- 1Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 2School of Nursing, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3School of Nursing, Hebei University, Baoding, China
- 4Johns Hopkins University School of Nursing, Baltimore, MD, United States
- 5Department of Nutrition and Food Hygiene, Hubei Key Laboratory of Food Nutrition and Safety, Ministry of Education Key Laboratory of Environment and Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 6Department of Pulmonary and Critical Care Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 7Qingdao University School of Nursing, Qingdao, Shandong, China
- 8Department of Natural Sciences, University of Houston-Downtown, Houston, TX, United States
Introduction: Either exposure to secondhand smoke (SHS) or frailty has been linked to adverse health outcomes in nonsmoking adults. However, their relationship is rarely studied. The purpose of this study is to examine the association between serum cotinine level and frailty status among non-smoking older adults.
Method: The study population consisted of 2,703 older adults aged ≥60 from the National Health and Nutrition Examination Survey 2011–2014. Non-smokers were included based on (1) a serum cotinine level ≤ 10 ng/mL and 2) a response of “no” to the question, “Do you currently smoke?” Frailty status was measured based on the Fried Phenotype and had three groups- robust, pre-frailty, and frailty. Multinomial logistic regression models were constructed to examine the association between serum cotinine level quartile and frailty status, controlling for age, sex, race/ethnicity, education, depressive symptoms, alcohol use, and systolic blood pressure.
Results: About half of the participants (median age 70.0 years, range 64–78) were female (53.6%), non-Hispanic White (48.3%), and completed some college and above (50.1%). Multinomial logistic regression with a reference group being those in the 1st quantile (the lowest) of serum cotinine level showed that participants in the 4th quartile (the highest) of serum cotinine level had increased odds of pre-frailty vs. robust (OR 1.522, 95% confidence interval [CI] 1.060, 2.185, P = 0.023) as well as increased odds of frailty vs. robust (OR 2.349, 95% CI 1.081, 5.107, P = 0.031).
Conclusions: Higher serum cotinine level is associated with increased risk of pre-frailty and frailty versus robust in non-smoking older adults. Prevention and reduction of SHS in older adults may help protect them from developing pre-frailty or frailty.
Introduction
Frailty and pre-frailty is a prominent aging-related symptom and is very common in older adults (1). Frailty is caused by the lifetime cumulative degradation in multiple physiological systems and is characterized by poor resolution of homeostasis following an external stressor (2). Pre-frailty is a condition predisposing a person to frailty and usually preceding the onset of frailty. According to a systematic review, the overall weighted prevalence of frailty in community-dwelling older adults aged 65 and older was 10.7%, ranging widely from 4.0 to 59.1% in various communities (1). Frailty is frequently referred to as a stage in between successful aging and disability (3). With frailty, the physical, mental, and social engagement of older persons are significantly compromised along with a number of other health indicators (4). As a result, older adults with frailty have an increased risk of falling, disability, immobility, hospitalizations, and lower quality of life (3). Early detection and intervention are crucial to avoid or reverse the development of frailty and its detrimental effects because frailty is a dynamic process and can be seen as a controllable state, especially in the early stages (5–7).
Another prevalent health threat in older adults is exposure to secondhand smoke (SHS). SHS refers to situations when a nonsmoker is exposed to either side-stream or mainline smoke and thus inhales other's smoke (8). SHS is common in older adults (9) and exposure to SHS causes an estimated 41,000 deaths each year among adults in the United States (10). Older adults are particularly vulnerable to the effects of tobacco exposure due to physiologic changes associated with aging and complex medical conditions (11, 12). The associations between active smoking and frailty has suggested smoking as a predictor of worsening frailty status in community-dwelling populations (13). Although many studies have examined the relationship between active smoking and frailty (13), the effect of SHS on frailty is poorly studied (11). To the best of our knowledge, there is only one published study on this topic (11). Therefore, a research gap exists on the impact of SHS on frailty in older adults. In this study, we examined the association between serum cotinine level, a biomarker of tobacco exposure, and frailty status in a group of non-smoking older adults using the National Health and Nutrition Examination Study (NHANES) from 2011 to 2014. The results of this study will provide implications for clinical practice and policy development aimed at preventing frailty among the growing number of older adults.
Methods
The parent study design
The NHANES is a continuous cross-sectional survey of civilian, non-institutionalized, America-residing adults and children led by the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC) (45). Participants with wide sociodemographic regions in the US are recruited using a complex, multistage probability strategy every 2 years (14). Participants' sociodemographic, health, and nutritional status are evaluated using in-person home interviews as well as health exams conducted at mobile exam centers.
