Krautheim JT, Steines M, Dannlowski U, Neziroglu G, Acosta H, Sommer J, et al. Emotion specific neural activation for the production and perception of facial expressions. Cortex. (2020) 127:17–28. doi: 10.1016/j.cortex.2020.01.026
25. Schmidt SNL, Sojer CA, Hass J, Kirsch P, Mier D. fMRI adaptation reveals: the human mirror neuron system discriminates emotional valence. Cortex. (2020) 128:270–80. doi: 10.1016/j.cortex.2020.03.026
26. Eddy CM, Cavanna AE, Hansen PC. Empathy and aversion: the neural signature of mentalizing in Tourette syndrome. Psychol Med. (2017) 47:507–17. doi: 10.1017/S0033291716002725
27. Neuner I, Kellermann T, Stöcker T, Kircher T, Habel U, Shah JN, et al. Amygdala hypersensitivity in response to emotional faces in Tourette's patients. World J Biol Psychiatry. (2010) 11:858–72. doi: 10.3109/15622975.2010.480984
28. Lehmann A, Bahçesular K, Brockmann EM, Biederbick SE, Dziobek I, Gallinat J, et al. Subjective experience of emotions and emotional empathy in paranoid schizophrenia. Psychiatry Res. (2014) 220:825–33. doi: 10.1016/j.psychres.2014.09.009
29. Brosnan M, Ashwin C, Walker I, Donaghue J. Can an “Extreme Female Brain” be characterised in terms of psychosis? Pers Indiv Differ. (2010) 49:738–42. doi: 10.1016/j.paid.2010.06.018
30. Jalal B, Ramachandran VS. “I feel your disgust and relief”: can the action understanding system (mirror neuron system) be recruited to induce disgust and relief from contamination vicariously, in individuals with obsessive–compulsive disorder symptoms? Neurocase. (2017) 23:31–5. doi: 10.1080/13554794.2017.1279638
31. Helt MS, Fein DA, Vargas JE. Emotional contagion in children with autism spectrum disorder varies with stimulus familiarity and task instructions. Dev Psychopathol. (2020) 32:383–93. doi: 10.1017/S0954579419000154
32. Hadjikhani N, Zürcher NR, Rogier O, Hippolyte L, Lemonnier E, Ruest T, et al. Emotional contagion for pain is intact in autism spectrum disorders. Transl Psychiatry. (2014) 4:e343. doi: 10.1038/tp.2013.113
33. Herpertz SC, Bertsch K. The social-cognitive basis of personality disorders. Curr Opin Psychiatry. (2014) 27:73–7. doi: 10.1097/YCO.0000000000000026
34. Matzke B, Herpertz SC, Berger C, Fleischer M, Domes G. Facial reactions during emotion recognition in borderline personality disorder: a facial electromyography study. Psychopathology. (2014) 47:101–10. doi: 10.1159/000351122
35. Czarna AZ, Zajenkowski M, Maciantowicz O, Szymaniak K. The relationship of narcissism with tendency to react with anger and hostility: the roles of neuroticism and emotion regulation ability. Curr Psychol. (2021) 40:5499–514. doi: 10.1007/s12144-019-00504-6
36. Eddy CM, Macerollo A, Martino D, Cavanna AE. Interpersonal reactivity differences in Tourette syndrome. Psychiatry Res. (2015) 228:932–5. doi: 10.1016/j.psychres.2015.05.070
37. Montag C, Heinz A, Kunz D, Gallinat J. Self-reported empathic abilities in schizophrenia. Schizophr Res. (2007) 92:85–9. doi: 10.1016/j.schres.2007.01.024
38. Wang W, Zhou Y, Wang J, Xu H, Wei S, Wang D, et al. Prevalence, clinical correlates of suicide attempt and its relationship with empathy in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2020) 99:109863. doi: 10.1016/j.pnpbp.2020.109863
39. Fontenelle LF, Soares ID, Miele F, Borges MC, Prazeres AM, Rangé BP, et al. Empathy and symptoms dimensions of patients with obsessive-compulsive disorder. J Psychiatric Res. (2009) 43:455–63. doi: 10.1016/j.jpsychires.2008.05.007
40. Kang JI, Namkoong K, Yoo SW, Jhung K, Kim SJ. Abnormalities of emotional awareness and perception in patients with obsessive-compulsive disorder. J Affect Dis. (2012) 141:286–93. doi: 10.1016/j.jad.2012.04.001
41. Rogers K, Dziobek I, Hassenstab J, Wolf OT, Convit A. Who cares? Revisiting empathy in Asperger syndrome. J Autism Dev Disord. (2007) 37:709–15. doi: 10.1007/s10803-006-0197-8
42. Brett JD, Maybery MT. Understanding oneself to understand others: the role of alexithymia and anxiety in the relationships between autistic trait dimensions and empathy. J Autism Dev Disord. (2021) 26:1–3. doi: 10.1007/s10803-021-05086-6
43. Dziobek I, Preissler S, Grozdanovic Z, Heuser I, Heekeren HR, Roepke S. Neuronal correlates of altered empathy and social cognition in borderline personality disorder. Neuroimage. (2011) 57:539–48. doi: 10.1016/j.neuroimage.2011.05.005
44. Flasbeck V, Enzi B, Brüne M. Childhood trauma affects processing of social interactions in borderline personality disorder: An event-related potential study investigating empathy for pain. World J Biol Psychiatry. (2019) 20:278–88. doi: 10.1080/15622975.2017.1333147
45. Harari H, Shamay-Tsoory SG, Ravid M, Levkovitz Y. Double dissociation between cognitive and affective empathy in borderline personality disorder. Psychiatry Res. (2010) 175:277–9. doi: 10.1016/j.psychres.2009.03.002
46. New AS, aan het Rot M, Ripoll LH, Perez-Rodriguez MM, Lazarus S, Zipursky E, et al. Empathy and alexithymia in borderline personality disorder: clinical and laboratory measures. J Pers Disord. (2012) 26:660–75. doi: 10.1521/pedi.2012.26.5.660
47. Petersen R, Brakoulias V, Langdon R. An experimental investigation of mentalization ability in borderline personality disorder. Compr Psychiatry. (2016) 64:12–21. doi: 10.1016/j.comppsych.2015.10.004
48. De Panfilis C, Antonucci C, Meehan KB, Cain NM, Soliani A, Marchesi C, et al. Facial emotion recognition and social-cognitive correlates of narcissistic features. J Pers Disord. (2019) 33:433–49. doi: 10.1521/pedi_2018_32_350
49. Rogoza R, Zemojtel-Piotrowska M, Kwiatkowska MM, Kwiatkowska K. The bright, the dark, and the blue face of narcissism: the spectrum of narcissism in its relations to the metatraits of personality, self-esteem, and the nomological network of shyness, loneliness, and empathy. Front Psychol. (2018) 9:343. doi: 10.3389/fpsyg.2018.00343
50. Quast LF, Rosenthal LD, Cushman GK, Gutiérrez-Colina AM, Braley EI, Kardon P, et al. Relations between tic severity, emotion regulation, and social outcomes in youth with tourette syndrome. Child Psychiatry Hum Dev. (2020) 51:366–76. doi: 10.1007/s10578-019-00948-8
51. Hagstrøm J, Spang KS, Vangkilde S, Maigaard K, Skov L, Pagsberg AK, et al. An observational study of emotion regulation in children with Tourette syndrome. J Child Psychol Psychiatry. (2021) 62:790–7. doi: 10.1111/jcpp.13375
52. Liu J, Subramaniam M, Chong SA, Mahendran R. Maladaptive cognitive emotion regulation strategies and positive symptoms in schizophrenia spectrum disorders: The mediating role of global emotion dysregulation. Clin Psychol Psychother. (2020) 27:826–36. doi: 10.1002/cpp.2466
53. Liu J, Chan T, Chong SA, Subramaniam M, Mahendran R. cognitive insight on psychotic and depressive symptoms during the early course of schizophrenia spectrum disorders. Early Interv Psychiatry. (2020) 14:691–7. doi: 10.1111/eip.12895
54. Jansen M, Overgaauw S, De Bruijn ERA. Social cognition and obsessive-compulsive disorder: a review of subdomains of social functioning. Front Psychiatry. (2020) 11:118. doi: 10.3389/fpsyt.2020.00118
55. Samson AC, Phillips JM, Parker KJ, Shah S, Gross JJ, Hardan AY. Emotion dysregulation and the core features of autism spectrum disorder. J Autism Dev Disord. (2014) 44:1766–72. doi: 10.1007/s10803-013-2022-5
56. Gormley E, Ryan C, McCusker C. Alexithymia is associated with emotion dysregulation in young people with autism spectrum disorder. J Dev Phys. (2021) 7:1–6. doi: 10.1007/s10882-021-09795-9
57. Mazefsky CA Yu L, White SW, Siegel M, Pilkonis PA. The emotion dysregulation inventory: Psychometric properties and item response theory calibration in an autism spectrum disorder sample. Autism Res. (2018) 11:928–41. doi: 10.1002/aur.1947
58. Tani M, Kanai C, Ota H, Yamada T, Watanabe H, Yokoi H, et al. Mental and behavioral symptoms of person's with Asperger's syndrome: Relationships with social isolation and handicaps. Res Autism Spectrum Disord. (2012) 6:907–12. doi: 10.1016/j.rasd.2011.12.004
59. Sharp C, Pane H, Ha C, Venta A, Patel AB, Sturek J, et al. Theory of mind and emotion regulation difficulties in adolescents with borderline traits. J Am Acad Child Adolesc Psychiatry. (2011) 50:563–73. doi: 10.1016/j.jaac.2011.01.017
60. Cheshure A, Zeigler-Hill V, Sauls D, Vrabel JK, Lehtman MH. Narcissism and emotion dysregulation: Narcissistic admiration and narcissistic rivalry have divergent associations with emotion regulation difficulties. Pers Individ Diff. (2020) 154:109679. doi: 10.1016/j.paid.2019.109679
61. Zhang H, Wang Z, You X, Lü W, Luo Y. Associations between narcissism and emotion regulation difficulties: Respiratory sinus arrhythmia reactivity as a moderator. Biol Psychol. (2015) 110:1–11. doi: 10.1016/j.biopsycho.2015.06.014
62. Ponzoni S, Beomonte Zobel S, Rogier G, Velotti P. Emotion dysregulation acts in the relationship between vulnerable narcissism and suicidal ideation. Scand J Psychol. (2021) 62:468–75. doi: 10.1111/sjop.12730
63. Di Pierro R, Di Sarno M, Madeddu F. Investigating the relationship between narcissism and emotion regulation difficulties: The role of grandiose and vulnerable traits. Clin Neuropsychiatry: J Treat Eval. (2017) 14:209–15.
