- 1Department of Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
- 2Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada
- 3Snyder Institute for Chronic Diseases, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Introduction: Irritable bowel syndrome and fibromyalgia share similar pathophysiologic mechanisms including sensitization of peripheral and central pain pathways, autonomic dysfunction and are often co-diagnosed. Co-diagnosed patients experience increased symptom severity, mental health comorbidities, and decreased quality of life. The role of mind-body interventions, which have significant effects on central pain syndromes and autonomic dysregulation, have not been well-described in co-diagnosed patients. The aim of this state-of-the art narrative review is to explore the relationship between irritable bowel syndrome and fibromyalgia, and to evaluate the current evidence and mechanism of action of mind-body therapies in these two conditions.
Methods: The PubMed database was searched without date restrictions for articles published in English using the following keywords: fibromyalgia, irritable bowel syndrome, mind-body interventions, cognitive behavioral therapy, mindfulness based stress reduction, and yoga.
Results: Mind-body interventions resulted in improved patient-reported outcomes, and are effective for irritable bowel syndrome and fibromyalgia individually. Specifically, cognitive behavioral therapy and yoga trials showed decreased symptom severity, improved mental health, sleep and quality of life for both conditions individually, while yoga trials demonstrated similar benefits with improvements in both physical outcomes (gastrointestinal symptoms, pain/tenderness scores, insomnia, and physical functioning), mental health outcomes (anxiety, depression, gastrointestinal-specific anxiety, and catastrophizing), and quality of life, possibly due to alterations in autonomic activity.
Conclusion: Mind-body interventions especially CBT and yoga improve patient-reported outcomes in both irritable bowel syndrome and fibromyalgia individually. However, limited available data in co-diagnosed patients warrant high quality trials to better tailor programs to patient needs.
1 Introduction
Irritable Bowel Syndrome (IBS) is a prevalent disorder that affects 7–21% of the population worldwide, and 12% of Canadians (1, 2). IBS is characterized by abdominal pain and altered bowel habits and is classified according to the primary bowel habit: IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed), with some patients migrating between subtypes (1, 2). The etiology of IBS is multifactorial with aberrant brain-gut interactions (1) at its core. Patients with IBS have a poor quality of life owing to the severity of gut symptoms as well as associated comorbidities, including somatic pain disorders and psychiatric disorders (1). High symptom burden in IBS is associated with lost productivity and work absenteeism, accounting for at least $20 billion a year and cost of $9,993 per patient, and 3.5 million physician visits in the United States (3–5).
Current literature suggests that a strong relationship exists between fibromyalgia (FM) and IBS (6). FM is characterized by chronic widespread pain, headaches, sleep disturbances, difficulty concentrating, depression, and fatigue (7, 8). FM has a global prevalence of 2.7% [range 2–8% (9)]. Like IBS [3:1 ratio (4)], FM is more prevalent in women compared to men [6:1 (9)]. FM costs $8,561 per patient per year in lost productivity and work absenteeism, with direct medical costs that are three times higher than in patients without FM, highlighting its significant burden (10, 11).
FM and IBS have substantial symptom overlap and are frequently co-diagnosed (6). They have common comorbidities including other functional gastrointestinal disorders, pain syndromes (12) and psychiatric conditions including depression (13), suggesting that they share a common pathogenesis. Both disorders are difficult to treat with conventional pharmacotherapies (14–16). Up to 50% of IBS patients and 91% of FM patients seek non-pharmacologic or complementary and alternative treatments to manage their symptoms (17, 18). Thus it is critical to understand how evidence-based non-pharmacologic therapies can be used to treat these disorders.
Mind-body interventions (MBI) are effective in symptom improvement, stress relief, cognitive flexibility, and improved attention and concentration, suggesting these may modify central pain pathways, and/or autonomic dysfunction in both IBS and FM (3, 19–22). It is imperative to understand how MBIs can be used as adjunctive treatments in co-diagnosed IBS and FM. The aim of this study is to review the current literature to describe the prevalence, comorbidities, and shared pathophysiology of co-diagnosed IBS and FM. We also discuss the rationale and evidence for MBI as a therapeutic strategy in these disorders. We focus on mindfulness, cognitive behavioral therapy and yoga because of their popularity among patients and the quality of available clinical studies.
2 Methodology
Our methodology consisted of a PubMed search without date restrictions for articles published in English using the following keywords: Fibromyalgia, Irritable Bowel Syndrome, Mind-body interventions, Cognitive behavioral therapy (CBT), Mindfulness based stress reduction (MBSR), and Yoga. Variations of these keywords were also used; mindfulness, MBSR, MBI, IBS, and FM/Fibromyalgia Syndrome (FMS). Both primary and secondary articles were used to synthesize this review.
3 The relationship between IBS and FM
IBS patients report symptoms of bloating, abdominal pain, and altered bowel habits such as constipation or diarrhea (1). Extraintestinal symptoms include headache, insomnia, fatigue, and palpitations (4). FM presents with unexplained musculoskeletal and widespread pain along with fatigue, sleep disturbances, and altered bowel habits (4, 6, 12). Diagnostic criteria involves assessment of defined tender points using the 2016 fibromyalgia survey with widespread pain on both sides of the body (23), although variability exists in presentations (12).
A systematic review (n = 14 studies) reported the prevalence of IBS in FM to be 32.5% (range 28–59%), whereas 73% of patients with FM reported altered bowel habits (6). Despite shared comorbidities and symptoms, the prevalence of FM in IBS has not been well-defined. There is a discordance in prevalence estimates, ranging from 48% (range 32–77%) to 12.9% (95% CI 12.7–13.1) from a systematic review (n = 30 studies) and meta-analysis (n = 65 studies), likely as a consequence of differing study designs (24, 25).
Amongst FM patients, bowel symptoms occur frequently: bloating (65.4%), abdominal pain (57.1%), fecal incontinence (56%), constipation (52.9%), alternating diarrhea and constipation (21.3%), and diarrhea alone [6% (6)]. Interestingly, FM predominates in patients with IBS-C (6). Both FM and IBS affect women more and overlap with depression, anxiety, sleep difficulties, fatigue, and chronic headaches (1, 6, 12). Psychiatric disorders are highly prevalent in both conditions. For instance, 30–50% and 30% of patients with functional gastrointestinal disorders report anxiety, and depression, respectively (26). In IBS, a prevalence of 39.1 and 23.8% exists for anxiety and depression, respectively, affecting the IBS-C type most (27, 28). Moreover, 38% of IBS patients report suicidal ideation (29). In comparison, FM has a prevalence of 32% for mood disorders, 63% for depression, with 32.5% of patients reporting suicidal ideation (13, 24, 30).
