- Directorate, South African Brain Research Institute, Johannesburg, South Africa
Medication dosages are crucial–no single dose fits all. My paper compares the safety, scientific and practical applicability of fixed 25–50% concentrations of nitrous oxide (N2O) with the variable titrated concentrations of Psychotropic Analgesic N2O (PAN), as used in dentistry, and neuropsychiatry. A crucial difference is that PAN is always titrated, via an open circuit (nasal mask), to the minimum concentration (dose), which ensures full consciousness, cooperation, comfort and relaxation. With PAN, the goal is subject comfort, not dose. In contrast, fixed goal concentrations are usually given via relatively closed circuits (full facial mask/similar) without account for individual patient's dose-response. Hence, fixed concentrations, in N2O sensitive subjects, could result in unconsciousness and other adverse effects (nausea, vomiting, anxiety, aspiration, might occur; requiring an anaesthesiologist for patient safety. PAN is titrated using each subject's subjective and objective responses as the guide to the ideal concentration. Thus, when PAN is used, there is no fixed concentration even for a single subject, nor is an anaesthesiologist required. Furthermore, there is a greater scientific rationale for using PAN, because the receptor systems involved are better known, whilst those for fixed concentrations are not. The PAN or dental titration method has been safely used in general dentistry for over 70 years and as an investigative, diagnostic and therapeutic tool for neuropsychiatry for over 40 years. Clinical applications include substance abuse detoxification, ameliorating depression, and investigations of schizophrenia, human orgasm, pain perception and basic neuroscience. By contrast, the experience with fixed doses in psychiatry is limited.
Introduction
There is a sudden reawakening of interest in using subanaesthetic concentrations of N2O in psychiatry (1–6). Thus, we need answers to these questions. Particularly, as research has already shown the greater safety (7, 8) and wider usefulness of correctly titrated subanaesthetic N2O for psychiatry (9–12).
The technique recently advocated (1, 2, 4, 6), a fixed 50% concentration of N2O (5). Unfortunately, this is less safe (7, 8, 13), unless used in hospital practice, with an attending anaesthesiologist. Importantly, the only past experience with 50% N2O in psychiatry is anecdotal and limited to a few patients only (14).
In a later double-blind study, Nagele et al., using 50% found that 25% N2O was safer and yet as effective and reduced the unwanted side effects considerably (15). However, like 50%, 25% is not tailored to each subject's needs. As a result, those sensitive to the actions of N2O, will be unnecessarily exposed to side-effects (7, 8) including the undesirable psychomimetic states, found with ketamine (6, 16). Inadvertently, by using different dosages, these authors have underlined the dose-dependent variability of the effects of N2O.
The other technique, PAN (psychotropic analgesic nitrous oxide) refers to low subanaesthetic concentrations of N2O as used in modern dentistry (7, 8, 17). Inhalation sedation or minimal sedation refer to the identical dental technique as PAN (7, 8, 11, 17, 18). PAN concentrations are titrated to the point of the subjects' maximum comfort and effectiveness (and are not fixed at any specific level) and therefore vary, depending on the each subject's sensitivity to the gas.
The main objection to using fixed concentrations, whether 25% or 50% is that the majority of individuals inhaling N2O will be subjected unnecessarily (7) to unwanted side-effects such as nausea, vomiting, sleepiness and headache (6–8, 15, 16). Indeed, in hypersensitive people sleepiness could be converted to anesthesia (7, 8) as well as other undesirable psychomimetic effects, including delusions, anhedonia, mania and paranoia as well as distortions in perception, mental ineffectiveness and anhedonia (6, 15, 16). Likewise, at both the fixed 25% or 50% levels, many patients will not have received the optimal dosage. In both the latter scenarios, giving too high or low a dose is undesirable. In addition, only a fraction of patients are likely to receive the correct concentration.
This paper will briefly discuss some of the research conducted with PAN and fixed concentrations and then compare their advantages and disadvantages (see Table 1).
