
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 20 December 2022
Sec. Psychological Therapy and Psychosomatics
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.1062426
Objective: The Multidimensional Fatigue Inventory (MFI-20) is commonly used, but its factor structure remains unclear. The MFI-20 consists of five subscales (general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue). This study investigates the psychometric properties, including the factor structure, of a general German population sample and tests group hypotheses on gender and age. Another objective is to provide normative data by gender and age groups.
Methods: Using data from a representative German sample (n=2,509), reliability and convergent validity measures, group hypothesis testing, and confirmatory/exploratory factor analyses were conducted.
Results: The MFI-20 demonstrated satisfactory internal consistency and showed adequate convergent validity with the SF-36. All subscales of the MFI-20 were significantly correlated (0.71–0.85). Physical fatigue exhibited the highest (0.42) and mental fatigue had the lowest (0.19) correlation with age. Fatigue scores were significantly higher for women and significantly increased with age. A five-factor structure showed poor model fit; using an exploratory factor analysis, a two-factor structure emerged (a general factor and a mental/motivational factor).
Conclusion: The MFI-20 is a reliable and valid instrument for measuring fatigue in the general population, but the five-factor structure is not supported. The subscale general fatigue or the MFI-20 total score might measure fatigue sufficiently. The provided norms can be used for further research and individual assessment.
Definitions of fatigue often include “general exhaustion, extreme tiredness, weakness, and lack of energy” (1) and “impaired physical and/or cognitive functioning” (2). It is both a normal, transient phenomenon in the general population and one of the most-stated symptoms in (chronic) pathological conditions. Chronic fatigue, as an enduring state with pathological significance that does not reduce through common mechanisms of regeneration, is stated as one of the most frequent symptoms in cancer, lupus, chronic inflammation, and patients with multiple sclerosis (3, 4). It is also a prominent symptom in psychiatric disorders, especially in major depression (5, 6). Recently, fatigue has been described as part of the post-COVID-19 syndrome (7). Interestingly, research on myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) shows heterogeneous but converging evidence for a “neuropsychological profile” (8), i.e., substantial differences to healthy controls using different neuroimaging techniques (9). Fatigue is different from fatigability, an objective change in performance that can be measured electrophysiologically but is not necessarily correlated with the subjective experience of fatigue (10). Objective somatic markers or correlates of fatigue have yet to be found (2) which shows the importance of valid and reliable self-report instruments, e.g., patient-reported outcome measures (PROMs). There is an ongoing discussion on the dimensionality of fatigue. It is often described as two-dimensional (mental and physical) or three-dimensional (physical, emotional, and cognitive) (11). More than 40 fatigue measurements are in general use (12). Instruments to directly compare the results of different fatigue instruments have been developed (13). Alongside unidimensional fatigue inventories, the most studied multidimensional fatigue inventories are the Checklist Individual Strength, the Chalder Fatigue Scale, the Multidimensional Assessment of Fatigue, the Piper Fatigue Scale, and the Multidimensional Fatigue Inventory (MFI-20). The latter is the most used of those consisting of three or more subscales (14) and is further investigated in this study.
The MFI-20 was originally developed focusing on patients with cancer and initially proposed a five-factor structure, namely, general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue (15). This structure is strongly questioned by many studies, finding a three-, four- or five-factor structure with varying item loadings [refer to (11) for an overview]. A recent German study of patients (n = 140) with spinal muscular atrophy, however, confirmed the five-factor structure using principal component analysis (16). They explained 62.2% of the variance, and only five of the 20 items did not load on the components that otherwise matched the original MFI-20 subscales. Contrary to validation studies of the MFI-20 in disease-specific populations, reliability and validity of the MFI-20 in large general population samples are scarce. Large German, US-American, and Dutch studies show subscale-specific age and gender differences (1, 11, 17). The last German validation study was published in 2003 and relied on data from 1998. Hence, updated and more detailed results are warranted for research and practice. This study uses a large representative German population sample to (a) investigate the psychometric properties of the MFI-20, including the factor structure, (b) test group differences between age and gender, and (c) provide German population-based norms.