4 Common pathophysiologic basis in IBS and FM
4.1 Central sensitization and altered neurotransmission
An altered central pain state, characterized by increased neuronal excitability resulting in hyperalgesia (increased pain intensity from a painful stimulus), as well as allodynia (pain caused by a non-painful stimulus), is the first proposed common mechanism underpinning FM and IBS (31). Both FM and IBS patients show enhanced activation of ascending excitatory pain pathways, and dampening of descending inhibitory pain pathways (12, 32, 33). This results in heightened activation of central pain circuits and in the processing of negative emotions in the brain. Patients with IBS and FM individually show greater activation of brain areas associated with pain, negative emotions, memory retrieval, and attention to sensory stimuli compared to healthy participants (26, 34–37). In FM, functional MRI studies demonstrate heightened pain processing in subcortical and cortical regions in response to mild pressure that is perceived as normal touch for those without FM (38). In IBS, MRI studies demonstrate abnormal brain responses to painful visceral stimuli, such as rectal distention (39) as well as abnormal brain activity and connectivity at rest (26, 40) suggesting that abnormal central pain processing is a key component of both IBS and FM.
4.2 Somatic/visceral hypersensitivity
IBS is characterized by visceral hypersensitivity while FM is characterized by somatic hypersensitivity. Those with co-diagnosed IBS and FM show somatic hyperalgesia with lower pain thresholds and higher pain frequency and severity, whilst those with only IBS demonstrate somatic hypoalgesia (41).
Peripheral sensitization of nociceptors (pain-sensing neurons) contributes to hypersensitivity in both IBS (35) and FM (7). Peripheral nociceptors, either at the level of the gut wall, or at the level of the skin and joints, express receptors for mediators (e.g., proteases, cytokines, histamine, and bradykinin) which are released in response to cell damage or injury. These mediators can sensitize nociceptors, leading to increased neuronal excitability. In turn, nociceptors release substance P and calcitonin gene related peptide, which augment the inflammatory response at the level of the periphery and activate central pain pathways, thus contributing to central sensitization (7, 35).
4.3 Autonomic dysfunction
Both IBS and FM are associated with increased sympathetic tone and activation of the hypothalamic-pituitary-adrenal (HPA) axis, which is associated with disturbances in gut motility (6, 24, 42, 43). This suggests why MBIs may be effective for both disorders as they are thought to increase parasympathetic activity (3) and dampen sympathetic outflow.
4.4 Gut microbial dysbiosis
An altered gut microbiome is hypothesized to contribute to the pathophysiology of IBS and FM, although is more extensively characterized in IBS. Dysbiosis, or a change in the gut microbiome composition, has been shown in both disorders (44–46), with an altered Firmicutes to Bacteroidetes ratio observed at a phyla level, although the data are heterogeneous. IBS is associated with a high Firmicutes to Bacteroidetes ratio (45, 46) whereas in a study comparing 54 FM patients and 36 healthy individuals, a low Firmicutes to Bacteroidetes ratio (44) was observed. A decrease in Firmicutes has also been associated with major depressive disorder which is comorbid in IBS and FM (47). However, it is unknown whether these changes in the gut microbiome are a cause or consequence of altered gut motility. Further studies are warranted to understand the causative role of dysbiosis in both conditions.
4.5 Psychological basis
There is strong evidence that psychological comorbidities in IBS increase stress reactivity and amplify somatic sensations (24). Patients with IBS or FM report increased adverse early life events (48), a perceived lack of social support, and increased association of stressful life events to symptoms (26, 49). In addition, IBS and FM share a behavioral component called “catastrophization” (envisioning the worst possible scenario for an action or exaggerating a painful experience) which correlates with pain severity, presenting a potential therapeutic target for MBIs (1, 24).
5 Impact on quality of life
5.1 Psychological
A meta-analysis found a strong correlation between medically unexplained symptoms and increased depression/anxiety in IBS and FM (50). In IBS, a positive correlation was seen between somatic and psychiatric comorbidities, increased health care seeking, and reduced quality of life (51). Major depressive disorder is the most common psychiatric comorbidity in FM and IBS (4, 13). However, FM is characterized by lower anxiety scores than IBS (50).
5.2 Sleep and fatigue
Sleep disturbances contribute to pain, as lack of sleep impairs descending pain inhibitory pathways, impairing an individual’s ability to cope with pain (52). Sleep disorders are highly common in IBS and FM, with studies estimating a prevalence of 33% in IBS (48) and 92.9% in FM (53). More than 50% of FM patients meet criteria for insomnia; non-restorative sleep in these patients is associated with heightened pain, cognitive arousal and catastrophization (32). FM patients report morning stiffness, fatigue, and pain; hence improving the sleep quality by employing exercise is effective (54). In addition, fatigue contributes to poor health in both conditions. There is a median comorbidity of 51% for chronic fatigue syndrome in IBS and 76% in FM. Patients with comorbid chronic fatigue have 57% loss of productivity and 37% decline in household income (48, 55, 56) compared to those without. Furthermore, patients with co-diagnosed FM and IBS experience increased fatigue and symptom severity compared to those with FM or IBS alone (6). Taken together, a co-diagnosis of both FM and IBS results in significantly increased fatigue, poor sleep, and impaired quality of life, suggesting a need for therapies aimed at improving these common symptoms. Given the role of stress and anxiety in exacerbating chronic pain in both conditions, it would be important to engage patients in therapies which address these concerns.
