- 1Department of Child and Adolescent Healthcare, Children’s Hospital of Soochow University, Suzhou, Jiangsu, China
- 2Department of Developmental and Behavioral Pediatric & Child Primary Care, Brain and Behavioral Research Unit of Shanghai Institute for Pediatric Research, MOE-Shanghai Key Laboratory for Children’s Environmental Health, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Psychology and Neuroscience of Cognition Research Unit, University of Liège, Liège, Belgium
Background: Children with autism spectrum disorder (ASD) and developmental delay (DD; ASD + DD) have more severe clinical symptoms than those with ASD without DD (ASD-only). However, little is known about the underlying neuroimaging mechanisms. The aim of this study was to explore the volumetric difference between patients with ASD + DD and ASD-only and investigate the relationship between brain alterations and clinical manifestations.
Materials and methods: A total of 184 children with ASD aged 2–6 years were included in this study, who were divided into two groups according to their cognitive development: ASD + DD and ASD-only. Clinical symptoms and language development were assessed using the Autism Diagnostic Observation Schedule (ADOS), Childhood Autism Rating Scale (CARS), and the Putonghua Communicative Development Inventory. Of the 184 children, 60 age-matched males (30 ASD + DD and 30 ASD-only patients) with high-resolution structural neuroimaging scans were included for further voxel-based morphometry analysis to examine the relationship between clinical symptoms and gray matter volumes.
Results: The ASD + DD group had higher CARS and ADOS scores, lower gesture scores, and poorer performance in “responding to joint attention” (RJA) and “initiating joint attention” than the ASD-only group. Larger gray matter volumes in the temporal poles of the right and left middle temporal gyri were associated with the co-occurrence of DD in patients with ASD. Moreover, temporopolar volumes were correlated with CARS and ADOS scores, gesture scores, and RJA ability. Pre-language development significantly mediated the relationship between temporopolar volumes and both CARS and ADOS scores; RJA ability, but not gesture development, contributed to this mediating effect.
Conclusion: In this study, we found that temporopolar volumes were enlarged in patients with ASD who had comorbid DD, and these patients showed an association between symptom severity and language ability during the pre-language stage. Offering early interventions focused on RJA and the temporal pole may help improve clinical symptoms.
Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by social communication deficits, repetitive behaviors, and restricted interests. The previous several decades have seen a significant increase in the prevalence of ASD. The most recent report in 2021 suggested that the prevalence of autism had reached 1/44 in the United States (1). Currently, ASD is one of the most common neurodevelopmental disorders, affecting approximately 78 million people and their families worldwide. ASD is a highly heterogeneous condition with differential manifestations of language development, intelligence, and comorbidity, which influence intervention plans. A recently published Lancet commission proposed the concept of “profound autism”, referring to autism with severe to profound intellectual disability (ID) or minimal language, for which additional support is required because of the patient’s inability to live independently (2). The commission suggested that more studies focused on autism with ID and the underlying mechanisms are needed, which would be more valuable for improving clinical and societal outcomes.
To study the early clinical manifestation of profound autism and understand the behavioral heterogeneity of ASD, there is no better example than variability in early language development (3). For toddlers and pre-schoolers, delays in language development are one of the primary concerns that motivate parents to seek ASD diagnostic evaluation for their children, especially those comorbid with DD/ID (3, 4). Moreover, deficits in early language developmental trajectories, such as the use of gestures and the ability to engage in joint attention, have been observed in children with ASD (5, 6). Gestures are a form of pre-verbal communication through body movements (such as pointing and showing), providing a foundation upon which communication skills can further develop; while joint attention involves sharing attention with a partner to a third entity by integrating gestures and eye gaze, which include the ability to follow others’ attention (response to joint attention, RJA) and initiating joint attention (IJA) (7–9). Therefore, pre-language developmental discrepancy may be an important contributor to phenotypic heterogeneity between young ASD with and without DD/ID (10), and the assessment of gesture and joint attention in this critical period is crucial either to understanding their early communicative skills and for planning prompt intervention when the brains are still in development (11–13).
Neurobiological explanations for why toddlers and pre-schoolers with ASD exhibit striking differences between those with and without comorbid DD/ID are lacking. Non-invasive imaging techniques are promising tools to investigate the neurological underpinnings of ASD; and these neuroimaging characters may be served as potential biomarkers to discriminate patient subgroups and intervention targets. In patients with autism aged 2–65 years, cortical thickness across the frontal, temporal, and occipital cortices has been shown to be negatively associated with the full intelligence quotient score and overall clinical symptom severity (14). Moreover, a functional connectivity study showed lower network segmentation and integration in patients with ASD whose ages ranged from 7 to 17 with low verbal and cognitive performance than in those with high verbal and cognitive performance (15). The current pieces of evidence from ASD patients with a broad age range indicate potential neuroimaging characteristics of ASD with differential cognitive function; however, considering the high plasticity of the brain in development, such neuroimaging evidence on young children with ASD and the relationship between brain alterations and impaired early language development remains scarce.
In the present study, we aimed to compare the features of early-language development profiles between the two subgroups of ASD with DD (ASD + DD) and ASD-only and identify brain volume differences between these two groups. We also examined the relationship between behavioral heterogeneity and brain structural alterations. It provides an important complement to previous related research. This study serves as an important step toward parsing factors that influence neuroanatomical heterogeneity in ASD and is a potential step toward establishing precise medical biomarkers.
Materials and methods
Participants
Autism spectrum disorder participants were enrolled in the Shanghai Xinhua ASD Registry in Shanghai, China. Participants were recruited from January 2016 to December 2017. Children aged 2–6 years were diagnosed with ASD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) (16). Diagnoses were confirmed with the Autism Diagnostic Observation Schedule (ADOS), the Autism Diagnostic Interview-Revised (ADI-R), and a Childhood Autism Rating Scale (CARS) total score of no less than 30. In addition, children were excluded if they met any of the following criteria: chromosomal or genetic abnormalities; hearing or visual impairments; diagnosed with neurological disease (e.g., epilepsy and Rett syndrome); and diagnostic imaging anomalies if the patient had undergone magnetic resonance imaging (MRI) scanning.
