- 1Division of Child and Adolescent Psychiatry, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA, United States
- 2Center for Interdisciplinary Brain Sciences Research, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA, United States
- 3Research Imaging Institute, University of Texas Health Science Center, San Antonio, TX, United States
- 4Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center, San Antonio, TX, United States
Background: Little is known about the effects of social exclusion on youth with bipolar disorder (BD). Understanding these effects and the functional neural correlates of social exclusion in youth with BD may establish differences from healthy youth and help identify areas of intervention.
Methods: We investigated brain function in 19 youth with BD and 14 age and gender matched healthy control (HC) participants while performing Cyberball, an fMRI social exclusion task. Whole brain activation, region-of-interest, and functional connectivity were compared between groups and examined with behavioral measures.
Results: Compared with the HC group, youth with BD exhibited greater activation in the left fusiform gyrus (FFG) during social exclusion. Functional connectivity between the left FFG and the posterior cingulate/precuneus was significantly greater in the HC compared with the BD group. For the HC group only, age and subjective distress during Cyberball significantly predicted mean FFG activation. No significant differences in distress during social exclusion were found between groups.
Conclusion: Although preliminary due to small sample size, these data suggest that youth with BD process social exclusion in a manner that focuses on basic visual information while healthy youth make use of past experiences to interpret current social encounters. This difference may account for the social cognitive issues experienced by youth with BD, which can lead to more severe anxiety and mood symptoms.
Introduction
Bipolar disorder (BD) with comorbid anxiety is associated with poorer response to treatment, more severe depression, rapid cycling, substance abuse, and suicide attempts (1–5). Emerging longitudinal evidence suggests that youth at high-risk for BD that develop any mood disorder experience an anxiety disorder as an early antecedent (2, 5, 6). In fact, the risk of a mood disorder diagnosis was over two times higher in those with an anxiety disorder than those without, with social anxiety disorder and generalized anxiety disorder the most predictive (5). Anxiety is therefore an important symptom in the developmental trajectory of BD, both as a comorbidity and as a potential risk factor for the development of a mood disorder.
One of the largest sources of anxiety in youth is the quality of social relationships, which greatly influence youth’s perceived quality of life (7–9). Youth with BD demonstrate deficits in interpersonal functioning that contribute to anxiety (9, 10) and undermine emotion regulation, potentially leading to mood episodes (10–13). A major source of anxiety for youth is social exclusion (14). Therefore, better understanding of responses to social exclusion in youth with BD could lead to interventions that prevent mood symptom development.
No previous studies have examined the neural underpinnings of social exclusion in youth with BD. For youth with unipolar depression, previous studies of social exclusion have reported abnormal hyperactivation of the anterior insula and subgenual cingulate cortex (sgACC) (11, 12, 15–18), which was correlated with greater feelings of distress compared with healthy controls (HC) (12). Additionally, hyperactivation in the sgACC and medial prefrontal cortex (PFC) during social exclusion was predictive of depressive symptoms one year later (13). FMRI studies also suggest the ventral PFC and ventral striatum regulate areas hyperactivated during social exclusion (12, 15). In fact, a recent coordinate-based meta-analysis found that activation in the right ventral striatum and left ventrolateral prefrontal cortex (VLPFC) is consistently reported in studies of developmental samples during a social exclusion fMRI task called “Cyberball” (19). Taken together, these studies suggest that the neural response to social exclusion involves structures associated with internal perception (anterior insula) and emotional experience (sgACC) regulated by the VLPFC. We therefore hypothesized that youth with BD would exhibit greater activation of this neural circuitry and report significantly greater distress during social exclusion when compared with HC.
Materials and Methods
Participants and Assessments
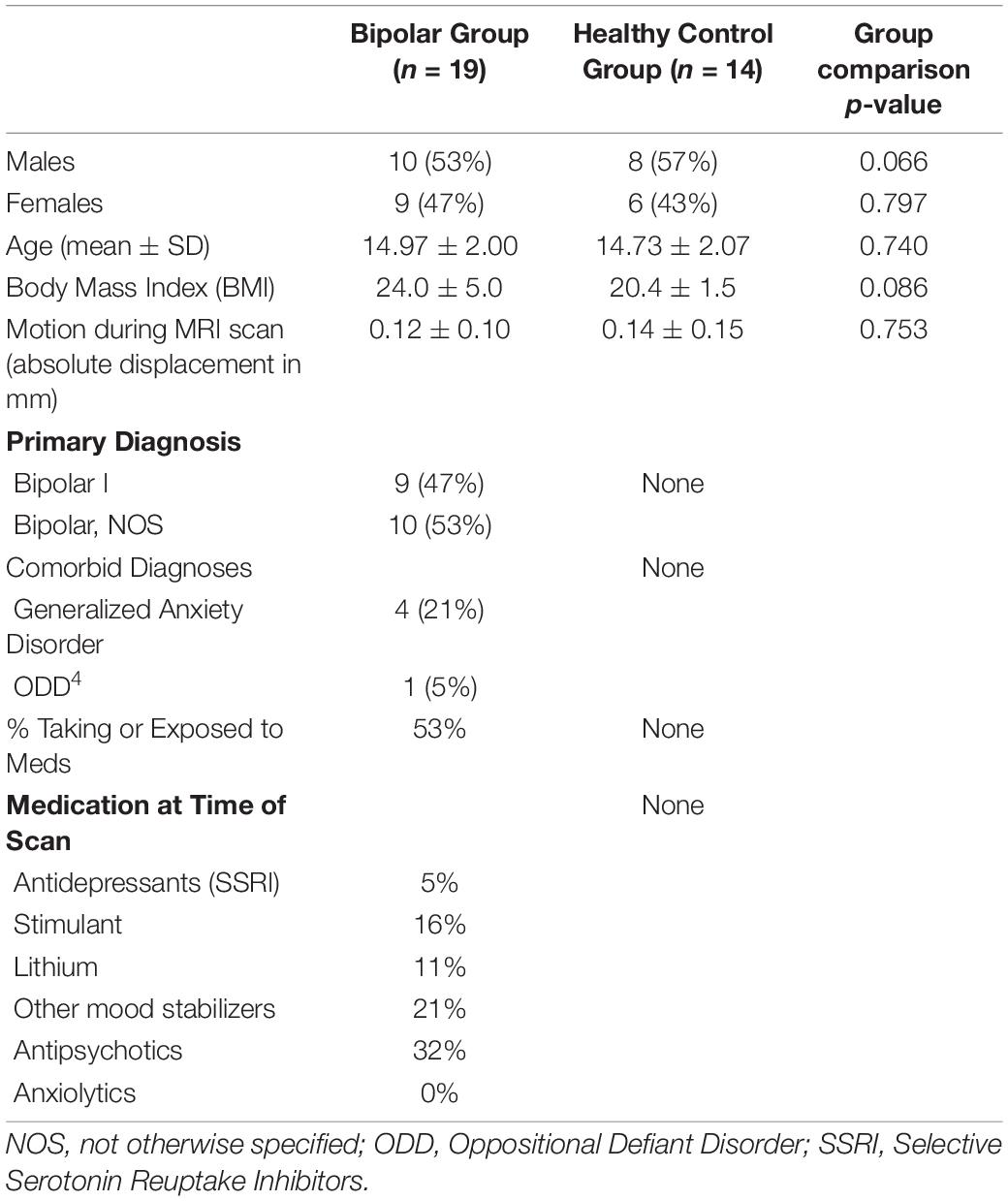
The Stanford University Administrative Panel of Medical Research in Human Subjects approved the protocol. We recruited 19 youth fulfilling DSM-IV-TR criteria for BD I, II, or not otherwise specified (NOS) from a pediatric bipolar disorders clinic and 16 gender and age matched healthy controls (HC) from the surrounding community. We examined the bipolar spectrum of disease owing to the fact that longitudinal studies have shown that within 2.5 years, youth with BD, NOS convert to BD II or I, and youth with BD II convert to BD I (1). All participants were between the ages of 10–18. We obtained written informed consent and assent from the parents and children, respectively. Children were administered the Young Mania Rating Scale (YMRS) (20) and the Children’s Depression Rating Scale-Revised Version (CDRS-R) (21) by raters with established inter-rater reliability (ICC > 0.9). Participants were also administered the children’s Rejection Sensitivity Questionnaire (RSQ), a validated scale measuring the severity of anxiety and anger that might be experienced with regards to the likelihood of being accepted in various social exclusion scenarios (22). Participants were also administered the Need Threat Scale (NTS), a validated scale used to assess the severity of subjective distress felt during the fMRI social exclusion task (23, 24). Subjective distress, as defined by the NTS, assesses the degree of threat someone feels during social exclusion to their needs for belonging, control, self-esteem, and meaningful existence (23, 24). The NTS is scored such that higher scores indicate lower levels of subjective distress, or threat to need, and lower scores indicate higher subjective distress.
