
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 31 May 2022
Sec. Molecular Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.788153
This article is part of the Research Topic Role of the Serotonergic System in Pathology of Major Depressive Disorders View all 9 articles
Background: Serotonin plays an important role in mood regulation and depression. However, it is not clear whether the levels of serotonin in saliva are related to current mood.
Aim: To test the association of salivary serotonin concentrations with mood, as well as cardiovascular and autonomic parameters.
Materials and Methods: Saliva samples were obtained from collegiate runners and output parameters were examined before and after physical activity.
Results: Salivary serotonin concentration was negatively associated with current mood (β = −0.32, 95%CI −0.62 to −0.02, p = 0.037, analysis adjusted for potential confounders), but insignificantly with measured cardiovascular and autonomic parameters.
Conclusions: Salivary serotonin may reflect current mood. The results are preliminary and require further evaluation.
Serotonin (5-hydroxytryptamine, 5-HT) is a hormone and neurotransmitter of multiple biological functions. The majority of 5-HT is synthesized in the periphery; this happens in enterochromaffin cells of the gut and 5-HT is stored in the blood platelets. By contrast, only a small portion of bodily 5-HT functions in the central nervous system (CNS) (1), which represents a distinct pool of 5-HT (2, 3). Despite that, 5-HT in the CNS contributes to many neuropsychological processes (1, 4). Specifically, the link between central serotonergic neurotransmission and depression or mood regulation can be recognized in the depressogenic effect of 5-HT depleting agents such as reserpine, the antidepressant action of serotonergic medications, and the transient drops in mood resulting from tryptophan depletion in susceptible individuals (5). However, the serotonergic hypothesis of depression has not been well substantiated (5–8). Particularly, conflicting results have been reported regarding the association between 5-HT levels in peripheral body fluids and depressive symptoms or mood. For example, in relation to healthy controls, both decreased (9, 10) and increased (11) plasma 5-HT levels have been reported in depressed patients.
5-HT also appears to be involved in autonomic and cardiovascular function (12, 13). Peripheral 5-HT maintains blood vessel tone by vasoconstriction and endothelial-mediated vasodilatation (14), and is responsible for positive chronotropic and inotropic effects on the heart (15). In the CNS, the raphe nuclei serotonergic projections to autonomic brain areas mediate receptor-dependent tachy- or bradycardia as well as vascular tone regulation (12, 13). Previous human studies suggested plasma and platelet (16) as well as salivary 5-HT (17) to be positively linked to heart rate, but little attention has been paid on temporal changes within these parameters.
Among peripheral body fluids, saliva offers an attractive and easily accessible source of biomarkers with a potential for diagnostic and prognostic application in neuroscience (18, 19). 5-HT has been detected in saliva, being likely derived from the plasma (19, 20). In line, some studies attributed salivary 5-HT to peripheral indices (17, 21, 22), but no clear plasma-saliva relationship has been demonstrated for 5-HT (23, 24). On the other hand, although no solid evidence for the CNS origin of salivary 5-HT exists, saliva appears to reflect, to some extent, the CNS serotonergic status (23, 25, 26). It is not clear, however, whether salivary 5-HT measurement is relevant to mood assessment. Also, limited evidence exists regarding the relation of salivary 5-HT to autonomic or cardiovascular function (17).
The primary aim of the present study was to evaluate whether salivary 5-HT concentration is associated with current mood. Additionally, the study sought to examine the relationships between salivary 5-HT and several cardiovascular (blood pressure and heart rate) and autonomic parameters (body temperature and pupil diameter).
This was a secondary analysis of a dataset obtained from an observational cohort study employing a convenience sample of collegiate distance runners (27). The Independent Review Board at Marist College (Poughkeepsie, NY, USA) approved the study. Prior to enrolment, all the volunteers expressed their consent in a written form to participate in the study.
Collegiate distance runners of both sexes, aged 18-23, were approached during their weekly physical training. Runners suffering from acute illness or experiencing orthopedic injury in the previous week were excluded.