A total of 184 children were enrolled in the study. Children were classified as ASD with DD (ASD + DD) or ASD-only according to whether they presented with DD/ID (developmental quotient [DQ] and/or IQ score < 75). Since the sex/gender differences in ASD have been observed in previous neuroimaging studies (17, 18), and the sample size of the ASD female participants who had MRI scans was limited in our study, we focused on the ASD men for further neuroimaging analysis; 60 age-matched male participants (30 ASD + DD and 30 patients with ASD-only) scanned with the Siemens Version 3.0-Tesla MRI scanner were included.
The parents or guardians of all participants provided written informed consent according to the Declaration of Helsinki. This study was approved by the Ethical Committee of Shanghai Jiao Tong University School of Medicine, affiliated with Xinhua Hospital.
Behavioral assessments
Autistic symptoms
Autism Diagnostic Observation Schedule and CARS were used for the diagnosis of ASD alongside the DSM-V and ADI-R by a qualified and experienced assessor. These scales were also used to evaluate the clinical severity of autistic symptoms, where a higher ADOS score and CARS total score indicated more severe symptoms. For ADOS, a calibrated ASD severity score (CSS) was also calculated in the social affect (SA) domain to represent the severity of social communication.
Cognitive function
The average DQ in children aged 2–4 years was evaluated using the Gesell Developmental Diagnosis Scale (GDDS), whereas the IQ of children aged 4–6 years was assessed using the Weschler pre-school and primary scale of intelligence (19). We have compared the language development between the two groups using the language DQ in GDDS for children under 4 years.
Gesture development
The Putonghua Communicative Development Inventory (PCDI), adapted for Mandarin Chinese, was used to assess the early language and communication development of children aged 8–30 months, and it can be used to assess older children with developmental disorders (20, 21). The form of “Words and Gestures” was used to assess children’s early communication ability according to their actual mental age and language ability. “Words and Gestures” was divided into two sections, of which the second section was used to evaluate the use of gestures. This section included three items: early gestures, late gestures, and total gestures. Gestures on the PCDI that appear early reflect social communication, whereas gestures that appear later reflect symbolic ones (6). A higher score indicated a better level of gesture development.
Joint attention
“Responding to joint attention” (RJA) and “initiating joint attention” (IJA) were evaluated within the ADOS assessment. For the scenario of RJA, the evaluator assessed the child’s response to the use of gaze coordinated with facial orientation, vocalization, and pointing to draw attention to a distant object. When rating the IJA item, the evaluator assessed whether the child used eye-tracking or oral language to draw adults’ attention. Scores (0, 1, 2, or 3) represented different degrees of engagement (e.g., coordinated joint engagement with gaze shift, joint engagement with gaze shift and pointing, and unengaged) and initiation (e.g., child-initiated) (22).
Image acquisition and pre-processing
Image acquisition
Participants were scanned using a Siemens Version 3.0-Tesla MRI scanner (Siemens Medical Solutions, Munich, Germany) with a 32-channel head coil and a four-channel neck coil. Given their young age and inability to cooperate during the examination, our subjects were under sedation (50 mg/kg chloral hydrate at a maximum dose of 1 g administered rectally). Additionally, earplugs, earphones, and extra foam padding were provided to the participants to reduce the impact of the sound of the scanner during the scan. Through these efforts, the images of all patients were clear and usable. The high-resolution anatomical T1-weighted magnetization-prepared rapid gradient echo image (192 sagittal slices; voxels = 1 × 1 × 1 mm; repetition time [TR] = 2,300 ms; echo time [TE] = 2.28 ms; inversion time = 1,100 ms; flip angle = 8°; and field of view = 192 × 192 × 192 mm) was acquired.
Image pre-processing
T1-weighted images were processed using SPM121 and the CAT12 toolbox, which incorporates the DARTEL toolbox. Pre-processing steps included segmentation, registration, normalization, and smoothing. Notably, customized tissue probability maps (TPMs) of children were created using the Template-O-Matic Toolbox.2 These customized TPMs were used for the initial spatial registration and segmentation processes. We used the standard optimized method for iterative tissue segmentation and spatial normalization, which uses both linear (12-parameter affine) and non-linear transformations. To ensure that the residuals in later analyses conform to a Gaussian distribution and to account for individual differences in brain anatomy, the modulated gray matter images in Montreal Neurological Institute (MNI) space were smoothed using an isotropic Gaussian kernel of 8 mm full width at half maximum. The resulting voxel size was 1.5 × 1.5 × 1.5 mm3.
Statistical analyses
Analysis of clinical symptoms
The SPSS version 21.0 statistical software (Chicago, IL, United States) was used for clinical data analysis. After testing for data distribution and variance homogeneity, we calculated the means ± standard deviations for all measurement data, which included age, the GDDS average DQ, the ADOS social + communication score, the CARS total score, and the scores of the PCDI. Comparisons between the two groups were conducted using independent-sample t-tests. Classification data, such as sex, RJA, and IJA item scores, are expressed as numbers and were compared using χ2 tests. A p-value <0.05 was considered significant.
Analysis of brain volumes
Structure association analysis
A linear regression model was used to investigate the relationship between gray matter volume (GMV) and ASD with/without DD, with age and total intracranial volume as covariates of no interest. For the neuroimaging analysis, we conducted a permutation-based cluster-level correction (5,000 times) for multiple comparisons (23). At the voxel level, we used a two-sided test with a significance level of α = 0.001, whereas at the cluster level, we used a permutation-based family-wise error correction with a significance level of α = 0.05. Significant GMVs were defined as clusters with more than 217 voxels that fell within the 90% confidence interval (CI) of the smoothing kernel voxels (24, 25). An association analysis was then conducted between significant GMVs and clinical symptoms of autism and language development scores.
Mediation analysis
We tested the mediating effect of language development scores on the association between significant GMVs and clinical symptoms of autism while controlling for age and total intracranial volume as covariates of no interest. We applied a bootstrap procedure provided by PROCESS in SPSS3 to test the mediation (indirect) effect. The indirect effect was considered significant if the bootstrap CI did not include zero.