A random sample of German residents aged 16 and older was recruited as part of a broader cross-sectional questionnaire survey between October and December 2021. Since there is no directory available that contains the addresses of all private households or individuals in Germany, the “ADM Sampling System for face-to-face surveys” (18) was used to draw a representative German sample. A market and social research company (USUMA GmbH, Berlin, Germany) performed the subject acquisition and face-to-face interviews with trained interviewers. Overall, 5,901 persons were contacted, 2,526 participated (others did not respond or were ill or otherwise unavailable at the appointed interview date; all reasons for non-participation are listed at the end of the manuscript), and 2,509 completed the interview and the self-report battery. Data for comparison on demographics between participants and non-participants were not available, but the participants are representative regarding gender, age, and regional distribution (16 German federal states). All data were fully anonymized by USUMA prior to data analysis. All participants gave their verbal informed consent in accordance with the Helsinki declaration, which was documented by USUMA, and followed a structured face-to-face interview to collect the statistical and sociodemographic data. Participants were ≥ 16 years and are legally competent in Germany to participate in a study without the need for others' consent. The self-report questionnaires, such as the MFI-20, were then completed via paper and pencil independently by the subjects themselves, so as to not bias their personal mental and physical health answers. The study was approved by the Ethical Review Committee of the University of Leipzig (298/21-ek).
The Multidimensional Fatigue Inventory (MFI-20) is a 20-item self-report instrument, which consists of five subscales: 1. general fatigue (items 1, 5, 12, 16), 2. physical fatigue (items 2, 8, 14, 20), 3. reduced activity (items 3, 6, 10, 17), 4. reduced motivation (items 4, 9, 15, 18), and 5. mental fatigue (items 7, 11, 13, 19). Each subscale consists of four items with possible answers on a 5-point Likert scale (1 = yes, that is true; 5 = no, this is not true). Higher scores indicate higher levels of fatigue. Convergent validity with a Visual Analog Scale (VAS) assessing fatigue showed significant correlations ranging from 0.77 (general fatigue) to 0.23 (mental fatigue) (15), with the Fatigue Severity Scale ranging from 0.73 (general fatigue) to 0.16 (reduced activity) (19). The previous German validation study argued for using the total score of the 20 items because of the empirically uncertain factorial structure and the strong correlations between the subscales (1).
The Short Form-36 Health Survey (SF-36) (20) was used to provide a convergent validity criterion for the MFI-20. The 36 questions on the SF-36 are meant to reflect eight domains of health, including physical functioning, physical role, pain, general health, vitality, social functioning, emotional role, and mental health (range 0 to 100). Additionally, a Physical Composite Score (PCS) and a Mental Composite Score (MCS) can be calculated. These scores are T-values (mean = 50, SD = 10) in reference to a US norm sample. Especially, the SF-36 domain vitality has been widely used as a fatigue marker (21, 22).
IBM SPSS Statistics version 28 (23) was used for the statistical analyses. Negatively phrased items were inverted prior to further analyses. Because the questionnaire scores were not normally distributed (Shapiro-Wilk tests for all fatigue scales, p < 0.001), median and mode values are reported in addition to means and standard deviations (SD). Spearman's ρ correlations were calculated to investigate the association among the MFI-20 subscales and between those, the SF-36 domains, and age. Mann–Whitney U tests were calculated to compare MFI-20 scores between male and female subjects. Kruskal-Wallis tests with Dunn-Bonferroni post-hoc tests were conducted to compare seven age groups (≤ 24, 25–34, 35–44, 45–54, 55–64, 65–74, and ≥ 75 years, in accordance with other studies using this survey). A confirmatory factor analysis was computed using the lavaan R package for SPSS (24). The norm tables were conducted as percentile ranks. McDonald's omega (ωt), an alternative measure of internal consistency that does not assume a tau-equivalent but a congeneric model, was computed using the SPSS Macro OMEGA (25).