Before the run, all the participants self-reported some basal characteristics: socio-demographic (age, sex, ethnicity), physical parameters (height, body mass), substance use history (alcohol, nicotine, cannabinoid and opioid), depressive and anxiety symptoms over 2 weeks [assessed with Patient Health Questionnaire-9, PHQ-9 Scale (28) and Generalized Anxiety Disorder-7, GAD-7 Scale (29), respectively; all the participants regardless of depression/anxiety status were enrolled to the study], and oropharyngeal factors relevant to saliva collection (time since last tooth brushing, time since last meal, dietary restrictions) via written survey.
Saliva collection (to assess 5-HT concentration) and measurement of output parameters (mood, systolic and diastolic blood pressure, heart rate, body temperature and pupil diameter) were all performed at the same time twice: 10-15 min before the run (about 8.30 a.m.) and 10-15 min after the run (about 10.00 a.m.). The timeline of the study was presented in Figure 1A.
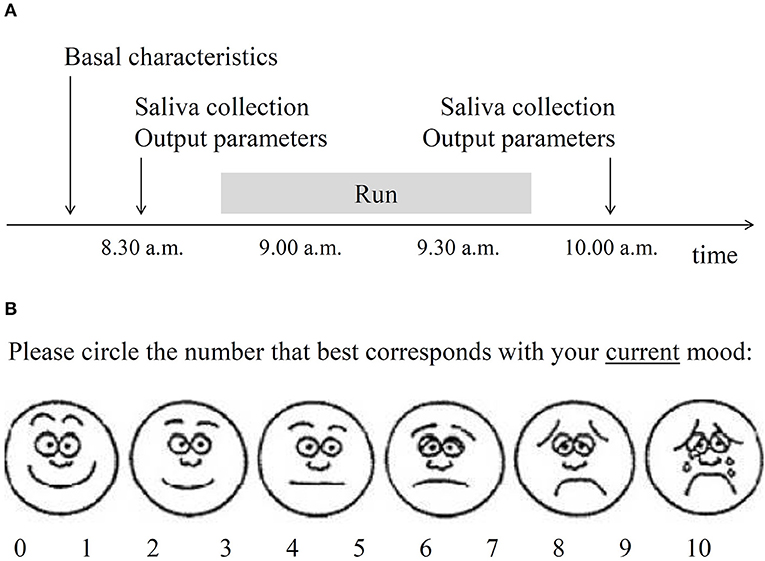
Figure 1. Selected methodological details of the study. (A) Timeline of the study. Basal characteristics included sociodemographic and physical parameters, substance use history, depressive, and anxiety symptoms over 2 weeks and oropharyngeal factors relevant to saliva collection. Saliva was collected to assess serotonin level. Output parameters included current mood (B), blood pressure, heart rate, body temperature, and pupil diameter. (B) Visual analog mood scale used in the present study. The obtained score was reverse-coded to operationalize the construct of mood.
Collection of whole saliva (at least 1 ml for a sample) was performed through active expectoration into 50 ml Falcon conical centrifuge tubes (Fisher Scientific; Waltham, MA, USA) following a water mouth rinse. The samples were kept on ice until they were transferred to be stored at −20°C until analysis.
Mood was assessed with the use of a pictogram-enhanced single-item 10-point visual analog mood scale (VAMS), illustrated by six facial expressions anchored between happy and sad. A participant was given a piece of paper with the VAMS and was asked to circle the number that best corresponded to her/his mood at that time (Figure 1B). Pictogram-enhanced visual analog scales are very brief tools that require minimum cognitive and linguistic involvement, produce negligible questionnaire burden, and enable respondents to express freely (30, 31); thus such tools appear suitable for sport research settings. Different formats of VAMS assessment have been successfully used in several other studies across age groups (32–34). Convergent validity of VAMS tools were confirmed against several standard mood assessing questionnaires and their test-retest reliability were satisfactory (30, 33, 35, 36).
Blood pressure was obtained in a seated position from the left arm using an adult-sized standard blood pressure cuff according to American Heart Association recommendations (37). Heart rate was measured by manually counting the radial pulse for 20 s. Pupil diameter was manually estimated by research staff using a standardized pupil diameter chart. Pupil diameter was measured indoors to ensure consistent ambient lighting conditions; participants were instructed to gaze directly at a blank wall 6 m away for 20 s before the examination to allow for pupillary accommodation. Body temperature was measured with a hospital-grade temporal artery thermometer (Exergen; Watertown, MA, USA) according to the manufacturer instructions. Excessive sweat was pat dry before post-run temperature assessment to avoid cooling resulted from evaporation.