6 Mind-body interventions
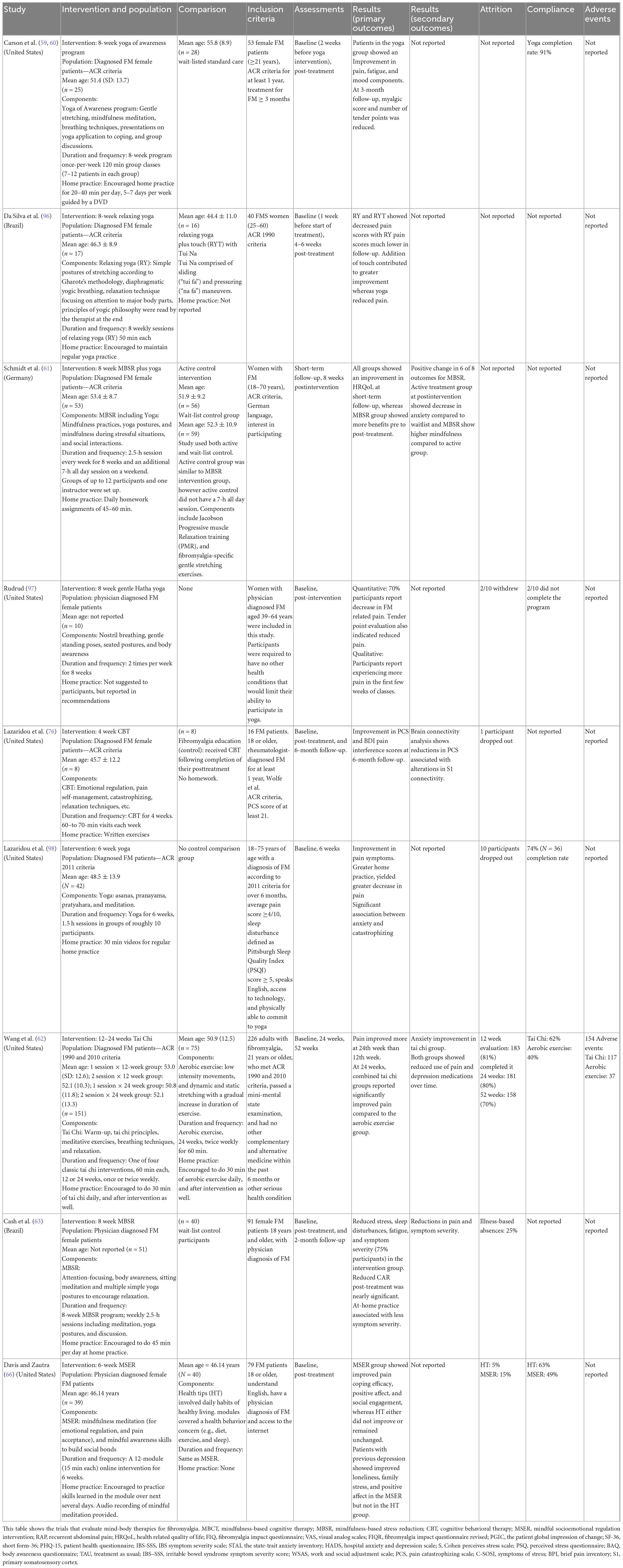
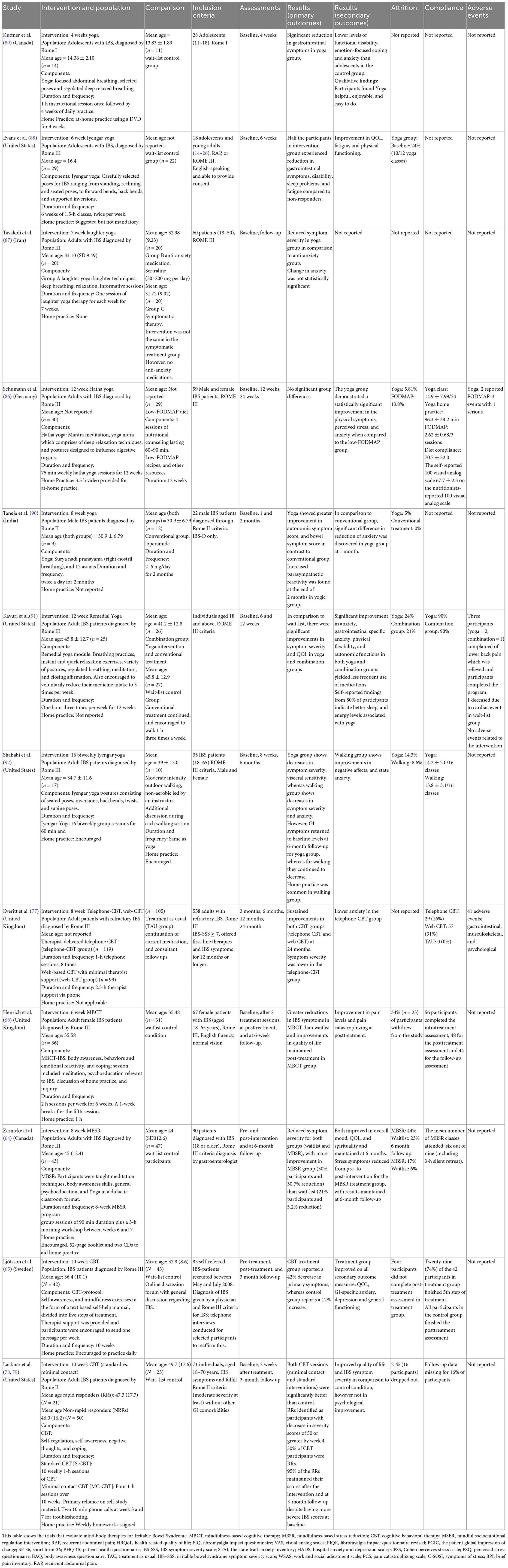
Mind-body interventions (MBI) are alternative treatment options that allow active participation of patients in their health. This is done through introspective practices that involve self-observation, meditation, relaxation exercises such as breathing, and non-judgmental acceptance of both internal (emotions, breathing, etc.) and external events (noises, smells, etc.) known as mindfulness (57). This review will focus on: (a) Mindfulness MBIs such as Mindfulness-based stress reduction, Mindfulness-Based Cognitive Therapy, Mindful Socioemotional Regulation Intervention, and Tai Chi; (b) Cognitive Behavioral Therapy (CBT); and (c) Yoga. A summary of randomized controlled trials examining MBIs for FM and IBS is found in Tables 1, 2, respectively.
6.1 Mindfulness
Mindfulness is used to treat both IBS and FM (20, 58–66). A recent meta-analysis suggests mindfulness and acceptance-based interventions result in moderate improvements in pain, sleep, quality of life, anxiety and depression in FM (67). In IBS, a recent systematic review highlights improvements in psychological wellbeing, catastrophizing, and pain coping efficacy with mindfulness (20). Another online mindfulness trial demonstrated a significant improvement in the IBS quality of life and GI-Specific Anxiety, with 42% of intervention participants reporting decreased IBS symptoms compared to a 12% increase in controls (65). Other trials show significant improvements in IBS symptom severity, quality of life and anxiety with mindfulness therapy, compared to controls (64, 65, 68) (Table 2). Moreover, the improvement in symptom severity was maintained at a 6 month follow-up in the intervention group (64).
