- 1Department of Psychiatry, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 2Neuroscience Research Institute, Gachon University, Incheon, South Korea
- 3Geumsan-gun Public Health Center, Seoul, South Korea
- 4Department of Psychiatry, Seoul National Hospital, Seoul, South Korea
- 5Department of Psychiatry, National Medical Center, Seoul, South Korea
- 6Department of Psychiatry and Center for Sleep and Chronobiology, Seoul National University Hospital, Seoul, South Korea
The current study investigated differences in the regional gray matter (GM) volume of specific thalamic nuclei between North Korean (NK) refugees and South Korean (SK) residents. It also investigated associations between thalamic GM volume changes and psychological symptoms. Psychological evaluations and magnetic resonance imaging were conducted on 50 traumatized NK refugees and 55 non-traumatized SK residents. The regional GM volume ratios in the bilateral thalami were calculated for all participants using voxel-based morphometry. NK refugees showed greater GM volume ratios in the right medial-posterior nuclei and left medial nuclei compared with SK residents. NK refugees also exhibited more depressive symptoms than SK residents. However, increased GM volume ratios in both right medial-posterior nuclei and left medial nuclei were correlated with fewer depressive symptoms in NK refugees, but not in SK residents. The findings indicate that traumatized NK refugees had increased GM volumes in the right medial-posterior nuclei and left medial nuclei, which were associated with fewer depressive symptoms. The enlarged specific thalamic nuclei presented among refugees in the current study might be associated with a neurobiological compensatory mechanism that prevents the development or progression of depression in refugees after repetitive traumatic experiences.
Introduction
The thalamus has been presumed to play a pivotal role in fear processing, which is crucial for understanding trauma-related disorders [e.g., posttraumatic stress disorder (PTSD)] (1, 2). The hippocampal-prefrontal-thalamic circuitry functions in contextual fear conditioning; its disruption is presumed to be a core component of PTSD pathophysiology (2). The thalamus regulates fear learning and expression in the central amygdala by modulating the functions of neurons through the activities of brain-derived neurotrophic factor receptor tropomyosin-related kinase B (3). Alternating bilateral sensory stimulation reportedly led to long-lasting fear attenuation via thalamic activation in mice (4).
Trauma itself is known to affect brain structure and function regardless of PTSD development (5, 6). These brain changes include the change of thalamus after trauma (5). In addition, trauma can induce non-PTSD psychiatric symptoms, which are also related to brain structure and function (7–13). Therefore, we intended to explore the effects of trauma itself on thalamus.
Several previous studies have shown structural changes in the thalamus after trauma or severe stress (6, 14). Changes in thalamic volume have been observed in victims of childhood maltreatment or earthquakes (15, 16). The thalamus consists of various nuclei, each of which has a distinct location and function. Many thalamic nuclei are involved in fear conditioning and emotional regulation, which may be related to structural or functional changes of the thalamus after traumatic experiences (17–19). To our knowledge, no study has specifically investigated changes in the thalamic nuclei of traumatized people. In addition, the functional meaning of the structural changes in specific thalamic nuclei has not been reported. In the present study, we investigated which thalamic nuclei exhibit volumetric alterations in traumatized refugees and the clinical implications.
Among traumatized victims, refugees are an important population for examining the effect of chronic, repetitive, and severe trauma on brain structures such as the thalamus. Most refugees fled their communities because of war, civil conflict, disaster, oppression, or persecution (20). They may have experienced imprisonment, torture, physical assault, rape, and forced separation from family prior to fleeing (21, 22). Although brain structures and functions have been widely studied in other traumatized populations, only a few studies have been performed on alterations of brain structure in traumatized refugees. North Korean (NK) refugees reportedly experience chronic and repetitive trauma after defection from NK, prior to their arrival in South Korea (23–25). Structural and functional brain changes in NK refugees have been characterized in previous studies (26–28).
In this study, we compared regional gray matter volumes (GM volumes) in thalamic nuclei between NK refugees and South Korean (SK) residents. This study also explored associations between structural changes in thalamic nuclei and common psychological symptoms in traumatized people (e.g., depression). Based on previous studies, we hypothesized that GM volumes in specific thalamic nuclei would differ between NK refugees and SK residents. Additionally, we hypothesized that changes in thalamic GM volumes in NK refugees would be correlated with their psychological symptoms.
Materials and Methods
Participants
North Korean refugees staying in South Korea and native SK residents were recruited through advertisements. Participants with the following conditions were excluded: history of head injury, neurological disorder, untreated serious medical illness, and/or neurodevelopmental disorder, and any metal or electronic implants that violated magnetic resonance imaging (MRI) safety standards. The presence of a psychiatric disorder was an additional exclusion criterion for SK residents but not NK refugees. Among the initially recruited participants, three NK refugees were excluded because of structural abnormalities in the brain; one NK refugee was excluded because of poor image quality. Finally, 50 NK refugees (11 men and 39 women; mean age, 35.96 ± 11.05 years) and 55 SK residents (19 men and 36 women; mean age, 35.96 ± 11.07 years) participated in the study. All NK refugees had at least one traumatic experience, as defined by the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV) criteria. Six NK refugees used psychotropic medications including antidepressants and sedatives. The psychiatric disorders of the NK refugees are shown in Table 1. Twenty-three NK refugees were diagnosed with current Axis I psychiatric disorders, according to the Structured Clinical Interview for DSM-IV (SCID-IV). Among these 23 NK refugees, the psychiatric disorders were as follows (some refugees had multiple disorders): PTSD (n = 3), depressive disorders (n = 11), anxiety disorders (n = 7), adjustment disorders (n = 3), somatoform disorders (n = 6), and eating disorders (n = 1). This study was approved by the Institutional Review Board of Seoul National University Hospital; all participants provided written informed consent before inclusion in the study.