The affective module of the Washington University in St. Louis Kiddie-Schedule for Affective Disorders and Schizophrenia (WASH-U KSADS) (kappa > 0.9 for diagnostic reliability) (25, 26) and the Kiddie–Schedule for Affective Disorders and Schizophrenia, Present and Lifetime (kappa 0.77–1.00 for diagnostic reliability) (27) were administered to parents and children in separate interviews by a trained masters-level clinician and/or board-certified psychiatrist. DSM-IV-TR criteria were used to determine current and lifetime psychiatric diagnoses. BD-NOS criteria was defined as a minimum of either (1) two lifetime episodes of at least four hours duration each of criterion A: either elevated mood plus two associated symptoms or irritable mood plus three associated symptoms, but not meeting threshold BD I or II criteria or (2) 2–3 days of criterion A. Participants taking medications were stable on medications, defined as three weeks at the same dosage if taking a selective serotonin reuptake inhibitor (SSRI), and 2 weeks if taking a mood stabilizer, antipsychotic, and/or stimulant.
Youth were excluded from the BD group if they had diagnoses of pervasive development disorder, intellectual disability, obsessive-compulsive disorder, panic disorder, post-traumatic stress disorder, a history of head trauma with loss of consciousness, or Tourette’s syndrome. Participants in the healthy control group were excluded if they were taking psychotropic medications or if they or any of their first-degree relatives had a current or lifetime DSM-IV-TR diagnosis. Further excluded from either group were any children with a neurologic condition (e.g., seizure disorder), substance use disorder, or the presence of metallic implants or braces.
“Cyberball” Task During fMRI
Participants were scanned while playing Cyberball, a computer game used to study the effects of social exclusion that has been adapted for use in the fMRI scanner (23, 24, 28). In this game, the participant played a virtual ball-tossing game with two other players. To enhance the interpersonal nature of the game, the participant was told s/he was playing with two other players and that each player was in a separate scanner. These two other players were shown as cartoon figures on the projection screen viewed by the participant via a mirror attached to the headcoil. The participant was represented by a cartoon hand at the bottom of the screen.
The cyberball task was designed to replicate that used in previous studies (12). The task is a block design containing “inclusion” and “exclusion” blocks. During inclusion blocks, a cartoon ball was thrown to the participant, who could then throw the ball to one of the two other (cartoon) players by pressing the left or right button on the button box. During exclusion blocks, the ball was thrown to one of the other (cartoon) players, and the participant was excluded from all throws. For all blocks, each throw had a duration of 5–6 s (depending on how quickly the participant threw the ball) with an inter-throw interval of 0.5 s. The order of blocks was inclusion–inclusion–exclusion. The first inclusion block contained 55 throws, the second inclusion block contained 30 throws, and the exclusion block contained 27 throws. Overall, the task duration was 4:29”.
After the scan, participants were administered the Need Threat Scale to assess the severity of subjective distress experienced during the game (23, 24).
fMRI Acquisition and Preprocessing
Magnetic resonance imaging scans were conducted at the Stanford University Richard M. Lucas Center for Imaging. Images were acquired using a 3.0T General Electric MR750 scanner (General Electric, Milwaukee, WI, United States) using an 8-channel head coil. The following pulse sequence parameters were used for the fMRI scans: spiral in-out, echo time (TE)/repetition time (TR) = 30/2,000 ms, flip angle = 89° and 1 interleave, matrix size 64 × 64, field of view (FOV) = 240 mm, 31 slices, slice thickness 4 mm, skip 0.5 mm; entire brain and cerebellum. An individually calculated high-order shim for spiral acquisitions was used to reduce field inhomogeneity. A high resolution fast spoiled grass (FSPGR) anatomical scan also was collected to optimize registration of fMRI data to standard space.
fMRI Data Preprocessing
Functional MRI data were analyzed using SPM8 software.1 Images were realigned to the third volume and motion was corrected using the ArtRepair toolbox (cibsr.stanford.edu/tools/ArtRepair). Volumes with motion artifact (slope > 1.5 mm/volume) were replaced with a volume that was interpolated from the nearest surrounding unaffected volumes. Scans were rejected from further analysis for motion spikes greater than 4 mm translation or if more than 20% of volumes required motion correction. Images were normalized to the MNI152 template using each subject’s anatomical scan and resampled to a 2-cubic mm matrix using sinc interpolation, smoothed with a 5 mm FWHM Gaussian filter, and high pass filtered at 120 s.
Group Differences in Ratings of Distress and Rejection Sensitivity
An independent-sample t-test performed in IBM SPSS v26.02 was used to examine differences between BD and HC group mean NTS scores. Spearman’s correlations within each group were used to examine the association between NTS and RSQ scores. RSQ scores comprised two scores, an anger and an anxiety domain. NTS scores for each group were therefore correlated with each domain separately. Thresholds for significance were set at q = 0.05, after FDR correction for multiple comparisons.
Whole Brain Analyses
For each subject, a fixed-effects analysis in SPM8 using the general linear model was performed to calculate voxel-wise statistical maps for each subject, for the contrast of exclusion minus inclusion blocks. Between group voxel-wise comparisons were conducted using an independent groups t-test, while covarying for age.
Inference was conducted using a cluster-forming threshold of p < 0.005, combined with family-wise error correction of p < 0.05 at the cluster level. While our cluster-forming threshold of p = 0.005 is somewhat more liberal than the traditional setting of p = 0.001 (29), it is recommended for reducing Type II error in fMRI studies of social and affective processes, which have small effect sizes and weak statistical power due to the complexity of these psychological processes (30,31). In addition, this threshold is similar to previous studies examining the effects of Cyberball in youth (19, 30). Age was covaried given previous findings that brain regions activated by Cyberball were age dependent (19).
Region-of-Interest Analyses
Regions of interest (ROI) were defined using the Automated Anatomic Labeling (AAL) atlas (32) for the anterior insula and the anterior cingulate cortex. For the ventral striatum and ventral PFC, coordinates were taken from a meta-analysis of developmental Cyberball studies and a 5 mm sphere was created for each a priori region using MarsBar3 from which mean activation was extracted (19).
Functional Connectivity
The generalized Psychophysiological Interaction (gPPI) toolbox (33) was used to examine whole brain functional connectivity with seeds placed at each of the significant activation clusters. The resulting voxel-wise connectivity maps were contrasted between the BD and HC groups using independent groups t-tests in SPM8. Inference was conducted using a cluster-forming threshold of p < 0.005, combined with Family-wise error correction of p < 0.05 at the cluster level, as justified in the section “Whole Brain Analyses.”