For this analysis, the NHANES 2011-2012 (n = 9,338) and 2013–2014 (n = 9,813) were merged. People who (1) aged ≥ 60, (2) were not actively smokers at the time of the survey, and (3) had no missing information on serum cotinine level and frailty were included. We excluded people (1) who aged <60 (n = 15,679), (2) responded “yes” to the question, “Do you currently smoke?” (n = 444), (3) had a serum cotinine level >10 ng/mL (n = 325), or had missing values on serum cotinine level or frailty status (n = 0). People with serum cotinine levels >10 ng/mL were eliminated from the analysis since this level is almost always present in active smokers (15). Finally, the study population consisted of 2,703 non-smoking older adults aged 60 and above.
Ethical considerations
The NHANES has received ethical permission from the National Center for Health Statistics Research Ethics Review Board. Because we solely used de-identified, publicly accessible data, the University of Houston-Downtown Committee for the Protection of Human Subjects gave this study an exemption.
Measures
Independent variable: Serum cotinine level quartile (1st, 2nd, 3rd, and 4th quartile)
As the most commonly used biomarker for smoking, cotinine is the main metabolite of nicotine, with a half-life of 15 to 20 h. Serum or urinary cotinine level may be used to detect both active smoking and SHS (16). Serum samples from participants were collected during physical exams, aliquoted, and stored at−20°C until being analyzed. Serum cotinine level was measured using the isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometric (ID HPLC-APCI MS/MS) method. The thorough procedure has been published in another study (17). A blank and two quality-control pools were used in each analytical run. Strict quality control and quality assurance were conducted by the Division of Laboratory Sciences, National Center for Environmental Health, CDC (18). This method for measuring serum cotinine has a lower detection limit (LLOD) of 0.015 ng/mL using the variation from the repeated analysis of a 0.2 ml spiked serum sample.
Dependent variable: Frailty status (robust, pre-frailty, or frailty)
Frailty status was classified in accordance with a previous NHANES-based study (19). Based on the Fried Phenotype (20), when three or more of the following conditions were met, the person was determined to be frail, including unintended weight loss, sluggish walking, weakness, fatigue, and a lack of physical activity. One or two of the conditions must be present for pre-frailty to exist, but none of the criteria must be present for robustness. Our definition followed the Fried Phenotype's five frailty dimensions; however, we modified the standards for use based on the NHANES data, which is consistent with a previously published study (19). All the following variables were operated as binary variables (yes or no).
1) Unintentional weight loss was determined by participants' responses to three questions: (a) “How much do you weigh without clothes or shoes?” (b) “How much did you weigh a year ago?” and (c) “was the change between your current weight and weight a year ago intentional?” Low body weight for height was defined as having a body mass index (BMI) less or equal to 22.5 kg/m2 or at least 5% unintentional weight loss (responding that their weight loss was not intentional) over the previous year. Other published studies have used the same criteria for determining unintentional weight loss (21, 22).
2) Sluggish walking. With no direct information on walking speed in NHANES, we used similar information to assess whether participants had slow walking speed. When asked, “By yourself and without using any special equipment, how much difficulty do you have walking from one room to another on the same level?” Participants who responded, “with some difficulty,” “with significant difficulty,” or “unable to do” were categorized as having slow walking speed.
3) Weakness. If participants responded, “with some difficulty,” “great difficulty,” or “unable to do” to the question “by yourself and without using any special equipment, how much difficulty do you have lifting or carrying something as heavy as 10 pounds?” they would be categorized as having weakness.
4) Fatigue. If participants responded, “with some difficulty,” “great difficulty,” or “unable to do” to the question “by yourself and without using any special equipment, how much difficulty do you have walking for a quarter of a mile?” they would be categorized as having exhaustion.
5) Lack of physical activity. Participants reported an average amount of vigorous and moderately intense activity in minutes for three kinds of activities- work, going to and from locations, and recreation. After that, metabolic equivalent (MET) minutes were computed. One MET minute is equal to 3.5 ml of O2 per kilogram of body weight multiplied by the length of time in minutes spent sitting at rest. It indicates the amount of oxygen consumed while sitting at rest (23). Participants would be categorized as having low physical activity if their MET minutes per week were <600.
Covariates
To control for potential confounding between serum cotinine level and frailty status, the following covariates were included in the study- age (years), sex (male or female), race/ethnicity (Mexican Americans, other Hispanics, non-Hispanic White, or non-Hispanic Black), education (below high school, high school graduate, or some college or above), alcohol use (0–1 drink per day, two drinks per day, or three and more drinks per day), depressive symptoms, total cholesterol (mg/dL), and systolic blood pressure (mmHg). All the above information was collected via face-to-face interviews or health exams. The Patient Health Questionnaire (PHQ-9) total score was used to represent depressive symptoms (24). The PHQ-9 has shown good reliability and validity among the general population (25).