Table 1 summarizes the sociodemographic characteristics, shows the MFI-20 subscale and total scores, and the SF-36 scores of the total sample. The descending order of the MFI-20 subscales, with general fatigue exhibiting the highest scores and mental fatigue the lowest scores, is comparable to a recent Dutch norm sample and other studies (11). The five MFI-20 subscales, the total score, and age did not follow a normal distribution. The MFI-20 total score had a profound right-tailed skewness (0.82) and a slight flat kurtosis (−0.13). The five subscales showed a strong floor effect, i.e., between 15% (e.g., general fatigue) and 20% (e.g., mental fatigue) of the subjects exhibited the lowest possible score.
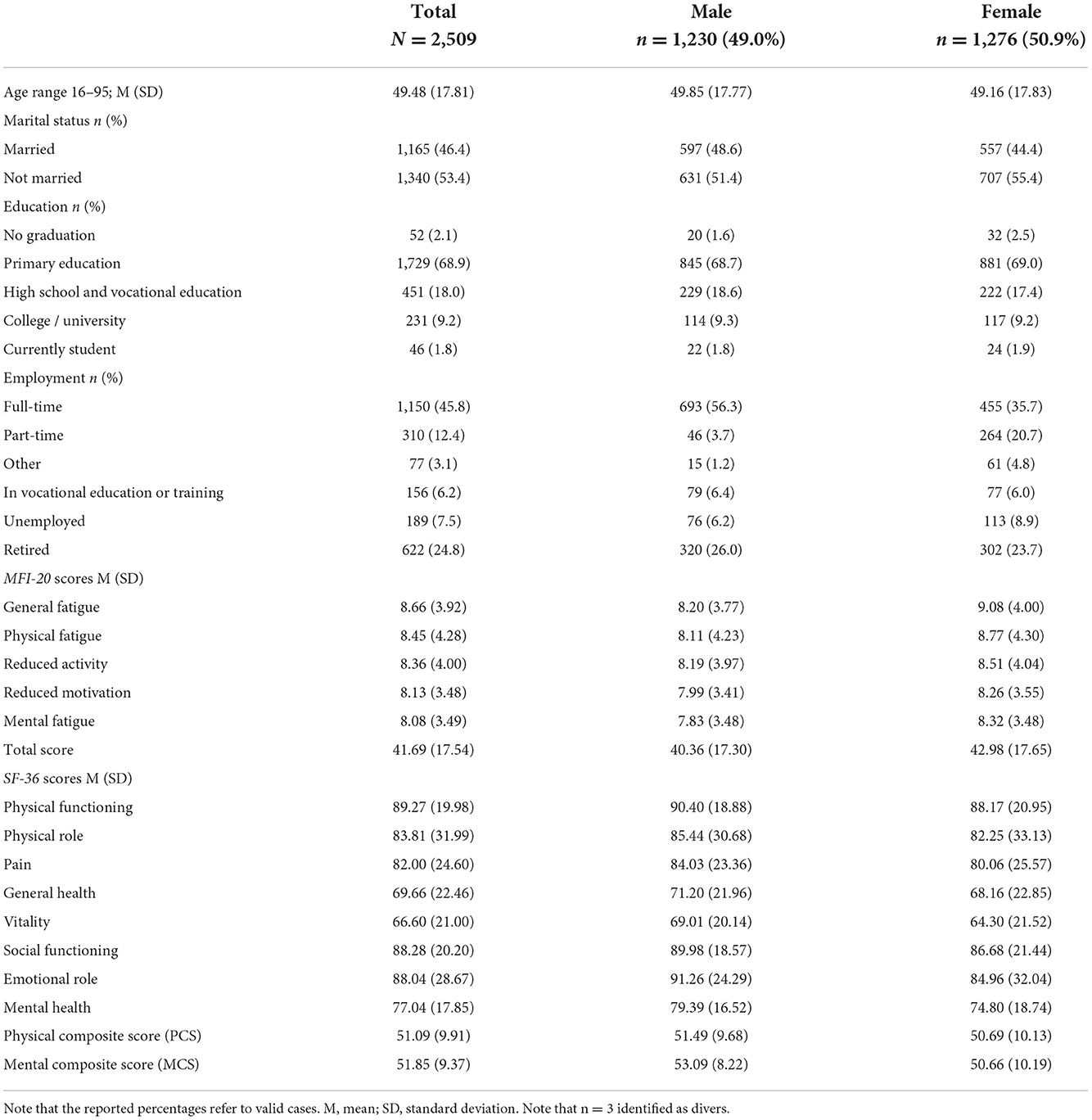
Table 1. Sociodemographic characteristics, MFI-20 and SF-36 scores for the total sample (N = 2,509), and for the male participant and female participant.