In FM, a web-based mindful socioemotional regulation intervention improved pain, stress coping, social engagement, and loneliness in comparison to a health education control group (20, 66) (Table 1). Another randomized controlled trial found significant decreases in stress, and sleep disturbances, suggesting those with greater at-home practice had decreased symptom severity (63). The proposed mechanisms of action of mindfulness is through decreased sympathetic outflow and HPA axis activation (57), with associated changes in brain connectivity resulting in enhanced self-regulation through the modulation of emotions, self-compassion, and body awareness (69, 70).
6.2 Cognitive behavioral therapy
Cognitive Behavioral Therapy (CBT) involves altering unhelpful patterns of thinking (cognitive bias) to alleviate stress, and improve self-regulation (68). CBT has also shown promising outcomes with reducing catastrophizing through Acceptance and Commitment Therapy. This allows participants to reflect on their thoughts and sensations, effectively reducing psychological symptoms and facilitating pain acceptance, thus improving quality of life (71, 72). Although the mechanism behind such psychological interventions is unclear, an improvement in illness-specific thoughts, beliefs and perceptions or cognitive bias has been postulated (73). In both IBS and FM, CBT results in decreased symptom severity, improved mental health and quality of life (32, 65, 68, 74–79).
The Cognitive Activation Theory of Stress hypothesizes that insomnia causes changes in the HPA axis, the central nervous system, and increases sympathetic activity, causing higher pain sensitivity (32). In turn, pain prevents restful sleep. Patients can undergo CBT specifically aimed at treating insomnia to reduce chronic arousal, improve sleep quality, and consequently pain.
In IBS, there have been four trials of CBT which reported benefits on symptom severity (65, 68, 77–79) (Table 2). A 24-month follow up comparing telephone CBT, web CBT and a treatment as usual group found greatest reduction of symptom severity in the telephone-CBT group (77). Patients receiving a 10 week course of CBT who achieved a positive response by week 4 (termed as rapid responders) experienced symptomatic reduction that was maintained at 3 month follow up (78). Similarly, a trial of CBT in female IBS patients found reduced pain catastrophizing, and improved quality of life compared to waitlist controls (68). A meta-analysis demonstrates CBT was most effective with long-term or continuous home practice (80, 81).
6.3 Yoga
Yoga combines techniques of different MBIs including breath work, movement, and meditation, showing promising benefits in chronic diseases such as cancer, IBS, as well as mental illnesses (82–84). Yoga improves balance, strength, and mobility, and allows non-judgmental observation of thoughts. Schumann et al. suggest it is a safe and feasible therapy for IBS because it improves symptom severity, quality of life, physical functioning and anxiety (85) (Table 2), however, more high quality clinical trials are needed to determine efficacy (85–92). The proposed mechanisms includes changes in autonomic outflow, as well as changes in central connectivity in the brain (69, 93–95). Moreover, breathing influences autonomic activity; in yoga, this is demonstrated through decreased sympathetic and increased parasympathetic activity (3, 59). In comparison to other therapies such as Mindfulness-based stress reduction, a low-FODMAP diet, and physical exercise (but not CBT), yoga was shown to be superior in improving quality of life, GI symptom severity and reducing stress and anxiety (3). Yoga programs inclusive of different breathing exercises, postures and meditation have beneficial effects on symptom severity in comparison to CBT; thus yoga programs with multiple modalities of mindfulness may provide more benefits (3). Although larger studies are needed, preliminary studies in adults and adolescents suggest that clinically meaningful improvement in IBS symptoms and sleep quality is experienced from yoga (86–89). However, qualitative studies demonstrate the need for better adherence strategies, social support, and yoga programs tailored for IBS (88, 89). For example, yoga delivered in a group setting was found to be more effective with engaged participants (71).
Yoga also demonstrates benefits in FM (59, 96–98) (Table 1). A trial with female FM patients comparing a Yoga Awareness program to a wait-listed control showed decreased anxiety (by 42.2%), depression (41.5%), emotional distress (30%), and fatigue (29.9%) in the intervention group (31, 59, 60). Sustained improvements were seen at 3 month follow-up, with greater impact when adhering to at-home yoga practice (59, 60). A pilot study with daily home practice showed reductions in catastrophization and pain, which were maintained at 6 month follow-up (98). A gentle Hatha Yoga program improved FM physical symptoms, assessed with the Fibromyalgia Impact Questionnaire (97). Interestingly, Yoga in combination with Tui Na massage (targeting meridians and acupuncture points on the body) showed promising results in pain reduction (96). Thus, multiple modalities of yoga demonstrate clinical benefit in FM.
7 Limitations and future directions
A strong relationship between FM and IBS is evident through their pathogenesis. The current evidence base for MBIs in the treatment of IBS and FM is growing. Studies have demonstrated multiple physical and mental health benefits, along with safety and feasibility. To our knowledge, high quality studies such as large randomized control trials assessing the efficacy of MBIs in co-diagnosed patients with IBS and FM are lacking. Therefore, we recommend that future studies testing the feasibility and efficacy of MBIs should use an active comparator groups and be tailored toward the patient to increase intervention effectiveness. Gaps in the literature include assessment of optimal MBI duration, frequency, components (single vs. multimodal) and delivery (online vs. in-person).
Our review has several limitations. First, the heterogeneity of the MBIs chosen for discussion included only the most investigated interventions among IBS and FM patients. Second, assessing MBI efficacy is challenging given the examined studies differ greatly in their methodologies. This limits the generalizability of the results, and the specific recommendations (MBI type, dose, and frequency) that can be made for co-diagnosed IBS and FM.
Until further data from high-quality trials are available to inform a definitive approach to yoga interventions in co-diagnosed patients, yoga practice involving postures, breathing, and meditation may be recommended at a dose of 30 min daily, five times weekly. These recommendations are in parallel to widely accepted physical activity guidelines and from studies that demonstrate similar integrated approach to yoga intervention and dosage achieve improved outcomes (99, 100).
Lastly, studies should also evaluate potential mechanisms of action of MBIs such as microbiome alteration, neuroendocrine/neuroimmune responses, and autonomic outflow.