Associations Between Significant Clusters and Self-Reported Distress and Mood Symptomatology
Within each group separately, linear regression in SPSS was used to predict NTS score from mean activation in each significant cluster, adjusted for age. A second model was used to predict RSQ from mean activation in significant clusters.
CDRS-R scores measuring depression symptoms were correlated with each individual’s mean activation for each significant cluster using Spearman’s rho. Thresholds for significance were set at q = 0.05, after FDR correction for multiple comparisons. The same was performed for mania symptoms using YMRS scores.
Whole-brain linear regression was also performed for each group twice using activation and functional connectivity each as dependent variables in separate models in SPM8. Total NTS scores and age were the independent variables in these models. We used a cluster forming threshold of p = 0.005 and thresholds of inference set at p ≤ 0.05, FWE corrected.
Results
Demographics and Clinical Characteristics
Two HC scans were not usable, one due to artifact during the exclusion run and the second due to incomplete capture of superior portions of the brain. A total of 19 scans in the BD group and 14 scans in the HC group were included in fMRI analysis. There were no group differences in age [t(30) = 0.540, p = 0.74] or proportion of females to males (χ2 = 0.07, p = 0.80). Nine youth were diagnosed with BD I and ten with BD, NOS. Four had Generalized Anxiety Disorder and one had Oppositional Defiant Disorder. Table 1 provides additional demographic and clinical characteristics.
Group Differences in Mood Symptoms and Distress During Exclusion
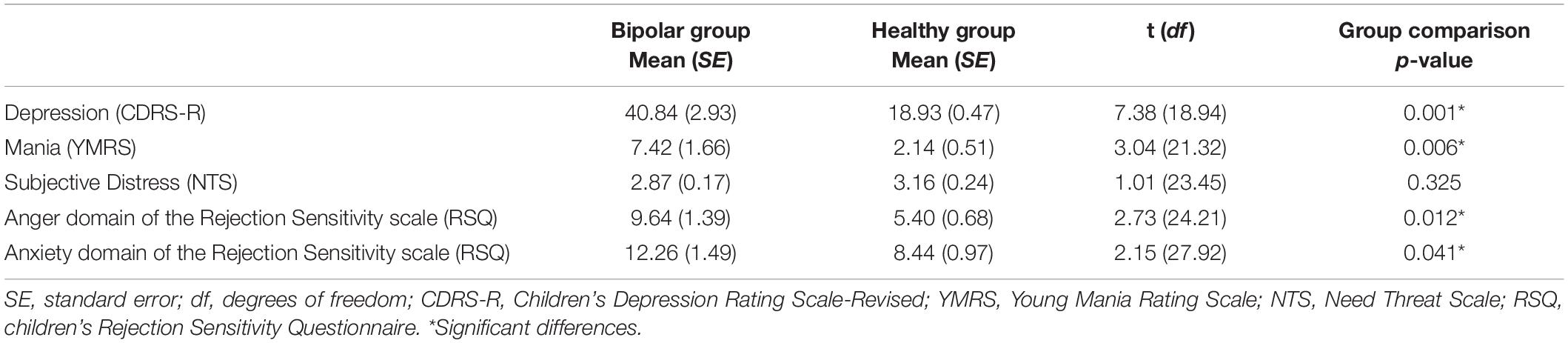
Table 2 depicts CDRS-R, YMRS, NTS, and RSQ scores for each group. No significant difference was found between the BD and HC groups for mean NTS scores (p = 0.33). The BD group had significantly higher scores when compared with HC for the anger domain [t(24) = 2.73, p = 0.012] and the anxiety domain [t(28) = 2.15, p = 0.041] of the RSQ. As expected, YMRS and CDRS-R scores were significantly higher in the BD group [YMRS: t(21) = 3.04, p = 0.006; CDRS-R: t(19) = 7.38, p < 0.001]. Within the BD group, NTS score was significantly correlated with RSQ scores in the anger domain (rho = –0.65, p = 0.012, q = 0.025, FDR corrected; Figure 1) and a near significant correlation was found between NTS and RSQ scores in the anxiety domain (rho = –0.55, p = 0.041, q = 0.05, FDR corrected). None of the correlations within the HC group were significant.
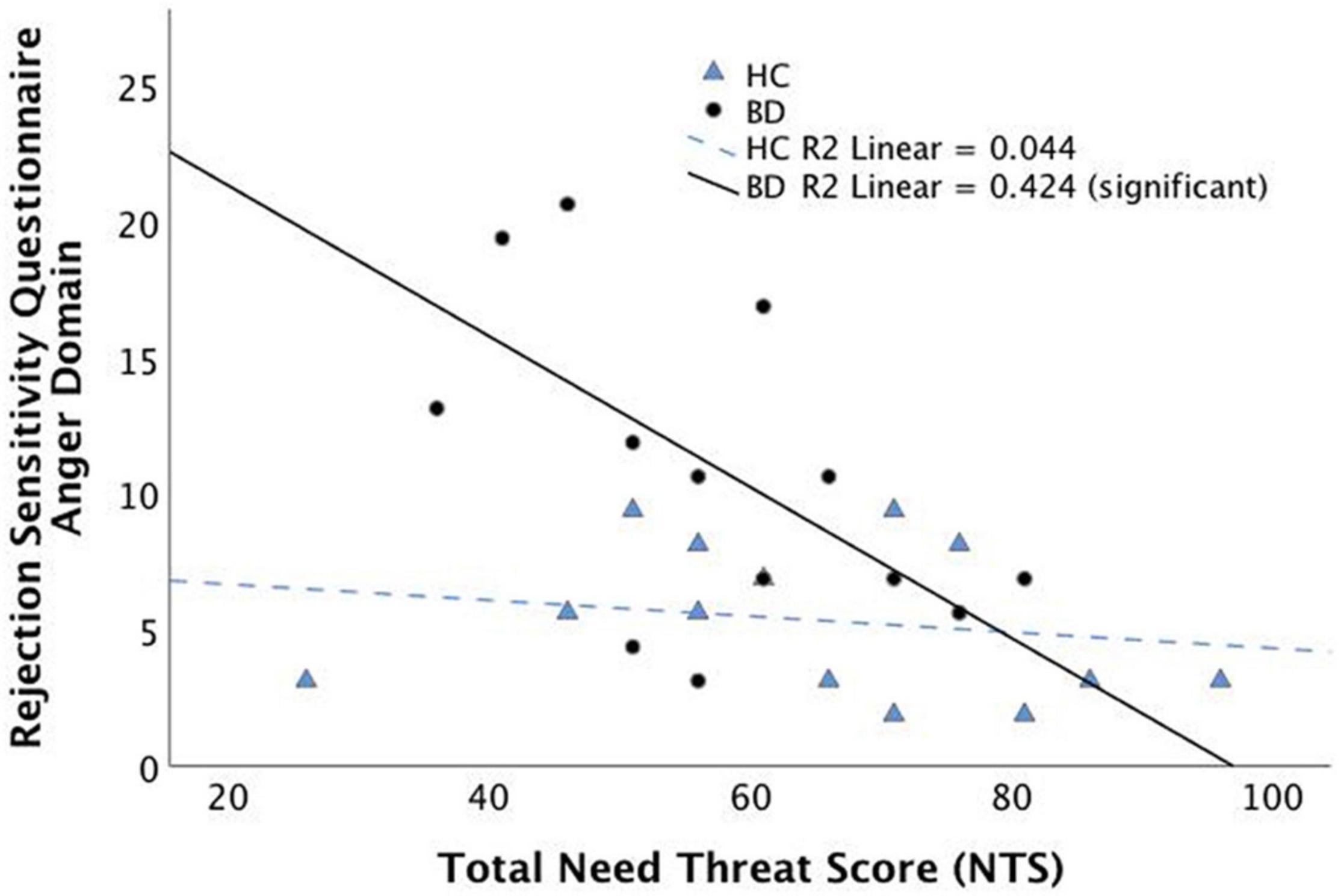
Figure 1. Spearman’s correlation between subjective distress during social exclusion (as measured by the Need Threat Scale) and the anger domain of the Rejection Sensitivity Questionnaire (RSQ). Correlation is significant within the Bipolar Disorder group but not the Healthy Control group.