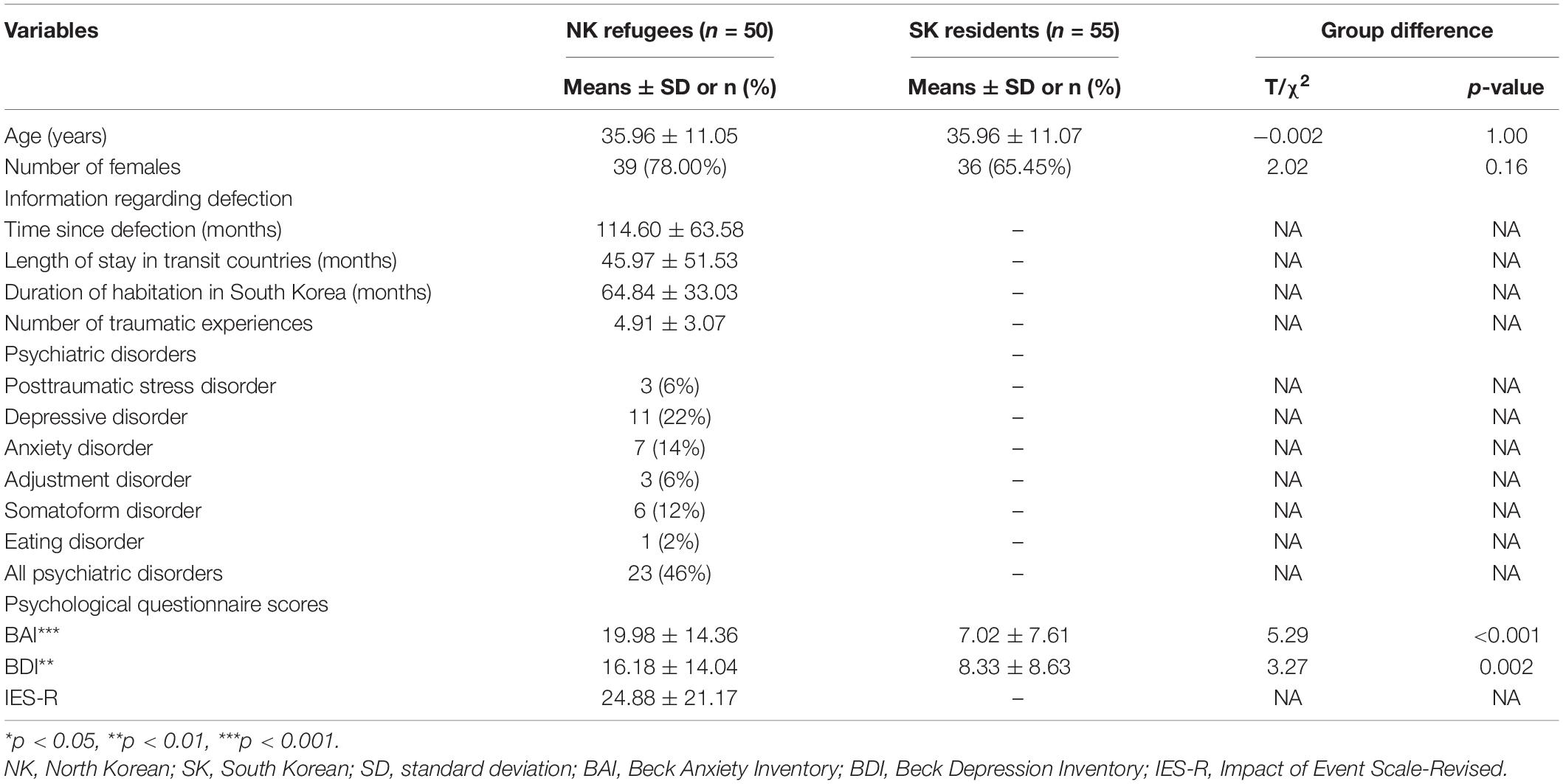
Table 1. Demographic and clinical characteristics of North Korean (NK) refugees and South Korean (SK) residents.
Evaluation for Psychological Symptoms
All participants were assessed using the Korean version of the SCID-IV to diagnose psychiatric disorders. Korean versions of the Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI) were administered to each participant to estimate the severity of anxiety and depression, respectively. Using the Impact of Event Scale-Revised (IES-R), the NK refugees were evaluated in terms of symptom severity in three PTSD domains (intrusion, avoidance, and hyperarousal). The Korean versions of the BAI (Cronbach α = 0.91) (29), BDI (Cronbach α = 0.89) (30), and IES-R (Cronbach α = 0.93) (31) have demonstrated excellent internal consistency and strong correlations with other inventories of anxiety, depression, and PTSD, respectively. The significant anxiety can be suspected when BAI scores were over 21 (32). According to the BDI, 14 is the cut-off point for significant depressive symptoms (30). The presence of PTSD symptoms was defined as IES-R≥24 (31).
The Trauma Exposure Check List for North Korean Refugees was used to evaluate the types and numbers of traumatic experiences reported by NK refugees (33). The checklist consists of 13 types of traumatic events that could be experienced in North Korea, as well as 16 types of traumatic events that can occur during defection to South Korea. These events include torture, severe battery, life-threatening starvation/cold/accidents, rape, human trafficking, arrest, imprisonment, and observation of violent death. The period of time after defection, length of stay in transit countries, duration of habitation in South Korea, and use of psychotropic medication were also assessed in interviews with the refugees.
Magnetic Resonance Imaging Data Acquisition
Structural MRI data were acquired using a 3T MRI system (Trio Tim, Siemens; Erlangen, Germany) with a 12-channel birdcage head coil. Sagittal T1-weighted, 3D magnetization-prepared rapid gradient echo imaging was performed with the following parameters: repetition time = 1,670 ms, echo time = 1.89 ms, inversion time = 900 ms, flip angle = 9°, slice thickness = 1.0 mm, in-plane resolution = 1.0 mm × 1.0 mm, field of view = 250 mm, and matrix size = 256 × 256.
Voxel-Based Morphometry
Voxel-based morphometry was conducted using the Diffeomorphic Anatomical Registration Through Exponentiated Lie (DARTEL) tool in SPM12.1 First, T1-weighted images were segmented (unified segmentation) into probability maps of gray and white matter, cerebrospinal fluid, skull and soft tissue outside the brain. A population gray matter template was created by high-dimensional non-linear warping, by applying DARTEL algebra to the probability maps of gray and white matter. Then, the gray matter probability maps of each participant were normalized to the population template. The population template was normalized to Montreal Neurological Institute (MNI) space using affine transformation; all individual gray matter maps normalized to the template space were then transformed into MNI space using the same transformation parameter with Jacobian modulation. Finally, images that had been normalized to MNI space were smoothed with a Gaussian kernel of 8-mm full-width at half-maximum (34).
Bilateral thalamic regions of interest were generated using the AAL template of Marsbar.2 GM volume in the thalamus was calculated, with small volume correction (SVC) done in SPM12.
Since VBM based methods may have low sensitivity to small structures and probability of misidentification of the nuclei, we conducted direct thalamic nuclei segmentation using Freesurfer (35). To identify areas with significant groupwise differences in GM volume, thalamic nuclei were separated and defined in accordance with the atlas in Freesurfer. The segmentation of thalamic nuclei was done after completing cortical segmentation (e.g., recon-all). The atlas consisted of 26 human thalamic nuclei based on ex vivo MRI and histology. It segmented the thalamic nuclei into anterior group (anteroventral nucleus), lateral group (laterodorsal, lateral posterior), ventral group (ventral anterior, ventral anterior magnocellular, ventral lateral anterior, ventral lateral posterior, ventral posterolateral, ventromedial), intralaminar group (central medial, central lateral, paracentral, centromedian, parafasciculuar), medial group (paratenial, reuniens [medial ventral], mediodorsal medial magnocellular, mediodorsal lateral parvocellular), and posterior group (lateral geniculate, medial geniculate, limitans [suprageniculate], pulvinar anterior, pulvinar medial, pulvinar lateral, pulvinar inferior). The volume of thalamic nuclei between NK refugees and SK residents was statistically tested using independent two-sample t-test. For controlling for multiple comparison, additional analysis with Bonferroni correction was also conducted.
Data Analyses
To evaluate groupwise differences in thalamic GM volume, two-sample t-tests of bilateral thalami were conducted with SVC. Age, sex, and intracranial content volumes were entered as nuisance covariates. The significant level was set to a familywise error rate-corrected cluster level of p < 0.05, and an uncorrected peak level of p < 0.001. To further evaluate potential associations between clinical variables (e.g., BAI, BDI, and IES-R) and regional GM volumes in thalamic nuclei after the identification of significant groupwise differences, two-sample t-tests were used.
To compare the demographic and clinical data between the two groups, independent t-tests were used to analyze continuous variables and chi-squared tests to analyze categorical variables. Partial correlation analyses, adjusted for age, sex, and the number of traumatic experiences, were performed within each study group to identify associations between GM volume in the identified cluster and each clinical variable. These analyses were repeated with additional adjustment for psychotropic medication and psychiatric disorders. All statistical analyses were performed using R software (R Development Core Team, Vienna, Austria) and p-values < 0.05 were considered to indicate statistical significance.
In the additional analysis with direct thalamic nuclei segmentation, ANCOVA was used to compare GM volume ratios of thalamic nuclei adjusted for intracranial volume between the two groups. Age, sex, psychotropic medication, and psychiatric disorders were defined as covariates.