8 Conclusion
Negative impacts on patient quality of life and mental health arising from comorbid FM and IBS, and limited data on co-diagnosed patients warrant study of effective interventions. MBIs such as CBT and yoga are impactful and leverage one of many potential pathophysiological mechanisms. Future interventions should aim toward tailoring yoga programs in combination with other MBIs to meet the needs of IBS and FM patients.
Author contributions
ZI drafted the manuscript. AD’S, MR, and YN critically revised the manuscript for important intellectual content. All authors have reviewed and approved the final manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research and the Weston Family Microbiome Initiative (to YN).
Conflict of interest
YN has received speaker fees, honoraria, and grant funding from Abbvie/Allergan. MR has received speaker fees from Abbvie/Allergan and Lupin.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IBS, irritable bowel syndrome; FM, fibromyalgia; MBI, mind-body interventions; HPA, hypothalamic-pituitary-adrenal axis; CBT, cognitive behavioral therapy.
References
1. Chey W, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. (2015). 313:949–58. doi: 10.1001/jama.2015.0954
2. Laskaratos F, Goodkin O, Thoua N, Murray C. Irritable bowel syndrome. Medicine. (2015) 43:266–70. doi: 10.1016/j.mpmed.2015.02.010
3. D’Silva A, MacQueen G, Nasser Y, Taylor L, Vallance J, Raman M. Yoga as a therapy for irritable bowel syndrome. Dig Dis Sci. (2020) 65:2503–14. doi: 10.1007/s10620-019-05989-6
4. Warnock J, Clayton A. Chronic episodic disorders in women. Psychiatr Clin North Am. (2003) 26:725–40. doi: 10.1016/S0193-953X(03)00042-X
5. Buono J, Carson R, Flores N. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. (2017) 15:1–8. doi: 10.1186/s12955-017-0611-2
6. Erdrich S, Hawrelak J, Myers S, Harnett J. A systematic review of the association between fibromyalgia and functional gastrointestinal disorders. Therap Adv Gastroenterol. (2020) 13:1756284820977402. doi: 10.1177/1756284820977402
7. Sluka K, Clauw D. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. (2016) 338:114. doi: 10.1016/j.neuroscience.2016.06.006
8. Ranum R, Toussaint L, Whipple M, Vincent A. Predictive bidirectional relations between pain, fatigue, and dyscognition in fibromyalgia. Mayo Clin Proc Innov Qual Outcomes. (2022) 6:143–7. doi: 10.1016/j.mayocpiqo.2021.12.007
9. Queiroz L. Worldwide epidemiology of fibromyalgia topical collection on fibromyalgia. Curr Pain Headache Rep. (2013) 17:356. doi: 10.1007/s11916-013-0356-5
10. Rusu C, Gee M, Lagacé C, Parlor M. Chronic fatigue syndrome and fibromyalgia in Canada: prevalence and associations with six health status indicators. Heal Promot Chronic Dis Prev Can. (2015) 35:3–11. doi: 10.24095/hpcdp.35.1.02
11. Lacasse A, Bourgault P, Choinière M. Fibromyalgia-related costs and loss of productivity: a substantial societal burden. BMC Musculoskelet Disord. (2016) 17:168. doi: 10.1186/s12891-016-1027-6
13. Kleykamp B, Ferguson M, McNicol E, Bixho I, Arnold L, Edwards R, et al. The prevalence of psychiatric and chronic pain comorbidities in fibromyalgia: an acttion systematic review. Semin Arthritis Rheum. (2021) 51:166–74. doi: 10.1016/j.semarthrit.2020.10.006
14. Carbone F, Van Den Houte K, Besard L, Tack C, Arts J, Caenepeel P, et al. Diet or medication in primary care patients with IBS: the DOMINO study–a randomised trial supported by the Belgian health care knowledge centre (KCE trials programme) and the Rome foundation research institute. Gut. (2022) 71:2226–32. doi: 10.1136/gutjnl-2021-325821
15. Giorgi V, Sirotti S, Romano M, Marotto D, Ablin J, Salaffi F, et al. Fibromyalgia: one year in review 2022. Clin Exp Rheumatol. (2022) 40:1065–72. doi: 10.55563/clinexprheumatol/if9gk2
16. Paine P. Review article: current and future treatment approaches for pain in IBS. Aliment Pharmacol Ther. (2021) 54(Suppl. 1):S75–88. doi: 10.1111/apt.16550
17. Pioro-Boisset M, Esdaile J, Fitzcharles M. Alternative medicine use in fibromyalgia syndrome. Arthritis Care Res. (1996) 9:13–7. doi: 10.1002/art.1790090105
18. Kong S, Hurlstone D, Pocock C, Walkington L, Farquharson N, Bramble M, et al. The incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseases. J Clin Gastroenterol. (2005) 39:138–41. doi: 10.1097/01.mcg.0000177234.36640.68
19. Magge S, Wolf J. Complementary and alternative medicine and mind-body therapies for treatment of irritable bowel syndrome in women. Womens Health (Lond). (2013) 9:557–67. doi: 10.2217/WHE.13.57
20. Toivonen K, Zernicke K, Carlson L. Web-based mindfulness interventions for people with physical health conditions: systematic review. J Med Internet Res. (2017) 19:1–35. doi: 10.2196/jmir.7487
21. Bulzacka E, Lavault S, Pelissolo A, Bagnis Isnard C. Mindful neuropsychology: mindfulness-based cognitive remediation. Encephale. (2018) 44:75–82. doi: 10.1016/j.encep.2017.03.006
22. McClintock A, McCarrick S, Garland E, Zeidan F, Zgierska A. Brief mindfulness-based interventions for acute and chronic pain: a systematic review. J Alternat Complement Med. (2019) 25:265–78. doi: 10.1089/acm.2018.0351
23. Wolfe F, Clauw D, Fitzcharles M, Goldenberg D, Häuser W, Katz R, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. (2016) 46:319–29. doi: 10.1016/j.semarthrit.2016.08.012
24. Whitehead W, Palsson O, Jones K. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. (2002) 122:1140–56. doi: 10.1053/gast.2002.32392
25. Heidari F, Afshari M, Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int. (2017) 37:1527–39. doi: 10.1007/s00296-017-3725-2