fMRI Results
Group Differences in Activation to Exclusion vs Inclusion
For the whole brain voxel-wise analysis, youth with BD showed significantly greater activation than HC in the left fusiform gyrus [FFG, Brodmann’s Area (BA) 37, peak X = –42, Y = –56, Z = –12, z = 3.71, cluster size = 270, p = 0.037, Figure 2]. For the ROI analysis, no significant differences were found between BD and HC for the ventral striatum ROI, ventral PFC ROI, and anterior insula ROI.
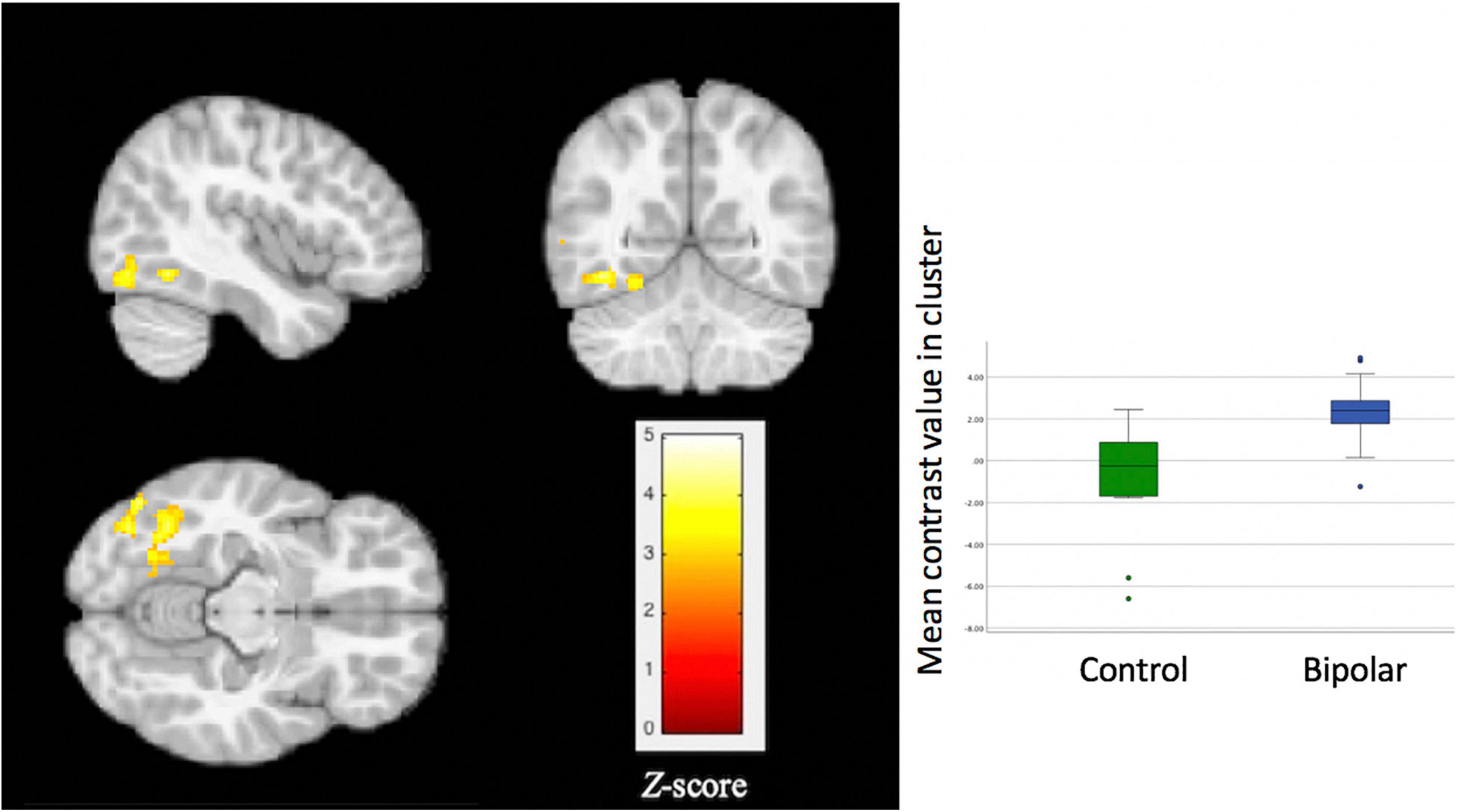
Figure 2. Significant group differences in activation of the left fusiform gyrus during a social exclusion task. Compared to healthy controls, youth with bipolar disorder showed significantly greater activation (p = 0.037) for the contrast of exclusion > inclusion. Thresholds for inference were set at p < 0.05, FWE corrected at the cluster level.
Functional Connectivity
The BD group, compared with the HC group, showed significantly lower functional connectivity between the left FFG cluster and two clusters: (1) posterior cingulate (PCC), precuneus, and cuneus (BA 23, 30, 31, 17 and 18; peak X = 6, Y = –66, Z = 18, z = 3.92, cluster size = 1617, p < 0.001) and (2) the postcentral gyrus (BA 3, 4; peak X = 24, Y = –32, Z = 64, z = 3.60, cluster size = 411, p = 0.006). These results are shown in Figure 3.