Statistical analysis
For descriptive statistics, means (standard deviation) were used to describe continuous data with normal distribution and medians (interquartile range) for continuous data not following a normal distribution. Frequency (percentages) was used to describe categorical data. Multinomial logistic regression models were used to examine the independent relationship between serum cotinine level quartile (reference: 1st quartile, the lowest) and frailty (pre-frailty, frailty, or robust) (reference: robust), controlling the covariates mentioned above. Prior to constructing the regression model, we examined whether multicollinearity existed among the covariates with the resulting variance inflation factor (VIF) less than ten, indicating no multicollinearity (26). A 95% confidence interval (CI) (11) excluding one or a P-value < 0.05 was considered as statistical significance. All analyses were performed using SPSS 25.0.
Results
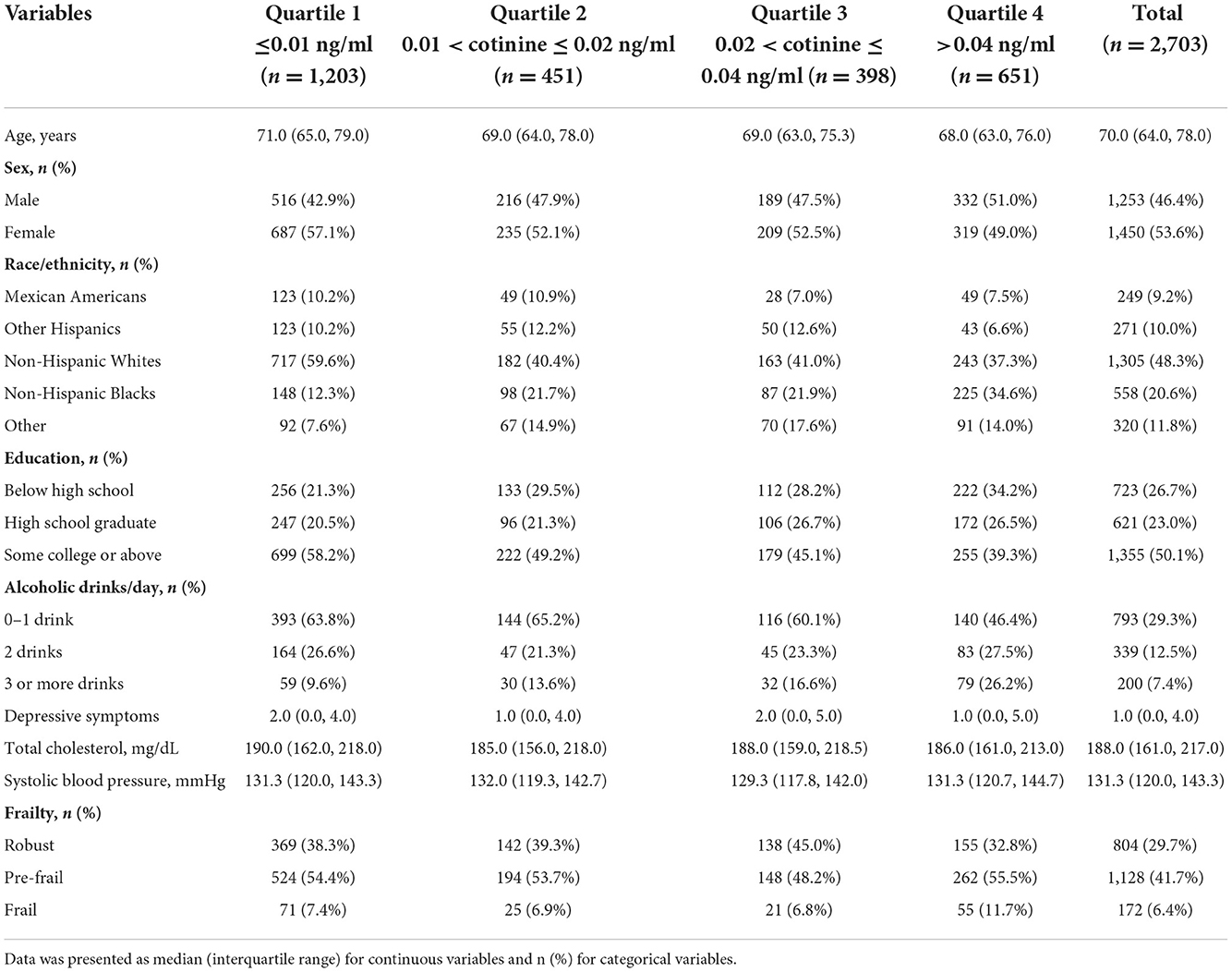
The characteristics of the study population were presented in Table 1. The 2,703 participants had a median age of 70.0 years, ranging from 64 to 78. About half of them were female (53.6%), non-Hispanic White (48.3%), completed some college or above (50.2%), had a BMI ≥ 25 kg/m2 (73.4%), had 0–1 drink of alcohol use per day (29.3%), and were never smokers (58.2%). The participants had a median of 188.0 mg/dL total cholesterol (range 161.0, 217.0) and 131.3 mmHg systolic blood pressure (range 120.0, 143.3). Their mean serum cotinine level (ng/mL) was 0.13 (SD 0.60), ranging from 0.01 to 9.90. In terms of their frailty status, most of them had pre-frailty (41.7%), followed by robust (29.7%), and frailty (6.4%).