The technique of enzyme-linked immunosorbent assay (ELISA) was applied to determine 5-HT concentration in salivary samples. A ready-made kit Serotonin ELISA Fast Track (Rocky Mountain Diagnostics; Colorado Springs, CO, USA) was used according to the manufacturer instructions. The samples were thawed, centrifuged and supernatants were subjected to ELISA procedure in triplicates. The experimentally set intra- and inter-assay coefficients of variation were 10.5 and 13.2%, respectively, suggesting an acceptable technical performance for this assay.
As both salivary 5-HT and all output characteristics were measured twice: before and after the run (Figure 1A), pre- and post-run data for each participant was split into separate cases. As a result, there were twice as many cases as there were participants. This was done to increase statistical power of the analyses and was justified by different biological conditions of pre- and post-run measurements. To overcome a problem of inclusion of replicated cases, the analyses were confirmed using a bootstrap technique with 10,000 dataset resamples (38). General linear modeling was used to examine the association between salivary serotonin concentration and output parameters. As multiple hypotheses were tested, the Benjamini and Hochberg (B-H) procedure was used to control the false discovery rate at the level of 0.25 due to the exploratory nature of the analyses. P-values below the B-H corrected significance level were considered statistically significant. The analyses were performed using STATISTICA software version 13.3 (StatSoft; Tulsa, OK, USA).
Twenty-five athletes participated in the study. Their basal characteristics are presented in Table 1. The mean (± standard deviation) pre-run level of salivary 5-HT was 1,286 ± 873 ng/ml. Pre-run salivary 5-HT concentration was insignificantly related to socio-demographic data and physical characteristics (age p = 0.36, sex p = 0.20, body mass index p = 0.48).
The mood of athletes was found to be inversely correlated with their salivary 5-HT concentration and the extent of association was not affected by adjusting for potential confounders. The effect size of the association was similar in pre- as well as post-run settings, and there was no significant difference in the extent of association between sexes. The bootstrap analysis performed with 10,000 dataset resamples yielded similarly significant results. Heart rate was also linked to salivary 5-HT in a negative way, but adjusted analysis returned insignificant result. Other output characteristics were found not to be significantly related to salivary 5-HT. See detailed results in Table 2; Figure 2.

Figure 2. Correlation between salivary serotonin concentration and the output parameters in adjusted analyses. Individual data points are presented with best fitted least-squares regression lines and their 95% confidence intervals. Regression coefficients, p-values for the associations and effect sizes expressed as partial eta-squared values are presented above each scatter plot. (A) Mood, (B) systolic blood pressure, (C) diastolic blood pressure, (D) heart rate, (E) body temperature, and (F) pupil diameter. SBP, systolic blood pressure; DPB, diastolic blood pressure; HR, heart rate; Temp, body temperature; PD, pupil diameter; pη2, partial eta-squared.
The role of 5-HT in the pathogenesis and therapy of depressive disorders has been originally appreciated (5–8). However, the literature reports inconsistent results regarding the link between 5-HT in the periphery, including the saliva, and depression or mood (21, 26). Here, we show that salivary 5-HT may be inversely associated with an individual's current mood.