Results
Demographic and Clinical Characteristics
The demographic and clinical characteristics of the participants are shown in Table 1. The mean age did not significantly differ between NK refugees (35.96 ± 11.05 years) and SK residents (35.96 ± 11.07 years; t = −0.002, p = 1.00). Additionally, there were no significant differences in the sex ratio between NK refugees (39 women, 78%) and SK residents (36 women, 65.45%; chi = 2.02, p = 0.16).
The BAI and BDI scores were higher in NK refugees (BAI = 19.98 ± 14.36; BDI = 16.18 ± 14.04) than SK residents (BAI = 7.02 ± 7.61, t = 5.29, p < 0.001; BDI = 8.33 ± 8.63, t = 3.27, p = 0.002, respectively). Following adjustment for age and sex, NK refugees still showed significantly higher BAI and BDI scores compared with SK residents (BAI: t = 6.09, p < 0.001; BDI: t = 3.74, p < 0.001).
All NK refugees had at least one traumatic experience. The mean number of traumatic experiences was 4.91 ± 3.07. The mean interval after defection was 114.60 ± 63.58 months. The mean length of stay in transit countries (neither North Korea nor South Korea) was 45.97 ± 51.53 months. The mean duration of habitation in South Korea was 64.84 ± 33.03 months. The mean IES-R score of the NK refugees was 24.88 ± 21.17.
Comparison of Thalamic Gray Matter Volume Ratio Between North Korean Refugees and South Korean Residents
Compared with SK residents, NK refugees showed greater thalamic GM volumes in the right medial-posterior nuclei (x = 5, y = −23, z = 2, cluster size = 546, Z-score = 3.61) and left medial nuclei (x = −9, y = −6, z = 5, cluster size = 132, Z-score = 3.69) (Table 2). As the significant cluster overlied both parts of right medial and posterior nuclei, we designated the cluster as right medial-posterior nuclei. Figure 1 shows the brain areas with increased thalamic GM volume in the NK refugees compared with SK residents. The difference in cluster volume ratio remained statistically significant after adjustment for age and sex (right medial-posterior nuclei: t = 8.75, p < 0.001; left medial nuclei: t = 9.57, p < 0.001, respectively). After additional adjustment for psychotropic medication and psychiatric disorders, the GM volume ratios of the above-mentioned clusters remained greater in NK refugees than SK residents (right medial-posterior nuclei: t = 8.04, p < 0.001; left medial nuclei: t = 8.33, p < 0.001).
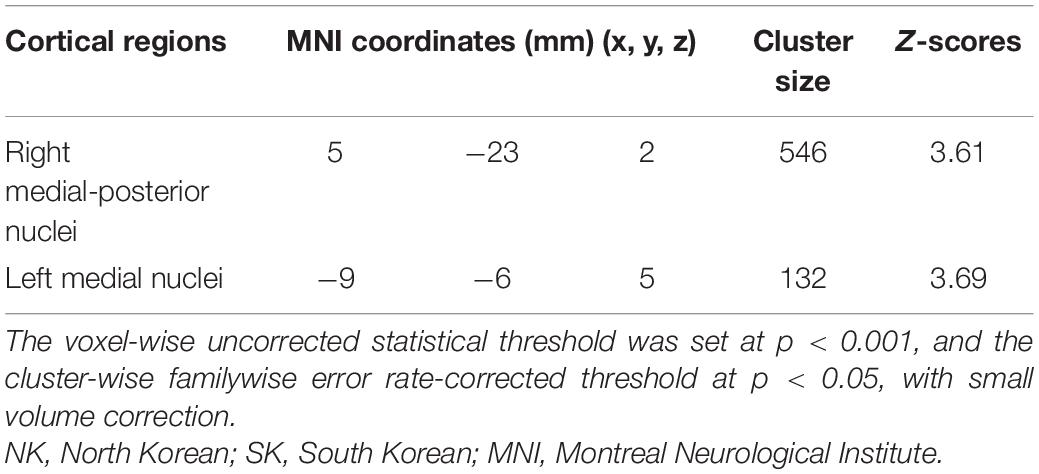
Table 2. Coordinates of thalamic areas showing increased gray matter volume in NK refugees compared with SK residents.

Figure 1. Thalamic areas showing increased gray matter volume in NK refugees compared with SK residents. Thalamic areas showing increased gray matter volume in NK refugees, compared with SK residents, are shown in the (A) axial plane, (B) coronal plane, and (C) 3D rendering. These areas correspond to the left medial nuclei (green arrow) and right medial-posterior nuclei (yellow arrow). The voxel-wise uncorrected statistical threshold was set at p < 0.001, and the cluster-wise familywise error rate-corrected threshold at p < 0.05, with small volume correction. NK, North Korean; SK, South Korean.
Additional analysis was conducted to investigate the associations between GM volumes and psychiatric disorders in NK refugees. There were no significant differences in GM volumes in the right medial-posterior nuclei and left medial nuclei between NK refugees with and without psychiatric disorders after adjustment for age and sex. Additionally, in order to investigate the associations between GM volumes and psychiatric symptoms, we compared GM volumes in the clusters between NK refugees with and without psychiatric symptoms. There were no significant differences in GM volumes in the right medial-posterior nuclei and left medial nuclei between NK refugees with and without anxiety, depression or PTSD symptoms.
In the additional analysis with false discovery rate (FDR) corrected p-value, compared with SK residents, NK refugees showed greater GM volumes in the right medial-posterior nuclei (x = 5, y = −23, z = 2, cluster size = 546, Z-score = 3.61). In whole brain analysis with same threshold, NK refugees showed greater GM volumes than SK residents in the left middle frontal lobe (x = −45, y = 6, z = 51, cluster size = 1,394, Z-score = 4.2). Although left middle frontal GM volume was significantly associated with GM volumes of right medial-posterior nuclei (r = 0.56, p < 0.001) and left medial nuclei (r = 0.49, p < 0.001) in all participants, this significance disappeared after controlling the NK-SK group effects.
In the direct thalamic nuclei segmentation using Freesurfer, NK refugees showed greater GM volumes in the left central medial (p = 0.02), suprageniculate (p < 0.001), mediodorsal lateral parvocellular (p = 0.006), mediodorsal medial magnocellular (p = 0.03), medial geniculate (p < 0.001), reuniens (p < 0.001), paracentral (p = 0.02), ventral anterior (p = 0.01), ventral anterior magnocellular nucleus (p = 0.03) and right suprageniculate (p = 0.002), lateral geniculate (p < 0.001), mediodorsal lateral parvocellular (p = 0.046), mediodorsal medial magnocellular (p = 0.04), medial geniculate (p < 0.001), reuniens (p = 0.02), paracentral (p = 0.02), pulvinar anterior (p = 0.002), pulvinar inferior (p = 0.04), pulvinar medial nucleus (p = 0.002) after controlling ICV than SK residents (Figure 2). After adjustment for age, sex, psychotropic medication and psychiatric disorders, the differences in GM volumes in the left central medial (p = 0.004), suprageniculate (p < 0.001), mediodorsal lateral parvocellular (p = 0.007), mediodorsal medial magnocellular (p = 0.02), medial geniculate (p < 0.001), reuniens (p < 0.001), paracentral (p = 0.003), ventral anterior (p < 0.001), ventral anterior magnocellular nucleus (p = 0.002) and right suprageniculate (p = 0.01), lateral geniculate (p < 0.001), mediodorsal lateral parvocellular (p = 0.04), mediodorsal medial magnocellular (p = 0.03), medial geniculate (p = 0.002), reuniens (p = 0.007), paracentral (p = 0.002), pulvinar anterior (p = 0.02), pulvinar inferior (p = 0.04), pulvinar medial nucleus (p = 0.009) remained statistically significant.