Item scores ranged from 1.75 (item 18) to 2.38 (item 20). The selectivity (item-scale correlations) varied between 0.545 (item 9) and 0.814 (item 14), and the homogeneity (inter-item-correlations) varied between 0.362 (item 9 x item 15) and 0.733 (item 8 x item 20). The mean value of missing answers per item was 6 (SD = 2.77) or 0.24%, indicating a highly accepted questionnaire. Item characteristics are shown in Table 2.
Ascertaining reliability, Cronbach's α values for the subscales were 0.87 (General fatigue), 0.90 (Physical fatigue), 0.88 (Reduced activity), 0.79 (Reduced motivation), and 0.83 (Mental fatigue). These values are similar to the developers' 1995 values (15) and follow the identical ordinal ranking found in the first German 2003 validation study (1). The omission of items did not increase any Cronbach's α considerably. McDonald's ωt for all items was 0.964, indicating high reliability (26, 27).
The convergent validity of the MFI-20 was examined by correlating the MFI-20 subscales/total score with the SF-36 domains and its PCS and MCS. All correlations between the MFI-20 subscales/total score and the SF-36 domains/PCS/MCS were significant (p < 0.001), using Spearman's ρ correlations due to the non-parametric distribution. Correlations between 0.40 and 0.60 can be seen as a medium in convergent validity. As expected, vitality exhibited the highest correlations with three of the five MFI-20 subscales and the total score, highlighted in bold in Table 3. The PCS correlated the most with physical fatigue (−0.72) and the lowest with mental fatigue (−0.41). The MCS correlated the most with the MFI-20 total score (−0.64), followed by general fatigue (−0.63) and mental fatigue (−0.61). Pairwise correlations of the MFI-20 subscales ranged between 0.71 and 0.85 and are slightly lower when controlled for age (refer to Table 3, values labeled p). The correlation between the MFI-20 total score and age was rs = 0.35, with physical fatigue having the highest (rs = 0.42) and mental fatigue having the lowest (rs = 0.19) subscale correlation with age (all p < 0.001).
Mann–Whitney U tests showed significantly higher fatigue scores in females for all but one subscale and the total score (all p < 0.001, reduced activity p = 0.04) but not for reduced motivation (p = 0.08). The Kruskal-Wallis test showed significant differences between age groups for the different subscales and the total score: general fatigue [H(2) = 269.969], physical fatigue [H(2) = 466.147], reduced activity [H(2) = 318.654], reduced motivation [H(2) = 273.402], mental fatigue [H(2) = 118.925], and the total score [H(2) = 342.471]; all p < 0.001, df = 6. Following post-hoc tests (Dunn-Bonferroni) revealed which age groups significantly differed from each other (Table 4).