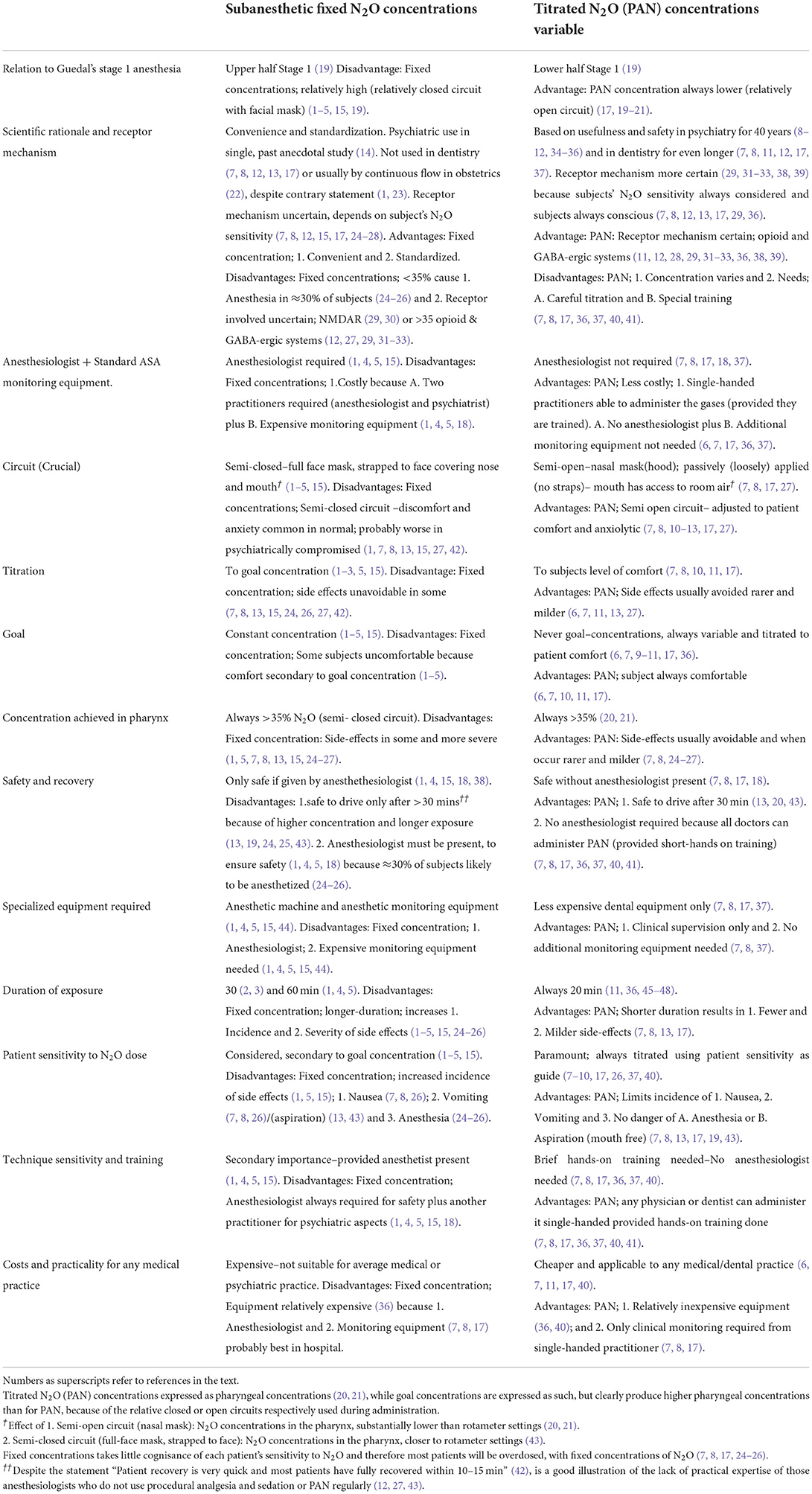
Table 1. Comparison of fixed and variable titrated (PAN) concentrations of subanaesthetic N2O for psychiatry.
Psychotropic analgesic nitrous oxide (titrated variable concentrations) in psychiatry
PAN has been used as an investigative, diagnostic and therapeutic tool in psychiatry (11) for:-
1. Pain perception. Here, research uncovered an endogenous algesic opioid system that counterbalanced the well-known analgesic system (11, 31–33). An algesic opioid system in dog pontine-medullary area was located by others (33).
2. Human sexual research. It gave tentative evidence of opioid system involvement in human sexual response (49), which was later confirmed more rigorously by others (50). Our work indicated that N2O might be useful for treating and researching female sexual dysfuction (49). Further, the action of these two opposing opioid systems uncovered a possible physiological link on the pain-pleasure continuum. The existence of such a continuum was first postulated by Aristotle (51) and later espoused by Descartes and Spinoza (52). The pain pleasure system also seemed involved in substance abuse (53–56), as well as the placebo response (34, 45, 57, 58). Moreover, the opioid system could be on a common pathway underlying all substances of abuse (59). Interestingly, later researchers have suggested the opioids, the placebo response, drug addiction and learning are all linked as part of reward and punishment continuum (60).
3. Depression research, both with (10) and without substance abuse (9). We have also showed that the gas could be used to treat depression during the latent period, before conventional antidepressants become effective (9, 61). Further, we have showed the role of the placebo response in the action of antidepressants (62). It is pleasing that Nagele et al. (1, 4, 15) have confirmed our observations that N2O is an antidepressant.
4. Investigating various psychiatric conditions. These include inpatient therapy for alcohol abuse (10, 63) as well as the likely possibility that the gas has potential for outpatient alcohol withdrawal treatment (64). Moreover, we have also demonstrated that N2O can ameliorate withdrawal from opioids (65), cocaine (46), cannabis (47, 63), nicotine (63), and methaqualone (47). We have also used N2O for other conditions such as anxiety (11) stress (48) psychosis (66), eating (67, 68) and movement disorders. The movement disorders studied, include neuroleptic-induced akathisia (69), Tourette Syndrome (70) spasmodic torticollis (71) and hyperactivity (72).
5. Finding a double-blind method of applying rapidly acting agents like N2O for future research (35, 73).
6. For discovering in 1983, that N2O (31, 32, 38, 39, 74, 75), was the first gas identified as a gaseous neurotransmitter, whereas nitric oxide (NO) was only shown to be a gaseous neurotransmitter in 1990, i.e., 7 years later (76).