26. Van Oudenhove L, Levy R, Crowell M, Drossman D, Halpert A, Keefer L, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. (2016) 150:1355–1367.e2. doi: 10.1053/j.gastro.2016.02.027
27. Hu Z, Li M, Yao L, Wang Y, Wang E, Yuan J, et al. The level and prevalence of depression and anxiety among patients with different subtypes of irritable bowel syndrome: a network meta-analysis. BMC Gastroenterol. (2021) 21:23. doi: 10.1186/s12876-020-01593-5
28. Zamani M, Alizadeh-Tabari S, Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment Pharmacol Ther. (2019) 50:132–43. doi: 10.1111/apt.15325
29. Miller V, Hopkins L, Whorwell P. Suicidal ideation in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. (2004) 2:1064–8. doi: 10.1016/S1542-3565(04)00545-2
30. Duque L, Fricchione G. Fibromyalgia and its new lessons for neuropsychiatry. Med Sci Monit Basic Res. (2019) 25:169–78. doi: 10.12659/MSMBR.915962
31. Bravo C, Skjaerven L, Guitard Sein-Echaluce L, Catalan-Matamoros D. Effectiveness of movement and body awareness therapies in patients with fibromyalgia: a systematic review and meta-analysis. Eur J Phys Rehabil Med. (2019) 55:646–57. doi: 10.23736/S1973-9087.19.05291-2
32. McCrae C, Curtis A, Craggs J, Deroche C, Sahota P, Siva C, et al. Protocol for the impact of CBT for insomnia on pain symptoms and central sensitisation in fibromyalgia: a randomised controlled trial. BMJ Open. (2020) 10:e033760. doi: 10.1136/bmjopen-2019-033760
33. Staud R, Robinson M, Price D. temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. (2007) 8:893–901. doi: 10.1016/j.jpain.2007.06.006
34. Chang L, Mayer E, Munakata J, Silverman D, Berman S, Gilmore S, et al. Differences in left prefrontal activation to visceral and somatic stimuli assessed by O-15-water PET in female patients with irritable bowel syndrome (IBS) and fibromyalgia. Gastroenterology. (1998) 114:A732. doi: 10.1016/S0016-5085(98)83000-X
35. Vanner S, Greenwood-Van Meerveld B, Mawe G, Shea-Donohue T, Verdu E, Wood J, et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. (2016) 150:1280–91. doi: 10.1053/j.gastro.2016.02.018
36. Trucharte A, Leon L, Castillo-Parra G, Magán I, Freites D, Redondo M. Emotional regulation processes: influence on pain and disability in fibromyalgia patients. Clin Exp Rheumatol. (2020) 38:40–6.
37. Ichesco E, Puiu T, Hampson J, Kairys A, Clauw D, Harte S, et al. Altered fMRI resting-state connectivity in individuals with fibromyalgia on acute pain stimulation. Eur J Pain. (2016) 20:1079–89. doi: 10.1002/ejp.832
38. Gracely R, Petzke F, Wolf J, Clauw D. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. (2002) 46:1333–43. doi: 10.1002/art.10225
39. Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. (2000) 118:842–8. doi: 10.1016/S0016-5085(00)70170-3
40. Hong J, Kilpatrick L, Labus J, Gupta A, Jiang Z, Ashe-McNalley C, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. (2013) 33:11994–2002. doi: 10.1523/JNEUROSCI.5733-12.2013
41. Chang L, Mayer E, Johnson T, Fitzgerald L, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. (2000) 84:297–307. doi: 10.1016/S0304-3959(99)00215-8
42. Mazur M, Furgała A, Jabłoński K, Mach T, Thor P. Autonomic nervous system activity in constipation-predominant irritable bowel syndrome patients. Med Sci Monit. (2012) 18:CR493. doi: 10.12659/MSM.883269
43. Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. (2011) 140:761–5. doi: 10.1053/j.gastro.2011.01.032
44. Albayrak B, Süsgün S, Küçükakkaş O, Akbaş F, Yabaci A, Özçelik S. Investigating of relation between fibromyalgia syndrome and intestinal microbiota. Mikrobiyol Bull. (2021) 55:146–60. doi: 10.5578/mb.20219903
45. Duan R, Zhu S, Wang B, Duan L. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16s rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol. (2019) 10:e00012. doi: 10.14309/ctg.0000000000000012
46. Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. (2020) 12:1474. doi: 10.3390/nu12051474
47. Huang Y, Shi X, Li Z, Shen Y, Shi X, Wang L, et al. Possible association of firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr Dis Treat. (2018) 14:3329–37. doi: 10.2147/NDT.S188340
48. Riedl A, Schmidtmann M, Stengel A, Goebel M, Wisser A, Klapp B, et al. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res. (2008) 64:573–82. doi: 10.1016/j.jpsychores.2008.02.021
49. Yavne Y, Amital D, Watad A, Tiosano S, Amital H. A systematic review of precipitating physical and psychological traumatic events in the development of fibromyalgia. Semin Arthritis Rheum. (2018) 48:121–33. doi: 10.1016/j.semarthrit.2017.12.011
50. Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. (2003) 65:528–33. doi: 10.1097/01.PSY.0000075977.90337.E7
51. Vandvik P, Wilhelmsen I, Ihlebæk C, Farup P. Comorbidity of irritable bowel syndrome in general practice: a striking feature with clinical implications. Aliment Pharmacol Ther. (2004) 20:1195–203. doi: 10.1111/j.1365-2036.2004.02250.x
52. Choy E. The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol. (2015) 11:513–20. doi: 10.1038/nrrheum.2015.56
53. Andrade A, Vilarino G, Sieczkowska S, Coimbra D, Bevilacqua G, Steffens R. The relationship between sleep quality and fibromyalgia symptoms. J Health Psychol. (2020) 25:1176–86. doi: 10.1177/1359105317751615
54. Bigatti S, Hernandez A, Cronan T, Rand K. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Care Res. (2008) 59:961–7. doi: 10.1002/art.23828
