
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 09 May 2022
Sec. Computational Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.754165
Background: Obsessive–compulsive disorder (OCD) a complex neuropsychiatric disorder, is characterized by irresistible obsessive thinking and compulsive behavior. Folate is a member of water-soluble vitamins in the human body and sustains many normal daily activities (e.g., exercise, sleep, and memory). Homocysteine, a sulfur-containing non-essential amino acid, has been investigated in numerous psychiatric disorders (e.g., OCD). Vitamin B12 is a type of complex organic compound with cobalt contained. Moreover, vitamin B12 and folate deficiency and high levels of homocysteine were found to have an effect on brain functions and also lead to non-specific psychiatric symptoms.
Objectives: This study aimed to confirm the epidemiological evidence of OCD and investigate whether vitamin B12, folate, and homocysteine have an effect on the etiology of OCD.
Methods: A systematic search was conducted on eight databases (i.e., PubMed, Embase, Web of Science, the Cochrane Library, China Biology Medicine disc, China National Knowledge Infrastructure, Wanfang Database, China Science and Technology Journal Database), and the retrieval time was up to March 2021. The available articles involving patients with OCD with/without abnormal serum levels of vitamin B12, folate, and homocysteine were comprehensively reviewed and analyzed.
Results: A total of 5 studies involving 309 patients were included in this meta-analysis, including 172 cases in the experimental group and 137 in the control group. The content of folate in the OCD group was not significantly different from that in the control group (SMD = −0.089, 95%CI −0.755 to 0.577, p = 0.794). And serum homocysteine was significantly higher in the patients with OCD (SMD = 1.132, 95%CI 0.486 to 1.778, p = 0.001). Vitamin B12 was significantly lower in patients with OCD (SMD = −0.583, 95%CI −0.938 to −0.229, p = 0.001).
Conclusions: This meta-analysis shows serum high levels of homocysteine, low levels of vitamin B12, and normal folate level are closely correlated with OCD. However, high-quality case-control studies should be further conducted to explore the correlation between serum levels of vitamin B12, folate, homocysteine, and OCD.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021262161; PROSPERO (Number CRD#42021262161).
According to the latest revision of diagnostic criteria for OCD1 in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (1), compulsions can be observable behaviors. The prevalence rate of OCD in the populations ranges from 0.88 to 4% (2), and regional differences lead to a change in prevalence rate to a certain extent. The mean age at onset of OCD was 20.9 to 25.4 years, and the mean lifetime duration of the disease was 11.8 years. OCD is equally common in men and women, whereas it is more common in young people, divorced or separated people, and unemployed people (3). OCD is characterized by intrusive thoughts or images (obsessions), increased anxiety, and repetitive or ritualistic behaviors (compulsions) (4). According to some researches, there are abnormalities of Cortico-Striatal-Thalamic-Cortical dysfunction and impaired inhibition in patients with OCD (5).
A series of articles primarily demonstrated that a subset of the different forms of OCD is familial and thus might arise from genetic factors (6–8). A meta-analysis (9) including 37 groups of samples with 24,161 pairs of twin from 14 articles examined the heritability of obsessive-compulsive behaviors. Findings from this meta-analysis supported the hypothesis that genetic factor is critical for manifesting obsessive-compulsive behaviors. In addition, some researchers approved that OCD was because of the abnormal expression and function of some genes in the serotonergic and dopaminergic pathways (10). The “serotonergic hypothesis” (11), which proposed that OCD was primarily a disorder of the serotonin system, has received scientific support. The role of dopaminergic mechanisms in the manifestation of OCD has been confirmed (12–15). Furthermore, it is progressively confirmed that glutamate and GABA2 also have an effect on the occurrence of OCD (16–18). In addition, the caudate nucleus has been involved in the pathophysiologic process of OCD, and lots of studies reported hyperactivity of the bilateral caudate nucleus bilaterally in both the adult and pediatric patients (19–21). However, despite all these cognitive causes, the etiology of OCD is not completely clear.
Amounts of evidence support the cognitive behavioral therapy and selective serotonin reuptake inhibitors (SSRIs) have been as the treatment of OCD (22–26).
As revealed, homocysteine is correlated with both current depression and a history of depression, and a higher concentration of homocysteine might increase the risk of depression (27). Amounts of evidences show that folate and vitamin B12 are important nutrient elements involved in the physiological and pathological functions of the neuropsychiatric system (28–30). The incidence of neuropsychiatric findings in patients with vitamin B12 deficiency has been reported to be 4–50% (31). More recently, folate and vitamin B12 have been reported in a large amount of literature as treatments or supplements for the treatment of psychiatry disease (32–36). The interaction among folate, vitamin B12, and homocysteine, genetic factors, and metabolism of neurotransmitters have significance in patients with OCD (37–39). As the research on folate and its carbon unit metabolism, homocysteine and other psychiatric disorders has been progressed, researchers have been attempting to determine the correlation between them with OCD, whereas inconsistent results were achieved (37–40). Thus, this meta-analysis was conducted to investigate the epidemiological evidence of serum levels of folate, homocysteine, and vitamin B12 in OCD.
