- 1Department of Psychiatry, Renmin Hospital, Wuhan University, Wuhan, China
- 2Department of Psychiatry, The Second Affiliated Hospital, Guangxi Medical University, Nanning, China
- 3Department of Psychology, The Fourth Affiliated Hospital, Xinjiang Medical University, Urumqi, China
- 4Department of Psychiatry, The Second Xiangya Hospital, Central South University, Changsha, China
- 5Global Clinical and Translational Research Institute, Bethesda, MD, United States
Editorial on the Research Topic
Temporal lobe dysfunction in neuropsychiatric disorder
The temporal lobe is a significant part of the human brain forming the cerebral cortex with other lobes. It spans several cortical regions paralleling five temporal gyri, including the superior temporal gyrus (STG), the middle temporal gyrus (MTG), and inferior temporal gyrus (ITG), the fusiform gyrus, and the parahippocampal gyrus/entorhinal gyrus (1). The temporal lobe directly interacts with the limbic system, which plays essential roles in cognitive systems, emotional regulation, autonomic nervous systems, and information relay. Limbic circuits are involved in multiple neuropsychiatric disorders (2). However, the underlying neural mechanisms remain to be elucidated.
This Research Topic published 21 articles that include 20 original research using resting-state fMRI (rs-fMRI) in patients with neuropsychiatric and related disorders relative to healthy controls (HCs). Seven studies focused on temporal lobe epilepsy (TLE); Five on major depressive disorder (MDD), two of which patients underwent antidepressant treatment; Two studies about schizophrenia (SCZ); Others focused on monocular blindness (MCB), congenital blindness (CB), primary nocturnal enuresis (PNE), acoustic neuroma, sudden sensorineural hearing loss (SSHL), premenstrual syndrome (PMS), and premenstrual dysphoric disorder (PMDD).
Temporal lobe epilepsy
Two studies provided consistent evidence that patients with TLE had decreased FC in the bilateral MTG. Based on the voxel-mirrored homotopic connectivity (VMHC) (3), Wu et al. found that patients with lTLE exhibited a decreased VMHC in the bilateral MTG and middle cingulum gyrus (CG). With a similar study design, Chu et al. found that patients with the right TLE (rTLE) had a decreased VMHC in the bilateral superior temporal pole (STP), middle temporal pole (MTP), MTG, ITG, and inferior frontal gyrus (IFG). In contrast, they exhibited an increase of VMHC in the bilateral precentral gyrus (PreCG), the postcentral gyrus (PoCG), and the supplemental motor area (SMA).
Patients with TLE may have altered network connectivity. Li et al. studied network homogeneity (NH) of the ventral somatomotor network (VSN) in patients of lTLE and rLTE and found reduced NH in the bilateral Rolandic operculum and STG, but increased in PoCG. Huang et al. showed rTLE patients had a decreased NH of default mode network (DMN) in the R-ITG and L-MTG, but an increase in the bilateral PCu and R-inferior parietal lobe. Based on the degree of centrality (DC) (4), Guo et al. showed that patients with rTLE had a reduced DC in the R-caudate, but increased network connectivity in the L-MTG, superior parietal gyrus (SPG), inferior parietal gyrus (IPG), superior frontal gyrus (SFG), medial SFG, IFG, and R-precuneus (PCu).
The basal forebrain (BF) is a cluster of subcortical structures providing axonal projection to the entire cerebral cortex and comprises four nuclei of the medial septal (MS, Ch1), the vertical limb of the diagonal band of Broca (vDbB, Ch2), the horizontal limb of the diagonal band of Broca (hDbB, Ch3), and the nucleus basalis of Meynert (nBM, Ch4) (5). Fan et al. studied TLE patients with and without focal to bilateral tonic-clonic seizure (FBTCS), using four basal forebrain subregions to examine the resting-state FC (rsFC). Regardless of FBTCS status, the TLE patients had reduced rsFC of BF subregions with the cerebellum, striatum, DMN, frontal and occipital lobes. Decreased rsFC of Ch1-3 with bilateral striatum and left cerebellum posterior lobe and Ch4 with bilateral amygdala was associated with FBTCS.