55. Vincent A, Benzo R, Whipple M, McAllister S, Erwin P, Saligan L. Beyond pain in fibromyalgia: insights into the symptom of fatigue. Arthritis Res Ther. (2013) 15:221. doi: 10.1186/ar4395
56. Reynolds K, Vernon S, Bouchery E, Reeves W. The economic impact of chronic fatigue syndrome. Cost Eff Resour Alloc. (2004) 2:4. doi: 10.1186/1478-7547-2-4
57. Wahbeh H, Elsas S, Oken B. Mind–body interventions: applications in neurology. Neurology. (2008) 70:2321. doi: 10.1212/01.wnl.0000314667.16386.5e
58. Shorey S, Demutska A, Chan V, Siah K. Adults living with irritable bowel syndrome (IBS): a qualitative systematic review. J Psychosom Res. (2021) 140:110289. doi: 10.1016/j.jpsychores.2020.110289
59. Carson J, Carson K, Jones K, Bennett R, Wright C, Mist SD. A pilot randomized controlled trial of the yoga of awareness program in the management of fibromyalgia. Pain. (2010) 151:530–9. doi: 10.1016/j.pain.2010.08.020
60. Carson J, Carson K, Jones K, Mist S, Bennett R. Follow-up of yoga of awareness for fibromyalgia: results at 3 months and replication in the wait-list group. Clin J Pain. (2012) 28:804–13. doi: 10.1097/AJP.0b013e31824549b5
61. Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness-based stress reduction: results from a 3-armed randomized controlled trial. Pain. (2011) 152:361–9. doi: 10.1016/j.pain.2010.10.043
62. Wang C, Schmid C, Fielding R, Harvey W, Reid K, Price L, et al. Effect of tai chi versus aerobic exercise for fibromyalgia: comparative effectiveness randomized controlled trial. BMJ. (2018) 360:1–14. doi: 10.1136/bmj.k851
63. Cash E, Ph D, Salmon P, Ph D, Weissbecker I, Ph D, et al. Mindfulness meditation alleviates fibromyalgia symptoms in women: results of a randomized clinical trial. Ann Behav Med. (2015) 49:319–30. doi: 10.1007/s12160-014-9665-0
64. Zernicke K, Campbell T, Blustein P, Fung T, Johnson J, Bacon S, et al. Mindfulness-based stress reduction for the treatment of irritable bowel syndrome symptoms: a randomized wait-list controlled trial. Int J Behav Med. (2013) 20:385–96. doi: 10.1007/s12529-012-9241-6
65. Ljótsson B, Falk L, Vesterlund A, Hedman E, Lindfors P, Rück C, et al. Internet-delivered exposure and mindfulness based therapy for irritable bowel syndrome–a randomized controlled trial. Behav Res Ther. (2010) 48:531–9. doi: 10.1016/j.brat.2010.03.003
66. Davis M, Zautra A. An online mindfulness intervention targeting socioemotional regulation in fibromyalgia: results of a randomized controlled trial. Ann Behav Med. (2013) 46:273–84. doi: 10.1007/s12160-013-9513-7
67. Haugmark T, Hagen K, Smedslund G, Zangi H. Mindfulness- and acceptance-based interventions for patients with fibromyalgia – a systematic review and meta-analyses. PLoS One. (2019) 14:e0221897. doi: 10.1371/journal.pone.0221897
68. Henrich J, Gjelsvik B, Surawy C, Evans E, Martin M. A randomized clinical trial of mindfulness-based cognitive therapy for women with irritable bowel syndrome-effects and mechanisms. J Consult Clin Psychol. (2020) 88:295–310. doi: 10.1037/ccp0000483
69. Hölzel B, Lazar S, Gard T, Schuman-Olivier Z, Vago D, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. (2011) 6:537–59. doi: 10.1177/1745691611419671
70. Alsubaie M, Abbott R, Dunn B, Dickens C, Keil T, Henley W, et al. Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: a systematic review. Clin Psychol Rev. (2017) 55:74–91. doi: 10.1016/j.cpr.2017.04.008
71. Adler-Neal A, Zeidan F. Mindfulness meditation for fibromyalgia: mechanistic and clinical considerations. Curr Rheumatol Rep. (2017) 19:59. doi: 10.1007/s11926-017-0686-0
72. Galvez-Sánchez C, Montoro C, Moreno-Padilla M, Reyes Del Paso G, De La Coba P, Merwin R, et al. Clinical medicine effectiveness of acceptance and commitment therapy in central pain sensitization syndromes: a systematic review. J Clin Med. (2021) 10:2706. doi: 10.3390/jcm10122706
73. Windgassen S, Moss-Morris R, Chilcot J, Sibelli A, Goldsmith K, Chalder T. The journey between brain and gut: a systematic review of psychological mechanisms of treatment effect in irritable bowel syndrome. Br J Health Psychol. (2017) 22:701–36. doi: 10.1111/bjhp.12250
74. Hernando-Garijo I, Jiménez-Del-Barrio S, Mingo-Gómez T, Medrano-De-La-Fuente R, Ceballos-Laita L. Effectiveness of non-pharmacological conservative therapies in adults with fibromyalgia: a systematic review of high-quality clinical trials. J Back Musculoskelet Rehabil. (2022) 35:3–20. doi: 10.3233/BMR-200282
75. Samami E, Shahhosseini Z, Elyasi F. The effect of psychological interventions on the quality of life in women with fibromyalgia: a systematic review. J Clin Psychol Med Settings. (2021) 28:503–17. doi: 10.1007/s10880-021-09794-0
76. Lazaridou A, Kim J, Cahalan C, Loggia M, Franceschelli O, Berna C, et al. Effects of cognitive-behavioral therapy (CBT) on brain connectivity supporting catastrophizing in fibromyalgia. Clin J Pain. (2017) 33:215–21. doi: 10.1097/AJP.0000000000000422
77. Everitt H, Landau S, O’Reilly G, Sibelli A, Hughes S, Windgassen S, et al. Cognitive behavioural therapy for irritable bowel syndrome: 24-month follow-up of participants in the ACTIB randomised trial. Lancet Gastroenterol Hepatol. (2019) 4:863–72. doi: 10.1016/S2468-1253(19)30243-2
78. Lackner J, Gudleski G, Keefer L, Krasner S, Powell C, Katz L. Rapid response to cognitive behavior therapy predicts treatment outcome in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. (2010) 8:426–32. doi: 10.1016/j.cgh.2010.02.007
79. Lackner J, Jaccard J, Krasner S, Katz L, Gudleski G, Holroyd K. Self administered cognitive behavior therapy for moderate to severe IBS: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. (2008) 6:899. doi: 10.1016/j.cgh.2008.03.004