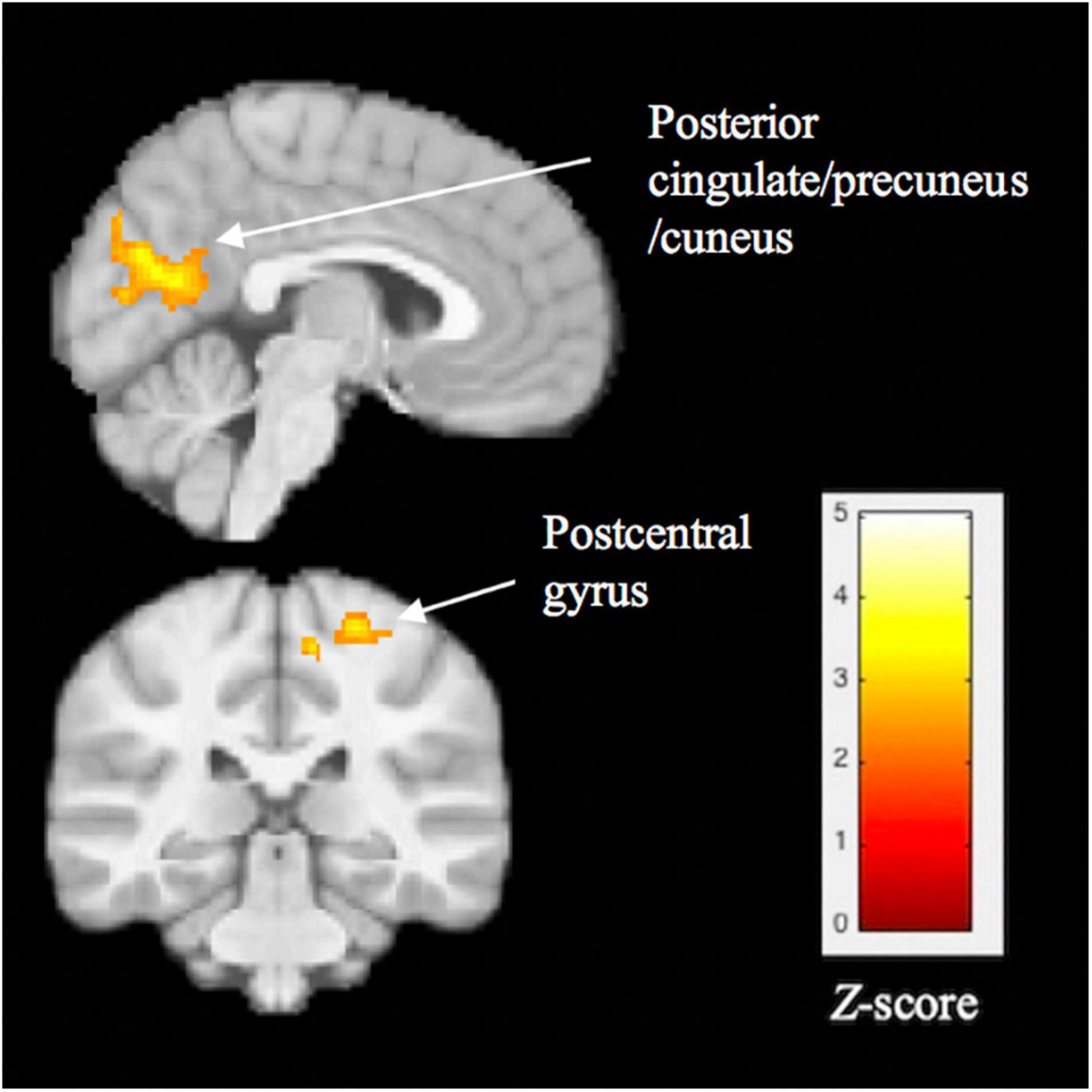
Figure 3. Significant group differences in task-related functional connectivity of the left fusiform gyrus, assessed using psychophysiological interaction analysis. Compared with healthy controls, youth with BD showed lower connectivity between the fusiform cluster and 3 regions: posterior cingulate, precuneus/cuneus, and postcentral gyrus. Thresholds were set at p < 0.05, FWE corrected at the cluster level.
Associations Between Activation and Distress During Exclusion
Within the HC group, fusiform gyrus activation was significantly associated with subjective distress during exclusion (total NTS score), after adjusting for age (model R square = 0.53, p = 0.020), such that lower distress during exclusion was associated with higher levels of FFG activation. Within the BD group, the association between subjective distress during exclusion and FFG activation was not significant (R square = 0.006, p = 0.961). A scatterplot of these associations is shown in Figure 4, for each group separately. No significant correlations were found between FFG activation and CDRS-R or YMRS scores within either group.
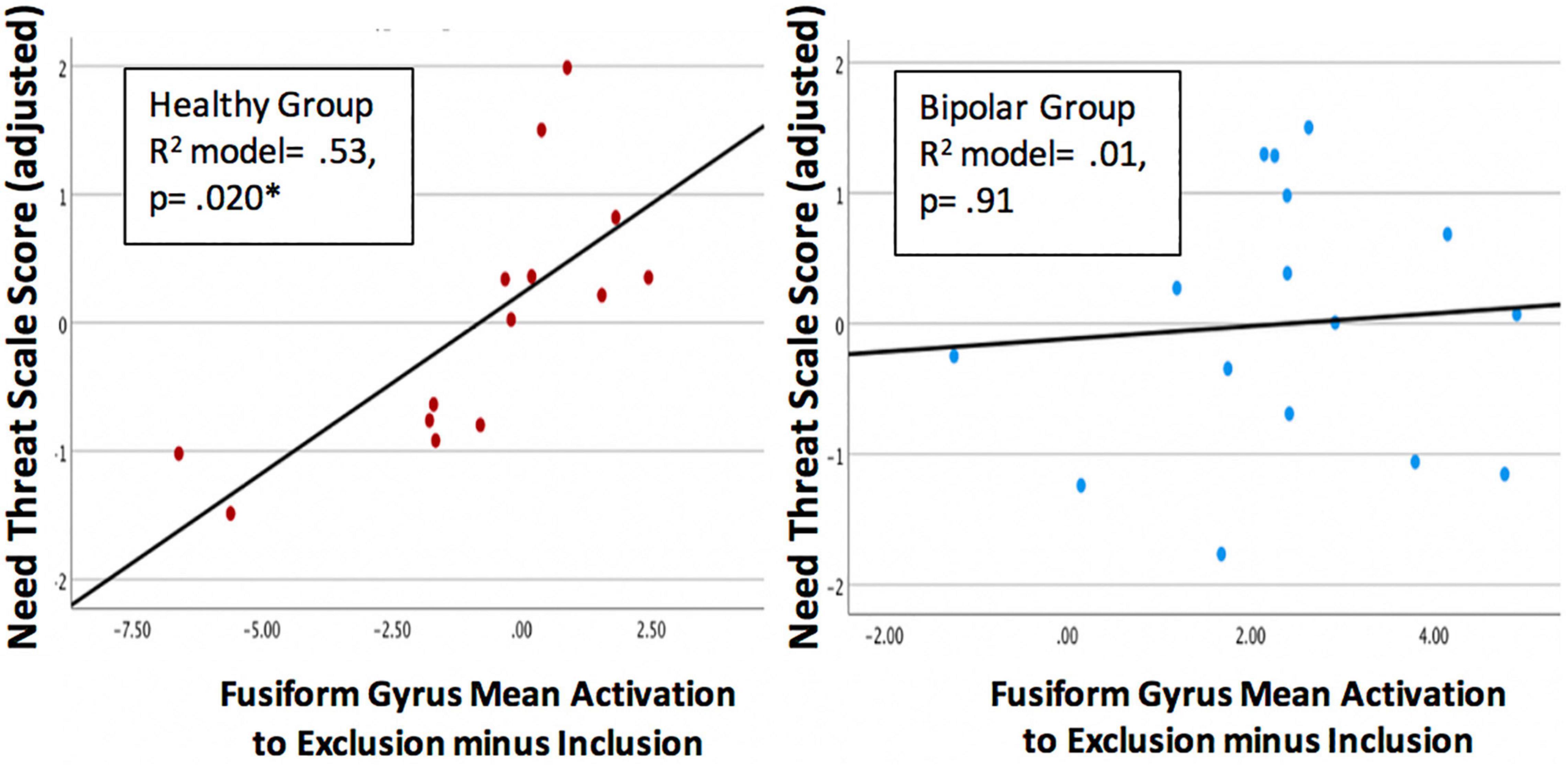
Figure 4. Scatterplots showing the association between activation of the left fusiform gyrus (FFG) and subjective distress during exclusion (Need Threat Scale total score adjusted for age) within the each group. Regression models were significant for the healthy control group (p = 0.020, left) but not the bipolar group (p = 0.91, right).
Whole Brain Associations Between Functional Connectivity and Distress During Exclusion
Within the BD group, subjective distress during exclusion was not significantly associated with connectivity of the FFG. Within the HC group, greater distress during exclusion was significantly associated with lower connectivity between the left FFG and left posterior cerebellum (p = 0.004) and greater connectivity between the left FFG and four regions: (1) left cuneus (p < 0.001), (2) left precuneus (p = 0.008), (3) right anterior insula (p = 0.001), and (4) right premotor cortex (p = 0.001). These results are shown in Figure 5.