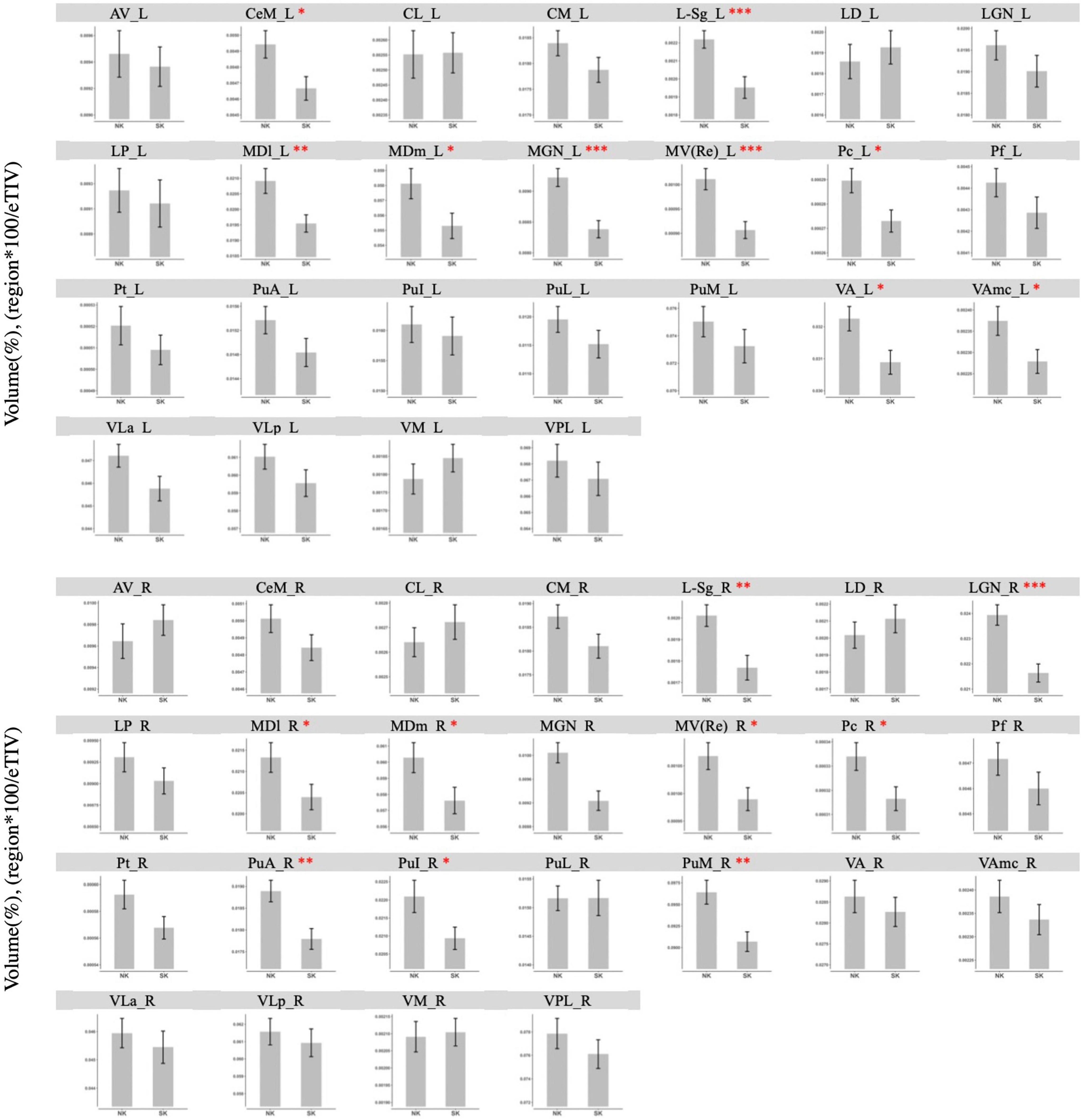
Figure 2. Comparison of gray matter volume of thalamic nuclei between NK refugees and SK residents in additional analysis using Freesurfer. In the direct thalamic nuclei segmentation using Freesurfer, NK refugees showed greater GM volumes in the left central medial, suprageniculate, mediodorsal lateral parvocellular, mediodorsal medial magnocellular, medial geniculate, reuniens, paracentral, ventral anterior, ventral anterior magnocellular nucleus and right suprageniculate, lateral geniculate, mediodorsal lateral parvocellular, mediodorsal medial magnocellular, medial geniculate, reuniens, paracentral, pulvinar anterior, pulvinar inferior, pulvinar medial nucleus than SK residents. *p < 0.05, **p < 0.01, ***p < 0.001. AV, Anteroventral; LD, Laterodorsal; LP, Lateral posterior; VA, Ventral anterior; VAmc, Ventral anterior magnocellular; VLa, Ventral lateral anterior; VLp, Ventral lateral posterior; VPL, Ventral posterolateral; VM, Ventromedial; CeM, Central medial; CL, Central lateral; Pc, Paracentral; CM, Centromedian; Pf, Parafascicular; Pt, Paratenial; MV-re, Reuniens (medial ventral); MDm, Mediodorsal medial magnocellular; MDl, Mediodorsal lateral parvocellular; LGN, Lateral geniculate; MGN, Medial Geniculate; L-sg, Limitans (suprageniculate); PuA, Pulvinar anterior; PuM, Pulvinar medial; PuL, Pulvinar lateral; PuI: Pulvinar inferior.
In the additional analysis with grouping nuclei into anterior, lateral, ventral, intralaminar, medial, and posterior groups of nuclei, NK refugees showed greater GM volumes in the left medial group (p = 0.02) and right medial (p = 0.03), posterior group (p < 0.001) after controlling ICV than SK residents (Figure 3). After additional adjustment for age, sex, psychotropic medication and psychiatric disorders, the differences in GM volumes in the left medial group (p = 0.01) and right medial (p = 0.02), posterior group (p < 0.001) remained statistically significant. With Bonferroni corrected p-value, NK refugees showed greater GM volumes in the posterior group (p = 0.006) after controlling ICV, age, sex, psychotropic medication, and psychiatric disorders than SK residents. There were no significant between group differences in the left medial group (p = 0.06) and right medial (p = 0.10) with Bonferroni correction.”
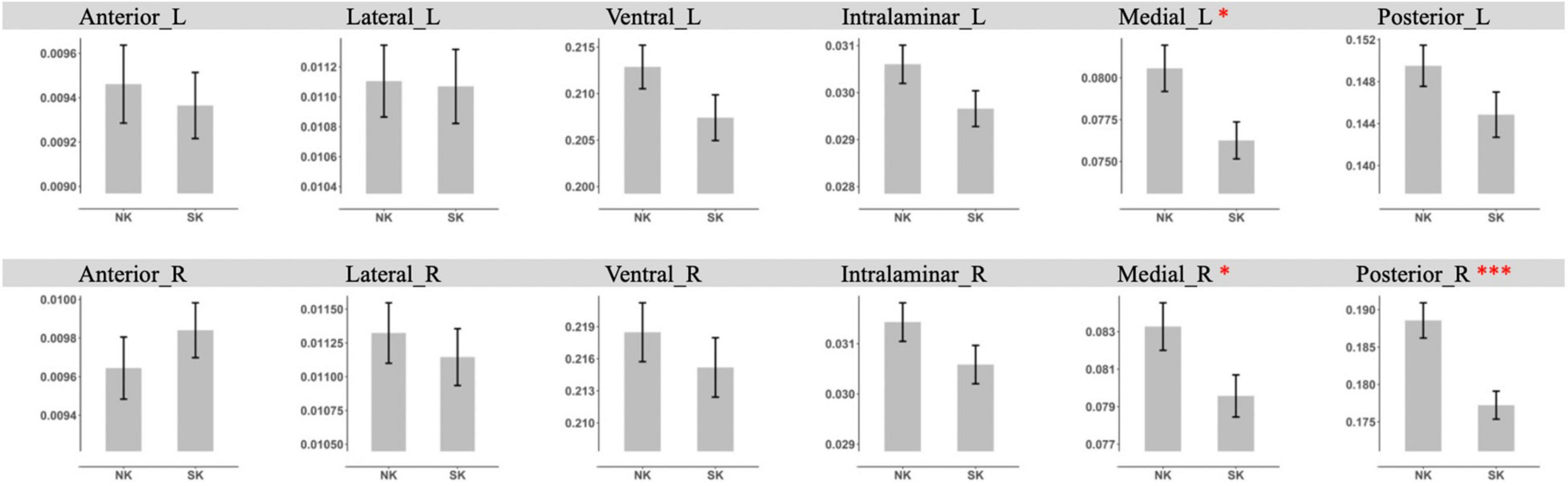
Figure 3. Comparison of gray matter volume of thalamic nuclei group between NK refugees and SK residents in additional analysis using Freesurfer. NK refugees showed greater GM volumes in the left medial group and right medial, posterior than SK residents. *p < 0.05, **p < 0.01, ***p < 0.001.