Major depressive disorder
MD is highly heterogeneous and associated with multi-faceted risk factors (6, 7). While neuroimaging studies have shown that patients with MDD tend to have altered brain structure and FC (8, 9), there is significant inconsistency. Five studies focused on brain activity and functional connectivity in MDD using various neuroimaging techniques (Supplementary Box).
Two studies examined brain activities in patients with MDD relative to HCs and after antidepressant treatment. Wang et al. studied drug-naïve first episode of adolescent MDD who also had 2-week electroconvulsive therapy (ECT). Whole brain voxel analysis showed increased activity in the R-orbital IFG, inferior occipital gyrus (IOG), and L-middle frontal gyrus (MFG) in patients with MDD. Meanwhile, patients treated with ECT significantly increased brain activity in the R-medial SFG, anterior cingulate gyrus (ACG), paracingulate gyrus (paraCG), medial CG and PCG, dorsolateral SFG, and L-MFG. Also, the increased brain activity in the frontal gyri seemed to be significantly associated with reducing clinical severity. Xiong et al. detected a high activity in the bilateral MFG, CG, and MTG in MDD; patients treated with vortioxetine for 2 weeks had decreased brain activity in the R-ITG, but increased activity in the L-low cerebellum, R-CG, and central posterior gyrus.
Three studies focused on regional homogeneity and network connectivity. Song et al. found that patients with drug-naïve first-episode MDD had an increase of ReHo in the L-anterior cingulate cortex (ACC) but decreased ReHo in the L-PreCG. However, none of these were significantly associated with the HAMD score. Luo et al. studied treatment-naïve first-episode MDD and HCs matched by gender, age, and education. They found a decreased NH in the R-PCu and abnormal executive control reaction time relative to HCs; the decreased NH was not significantly correlated to the clinical severity. In addition, Lin et al. conducted a larger-size study of 198 cases with MDD and 234 HCs using network centrality measure. Patients with MDD exhibited an elevated level of DC in the L-anterior cerebellar lobe, vermis, L-hippocampus, L-caudate, but a reduced DC in the L-posterior cerebellar lobe, L-insula, and R-caudate.
Han et al. conducted a clinical study of patients with MDD. They found that the frequency of negative evaluation and emotional words were significantly associated with the severity of MDD measured by HAMD-17.
Schizophrenia
Activation of auditory-related brain regions is one of the neuropathological mechanisms in SCZ. Disruption of functional connectivity in the L-temporal lobe, particularly L-STG has been indicated in AVH (10). Xue et al. studied voxel-wised dynamic FC (dFC) of the primary auditory cortex—Heschl's gyrus (HES) and auditory association cortex (AAC) in drug-naïve first episode SCZ with and without AVH. They found SCZ patients with AVH had an increased dFC of L-AAC with R-MTG and middle occipital gyrus (MOG) but a decreased dFC of L-HES gyrus with L-superior CG, L-cuneus, and L-PCu gyri, and R-HES gyrus with posterior CG.
Inter-hemispheric disconnection has been a notable pathological finding in SCZ (11, 12). Chen et al. studied inter-hemispheric connectivity, focusing on the STG cluster (extending into Heschl's gyrus, insula, and Rolandic operculum) and fusiform cluster (growing into the para-hippocampus). SCZ with AVH showed a reduced VMHC in the fusiform cluster; A decreased VMHC in the STG cluster was observed in both SCZ with and without AVH compared to HCs.
Acoustic neuromas and sudden sensorineural hearing loss
Acoustic neuroma (AN) is a type of tumor that develops from the sheath of Schwann cells and grows in the ear and can affect hearing and body balance function. While often described as transient, the psychiatric symptoms in patients with ANs may include mood change, memory loss, hallucination, and delusion (13). Deng et al. performed a clinical neuropsychological study of patients with ANs, and then an rs-fMRI investigation. Patients with ANs exhibited a cognitive decline, reduced ReHo, and decreased connectivity in the frontal lobe. Liu et al. found a reduced ReHo in the L-cerebellum, B-ITG, L-STP, R-para-hippocampal gyrus, L-PCC, and R-SFG in patients with SSHLcompared to HCs.