This meta-analysis was based on the statement of the Preferred Report Item for Systematic Reviews and Meta-analysis (PRISMA) (41). A systematic search was conducted on eight databases (i.e., PubMed, Embase, Web of Science, the Cochrane Library, CBM, CNKI, WANFANG DATA, China Science and Technology Journal Database). The search strategy included the search terms: (1) “OCD” or “obsessive-compulsive” or “Obsessive-Compulsive Neuroses” or “Anankastic Personality”; (2) “Vitamin M” or “Vitamin B9” or “Pteroylglutamic Acid” or “Folic Acid, Monopotassium Salt” or “Folic Acid,” (DL)-Isomer” or “Folvite” or “Folacin” or “Folate”; (3) “Homocysteine” or “2-amino-4-mercaptobutyric acid” or “L-Isomer Homocysteine”; (4) “Vitamin B12” or “Cyanocobalamin” or “Cobalamin” or “Eritron.” Date search was limited from inception to 1 March 2021. All the abstracts were evaluated in accordance with the inclusion and exclusion criteria to verify the suitability for meta-analysis, and articles were added by screening references of included articles conditionally (Table 1).
Original articles matching the following eligibility criteria were included: (1) cross-sectional, case-control, or cohort study design; (2) involving a sample of patients with clinically diagnosed OCD, with/without a comparison group (at least 10 people in each group); (3) reported vitamin B12, folate, and homocysteine as outcomes and diagnosed with the Structured Clinical Interview for DSM-4/DSM-5; (4) provided sufficient raw data for calculation; (5) had clear inclusion and exclusion criteria (intellectual disability, growth disorders, psychotic disorders, substance and alcohol abuse, history of endocrine disorders and pregnant or breastfeeding patients or more than 60 years old, taking supplements or drugs in recent 1 year, not have a history of major mood disorder, dementia, intellectual disability, or psychosis in their first-degree relatives) for participants; (6) published in English or Chinese. The exclusion criteria included: (1) reviews, letters, case reports, or conference abstracts; (2) duplicated or overlapping data. All sifting process was conducted by two researchers (i.e., SiRui Yan and Hailong Liu) independently. Their respective evaluation results were compared, and any discrepancies were resolved by discussion.
In total, three independent researchers (i.e., SiRui Yan, Hailong Liu and Yaqiong Yu) extracted the information from eligible articles. Baseline data extracted from the respective manuscripts and supplements are presented later: the first name of the author, year of publication, country, ethnicity, study design, sample size, mean age, gender ratio, comparison group, diagnostic scales, and also diagnostic criteria. To accurately evaluate the methodological quality of eligible articles, three researchers (i.e., SiRui Yan and Yaqiong Yu and Nashu Han) employed the Newcastle-Ottawa Scale (NOS) (42) for the evaluation. Article quality was evaluated on the aspects of the participant selection, comparability, outcome ascertainment, and data processing. Scale score ≥7 was identified as high quality, moderate quality = 4–7, while a score below 4 was classified as poor quality.
The odds ratio (ORs) for dichotomous data was estimated, and standard mean differences (SMDs) for continuous data with the corresponding 95% CIs were also estimated. The chi-squared test and I2 statistic were performed to identify heterogeneity among articles. Under insignificant heterogeneity (I2 <50% or p > 0.1), a fixed-effect model was used to estimate the pooled effect size; otherwise, the random-effect model was used. To determine potential sources of heterogeneity between articles, sensitivity analysis, and meta-regression were conducted using prespecified variables (e.g., study design, ethnicity of the participants, outcome measurement tool). The Egger's test and Begg's test were performed to detect publication bias. Furthermore, all the data synthesis and analysis were conducted using Stata version 11.0 (StataCorp, College Station, TX, USA).
A total of 1,426 records were found in the initial search strategy (PubMed 1229, Embase 165, Web of Science 20, the Cochrane Library 5, CBM 4, CNKI 3, WanFang Data 0, Technology Journal Database 0), and 127 duplicates were removed. A total of 1,299 records were excluded for irrelevant themes or unsuitable types of articles after the titles and the abstracts were screened. In accordance with the eligibility criteria, 40 articles were excluded through a careful review of the full texts. In summary, 5 articles involving 172 cases of OCD were selected for the meta-analysis (Figure 1).