Relationships Between Thalamic Gray Matter Volume Ratios and Clinical Variables
In the NK refugees, the GM volume ratio in the left medial nuclei was negatively correlated with the BDI score after adjustment for age, sex and the number of traumatic experiences [r = −0.43, p = 0.009) (Figure 4A)]. This correlation between the GM volume ratio in the left medial nuclei and the BDI score remained statistically significant despite additional adjustment for psychotropic medication and psychiatric disorders (r = −0.43, p = 0.02). Moreover, the GM volume ratio in the right medial-posterior nuclei was significantly correlated with the BDI score after adjustment for age, sex, and the number of traumatic experiences (r = −0.33, p = 0.049) (Figure 4B)]. However, this correlation between the GM volume ratio in the right medial-posterior nuclei and the BDI score did not remain statistically significant after additional adjustment for psychotropic medication and psychiatric disorders. In contrast to the findings in the NK refugees, the GM volume ratios in both clusters were not significantly correlated with any clinical variables in the SK residents.
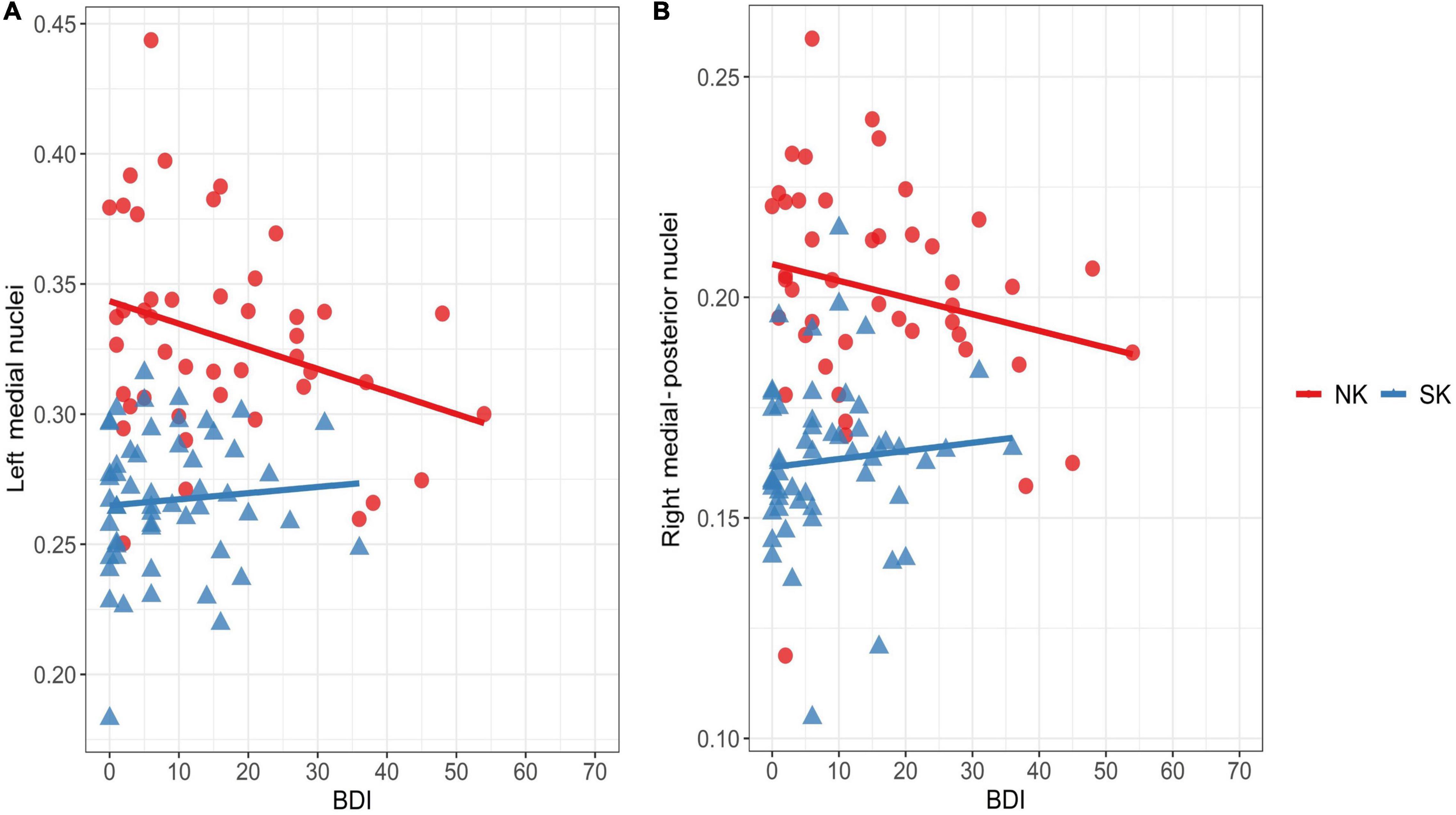
Figure 4. Correlations between depressive symptoms and thalamic areas showing increased gray matter volume in the NK refugees (red) SK residents (blue). Correlations are shown between the BDI score and gray matter volume ratio in the left medial nuclei (A) and right medial-posterior nuclei (B) is shown above. In the NK refugees alone, gray matter volume ratios in the left medial nuclei (r = –0.43, p = 0.009) and right medial-posterior nuclei (r = –0.33, p = 0.049) were negatively correlated with the BDI score after adjustment for age, sex, and the number of traumatic experiences. NK, North Korean; SK, South Korean; BDI, Beck Depression Inventory.
Discussion
The present study demonstrated structural differences in thalamic nuclei between NK refugees and SK residents. Specifically, NK refugees exhibited greater thalamic GM volume ratios in the right medial-posterior nuclei and left medial nuclei compared with SK residents. Additionally, greater GM volume ratios in both clusters were associated with less severe depression in NK refugees, but not in SK residents.
Consistent with our hypothesis, GM volumes in specific thalamic nuclei differed between NK refugees and SK residents. Thalamic structure and function are reportedly affected by genetic (36) and environmental factors (37). The populations of NK and SK share an ethnic background, language, history, and culture. Exposure to trauma, which is much more common in NK refugees, may therefore play an important role in their thalamic alterations.