Multinomial logistic regression (Tables 2, 3) with a reference group being those in the 1st quartile (the lowest) of serum cotinine level showed that participants in the 4th quartile (the highest) of serum cotinine level had increased odds of pre-frailty vs. robust (OR 1.522, 95% CI 1.060, 2.185, P = 0.023) as well as increased odds of frailty vs. robust (OR 2.349, 95% CI 1.081, 5.107, P = 0.031). No significance was found in pre-frailty or frailty for the 2nd and 3rd quartiles, compared with the 1st quartile.
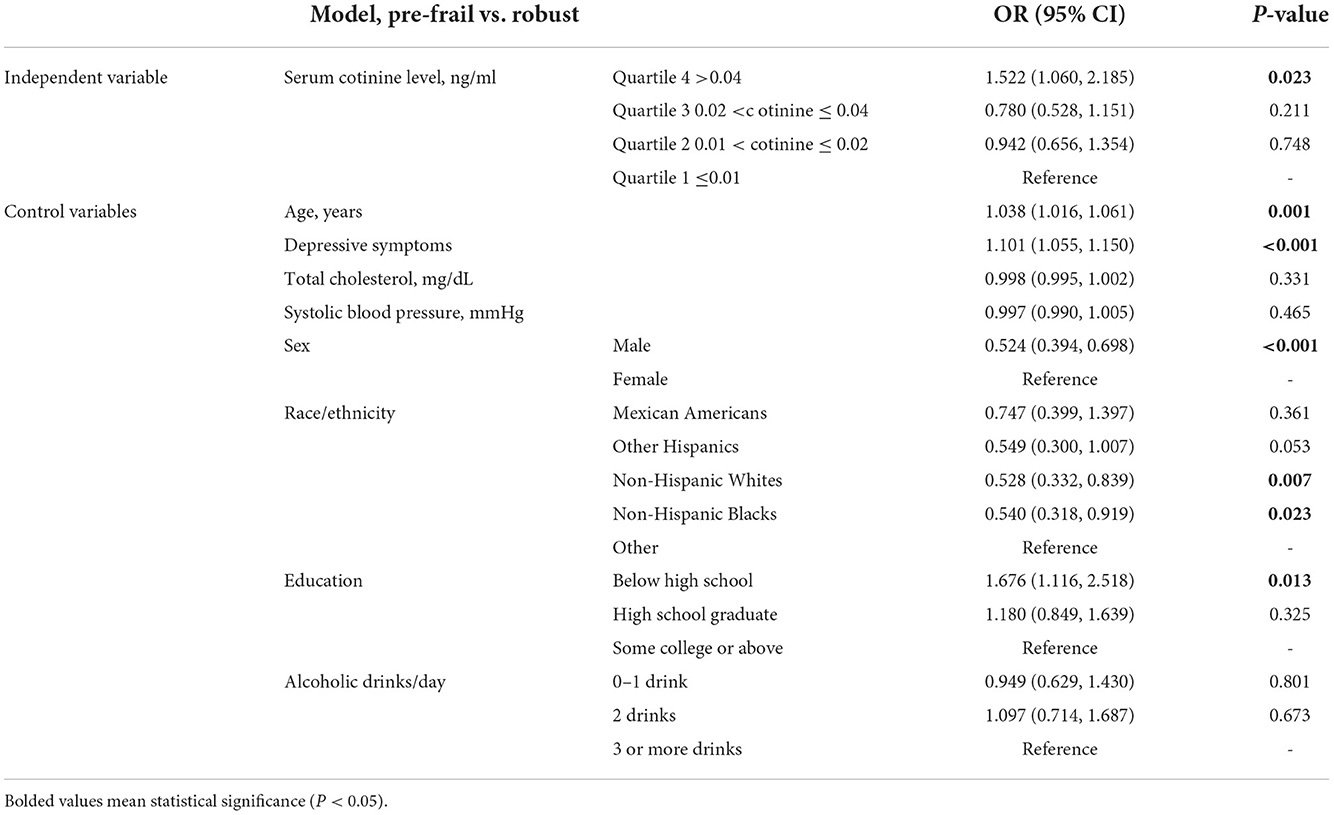
Table 2. The independent associations of serum cotinine level quartile (reference: ≤0.01 ng/ml) with pre-frailty.