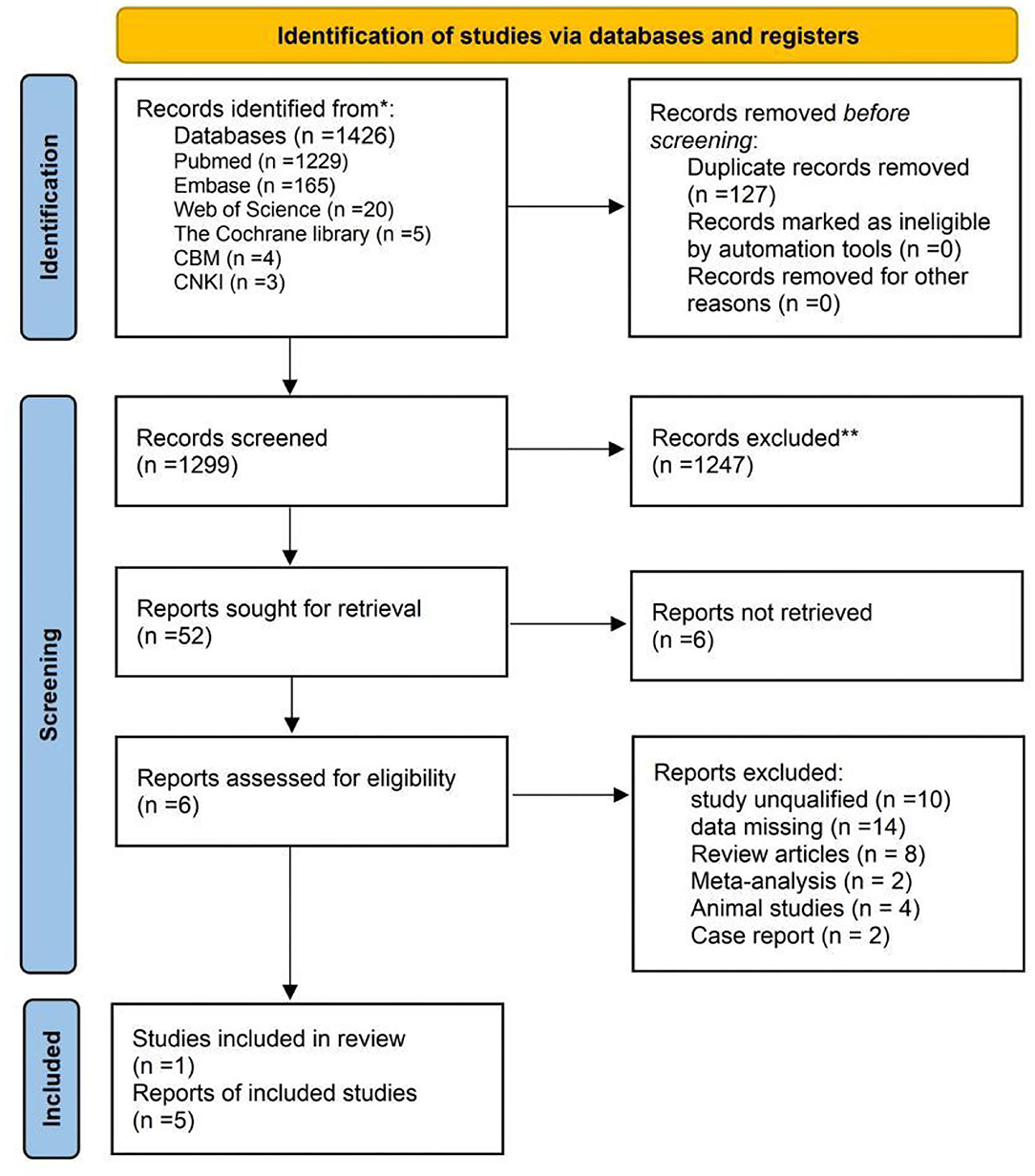
Figure 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
The last remaining 5 articles (37–40, 43) were included in this study. Table 2 lists an overview of the characteristics for 5 eligible studies, with 1 study only reporting the number of participants, and all of them were published in English. All the studies were case-control published from 1988 to 2021, 3 were conducted in Turkey (37–39), one in Iran (40) and one in Israel (43). All the cases of patients with OCD were recruited by the clinical hospitals. All of the participants were Caucasian. Sample sizes of participants ranged from 23 to 52 people, 309 in total, and men and women were represented similarly (172:137). Most of the mean age of patients with OCD ranged from 12.4 to 44.5 years. The control groups in the 5 articles were all healthy people. A total of 309, 185, and 110 participants were included about folate (172 in OCD group and 137 in control group), homocysteine (110 in OCD group and 75 in control group), and vitamin B12 (149 in OCD group and 114 in control group), respectively. Samples in all 5 studies were venous blood after overnight, and 2 studies reported the methods used to analyze the samples (37, 38), while 3 did not (39, 40, 43). Fasting Scores of the NOS or the AHRQ-11 in most articles (n = 4) reached 6 or higher (37–40), suggesting an overall good/moderate quality of the included articles (Table 3).
Three clinical diagnostic criteria, i.e., DSM-5 (The Diagnostic and Statistical Manual of Intellectual Disability, 5th ed), DSM-4, and DSM-4-TR, were employed separately in included articles to evaluate anxiety disorder. On the whole, 7 different validated screening scales were used for evaluating obsessive symptoms. The Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) is the most common tool. Furthermore, there was a problem that thresholds adopted to screening scales might differ from each other in different articles when defining anxiety symptoms.