CNS and periphery constitute two distinct 5-HT pools (2, 3) and saliva appears to reflect peripheral more than central 5-HT content (17, 20–22, 24). Nevertheless, Matsunaga et al. (25) found salivary 5-HT to be linked with happiness in socially-positive settings. As happiness appears to be constituted by higher CNS functioning (39), this may support the hypothesis of salivary 5-HT relevance to CNS processes. Importantly, the association between happiness and salivary 5-HT level discovered by Matsunaga et al. (25) was inverse, which is in line with the present findings; yet happiness, positive mood or just subjective well-being are all similar and overlapping constructs (40–42). Moreover, Tan et al. (26) revealed that diurnal rhythm of salivary 5-HT concentration differs between healthy subjects and patients suffering from depression. In their report, healthy individuals tended to display lower morning levels of salivary 5-HT than depressed individuals before and after pharmacological treatment (26). This finding is consistent with the current study, which also relied on morning saliva samples. Additionally, following acute treatment of depressed individuals with a selective serotonin re-uptake inhibitor, a slight increase in plasma 5-HT (from which salivary 5-HT likely arises) has been reported (43), whereas long-term treatment of more than 4 weeks (time relevant to clinical improvement) leads to a decrease in plasma 5-HT (9, 44). This decrease is more pronounced in patients whose depressive symptoms ameliorate with treatment (9). Collectively, the current findings align with literature reports relevant to peripheral 5-HT.
The reported negative association between salivary 5-HT level and current mood might be somewhat specific to the athletes. It appears that increased extracellular brain 5-HT contributes to development of fatigue during prolonged exercise (45). In laboratory animals, serotonergic 5-HT1A receptor stimulation diminished physical activity endurance, particularly in highly active animals (46). Therefore, the increase in salivary 5-HT may reflect accumulating fatigue during training, which may lead to lowered mood (47, 48). The midbrain 5-HT levels may adaptively increase following chronic endurance exercise (49). It will be interesting to see whether the negative relationship between salivary serotonin and mood, reported here, can be replicated in individuals with a sedentary lifestyle.
Although salivary 5-HT was found statistically significantly associated with mood, this measure is unlikely to serve as a stand-alone mood biomarker. To be such, a biomarker should be characterized by a substantial effect size in the link to the outcome as reflected by satisfactory discriminatory accuracy (19). Salivary 5-HT level in the present study could explain only 11% of variation in mood, leaving great majority potentially attributable to other factors. Biological processes are too much complex for a single molecular entity to accurately characterize an outcome. Instead, composite biomarkers may enable better predictions (50). Future studies should additionally investigate other salivary molecules potentially relevant to mental health (51, 52).
The reported negative association between salivary 5-HT and current mood was found to be stable irrespective of adjustment for confounding factors, particularly, post-exercise (27) and recent substance use (53), as these states may reduce salivary 5-HT and improve mood themselves. The effect size of the 5-HT-mood link in pre- and post-run states were similar to that observed in the combined dataset, which supports the veracity of the results. In comparison, heart rate, which was negatively linked to salivary serotonin in raw analysis, became insignificantly associated following adjustment for post-run status. Moreover, for heart rate, separate raw analyses in pre- and post-run states yielded much lower effect sizes than that of combined. On the other hand, despite insignificant raw analyses, diastolic blood pressure and pupil diameter presented some trends in adjusted analyses toward positive and negative association with salivary 5-HT, respectively. It is possible that cardiovascular and autonomic measures in the present study could be confounded by physical activity as the training and related increase in oxygen demand is well recognized to enhance these physiologic parameters (54). The existing literature suggests a positive link between peripheral 5-HT status and adrenergic functioning (16, 17, 55), and the use of serotonergic medications was associated with mydriasis (56), but no link was reported between peripheral 5-HT levels and blood pressure (16). Due to largely insignificant results in the present study, little coherence between raw and adjusted analyses and dissimilar literature reports, our results of cardiovascular and autonomic function may be regarded inconclusive.