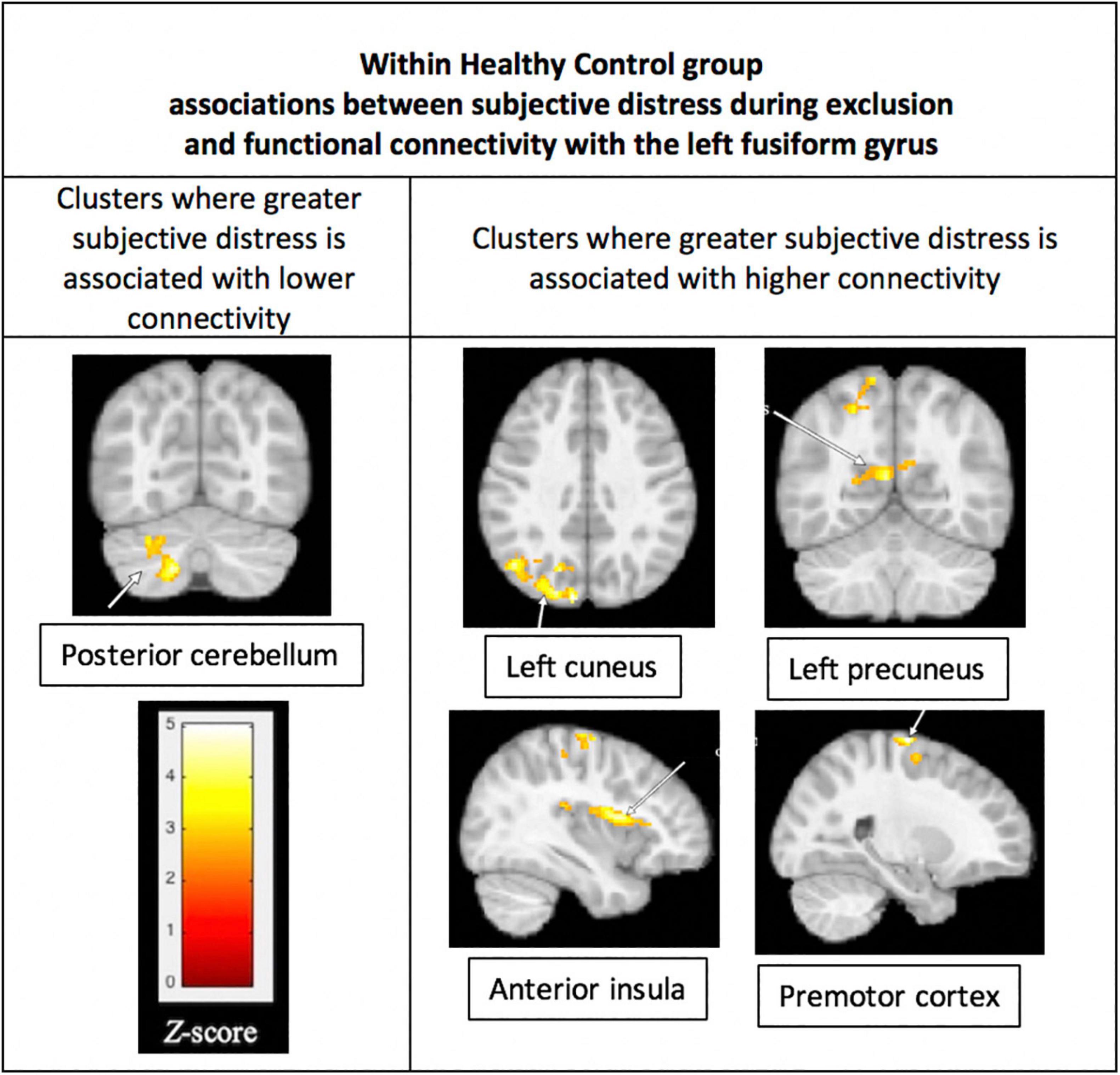
Figure 5. Associations between subjective distress and functional connectivity with the left fusiform gyrus (FFG). Within the HC group, greater distress during social exclusion was associated with lower connectivity between the fusiform gyrus and the left posterior cerebellum (p = 0.004), shown in the (left) column of the figure. Also for the HC group, greater distress was associated with higher connectivity of the cuneus, precuneus, insula, and premotor cortex, as shown in the (right) column. Functional connectivity was performed using the left FFG cluster as the seed in a generalized PPI analysis, with a threshold of p < 0.05, FWE corrected at the cluster level. Results were not significant within the Bipolar Disorder group.
Discussion
During a social exclusion task, youth with BD showed greater activation in the left FFG compared with HC. The HC group had greater functional connectivity over BD between the left FFG and the PCC compared to the BD group. Interestingly, there was no significant difference between the BD and HC groups in severity of distress during the social exclusion task. We found that distress significantly predicted FFG activation in the HC group but not in the BD group. In functional connectivity analysis for the HC group using the left FFG as the seed, greater connectivity with the right anterior insula, right premotor cortex, and left middle occipital cortex was also significantly correlated with greater feelings of distress. Taken together, these results suggest that youth with BD process social exclusion differently than healthy youth and that distress from social exclusion may correlate with alternate pathways not typically seen in healthy youth. These results are considered preliminary due to small sample size.
To our knowledge, this is the first fMRI study examining social exclusion in youth with BD. While we hypothesized we would see greater activation in the BD group, when compared with HC, in areas previously shown to hyperactivate in adolescent samples experiencing Cyberball, our study did not produce these results using ROI analysis. Regions in the HC group that correlated with greater distress in social exclusion, however, were the same that were hyperactivated in previous studies of Cyberball in healthy developmental samples (19). A recent meta-analysis of 53 cyberball neuroimaging studies including both adult and child samples, reported consistent recruitment of ventral anterior cingulate, posterior cingulate, inferior and superior frontal, insula and occipital cortex (34). These findings overlap with the 2017 meta-analysis (19) that also found consistent recruitment of the posterior cingulate and ventrolateral frontal corticies. While these meta-analyses do not include comparisons between clinical and healthy groups, they are relevant to the present findings of lower connectivity between posterior cingulate and fusiform gyrus in BD versus HC groups, suggesting that the fusiform is relevant to social exclusion through its connectivity to the posterior cingulate. We also note that the current findings of a correlation between subjective distress and functional connectivity of insula with fusiform gyrus within the healthy control group further suggest that the fusiform gyrus is clinically relevant because of its connectivity with regions that are consistently reported across previous studies of the cyberball paradigm.
To explore whether the BD group’s distress from social rejection was correlated with other brain regions, we conducted an exploratory whole brain voxel-wise correlation with NTS scores, but results were not significant. This could suggest the processing of distress after social rejection is not localized to a particular region or regions in the brain, or that our sample size was too small to detect an effect. However, the effect for the HC group was found with a smaller sample than the BD group.
The BD group did report some subjective distress but it was not significantly different than the HC group. However, the BD group had significantly higher ratings of anger and depression than the HC group, which were negatively correlated with subjective distress. This suggests that symptoms of anger and depression experienced by the BD group may have diminished or interfered with reporting subjective distress related to social exclusion in the BD group. If so, higher fusiform activation in BD may reflect an altered way of processing social exclusion that is not on a continuum with the HC group, e.g., not simply a more extreme level of subjective distress from social exclusion, but a different strategy, perhaps involving the visual system.