Data regarding serum levels of folate were provided in 4 studies with a total of 309 patients were analyzed (37–40, 43). The content of serum levels of folate in the OCD group was not significantly different from that in the control group (SMD = −0.089, 95% CI −0.755 to 0.577, p = 0.794, Figure 2) with obvious heterogeneity (p = 0.000, I2 = 87.6%, Figure 2).
Data regarding serum levels of homocysteine provided in 3 studies with a total of 185 patients were analyzed (37–39). The content of serum levels of homocysteine in the OCD group was not significantly different from that in the control group (SMD = 1.132, 95% CI 0.486 to 1.778, p = 0.001, Figure 3) with significant heterogeneity (p = 0.020, I2 = 74.3%, Figure 3).
Data regarding serum levels of vitamin B12 provided in 4 studies with a total of 110 patients were analyzed (38–40, 43). The content of serum levels of vitamin B12 in the OCD group was significantly lower than that in the control group (SMD = −0.583, 95% CI−0.938 to−0.229, p = 0.001, Figure 4) and there was no obvious heterogeneity (p = 0.117, I2 = 49.1%, Figure 4).
There was no obvious influence of one individual study on the pooled SMDs of serum levels of folate, vitamin B12, and homocysteine in groups (Figures 5–7).
Obsessive–compulsive disorder is a complex neuropsychiatric disorder with not fully elucidated etiology (6, 7, 11, 21). Studies have shown that anomalous changes in serum levels of vitamin B12, folate, and homocysteine may contribute to the development of OCD (37–40). In this meta-analysis, we found a statistically significant higher homocysteine level and lower concentration of B12 vitamins in patients with OCD. This result is in line with most of the findings (37–40). However, there was not a statistically significant correlation between folate deficiency and OCD. This is consistent with the results of the 3 studies (38, 40, 43) and contradicts the results of the other 2 studies (37, 39). Although there were differences in the content of serum levels of vitamin B12 and homocysteine between the patients with OCD and normal individuals, whether these potential influences could be proved in the future by well-designed RCTs is still unknown.
The synthesis of methionine from homocysteine requires a supply of methyl groups from methyl folate. Folate and vitamin B12 are required in the synthesis of SAM, the sole methyl donor in numerous methylation reactions involving proteins, phospholipids, and biogenic amines. In the case of folate or vitamin B deficiency, the S-adenosylmethionine synthesis may become severely impaired, which might be the cause of many neuropsychiatries (28, 44–46). Reduced synthesis of S-adenosylmethionine could be due to a hypo-methylation state, leading in turn to impaired synthesis of proteins and neurotransmitters necessary for the brain structural integrity, and OCD was further induced (47). Decreased folate levels will elevate homocysteine levels. In addition, the methyl producing is indirectly involved in nucleotide, DNA, and RNA synthesis and is critical for many genomic and non-genomic methylation reactions. Disruption of methylation (single carbon transfer) responses leads to the production of monoamine neurotransmitters, phospholipids, and nucleotides in the central nervous system, which might be a pathologic mechanism for depression. In addition, folate deficiency may help mediate some of its neuropsychiatric complications by generating elevated levels of Sadenosyl-homocysteine, which broadly inhibits methylation reactions, and possibly exert direct excitotoxic effects via activity at the N-methyl-D-aspartate glutamate receptors (48, 49). Furthermore, this hypomethylation state can lead to impaired synthesis of proteins and neurotransmitters required for the brain's structural integrity (47, 50).
Observations on the antidepressant effects of folate supplementation may suggest the effect of these nutrients in psychopathology (51, 52). Folate also considerably influences the rate of synthesis of tetrahydrobiopterin (53–55), a cofactor in the hydroxylation of phenylalanine and tryptophan, rate-limiting steps in the biosynthesis of dopamine, norepinephrine, and serotonin, neurotransmitters postulated to impact the monoamine hypothesis of affective disorders. In addition, there is a study supporting that genetic modifications affecting the MTFHR level or function decreases the 5-methyltetrahydrofolate level further results in increased homocysteine (49, 56, 57). There are many meta-analyses in the literature that reveal the correlation between psychiatric diseases and MTHFR gene polymorphisms (58–61). It is also a non-negligible reason for OCD. SAM3 and methyl folate have been shown to exert stronger antidepressant effects than placebos when administered in parenteral and certain oral forms, and are even more effective than tricyclic antidepressants (62–64). Total plasma homocysteine can be as a sensitive marker of functional deficiency of folate and vitamin B12 (65, 66). Total homocysteine level changes have also been suggested to be correlated with numerous psychiatric disorders (e.g., schizophrenia and affective disorders) (61, 67–72). Also, some literature reported neuropsychiatric symptoms in patients with vitamin B12 deficiency, including ataxia, mania, hallucinations, memory loss, depression, paresthesias, proprioception loss, delirium, dementia, personality change, and abnormal behavior (73–76). As mentioned earlier, folate and vitamin B12 deficiencies inhibit the methylation reactions, as well as elevate homocysteine levels, which results in a drop in neurotransmitter levels and affects other biochemical pathways in the cell to varying adversely degrees (77, 78).