Congenital and monocular blindness
Lack of visual experience may affect the development of brain structure and functions development. Hu J-J. et al. found that patients with CB decreased ReHo in the R-orbital MFG, bilateral MOG, and R-dorsolateral SFG, but an increase in the L-paracentral lobule, R-insula, and bilateral thalamus. Using the percentage of amplitude fluctuations (perAL), Hu Q. et al. found that patients with MCB had decreased brain activity in the middle OL and the L-middle cingulate lobe but increased activity in the frontal and temporal lobes.
Meibomian gland dysfunction in severely obese population
Meibomian gland dysfunction is a chronic and diffuse abnormality of meibomian glands characterized by terminal duct obstruction. Xu et al. found that Meibomian gland dysfunction (MGD) in severely obese people exhibited decreased brain activity in the R-cerebellum, L-fusiform gyrus, R-medial orbitofrontal gyrus, L-triangle IFG, and L-IPG, but increased activity in the left lingual gyrus, R-globus pallidus, right ACG and para-CG, and L-MOG. The increased brain activity in R-cerebellum and left triangle IFG seemed to be associated with a high anxiety and depression score. Notably, this analysis was based on a tiny sample (n = 12).
Primary nocturnal enuresis
The development of chidrlem with PNE may involve a brain-bladder control network. Zhong et al. performed a seed-based rsFC of PNE and HCs with the first attempt to focus on four regions of the left and right rostral hippocampus (rHipp) and caudal hippocampus (cHipp) as of interest (ROI). Children with PNE exhibited a decreased rsFC in L-rHipp with R-fusiform gyrus, R-Rolandic operculum, L-inferior parietal lobe (IPL), and R-PreCG, but a reduced rsFC in L-cHipp with R-fusiform gyrus and R-SMA. The reduced FC of L-rHipp with R-Rolandic operculum and left cHipp with fusiform was also associated with the disease duration. The altered functional connectivity in these brain regions may involve multiple brain networks, including the limbic system, sensorimotor, DMN, and frontoparietal network. In addition to the brain-bladder control network, these brain regions are important in cognitive function and emotion.
Premenstrual syndrome and premenstrual dysphoric disorder
Premenstrual syndrome (PMS) is a group of clinically significant somatic and psychological symptoms or distress that women develop during the luteal phase of the menstrual cycle (about a week or two before the period) (14). Its severe form, premenstrual dysphoric disorder (PMDD), is classified as a subcategory of depression. Long et al. contributed a mini-review of neuroimaging studies of PMS and PMDD. Neuroimaging studies show more robust evidence of structural and functional alterations in the amygdala and the hippocampus's medial temporal lobe (MTL). Also, an fMRI study indicated abnormal fusiform gyrus activity in patients with PMS and PMDD.
Summary
The studies indicated temporal lobe dysfunction in neuropsychiatric and related disorders. In TLE, decreased FC in the MTG, ITG, and Rolandic operculum were identified, and reduced FCs of BF subregions with the striatum and cerebellum were associated with both TLE and FBTCS. However, increased connectivity in the PreCG and PoCG was associated with TLE (Table 1). These findings consistently appear in at least one independent studies or samples within the same study. The reduced FC between the left rHipp and the right Rolandic operculum was associated with PNE. Moreover, AVH in SCZ might be related to increased dsFC in L-AAC, decreased connectivity in the HES gyrus, and reduced inter-hemispheric connectivity in the fusiform cluster.