In the current study, NK refugees had increased GM volume in thalamic nuclei. Previous studies have suggested that increased thalamic volume is associated with resilience to traumatic experiences. An association between reduced thalamic volume and increased social avoidance has been observed in mice experiencing social defeat stress (38). Reduced thalamic volume is reportedly associated with the frequency and duration of re-experience of trauma in traumatized humans (39). Thalamic volume is also reportedly associated with childhood maltreatment among high school students and exposure to trauma among deployed soldiers (15, 40). While reduced thalamic volume is reportedly associated with psychological disturbances after trauma, increased thalamic GM volume might reflect neurobiological resilience to trauma.
Consistent with this notion of resilience, the current study found that NK refugees with fewer depressive symptoms tended to have increased thalamic GM volume. Many previous studies have also reported a relationship between depression and thalamus. Reduced volume and shape deformation have been reported in patients with depressive symptoms (9–11). Thalamic structural changes in patients with depressive symptoms were reported to recover after remission of depression (41). As brain structural changes have been reported to represent resilience to traumatic experiences by reducing psychiatric symptoms (26, 27, 42), increased thalamic GM volume is associated with fewer depressive symptoms in NK refugees.
In the present study, the NK refugees were more depressed, but had increased thalamic GM volume, compared with SK residents. The association was significant after controlling ICV, age, sex, and the number of traumatic experiences. This appears counterintuitive because greater depression in NK refugees was correlated with a “reduction” of thalamic GM volume. The increased thalamic GM volume in NK refugees (compared with SK residents) may either be a compensatory mechanism to prevent depression after trauma or a pre-existing factor protecting against depression before trauma. However, because this study used a cross-sectional design, the causal relationship between thalamic changes and trauma remains unclear. Because thalamic volume has been reported to predict the treatment response of patients with major depressive disorder (43), NK refugees with greater thalamic GM volume may be more resistant to the development or progression of depression compared with NK refugees, who have smaller thalamic GM volumes. Despite their higher depression levels, our NK refugees had greater thalamic GM volume than SK residents; therefore, a compensatory mechanism after trauma appears more plausible. The lack of a correlation between depression and thalamus in SK residents (in contrast to NK refugees) also supports the existence of a compensatory mechanism after trauma. Because thalamic structure has been reported to change after trauma (39), long-term and repeated trauma among NK refugees may induce the compensatory thalamic changes, while in non-traumatized SK residents, no such compensatory alteration is required.
In the present study, the NK refugees had increased thalamic GM volumes in the right medial-posterior nuclei and left medial nuclei. Posterior group includes the pulvinar and the geniculate nuclei, where the additional analysis showed increased GM volumes in the NK refugees. The pulvinar nucleus has been identified as a key structure for modulating both emotional and attentional processes (44, 45). The pulvinar nucleus is also considered important for fearful stimuli processing (5, 46). The recruitment of a subcortical pathway from the pulvinar nucleus to the amygdala facilitates fear recognition (47, 48). In particular, explicit fear processing elicits greater activation of the pulvinar nucleus, which suggests the importance of visual attention during explicit fear processing (49). Additionally, the medial geniculate nucleus has been identified as a critical component for fear conditioning (50, 51). It is also involved in emotional behavior by projecting to several subcortical areas (52). The lateral geniculate (53) and suprageniculate nuclei (54) have also been suggested to participate in fear processing. The lateral geniculate nucleus is involved in selective attention (55), whereas the suprageniculate nucleus is involved in emotional processing (56). Based on previous studies, we can speculate that the posterior nuclei play a crucial role in enhancing regulation of fear and emotion after trauma exposure. Consistent with previous findings regarding an association between depression and structural deficits in the posterior nuclei (57, 58), reduced GM volume in the right posterior nuclei in our traumatized NK refugees was associated with severer depressive symptoms.
The medial group includes the mediodorsal and the reuniens nuclei, where NK refugees showed greater GM volumes than SK residents, although the statistical significance is not enough for Bonferroni correction for multiple comparison. The mediodorsal nucleus plays a role in emotional processing and fear extinction through connections to limbic circuitry (59, 60). The reuniens nucleus, as a part of the medial group, also mediates contextual fear conditioning and promotes resilience to depression (61, 62). The current study revealed increased thalamic GM volumes in the bilateral medial nuclei of NK refugees. Additionally, the increased thalamic GM volumes in the bilateral medial nuclei were associated with decreased depression. The increased medial nuclei volumes might also reflect neurobiological resilience to trauma and serve as a compensatory mechanism for depression through modulation of emotion and fear after trauma.
To our knowledge, this is the first study to investigate thalamic GM volume changes in traumatized refugees. However, this study had several limitations. Various trauma characteristics (e.g., severity, duration, and time since trauma) were not evaluated in detail. The chronic and repetitive nature of the traumas experienced by refugees makes it difficult to assess these features. We attempted to address this limitation by counting the number of traumatic experiences to estimate trauma burden. Second, because the study used a cross-sectional design, causal relationships could not be determined. The retrospective nature of cross-sectional studies is also vulnerable to recall bias and decreases the reliability of the results. Longitudinal analysis may be needed to clarify specific causal relationships without recall bias. Third, handedness could not be explored, as handedness data were not present for some participants. Although some previous studies reported the absence of the associations between thalamic GM volumes and handedness (63, 64), handedness should be considered in future studies. Another limitation of the present study is that it included SK residents, rather than NK residents, as a control group. Generally, it is not possible to recruit people currently residing in North Korea, and non-traumatized NK refugees are very rare. Because SK residents share a similar genetic and historical background with NK residents, we considered SK residents to be the best alternative control group.