This meta-analysis has the following limitations that should be considered: (1) some articles' measures data are not distributed normally and were not reported in the form of median and quartile, and therefore, they could not be included in the meta-analysis; (2) only English and Chinese language reports have been searched and maybe consequently leads to missing data from other vital high-quality articles published in other languages; (3) since all the included studies were retrospective with small sample sizes, high-quality RCTs with large sample is lacking; (4) the results were based on unadjusted estimates, more accurate outcomes would be achieved due to adjustments for other confounding factors (e.g., gender, age, body mass index, and lifestyle); (5) some factors had not been taken consider into study, e.g., renal function of participants, dietary habits changes correlated with symptoms in patients with OCD, reduced ultraviolet exposure required for vitamin D synthesis; (6) one set of trails from the last century has not mentioned the details about the diagnosis and detection methods (43); (7) most of the participants in 1 study were women (40); (8) there are also some differences in the laboratory examination methods for each index in each group due to time. Various factors aforementioned may contribute to the heterogeneity of the data. In the future, more standardized patients should be involved and the principle of randomization and double blindness should be strictly followed to achieve the higher experimental effect.
In conclusion, this meta-analysis found that OCD might be correlated with a low level of vitamin B12 and high level of homocysteine, whereas the sample here was too small to conclude that this change might be a vital biological indicator for OCD. Abnormal serum levels of vitamin B12 and homocysteine expression might be a risk factor for the development of OCD. Clinicians should be vigilant about serum levels of vitamin B12 and homocysteine in patients with OCD, which should be investigated in more in-depth and high-quality studies. These findings can provide a starting point for further research.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
SY: conceptualization, methodology, article selection, data extraction, data analysis, quality assessment, and writing—original draft preparation. HL and YY: data extraction and quality assessment. NH: quality assessment. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank everyone who contributed to the article.
1. ^OCD, obsessive-compulsive disorder.
2. ^GABA, γ-aminobutyric acid.
3. ^SAM, S-adenosylmethionine.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5 (Vol. 5). Washington, DC: American Psychiatric Association (2013).
2. Nazeer A, Latif F, Mondal A, Azeem MW, Greydanus DE. Obsessive-compulsive disorder in children and adolescents: epidemiology, diagnosis and management. Transl. Pediatr. (2020) 9:S76. doi: 10.21037/tp.2019.10.02
3. Fontenelle LF, Mendlowicz MV, Versiani M. The descriptive epidemiology of obsessive-compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. (2006) 30:327–37. doi: 10.1016/j.pnpbp.2005.11.001
4. Salkovskis PM. Understanding and treating obsessive-compulsive disorder. Behav Res Ther. (1999) 37:S29–52. doi: 10.1016/S0005-7967(99)00049-2
5. Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological deficits in obsessive-compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch Gen Psychiatry. (1998) 55:415–23. doi: 10.1001/archpsyc.55.5.415
6. Nestadt G, Samuels J, Riddle M, Bienvenu OJ, Liang KY, LaBuda M, et al. A family study of obsessive-compulsive disorder. Arch Gen Psychiatry. (2000) 57:358–63. doi: 10.1001/archpsyc.57.4.358
7. Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology. (2004) 174:530–8. doi: 10.1007/s00213-004-1847-1
8. Shugart YY, Samuels J, Willour VL, Grados MA, Greenberg BD, Knowles JA, et al. Genomewide linkage scan for obsessive-compulsive disorder: evidence for susceptibility loci on chromosomes 3q, 7p, 1q, 15q, and 6q. Mol Psychiatry. (2006) 11:763–70. doi: 10.1038/sj.mp.4001847
9. Taylor S. Etiology of obsessions and compulsions: a meta-analysis and narrative review of twin studies. Clin Psychol Rev. (2011) 31:1361–72. doi: 10.1016/j.cpr.2011.09.008
10. Pauls DL. The genetics of obsessive-compulsive disorder: a review. Dialogues Clin Neurosci. (2010) 12:149. doi: 10.31887/DCNS.2010.12.2/dpauls