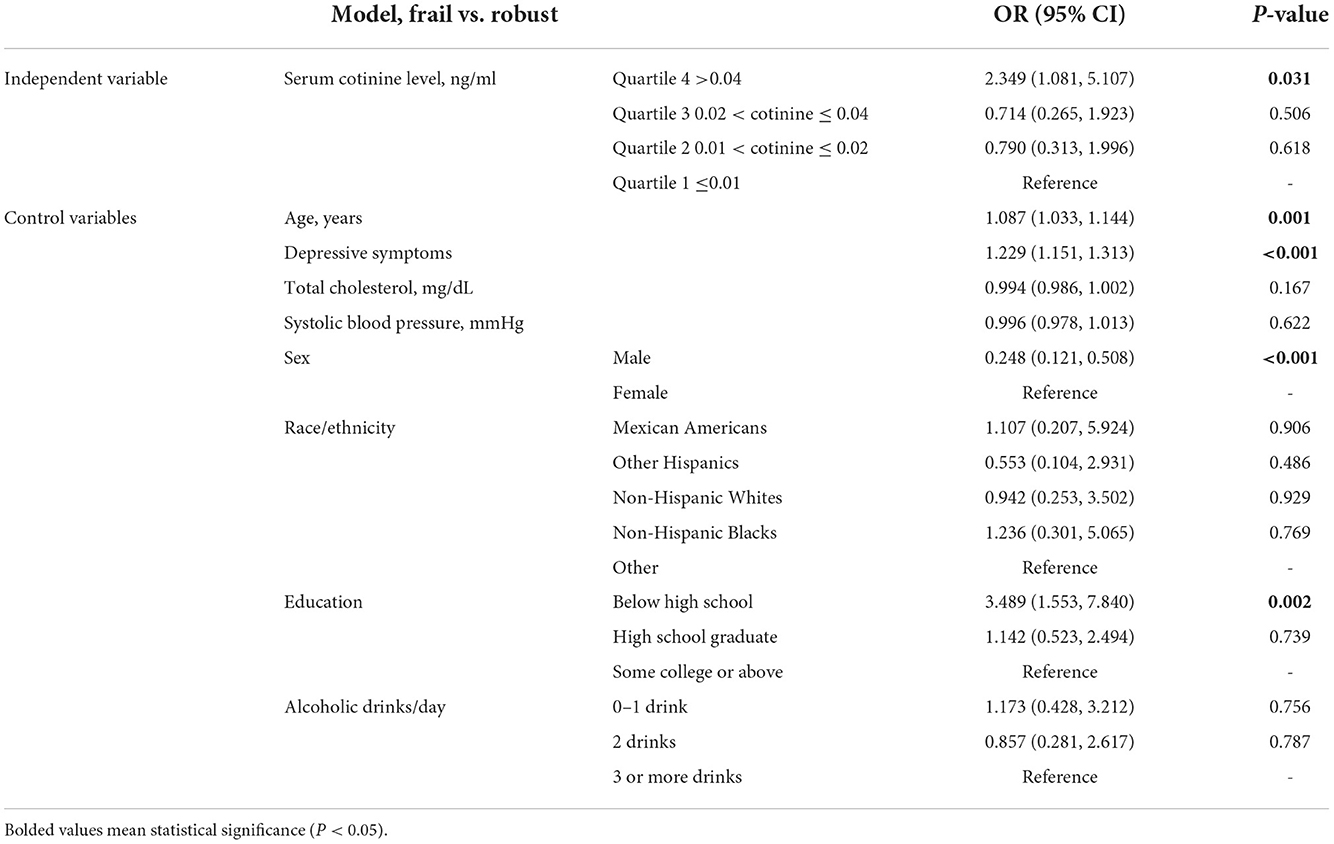
Table 3. The independent associations of serum cotinine level quartile (reference: ≤0.01 ng/ml) with frailty.
Discussion
In this group of 2,703 non-smoking older adults, we found that almost half of them had either pre-frailty or frailty. Higher serum cotinine level (>0.04 ng/ml) is associated with an increased risk of pre-frailty and frailty vs. robust. Although our results need to be validated by future prospective studies, they indicate that prevention and reduction of SHS in older adults may prevent them from developing pre-frailty or frailty. Our findings provided implications for clinical practice and policy development aiming at preventing frailty among the growing number of older adults.