Fixed concentrations of subanesthetic N2O in psychiatry
Recently, a group in the USA, showed single-blind (N = 20) (1) that N2O ameliorates treatment resistant depression and that in a single case (4) that it might have more lasting effects. In 2016, British investigators found evidence that 50% N2O might be useful in suppressing traumatic thoughts (2). The latter also observed that the subjective response to 50% N2O might be a marker for future alcoholism (3). Later, the Americans published a three-patient case study showing the gas might decrease intrusive traumatic thoughts in post-traumatic stress disorder (5). In 2021 the same USA group (15), demonstrated that 50% N2O in oxygen ameliorated depression. They listed 15 unwanted side-effects, among others; dizziness, uncontrolled laughter, feeling of disconnection, paranoia as well as nausea and vomiting. In an effort to reduce the side-effects they tried 25% and found less than half the side effects. When side-effects occurred, these were considerably reduced as compared to the higher concentration (15). For instance, in a single subject only nausea occurred without vomiting (15). However, as will be seen later, some sensitive subjects may also vomit at fixed concentrations of 22.5% or less (7).
For these reasons fixed 50% concentrations may be hazardous in general medical or psychiatric practice, unless practitioners are trained anaesthesiologists (1, 5, 6). Indeed, even a 25% concentration may also be undesirable among those untrained as specialist anesthesthesiologists. It is therefore significant that the dental titration technique has been safely used since the 1940's, in routine general dental practice (7–9, 11, 17) and since the 1980's in psychiatry (8–12, 34, 45, 57). Concentrations used in dentistry are almost always lower (7–11, 17) than those recommended in the most recent research (1–6, 15, 16).
Differentiating subanaesthetic N2O from PAN
What is PAN
The term PAN was initially introduced to avoid confusion when speaking to medical professionals, about its use in neuropsychiatry and dentistry (12). Most physicians, apart from obstetricians (22), seldom, if ever, use the low concentrations favored in dentistry. And for labor analgesia, 50% N2O is mainly administered briefly, intermittently, and on demand only (22). Dentists use low titrated concentrations of the gas (i.e., PAN), as an anxiolytic, while the patient is conscious and fully co-operative (7, 8, 12, 13). Any fixed concentration of N2O is discouraged in modern dentistry (7, 8, 12, 17). Confusion occurs, because other medical professionals including anaesthesiologists, usually know the gas as part of balanced anesthesia only. Further, few anaesthesiologists have regular experience of using N2O for minimal sedation in conscious patients, apart from those who regularly use procedural sedation and analgesia (44). And then, N2O is usually part of a cocktail of other agents.
Subtle but important differences between PAN and fixed concentrations
The differences are subtle. But are of paramount importance for ethical and practical reasons. Thus, readers must clearly distinguish subanaesthetic N2O and PAN, although both fall within Stage 1 Anesthesia. PAN lies within the first half of Stage 1, while a 50% concentration lies in the second half of Stage 1 (19). Indeed, in some N2O-sensitive individuals 25% may also lie within the second half of Stage 1 (19). The difference is more relevant now, since the latest work, features the administration of relatively high concentrations of subanaesthetic N2O (1–6, 13, 15, 16, 19), not consistent with PAN (7, 8, 17, 33).
Unlike PAN these relatively high concentrations are administered via a full face-mask i.e., a relatively closed system for 30 (2, 3) or 60 min (1, 4, 5, 15). [I]In addition, the goal concentration seems more important than patient comfort (1–6, 15). In contrast, and by definition, PAN implies titrating N2O to the lowest levels through a relatively open system (nasal mask) to each subjects clinical comfort, while fully conscious (7, 8, 10–12, 17, 47). There is definitely no goal concentration. Rather, the goal is maximum patient comfort and anxiolysis (7, 8, 12). For psychiatry, excellent results are obtained after 20 min only (9–11, 47). As a result, there are fewer side-effects, which when they occur, are milder (7–11, 13, 17).
N2O administration via open circuit avoids anesthesia and unpleasant side-effects
A crucial result of these difference is that while using a similar semi-closed circuit (1–5), ~30% of subjects become unconscious while breathing 35–45% N2O (24–26). Indeed, unconsciousness results even if the gas is applied for less than an hour (13, 24, 25). Further, in those not anesthetised while on a semi-closed facial mask system; and inhaling for considerably shorter periods than 60 min (2, 3), individuals suffer unpleasant side-effects like nausea, vomiting etc. while inhaling <50% (1, 5, 13, 24, 25), or 25% (7, 8). In contrast, correctly applied PAN does not result in anesthesia (7, 8, 13, 17, 19), while other side-effects are milder and less frequent (7, 8, 13). This because, the optimum dosage is evaluated by the practitioner on a case by case basis.
Importantly, like procedural sedation and analgesia (44) the gas is titrated to a clinical state (not a predetermined goal concentration) thus, there is no actual or finite concentration, even in the same subject (25) when PAN is correctly used (7, 8, 12, 13, 17, 44). But, once again these concentrations are lower than 50% (20, 21), and fall within the first half of Stage 1 Anesthesia (19).