11. Zohar J, Greenberg B, Denys D. Obsessive-Compulsive Disorder. Amsterdam: Elsevier Science Publishers BV (2012).
12. Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. (2013) 7:201. doi: 10.3389/fnins.2013.00201
13. Denys D, van der Wee N, Janssen J, De Geus F, Westenberg HG. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biol Psychiatry. (2004) 55:1041–5. doi: 10.1016/j.biopsych.2004.01.023
14. Moresco RM, Pietra L, Henin M, Panzacchi A, Locatelli M, Bonaldi L, et al. Fluvoxamine treatment and D2 receptors: a pet study on OCD drug-naive patients. Neuropsychopharmacology. (2007) 32:197–205. doi: 10.1038/sj.npp.1301199
15. Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, et al. In vivo PET study of 5HT2A serotonin and D2 dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. (2008) 42:306–14. doi: 10.1016/j.neuroimage.2008.04.233
16. Ting JT, Feng G. Neurobiology of obsessive-compulsive disorder: insights into neural circuitry dysfunction through mouse genetics. Curr Opin Neurobiol. (2011) 21:842–8. doi: 10.1016/j.conb.2011.04.010
17. Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. (2007) 448:894–900. doi: 10.1038/nature06104
18. Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. (2006) 9:119–26. doi: 10.1038/nn1609
19. Bolton J, Moore GJ, MacMillan S, Stewart CM, Rosenberg DR. Case study: caudate glutamatergic changes with paroxetine persist after medication discontinuation in pediatric OCD. J Am Acad Child Adolesc Psychiatry. (2001) 40:903–6. doi: 10.1097/00004583-200108000-00011
20. Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. (2008) 32:525–49. doi: 10.1016/j.neubiorev.2007.09.005
21. Abramovitch A, Mittelman A, Henin A, Geller D. Neuroimaging and neuropsychological findings in pediatric obsessive-compulsive disorder: a review and developmental considerations. Neuropsychiatry. (2012) 2:313. doi: 10.2217/npy.12.40
22. Pallanti S, Quercioli L. Treatment-refractory obsessive-compulsive disorder: methodological issues, operational definitions and therapeutic lines. Prog Neuro-Psychopharmacol Biol Psychiatry. (2006) 30:400–12. doi: 10.1016/j.pnpbp.2005.11.028
23. Öst LG, Havnen A, Hansen B, Kvale G. Cognitive behavioral treatments of obsessive-compulsive disorder. A systematic review and meta-analysis of studies published 1993-2014 Clinical psychology review. (2015) 40:156–69. doi: 10.1016/j.cpr.2015.06.003
24. Skapinakis P, Caldwell DM, Hollingworth W, Bryden P, Fineberg NA, Salkovskis P, et al. Pharmacological and psychotherapeutic interventions for management of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry. (2016) 3:730–9. doi: 10.1016/S2215-0366(16)30069-4
25. Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB. Practice Guideline for the Treatment of Patients With Obsessive-Compulsive Disorder. New York, NY: American Psychiatric Association (2007).
26. Bandelow B, Zohar J, Hollander E, Kasper S, Möller HJ, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders-first revision. World J Biol Psychiatry. (2008) 9: 248–312. doi: 10.1080/15622970802465807
27. Almeida OP, McCaul K, Hankey GJ, Norman P, Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry. (2008) 65:1286–94. doi: 10.1001/archpsyc.65.11.1286
28. Bottiglieri T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev. (1996) 54:382–90. doi: 10.1111/j.1753-4887.1996.tb03851.x
29. Castillo-Lancellotti C, Margozzini P, Valdivia G, Padilla O, Uauy R, Rozowski J, et al. Serum folate, vitamin B12 and cognitive impairment in Chilean older adults. Public Health Nutr. (2015) 18:2600–8. doi: 10.1017/S1368980014003206
30. Engelborghs S, Vloeberghs E, Maertens K, Mariën P, Somers N, Symons A, et al. Correlations between cognitive, behavioural and psychological findings and levels of vitamin B12 and folate in patients with dementia. Int J Geriatr Psychiatry. (2004) 19:365–70. doi: 10.1002/gps.1092
31. Goebels N, Soyka M. Dementia associated with vitamin B12 deficiency: presentation of two cases and review of the literature. J Neuropsychiatry Clin Neurosci. (2000) 12:389–94. doi: 10.1176/jnp.12.3.389
32. Lazarou C, Kapsou M. The role of folic acid in prevention and treatment of depression: an overview of existing evidence and implications for practice. Complement Ther Clin Pract. (2010) 16:161–6. doi: 10.1016/j.ctcp.2010.01.003