We hypothesized that the BD group would show greater activation in regions previously implicated in social exclusion in healthy controls (e.g., anterior cingulate cortex, VLPFC, and ventral striatum) but our results showed group differences in the fusiform gyrus. The fusiform gyrus is salient to higher level visual processing and implicated in facial perception, which are important components in the social cognitive circuit (35, 36). While some debate surrounds the function of the FFG, studies agree the area is recruited in the processing of faces (37). The role of facial processing and perception is important to understand the intention and emotions of others and therefore, to social interaction (37). In fact, a recent meta-analysis of the neural network of face processing in healthy adults showed the left posterior FFG was specifically involved in face processing tasks that required emotion evaluation (38). The posterior FFG, where our findings are located, encompasses the fusiform face area. Studies have shown this area to have higher activation when healthy adolescents are viewing fearful compared to neutral faces (39). Perhaps the BD group finds the depiction of cartoon faces in Cyberball emotionally evocative. This finding is consistent with previous literature suggesting adolescents with BD misinterpret neutral faces as fearful with greater hostility (40). Aberrant face emotion processing is well established in bipolar disorder, and so it is perhaps not surprising this marker of illness may be involved in any sort of social evaluative process (41).
Our connectivity analysis using the FFG as the seed showed the HC group had greater functional connectivity between the left FFG and the left PCC, specifically the caudal left PCC, which is an area associated with autobiographical memory. The left PCC is activated during successful autobiographical memory recollection in healthy adults (42). This may suggest that the HC group recalls social experiences more than the BD group in the context of social exclusion. The PCC is also implicated in tasks of emotional salience. Studies have shown hyperactivation of the PCC in tasks of both positive and negative emotional stimuli (43). These studies have postulated the strength of successful recall of autobiographical memories to be dependent on their emotional importance, and the PCC consistently hyperactivates on successful recall of such memories. This suggests the PCC moderates the interaction between memory and emotion (42). Healthy youth may be able to interpret social exclusion in the larger context of positive autobiographical memories of social experiences. Youth with BD, however, are known to have structural and functional abnormalities in the PCC, which may suggest they do not have the same ability to recall autobiographical experiences in the same way as healthy youth (44, 45).
Lastly, for the HC group only, lower distress during Cyberball was correlated with greater functional connectivity between the posterior cerebellum and the FFG. Studies suggest the posterior cerebellum connects with the limbic system and participates in the limbic related functions of emotion (46). The posterior cerebellum has been shown to have abnormal function and structure in youth with bipolar disorder (47). Lesions in the cerebellum have been implicated in causing manic states (48) and with problems with social interaction (49). It may be, then, that the HC group has a more intact emotional circuit during the social exclusion experience, unlike the BD group. Areas known to have structural and functional abnormalities in BD that overlap with our findings, specifically the PCC and the posterior cerebellum, are therefore associated with aberrant processing of social exclusion when compared with healthy youth.
Limitations of this study include a small sample size. However, this is the only published study to date to examine the functional neuroanatomy of youth with bipolar disorder using a social cognitive paradigm. The fMRI block design which provided only four minutes of game time data also is a limitation of this study. Current Cyberball fMRI studies have extended this model to provide more data points by using an alternating block design and multiple games in one scan (19). We did use FWE for fMRI analysis, which is a stringent thresholding method, but may have missed some relevant between group differences as a result. Future studies should examine whether domains of anxiety, affective lability, and coping skills moderate responses to social exclusion. We did find greater scores in the anger domain for the RSQ to significantly correlate with greater distress in the BD but not the HC group. A similar finding was discovered for the anxiety domain of the RSQ, though this finding did not reach significance. This suggests an emotional and anxious component in youth with BD that may predict the reaction to social exclusion that should be further explored.
In summary, despite aberrant neural processing, the BD group did not show significant differences in distress during social exclusion when compared with the HC group. Youth with BD may therefore process social exclusion in a manner different from the HC group that focuses on visual processes early in the social cognitive circuit while HC uses past social experiences to inform current social encounters. This difference in processing may pose clinical implications for improving social cognition in youth with BD and preventing mood symptoms.
Data Availability Statement
Due to difficulties in de-identifying MRI images of this vulnerable child population, raw data are not available. Processed data are available upon request to the corresponding author, with a data sharing agreement.
Ethics Statement
This study was reviewed and approved by the Institutional Review Board of Stanford University School of Medicine. Written informed assent and consent were provided by the youth and parent/guardian, respectively.
Author Contributions
DR, KC, and AG designed the study. DR wrote the protocol and performed literature searches. DR, RML, and BN conducted the statistical analyses. DR, AG, RS, and RK conducted scans, pre-processed neuroimaging data, and analyzed neuroimaging data. All authors contributed to and approved the final manuscript.
Funding
This work was supported by funding awarded to DR from the American Academy of Child and Adolescent Psychiatry/Lilly Pilot Research Award.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Jennifer Pearlstein, Paige Staudemaier, and Sherrie Li for their assistance with recruiting and scanning participants.
Footnotes
- ^ http://www.fil.ion.ucl.ac.uk/spm
- ^ https://www.ibm.com/products/spss-statistics
- ^ http://marsbar.sourceforge.net/
References
1. Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the pittsburgh bipolar offspring study. Arch Gen Psychiatry. (2009) 66:287–96. doi: 10.1001/archgenpsychiatry.2008.546
2. Duffy A. The early natural history of bipolar disorder: what we have learned from longitudinal high-risk research. Can J Psychiatry. (2010) 55:477–85. doi: 10.1177/070674371005500802
3. Sala R, Axelson DA, Castro-Fornieles J, Goldstein TR, Ha W, Liao F, et al. Comorbid anxiety in children and adolescents with bipolar spectrum disorders: prevalence and clinical correlates. J Clin Psychiatry. (2010) 71:1344–50. doi: 10.4088/JCP.09m05845gre
4. Sala R, Strober MA, Axelson DA, Gill MK, Castro-Fornieles J, Goldstein TR, et al. Effects of comorbid anxiety disorders on the longitudinal course of pediatric bipolar disorders. J Am Acad Child Adolesc Psychiatry. (2014) 53:72–81. doi: 10.1016/j.jaac.2013.09.020
5. Duffy A, Horrocks J, Doucette S, Keown-Stoneman C, McCloskey S, Grof P. Childhood anxiety: an early predictor of mood disorders in offspring of bipolar parents. J Affect Disord. (2013) 150:363–9. doi: 10.1016/j.jad.2013.04.021
6. Duffy A, Horrocks J, Doucette S, Keown-Stoneman C, McCloskey S, Grof P. The developmental trajectory of bipolar disorder. Br J Psychiatry. (2014) 204:122–8.
7. Hecht DB, Inderbitzen HM, Bukowski AL. The relationship between peer status and depressive symptoms in children and adolescents. J Abnorm Child Psychol. (1998) 26:153–60. doi: 10.1023/a:1022626023239
8. Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. (2005) 35:163–74. doi: 10.1017/s0033291704003915
9. Siegel RS, Hoeppner B, Yen S, Stout RL, Weinstock LM, Hower HM, et al. Longitudinal associations between interpersonal relationship functioning and mood episode severity in youth with bipolar disorder. J Nerv Ment Dis. (2015) 203:194–204. doi: 10.1097/NMD.0000000000000261
10. Goldstein TR, Miklowitz DJ, Mullen KL. Social skills knowledge and performance among adolescents with bipolar disorder. Bipolar Disord. (2006) 8:350–61. doi: 10.1111/j.1399-5618.2006.00321.x
11. Sebastian CL, Roiser JP, Tan GCY, Viding E, Wood NW, Blakemore SJ. Effects of age and MAOA genotype on the neural processing of social rejection. Genes Brain Behav. (2010) 9:628–37. doi: 10.1111/j.1601-183X.2010.00596.x
12. Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. (2009) 4:143–57. doi: 10.1093/scan/nsp007
13. Masten CL, Eisenberger NI, Borofsky LA, Mcnealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Dev Psychopathol. (2011) 23:283–92. doi: 10.1017/S0954579410000799
14. Leary MR. Responses to social exclusion: social anxiety, jealousy, loneliness, depression, and low self-esteem. J Soc Clin Psychol. (1990) 9:221–9.