64. Thibert AL, Day HI, Sandor P. Self-concept and self-consciousness in adults with Tourette syndrome. Can J Psychiatry. (1995) 40:35–9. doi: 10.1177/070674379504000109
65. Pile V, Robinson S, Topor M, Hedderly T, Lau JYF. Attention bias for social threat in youth with tic disorders: Links with tic severity and social anxiety. Child Neuropsychol. (2019) 25:394–409. doi: 10.1080/09297049.2018.1480754
66. Harvey PO, Bodnar M, Sergerie K, Armony J, Lepage M. Relation between emotional face memory and social anhedonia in schizophrenia. J Psychiatry Neurosci. (2009) 34:102–10.
67. Lecomte T, Théroux L, Paquin K, Potvin S, Achim A. Can Social Anxiety Impact Facial Emotion Recognition in Schizophrenia? J Nerv Ment Dis. (2019) 207:140–4. doi: 10.1097/NMD.0000000000000934
68. Berger P, Bitsch F, Jakobi B, Nagels A, Straube B, Falkenberg I. Cognitive and emotional empathy in patients with schizophrenia spectrum disorders: a replication and extension study. Psychiatry Res. (2019) 276:56–9. doi: 10.1016/j.psychres.2019.04.015
69. Çeşmeci U, Yüksel RN, Kaya H, Dilbaz N. Schizotypality and neurological soft signs in patients with obsessive–compulsive disorder. Psychiatry Clin Psychopharmacol. (2017) 27:234–40. doi: 10.1080/24750573.2017.1342752
70. Xia J, Fan J, Du H, Liu W, Li S, Zhu J, et al. Abnormal spontaneous neural activity in the medial prefrontal cortex and right superior temporal gyrus correlates with anhedonia severity in obsessive-compulsive disorder. J Affect Disord. (2019) 259:47–55. doi: 10.1016/j.jad.2019.08.019
71. Bejerot S, Eriksson JM, Mörtberg E. Social anxiety in adult autism spectrum disorder. Psychiatry Res. (2014) 220:705–7. doi: 10.1016/j.psychres.2014.08.030
72. Pickard H, Hirsch C, Simonoff E, Happé F. Exploring the cognitive, emotional and sensory correlates of social anxiety in autistic and neurotypical adolescents. J Child Psychol Psychiatry. (2020) 61:1317–27. doi: 10.1111/jcpp.13214
73. Chevallier C, Grèzes J, Molesworth C, Berthoz S, Happé F. Brief report: selective social anhedonia in high functioning autism. J Autism Dev Disord. 42:1504–9. doi: 10.1007/s10803-011-1364-0
74. Weinbrecht A, Roepke S, Renneberg B. Fear of positive evaluation in borderline personality disorder. PLoS ONE. (2020) 15:e0237944. doi: 10.1371/journal.pone.0237944
75. Chabrol H, Valls M, van Leeuwen N, Bui E. Callous-unemotional and borderline traits in nonclinical adolescents: Personality profiles and relations to antisocial behaviors. Pers Individ Diff. (2012) 53:969–73 doi: 10.1016/j.paid.2012.07.017
76. Stanton K, Zimmerman M. Clinician ratings of vulnerable and grandiose narcissistic features: Implications for an expanded narcissistic personality disorder diagnosis. Personal Disord. (2018) 9:263–72. doi: 10.1037/per0000272
77. Eddy CM, Cavanna AE. Triangles, tricks and tics: Hyper-mentalizing in response to animated shapes in Tourette syndrome. Cortex. (2015) 71:68–75. doi: 10.1016/j.cortex.2015.06.003
78. Silvestri PR, Chiarotti F, Giustini S, Cardona F. Alexithymia and tic disorders: a study on a sample of children and their mothers. Eur Child Adolesc Psychiatry. (2019) 28:461–70. doi: 10.1007/s00787-018-1209-x
79. Ospina LH, Shanahan M, Perez-Rodriguez MM, Chan CC, Clari R, Burdick KE. Alexithymia predicts poorer social and everyday functioning in schizophrenia and bipolar disorder. Psychiatry Res. (2019) 273:218–26. doi: 10.1016/j.psychres.2019.01.033
80. Irani F, Platek SM, Panyavin IS, Calkins ME, Kohler C, Siegel SJ, et al. Self-face recognition and theory of mind in patients with schizophrenia and first-degree relatives. Schizophr Res. (2006) 88:151–60. doi: 10.1016/j.schres.2006.07.016
81. Suslow T, Roestel C, Arolt V. Affective priming in schizophrenia with and without affective negative symptoms. Eur Arch Psychiatry Clin Neurosci. (2003) 253:292–300. doi: 10.1007/s00406-003-0443-4
82. Lindner C, Dannlowski U, Bauer J, Ohrmann P, Lencer R, Zwitserlood P, et al. Affective flattening in patients with schizophrenia: differential association with amygdala response to threat-related facial expression under automatic and controlled processing conditions. Psychiatry Investig. (2016) 13:102–11. doi: 10.4306/pi.2016.13.1.102
83. Lee JS, Chun JW, Yoon SY, Park HJ, Kim JJ. Involvement of the mirror neuron system in blunted affect in schizophrenia. Schizophr Res. (2014) 152:268–74. doi: 10.1016/j.schres.2013.10.043
84. Grabe HJ, Ruhrmann S, Ettelt S, Muller A, Buhtz F, Hochrein A, et al. Alexithymia in obsessive-compulsive disorder—results from a family study. Psychother Psychosom. (2006) 75:312–8. doi: 10.1159/000093954
85. Roh D, Kim WJ, Kim CH. Alexithymia in obsessive-compulsive disorder: clinical correlates and symptom dimensions. J Nerv Ment Dis. (2011) 199:690–5. doi: 10.1097/NMD.0b013e318229d209
86. Khosravani V, Samimi Ardestani M, Sharifi Bastan F, Kamali Z. The relationship between alexithymia and symptom dimensions in patients with obsessive-compulsive disorder. J Obsessive-Compulsive Relat Disord. (2017) 14:127–33. doi: 10.1016/j.jocrd.2017.04.001
87. Kim H, Seo J, Namkoong K, Hwang EH, Sohn SY, Kim SJ, et al. Alexithymia and perfectionism traits are associated with suicidal risk in patients with obsessive-compulsive disorder. J Affect Disord. (2016) 192:50–5. doi: 10.1016/j.jad.2015.12.018
88. Milosavljevic B, Carter Leno V, Simonoff E, Baird G, Pickles A, Jones CR, et al. Alexithymia in adolescents with autism spectrum disorder: its relationship to internalising difficulties, sensory modulation and social cognition. J Autism Dev Disord. (2016) 46:1354–67. doi: 10.1007/s10803-015-2670-8
89. Stagg SD, Slavny R, Hand C, Cardoso A, Smith P. Does facial expressivity count? How typically developing children respond initially to children with autism. Autism. (2014) 18:704–11. doi: 10.1177/1362361313492392
90. Trevisan DA, Hoskyn M, Birmingham E. Facial expression production in autism: a meta-analysis. Autism Res. 11:1586–601. doi: 10.1002/aur.2037
91. Lind M, Thomsen DK, Bøye R, Heinskou T, Simonsen S, Jørgensen CR. Personal and parents' life stories in patients with borderline personality disorder. Scand J Psychol. (2019) 60:231–42. doi: 10.1111/sjop.12529
92. Németh N, Péterfalvi Á, Czéh B, Tényi T, Simon M. Examining the relationship between executive functions and mentalizing abilities of patients with borderline personality disorder. Front Psychol. (2020) 11:1583. doi: 10.3389/fpsyg.2020.01583
93. De Panfilis C, Ossola P, Tonna M, Catania L, Marchesi C. Finding words for feelings: The relationship between personality disorders and alexithymia. Pers Individ Diffs. (2015) 74:285–91. doi: 10.1016/j.paid.2014.10.050
94. Derks YPMJ, Westerhof GJ, Bohlmeijer ET. A meta-analysis on the association between emotional awareness and borderline personality pathology. J Pers Disord. (2017) 31:362–84. doi: 10.1521/pedi_2016_30_257
95. Sleuwaegen E, Houben M, Claes L, Berens A, Sabbe B. The relationship between non-suicidal self-injury and alexithymia in borderline personality disorder: “actions instead of words”. Compr Psychiatry. (2017) 77:80–8. doi: 10.1016/j.comppsych.2017.06.006
96. Renneberg B, Heyn K, Gebhard R, Bachmann S. Facial expression of emotions in borderline personality disorder and depression. J Behav Ther Exp Psychiatry. (2005) 36:183–96. doi: 10.1016/j.jbtep.2005.05.002
97. Fan Y, Wonneberger C, Enzi B, de Greck M, Ulrich C, Tempelmann C, et al. The narcissistic self and its psychological and neural correlates: an exploratory fMRI study. Psychol Med. (2011) 41:1641–50. doi: 10.1017/S003329171000228X
98. Ritzl A, Csukly G, Balázs K, Égerházi A. Facial emotion recognition deficits and alexithymia in borderline, narcissistic, and histrionic personality disorders. Psychiatry Res. (2018) 270:154–9. doi: 10.1016/j.psychres.2018.09.017
99. Krystal H. Affect regulation and narcissism: Trauma, alexithymia and psychosomatic illness in narcissistic patients. In: Ronningtam E, editors. Disorders of narcissism: diagnostic, clinical and empirical implications. (1998). p. 229–325. New York: American Psychiatric Press.