The present study holds important limitations. First, it is a secondary analysis of existing data, which diminishes certainty of the obtained results (57). Second, the methods used are of limited validity; cardiovascular and autonomic functions were partially examined with subjective measures, however, the participants were accustomed to frequent manual heart rate assessment, as part of their training, and were likely to provide more accurate heart rate estimates than novice athletes. Mood was assessed with single-item pictogram-based VAMS questionnaire. Although the validity of such VAMS tools was proven (30, 33, 35, 36), rapid measuring may not be capable of distinguishing whether the assessed construct is more relevant to mood or emotion (34). On the other hand, as the assessment was related to the current state, it could track temporal changes secondary to possibly rapid fluctuation in salivary 5-HT (27, 58). Monitoring salivary 5-HT and mood “at the same moment” is a clear advantage of this study. Lack of association between depressive and anxiety symptoms with salivary 5-HT reported elsewhere (17), and only minimal and insignificant herein (PHQ-9 and GAD-7 scores), could be attributed to wide time-frame of assessed mood or not taking measurements at the same time. Third, saliva collection in this study was not preceded with sufficient instructions aimed at eliminating possible confounding effects of diet (59), brushing the teeth and other behaviors, as some other studies have done (17, 24–26); however, some relevant data was gathered to adjust the analysis for these confounders. Finally, the major barrier to generalization of the obtained results is high heterogeneity of reported salivary 5-HT concentration in humans, ranging from fractions of a nanogram per milliliter (21), through several dozens (17, 24–26), up to hundreds or even thousands of nanograms per milliliter (27, 60). High values of salivary 5-HT concentration are observed in the present study. This problem calls for validation of salivary 5-HT measurement techniques. Nevertheless, although these preliminary findings require reassessment in more valid research settings, the results add to a growing body of literature regarding the relevance of salivary 5-HT biomarker to neuroscience.
Salivary 5-HT concentration appears negatively associated with current mood. The evidence of salivary 5-HT association with some cardiovascular and autonomic functions, such as blood pressure, heart rate, body temperature and pupil diameter measurement, is inconclusive. Although the result for salivary 5-HT and mood finds literature support, it is preliminary and requires further testing. Salivary 5-HT measures require standardization and validation before firm conclusions can be drawn.
The dataset for this study can be found in the Mendeley Data repository (http://dx.doi.org/10.17632/wkyrf5prjc.1).
The study was reviewed and approved by Independent Review Board at Marist College. All the participants provided their written informed consent to participate in this study.
MK: conceptualization, formal analysis, visualization, and writing—original draft. MK and SH: data curation, funding acquisition, methodology, supervision, validation, and writing—review and editing. SH: investigation, project administration, and resources. Both authors have read and agreed to the published version of the manuscript.
The research was supported by Quadrant Biosciences (Syracuse, NY, USA), Penn State College of Medicine (Hershey, PA, USA), Marist College (Poughkeepsie, NY, USA), and Medical University of Lodz (Łódz, Poland, grant number 503/5-108-03/503-51-001-19-00).
SH has served as a paid consultant and scientific advisory board member for Quadrant Biosciences.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the volunteer runners participating in the study. We also thank Zofia Gagnon, Paige Jacob, Omar Perez, Saad Baig, and Matthew Baffuto for sample collection and serotonin processing.
1. Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. (2008) 31:187–99. doi: 10.1111/j.1365-2885.2008.00944.x
2. Bektaş A, Erdal H, Ulusoy M, Uzbay IT. Does seratonin in the intestines make you happy? Turkish J Gastroenterol. (2020) 31:721–723. doi: 10.5152/tjg.2020.19554
3. El-Merahbi R, Löffler M, Mayer A, Sumara G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. (2015) 589:1728–34. doi: 10.1016/j.febslet.2015.05.054
4. Siotto M, Germanotta M, Santoro M, Cipollini V, Guardati G, Papadopoulou D, et al. Serotonin levels and cognitive recovery in patients with subacute stroke after rehabilitation treatment. Brain Sci. (2021) 11:642. doi: 10.3390/brainsci11050642
5. Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. (2015) 14:158–60. doi: 10.1002/wps.20229
6. Albert PR, Benkelfat C, Descarries L. The neurobiology of depression—Revisiting the serotonin hypothesis. I Cellular and molecular mechanisms. Philosoph Transact Royal Society B Biol Sci. (2012) 367:2378–81. doi: 10.1098/rstb.2012.0190
7. Albert PR, Benkelfat C. The neurobiology of depression–Revisiting the serotonin hypothesis. II Genetic, epigenetic and clinical studies. Philosoph Transact Royal Society B Biol Sci. (2013) 368:20120535. doi: 10.1098/rstb.2012.0535
8. Sharp T. Molecular and cellular mechanisms of antidepressant action. Curr Top Behav Neurosci. (2013) 14:309–25. doi: 10.1007/7854_2012_216
9. Holck A, Wolkowitz OM, Mellon SH, Reus VI, Nelson JC, Westrin Å, et al. Plasma serotonin levels are associated with antidepressant response to SSRIs. J Affect Disord. (2019) 250:65–70. doi: 10.1016/j.jad.2019.02.063
10. Sarrias MJ, Artigas F, Martínez E, Gelpí E, Alvarez E, Udina C, et al. Decreased plasma serotonin in melancholic patients: a study with clomipramine. Biol Psychiatry. (1987) 22:1429–38. doi: 10.1016/0006-3223(87)90100-4
11. Lechin F, van der Dijs B, Orozco B, Lechin ME, Báez S, Lechin AE, et al. Plasma neurotransmitters, blood pressure, and heart rate during supine-resting, orthostasis, and moderate exercise conditions in major depressed patients. Biol Psychiatry. (1995) 38:166–73. doi: 10.1016/0006-3223(94)00258-5
12. Côté F, Fligny C, Fromes Y, Mallet J, Vodjdani G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol Med. (2004) 10:232–8. doi: 10.1016/j.molmed.2004.03.007
13. Villalón CM. Chapter Three—The role of serotonin receptors in the control of cardiovascular function. In: Tricklebank MD, Daly E, editors. The Serotonin System. Boston, MA: Academic Press (2019). p. 45-61.
14. Cohen RA, Shepherd JT, Vanhoutte PM. 5-Hydroxytryptamine can mediate endothelium-dependent relaxation of coronary arteries. Am J Physiol. (1983) 245:H1077–80. doi: 10.1152/ajpheart.1983.245.6.H1077
15. Ouadid H, Seguin J, Dumuis A, Bockaert J, Nargeot J. Serotonin increases calcium current in human atrial myocytes via the newly described 5-hydroxytryptamine4 receptors. Mol Pharmacol. (1992) 41:346–51.
16. Missouris CG, Cappuccio FP, Varsamis E, Barron JL, Carr E, Markandu ND, et al. Serotonin and heart rate in hypertensive and normotensive subjects. Am Heart J. (1998) 135:838–43. doi: 10.1016/S0002-8703(98)70043-2
17. Karbownik MS, Kreczyńska J, Wiktorowska-Owczarek A, Kwarta P, Cybula M, Stilinović N, et al. Decrease in salivary serotonin in response to probiotic supplementation with saccharomyces boulardii in healthy volunteers under psychological stress: secondary analysis of a randomized, double-blind, placebo-controlled trial. Front Endocrinol. (2022) 12:800023. doi: 10.3389/fendo.2021.800023
18. Wormwood KL, Aslebagh R, Channaveerappa D, Dupree EJ, Borland MM, Ryan JP, et al. Salivary proteomics and biomarkers in neurology and psychiatry. Proteom Clin Appl. (2015) 9:899–906. doi: 10.1002/prca.201400153
19. Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DTW. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev. (2013) 26:781–91. doi: 10.1128/CMR.00021-13
20. Ferreira JN, Hoffman MP. Interactions between developing nerves and salivary glands. Organogenesis. (2013) 9:199–205. doi: 10.4161/org.25224
21. Leung J, Selvage C, Bosdet T, Branov J, Rosen-Heath A, Bishop C, et al. Salivary serotonin does not correlate with central serotonin turnover in adult phenylketonuria (PKU) patients. Mol Gen Metabolism Rep. (2018) 15:100–5. doi: 10.1016/j.ymgmr.2018.03.008
22. Scarsella E, Cintio M, Iacumin L, Ginaldi F, Stefanon B. Interplay between neuroendocrine biomarkers and gut microbiota in dogs supplemented with grape proanthocyanidins: results of dietary intervention study. Animals. (2020) 10:531. doi: 10.3390/ani10030531
23. Audhya T, Adams JB, Johansen L. Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochim Biophys Acta. (2012) 1820:1496–501. doi: 10.1016/j.bbagen.2012.05.012
24. Egri C, Dunbar M, Horvath GA. Correlation between salivary, platelet and central serotonin levels in children. Can J Neurol Sci. (2020) 47:214–8. doi: 10.1017/cjn.2019.334
25. Matsunaga M, Ishii K, Ohtsubo Y, Noguchi Y, Ochi M, Yamasue H. Association between salivary serotonin and the social sharing of happiness. PLoS ONE. (2017) 12:e0180391. doi: 10.1371/journal.pone.0180391
26. Tan Z-L, Bao A-M, Tao M, Liu Y-J, Zhou J-N. Circadian rhythm of salivary serotonin in patients with major depressive disorder. Neuro Endocrinol Lett. (2007) 28:395–400.