Conclusion
In conclusion, our study demonstrated that NK refugees had increased thalamic GM volumes in right medial-posterior nuclei and left medial nuclei. Increased GM volumes in these nuclei were associated with reduced depression in NK refugees. These results suggest that the medial and posterior thalamic nuclei might play a role in neurobiological resilience after trauma through enhanced emotional regulation.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Seoul National University Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JJ, SY, SL, YL, and SK: conceptualization. JiL, NK, and HJ: methodology and data curation. JoL, YL, and SK: validation and writing – review and editing. JiL and NK: formal analysis. SK: investigation and funding acquisition. JJ, SY, and SL: resources. JiL: writing – original draft preparation. NK: visualization. YL and SK: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (Nos. 2020R1F1A1049200 and 2016R1A2B4011561), the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2020M3E5D9080561), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, South Korea (No. HR21C0885).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Burra N, Hervais-Adelman A, Celeghin A, de Gelder B, Pegna AJ. Affective blindsight relies on low spatial frequencies. Neuropsychologia. (2019) 128:44–9. doi: 10.1016/j.neuropsychologia.2017.10.009
2. Maddox SA, Hartmann J, Ross RA, Ressler KJ. Deconstructing the gestalt: mechanisms of fear, threat, and trauma memory encoding. Neuron. (2019) 102:60–74. doi: 10.1016/j.neuron.2019.03.017
3. Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature. (2015) 519:455–9. doi: 10.1038/nature13978
4. Baek J, Lee S, Cho T, Kim S-W, Kim M, Yoon Y, et al. Neural circuits underlying a psychotherapeutic regimen for fear disorders. Nature. (2019) 566:339–43. doi: 10.1038/s41586-019-0931-y
5. Yoshii T. The role of the thalamus in post-traumatic stress disorder. Int J Mol Sci. (2021) 22:1730. doi: 10.3390/ijms22041730
6. Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. (2010) 30:7466–72. doi: 10.1523/JNEUROSCI.0859-10.2010
7. Chan D, Cheadle AD, Reiber G, Unützer J, Chaney EF. Health care utilization and its costs for depressed veterans with and without comorbid PTSD symptoms. Psychiatr Serv. (2009) 60:1612–7. doi: 10.1176/ps.2009.60.12.1612
8. Vitriol V, Cancino A, Weil K, Salgado C, Asenjo MA, Potthoff S. Depression and psychological trauma: an overview integrating current research and specific evidence of studies in the treatment of depression in public mental health services in chile. Depress Res Treat. (2014) 2014:608671. doi: 10.1155/2014/608671
9. Nugent AC, Davis RM, Zarate CA, Drevets WC. Reduced thalamic volumes in major depressive disorder. Psychiatry Res Neuroimaging. (2013) 213:179–85. doi: 10.1016/j.pscychresns.2013.05.004
10. Espinoza Oyarce DA, Shaw ME, Alateeq K, Cherbuin N. Volumetric brain differences in clinical depression in association with anxiety: a systematic review with meta-analysis. J Psychiatry Neurosci. (2020) 45:406–29. doi: 10.1503/jpn.190156
11. Lu Y, Liang H, Han D, Mo Y, Li Z, Cheng Y, et al. The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. Neuroimage Clin. (2016) 11:658–66. doi: 10.1016/j.nicl.2016.04.008
12. Shang J, Fu Y, Ren Z, Zhang T, Du M, Gong Q, et al. The common traits of the ACC and PFC in anxiety disorders in the DSM-5: meta-analysis of voxel-based morphometry studies. PLoS One. (2014) 9:e93432. doi: 10.1371/journal.pone.0093432
13. van Tol M-J, van der Wee NJA, van den Heuvel OA, Nielen MMA, Demenescu LR, Aleman A, et al. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. (2010) 67:1002–11. doi: 10.1001/archgenpsychiatry.2010.121
14. Chen Y, Fu K, Feng C, Tang L, Zhang J, Huan Y, et al. Different regional gray matter loss in recent onset PTSD and non PTSD after a single prolonged trauma exposure. PLoS One. (2012) 7:e48298. doi: 10.1371/journal.pone.0048298
15. Liao M, Yang F, Zhang Y, He Z, Song M, Jiang T, et al. Childhood maltreatment is associated with larger left thalamic gray matter volume in adolescents with generalized anxiety disorder. PLoS One. (2013) 8:e71898. doi: 10.1371/journal.pone.0071898
16. Bruno F, Splendiani A, Tommasino E, Conson M, Quarantelli M, Saporito G, et al. Multimodal MRI assessment of thalamic structural changes in earthquake survivors. Diagnostics. (2021) 11:70. doi: 10.3390/diagnostics11010070
17. Lee J-H, Latchoumane C-FV, Park J, Kim J, Jeong J, Lee K-H, et al. The rostroventral part of the thalamic reticular nucleus modulates fear extinction. Nat Commun. (2019) 10:4637. doi: 10.1038/s41467-019-12496-9
18. Bengoetxea X, Goedecke L, Blaesse P, Pape H-C, Jüngling K. The μ-opioid system in midline thalamic nuclei modulates defence strategies towards a conditioned fear stimulus in male mice. J Psychopharmacol (Oxf). (2020) 34:1280–8. doi: 10.1177/0269881120940919
19. Quet E, Majchrzak M, Cosquer B, Morvan T, Wolff M, Cassel J-C, et al. The reuniens and rhomboid nuclei are necessary for contextual fear memory persistence in rats. Brain Struct Funct. (2020) 225:955–68. doi: 10.1007/s00429-020-02048-z
20. Hollifield M, Warner TD, Lian N, Krakow B, Jenkins JH, Kesler J, et al. Measuring trauma and health status in refugees: a critical review. JAMA. (2002) 288:611. doi: 10.1001/jama.288.5.611
21. Bryant RA, Edwards B, Creamer M, O’Donnell M, Forbes D, Felmingham KL, et al. A population study of prolonged grief in refugees. Epidemiol Psychiatr Sci. (2020) 29:e44. doi: 10.1017/S2045796019000386
22. Regev S, Slonim-Nevo V. Trauma and mental health in Darfuri asylum seekers: the effect of trauma type and the mediating role of interpersonal sensitivity. J Affect Disord. (2019) 246:201–8. doi: 10.1016/j.jad.2018.12.024
23. Lee CW, Lee J, Jun JY, Lee SH, Yu SY, Park J, et al. Associations between defense mechanisms and life satisfaction among North Korean refugees. Ann Gen Psychiatry. (2021) 20:18. doi: 10.1186/s12991-021-00339-1
24. Jeon S, Lee J, Jun JY, Park YS, Cho J, Choi J, et al. The effectiveness of cognitive behavioral therapy on depressive symptoms in north Korean refugees. Psychiatry Investig. (2020) 17:681–7. doi: 10.30773/pi.2019.0134
25. Lee J, Jeon S, Kim S, Seo Y, Park J, Lee YJ, et al. Polysomnographic sleep and attentional deficits in traumatized North Korean Refugees. Nat Sci Sleep. (2021) 13:635–45. doi: 10.2147/NSS.S308968
26. Jeon S, Lee YJ, Park I, Kim N, Kim S, Jun JY, et al. Resting state functional connectivity of the thalamus in North Korean refugees with and without posttraumatic stress disorder. Sci Rep. (2020) 10:3194. doi: 10.1038/s41598-020-59815-5
27. Jeong H, Lee YJ, Kim N, Jeon S, Jun JY, Yoo SY, et al. Increased medial prefrontal cortical thickness and resilience to traumatic experiences in North Korean refugees. Sci Rep. (2021) 11:14910. doi: 10.1038/s41598-021-94452-6
28. Kim N, Park I, Lee YJ, Jeon S, Kim S, Lee KH, et al. Alexithymia and frontal–amygdala functional connectivity in North Korean refugees. Psychol Med. (2020) 50:334–41. doi: 10.1017/S0033291719000175
29. Lee H-K, Kim J, Hong S, Lee E-H, Taeg HS. Psychometric properties of the beck anxiety inventory in the community-dwelling sample of Korean adults. Korean J Clin Psychol. (2016) 35:822–30. doi: 10.1007/s10823-016-9302-4
30. Sung HM, Kim J-B, Park Y-N, Bai D, Lee S-H, Ahn H-NA. Study on the reliability and the validity of Korean version of the beck depression inventory-II(BDI-II). J Korean Soc Biol Ther Psychiatry. (2008) 14:201–12.
31. Lim H-K, Woo J-M, Kim T-S, Kim T-H, Choi K-S, Chung S-K, et al. Reliability and validity of the Korean version of the impact of event scale-revised. Compr Psychiatry. (2009) 50:385–90. doi: 10.1016/j.comppsych.2008.09.011
32. Jeong HJ, Jo H, Jeong D, Lim S, Kim J, Bae S, et al. Study on validity and development of the Korean version of beck anxiety inventory-sign language version (K-BAI-SL). J Disability Welfare. (2017) 35:279–301.