Lower titrated N2O concentrations prevent anesthesia
There is another basis for the rarity of anesthesia and other untoward side-effects with PAN. Simply, the concentrations of N2O are always lower than a 50% (1, 4–6, 15) or 25% (15) fixed mixture administered through a strapped facial mask, because dental flowmeters use a semi-open nasal mask system (7, 8, 13, 14) Further, dental flowmeters ensure that the maximum concentration, as per the rotameters is 70% (7, 8). And typical dental nasal-mask systems (7, 8, 12, 17), produce concentrations of N2O reaching the pharynx less than half than those shown on the rotameters (20, 21). With PAN, <1%, of subjects require rotameter setting of 70%, which is the maximum concentration possible with a dental flowmeter. Here, the actual alveolar concentration of N2O is <35%, in O2. In terms of these lower rotameter to pharyngeal concentrations, Malamed indicates that 91% of subjects given 22.5% N2O or less will be adequately sedated (7). Consequently, any fixed concentration of 25% (15) or 50% (1–5, 15) will cause numerous individuals to be over-sedated (7), resulting in avoidable side effects (7, 8).
Obviously, for balanced anesthesia, these variable concentration effects when N2O is used are of limited import. Here the goal endpoint is rapid unconsciousness and not consciousness. Thus, for anesthesia, virtually all subjective effects can be discounted. Of course, this presupposes that the patient has good intra-operative analgesia and there is an absence of awareness, as well as post-operative recall. Clearly, where the gas is used on conscious people their subjective responses are of paramount importance.
Bell-shaped N2O sensitivity curve
N2O, like any pharmacological agent used in procedural sedation and analgesia, manifests a normal bell-shaped distribution curve (7, 8) reflecting the sensitivity of subjects to the agent (7, 8, 12, 19, 44), when given during consciousness (7, 8, 11, 17). And, the dose-response curve is of central importance when administering rapid onset and offset agents like N2O to conscious subjects (7, 8, 12, 17, 19, 25, 26). Furthermore, the dose-response curve in any individual, can vary even from day to day, because it is state rather than trait dependant (25, 77).
Differences in receptor actions of titrated vs. fixed concentration N2O
Apart from dose response differences, there is another important distinction. The mechanisms underlying PAN differs from higher subanaesthetic concentrations. For instance, the analgesic properties of subanaesthetic N2O and PAN are mediated mainly by the endogenous opioid and GABA-ergic system (10, 11, 29, 31, 32, 38, 39). In contrast, the anesthetic actions of the gas occur mainly through N-methyl-D-aspartate receptor (NMDAR) (29, 30). As we have seen, some patients may be inadvertently anesthetised, when using a fixed 50:50 mixtures of N2O (7, 8, 13, 17, 24–26). Thus, the underlying mechanisms governing the consciously experienced psychotropic actions of N2O, are different to those where pre-anesthesia or anesthesia supervene. Consequently, one can see how the antidepressant actions of N2O could wrongly be attributed to the NMDAR blockade (1–5, 15, 27, 78). Indeed, the same error has been made regarding ketamine, which until recently (27, 28, 79), was purported to have been due to NMDARs (1–5, 42).
Although convenient, and superficially more scientific to use fixed concentrations of 25% (15) or 50% (1, 2, 4, 5, 15) N2O in oxygen, it may be imprudent to generalize findings with these concentrations. Particularly, because of the higher concentrations delivered by a relatively closed system. A factor complicated by the varying sensitivity of patients to N2O (7, 8, 17, 24–27). These variables are virtually eliminated where the concentration is correctly titrated to patient comfort levels (PAN) (11, 12, 17, 27) as compared to higher subanaesthetic goal concentrations (1–5, 15). Moreover, the mechanism involved underlying observations (1–5, 15) may also be questionable (27, 78), even where the operator chooses to titrate to a highish goal concentration (1, 5, 24, 27, 28, 78). For these reasons, the use of continuous-flow, fixed concentrations of 25% (15) or 50% N2O (1–6, 15), are inappropriate (7, 8, 12, 13, 24–27, 77).
Side effects prevented by titration
Almost all these unwanted side effects, particularly inadvertent unconsciousness and/or nausea and vomiting are avoided by using the correct dental technique (7, 8, 13, 17). Here, the gases are titrated to each subjects' unique requirements, always using a nasal (7–12, 17), rather than a full-face (1–5, 15) mask. To use the dental technique safely, effectively, and correctly, a short hands-on training (lasting a few hours), is essential (36, 37, 40, 41).
From the foregoing it should be clear, that those using a 25% or 50% goal gas concentration mixture (1–5, 15) for periods above a few minutes, must therefore accept that unpleasant side-effects, such as inadvertent anesthesia, nausea, vomiting and inappropriate affective changes as inevitable in some cases (1, 7, 8, 13, 24–26, 43). A fact borne out in two studies, where nausea and vomiting has already been noted in subjects breathing 50% N2O (1, 5, 15). These problems are almost always avoided when N2O is titrated to each subjects' requirements (7, 8, 11, 12, 17, 40), so that the subject is always conscious and co-operative. For these reasons, readers will understand the practical importance of distinguishing between subanaesthetic and psychotropic concentrations of N2O.