Results
Demographic and clinical characteristics of the participants
A total of 184 participants were enrolled from the Shanghai Xinhua Registry, which consisted of 103 patients with ASD + DD and 81 patients with ASD-only. There was no significant difference in age (t = −0.045, p = 0.964) or sex (χ2 = −0.990, p = 0.569) between the two groups. The CARS total score, ADOS social + communication score differed significantly between the ASD + DD and ASD-only groups (CARS: t = 7.029, p < 0.001; ADOS: t = −2.548, p = 0.012). The ASD + DD group (CARS: 38.81 ± 3.86; ADOS: 17.16 ± 3.59) had higher CARS, and ADOS scores than the ASD-only group (CARS: 34.60 ± 4.17; ADOS: 15.86 ± 3.17). In addition, compared with ASD-only, higher ADOS-SA CSS were observed in patients with ASD + DD (t = −2.207, p = 0.029) (Table 1). The language DQ in GDDS between the two groups was different (t = 12.988, p < 0.001).
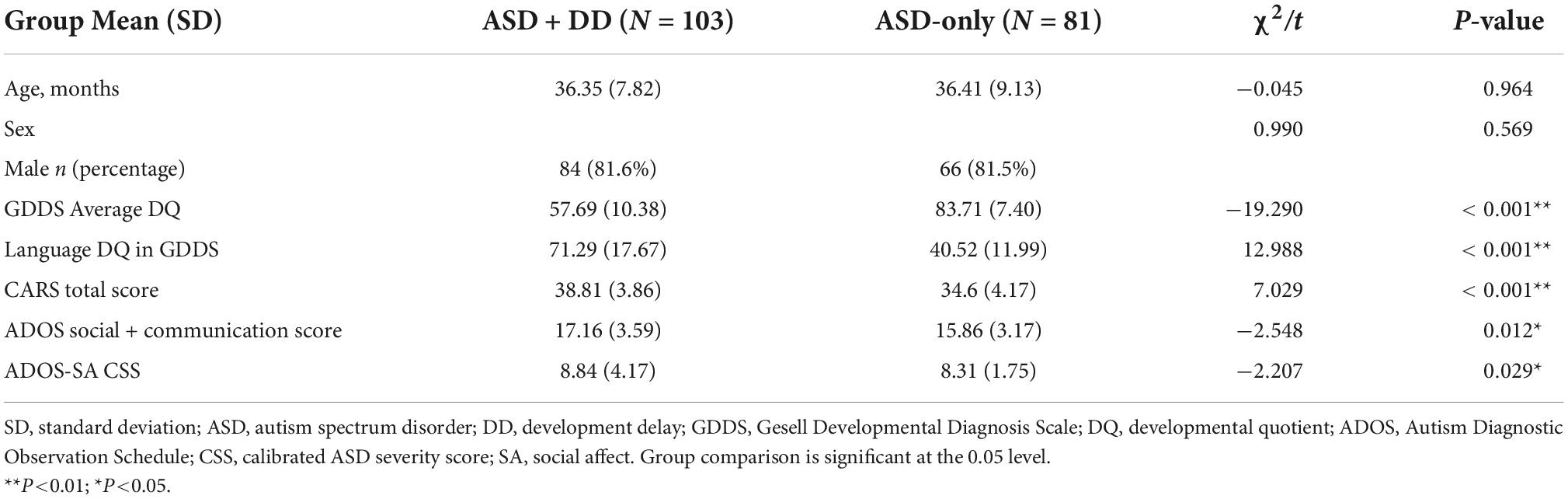
Table 1. Comparisons of demographic characteristics and symptom severity in autism spectrum disorder with or without developmental delay.
Comparisons of gesture development between autism spectrum disorder patients with and without developmental delay
The early, later, and total gesture scores were significantly different between the two groups (p < 0.05). Compared with the ASD-only group, the ASD + DD group’s scores for early, later, and total gestures were lower (Table 2).

Table 2. Comparisons of gesture development between autism spectrum disorder patients with and without gesell developmental diagnosis scale.
Comparisons of responding to joint attention and initiating joint attention ability between autism spectrum disorder patients with and without developmental delay
The comparisons of RJA and IJA ability showed that the ASD + DD group was poorer than the ASD-only group (Z = 27.630, p < 0.001; Z = 15.981, p < 0.001). For the RJA scores, 3.9% of patients in the ASD + DD group and 17.3% of patients in the ASD-only group had a score of zero.
However, 62.1 and 25.9% of the ASD + DD and ASD-only patients, respectively (Figure 1A), had higher RJA scores (i.e., two or three), which indicate more severe symptoms, and 68.9 and 38.3% of the patients with ASD + DD and ASD-only, respectively, had higher IJA scores (Figure 1B).
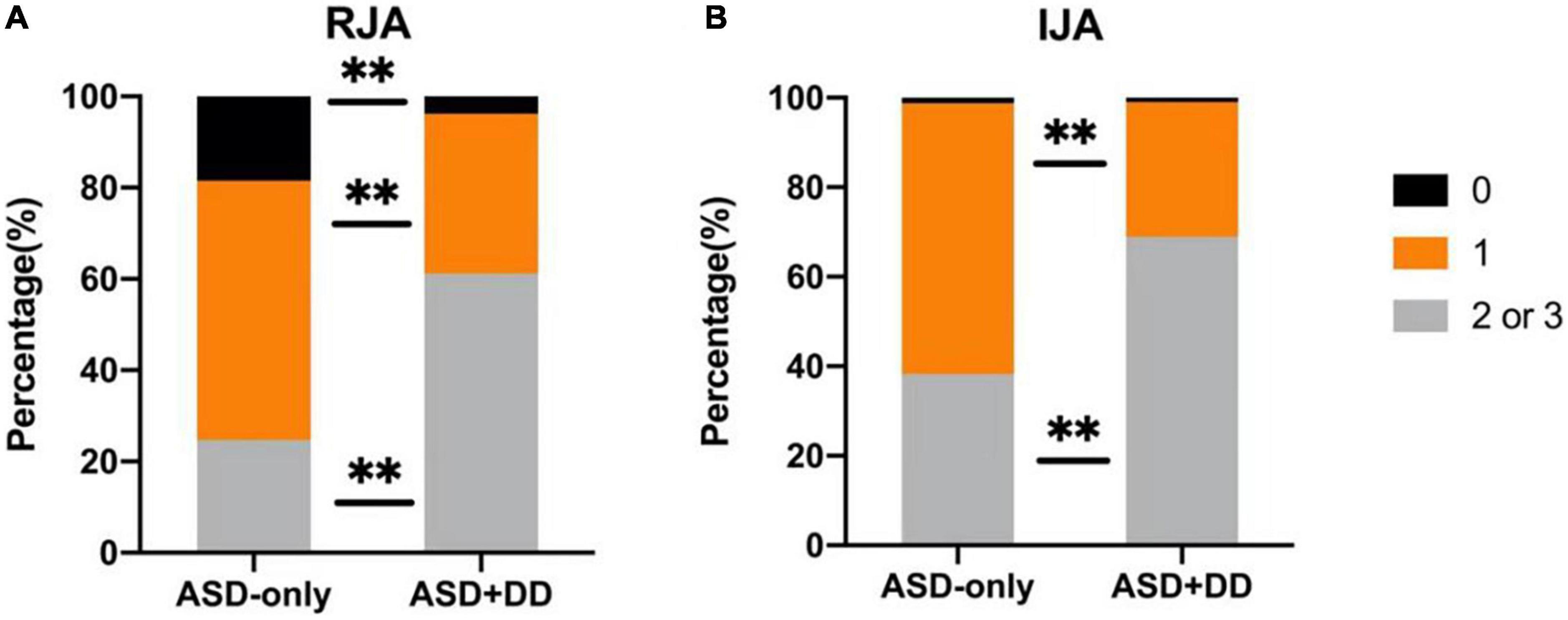
Figure 1. Comparisons of responding to joint attention (RJA) and initiating joint attention (IJA) ability between autism spectrum disorder (ASD) patients with and without developmental delay (DD). (A) and (B) represent the percentages of RJA and IJA scores. Comparisons between the two groups were performed using an analysis of variance. Different colors represent different scores. Black indicates an alerting score of 0, orange a score of 1, and gray a score of 2 or 3. **p < 0.01.