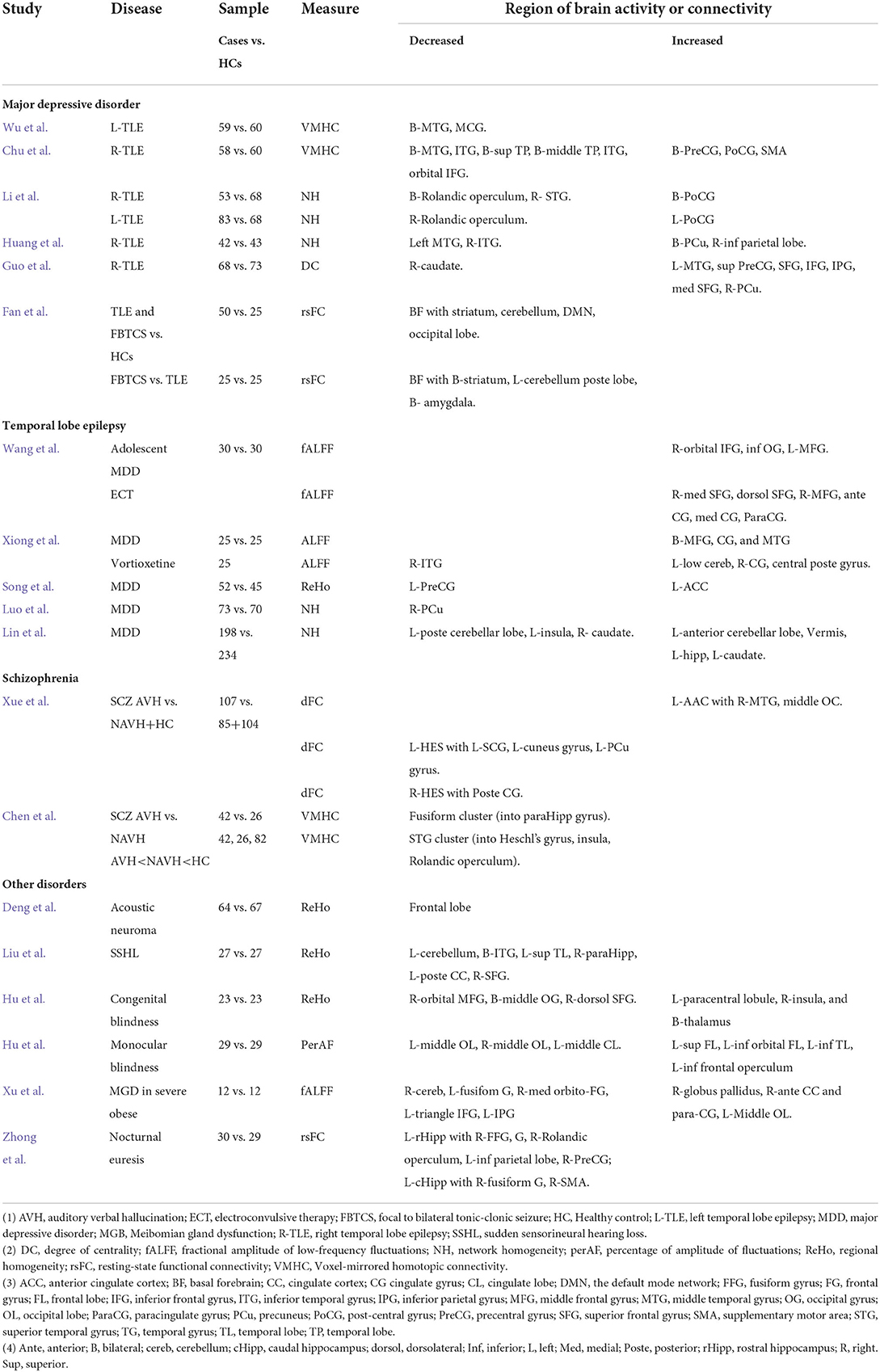
Table 1. rs-fMRI study of temporal lobe dysfunctions in temporal lobe epilepsy, major depression, schizophrenia and other disorders.
By contrast, findings from five studies of MDD seemed lacking consistency. Two studies with drug-naïve first episode MDD treated with antidepressant therapy did not find that antidepressant treatment significantly impacted the baseline brain activity associated with MDD. The inconsistent findings between disease association and response to treatment may pose a challenging question for interpretation. Part of the causes might be that patient heterogeneity had reduced the power to cause false-positive discovery.
The fMRI techniques have made it feasible to study the entire connectome at a large scale in humans. However, studies should move beyond the exploratory analysis of FC. Therefore, it calls for the rigor of study and advanced analytic techniques to maximize the critical tools of fMRI for neuroscience research. Furthermore, it might be more promising when fMRI combines with radionuclide scans such as positron emission tomography (PET) and structure MRI (sMRI), which can reveal the brain structure and functions at a circuit level. Multimodal neuroimaging may have a greater demand and challenge in visual data inspection, integration, and fusion for analysis (15).