Confusion of PAN with discontinued use of 100% N2O for anesthesia
In the 19th and early 20th Centuries, 100% (hypoxic) N2O was used for anesthesia (13, 80) which was potentially fatal, (80) possibly producing some deaths. Although, since the 1st years of the last century it was abandoned in medicine and dentistry, the confusion exists today. After training dentists and physicians in the PAN technique over 40 years, I am regularly told by potential trainees or anesthetists that the technique is dangerous. These people invariably confuse PAN with using 100% N2O for anesthesia. It would be a pity, if PAN wrongly fell into disrepute, because an untrained practitioner, using 25% or 50% N2O goal concentrations plus a full-face mask, produced a fatal pulmonary aspiration (43).
Although deaths are unlikely even when used at 50% or 25% (with a relatively closed circuits), unpleasant affective and physical side-effects are unavoidable in a certain percentage of patients (7, 8, 13, 15, 17, 24–26, 43), including vomiting, nausea and others, as confirmed recently (1, 5, 6, 15).
As discussed in some detail above, these high subanesthetic concentrations can and do, produce unconsciousness, vomiting, nausea and other disagreeable effects (1, 5, 6, 13, 24–26). Until the single controlled double-blind study (15) is repeated by others, these studies show promise only. Nonetheless, they do support the earlier work showing that PAN is antidepressant (9, 10). Interestingly, the same American group have published 3 reviews (23, 42, 81), heavily favoring the NMDAR and practically ignoring the other neurotransmitter systems, notably the opioid system (27).
Sadly, even if more studies are undertaken and confirm the north American work (15), the possibility of using fixed concentrations of 50% N2O in general psychiatric or medical practice are limited. Such studies are limited unless an anesthesthesiologist administers the gas and we accept that many subjects will be oversedated. The evidence mentioned above, also clearly indicates that a 25% fixed dose is also unsuitable. Nonetheless, the realization that N2O 25% (15) is probably better than 50% (15) seems an advance, particularly if these researchers realize the advantages of the PAN titration technique and to begin to use it. However, at this stage, the authors (1, 4, 6, 15) are still using arbitrary dosage rather than a dose optimized to each specific patient's needs.
Conclusions
At the moment, there is little controlled evidence showing the efficacy (42) and safety of subanesthetic fixed doses of N2O in in mood disorders. Nonetheless, the gas does show promise for mood and other psychiatric disorders (11, 15). Indeed, it is only comparatively recently that an adequate double-blind method has been devised using N2O (35, 73). The latter method is able to prevent the identification of the gas by both subjects and investigators (1, 15).
Compelling ethical and practical reasons make it unwise or even dangerous for psychiatrists or any other practitioner, unqualified as an anesthesthesiologists, to use fixed concentrations of 50% N2O (with full face mask), by continuous flow. The only way that continuous flow N2O at 50% can be safely used is when an anesthesiologist is present. The use of fixed 25% N2O should also be avoided, to prevent unnecessary suffering (7, 8, 15).
In closing, I leave the reader with this question: Is the current method, where fixed goal concentration are advocated (1–6, 15), going to result in a useful agent falling into disrepute because of unnecessary patient suffering or other unintended harmful consequence? A particular problem in the hands of those not qualified as anesthesiologists.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Acknowledgments
My thanks to my son Luis F Gillman for assisting me with the necessary it that enabled me to submit this paper, and David Silman for help with style and grammar.
Conflict of interest
MG is a medical adviser to Sedatek.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nagele P, Duma A, Kopec M, Gebara MA, Parsoei A, Walker M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. (2015) 78:10–18. doi: 10.1016/j.biopsych.2014.11.016
2. Das RK, Tamman A, Nikolova V, Freeman TP, Bisby JA, Lazzarino AI, et al. Nitrous oxide speeds the reduction of distressing intrusive memories in an experimental model of psychological trauma. Psychol Med. (2016) 46:1749–59. doi: 10.1017/S003329171600026X
3. Walsh K, Das RK, Kamboj SK. The subjective response to nitrous oxide is a potential pharmaco-endophenotype for alcohol use disorder: A Preliminary Study with Heavy Drinkers. Int J Neuropsychopharmacol. (2017) 20:346–50. doi: 10.1093/ijnp/pyw063
4. Nagele P, Brown F, Yohanna D. Prolonged remission of major depressive disorder after single nitrous oxide inhalation treatment. Front Psychiatry. (2020) 11:692. doi: 10.3389/fpsyt.2020.00692
5. Varias V, van Roessel P, Parsiani M, Filippou-Frye M; Neylan TC; Nagele P, Yesavage J, Clark JD, et al. Does nitrous oxide help veterans with posttraumatic stress disorder? A case series. J Clin Psychiat. (2020) 81:2013393. doi: 10.4088/JCP.20l13393
6. Desmidt T, Gissot V, Dujardin PA, Andersson F, Barantin L, Brizard B, et al. A case of sustained antidepressant effects and large changes in the brain with a single brief exposure to nitrous oxide. Am J Geriatr Psychiatry. (2021) 29:1298–300. doi: 10.1016/j.jagp.2021.01.138
9. Gillman MA, Matussek N, Lichtigfeld FJ. Effect of nitrous oxide on depressive patients and volunteers In: Pichot P, Berner P, Wolf R, Thau K, editors. Biological Psychiatry, Higher Nervous Activity. Springer. (1985). doi: 10.1007/978-1-4684-8329-1_58
10. Lichtigfeld FJ, Gillman MA. The treatment of alcoholic withdrawal states with oxygen and nitrous oxide. S Afr Med J. (1982) 61:349–51.