100. Eddy CM, Mitchell IJ, Beck SR, Cavanna AE, Rickards HE. Altered attribution of intention in Tourette's syndrome. J Neuropsychiatry Clin Neurosci. (2010) 22:348–51. doi: 10.1176/jnp.2010.22.3.348
101. Comings DE, Comings BG. A controlled study of Tourette syndrome. IV Obsessions, compulsions, and schizoid behaviors. Am J Hum Genet. (1987) 41:782–803.
102. Cavanna AE, Robertson MM, Critchley HD. Schizotypal personality traits in Gilles de la Tourette syndrome. Acta Neurol Scand. (2007) 116:385–91. doi: 10.1111/j.1600-0404.2007.00879.x
103. Russell TA, Reynaud E, Herba C, Morris R, Corcoran R. Do you see what I see? Interpretations of intentional movement in schizophrenia. Schizophr Res. (2006) 81:101–11. doi: 10.1016/j.schres.2005.10.002
104. Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. (2003) 160:1768–74. doi: 10.1176/appi.ajp.160.10.1768
105. Backasch B, Straube B, Pyka M, Klöhn-Saghatolislam F, Müller MJ, Kircher TT, et al. Hyperintentionality during automatic perception of naturalistic cooperative behavior in patients with schizophrenia. Soc Neurosci. (2013) 8:489–504. doi: 10.1080/17470919.2013.820666
106. Wastler HM, Lenzenweger MF. Self-referential hypermentalization in schizotypy. Personal Disord. (2019) 10:536–44. doi: 10.1037/per0000344
107. Fonseca-Pedrero E, Lemos-Giráldez S, Paíno-Piñeiro M, Villazón-García U, Muñiz J. Schizotypal traits, obsessive-compulsive symptoms, and social functioning in adolescents. Compr Psychiatry. (2010) 51:71–7. doi: 10.1016/j.comppsych.2009.02.003
108. Tellawi G, Williams MT, Chasson GS. Interpersonal hostility and suspicious thinking in obsessive-compulsive disorder. Psychiatry Res. (2016) 243:295–302. doi: 10.1016/j.psychres.2016.06.038
109. Neave N, Jackson R, Saxton T, Hönekopp J. The influence of anthropomorphic tendencies on human hoarding behaviors. Pers Individ Diffs. (2015) 72:214–9. doi: 10.1016/j.paid.2014.08.041
110. Caruana N, White RC, Remington A. Autistic traits and loneliness in autism are associated with increased tendencies to anthropomorphise. Q J Exp Psychol. (2021) 74:1295–304. doi: 10.1177/17470218211005694
111. Atherton G, Cross L. Seeing more than human: autism and anthropomorphic theory of mind. Front Psychol. (2018) 9:528. doi: 10.3389/fpsyg.2018.00528
112. Blackshaw AJ, Kinderman P, Hare DJ, Hatton C. Theory of mind, causal attribution and paranoia in Asperger syndrome. Autism. (2001) 5:147–63. doi: 10.1177/1362361301005002005
113. Pinkham AE, Sasson NJ, Beaton D, Abdi H, Kohler CG, Penn DL. Qualitatively distinct factors contribute to elevated rates of paranoia in autism and schizophrenia. J Abnorm Psychol. (2012) 121:767–77. doi: 10.1037/a0028510
114. Kernberg OF. Projection and projective identification: developmental and clinical aspects. J Am Psychoanal Assoc. (1987) 35:795–819. doi: 10.1177/000306518703500401
115. Muñoz-Negro JE, Prudent C, Gutiérrez B, Cervilla JA. Paranoia and risk of personality disorder in the general population. Personal Ment Health. (2019) 13:107–16. doi: 10.1002/pmh.1443
116. Oliva F, Dalmotto M, Pirfo E, Furlan PM, Picci RL. A comparison of thought and perception disorders in borderline personality disorder and schizophrenia: psychotic experiences as a reaction to impaired social functioning. BMC Psychiatry. (2014) 14:239. doi: 10.1186/s12888-014-0239-2
117. Joiner TE, Petty S, Perez M, Sachs-Ericsson N, Rudd MD. Depressive symptoms induce paranoid symptoms in narcissistic personalities (but not narcissistic symptoms in paranoid personalities). Psychiatry Res. (2008) 159:237–44. doi: 10.1016/j.psychres.2007.05.009
118. Poggi A, Richetin J, Preti E. Trust and rejection sensitivity in personality disorders. Curr Psychiatry Rep. (2019) 21:69. doi: 10.1007/s11920-019-1059-3
119. Goldner-Vukov M, Moore LJ. Malignant Narcissism: from fairy tales to harsh reality. Psychiatr Danub. (2010) 22:392–405.
120. Garfield D, Havens L. Paranoid phenomena and pathological narcissism. Am J Psychother. (1991) 45:160–72. doi: 10.1176/appi.psychotherapy.1991.45.2.160
121. Hanks CE, McGuire JF, Lewin AB, Storch EA, Murphy TK. Clinical correlates and mediators of self-concept in youth with chronic tic disorders. Child Psychiatry Hum Dev. (2016) 47:64–74. doi: 10.1007/s10578-015-0544-0
122. Moe AM, Docherty NM. Schizophrenia and the sense of self. Schizophr Bull. (2014) 40:161–8. doi: 10.1093/schbul/sbt121
123. Vodušek VV, Parnas J, Tomori M, Škodlar B. The phenomenology of emotion experience in first-episode psychosis. Psychopathology. (2014) 47:252–60. doi: 10.1159/000357759
124. Nelson B, Raballo A. Basic self-disturbance in the schizophrenia spectrum: taking stock and moving forward. Psychopathology. (2015) 48:301–9. doi: 10.1159/000437211
125. Doron G, Moulding R, Kyrios M, Nedeljkovic M. Sensitivity of self-beliefs in obsessive compulsive disorder. Depress Anxiety. (2008) 25:874–84. doi: 10.1002/da.20369
126. Doron G, Kyrios M, Moulding R. Sensitive domains of self-concept in obsessive-compulsive disorder (OCD): further evidence for a multidimensional model of OCD. J Anxiety Disord. (2007) 21:433–44. doi: 10.1016/j.janxdis.2006.05.008
127. Ferrier S, Brewin C. Feared identity and obsessive compulsive disorder. Behav Res Ther. (2005) 43:1363–74. doi: 10.1016/j.brat.2004.10.005
128. Berna F, Göritz AS, Schröder J, Coutelle R, Danion JM, Cuervo-Lombard CV, et al. Self-disorders in individuals with autistic traits: contribution of reduced autobiographical reasoning capacities. J Autism Dev Disord. (2016) 46:2587–98. doi: 10.1007/s10803-016-2797-2
129. Coutelle R, Goltzene MA, Bizet E, Schoenberger M, Berna F, Danion JM. Self-concept clarity and autobiographical memory functions in adults with autism spectrum disorder without intellectual deficiency. J Autism Dev Disord. (2020) 50:3874–82. doi: 10.1007/s10803-020-04447-x
130. Skirrow P, Jackson P, Perry E, Hare DJ. I Collect Therefore I am—autonoetic consciousness and hoarding in Asperger syndrome. Clin Psychol Psychother. (2015) 22:278–84. doi: 10.1002/cpp.1889
131. Lind M, Vanwoerden S, Penner F, Sharp C. Inpatient adolescents with borderline personality disorder features: Identity diffusion and narrative incoherence. Personal Disord. (2019) 10:389–93. doi: 10.1037/per0000338
132. Meares R, Gerull F, Stevenson J, Korner A. Is self disturbance the core of borderline personality disorder? An outcome study of borderline personality factors. Aust N Z J Psychiatry. (2011) 45:214–22. doi: 10.3109/00048674.2010.551280
133. Bender DS, Skodol AE. Borderline personality as a self-other representational disturbance. J Pers Disord. (2007) 21:500–17. doi: 10.1521/pedi.2007.21.5.500
134. Fuchs T. Fragmented selves: temporality and identity in borderline personality disorder. Psychopathology. (2007) 40:379–87. doi: 10.1159/000106468
135. Gunderson JG, Herpertz SC, Skodol AE, Torgersen S, Zanarini MC. Borderline personality disorder. Nat Rev Dis Primers. (2018) 4:18029. doi: 10.1038/nrdp.2018.29
136. Diamond D, Yeomans F, Keefe JR. Transference-Focused Psychotherapy for Pathological Narcissism and Narcissistic Personality Disorder (TFP-N). Psychodyn Psychiatry. (2021) 49:244–72. doi: 10.1521/pdps.2021.49.2.244
137. Eddy CM, Cavanna AE. Do patients with Tourette syndrome jump to conclusions? J Neuropsychiatry Clin Neurosci. (2014) 26:396–9. doi: 10.1176/appi.neuropsych.13090218
138. Robertson MM, Cavanna AE. The disaster was my fault! Neurocase. (2008) 13:446–51. doi: 10.1080/13554790802001395
139. Kabakci E, Demir B, Demirel H, Sevik AE. Thought-action fusion: is it present in schizophrenia? Behavi Change. (2008) 25:169–77. doi: 10.1375/bech.25.3.169