Author contributions
FZ reviewed all articles, summarized individual studies' findings, and drafted the manuscript. All other editors participated in editing articles and reviewed and had access to the manuscript and related Supplementary materials. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all authors who contributed to this Research Topic; HY provided coordination for launching this Research Topic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1077398/full#supplementary-material
References
1. Baker CM, Burks JD, Briggs RG, Milton CK, Conner AK, Glenn CA, et al. A Connectomic Atlas of the human cerebrum-chapter 6: the temporal lobe. Oper Neurosurg. (2018) 15:S245–94. doi: 10.1093/ons/opy260
2. Byrum CE, Thompson JE, Heinz ER, Krishnan KR, Tien RD. Limbic circuits and neuropsychiatric disorders. Functional anatomy and neuroimaging findings. Neuroimaging Clin N Am. (1997) 7:79–99.
3. Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. (2010) 30:15034–43. doi: 10.1523/JNEUROSCI.2612-10.2010
4. Zuo X-N, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, et al. Network centrality in the human functional connectome. Cereb Cortex. (2012) 22 8:1862–75. doi: 10.1093/cercor/bhr269
5. Hedreen JC, Struble RG, Whitehouse PJ, Price DL. Topography of the Magnocellular basal forebrain system in human brain. J Neuropathol Exp Neurol. (1984) 43:1–21. doi: 10.1097/00005072-198401000-00001
6. Li Y, Lu J. Childhood adversity and depression among older adults: results from a longitudinal survey in China. Glob Clin Transl Res. (2019) 1:53–7. doi: 10.36316/gcatr.01.0007
7. Chen Y-Y, Yu S, Hu Y-H, Li C-Y, Artaud F, Carcaillon-Bentata L, et al. Risk of suicide among patients with Parkinson disease. JAMA Psychiatry. (2021) 78:293–301. doi: 10.1001/jamapsychiatry.2020.4001
8. Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. (2017) 22:1455–63. doi: 10.1038/mp.2016.72
9. Demirtas M, Tornador C, Falcon C, Lopez-Sola M, Hernandez-Ribas R, Pujol J, et al. Dynamic functional connectivity reveals altered variability in functional connectivity among patients with major depressive disorder. Hum Brain Mapp. (2016) 37:2918–30. doi: 10.1002/hbm.23215
10. Alderson-Day B, McCarthy-Jones S, Fernyhough C. Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci Biobehav Rev. (2015) 55:78–87. doi: 10.1016/j.neubiorev.2015.04.016
11. Lang X, Wang L, Zhuo CJ, Jia F, Wang LN, Wang CL. Reduction of interhemispheric functional connectivity in sensorimotor and visual information processing pathways in schizophrenia. Chin Med J. (2016) 129:2422–6. doi: 10.4103/0366-6999.191758
13. Kalayam B, Young RC, Tsuboyama GK. Mood disorders associated with acoustic neuromas. Int J Psychiatry Med. (1994) 24:31–43. doi: 10.2190/50PT-T9YD-ACKJ-1GUX
14. Yesildere Saglam H, Orsal O. Effect of exercise on premenstrual symptoms: a systematic review. Complement Ther Med. (2020) 48:102272. doi: 10.1016/j.ctim.2019.102272
Keywords: temporal lobe dysfunction, neuropsychiatric disorder, functional connectivity, temporal lobe epilepsy (TLE), major depressive disorder, schizophrenia, temporal lobe (TL), resting-state functional MRI
Citation: Gao Y, Su Q, Liang L, Yan H and Zhang F (2022) Editorial: Temporal lobe dysfunction in neuropsychiatric disorder. Front. Psychiatry 13:1077398. doi: 10.3389/fpsyt.2022.1077398
Received: 22 October 2022; Accepted: 27 October 2022;
Published: 07 November 2022.
Edited and reviewed by: Stefan Borgwardt, University of Lübeck, Germany
Copyright © 2022 Gao, Su, Liang, Yan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengyu Zhang, emhhbmdmeUBnY2F0cmVzZWFyY2gub3Jn
 Yujun Gao
Yujun Gao Qinji Su
Qinji Su Liang Liang
Liang Liang Haohao Yan
Haohao Yan Fengyu Zhang
Fengyu Zhang