11. Gillman MA, Lichtigfeld FJ. Analgesic nitrous oxide in neuropsychiatry: Past, present future. Int J Neurosci. (1989) 49:75–81. doi: 10.3109/00207458909087041
12. Gillman MA, Lichtigfeld FJ. Opioid properties of psychotropic analgesic nitrous oxide (Laughing Gas). Perspect Biol Med. (1994) 38:125–38. doi: 10.1353/pbm.1994.0026
13. Smith RA, Beirne OR. The use of nitrous oxide by dentists. In: Eger EI, editor. Nitrous oxide/N2O. New York, NY: Elsevier (1985).
14. MacDonald IJ. Entonox as a psychotherapeutic aid. BMJ. (1970) 1:483. doi: 10.1136/bmj.2.5707.483-a
15. Nagele P, Palanca BJ, Got B, Brown F, Barnes L, Nguyen T, et al. A phase 2 trial of inhaled nitrous oxide for treatment-resistant major depression. Sci Transl Med. (2021) 13:eabe1376. doi: 10.1126/scitranslmed.abe1376
16. Piazza GG, Iskandar G, Hennessy V, Zhao H, Walsh K, McDonnell J, et al. Pharmacological modelling of dissociation and psychosis: an evaluation of the Clinician Administered Dissociative States Scale and Psychotomimetic States Inventory during nitrous oxide ('laughing gas')-induced anomalous states. Psychopharmacology (Berl). (2022) 239:2317–29. doi: 10.1007/s00213-022-06121-9
17. Langa H. Relative Analgesia in Dental Practice: Inhalation Analgesia With Nitrous Oxide. Philadelphia, PA: WB Saunders (1968).
18. American Society of Anesthesiologists. Task force on sedation and analgesia by non-anesthesiologists. Practice guidelines for non-anesthesiologists. Anesthesiology. (2002) 96:1004–17. doi: 10.1097/00000542-200204000-00031
19. Parbrook GD. The levels of nitrous oxide analgesia. Br J Anaesth. (1967) 39:974–82. doi: 10.1093/bja/39.12.974
20. Sher AM, Braude BM, Cleaton-Jones PE, Moyes DG, Mallett J. Nitrous oxide sedation in dentistry. A comparison between rotameter setting, pharyngeal concentrations and blood levels of nitrous oxide. Anesthesia. (1984) 39:236–9. doi: 10.1111/j.1365-2044.1984.tb07233.x
21. Klein U, Bucklin B, Bucklin T, Poulton J, Bozinov D. Nitrous oxide concentrations in the posterior nasopharynx during administration by nasal mask. Pediatr Dent. (2004) 26:410–6.
22. Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. (2012) 5:e126–31.
23. Zorumski CF, Peter Nagele P, Mennerick S, Conway CR. Treatment-resistant major depression: Rationale for NMDA receptors as targets and nitrous oxide as therapy. Charles F. Zorumski, Peter. Front Psychiatry. 6:172. doi: 10.3389/fpsyt.2015.00172
24. Seevers MH, Bennett JH, Pohle HW, Reinardy EW. The analgesia produced by nitrous oxide, ethylene and cyclopropane in the normal human subject. J Pharmacol Exp Ther. (1937) 59:291–300.
25. Robson JG, Burns BD, Welt PJL. The effect of inhaling dilute nitrous oxide upon recent memory and time estimation. Can Anaes Soc J. (1960) 7:399–410. doi: 10.1007/BF03021298
26. Cook TL, Starkweather JA, Winter PM, Eger EI. Behavioral effects of trace and subanesthetic halothane and nitrous oxide in man. Anesthesiology. (1978) 49:419–24. doi: 10.1097/00000542-197812000-00007
27. Gillman MA. Words of caution on using fixed 50% concentrations of nitrous oxide in psychiatry. J Clin Psychopharmacol. (2019) 39:421–2. doi: 10.1097/JCP.0000000000001067
28. Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. (2018) 175:1205–15. doi: 10.1176/appi.ajp.2018.18020138
29. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. (2007) 54:9–18. doi: 10.2344/0003-3006 (2007) 54[9:AIUTAO]2.0.CO;2
30. Jevtovic-Todorovic V, Todorovic SM, Mennerick S, Powell S, Dikranian K, Benshoff N, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. (1998) 4:460–3. doi: 10.1038/nm0498-460
31. Gillman MA, Kok L, Lichtigfeld FJ. Paradoxical effect of naloxone on nitrous oxide analgesia in man. Eur J Pharmacol. (1980) 61:175–7. doi: 10.1016/0014-2999(80)90160-0
32. Gillman MA, Lichtigfeld FJ. A comparison of the effect of morphine sulphate and nitrous oxide analgesia on chronic pain states in man. J Neurol Sci. (1981) 45:41–5. doi: 10.1016/0022-510X(81)90186-6
33. Gillman MA, Lichtigfeld FJ. A pharmacological overview of opioid mechanisms mediating analgesia and hyperalgesia. Neurological Res. (1985) 7:106–19 doi: 10.1080/01616412.1985.11739709
34. Gillman MA, Lichtigfeld FJ. Placebo and analgesic nitrous oxide for treatment of the alcohol withdrawal state. Brit J Psychiatry. (1991) 159:672–5. doi: 10.1192/bjp.159.5.672
35. Gillman MA, Lichtigfeld FJ. Randomized double-blind trial of psychotropic analgesic nitrous oxide compared with diazepam for alcohol withdrawal state. J Subst Abuse Treat. (2002) 22:129–34. doi: 10.1016/S0740-5472(02)00224-6
36. Gillman MA, Lichtigfeld FJ, Young T. Psychotropic analgesic nitrous oxide for alcoholic withdrawal states. Cochrane Library. (2008) 2008:CD005190. doi: 10.1002/14651858.CD005190.pub2
37. Gillman MA, Lang L. Guest Editorial. New guidelines for dentists in controlling anxiety using responsive sedation. S Afr Dent J. (2019) 70:219–20.