33. Taylor MJ, Carney SM, Geddes J, Goodwin G. Folate for depressive disorders. Cochr Datab Syst Rev. (2003) 2:CD003390. doi: 10.1002/14651858.CD003390
34. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. (2005) 19:59–65. doi: 10.1177/0269881105048899
35. Tural Ü, Çorapçioglu A, Boşgelmez S, Köroglu G, Ünver H, Duman C, et al. Double blind controlled study of adding folic acid to fluoxetine in the treatment of OCD. Psychiatr Danub. (2019) 31:69–77. doi: 10.24869/psyd.2019.69
36. Reynolds EH. Neurometabolic approach to treatment-resistant depression. Br J Psychiatry. (2019) 215:568–568. doi: 10.1192/bjp.2019.170
37. Atmaca M, Tezcan E, Kuloglu M, Kirtas O, Ustundag B. Serum folate and homocysteine levels in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. (2005) 59:616–20. doi: 10.1111/j.1440-1819.2005.01425.x
38. Esnafoglu E, Yaman E. Vitamin B12, folic acid, homocysteine and vitamin D levels in children and adolescents with obsessive compulsive disorder. Psychiatry Res. (2017) 254:232–7. doi: 10.1016/j.psychres.2017.04.032
39. Türksoy N, Bilici R, Yalçiner A, Özdemir YÖ, Örnek I, Tufan AE, et al. Vitamin B12, folate, and homocysteine levels in patients with obsessive-compulsive disorder. Neuropsychiatr Dis Treat. (2014) 10:1671. doi: 10.2147/NDT.S67668
40. Eslamzadeh M, Kamrani M, Googheri Z, Ziaee M, Anahitaarabi. Comparison of Vitamin B12 and folic acid serum levels in patients with obsessive-compulsive disorder versus healthy group. Pak J Med Health Sci. (2020) 14:959–62. Available online at: https://pjmhsonline.com/2020/july-sep/959.pdf
41. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group*. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
42. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
43. Hermesh H, Weizman A, Shahar A, Munitz H. Vitamin B12 and folic acid serum levels in obsessive compulsive disorder. Acta Psychiatr Scand. (1988) 78:8–10. doi: 10.1111/j.1600-0447.1988.tb06294.x
44. Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. (2004) 24:105–31. doi: 10.1146/annurev.nutr.24.012003.132306
45. Carmel R. (2011). Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr. 94, 348S−358S. doi: 10.3945/ajcn.111.013441
46. Ersan S, Bakir S, Ersan EE, Dogan O. Examination of free radical metabolism and antioxidant defence system elements in patients with obsessive-compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. (2006) 30:1039–42. doi: 10.1016/j.pnpbp.2006.03.034
47. Smulders YM, Smith DEC, Kok RM, Teerlink T, Swinkels DW, Stehouwer CDA, et al. Cellular folate vitamer distribution during and after correction of vitamin B12 deficiency: a case for the methylfolate trap. Br J Haematol. (2006) 132:623–9. doi: 10.1111/j.1365-2141.2005.05913.x
48. Shaw PJ. Excitatory amino acid receptors, excitotoxicity, and the human nervous system. Curr Opin Neurol Neurosurg. (1993) 6:414–22.
49. Durga J, van Boxtel MP, Schouten EG, Bots ML, Kok FJ, Verhoef P. Folate and the methylenetetrahydrofolate reductase 677C → T mutation correlate with cognitive performance. Neurobiol Aging. (2006) 27:334–43. doi: 10.1016/j.neurobiolaging.2005.01.003
50. Surtees R, Leonard J, Austin S. Association of demyelination with deficiency of cerebrospinal-fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet. (1991) 338:1550–4. doi: 10.1016/0140-6736(91)92373-A
51. Albert U, Maina G, Ravizza L, Bogetto F. An exploratory study on obsessive-compulsive disorder with and without a familial component: are there any phenomenological differences? Psychopathology. (2002) 35:8-16. doi: 10.1159/000056210
52. Dar SA, Wani RA, Haq I. A comparative study of aripiprazole, olanzapine, and L-methylfolate augmentation in treatment resistant obsessive-compulsive disorder. Psychiatric Q. (2021) 92:1413–24. doi: 10.1007/s11126-021-09892-0
53. Bottiglieri T, Hyland K, Laundy M, Godfrey P, Carney MWP, Toone BK, et al. Folate deficiency, biopterin and monoamine metabolism in depression. Psychol Med. (1992) 22:871–6. doi: 10.1017/S0033291700038447
54. Araújo JR, Martel F, Borges N, Araújo JM, Keating E. Folates and aging: role in mild cognitive impairment, dementia and depression. Ageing Res Rev. (2015) 22:9–19. doi: 10.1016/j.arr.2015.04.005
55. Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. (2008) 13:216–26.
56. Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci. (2001) 22:195–201. doi: 10.1016/S0165-6147(00)01675-8