A larger temporopolar cortex is associated with the co-occurrence of developmental delay in patients with autism spectrum disorder
Volumetric differences were observed between the ASD + DD and ASD-only groups in two brain regions: mainly distributed across the temporal poles of the right middle temporal gyrus (TPOmed.R) and the left middle temporal gyrus (TPOmed.L) (Figure 2 and Table 3).
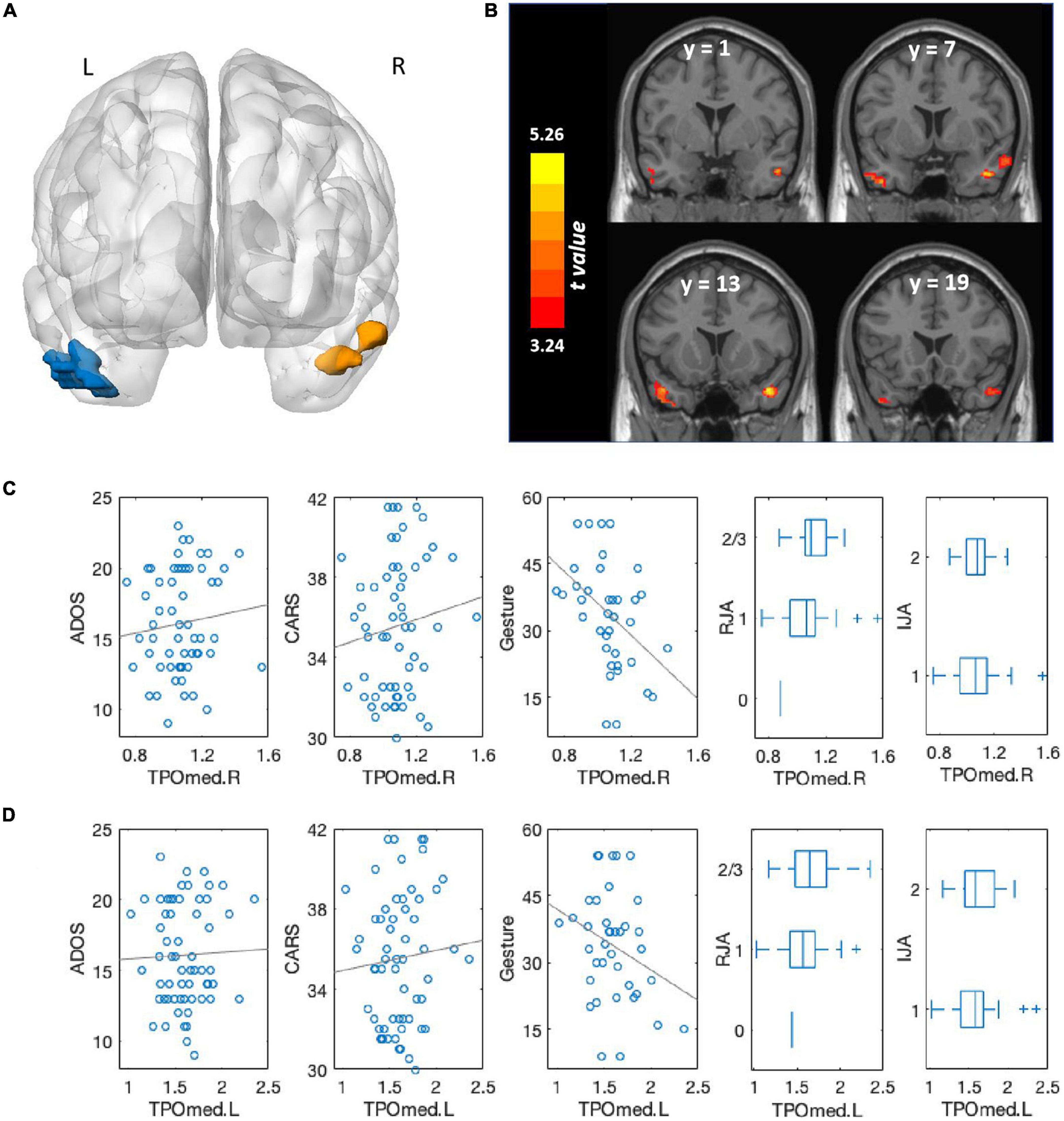
Figure 2. Comparison of brain volumes between autism spectrum disorder (ASD) patients with and without developmental delay (DD) and associations with clinical symptoms. (A) Brain regions that significantly differed between ASD patients with and without DD. The blue region comprised the TPOmed.L and left inferior temporal gyri; the orange region primarily comprised the TPOmed.R. Multiple comparison corrections were p < 0.001 at the voxel level and p < 0.05 cluster-level family-wise error correction. (B) Brain regions that significantly differed between ASD patients with and without DD, shown in the coronal view. The color bar represents t-values. (C) Association between TPOmed.R volume and ADOS scores, CARS scores, gestures scores, RJA ability, and IJA ability across the whole group. (D) Association between TPOmed.L volume and ADOS scores, CARS scores, gestures scores, RJA ability, and IJA ability across the whole group.