140. Randjbar S, Veckenstedt R, Vitzthum F, Hottenrott B, Moritz S. Attributional biases in paranoid schizophrenia: Further evidence for a decreased sense of self-causation in paranoia. Psychosis. (2011) 3:74–85. doi: 10.1080/17522431003717675
141. Asai T, Tanno Y. Highly schizotypal students have a weaker sense of self-agency. Psychiatry Clin Neurosci. (2008) 62:115–9. doi: 10.1111/j.1440-1819.2007.01768.x
142. Einstein DA, Menzies RG. The presence of magical thinking in obsessive compulsive disorder. Behav Res Ther. (2004) 42:539–49. doi: 10.1016/S0005-7967(03)00160-8
143. Shafran R, Thordarson DS, Rachman S. Thought-action fusion in obsessive compulsive disorder. J Anx Disord. (1996) 10:379–91. doi: 10.1016/0887-6185(96)00018-7
144. Obsessive Compulsive Cognitions Working Group. Cognitive assessment of obsessive-compulsive disorder. Behav Res Ther. (1997) 35:667–81. doi: 10.1016/S0005-7967(97)00017-X
145. Oren E, Friedmann N, Dar R. Things happen: individuals with high obsessive-compulsive tendencies omit agency in their spoken language. Conscious Cogn. (2016) 42:125–34. doi: 10.1016/j.concog.2016.03.012
146. Ciaramidaro A, Bölte S, Schlitt S, Hainz D, Poustka F, Weber B, et al. Schizophrenia and autism as contrasting minds: neural evidence for the hypo-hyper-intentionality hypothesis. Schizophr Bull. (2015) 41:171–9. doi: 10.1093/schbul/sbu124
147. Visuri I. Sensory supernatural experiences in autism. Religion Brain Behav. (2020) 10:151–65. doi: 10.1080/2153599X.2018.1548374
148. Lind M, Jørgensen CR, Heinskou T, Simonsen S, Bøye R, Thomsen DK. Patients with borderline personality disorder show increased agency in life stories after 12 months of psychotherapy. Psychotherapy. (2019) 56:274–84. doi: 10.1037/pst0000184
149. Brown AA, Freis SD, Carroll PJ, Arkin RM. Perceived agency mediates the link between the narcissistic subtypes and self-esteem. Pers Individ Diff. (2016) 90:124–9. doi: 10.1016/j.paid.2015.10.055
150. Schwarzkopf S, Schilbach L, Vogeley K, Timmermans B. “Making it explicit” makes a difference: evidence for a dissociation of spontaneous and intentional level 1 perspective taking in high-functioning autism. Cognition. (2014) 131:345–54. doi: 10.1016/j.cognition.2014.02.003
151. Grzegorzewski P, Kulesza M, Pluta A, Iqbal Z, Kucharska K. Assessing self-reported empathy and altruism in patients suffering from enduring borderline personality disorder. Psychiatry Res. (2019) 273:798–807. doi: 10.1016/j.psychres.2018.12.109
152. Eddy CM. Self-serving social strategies: a systematic review of social cognition in narcissism. Curr Psychol. (2021). doi: 10.1007/s12144-021-01661-3
153. Baskin-Sommers A, Krusemark E, Ronningstam E. Empathy in narcissistic personality disorder: from clinical and empirical perspectives. Personal Disord. (2014) 5:323–33. doi: 10.1037/per0000061
154. Ritter K, Dziobek I, Preissler S, Rüter A, Vater A, Fydrich T, et al. Lack of empathy in patients with narcissistic personality disorder. Psychiatry Res. (2011) 187:241–7. doi: 10.1016/j.psychres.2010.09.013
155. Kozáková E, Bakštein E, Havlíček O, Bečev O, Knytl P, Zaytseva Y, et al. Disrupted sense of agency as a state marker of first-episode schizophrenia: a large-scale follow-up study. Front Psychiatry. (2020) 11:570570. doi: 10.3389/fpsyt.2020.570570
156. Eddy CM, Cavanna AE. Altered social cognition in Tourette syndrome: nature and implications. Behav Neurol. (2013) 27:15–22. doi: 10.1155/2013/417516
157. Kurlan R, Daragjati C, Como PG, McDermott MP, Trinidad KS, Roddy S, et al. Non-obscene complex socially inappropriate behavior in Tourette's syndrome. J Neuropsychiatry Clin Neurosci. (1996) 8:311–7. doi: 10.1176/jnp.8.3.311
158. Najt P, Bayer U, Hausmann M. Atypical lateralisation in emotional prosody in men with schizotypy. Laterality. (2012) 17:533–48. doi: 10.1080/1357650X.2011.586702
159. Young E, Mason O. Psychosis-proneness and socially relevant reasoning. Psychiatry Res. (2007) 150:123–9. doi: 10.1016/j.psychres.2006.05.003
160. Amr M, Volpe FM. Relationship between anhedonia and impulsivity in schizophrenia, major depression and schizoaffective disorder. Asian J Psychiatr. (2013) 6:577–80. doi: 10.1016/j.ajp.2013.09.002
161. Belloch A, Roncero M, Perpiñá C. Ego-syntonicity and ego-dystonicity associated with upsetting intrusive cognitions. J Psychopathol Behav Assess. (2012) 34:94–106. doi: 10.1007/s10862-011-9255-4
162. Nurnberg HG, Raskin M, Levine PE, Pollack S, Siegel O, Prince R. The comorbidity of borderline personality disorder and other DSM-III-R axis II personality disorders. Am J Psychiatry. (1991) 148:1371–7. doi: 10.1176/ajp.148.10.1371
163. Nurnberg HG, Siegel O, Prince R, Levine PE, Raskin M, Pollack S. Axis II comorbidity of self-defeating personality disorder. J Personality Disord. (1993) 7:10–21. doi: 10.1521/pedi.1993.7.1.10
164. Tonello L, Giacobbi L, Pettenon A, Scuotto A, Cocchi M, Gabrielli F, et al. Problem behavior in autism spectrum disorders: a paradigmatic self-organized perspective of network structures. Behav Brain Sci. (2019) 42:e28. doi: 10.1017/S0140525X18001188
165. Williams DL, Siegel M, Mazefsky CA. Autism and Developmental Disorders Inpatient Research Collaborative (ADDIRC). Problem behaviors in autism spectrum disorder: association with verbal ability and adapting/coping skills. J Autism Dev Disord. (2018) 48:3668–77. doi: 10.1007/s10803-017-3179-0
166. Koenigsberg HW, Harvey PD, Mitropoulou V, New AS, Goodman M, Silverman J, et al. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Personality Disord. (2001) 15:358–70. doi: 10.1521/pedi.15.4.358.19181
167. Axelrod SR, Widiger TA, Trull TJ, Corbitt EM. Relations of five-factor model antagonism facets with personality disorder symptomatology. J Pers Assess. (1997) 69:297–313. doi: 10.1207/s15327752jpa6902_4
168. Mancini M, Stanghellini G. Values in persons with borderline personality disorder: their relevance for the therapeutic interview. Res Psychother. (2020) 23:449. doi: 10.4081/ripppo.2020.449
169. Lynam DR, Miller JD. The basic trait of antagonism: an unfortunately underappreciated construct. J Res Personality. (2019) 81:118–26. doi: 10.1016/j.jrp.2019.05.012
170. Miller JD, Lynam DR, Hyatt CS, Campbell WK. Controversies in narcissism. Ann Rev Clin Psychol. (2017) 13:291–315. doi: 10.1146/annurev-clinpsy-032816-045244
171. Miller JD, Campbell WK, Young DL, Lakey CE, Reidy DE, Zeichner A, et al. Examining the relations among narcissism, impulsivity, and self-defeating behaviors. J Pers. (2009) 77:761–94. doi: 10.1111/j.1467-6494.2009.00564.x
172. Trillini MO, Müller-Vahl KR. Patients with Gilles de la Tourette syndrome have widespread personality differences. Psychiatry Res. (2015) 228:765–73. doi: 10.1016/j.psychres.2015.04.043
173. Knowles R, McCarthy-Jones S, Rowse G. Grandiose delusions: a review and theoretical integration of cognitive and affective perspectives. Clin Psychol Rev. (2011) 31:684–96. doi: 10.1016/j.cpr.2011.02.009
174. Ardizzi M, Ambrosecchia M, Buratta L, Ferri F, Peciccia M, Donnari S, et al. Interoception and Positive Symptoms in Schizophrenia. Front Hum Neurosci. (2016) 10:379. doi: 10.3389/fnhum.2016.00379
175. Bulli F, Melli G, Cavalletti V, Stopani E, Carraresi C. Comorbid Personality Disorders in Obsessive-Compulsive Disorder and Its Symptom Dimensions. Psychiatr Q. (2016) 87:365–76. doi: 10.1007/s11126-015-9393-z
176. Strunz S, Dziobek I, Roepke S. Comorbid psychiatric disorders and differential diagnosis of patients with autism spectrum disorder without intellectual disability. Psychother Psychosom Med Psychol. (2014) 64:206–13. doi: 10.1055/s-0033-1358708