Studies have identified positive associations between SHS and cardiovascular diseases (27), stroke (28), lung cancer (29–32), diabetes (33), and all-cause mortality (34) among adults. In addition, a systematic review has found that active smoking predicts the worsening frailty status in various community-dwelling populations (13). However, to date, extremely limited research has been conducted to illustrate the association between SHS and frailty in older adults. To our knowledge, there is only one published study that examined the relationship between SHS and frailty using the NHANES III data collected from 1988 to 1994 (11). Similar to our findings, García-Esquinas et al. (11) found that ~6% of older adults aged 60 years and older had frailty and those in the 4th quartile of serum cotinine, compared with those in the lowest quartile of serum cotinine, were more likely to be frail (OR 2.51, 95% CI 1.06, 5.95). In addition to the findings similar to those of García-Esquinas et al. (11) our study provided additional evidence on the association between SHS and pre-frailty in older adults. In another study (n = 71), Teixeira-Gomes et al. (35) found a statistically higher prevalence of second-hand smokers in the pre-frailty group compared with that in the robust group; however, the relationship was insignificant for the frailty group. The findings of our study clarified the inconsistent findings of that study and addressed the knowledge gap on SHS and frailty pointed out by Teixeira-Gomes et al. (35). In another study on SHS, although researchers did not directly assess frailty, they examined grip strength and concluded that even low levels of exposure to SHS were associated with decreased grip strength (36).
The possible mechanism that explains the relationship between SHS and pre-frailty status is unclear but may be multifactorial. Tobacco smoke contains a mixture of multiple toxic chemicals and compounds and may impair almost every organ in the human body. Tobacco smoke has been linked to a variety of physical and mental illnesses (37), which may lead to the development of frailty. Specifically, the most prevalent explanation is that the toxic chemicals in tobacco smoke are associated with an increase in inflammatory markers (38). Chronic inflammation may lead to muscle wasting (39), weight loss, weakness, exhaustion, or slow gait, which are the main components of frailty (20). For example, elevated levels of interleukin-6, C-reactive protein, and tumor necrosis factor-α have been shown to decrease muscle mass, muscle strength, as well as other declined physical abilities (40). Research studies also have supported the positive association between interleukin-6 and C-reactive protein and prevalence and incidence of frailty (41, 42). Despite these findings, future studies are expected to clarify the mechanism.
This study has many strengths. Since there is only one study that examined the relationship between SHS and frailty status in non-smoking older adults, our study makes a novel contribution to the literature and fills in a research gap by providing solid evidence of the negative effect of SHS on frailty status in older adults. Our study has strong generalizability because of the size and representativeness of our study population. To ensure that the study population consisted of non-smokers, two criteria were applied to weed out active smokers. Several sociodemographic, lifestyle, mental health, and physical health factors were adjusted to reduce the risk of residual confounding. According to studies, when exposed to tobacco, older persons are more prone to develop diabetes, osteoporosis, cardiovascular disease, chronic renal disease, and respiratory problems with a poorer prognosis compared with the younger group (43). Thus, our study focused on a neglected problem in an at-risk population.
At the same time, several limitations are contained in this study. To start, because of the cross-sectional nature of this study, we are unable to determine the temporal connection between SHS and frailty status. Furthermore, cotinine is only a reflection of a person's recent exposure to tobacco and does not account for their long-term exposure (half-life 15–20 h). In addition, some excluded people with serum cotinine levels> 10 ng/mL may be non-smokers that are heavily exposed to SHS. Future studies are needed to examine the longitudinal relationship between other biomarkers of tobacco exposure that have a longer half-life, such as 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) (44) and frailty in non-smoking older adults, especially those residing in developing countries. The mechanisms explaining the relationship between the two variables should also be explored.
The following are the study's clinical implications: Serum cotinine level, a biomarker of tobacco exposure, was found to be a risk factor for both pre-frailty and frailty in non-smoking older adults. To educate the public on the harmful effects of SHS, officials should advocate smoking-free policies and use social media and other educational strategies to help minimize older adults' SHS exposure at home and in public settings. In clinical settings, clinicians should also talk about specific measures to minimize SHS exposure for their older patients. These approaches may help prevent older adults from developing pre-frailty and frailty.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The NHANES were reviewed and approved by the National Center for Health Statistics Research Ethics Review Board. The participants provided written informed consent to participate in this study.
Author contributions
ZF, WM, SG, FD, XL, and ML drafted the initial manuscript, designed the study, and searched for literature. TZ and YS conducted statistical analysis. All authors critically revised the manuscript, contributed to the article, and approved the submitted version.