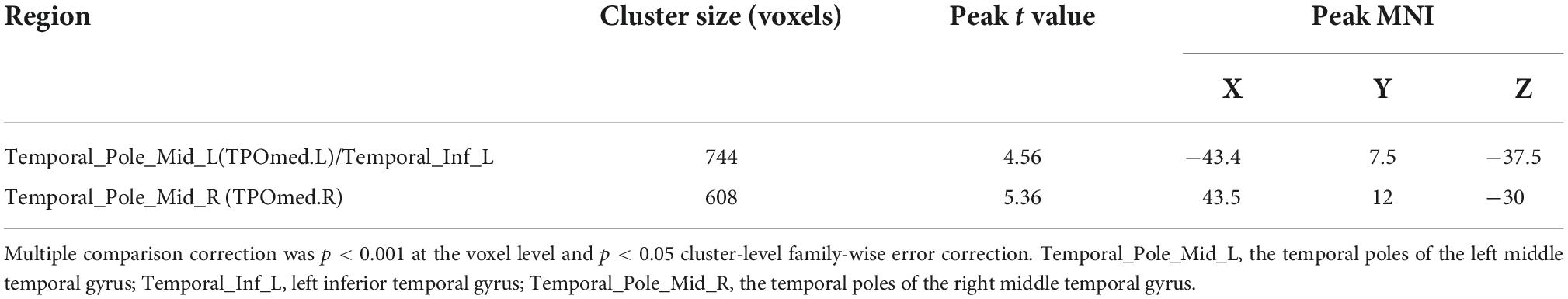
Table 3. Comparison of gray matter volume (GMV) between autism spectrum disorder (ASD) patients with and without developmental delay (DD).
For the association between the volumes of these two regions and clinical symptoms, we found that a larger TPOmed.R volume was correlated with greater severity of autistic symptoms as measured by both the ADOS score (r = 0.343, p = 0.008) and the CARS total score (r = 0.303, p = 0.021). A marginally significant association was observed between TPOmed.L volume and clinical symptoms (ADOS: r = 0.251, p = 0.057; CARS: r = 0.231, p = 0.082).
We also found that a larger TPOmed volume was correlated with a poorer gesture score (right: r = −0.624, p < 0.001; left: r = −0.443, p = 0.007) and RJA ability (right: r = 0.352, p = 0.007; left: r = 0.283, p = 0.031), whereas no correlation was observed between TPOmed volume and IJA ability (right: r = 0.140, p = 0.303; left: r = 0.248, p = 0.065).
Relationship among temporopolar volume, language development, and autistic symptoms
The mediation analysis was employed to examine whether language development mediated the relationship between TPOmed volume and autistic clinical symptoms. We observed a significant mediating effect of pre-language development on the association between TPOmed.R volume and both ADOS and CARS scores. In addition, we found that RJA ability, but not gesture development, contributed to this mediation effect. Similarly, the ability of RJA also linked TPOmed.L volume to poor clinical performance (Figure 3).
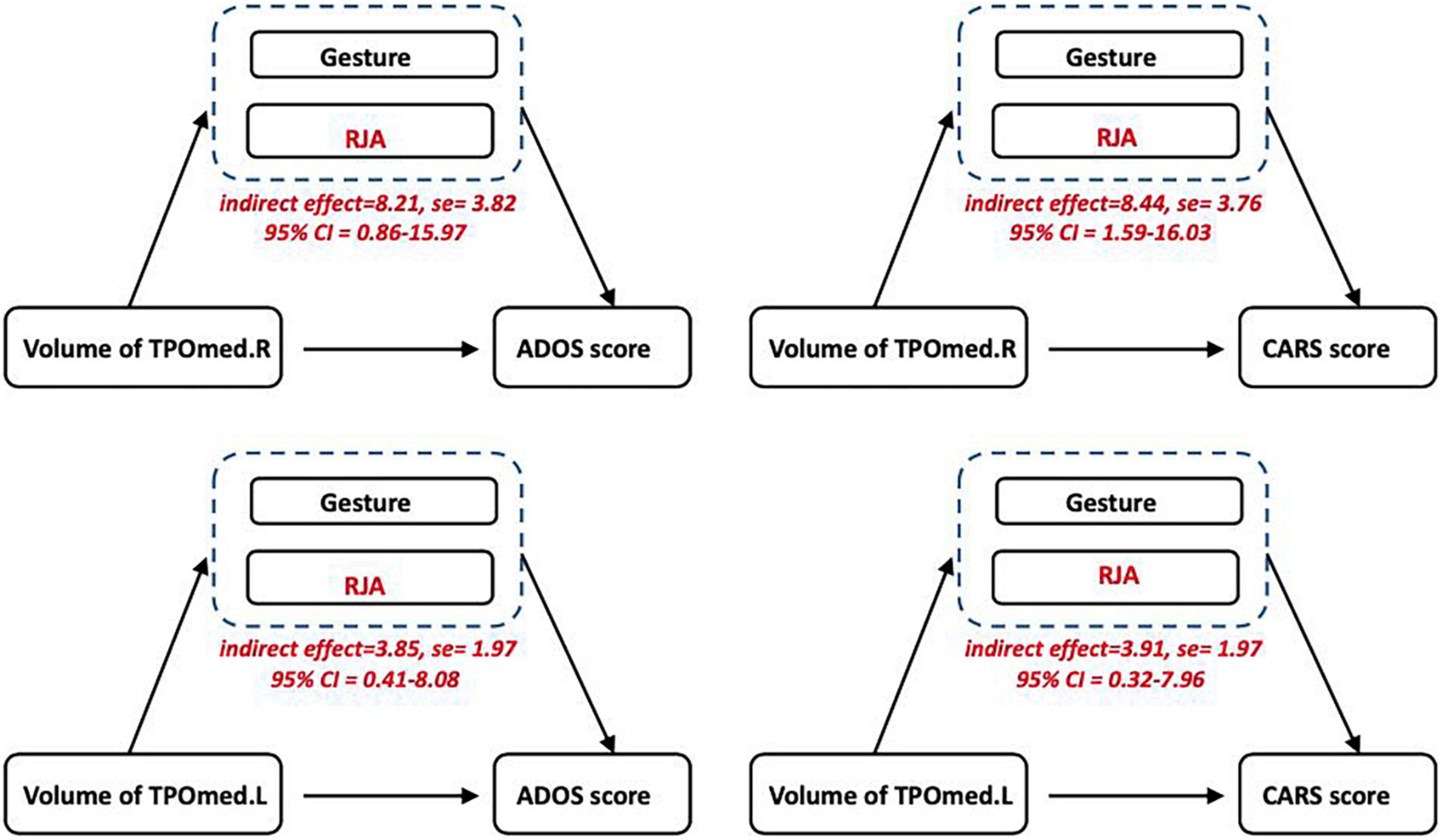
Figure 3. Relationship among temporopolar volume, language development, and autistic symptoms. The mediation analysis showed an association between the TPOmed volume and ASD severity (CARS and ADOS scores), which was mediated by RJA ability, but not gesture development.