177. Schriber RA, Robins RW, Solomon M. Personality and self-insight in individuals with autism spectrum disorder. J Pers Soc Psychol. (2014) 106:112–30. doi: 10.1037/a0034950
178. Euler S, Stöbi D, Sowislo J, Ritzler F, Huber CG, Lang UE, et al. Grandiose and vulnerable narcissism in borderline personality disorder. Psychopathology. (2018) 51:110–21. doi: 10.1159/000486601
179. Huczewska I, Rogoza R. Vulnerable narcissism and borderline personality in relation to personal values. Personality Individ Diff. (2021) 153:109636. doi: 10.1016/j.paid.2019.109636
180. Busmann M, Meyer AH, Wrege J, Lang UE, Gaab J, Walter et al. Vulnerable narcissism as beneficial factor for the therapeutic alliance in borderline personality disorder. Clin Psychol Psychother. (2021) 28:1222–9. doi: 10.1002/cpp.2570
181. Ackerman RA, Donnellan MB, Wright AGC. Current conceptualizations of narcissism. Curr Opin Psychiatry. (2019) 32:32–7. doi: 10.1097/YCO.0000000000000463
182. Ganos C, Ogrzal T, Schnitzler A, Münchau A. The pathophysiology of echopraxia/echolalia: relevance to Gilles de la Tourette syndrome. Mov Disord. (2012) 27:1222–9. doi: 10.1002/mds.25103
183. Finis J, Moczydlowski A, Pollok B, Biermann-Ruben K, Thomalla G, Heil M, et al. Echoes from childhood—imitation in Gilles de la Tourette Syndrome. Mov Disord. (2012) 27:562–5. doi: 10.1002/mds.24913
184. Eddy CM, Cavanna AE, Rickards HE, Hansen PC. Temporo-parietal dysfunction in Tourette syndrome: Insights from an fMRI study of Theory of Mind. J Psychiatr Res. (2016) 81:102–11. doi: 10.1016/j.jpsychires.2016.07.002
185. Jonas M, Thomalla G, Biermann-Ruben K, Siebner HR, Müller-Vahl K, Bäumer T, et al. Imitation in patients with Gilles de la Tourette syndrome-a behavioral study. Mov Disord. (2010) 25:991–9. doi: 10.1002/mds.22994
186. Quadrelli E, Bartoli B, Bolognini N, Cavanna AE, Zibordi F, Nardocci N, et al. Automatic imitation in youngsters with Gilles de la Tourette syndrome: a behavioral study. Child Neuropsychol. (2021) 27:782–98. doi: 10.1080/09297049.2021.1892050
187. Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. (2010) 25:1068–76. doi: 10.1002/mds.23050
188. Müller N, Riedel M, Zawta P, Günther W, Straube A. Comorbidity of Tourette's syndrome and schizophrenia—biological and physiological parallels. Prog Neuropsychopharmacol Biol Psychiatry. (2002) 26:1245–52. doi: 10.1016/S0278-5846(02)00260-9
189. Simonsen A, Fusaroli R, Skewes JC, Roepstorff A, Campbell-Meiklejohn D, Mors O, et al. Enhanced automatic action imitation and intact imitation-inhibition in schizophrenia. Schizophr Bull. (2019) 45:87–95. doi: 10.1093/schbul/sby006
190. Matthews N, Gold BJ, Sekuler R, Park S. Gesture imitation in schizophrenia. Schizophr Bull. (2013) 39:94–101. doi: 10.1093/schbul/sbr062
191. Rounis E, Banca P, Voon V. Deficits in limb praxis in patients with obsessive-compulsive disorder. J Neuropsychiatry ClinNeurosci. (2016) 28:232–5. doi: 10.1176/appi.neuropsych.15090233
192. Sajith SG, Liew SF, Tor PC. Response to electroconvulsive therapy in patients with autism spectrum disorder and intractable challenging behaviors associated with symptoms of catatonia. J ECT. (2017) 33:63–7. doi: 10.1097/YCT.0000000000000338
193. Williams JH, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. J Autism Dev Disord. (2004) 34:285–99. doi: 10.1023/B:JADD.0000029551.56735.3a
194. Spengler S, Bird G, Brass M. Hyperimitation of actions is related to reduced understanding of others' minds in autism spectrum conditions. Biol Psychiatry. (2010) 68:1148–55. doi: 10.1016/j.biopsych.2010.09.017
195. Hauschild S, Winter D, Thome J, Liebke L, Schmahl C, Bohus M, et al. Behavioural mimicry and loneliness in borderline personality disorder. Compr Psychiatry. (2018) 82:30–6. doi: 10.1016/j.comppsych.2018.01.005
196. Marcoux LA, Michon PE, Lemelin S, Voisin JA, Vachon-Presseau E, Jackson PL. Feeling but not caring: empathic alteration in narcissistic men with high psychopathic traits. Psychiatry Res. (2014) 224:341–8. doi: 10.1016/j.pscychresns.2014.10.002
197. Singer HS, Augustine F. Controversies surrounding the pathophysiology of tics. J Child Neurol. (2019) 34:851–62. doi: 10.1177/0883073819862121
198. Moretto G, Schwingenschuh P, Katschnig P, Bhatia KP, Haggard P. Delayed experience of volition in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry. (2011) 82:1324–7. doi: 10.1136/jnnp.2010.221143
199. Kim S, Jackson GM, Dyke K, Jackson SR. Impaired forward model updating in young adults with Tourette syndrome. Brain. (2019) 142:209–19. doi: 10.1093/brain/awy306
200. Zapparoli L, Seghezzi S, Devoto F, Mariano M, Banfi G, Porta M, et al. Altered sense of agency in Gilles de la Tourette syndrome: behavioural, clinical and functional magnetic resonance imaging findings. Brain Commun. (2020) 2:fcaa204. doi: 10.1093/braincomms/fcaa204
201. Frith C. The self in action: lessons from delusions of control. Conscious Cogn. (2005) 14:752–70. doi: 10.1016/j.concog.2005.04.002
202. Thakkar KN, Nichols HS, McIntosh LG, Park S. Disturbances in body ownership in schizophrenia: evidence from the rubber hand illusion and case study of a spontaneous out-of-body experience. PLoS ONE. (2011) 6:e27089. doi: 10.1371/journal.pone.0027089
203. Oren E, Eitam B, Dar R. Intentional binding and obsessive-compulsive tendencies: a dissociation between indirect and direct measures of the sense of agency. J Obsessive-Compulsive Relat Disord. (2019) 20:59–65. doi: 10.1016/j.jocrd.2017.11.002
204. Belayachi S, Van der Linden M. Feeling of doing in obsessive-compulsive checking. Conscious Cogn. (2010) 19:534–46. doi: 10.1016/j.concog.2010.02.001
205. Gentsch A, Schütz-Bosbach S, Endrass T, Kathmann N. Dysfunctional forward model mechanisms and aberrant sense of agency in obsessive-compulsive disorder. Biol Psychiatry. (2012) 71:652–9. doi: 10.1016/j.biopsych.2011.12.022
206. Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL, et al. An extended multisensory temporal binding window in autism spectrum disorders. Exp Brain Res. (2010) 203:381–9. doi: 10.1007/s00221-010-2240-4
207. Sperduti M, Pieron M, Leboyer M, Zalla T. Altered pre-reflective sense of agency in autism spectrum disorders as revealed by reduced intentional binding. J Autism Dev Disord. (2014) 44:343–52. doi: 10.1007/s10803-013-1891-y
208. Zalla T, Sperduti M. The sense of agency in autism spectrum disorders: a dissociation between prospective and retrospective mechanisms? Front. Psychol. (2015) 6:1278. doi: 10.3389/fpsyg.2015.01278
209. Bekrater-Bodmann R, Chung BY, Foell J, Gescher DM, Bohus M, Flor H. Body plasticity in borderline personality disorder: a link to dissociation. Compr Psychiatry. (2016) 69:36–44. doi: 10.1016/j.comppsych.2016.05.002
210. Möller TJ, Braun N, Thöne AK, Herrmann CS, Philipsen A. The senses of agency and ownership in patients with borderline personality disorder. Front Psychiatry. (2020) 11:474. doi: 10.3389/fpsyt.2020.00474
211. Neustadter ES, Fineberg SK, Leavitt J, Carr MM, Corlett PR. Induced illusory body ownership in borderline personality disorder. Neurosci Conscious. (2019) 2019:niz017. doi: 10.1101/628131
212. Hascalovitz AC, Obhi SS. Personality and intentional binding: an exploratory study using the narcissistic personality inventory. Front Hum Neurosci. (2015) 9:13. doi: 10.3389/fnhum.2015.00013
213. Vazire S, Funder DC. Impulsivity and the self-defeating behavior of narcissists. Pers Soc Psychol Rev. (2006) 10:154–65. doi: 10.1207/s15327957pspr1002_4
214. Palumbo D, Kurlan R. Complex obsessive compulsive and impulsive symptoms in Tourette's syndrome. Neuropsychiatr Dis Treat. (2007) 3:687–93.