27. Hicks SD, Jacob P, Perez O, Baffuto M, Gagnon Z, Middleton FA. The transcriptional signature of a runner's high. Med Sci Sports Exerc. (2019) 51:970–8. doi: 10.1249/MSS.0000000000001865
28. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
29. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
30. Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. (1969) 62:989–93. doi: 10.1177/003591576906201005
31. Klimek L, Bergmann K-C, Biedermann T, Bousquet J, Hellings P, Jung K, et al. Visual analogue scales (VAS): measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care. Allerg J Int. (2017) 26:16–24. doi: 10.1007/s40629-016-0006-7
32. Hall L, Hume C, Tazzyman S. Five degrees of happiness: effective smiley face likert scales for evaluating with children. In: Proceedings of the 15th International Conference on Interaction Design and Children. New York, NY: ACM (2016). doi: 10.1145/2930674.2930719
33. Lorish CD, Maisiak R. The face scale: a brief, nonverbal method for assessing patient mood. Arthritis Rheum. (1986) 29:906–9. doi: 10.1002/art.1780290714
34. Pérez-Sáez E, Cabrero-Montes EM, Llorente-Cano M, González-Ingelmo E. A pilot study on the impact of a pottery workshop on the well-being of people with dementia. Dementia. (2020) 19:2056–72. doi: 10.1177/1471301218814634
35. Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. (1973) 3:479–86. doi: 10.1017/S0033291700054283
36. Luria RE. The validity and reliability of the visual analogue mood scale. J Psychiatr Res. (1975) 12:51–7. doi: 10.1016/0022-3956(75)90020-5
37. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals. Hypertension. (2005) 45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e
38. Ranstam J. Repeated measurements, bilateral observations and pseudoreplicates, why does it matter? Osteoarthr Cartilage. (2012) 20:473–5. doi: 10.1016/j.joca.2012.02.011
39. Kringelbach ML, Berridge KC. The neuroscience of happiness and pleasure. Soc Res. (2010) 77:659–78.
40. Gamble A, Gärling T. The relationships between life satisfaction, happiness, and current mood. J Happiness Stud. (2012) 13:31–45. doi: 10.1007/s10902-011-9248-8
41. Heizomi H, Allahverdipour H, Asghari Jafarabadi M, Safaian A. Happiness and its relation to psychological well-being of adolescents. Asian J Psychiatr. (2015) 16:55–60. doi: 10.1016/j.ajp.2015.05.037
42. Yardley JK, Rice RW. The relationship between mood and subjective well-being. Soc Indic Res. (1991) 24:101–11. doi: 10.1007/BF00292653
43. Kotzailias N, Marker M, Jilma B. Early effects of paroxetine on serotonin storage, plasma levels, and urinary excretion: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. (2004) 24:536–9. doi: 10.1097/01.jcp.0000138765.08235.46
44. Gupta M, Neavin D, Liu D, Biernacka J, Hall-Flavin D, Bobo WV, et al. TSPAN5, ERICH3 and selective serotonin reuptake inhibitors in major depressive disorder: Pharmacometabolomics-informed pharmacogenomics. Mol Psychiatry. (2016) 21:1717–25. doi: 10.1038/mp.2016.6
45. Meeusen R, Watson P, Hasegawa H, Roelands B, Piacentini MF. Central fatigue: the serotonin hypothesis and beyond. Sports Med. (2006) 36:881–909. doi: 10.2165/00007256-200636100-00006
46. Claghorn GC, Fonseca IAT, Thompson Z, Barber C, Garland T. Serotonin-mediated central fatigue underlies increased endurance capacity in mice from lines selectively bred for high voluntary wheel running. Physiol Behav. (2016) 161:145–54. doi: 10.1016/j.physbeh.2016.04.033