Discussion
In this study, we noted that ASD children comorbid with DD/ID exhibited more severe clinical symptoms and more profound delay in gesture and joint attention than those without DD/ID, which indicated the importance of early surveillance of pre-language development. The volumetric alterations in the temporal poles of the middle temporal gyri worked as a driving neuroimaging factor for poor RJA ability and more severe clinical symptoms, indicating a potential brain intervention target to improve the clinical manifestations of ASD with DD/ID.
Currently, within the context of the novel concept of profound autism, increased attention has been drawn toward ASD children with comorbid DD/ID, since most of them are unable to live independently; the life-long cost of profound autism in education and healthcare was a heavy burden to both the families and the society (26). Although this concept is not appropriate to be applied to younger children, given their high plasticity of the brain and behavior, early recognition and intervention for disruptions in the developmental milestones associated with DD/ID in children with autism are necessary, which may improve the developmental trajectory pathways and prognosis of profound autism. We found that children with ASD + DD/ID showed more severe clinical symptoms compared with ASD-only (27), which manifested in the early developmental stage. Additionally, we focused on pre-verbal social communication skills and found widespread impairments in gesture, RJA, and IJA in children with ASD with comorbid DD/ID (5, 28). In typical development, the production of deictic gestures emerges early, around 8–10 months, and the engagement of joint attention can appear in the first year of life. Studies found that infants aged 15–18 months who were later diagnosed with autism used fewer gestures and joint attention than infants who were found to have DD and typical development (8). Therefore, deviation in early language development was pervasive in ASD + DD/ID and early language ability could be an important clinical feature and prognostic predictor of ASD with comorbid DD/ID (26).
Notably, the pre-language developmental discrepancy may be an important contributor to phenotypic heterogeneity between young ASD with and without DD/ID, and the assessment of gesture and joint attention in this critical period is crucial either in understanding their early communicative skills or developing suitable intervention when the brains are still in development. Early interventions targeting joint attention can help improve language and social skills in ASD with limited language (29). Some promising interventions, such as Joint Attention, Symbolic Play, Engagement and Regulation (known as JASPER), and the Pre-school Autism Communication Trial (known as PACT), which are interventions targeted at joint attention and parent-children synchrony, have been shown to alleviate both the core symptoms and clinical severity of ASD, and these effects can be maintained over time (30, 31). It is well-accepted that JASPER intervention could improve joint attention, joint engagement, and play directly in children with ASD (31). Another intervention, PACT, was also focused on joint attention and could result in long-term alleviation both in social communication and repetitive symptom domains for young children with ASD (30). These findings indicate that pre-language functions, including gesture and joint attention, and the related brain region may serve as a potential prognostic and therapeutic target, especially for ASD patients with comorbid DD/ID.
In our study, we also found that the differential brain region identified between ASD children with and without DD/ID was the temporal pole, and the ability of RJA mediated the relationship between temporopolar volume and symptom severity. This observation is in line with previous studies. The temporal pole is a cortex capable of higher-order cognitive functions and mentalizing processes, and the engagement of the temporal pole in joint attention has been observed in previous neuroimaging studies (32, 33). Previous studies revealed that temporal activation was associated with RJA ability (34). RJA and related behaviors appear to be closely associated with parietal and temporal cortical processes, which can regulate attention orienting to perceived objects or events (35). Furthermore, using a functional MRI eye-tracking paradigm, abnormal activation of the temporal pole was observed during a joint attention task (36). Compared with toddlers with ASD who had normal language development, those with poor language outcomes had hypoactive superior temporal cortices (37). Moreover, Meresse et al. found that cerebral perfusion in the temporal lobe of children with ASD was significantly negatively correlated with ADI-R scores, where the lower the cerebral perfusion in the temporal lobe, the more severe the autism symptoms (38). These present findings may have clinical implications that the temporal pole may serve as a potential prognostic and therapeutic target, especially for ASD patients with comorbid DD. In recent years, non-invasive neuromodulation techniques such as transcranial magnetic stimulation (TMS) have attracted particular interest in treating ASD, and existing evidence support that, via modulating certain brain areas or circuits, TMS could be a safe therapeutic option to treat the core symptoms of ASD (39). For example, repetitive TMS to the dorsomedial prefrontal cortex yielded a reduction in social impairment and social anxiety in patients with ASD (40). Therefore, our results indicate that for autistic children comorbid with DD, the temporal pole could be a candidate brain target for intervention and may produce a positive effect on joint attention.
Limitations
Our research has several limitations. First, the study design was cross-sectional, which limited the exploration of the trajectories of pre-language development in autistic children with language delays. Second, since the neuroimaging analysis was only performed on ASD male participants, the neuroimaging findings in this study could only be extrapolated to the male population. Finally, RJA and IJA abilities were recorded and coded when performing ADOS; combining tools, such as Short Play and Communication Evaluation (SPACE) or eye-tracking (5, 41), would be more informative in exploring joint attention in ASD.
Conclusion
Temporopolar volumes were significantly enlarged in children with minimal language children and ASD + DD, and were related to ASD severity and language ability during the pre-language stage. Our findings suggest that volumetric differences in the temporal pole may provide a biological basis for autism severity before the onset of clinical symptoms and offer clues for developing interventions targeting the temporal pole, which may improve the prognosis of ASD.