215. Mathews CA, Waller J, Glidden D, Lowe TL, Herrera LD, Budman CL, et al. Self injurious behaviour in Tourette syndrome: correlates with impulsivity and impulse control. J Neurol Neurosurg Psychiatry. (2004) 75:1149–55. doi: 10.1136/jnnp.2003.020693
216. Botteron HE, Richards CA, Nishino T, Ueda K, Acevedo HK, Koller JM, et al. The urge to blink in Tourette syndrome. Cortex. (2019) 120:556–66. doi: 10.1016/j.cortex.2019.07.010
217. Telgote SA, Pendharkar SS, Kelkar AD, Bhojane S. Very Early-onset Schizophrenia with Secondary Onset Tic Disorder. Indian J Psychol Med. (2017) 39:519–22. doi: 10.4103/0253-7176.211739
218. Russo M, Naro A, Mastroeni C, Morgante F, Terranova C, Muscatello MR, et al. Obsessive-compulsive disorder: a “sensory-motor” problem? Int. J Psychophysiol. (2014) 92:74–8. doi: 10.1016/j.ijpsycho.2014.02.007
219. Dayan-Riva A, Berger A, Anholt GE. Affordances, response conflict, and enhanced-action tendencies in obsessive-compulsive disorder: an ERP study. Psychol Med. (2021) 51:948–63. doi: 10.1017/S0033291719003866
220. Gadow KD, DeVincent CJ. Clinical significance of tics and attention-deficit hyperactivity disorder (ADHD) in children with pervasive developmental disorder. J Child Neurol. (2005) 20:481–8. doi: 10.1177/08830738050200060301
221. Uljarević M, Frazier TW, Jo B, Billingham WD, Cooper MN, Youngstrom EA, et al. Big data approach to characterize restricted and repetitive behaviors in autism. J Am Acad Child Adolesc Psychiatry. (2021) 61:p446–457. doi: 10.1016/j.jaac.2021.08.006
222. Maddox BB, Trubanova A, White SW. Untended wounds: non-suicidal self-injury in adults with autism spectrum disorder. Autism. (2017) 21:412–22. doi: 10.1177/1362361316644731
223. Kaplan B, Yazici Gulec M, Gica S, Gulec H. The association between neurocognitive functioning and clinical features of borderline personality disorder. Braz J Psychiatry. (2020) 42:503–9. doi: 10.1590/1516-4446-2019-0752
224. Terzi L, Martino F, Berardi D, Bortolotti B, Sasdelli A, Menchetti M. Aggressive behavior and self-harm in Borderline Personality Disorder: The role of impulsivity and emotion dysregulation in a sample of outpatients. Psychiatry Res. (2017) 249:321–6. doi: 10.1016/j.psychres.2017.01.011
225. McKay D, Kulchycky S, Danyko S. Borderline personality and obsessive-compulsive symptoms. J Pers Disord. (2000) 14:57–63. doi: 10.1521/pedi.2000.14.1.57
226. Zeigler-Hill V, Besser A, Gabay M, Young G. Narcissism and exercise addiction: the mediating roles of exercise-related motives. Int J Environ Res Public Health. (2021) 18:4243. doi: 10.3390/ijerph18084243
227. Efrati Y, Gerber Z, Tolmacz R. The relation of intra-psychic and relational aspects of the self to compulsive sexual behavior. J Sex Marital Ther. (2019) 45:618–31. doi: 10.1080/0092623X.2019.1599092
228. Wright A, Rickards H, Cavanna AE. Impulse-control disorders in gilles de la tourette syndrome. J Neuropsychiatry Clin Neurosci. (2012) 24:16–27. doi: 10.1176/appi.neuropsych.10010013
229. Wylie SA, Claassen DO, Kanoff KE, Ridderinkhof KR, van den Wildenberg WP. Impaired inhibition of prepotent motor actions in patients with Tourette syndrome. J Psychiatry Neurosci. (2013) 38:349–56. doi: 10.1503/jpn.120138
230. Atkinson-Clement C, Porte CA, de Liege A, Wattiez N, Klein Y, Beranger B, et al. Neural correlates and role of medication in reactive motor impulsivity in Tourette disorder. Cortex. (2020) 125:60–72. doi: 10.1016/j.cortex.2019.12.007
231. Bucci S, Birchwood M, Twist L, Tarrier N, Emsley R, Haddock G. Predicting compliance with command hallucinations: anger, impulsivity and appraisals of voices' power and intent. Schizophr Res. (2013) 147:163–8. doi: 10.1016/j.schres.2013.02.037
232. Martin S, Graziani P, Del-Monte J. Comparing impulsivity in borderline personality, schizophrenia and obsessional-compulsive disorders: Who is ahead? J. Clin Psychol. (2021) 77:1732–44. doi: 10.1002/jclp.23129
233. Denovan A, Dagnall N, Monk L. Schizotypy and risk-taking behaviour: the contribution of urgency. J Psychopathol Behav Assess. (2020) 42:1–12. doi: 10.1007/s10862-019-09769-4
234. Tumkaya S, Yucens B, Mart M, Tezcan D, Kashyap H. Multifaceted impulsivity in obsessive-compulsive disorder with hoarding symptoms. Nord J Psychiatry. (2021) 75:207–13. doi: 10.1080/08039488.2020.1838605
235. Chamberlain SR, Leppink EW, Redden SA, Grant JE. Are obsessive-compulsive symptoms impulsive, compulsive or both? Compr. Psychiatry. (2016) 68:111–8. doi: 10.1016/j.comppsych.2016.04.010
236. Richman DM, Barnard-Brak L, Bosch A, Thompson S, Grubb L, Abby L. Predictors of self-injurious behaviour exhibited by individuals with autism spectrum disorder. J Intellect Disabil Res. (2013) 57:429–39. doi: 10.1111/j.1365-2788.2012.01628.x
237. Laverty C, Oliver C, Moss J, Nelson L, Richards C. Persistence and predictors of self-injurious behaviour in autism: a ten-year prospective cohort study. Mol Autism. (2020) 11:8. doi: 10.1186/s13229-019-0307-z
238. Zanarini MC, Horwood J, Wolke D, Waylen A, Fitzmaurice G, Grant BF. Prevalence of DSM-IV borderline personality disorder in two community samples: 6,330 English 11-year-olds and 34,653 American adults. J Pers Disord. (2011) 25:607–19. doi: 10.1521/pedi.2011.25.5.607
239. Ditrich I, Philipsen A, Matthies S. Borderline personality disorder (BPD) and attention deficit hyperactivity disorder (ADHD) revisited—a review-update on common grounds and subtle distinctions. Borderline Personal Disord Emot Dysregul. (2021) 8:22. doi: 10.1186/s40479-021-00162-w
240. Gagnon J, Daelman S. An empirical study of the psychodynamics of borderline impulsivity: a preliminary report. Psychoanalytic Psychol. (2011) 28:341–62. doi: 10.1037/a0024358
241. Turner D, Sebastian A, Tüscher O. Impulsivity and Cluster B Personality Disorders. Curr Psychiatry Rep. (2017) 19:15. doi: 10.1007/s11920-017-0768-8
242. Edwards ER, Rose NLJ, Gromatsky M, Feinberg A, Kimhy D, Doucette JT, et al. Alexithymia, affective lability, impulsivity, and childhood adversity in borderline personality disorder. J Pers Disord. (2021) 35:114–31. doi: 10.1521/pedi_2021_35_513
243. Marissen MA, Arnold N, Franken IH. Anhedonia in borderline personality disorder and its relation to symptoms of impulsivity. Psychopathology. (2012) 45:179–84. doi: 10.1159/000330893
244. Cai H, Shi Y, Fang X, Luo YL. Narcissism predicts impulsive buying: phenotypic and genetic evidence. Front Psychol. (2015) 6:881. doi: 10.3389/fpsyg.2015.00881
245. Malesza M, Kaczmarek MC. Grandiose narcissism versus vulnerable narcissism and impulsivity. Personality Individ Diff. (2018) 126:61–5. doi: 10.1016/j.paid.2018.01.021
246. O'Reilly CA, Hall N. Grandiose narcissists and decision making: Impulsive, overconfident, and skeptical of experts-but seldom in doubt. Pers Individ Dif. (2021) 168:110280. doi: 10.1016/j.paid.2020.110280
247. Worbe Y, Marrakchi-Kacem L, Lecomte S, Valabregue R, Poupon F, Guevara P, et al. Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain. (2015) 138:472–82. doi: 10.1093/brain/awu311
248. Wan X, Zhang S, Wang W, Su X, Li J, Yang X, et al. Gray matter abnormalities in Tourette Syndrome: a meta-analysis of voxel-based morphometry studies. Transl Psychiatry. (2021) 11:287. doi: 10.1038/s41398-021-01394-8
249. McCormick LM, Brumm MC, Beadle JN, Paradiso S, Yamada T, Andreasen N. Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res. (2012) 201:233–9. doi: 10.1016/j.pscychresns.2012.01.004
250. Mitra S, Nizamie SH, Goyal N, Tikka SK. Mu-wave activity in schizophrenia: evidence of a dysfunctional mirror neuron system from an Indian study. Indian J Psychol Med. (2014) 36:276–81. doi: 10.4103/0253-7176.135380
251. Mehta UM, Thirthalli J, Aneelraj D, Jadhav P, Gangadhar BN, Keshavan MS. Mirror neuron dysfunction in schizophrenia and its functional implications: a systematic review. Schizophr Res. (2014) 160:9–19. doi: 10.1016/j.schres.2014.10.040