Acknowledgments
We thank all participants in the NHANES Study. We thank the NHANES research team for collecting and sharing the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. (2012) 60:1487–92. doi: 10.1111/j.1532-5415.2012.04054.x
2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. (2013) 381:752–62. doi: 10.1016/S0140-6736(12)62167-9
3. Chen CY, Gan P, How CH. Approach to frailty in the elderly in primary care and the community. Singapore Med J. (2018) 59:240. doi: 10.11622/smedj.2018052
4. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. doi: 10.1016/S0140-6736(19)31786-6
5. Lee JS, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc. (2014) 15:281–6. doi: 10.1016/j.jamda.2013.12.002
6. Park Y, Choi JE, Hwang HS. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2018) 108:1026–33. doi: 10.1093/ajcn/nqy214
7. Wei K, Thein FS, Nyunt MSZ, Gao Q, Wee SL, Ng T-P. Nutritional and frailty state transitions in the singapore longitudinal aging study. J Nutr Health Aging. (2018) 22:1221–7. doi: 10.1007/s12603-018-1096-3
8. Ling J, Heffernan T. The cognitive deficits associated with second-hand smoking. Front Psychiatry. (2016) 7:46. doi: 10.3389/fpsyt.2016.00046
9. Craciun OM, Ortolá R, Pascual JA, Pérez-Ortuño R, Galán Labaca I, Banegas JR, et al. Secondhand tobacco smoke and functional impairments in older adults living in the community. Nicotine Tob Res. (2022) 24:2026–34. doi: 10.1093/ntr/ntac131
10. World Health Organization. WHO Report on the Global Tobacco Epidemic, 2021: Addressing New and Emerging Products. World Health Organization (2021).
11. García-Esquinas E, Navas-Acien A, Rodríguez-Artalejo F. Exposure to secondhand tobacco smoke and the frailty syndrome in US older adults. Age. (2015) 37:1–9. doi: 10.1007/s11357-015-9762-4
12. Ge S, Tang X, Wei Z, Dune L, Liu T, Li J, et al. Smoking and cognitive function among middle-aged adults in China: findings from the China health and retirement longitudinal study baseline survey. J Addict Nurs. (2020) 31:E5–12. doi: 10.1097/JAN.0000000000000352
13. Kojima G, Iliffe S, Walters K. Smoking as a predictor of frailty: a systematic review. BMC Geriatr. (2015) 15:1–7. doi: 10.1186/s12877-015-0134-9
14. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszan-Moran D, Dohrmann SM, et al. National Health and Nutrition Examination Survey. Analytic guidelines 1999–2010 (2013).
15. Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. (2005) 57:79–115. doi: 10.1124/pr.57.1.3
16. Llewellyn DJ, Lang IA, Langa KM, Naughton F, Matthews FE. Exposure to secondhand smoke and cognitive impairment in non-smokers: national cross sectional study with cotinine measurement. BMJ. (2009) 338. doi: 10.1136/bmj.b462
17. Jacob III P, Yu L, Duan M, Ramos L, Yturralde O, et al. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography–tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. (2011) 879:267–76. doi: 10.1016/j.jchromb.2010.12.012
18. Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. (2008) 27:4094–106. doi: 10.1002/sim.3222
19. Baniak LM, Yang K, Choi J, Chasens ER. Long sleep duration is associated with increased frailty risk in older community-dwelling adults. J Aging Health. (2020) 32:42–51. doi: 10.1177/0898264318803470
20. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–57. doi: 10.1093/gerona/56.3.M146
21. Kamil RJ, Li L, Lin FR. Association of hearing impairment and frailty in older adults. Otolaryngol Head Neck Surg. (2014) 151:P195–6. doi: 10.1177/0194599814541629a183
22. Wilhelm-Leen ER, Hall YN, Tamura MK, Chertow GM. Frailty and chronic kidney disease: the third national health and nutrition evaluation survey. Am J Med. (2009) 122:664–71. doi: 10.1016/j.amjmed.2009.01.026
23. Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. (1990) 13:555–65. doi: 10.1002/clc.4960130809
24. Kroenke K, Spitzer R, Williams J. The patient health questionnaire (phq-9)–overview. J Gen Intern Med. (2001) 16:606–16. doi: 10.1046/j.1525-1497.2001.016009606.x
25. Martin A, Rief W, Klaiberg A, Braehler E. Validity of the brief patient health questionnaire mood scale (PHQ-9) in the general population. Gen Hosp Psychiatry. (2006) 28:71–7. doi: 10.1016/j.genhosppsych.2005.07.003