47. Lane AM, Terry PC, Stevens MJ, Barney S, Dinsdale SL. Mood responses to athletic performance in extreme environments. J Sports Sci. (2004) 22:886–97; discussion 897. doi: 10.1080/02640410400005875
48. Manning K, Garey L, Mayorga NA, Shepherd JM, Zvolensky MJ. The relation between fatigue severity and anxious arousal, negative affect, and emotion dysregulation among adult e-cigarette users. Fatigue Biomed Health Behav. (2019) 7:92–101. doi: 10.1080/21641846.2019.1626059
49. Brown BS, Payne T, Kim C, Moore G, Krebs P, Martin W. Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J Appl Physiol. (1979) 46:19–23. doi: 10.1152/jappl.1979.46.1.19
50. Califf RM. Biomarker definitions and their applications. Exp Biol Med. (2018) 243:213–21. doi: 10.1177/1535370217750088
51. Chojnowska S, Ptaszyńska-Sarosiek I, Kepka A, Knaś M, Waszkiewicz N. Salivary biomarkers of stress, anxiety and depression. J Clin Med. 10:517. doi: 10.3390/jcm10030517
52. Wiegand C, Heusser P, Klinger C, Cysarz D, Büssing A, Ostermann T, et al. Stress-associated changes in salivary microRNAs can be detected in response to the Trier Social Stress Test: an exploratory study. Sci Rep. (2018) 8:7112. doi: 10.1038/s41598-018-25554-x
53. Baltz JW, Le LT. Serotonin syndrome versus cannabis toxicity in the emergency department. Clin Pract Cases Emerg Med. (2020) 4:171–3. doi: 10.5811/cpcem.2020.1.45410
54. Fu Q, Levine BD. Exercise and the autonomic nervous system. Handb Clin Neurol. (2013) 117:147–60. doi: 10.1016/B978-0-444-53491-0.00013-4
55. Sugimoto Y, Yamada J, Yoshikawa T, Nishikawa F, Noma T, Horisaka K. The involvement of serotonin in the catecholamine release from the adrenal medulla. In: Filippini GA, Costa CVL, Bertazzo A, editors. Recent Advances in Tryptophan Research: Tryptophan and Serotonin Pathways. Springer US (1996). p. 561–3.
56. Gündüz GU, Parmak Yener N, Kilinçel O, Gündüz C. Effects of selective serotonin reuptake inhibitors on intraocular pressure and anterior segment parameters in open angle eyes. Cutan Ocul Toxicol. (2018) 37:36–40. doi: 10.1080/15569527.2017.1330270
57. Marler JR. Secondary analysis of clinical trials—a cautionary note. Prog Cardiovasc Dis. (2012) 54:335–7. doi: 10.1016/j.pcad.2011.09.006
58. Reilly JG, McTavish SFB, Young AH. Rapid depletion of plasma tryptophan: a review of studies and experimental methodology. J Psychopharmacol. (1997) 11:381–92. doi: 10.1177/026988119701100416
59. Blum I, Vered Y, Graff E, Grosskopf Y, Don R, Harsat A, et al. The influence of meal composition on plasma serotonin and norepinephrine concentrations. Metabolism. (1992) 41:137–40. doi: 10.1016/0026-0495(92)90141-V
Keywords: serotonin, salivary biomarkers, mood, depression, association
Citation: Karbownik MS and Hicks SD (2022) The Association of Salivary Serotonin With Mood and Cardio-Autonomic Function: A Preliminary Report. Front. Psychiatry 13:788153. doi: 10.3389/fpsyt.2022.788153
Received: 01 October 2021; Accepted: 02 May 2022;
Published: 31 May 2022.
Edited by:
Trevor Ronald Norman, The University of Melbourne, AustraliaReviewed by:
Philip Cowen, University of Oxford, United KingdomCopyright © 2022 Karbownik and Hicks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michał Seweryn Karbownik, bWljaGFsLmthcmJvd25pa0B1bWVkLmxvZHoucGw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.