252. Moran LV, Tagamets MA, Sampath H, O'Donnell A, Stein EA, Kochunov P, et al. Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol Psychiatry. (2013) 74:467–74. doi: 10.1016/j.biopsych.2013.02.029
253. Choe E, Lee TY, Kim M, Hur JW, Yoon YB, Cho KK, et al. Aberrant within- and between-network connectivity of the mirror neuron system network and the mentalizing network in first episode psychosis. Schizophr Res. (2018) 199:243–9. doi: 10.1016/j.schres.2018.03.024
254. Jung WH, Gu BM, Kang DH, Park JY, Yoo SY, Choi CH, et al. BOLD response during visual perception of biological motion in obsessive-compulsive disorder: an fMRI study using the dynamic point-light animation paradigm. Eur Arch Psychiatry Clin Neurosci. (2009) 259:46–54. doi: 10.1007/s00406-008-0833-8
255. Boedhoe PSW, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the ENIGMA Obsessive-Compulsive Disorder Working Group. Am J Psychiatry. (2018) 175:453–62. doi: 10.1176/appi.ajp.2017.17050485
256. Cheng B, Cai W, Wang X, Lei D, Guo Y, Yang X, et al. Brain gray matter abnormalities in first-episode, treatment-naive children with obsessive-compulsive disorder. Front Behav Neurosci. (2016) 10:141. doi: 10.3389/fnbeh.2016.00141
257. Weeland CJ, White T, Vriend C, Muetzel RL, Starreveld J, Hillegers MHJ, et al. Brain morphology associated with obsessive-compulsive symptoms in 2,551 children from the general population. J Am Acad Child Adolesc Psychiatry. (2021) 60:470–8. doi: 10.1016/j.jaac.2020.03.012
258. Schulte-Rüther M, Greimel E, Piefke M, Kamp-Becker I, Remschmidt H, Fink GR, et al. Age-dependent changes in the neural substrates of empathy in autism spectrum disorder. Soc Cogn Affect Neurosci. (2014) 9:1118–26. doi: 10.1093/scan/nst088
259. Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. (2006) 9:28–30. doi: 10.1038/nn1611
260. Enticott PG, Kennedy HA, Rinehart NJ, Tonge BJ, Bradshaw JL, Taffe JR, et al. Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biol Psychiatry. (2012) 71:427–33. doi: 10.1016/j.biopsych.2011.09.001
261. Juengling FD, Schmahl C, Hesslinger B, Ebert D, Bremner JD, Gostomzyk J, et al. Positron emission tomography in female patients with borderline personality disorder. J Psychiatr Res. (2003) 37:109–15. doi: 10.1016/S0022-3956(02)00084-5
262. Salavert J, Gasol M, Vieta E, Cervantes A, Trampal C, Gispert JD. Fronto-limbic dysfunction in borderline personality disorder: a 18F-FDG positron emission tomography study. J Affect Disord. (2011) 131:260–7. doi: 10.1016/j.jad.2011.01.001
263. Wolf RC, Sambataro F, Vasic N, Schmid M, Thomann PA, Bienentreu SD, et al. Aberrant connectivity of resting-state networks in borderline personality disorder. J Psychiatry Neurosci. (2011) 36:402–11. doi: 10.1503/jpn.100150
264. Kluetsch RC, Schmahl C, Niedtfeld I, Densmore M, Calhoun VD, Daniels J, et al. Alterations in default mode network connectivity during pain processing in borderline personality disorder. Arch Gen Psychiatry. (2012) 69:993–1002. doi: 10.1001/archgenpsychiatry.2012.476
265. Sosic-Vasic Z, Eberhardt J, Bosch JE, Dommes L, Labek K, Buchheim A, et al. Mirror neuron activations in encoding of psychic pain in borderline personality disorder. Neuroimage Clin. (2019) 22:101737. doi: 10.1016/j.nicl.2019.101737
266. Mao Y, Sang N, Wang Y, Hou X, Huang H, Wei D, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. (2016) 328:50–7. doi: 10.1016/j.neuroscience.2016.04.025
267. Yang C, Yao L, Liu N, Zhang W, Tao B, Cao H, et al. Microstructural Abnormalities of White Matter Across Tourette Syndrome: a Voxel-Based Meta-Analysis of Fractional Anisotropy. Front Neurol. (2021) 12:659250. doi: 10.3389/fneur.2021.659250
268. Zapparoli L, Porta M, Gandola M, Invernizzi P, Colajanni V, Servello D, et al. A functional magnetic resonance imaging investigation of motor control in Gilles de la Tourette syndrome during imagined and executed movements. Eur J Neurosci. (2016) 43:494–508. doi: 10.1111/ejn.13130
269. Brüne M, Ozgürdal S, Ansorge N, von Reventlow HG, Peters S, Nicolas V, et al. An fMRI study of “theory of mind” in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. Neuroimage. (2011) 55:329–37. doi: 10.1016/j.neuroimage.2010.12.018
270. Walter H, Ciaramidaro A, Adenzato M, Vasic N, Ardito RB, Erk S, et al. Dysfunction of the social brain in schizophrenia is modulated by intention type: an fMRI study. Soc Cogn Affect Neurosci. (2009) 4:166–76. doi: 10.1093/scan/nsn047
271. Fuentes-Claramonte P, Martin-Subero M, Salgado-Pineda P, Santo-Angles A, Argila-Plaza I, Salavert J, et al. Brain imaging correlates of self- and other-reflection in schizophrenia. Neuroimage Clin. (2020) 25:102134. doi: 10.1016/j.nicl.2019.102134
272. Patel GH, Arkin SC, Ruiz-Betancourt DR, Plaza FI, Mirza SA, Vieira DJ, et al. Failure to engage the temporoparietal junction/posterior superior temporal sulcus predicts impaired naturalistic social cognition in schizophrenia. Brain. (2021) 144:1898–910. doi: 10.1093/brain/awab081
273. Saito Y, Kubicki M, Koerte I, Otsuka T, Rathi Y, Pasternak O, et al. Impaired white matter connectivity between regions containing mirror neurons, and relationship to negative symptoms and social cognition, in patients with first-episode schizophrenia. Brain Imaging Behav. (2018) 12:229–37. doi: 10.1007/s11682-017-9685-z
274. Sun F, Zhao Z, Lan M, Xu Y, Huang M, Xu D. Abnormal dynamic functional network connectivity of the mirror neuron system network and the mentalizing network in patients with adolescent-onset, first-episode, drug-naïve schizophrenia. Neurosci Res. (2021) 162:63–70. doi: 10.1016/j.neures.2020.01.003
275. Zhang T, Wang J, Yang Y, Wu Q, Li B, Chen L, et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci. (2011) 36:23–31. doi: 10.1503/jpn.100006
276. Koh MJ, Seol J, Kang JI, Kim BS, Namkoong K, Chang JW, et al. Altered resting-state functional connectivity in patients with obsessive-compulsive disorder: a magnetoencephalography study. Int J Psychophysiol. (2018) 123:80–7. doi: 10.1016/j.ijpsycho.2017.10.012
277. Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and 'mirror neuron' functioning in autistic spectrum disorder. Neuropsychologia. (2006) 44:610–21. doi: 10.1016/j.neuropsychologia.2005.06.010
278. Pantelis PC, Byrge L, Tyszka JM, Adolphs R, Kennedy DP. A specific hypoactivation of right temporo-parietal junction/posterior superior temporal sulcus in response to socially awkward situations in autism. Soc Cogn Affect Neurosci. (2015) 10:1348–56. doi: 10.1093/scan/nsv021
279. Ilzarbe D, Lukito S, Moessnang C, O'Daly OG, Lythgoe DJ, Murphy CM, et al. Neural correlates of theory of mind in autism spectrum disorder, attention-deficit/hyperactivity disorder, and the comorbid condition. Front Psychiatry. (2020) 11:544482. doi: 10.3389/fpsyt.2020.544482
280. Nijhof AD, Bardi L, Brass M, Wiersema JR. Brain activity for spontaneous and explicit mentalizing in adults with autism spectrum disorder: an fMRI study. Neuroimage Clin. (2018) 18:475–84. doi: 10.1016/j.nicl.2018.02.016
281. Eack SM, Wojtalik JA, Keshavan MS, Minshew NJ. Social-cognitive brain function and connectivity during visual perspective-taking in autism and schizophrenia. Schizophr Res. (2017) 183:102–9. doi: 10.1016/j.schres.2017.03.009
282. Lombardo MV, Chakrabarti B, Bullmore ET, MRC AIMS Consortium, Baron-Cohen S. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage. (2011) 56:1832–8. doi: 10.1016/j.neuroimage.2011.02.067
283. Quiñones-Camacho LE, Fishburn FA, Belardi K, Williams DL, Huppert TJ, Perlman SB. Dysfunction in interpersonal neural synchronization as a mechanism for social impairment in autism spectrum disorder. Autism Res. (2021) 14:1585–96. doi: 10.1002/aur.2513
284. Haas BW, Miller JD. Borderline personality traits and brain activity during emotional perspective taking. Personal Disord. (2015) 6:315–20. doi: 10.1037/per0000130
285. Beeney JE, Hallquist MN, Ellison WD, Levy KN. Self-other disturbance in borderline personality disorder: neural, self-report, and performance-based evidence. Personal Disord. (2016) 7:28–39. doi: 10.1037/per0000127