57. Carmel R, Jacobsen DW editors. Homocysteine in health and disease. Cambridge: Cambridge University Press (2001).
58. Pan CC, McQuoid DR, Taylor WD, Payne ME, Ashley-Koch A, Steffens DC. Association analysis of the COMT/MTHFR genes and geriatric depression: an MRI study of the putamen. Int J Geriatric Psychiatry. (2009) 24:847–55. doi: 10.1002/gps.2206
59. Lewis SJ, Zammit S, Gunnell D, Smith GD. A meta-analysis of the MTHFR C677T polymorphism and schizophrenia risk. Am J Med Genetics Part B: Neuropsychiatr Genet. (2005) 135:2–4. doi: 10.1002/ajmg.b.30170
60. Zintzaras E. C677T and A1298C methylenetetrahydrofolate reductase gene polymorphisms in schizophrenia, bipolar disorder and depression: a meta-analysis of genetic association studies. Psychiatr Genet. (2006) 16:105–15. doi: 10.1097/01.ypg.0000199444.77291.e2
61. Muntjewerff JW, Kahn RS, Blom HJ, den Heijer M. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Mol Psychiatry. (2006) 11:143–9. doi: 10.1038/sj.mp.4001746
62. Spillman M. S-adenosyl-methionine in psychiatric disorders: historical perspective and current status. CNS Drugs. (1996) 6:416. doi: 10.2165/00023210-199606060-00002
63. Lucock M. (2004). Is folic acid the ultimate functional food component for disease prevention? Bmj, 328(7433), 211-214. doi: 10.1136/bmj.328.7433.211
64. Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. ASH Educ Program Book. (2003) 2003:62–81. doi: 10.1182/asheducation-2003.1.62
65. Folstein M, Liu T, Peter I, Buel J, Arsenault L, Scott T, et al. The homocysteine hypothesis of depression. Am J Psychiatry. (2007) 164:861–7. doi: 10.1176/ajp.2007.164.6.861
66. Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B12 and folate. Clin Chem. (2000) 46:1277–83. doi: 10.1093/clinchem/46.8.1277
67. Johnson WG. DNA polymorphism-diet-cofactor-development hypothesis and the gene-teratogen model for schizophrenia and other developmental disorders. Am J Med Genet. (1999) 88:311–23. doi: 10.1002/(SICI)1096-8628(19990820)88:4<311::AID-AJMG6>3.0.CO;2-V
68. Regland B, Johansson BV, Grenfeldt B, Hjelmgren LT, Medhus M. Homocysteinemia is a common feature of schizophrenia. J Neural Trans General Section JNT. (1995) 100:165–9. doi: 10.1007/BF01271539
69. Ayesa-Arriola R, Pérez-Iglesias R, Rodríguez-Sánchez JM, Mata I, Gómez-Ruiz E, García-Unzueta M, et al. Homocysteine and cognition in first-episode psychosis patients. Eur Arch Psychiatry Clin Neurosci. (2012) 262:557–64. doi: 10.1007/s00406-012-0302-2
70. Delport D, Schoeman R, van der Merwe N, van der Merwe L, Fisher LR, Geiger D, et al. Significance of dietary folate intake, homocysteine levels and MTHFR 677 C> T genotyping in South African patients diagnosed with depression: test development for clinical application. Metab Brain Dis. (2014) 29:377–84. doi: 10.1007/s11011-014-9506-7
71. Lok A, Mocking RJT, Assies J, Koeter MW, Bockting CL, de Vries GJ, et al. The one-carbon-cycle and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in recurrent major depressive disorder; influence of antidepressant use and depressive state? J Affect Disord. (2014) 166:115–23. doi: 10.1016/j.jad.2014.04.048
72. Permoda-Osip A, Dorszewska J, Skibinska M, Chlopocka-Wozniak M, Rybakowski JK. Hyperhomocysteinemia in bipolar depression: clinical and biochemical correlates. Neuropsychobiology. (2013) 68:193–6. doi: 10.1159/000355292
73. McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke. (2002) 33:2351–6. doi: 10.1161/01.STR.0000032550.90046.38
74. Starr JM, Pattie A, Whiteman MC, Deary IJ, Whalley LJ. Vitamin B-12, serum folate, and cognitive change between 11 and 79 years. J Neurol Neurosurg Psychiatry. (2005) 76:291–2. doi: 10.1136/jnnp.2004.046219
75. Wahlin TBR, Wahlin Å, Winblad B, Bäckman L. The influence of serum vitamin B12 and folate status on cognitive functioning in very old age. Biol Psychol. (2001) 56:247–65. doi: 10.1016/S0301-0511(01)00079-5
76. Sharma V, Biswas D. Cobalamin deficiency presenting as obsessive compulsive disorder: case report. Gen Hosp Psychiatry. (2012) 34:578–e7. doi: 10.1016/j.genhosppsych.2011.11.006
77. BaşogIu C, Ateş MA, AIgüI A, IpçiogIu OM, Geçici Ö, Yilmaz O, et al. Adjuvant folate with escitalopram treatment and homocystein, folate, vitamin b-12 levels in patients with major depressive disorder. Klinik Psikofarmakoloji Bulteni. (2009) 19:2.
Keywords: obsessive-compulsive disorder, folic acid, homocysteine, vitamin B12, meta-analysis
Citation: Yan S, Liu H, Yu Y, Han N and Du W (2022) Changes of Serum Homocysteine and Vitamin B12, but Not Folate Are Correlated With Obsessive-Compulsive Disorder: A Systematic Review and Meta-Analysis of Case-Control Studies. Front. Psychiatry 13:754165. doi: 10.3389/fpsyt.2022.754165
Received: 10 November 2021; Accepted: 22 March 2022;
Published: 09 May 2022.
Edited by:
Paweł Krukow, Medical University of Lublin, PolandReviewed by:
Joshua Miller, Rutgers, The State University of New Jersey, United StatesCopyright © 2022 Yan, Liu, Yu, Han and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenzhi Du, MTE1MjAzNTg1QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.