Data availability statement
The data of this study are available under reasonable and ethically approved request to the corresponding authors.
Ethics statement
This study involving human participants was approved by the Ethical Committee of Shanghai Jiao Tong University School of Medicine Affiliated Xinhua Hospital. The parents or guardians of all participants provided written informed consent according to the Declaration of Helsinki in the study.
Author contributions
LZa, FL, YJ, and MX designed the study. YJ, XL, YD, and LZo collected the clinical information and brain imaging data. YJ and LZa carried out the analysis. YJ, MX, and LZa interpreted the results and wrote the manuscript. LZa and FL guided and supervised all work. YJ, MX, XL, YD, LZo, FL, and LZa read, provided the feedback, discussed and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 82125032, 81930095, 82001771, and 81761128035), the Science and Technology Commission of Shanghai Municipality (Grant Nos. 19410713500 and 2018SHZDZX01), the Shanghai Municipal Commission of Health and Family Planning (Grant Nos. GWV-10.1-XK07, 2020CXJQ01, and 2018YJRC03), the Shanghai Clinical Key Subject Construction Project (Grant No. shslczdzk02902), the Guangdong Key Project (Grant No. 2018B030335001), the Innovative Research Team of High-level Local Universities in Shanghai (Grant No. SHSMU-ZDCX20211100), and the Shanghai Municipal Commission of Health (Grant No. 20214Y0125).
Acknowledgments
We thank all clinicians and patients involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ http://www.fil.ion.ucl.ac.uk/spm/
- ^ https://neuro-jena.github.io/software.html#tom
- ^ https://www.processmacro.org/index.html
References
1. Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years – Autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. (2021) 70:1–16. doi: 10.15585/mmwr.ss7011a1
2. Lord C, Charman T, Havdahl A, Carbone P, Anagnostou E, Boyd B, et al. The Lancet Commission on the future of care and clinical research in autism. Lancet. (2022) 399:271–334. doi: 10.1016/S0140-673601541-5
3. Pickles A, Wright N, Bedford R, Steiman M, Duku E, Bennett T, et al. Predictors of language regression and its association with subsequent communication development in children with autism. J Child Psychol Psychiatry. (2022) 63:1243–51. doi: 10.1111/jcpp.13565
4. Shumway S, Farmer C, Thurm A, Joseph L, Black D, Golden C. The ADOS calibrated severity score: relationship to phenotypic variables and stability over time. Autism Res. (2012) 5:267–76. doi: 10.1002/aur.1238
5. Nyström P, Thorup E, Bölte S, Falck-Ytter T. Joint attention in infancy and the emergence of autism. Biol Psychiatry. (2019) 86:631–8. doi: 10.1016/j.biopsych.2019.05.006
6. Ellawadi AB, Ellis Weismer S. Assessing gestures in young children with autism spectrum disorder. J Speech Lang Hear Res. (2014) 57:524–31. doi: 10.1044/2013_JSLHR-L-12-0244
7. Mundy P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur J Neurosci. (2018) 47:497–514. doi: 10.1111/ejn.13720
8. Ye Q, Liu L, Lv S, Cheng S, Zhu H, Xu Y. The gestures in 2-4-year-old children with autism spectrum disorder. Front Psychol. (2021) 12:604542. doi: 10.3389/fpsyg.2021.604542
9. Mundy P, Kim K, McIntyre N, Lerro L, Jarrold W. Brief report: joint attention and information processing in children with higher functioning autism spectrum disorders. J Autism Dev Disord. (2016) 46:2555–60. doi: 10.1007/s10803-016-2785-6
10. Tager-Flusberg H. Risk factors associated with language in autism spectrum disorder: clues to underlying mechanisms. J Speech Lang Hear Res. (2016) 59:143–54. doi: 10.1044/2015_JSLHR-L-15-0146
11. Gulsrud AC, Hellemann GS, Freeman SF, Kasari C. Two to ten years: developmental trajectories of joint attention in children with ASD who received targeted social communication interventions. Autism Res. (2014) 7:207–15. doi: 10.1002/aur.1360
12. Mundy P, Novotny S, Swain-Lerro L, McIntyre N, Zajic M, Oswald T. Joint-attention and the social phenotype of school-aged children with ASD. J Autism Dev Disord. (2017) 47:1423–35. doi: 10.1007/s10803-017-3061-0
13. Palomo R, Ozonoff S, Young GS, Belinchón Carmona M. Social orienting and initiated joint attention behaviors in 9 to 12 month old children with autism spectrum disorder: a family home movies study. Autism Res. (2022) 15:1109–19. doi: 10.1002/aur.2695
14. Bedford SA, Park M, Devenyi GA, Tullo S, Germann J, Patel R, et al. Large-scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Mol Psychiatry. (2020) 25:614–28. doi: 10.1038/s41380-019-0420-6
15. Gabrielsen TP, Anderson JS, Stephenson KG, Beck J, King JB, Kellems R, et al. Functional MRI connectivity of children with autism and low verbal and cognitive performance. Mol Autism. (2018) 9:67. doi: 10.1186/s13229-018-0248-y
16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association (2013).