26. Miles J. Tolerance and Variance Inflation Factor. Wiley Statsref: Statistics Reference Online Wiley StatsRef (2014). doi: 10.1002/9781118445112.stat06593
27. Steenland K, Thun M, Lally C, Heath C. Environmental tobacco smoke and coronary heart disease in the American cancer society CPS-II cohort. Circulation. (1996) 94:622–8. doi: 10.1161/01.CIR.94.4.622
28. Zhang X, Shu XO, Yang G, Li HL, Xiang YB, Gao Y-T, et al. Association of passive smoking by husbands with prevalence of stroke among Chinese women nonsmokers. Am J Epidemiol. (2005) 161:213–8. doi: 10.1093/aje/kwi028
29. Hirayama T. Non-smoking wives of heavy smokers have a higher risk of lung cancer: a study from Japan. Br Med J. (1981) 282:183–5. doi: 10.1136/bmj.282.6259.183
30. Kim CH, Lee YCA, Hung RJ, McNallan SR, Cote ML, Lim WY, et al. Exposure to secondhand tobacco smoke and lung cancer by histological type: a pooled analysis of the international lung cancer consortium (ILCCO). Int J Cancer. (2014) 135:1918–30. doi: 10.1002/ijc.28835
31. Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol. (2007) 36:1048–59. doi: 10.1093/ije/dym158
32. Trichopoulos D, Kalandidi A, Sparros L, Macmahon B. Lung cancer and passive smoking. Int J Cancer. (1981) 27:1–4. doi: 10.1002/ijc.2910270102
33. Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2015) 3:958–67. doi: 10.1016/S2213-8587(15)00316-2
34. Öberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. (2011) 377:139–46. doi: 10.1016/S0140-6736(10)61388-8
35. Teixeira-Gomes A, Lage B, Esteves F, Sousa AC, Pastorinho MR, Valdiglesias V, et al. Frailty syndrome, biomarkers and environmental factors–a pilot study. Toxicol Lett. (2020) 330:14–22. doi: 10.1016/j.toxlet.2020.04.023
36. Carrasco-Rios M, Ortolá R, Rodríguez-Artalejo F, García-Esquinas E. Exposure to secondhand tobacco smoke is associated with reduced muscle strength in US adults. Aging. (2019) 11:12674. doi: 10.18632/aging.102594
37. Choices N. What Are the Health Risks of Smoking. In: Retrieved from the NHS Choices Website. (2007). Available online at: http://www.nhs.uk/chq/Pages/2344.aspx
38. Gonçalves R, Coletta R, Silvério K, Benevides L, Casati M, Da Silva J, et al. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. (2011) 60:409–24. doi: 10.1007/s00011-011-0308-7
39. Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. (2009) 122:605–13. doi: 10.1016/j.amjmed.2009.01.030
40. Wang C, Song X, Mitnitski A, Yu P, Fang X, Tang Z, et al. Gender differences in the relationship between smoking and frailty: results from the Beijing longitudinal study of aging. J Gerontol A Biomed Sci. (2013) 68:338–46. doi: 10.1093/gerona/gls166
41. Gale CR, Baylis D, Cooper C, Sayer AA. Inflammatory markers and incident frailty in men and women: the english longitudinal study of ageing. Age. (2013) 35:2493–501. doi: 10.1007/s11357-013-9528-9
42. Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol. (2005) 63:403–11. doi: 10.1111/j.1365-2265.2005.02355.x
43. Burns DM. Cigarette smoking among the elderly: disease consequences and the benefits of cessation. Am J Health Promot. (2000) 14:357–61. doi: 10.4278/0890-1171-14.6.357
44. Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. (2011) 13:202–8. doi: 10.1093/ntr/ntq237
45. NHANES. 011-2012 Data Documentation, Codebook, and Frequencies. (2017). Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/CFQ_G.htm
Keywords: cotinine, cognitive function, older adults, NHANES, secondhand smoke, tobacco
Citation: Fu Z, Zhou T, Dong F, Li M, Lin X, Ma W, Song Y and Ge S (2022) Secondhand smoke is positively associated with pre-frailty and frailty in non-smoking older adults. Front. Psychiatry 13:1095254. doi: 10.3389/fpsyt.2022.1095254
Received: 11 November 2022; Accepted: 28 November 2022;
Published: 15 December 2022.
Edited by:
Dan Song, Shenzhen Shekou People's Hospital, ChinaReviewed by:
Derong Zeng, Kyoto University, JapanQingxia Zhao, Chengdu University of Traditional Chinese Medicine, China
Copyright © 2022 Fu, Zhou, Dong, Li, Lin, Ma, Song and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weixia Ma, bXd4N0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zhenmei Fu1†
Zhenmei Fu1† Fanghong Dong
Fanghong Dong Weixia Ma
Weixia Ma Yuting Song
Yuting Song Song Ge
Song Ge