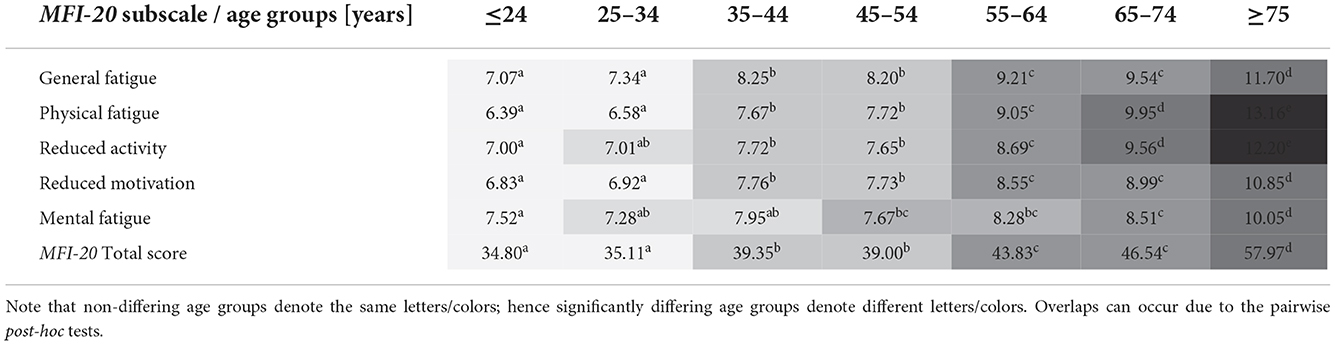
Table 4. Results of the Kruskal-Wallis Dunn-Bonferroni post-hoc tests for the MFI-20 subscales and the total score, by age groups, shown in the superscript table.
Two-way ANOVAs showed no interaction effect between age (seven age groups) and gender (male and female) on any subscale and the total score. However, this must be interpreted carefully due to non-parametric distributions. A descriptive, insignificant interaction effect of gender and age groups 3–44 and 45–54 years could be observed on all subscales (except mental fatigue) and the total score. Between these two age groups, the scores increase with age for men but decrease with age for women. Figure 1 shows the general fatigue scores to highlight the age and gender structure of the results. Figure 2 illustrates that in the oldest age group, reduced motivation and mental fatigue, two cognitive appraisal domains, show the relatively lowest scores. In the youngest age group (≤ 24 years), mental fatigue exhibited the highest and physical fatigue the lowest scores. In contrast, in the oldest age group (≥ 75 years), mental fatigue exhibited the lowest and physical fatigue the highest scores.
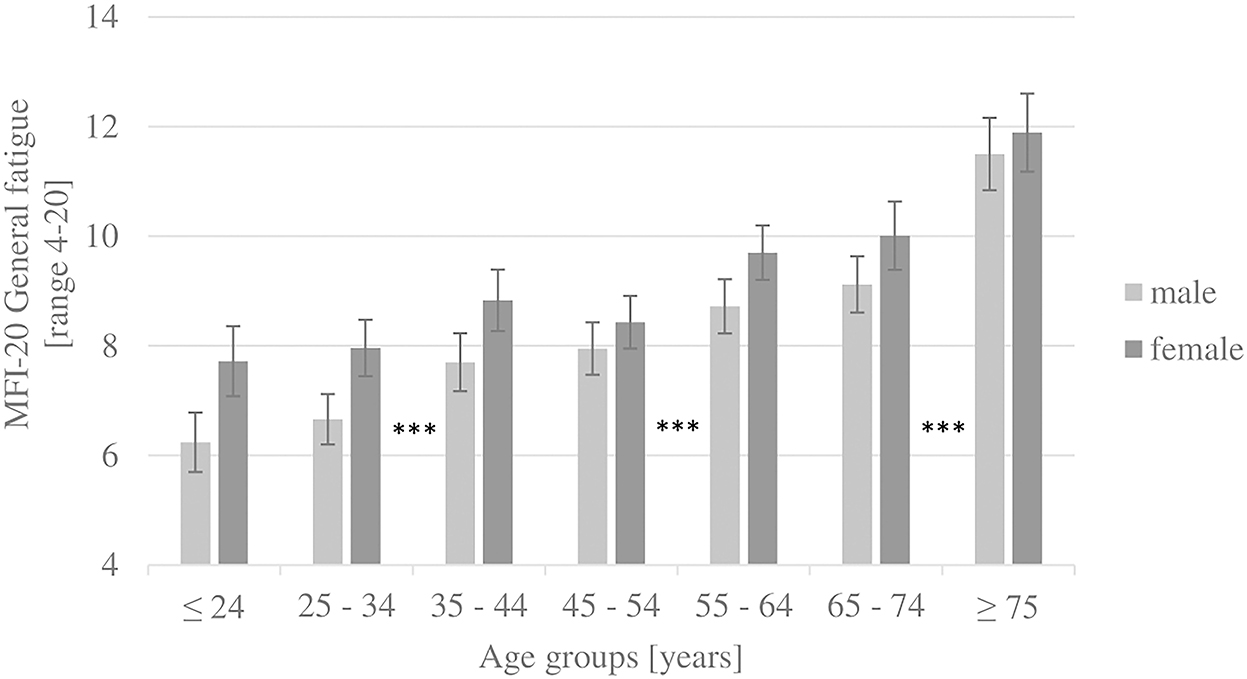
Figure 1. Results of the subscale general fatigue, by gender and age groups (whiskers show 95% confidence intervals). ***post-hoc tests (Dunn-Bonferroni): p < 0.001.