38. Daras C, Cantrill RC, Gillman MA. (3H)Naloxone displacement: evidence for nitrous oxide as opioid receptor agonist. Eur J Pharmacol. (1983) 89:177–8. doi: 10.1016/0014-2999(83)90626-X
39. Ori C, Ford-Rice F, London ED. Effects of nitrous oxide and halothane on mu and kappa opioid receptors in guinea-pig brain. Anesthesiology. (1989) 70:541–4. doi: 10.1097/00000542-198903000-00027
40. Gillman MA, Lichtigfeld FJ. The current status of analgesic nitrous oxide for treating alcoholic withdrawal states. Finnish Med J. (1997) 52:1055–58.
41. Crivello BJ, Reddy AA, Pazdernik VK, Davis JM. Impact of experiential learning on dental students' training in nitrous oxide inhalation sedation. J Dent Educ. (2020) 8:e12345. doi: 10.1002/jdd.12345
42. Nagele P, Zorumski CF, Conway CR. Exploring nitrous oxide as treatment for mood disorders: Basic concepts. J Clin Psychopharmacol. (2018) 38:144–8. doi: 10.1097/JCP.0000000000000837
43. Eger EI. Respiratory effects of nitrous oxide In: Eger EI, editor. Nitrous Oxide/N2O. New York, NY: Elsevier (1985). doi: 10.1097/00004669-198504000-00009
44. Miner JR, Krauss B. Procedural sedation and analgesia research: State of the Art. Acad Emer Med. (2007) 14:170–8. doi: 10.1197/j.aem.2006.10.101
45. Lichtigfeld FJ, Gillman MA. Analgesic nitrous oxide for alcohol withdrawal is better than placebo. Int J Neurosci. (1989) 49:71–4. doi: 10.3109/00207458909087040
46. Gillman MA, Lichtigfeld FJ, Harker N. Psychotropic analgesic nitrous oxide for acute cocaine withdrawal in man. Int J Neurosci. (2006) 116:847–57. doi: 10.1080/00207450600754038
47. Gillman MA, Harker N, Lichtigfeld FJ. Combined cannabis/methaqualone withdrawal treated with psychotropic analgesic nitrous oxide. Int J Neurosci. (2006) 116:859–69. doi: 10.1080/00207450600753998
48. Gillman MA, Katzeff IE. Anti-stress hormonal responses of analgesic nitrous oxide. Int J Neurosci. (1989) 49:199–202. doi: 10.3109/00207458909084825
49. Gillman MA, Lichtigfeld FJ. The effect of nitrous oxide and naloxone on orgasm in human females. A preliminary report. J Sex Res. (1983) 19:49–57. doi: 10.1080/00224498309551168
50. Murphy MR, Checkley SA, Seck JR, Lightman SL. Naloxone inhibits oxytocin release at orgasm in man. J Clin Endocrinol Metab. (1990) 71:1056–58. doi: 10.1210/jcem-71-4-1056
51. Aristotle's rhetoric Book I Chapter 11. In: Freese JH, editor. Aristotle, Rhetoric. http://www.perseus.tufts.edu/hopper/text?doc=Perseus:text:1999.01.0060:book=1:chapter=11 (accessed March 26, 2020).
52. Schmitter AM. 17th and 18th century theories of emotions. In: Zalta EN, editor. The Stanford Encyclopedia of Philosophy. (2020). https://plato.stanford.edu/archives/win2016/entries/emotions-17th18th (accessed November 6, 2020).