33. Park J, Jun JY, Lee YJ, Kim S, Lee S-H, Yoo SY, et al. The association between alexithymia and posttraumatic stress symptoms following multiple exposures to traumatic events in North Korean refugees. J Psychosom Res. (2015) 78:77–81. doi: 10.1016/j.jpsychores.2014.09.007
34. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. (2007) 38:95–113. doi: 10.1016/j.neuroimage.2007.07.007
35. Iglesias JE, Insausti R, Lerma-Usabiaga G, Bocchetta M, Van Leemput K, Greve DN, et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage. (2018) 183:314–26. doi: 10.1016/j.neuroimage.2018.08.012
36. Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. (2015) 54:57–75. doi: 10.1016/j.neubiorev.2015.01.013
37. Montero VM. C-fos induction in sensory pathways of rats exploring a novel complex environment: shifts of active thalamic reticular sectors by predominant sensory cues. Neuroscience. (1997) 76:1069–81. doi: 10.1016/s0306-4522(96)00417-4
38. Anacker C, Scholz J, O’Donnell KJ, Allemang-Grand R, Diorio J, Bagot RC, et al. Neuroanatomic differences associated with stress susceptibility and resilience. Biol Psychiatry. (2016) 79:840–9. doi: 10.1016/j.biopsych.2015.08.009
39. Mutluer T, Şar V, Kose-Demiray Ç, Arslan H, Tamer S, Inal S, et al. Lateralization of neurobiological response in adolescents with post-traumatic stress disorder related to severe childhood sexual abuse: the tri-modal reaction (T-MR) model of protection. J Trauma Dissociation. (2018) 19:108–25. doi: 10.1080/15299732.2017.1304489
40. Kühn S, Butler O, Willmund G, Wesemann U, Zimmermann P, Gallinat J. The brain at war: effects of stress on brain structure in soldiers deployed to a war zone. Transl Psychiatry. (2021) 11:247. doi: 10.1038/s41398-021-01356-0
41. Desmidt T, Andersson F, Brizard B, Cottier J-P, Patat F, Gissot V, et al. Cerebral blood flow velocity positively correlates with brain volumes in long-term remitted depression. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 81:243–9. doi: 10.1016/j.pnpbp.2017.09.018
42. Kim S, Lyoo I, Lee Y, Kim J, Sim M, Bae S, et al. Decreased cerebral blood flow of thalamus in PTSD patients as a strategy to reduce re-experience symptoms. Acta Psychiatr Scand. (2007) 116:145–53. doi: 10.1111/j.1600-0447.2006.00952.x
43. Batail JM, Coloigner J, Soulas M, Robert G, Barillot C, Drapier D. Structural abnormalities associated with poor outcome of a major depressive episode: the role of thalamus. Psychiatry Res Neuroimaging. (2020) 305:111158. doi: 10.1016/j.pscychresns.2020.111158
44. Arend I, Henik A, Okon-Singer H. Dissociating emotion and attention functions in the pulvinar nucleus of the thalamus. Neuropsychology. (2015) 29:191–6. doi: 10.1037/neu0000139
45. Gattass R, Soares JGM, Lima B. The role of the pulvinar in spatial visual attention. In: Gattass R, Soares JGM, Lima B editors. The Pulvinar Thalamic Nucleus of Non-Human Primates: Architectonic and Functional Subdivisions Advances in Anatomy, Embryology and Cell Biology. (Vol. 225), Cham: Springer International Publishing (2018). p. 57–60. doi: 10.1007/978-3-319-70046-5_12
46. Wei P, Liu N, Zhang Z, Liu X, Tang Y, He X, et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun. (2015) 6:6756.
47. McFadyen J, Mattingley JB, Garrido MI. An afferent white matter pathway from the pulvinar to the amygdala facilitates fear recognition. eLife. (2019) 8:e40766. doi: 10.7554/eLife.40766
48. Ward R, Calder AJ, Parker M, Arend I. Emotion recognition following human pulvinar damage. Neuropsychologia. (2007) 45:1973–8. doi: 10.1016/j.neuropsychologia.2006.09.017
49. Tao D, He Z, Lin Y, Liu C, Tao Q. Where does fear originate in the brain? A coordinate-based meta-analysis of explicit and implicit fear processing. Neuroimage. (2021) 227:117686. doi: 10.1016/j.neuroimage.2020.117686
50. Ferrara NC, Cullen PK, Pullins SP, Rotondo EK, Helmstetter FJ. Input from the medial geniculate nucleus modulates amygdala encoding of fear memory discrimination. Learn Mem. (2017) 24:414–21. doi: 10.1101/lm.044131.116
51. Orsini CA, Maren S. Glutamate receptors in the medial geniculate nucleus are necessary for expression and extinction of conditioned fear in rats. Neurobiol Learn Mem. (2009) 92:581–9. doi: 10.1016/j.nlm.2009.07.007
52. LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. (1984) 4:683–98. doi: 10.1523/JNEUROSCI.04-03-00683.1984
53. Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. (2006) 26:9264–71. doi: 10.1523/JNEUROSCI.1016-06.2006
54. Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. (1995) 15(3 Pt 2):2312–27. doi: 10.1523/JNEUROSCI.15-03-02312.1995
55. Kastner S, O’Connor DH, Fukui MM, Fehd HM, Herwig U, Pinsk MA. Functional imaging of the human lateral geniculate nucleus and pulvinar. J Neurophysiol. (2004) 91:438–48. doi: 10.1152/jn.00553.2003
56. Kim HF, Griggs WS, Hikosaka O. Long-term value memory in the primate posterior thalamus for fast automatic action. Curr Biol. (2020) 30:2901–11.e. doi: 10.1016/j.cub.2020.05.047
57. Tae W-S. Regional gray matter volume reduction associated with major depressive disorder: a voxel-based morphometry. Investig Magn Reson Imaging. (2015) 19:10. doi: 10.13104/imri.2015.19.1.10
58. Dorph-Petersen K-A, Caric D, Saghafi R, Zhang W, Sampson AR, Lewis DA. Volume and neuron number of the lateral geniculate nucleus in schizophrenia and mood disorders. Acta Neuropathol. (2009) 117:369–84. doi: 10.1007/s00401-008-0410-2
59. Timbie C, Barbas H. Pathways for emotions: specializations in the amygdalar. mediodorsal thalamic, and posterior orbitofrontal network. J Neurosci. (2015) 35:11976–87. doi: 10.1523/JNEUROSCI.2157-15.2015
60. Paydar A, Lee B, Gangadharan G, Lee S, Hwang EM, Shin H-S. Extrasynaptic GABAA receptors in mediodorsal thalamic nucleus modulate fear extinction learning. Mol Brain. (2014) 7:39. doi: 10.1186/1756-6606-7-39
61. Ramanathan KR, Maren S. Nucleus reuniens mediates the extinction of contextual fear conditioning. Behav Brain Res. (2019) 374:112114. doi: 10.1016/j.bbr.2019.112114
62. Kafetzopoulos V, Kokras N, Sotiropoulos I, Oliveira JF, Leite-Almeida H, Vasalou A, et al. The nucleus reuniens: a key node in the neurocircuitry of stress and depression. Mol Psychiatry. (2018) 23:579–86. doi: 10.1038/mp.2017.55
63. Jang H, Lee JY, Lee KI, Park KM. Are there differences in brain morphology according to handedness? Brain Behav. (2017) 7:e00730. doi: 10.1002/brb3.730
Keywords: thalamus, refugees, depression, trauma, magnetic resonance imaging
Citation: Lee J, Kim N, Jeong H, Jun JY, Yoo SY, Lee SH, Lee J, Lee YJ and Kim SJ (2022) Gray Matter Volume of Thalamic Nuclei in Traumatized North Korean Refugees. Front. Psychiatry 13:756202. doi: 10.3389/fpsyt.2022.756202
Received: 10 August 2021; Accepted: 08 April 2022;
Published: 28 April 2022.
Edited by:
Xenia Gonda, Semmelweis University, HungaryReviewed by:
Lin Shi, Capital Medical University, ChinaManojkumar Saranathan, University of Arizona, United States
Copyright © 2022 Lee, Kim, Jeong, Jun, Yoo, Lee, Lee, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seog Ju Kim, a3NqNzEyNkBza2t1LmVkdQ==
 Jiye Lee1
Jiye Lee1 Nambeom Kim
Nambeom Kim Hyunwoo Jeong
Hyunwoo Jeong Jin Yong Jun
Jin Yong Jun Jooyoung Lee
Jooyoung Lee Yu Jin Lee
Yu Jin Lee Seog Ju Kim
Seog Ju Kim