15. Bolling DZ, Pitskel NB, Deen B, Crowley MJ, Mayes LC, Pelphrey KA. Development of neural systems for processing social exclusion from childhood to adolescence. Dev Sci. (2011) 14:1431–44. doi: 10.1111/j.1467-7687.2011.01087.x
16. Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, et al. Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage. (2011) 54:2462–71. doi: 10.1016/j.neuroimage.2010.10.049
17. Sebastian CL, Blakemore SJ. Understanding the neural response to social rejection in adolescents with autism spectrum disorders: a commentary on Masten, et al., McPartland, et al. and Bolling, et al. Dev Cogn Neurosci. (2011) 1:256–9. doi: 10.1016/j.dcn.2011.03.006
18. Sebastian CL, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage. (2011) 57:686–94. doi: 10.1016/j.neuroimage.2010.09.063
19. Vijayakumar N, Cheng TW, Pfeifer JH. Neural correlates of social exclusion across ages: a coordinate-based meta-analysis of functional MRI studies. Neuroimage. (2017) 153:359–68. doi: 10.1016/j.neuroimage.2017.02.050
20. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. (1978) 133:429–35. doi: 10.1192/bjp.133.5.429
21. Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry. (1984) 23:191–7. doi: 10.1097/00004583-198403000-00011
22. Downey G, Lebolt A, Rincón C, Freitas AL. Rejection sensitivity and children’s interpersonal difficulties. Child Dev. (1998) 69:1074–91.
23. Williams KD, Cheung CKT, Choi W. Cyberostracism: effects of being ignored over the Internet. J Pers Soc Psychol. (2000) 79:748–62. doi: 10.1037//0022-3514.79.5.748
24. Williams KD, Govan CL, Croker V, Tynan D, Cruickshank M, Lam A. Investigations into differences between social- and cyberostracism. Group Dyn. (2002) 6:65–77.
25. Geller B, Williams M, Zimerman B, Frazier J. Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS). St. Louis, MO: Washington University (1996).
26. Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, Delbello MP, et al. Reliability of the Washington University in St. Louis Kiddie schedule for affective disorders and schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. (2001) 40:450–5. doi: 10.1097/00004583-200104000-00014
27. Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. (1997) 36:980–8. doi: 10.1097/00004583-199707000-00021
28. Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behav Res Methods. (2006) 38:174–80. doi: 10.3758/bf03192765
29. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. (2016) 113: 7900–905. doi: 10.1073/pnas.1602413113
30. Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. (2009) 4(:423–8. doi: 10.1093/scan/nsp052
31. Poldrack RA, Baker, CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafo MR, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. (2017) 18:115–26. doi: 10.1038/nrn.2016.167
32. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. (2002) 15:273–89.
33. McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. (2012) 61:1277–86. doi: 10.1016/j.neuroimage.2012.03.068
34. Mwilambwe-Tshilobo L, Spreng RN. Social exclusion reliably engages the default network: a meta-analysis of cyberball. Neuroimage. (2021) 227:117666. doi: 10.1016/j.neuroimage.2020.117666
35. Brothers L, Ring B, Kling A. Response of neurons in the macaque amygdala to complex social stimuli. Behav Brain Res. (1990) 41:199–213. doi: 10.1016/0166-4328(90)90108-q
37. Albohn DN, Adams RB. Social vision: at the intersection of vision and person perception. In: Absher JR, Cloutier J editors Neuroimaging Personality, Social Cognition, and Character. San Diego, CA: Academic Press (2016). p. 159–86.
38. Müller VI, Höhner Y, Eickhoff SB. Influence of task instructions and stimuli on the neural network of face processing: an ALE meta-analysis. Cortex. (2018) 103:240–55. doi: 10.1016/j.cortex.2018.03.011
39. Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. (2008) 65:1303–12. doi: 10.1001/archpsyc.65.11.1303
40. Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA. (2006) 103:8900–5. doi: 10.1073/pnas.0603246103
41. Kryza-Lacombe M, Brotman MA, Reynolds RC, Towbin K, Pine DS, Leibenluft E, et al. Neural mechanisms of face emotion processing in youths and adults with bipolar disorder. Bipolar Disord. (2019) 21:309–20. doi: 10.1111/bdi.12768
42. Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. (2001) 104:667–76. doi: 10.1016/s0306-4522(01)00108-7
43. Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci. (2011) 15:143–51. doi: 10.1016/j.tics.2011.02.002
44. Chang K, Garrett A, Kelley R, Howe M, Sanders EM, Acquaye T, et al. Anomalous prefrontal-limbic activation and connectivity in youth at high-risk for bipolar disorder. J Affect Disord. (2017) 222:7–13. doi: 10.1016/j.jad.2017.05.051
45. Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Monkul ES, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. (2005) 162:1637–43. doi: 10.1176/appi.ajp.162.9.1637
46. Blatt GJ, Oblak AL, Schmahmann JD. Cerebellar connections with limbic circuits: anatomy and functional implications. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N editors. Handbook of the Cerebellum and Cerebellar Disorders. Berlin: Springer (2013). p. 479–96.
47. Kim D, Byul Cho H, Dager SR, Yurgelun-Todd DA, Yoon S, Lee JH, et al. Posterior cerebellar vermal deficits in bipolar disorder. J Affect Disord. (2013) 150:499–506. doi: 10.1016/j.jad.2013.04.050
48. Lupo M, Olivito G, Siciliano L, Masciullo M, Molinari M, Cercignani M, et al. Evidence of cerebellar involvement in the onset of a manic state. Front Neurol. (2018) 9:774. doi: 10.3389/fneur.2018.00774
Keywords: bipolar, social exclusion, neuroimaging, anxiety, cyberball
Citation: Roybal DJ, Cosgrove VE, Kelley R, Smallwood Shoukry R, Larios RM, Novy B, Chang KD and Garrett AS (2022) Aberrant Neural Response to Social Exclusion Without Significantly Greater Distress in Youth With Bipolar Disorder: Preliminary Findings. Front. Psychiatry 13:687052. doi: 10.3389/fpsyt.2022.687052
Received: 08 April 2021; Accepted: 15 February 2022;
Published: 01 April 2022.
Edited by:
Jeffrey Robert Strawn, University of Cincinnati, United StatesReviewed by:
Gemma C. Monté-Rubio, University of Barcelona, SpainJennifer Barredo, Providence VA Medical Center, United States
Copyright © 2022 Roybal, Cosgrove, Kelley, Smallwood Shoukry, Larios, Novy, Chang and Garrett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donna J. Roybal, ZGp3ZHYzMUBnbWFpbC5jb20=
†Present addresses: Donna J. Roybal, Division of Child and Adolescent Psychiatry, Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center, San Antonio, TX, United States; Traditions Behavioral Health, Napa, CA, United States Amy S. Garrett, Division of Child and Adolescent Psychiatry, Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center, San Antonio, TX, United States Kiki D. Chang, Private Practitioner, Palo Alto, CA, United States
 Donna J. Roybal
Donna J. Roybal Victoria E. Cosgrove
Victoria E. Cosgrove Ryan Kelley2
Ryan Kelley2 Rachel Smallwood Shoukry
Rachel Smallwood Shoukry Rose Marie Larios
Rose Marie Larios Blake Novy
Blake Novy Amy S. Garrett
Amy S. Garrett