The data did not follow a multivariate normal distribution (28). A confirmatory factor analysis (CFA) for the five-factor structure showed poor model fit, comparative fit index (CFI) = 0.891, Tucker-Lewis index (TLI) = 0.871 (values > 0.95 indicate an acceptable fit), and root mean square error of approximation (RMSEA) = 0.105 (values < 0.08 indicate an acceptable fit). The Kaiser-Meyer-Olkin criterion of sampling adequacy was highly developed (0.972). The Bartlett test on sphericity was significant (p < 0.001, approximate χ2 = 39625.349, df = 190). An exploratory factor analysis (EFA using principal component analysis) showed two disparate components with an eigenvalue (λ) > 1. The first component with λ = 11.872 explained 59.36% of the variance, and the second component with λ = 1.302 explained 6.51% of the variance. Another EFA, using varimax rotation, a factor loading > 0.70, and a cross-loading of < 0.40, led to the following solution: a general factor with items from all five subscales (items 3, 8, 20, 4, 1, and 15 in descending loading order, all items phrased positively) and a mental/motivational factor with items from the subscales mental fatigue and reduced motivation (items 13, 9, 18, and 19 in descending order, all items phrased negatively). Additionally, the goodness-of-fit test for a unifactorial solution, computed with Hayes' macro (25) with all items, was significant (p < 0.001, χ2 = 5457.688, df = 170).
Given the significant differences between the MFI-20 subscales across gender and age, we calculated percentile ranks based on the MFI-20 subscale raw scores for gender and age separately. The percent rank norms as a non-linear and distribution-free test value transformation were applied since the distributions displayed a profound right-tailed skewness. A percent rank PRn shows the percentage of the norm sample that scored lower and exactly as the subject n, i.e., . The MFI-20 norm tables are shown in the Supplementary material for the subscales and the total score, separately by gender and age group.
The MFI-20 shows satisfying psychometric properties, including reliability and convergent validity. The construct validity was examined using CFA, which could not replicate the five-factor structure. An EFA suggests a strong general fatigue factor and a smaller mental/motivational factor. Kieffer et al. found a four-factor structure but allowed significant cross-loadings, “indicating that these items are unstable” (11). It has been shown that shorter versions, e.g., an MFI-10, resulted in comparable or better fit indices (29, 30). Regarding the reported factor structure, the above-mentioned clustering of positive and negative items was also noted by others (11, 30). The MFI-20 scores are overall comparable with other studies, showing higher fatigue scores for females and increasing scores with age. In comparison with a recent Dutch norm sample (11), the German scores are lower in all five subscales; most pronounced differences, mean (SD), were found for reduced activity, 9.3 (3.9) vs. 8.36 (4.00), and the total score 44.9 (16.7) vs. 41.69 (17.54). These differences may show overall lower fatigue values in the German population, but effects of language (item formulation), differences in introspection, acceptance of one's own experiences, social desirability, and/or the date of data acquisition (refer to limitations) may have contributed to these differences.