53. Gillman MA, Lichtigfeld FJ. The opioid and anti opioid system in addiction. S Afr Med J. (1984) 66:592.
54. Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. (1988) 85:5374–8. doi: 10.1073/pnas.85.14.5274
55. Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. (1992) 89:2046–50. doi: 10.1073/pnas.89.6.2046
56. Lichtigfeld FJ, Gillman MA. Role of dopamine mesolimbic system in opioid action of psychotropic analgesic nitrous oxide (PAN) in alcohol and drug withdrawal. Clin Neuropharmacol. (1996) 19:246–50. doi: 10.1097/00002826-199619030-00006
57. Lichtigfeld FJ, Gillman MA. The effect of placebo in the alcohol withdrawal state. Alc Alcohol. (1989) 24:109–12. doi: 10.1093/oxfordjournals.alcalc.a044873
58. Lichtigfeld FJ, Gillman MA. The opioid system and the placebo response. Am J Psychiatry. (1992) 149:276. doi: 10.1176/ajp.149.2.276-a
59. Gillman MA, Lichtigfeld FJ. The opioid effects of analgesic (Subanesthetic) nitrous oxide on the alcohol withdrawal state. Ann N Y Acad Sci. (1991) 625:784–5. doi: 10.1111/j.1749-6632.1991.tb33919.x
60. Leknes S, Tracey I. A common neurobiology for pain and pleasure Nature Reviews (2008). 9:314-320. doi: 10.1038/nrn2333
61. Lichtigfeld FJ, Gillman MA. Possible role of the endogenous opioid system in the placebo response in depression. Int J Neuropsychoph. (2002) 5:107–8. doi: 10.1017/S1461145701002735
62. Lichtigfeld FJ, Gillman MA. Another marker for depression. Int J Neuropsychoph. (2003) 5:91–2. doi: 10.1017/S1461145703003286
63. Daynes G, Gillman MA. Psychotropic analgesic nitrous oxide prevents craving after withdrawal from alcohol, cannabis and tobacco. Int J Neurosci. (1994) 76:13–6. doi: 10.3109/00207459408985987
64. Ojutkangas R, Gillman MA. Psychotropic analgesic nitrous Oxide for treating alcohol withdrawal in an outpatient setting. Int J Neurosci. (1994) 76:35–9. doi: 10.3109/00207459408985989
65. Gillman MA, Lichtigfeld FJ. Analgesic nitrous oxide: Adjunct to clonidine for opioid withdrawal. Am J Psychiatry. (1985) 142:784–5. doi: 10.1176/ajp.142.6.784b
66. Gillman MA, Sandyk R. Reversal of captopril-induced psychosis with naloxone. Am J Psychiatry. (1985) 142:270. doi: 10.1176/ajp.142.2.270a
67. Gillman MA, Lichtigfeld FJ. Naloxone in Anorexia Nervosa - The role of the opiate system. J Roy Soc Med. (1981) 74:631–2. doi: 10.1177/014107688107400820
68. Dafny N, Gillman MA, Lichtigfeld FJ. Cholecystokinin: induced suppression of feeding in fed, fasting and hypothalamic island rats. Brain Res Bull. (1988) 21:225–31. doi: 10.1016/0361-9230(88)90235-3
69. Gillman MA, Sandyk R, Lichtigfeld FJ. Evidence for underactivity of the opioid system in neuroleptic-induced akathisia. Psychiatry Res. (1984) 13:187. doi: 10.1016/0165-1781(84)90062-3
70. Gillman MA, Sandyk R. Tourette syndrome: Effect of analgesic concentrations of nitrous oxide and naloxone. Br Med J. (1984)288:114.
71. Gillman MA, Sandyk R. Nitrous oxide ameliorates spasmodic torticollis. Eur Neurol. (1985) 24:292–3. doi: 10.1159/000115811
72. de Wet J, Lichtigfeld FJ, Gillman MA. Psychotropic analgesic nitrous oxide (PAN) for hyperactivity. Aust N Z J Psychiatry. (2001) 35:543–4. doi: 10.1046/j.1440-1614.2001.0911e.x
73. Gillman MA, &Lichtigfeld F.J. Enlarged double-blind randomised trial of benzodiazepines against psychotropic analgesic nitrous oxide for alcohol withdrawal. Addictive Behav. (2004) 29:1183–7. doi: 10.1016/j.addbeh.2004.03.015
75. Gillman MA. Nitrous oxide as neurotransmitter. Lancet. (1992) 339:307. doi: 10.1016/0140-6736(92)91379-M
76. Bredt D, Hwang P, Snyder S. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. (1990) 347:768–70. doi: 10.1038/347768a0
77. Anonymous Editorial comment. Pain perception and the placebo response. S Afr J Sci. (1987) 83:519.
78. Zarate CA Jr, Machado-Vieira R. Potential pathways involved in the rapid antidepressant effects of nitrous oxide. Biol Psychiatry. (2015) 78:2–4. doi: 10.1016/j.biopsych.2015.04.007
79. Klein ME, J Chandra J, Sheriff S, Malinow R. Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc Natl Acad Sci USA. (2020) 117:2656–62. doi: 10.1073/pnas.1916570117
80. Frost EAM. A history of nitrous oxide. In: Eger EI, editor. Nitrous Oxide/N2O. New York, NY: Elsevier (1985).
Keywords: depression, psychiatry, titrated nitrous oxide, fixed concentrations nitrous oxide, ketamine, substance abuse, alcohol withdrawal
Citation: Gillman MA (2022) What is better for psychiatry: Titrated or fixed concentrations of nitrous oxide? Front. Psychiatry 13:773190. doi: 10.3389/fpsyt.2022.773190
Received: 09 September 2021; Accepted: 27 July 2022;
Published: 22 August 2022.
Edited by:
Roberto Ciccocioppo, University of Camerino, ItalyReviewed by:
Stefania Schiavone, University of Foggia, ItalyGeorge V. Rebec, Indiana University Bloomington, United States
Copyright © 2022 Gillman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark A. Gillman, bWFya2FnaWxsbWFuQGdtYWlsLmNvbQ==
 Mark A. Gillman
Mark A. Gillman