286. Sifneos PE. Problems of psychotherapy of patients with alexithymic characteristics and physical disease. Psychother Psychosom. (1975) 26:65–70. doi: 10.1159/000286912
287. Eddy CM, Hansen PC. Alexithymia is a key mediator of the relationship between magical thinking and empathy. Front Psychiatry. (2021) 12:719961. doi: 10.3389/fpsyt.2021.719961
288. Eddy CM, Cavanna AE. On being your own worst enemy: an investigation of socially inappropriate symptoms in Tourette syndrome. J Psychiatr Res. (2013) 47:1259–63. doi: 10.1016/j.jpsychires.2013.05.019
289. Silani G, Lamm C, Ruff CC, Singer T. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J Neurosci. (2013) 33:15466–76. doi: 10.1523/JNEUROSCI.1488-13.2013
290. Rymarczyk K, Zurawski Ł, Jankowiak-Siuda K, Szatkowska I. Neural correlates of facial mimicry: simultaneous measurements of EMG and BOLD responses during perception of dynamic compared to static facial expressions. Front Psychol. (2018) 9:52. doi: 10.3389/fpsyg.2018.00052
291. Rymarczyk K, Zurawski Ł, Jankowiak-Siuda K, Szatkowska I. Empathy in facial mimicry of fear and disgust: simultaneous EMG-fMRI recordings during observation of static and dynamic facial expressions. Front Psychol. (2019) 10:701. doi: 10.3389/fpsyg.2019.00701
292. Blakemore SJ, Wolpert DM, Frith CD. Abnormalities in the awareness of action. Trends Cogn Sci. (2002) 6:237–42. doi: 10.1016/S1364-6613(02)01907-1
293. Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci. (1998) 1:635–40. doi: 10.1038/2870
294. Sterzer P, Adams RA, Fletcher P, Frith C, Lawrie SM, Muckli L, et al. The predictive coding account of psychosis. Biol Psychiatry. (2018) 84:634–43. doi: 10.1016/j.biopsych.2018.05.015
295. Sterzer P, Mishara AL, Voss M, Heinz A. Thought insertion as a self-disturbance: an integration of predictive coding and phenomenological approaches. Front Hum Neurosci. (2016) 10:1–12. doi: 10.3389/fnhum.2016.00502
296. Uhlmann L, Pazen M, van Kemenade BM, Kircher T, Straube B. Neural correlates of self-other distinction in patients with schizophrenia spectrum disorders: the roles of agency and hand identity. Schizophr Bull. (2021) 47:1399–408. doi: 10.1093/schbul/sbaa186
297. Ebisch SJH, Aleman A. The fragmented self: imbalance between intrinsic and extrinsic self-networks in psychotic disorders. Lancet Psychiatry. (2016) 3:784–90. doi: 10.1016/S2215-0366(16)00045-6
298. Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacol. (2012) 37:1216–23. doi: 10.1038/npp.2011.308
299. Schleyken S, Baldermann J, Huys D, Franklin J, Visser-Vandewalle V, Kuhn J, et al. Deep brain stimulation and sensorimotor gating in Tourette syndrome and obsessive-compulsive disorder. J Psychiatr Res. (2020) 129:272–80. doi: 10.1016/j.jpsychires.2020.07.016
300. Horne O, Csipke E. From feeling too little and too much, to feeling more and less? A nonparadoxical theory of the functions of self-harm. Qual Health Res. (2009) 19:655–67. doi: 10.1177/1049732309334249
301. Kelly JR, Iannone NE, McCarty MK. Emotional contagion of anger is automatic: an evolutionary explanation. Br J Soc Psychol. (2016) 55:182–91. doi: 10.1111/bjso.12134
302. Prochazkova E, Kret ME. Connecting minds and sharing emotions through mimicry: a neurocognitive model of emotional contagion. Neurosci Biobehav Rev. (2017) 80:99–114. doi: 10.1016/j.neubiorev.2017.05.013
303. Kulke L, Johannsen J, Rakoczy H. Why can some implicit theory of mind tasks be replicated and others cannot? a test of mentalizing versus submentalizing accounts. PLoS ONE. (2019) 14:e0213772. doi: 10.1371/journal.pone.0213772
304. Salvatore G, Lysaker PH, Gumley A, Popolo R, Mari J, Dimaggio G. Out of illness experience: metacognition-oriented therapy for promoting self-awareness in individuals with psychosis. Am J Psychother. (2012) 66:85–106. doi: 10.1176/appi.psychotherapy.2012.66.1.85
305. Salvatore G, Procacci M, Popolo R, Nicolò G, Carcione A, Semerari A, et al. Adapted metacognitive interpersonal therapy for improving adherence to intersubjective contexts in a person with schizophrenia. Clin Case Stud. (2009) 8:473–88. doi: 10.1177/1534650109354916
306. Dimaggio G, Carcione A, Salvatore G, Nicolò G, Sisto A, Semerari A. Progressively promoting metacognition in a case of obsessive-compulsive personality disorder treated with metacognitive interpersonal therapy. Psychol Psychother. (2011) 84:70–83. doi: 10.1348/147608310X527240
307. Popolo R, MacBeth A, Lazzerini L, Brunello S, Venturelli G, Rebecchi D, Morales MF, Dimaggio G. Metacognitive interpersonal therapy in group versus TAU + waiting list for young adults with personality disorders: randomized clinical trial. Personal Disord. (2021). doi: 10.1037/per0000497
308. Cotelli M, Adenzato M, Cantoni V, Manenti R, Alberici A, et al. Enhancing theory of mind in behavioural variant frontotemporal dementia with transcranial direct current stimulation. Cogn Affect Behav Neurosci. (2018) 18:1065–75. doi: 10.3758/s13415-018-0622-4
309. Straube B, van Kemenade BM, Kircher T, Schülke R. Transcranial direct current stimulation improves action-outcome monitoring in schizophrenia spectrum disorder. Brain Commun. (2020) 2:fcaa151. doi: 10.1093/braincomms/fcaa151
310. Bitsch F, Berger P, Nagels A, Falkenberg I, Straube B. Impaired right temporoparietal junction—hippocampus connectivity in schizophrenia and its relevance for generating representations of other minds. Schizophr Bull. (2019) 45:934–45. doi: 10.1093/schbul/sby132
311. Uhlmann L, Pazen M, Van Kemenade BM, Steinsträter O, Harris LR, Kircher T, et al. Seeing your own or someone else's hand moving in accordance with your action : the neural interaction of agency and hand identity. Hum Brain Mapp. (2020) 41:2474–89. doi: 10.1002/hbm.24958
312. Fitzpatrick P, Frazier JA, Cochran DM, Mitchell T, Coleman C, Schmidt RC. Impairments of social motor synchrony evident in autism spectrum disorder. Front Psychol. (2016) 7:1323. doi: 10.3389/fpsyg.2016.01323
313. Khalil R, Tindle R, Boraud T, Moustafa AA, Karim AA. Social decision making in autism: On the impact of mirror neurons, motor control, and imitative behaviors. CNS Neurosci Ther. (2018) 24:669–76. doi: 10.1111/cns.13001
314. Kaur M, M Srinivasan S, N Bhat A. Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without Autism Spectrum Disorder (ASD). Res Dev Disabil. (2018) 72:79–95. doi: 10.1016/j.ridd.2017.10.025
315. Hamilton AF. Reflecting on the mirror neuron system in autism: a systematic review of current theories. Dev Cogn Neurosci. (2013) 91–105. doi: 10.1016/j.dcn.2012.09.008
316. Eddy CM, Cook JL. Emotions in action: the relationship between motor function and social cognition across multiple clinical populations. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 86:229–44. doi: 10.1016/j.pnpbp.2018.05.021
317. Marmolejo-Ramos F, Murata A, Sasaki K, Yamada Y, Ikeda A, Hinojosa JA, et al. Your face and moves seem happier when I smile. Exp Psychol. (2020) 67:14–22. doi: 10.1027/1618-3169/a000470
318. Craig SG, Goulter N, Moretti MM. A systematic review of primary and secondary callous-unemotional traits and psychopathy variants in youth. Clin Child Fam Psychol Rev. (2021) 24:65–91. doi: 10.1007/s10567-020-00329-x
Keywords: empathy, social cognition, self-other distinction, Tourette syndrome, obsessive-compulsive disorder, schizophrenia, autism, personality disorder
Citation: Eddy CM (2022) The Transdiagnostic Relevance of Self-Other Distinction to Psychiatry Spans Emotional, Cognitive and Motor Domains. Front. Psychiatry 13:797952. doi: 10.3389/fpsyt.2022.797952
Received: 19 October 2021; Accepted: 14 February 2022;
Published: 10 March 2022.
Edited by:
Frieder Michel Paulus, University of Lübeck, GermanyReviewed by:
Benjamin Straube, University of Marburg, GermanyKristína Czekóová, Masaryk University, Czechia
Copyright © 2022 Eddy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clare M. Eddy, Y2xhcmUuZWRkeTFAbmhzLm5ldA==; Yy5lZGR5QGJoYW0uYWMudWs=
 Clare M. Eddy
Clare M. Eddy