80. Black C, Thakur E, Houghton L, Quigley E, Moayyedi P, Ford A. Efficacy of psychological therapies for irritable bowel syndrome: systematic review and network meta-analysis. Gut. (2020) 69:1441–51. doi: 10.1136/gutjnl-2020-321191
81. Radziwon C, Quigley B, Vargovich A, Krasner S, Gudleski G, Mason S, et al. Do I really have to do my homework? The role of homework compliance in cognitive behavioral therapy for irritable bowel syndrome. Behav Res Ther. (2022) 152:104063. doi: 10.1016/j.brat.2022.104063
82. Glynn B, Khoo E, MacLeay H, Duong A, Cantave R, Poulin P. Exploring cancer patients’ experiences of an online mindfulness-based program: a qualitative investigation. Mindfulness (NY). (2020) 11:1666–77. doi: 10.1007/s12671-020-01380-z
83. Jindani F, Khalsa GFS. A yoga intervention program for patients suffering from symptoms of posttraumatic stress disorder: a qualitative descriptive study. J Altern Complement Med. (2015) 21:401–8. doi: 10.1089/acm.2014.0262
84. Ewais T, Begun J, Kenny M, Headey A, Kisely S. Mindfulness-based cognitive therapy experiences in youth with inflammatory bowel disease and depression: protocol for a mixed methods qualitative study. JMIR Res Protoc. (2019) 8:e14432. doi: 10.2196/14432
85. Schumann D, Anheyer D, Lauche R, Dobos G, Langhorst J, Cramer H. Effect of yoga in the therapy of irritable bowel syndrome: a systematic review. Clin Gastroenterol Hepatol. (2016) 14:1720–31. doi: 10.1016/j.cgh.2016.04.026
86. Schumann D, Langhorst J, Dobos G, Cramer H. Randomised clinical trial: yoga vs a low-FODMAP diet in patients with irritable bowel syndrome. Aliment Pharmacol Ther. (2018) 47:203–11. doi: 10.1111/apt.14400
87. Tavakoli T, Davoodi N, Jafar Tabatabaee T, Rostami Z, Mollaei H, Salmani F, et al. Comparison of laughter yoga and anti-anxiety medication on anxiety and gastrointestinal symptoms of patients with irritable bowel syndrome. Middle East J Dig Dis. (2019) 11:212–8. doi: 10.15171/mejdd.2019.151
88. Evans S, Seidman L, Lung K, Sternlieb B, Zeltzer L. Yoga for teens with irritable bowel syndrome: results from a mixed-methods pilot study. Holist Nurs Pract. (2018) 32:253–60. doi: 10.1097/HNP.0000000000000288
89. Kuttner L, Chambers C, Hardial J, Israel D, Jacobson K, Evans K. A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Res Manag. (2006) 11:217–23. doi: 10.1155/2006/731628
90. Taneja I, Deepak K, Poojary G, Acharya I, Pandey R, Sharma M. Yogic versus conventional treatment in diarrhea-predominant irritable bowel syndrome: a randomized control study. Appl Psychophysiol Biofeedback. (2004) 29:19–33. doi: 10.1023/B:APBI.0000017861.60439.95
91. Kavuri V, Raghuram N, Malamud A, Selvan S. Irritable bowel syndrome: yoga as remedial therapy. Evid Based Complement Alternat Med. (2015) 2015:398156. doi: 10.1155/2015/398156
92. Shahabi L, Naliboff B, Shapiro D. Self-regulation evaluation of therapeutic yoga and walking for patients with irritable bowel syndrome: a pilot study. Psychol Heal Med. (2016) 21:176–88. doi: 10.1080/13548506.2015.1051557
93. van Aalst J, Ceccarini J, Demyttenaere K, Sunaert S, Van Laere K. What has neuroimaging taught us on the neurobiology of yoga? A review. Front Integr Neurosci. (2020) 14:34. doi: 10.3389/fnint.2020.00034
94. Naveen G, Varambally S, Thirthalli J, Rao M, Christopher R, Gangadhar B. Serum cortisol and BDNF in patients with major depression-effect of yoga. Int Rev Psychiatry. (2016) 28:273–8. doi: 10.1080/09540261.2016.1175419
95. Mishra S, Singh S, Moheb N, Khosa S, Trikamji B. Changes in functional magnetic resonance imaging with yogic meditation: a pilot study. Ayu. (2017) 38:108. doi: 10.4103/ayu.AYU_34_17
96. Da Silva G, Lorenzi-Filho G, Lage LV. Effects of yoga and the addition of Tui Na in patients with fibromyalgia. J Altern Complement Med. (2007) 13:1107–13. doi: 10.1089/acm.2007.0615
97. Rudrud L. Gentle Hatha yoga and reduction of fibromyalgia-related symptoms: a preliminary report. Int J Yoga Therap. (2012) 22:53–7. doi: 10.17761/ijyt.22.1.hp278678261h5363
98. Lazaridou A, Koulouris A, Dorado K, Chai P, Edwards R, Schreiber K. The impact of a daily yoga program for women with fibromyalgia. Int J Yoga. (2019) 12:206–17. doi: 10.4103/ijoy.IJOY_72_18
99. Piercy K, Troiano R, Ballard R, Carlson S, Fulton J, Galuska D, et al. The physical activity guidelines for Americans. JAMA. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
Keywords: IBS–irritable bowel syndrome, fibromyalgia (FM), mind-body interventions, yoga, cognitive behavioral therapy (CBT)
Citation: Islam Z, D’Silva A, Raman M and Nasser Y (2022) The role of mind body interventions in the treatment of irritable bowel syndrome and fibromyalgia. Front. Psychiatry 13:1076763. doi: 10.3389/fpsyt.2022.1076763
Received: 24 October 2022; Accepted: 06 December 2022;
Published: 22 December 2022.
Edited by:
Vijaya Majumdar, Swami Vivekananda Yoga Anusandhana Samsthana, IndiaReviewed by:
Octavian Vasiliu, Dr. Carol Davila University Emergency Military Central Hospital, RomaniaVijaya Kavuri, Swami Vivekananda Yoga Anusandhana Samsthana, India
Copyright © 2022 Islam, D’Silva, Raman and Nasser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasmin Nasser, ✉ eW5hc3NlckB1Y2FsZ2FyeS5jYQ==
†Present address: Zarmina Islam, Department of Medicine, Dow Medical College, Dow University of Health Sciences, Karachi, Pakistan
‡These authors have contributed equally to this work
 Zarmina Islam1†
Zarmina Islam1† Maitreyi Raman
Maitreyi Raman Yasmin Nasser
Yasmin Nasser