17. Walsh MJM, Wallace GL, Gallegos SM, Braden BB. Brain-based sex differences in autism spectrum disorder across the lifespan: a systematic review of structural MRI, fMRI, and DTI findings. Neuroimage Clin. (2021) 31:102719. doi: 10.1016/j.nicl.2021.102719
18. Mo K, Sadoway T, Bonato S, Ameis SH, Anagnostou E, Lerch JP, et al. Sex/gender differences in the human autistic brains: a systematic review of 20 years of neuroimaging research. Neuroimage Clin. (2021) 32:102811. doi: 10.1016/j.nicl.2021.102811
19. Shan L, Feng JY, Wang TT, Xu ZD, Jia FY. Prevalence and developmental profiles of autism spectrum disorders in children with global developmental delay. Front Psychiatry. (2022) 12:794238. doi: 10.3389/fpsyt.2021.794238
20. Miniscalco C, Rudling M, Råstam M, Gillberg C, Johnels JÅ. Imitation (rather than core language) predicts pragmatic development in young children with ASD: a preliminary longitudinal study using CDI parental reports. Int J Lang Commun Disord. (2014) 49:369–75. doi: 10.1111/1460-6984.12085
21. Su YE, Naigles LR, Su LY. Uneven expressive language development in mandarin-exposed preschool children with ASD: comparing vocabulary, grammar, and the decontextualized use of language via the PCDI-toddler form. J Autism Dev Disord. (2018) 48:3432–48. doi: 10.1007/s10803-018-3614-x
22. Shih W, Shire S, Chang YC, Kasari C. Joint engagement is a potential mechanism leading to increased initiations of joint attention and downstream effects on language: JASPER early intervention for children with ASD. J Child Psychol Psychiatry Allied Discipl. (2021) 62:1228–35. doi: 10.1111/jcpp.13405
23. Shen C, Luo Q, Chamberlain SR, Morgan S, Romero-Garcia R, Du J, et al. What is the link between attention-deficit/hyperactivity disorder and sleep disturbance? A multimodal examination of longitudinal relationships and brain structure using large-scale population-based cohorts. Biol Psychiatry. (2020) 88:459–69. doi: 10.1016/j.biopsych.2020.03.010
24. Luo Q, Chen Q, Wang W, Desrivières S, Quinlan EB, Jia T, et al. Association of a schizophrenia-risk nonsynonymous variant with putamen volume in adolescents: a voxelwise and genome-wide association study. JAMA Psychiatry. (2019) 76:435–45. doi: 10.1001/jamapsychiatry.2018.4126
25. Luo Q, Zhang L, Huang CC, Zheng Y, Kanen JW, Zhao Q, et al. Association between childhood trauma and risk for obesity: a putative neurocognitive developmental pathway. BMC Med. (2020) 18:278. doi: 10.1186/s12916-020-01743-2
26. Hyman SL, Levy SE, Myers SM, Council on Children With Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. (2020) 145:e20193447. doi: 10.1542/peds.2019-3447
27. Thomas RP, Milan S, Naigles L, Robins DL, Barton ML, Adamson LB, et al. Symptoms of autism spectrum disorder and developmental delay in children with low mental age. Clin Neuropsychol. (2021) 36:1028–48. doi: 10.1080/13854046.2021.1998634
28. Roemer EJ, West KL, Northrup JB, Iverson JM. Word comprehension mediates the link between gesture and word production: examining language development in infant siblings of children with autism spectrum disorder. Dev Sci. (2019) 22:e12767. doi: 10.1111/desc.12767
29. Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, Rogers SJ. A prospective study of response to name in infants at risk for autism. Arch Pediatr Adolesc Med. (2107) 161:378–83. doi: 10.1001/archpedi.161.4.378
30. Pickles A, Le Couteur A, Leadbitter K, Salomone E, Cole-Fletche R, Tobin H, et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet. (2016) 388:2501–9. doi: 10.1016/S0140-673631229-6
31. Waddington H, Reynolds JE, Macaskill E, Curtis S, Taylor LJ, Whitehouse AJ. The effects of JASPER intervention for children with autism spectrum disorder: a systematic review. Autism. (2021) 25:2370–85. doi: 10.1177/13623613211019162
32. Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. (2005) 62:1366–76. doi: 10.1001/archpsyc.62.12.1366
33. Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. (2002) 59:184–92. doi: 10.1212/wnl.59.2.184
34. Mundy P, Jarrold W. Infant joint attention, neural networks and social cognition. Neural Netw. (2010) 23:985–97. doi: 10.1016/j.neunet.2010.08.009
35. Materna S, Dicke PW, Thier P. Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: a functional magnetic resonance imaging study. J Cogn Neurosci. (2008) 20:108–19. doi: 10.1162/jocn.2008.20.1.108
36. Oberwelland E, Schilbach L, Barisic I, Krall SC, Vogeley K, Fink GR, et al. Young adolescents with autism show abnormal joint attention network: a gaze contingent fMRI study. Neuroimage Clin. (2017) 14:112–21. doi: 10.1016/j.nicl.2017.01.006
37. Lombardo MV, Pierce K, Eyler LT, Carter Barnes C, Ahrens-Barbeau C, Solso S, et al. Different functional neural substrates for good and poor language outcome in autism. Neuron. (2015) 86:567–77. doi: 10.1016/j.neuron.2015.03.023
38. Gendry Meresse I, Zilbovicius M, Boddaert N, Robel L, Philippe A, Sfaello I, et al. Autism severity and temporal lobe functional abnormalities. Ann Neurol. (2015) 58:466–9. doi: 10.1002/ana.20597
39. Barahona-Corrêa JB, Velosa A, Chainho A, Lopes R, Oliveira-Maia AJ. Repetitive transcranial magnetic stimulation for treatment of autism spectrum disorder: a systematic review and meta-analysis. Front Integr Neurosci. (2018) 12:27. doi: 10.3389/fnint.2018.00027
40. Enticott PG, Fitzgibbon BM, Kennedy HA, Arnold SL, Elliot D, Peachey A, et al. A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimul. (2014) 7:206–11. doi: 10.1016/j.brs.2013.10.004
Keywords: autism spectrum disorder, developmental delay, gesture, joint attention, temporal pole
Citation: Ji Y, Xu M, Liu X, Dai Y, Zhou L, Li F and Zhang L (2022) Temporopolar volumes are associated with the severity of social impairment and language development in children with autism spectrum disorder with developmental delay. Front. Psychiatry 13:1072272. doi: 10.3389/fpsyt.2022.1072272
Received: 17 October 2022; Accepted: 07 November 2022;
Published: 01 December 2022.
Edited by:
Juehua Yu, The First Affiliated Hospital of Kunming Medical University, ChinaReviewed by:
Yanni Chen, Xi’an Children’s Hospital, ChinaLi Chen, Children’s Hospital of Chongqing Medical University, China
Copyright © 2022 Ji, Xu, Liu, Dai, Zhou, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingli Zhang, bHpoYW5nODkwMUBnbWFpbC5jb20=; Fei Li, ZmVpbGlAc2hzbXUuZWR1LmNu; Mingyu Xu, eHVtaW5neXVAeGluaHVhbWVkLmNvbS5jbg==
†These authors have contributed equally to this work
 Yiting Ji
Yiting Ji Mingyu Xu
Mingyu Xu Xin Liu2
Xin Liu2 Li Zhou
Li Zhou Fei Li
Fei Li Lingli Zhang
Lingli Zhang