Reduced motivation showed the lowest internal consistency, which is in line with many studies (16). Mental fatigue showed the lowest interscale correlations with the other subscales. The highest interscale correlations were found between physical fatigue and general fatigue, and physical fatigue and reduced activity, exactly as in other studies (1, 31). This is in line with reported difficulties to distinguish between general and physical fatigue (16) and argues for combining these two subscales or even for using the entire unidimensional structure of the MFI-20. Since even the lowest subscale × total score correlation is higher than any inter-subscale correlation in our data, it could be that “the MFI-total is a more valid score for fatigue than the single MFI-subscale scores” (31).
The right-tailed skewness of the distribution in the norm sample may be less pronounced if more items with varying difficulties per subscale existed. The observed floor effect of 15–20% is similar to a comparable general sample: Lin et al. (17) compared subsamples with chronic fatigue symptoms, who had moderate ceiling percentages (1.4–13.0%) and negligible floor percentages (0–3.8%) in the subscales, with a (not further defined) “well” subsample from the general population. They in turn showed negligible ceiling percentages (0–0.5%) but substantial floor percentages (10.3–26.5%), lying in a slightly broader but comparable range with our data. Varying floor/ceiling effects in fatigue norm samples have been observed (32), which could be of diagnostic interest in different pathological subgroups. In a sample of cancer patients receiving radiotherapy, 28.6% showed a ceiling effect in general fatigue and 17.3% showed a floor effect in mental fatigue (33). In a sample of patients with spinal muscular atrophy, 19.3% exhibited a floor effect in mental fatigue (16). These strong differences in percentages of floor and ceiling effects between the subscales, even within samples, question the homogeneity of the latent construct underlying the MFI-20. The lowest floor effect in the cited healthy US-American sample (17) was in the subscale of general fatigue, indicating the best diagnostically conclusive discrimination of this subscale. This supports arguments stating that the subscale general fatigue measures fatigue sufficiently valid and that the other subscales might measure neighboring constructs, such as physical or cognitive functioning (11). In comparison to other fatigue inventories, the items of the MFI-20 focus on cognitive appraisal rather than objectifiable fatigue impacts, and they do not express an explicit attribution of the stated experiences of fatigue. This is supported by the Mental Composite Score (MCS) of the SF-36, which correlates most strongly with the MFI-20 total score, followed by the general fatigue and mental fatigue subscales. Furthermore, general fatigue correlated higher with the MCS than with the PCS.
The strength of our study is the use of a large German population-based sample, which was representative regarding gender, age, and regional distribution. Representativeness regarding education level, employment, and marital status was not considered. Further study limitations include the lack of a systematic comparison between chronically ill people and the general population. For example, the age effect of fatigue in the general population could not be found in cancer patient samples (34), for whom the MFI-20 was originally developed. Data on somatic symptoms or psychopathology would have been interesting but are limited in validity as part of a self-report battery. A simultaneous standardization process for the general population and selected patient groups would provide suggestions as to whether different populations could profit from adapted versions of the MFI-20. Furthermore, more indices for determining convergent validity would have been preferable. Another limitation might be the acquisition of subjects during the COVID-19 pandemic. There could be a selection bias, as more vulnerable, more cautious, and potentially more fatigued subjects might have refused to participate in the study. This potential exclusion may partially explain the observed overall lower MFI-20 scores in comparison with other studies (11), where data acquisition occurred before the pandemic. One potential explanation is that the pandemic did not increase fatigue in the general population; another explanation is that the pandemic did indeed increase fatigue, but the aforementioned exclusion bias overshadowed this effect. Since the data are cross-sectional, no statements on sensitivity to (clinical) changes in fatigue can be made. The developers of the MFI-20, however, state that for healthy subjects, the subscale general fatigue is the most sensitive to change and, thus, state that “it could be argued that when a short instrument is required only this scale should be used” (15). Once again, this supports the assumption that the subscale general fatigue might measure fatigue sufficiently enough.
The kish selection grid was used to identify randomly assigned target persons. A total of 57.2% of the target population did not participate due to the following reasons: household did not respond within four attempts (n = 791, 13.4%); household refused participation (n = 1374, 23.3%); target person did not respond within four attempts (n = 288, 4.9%); target person was on a business or vacation trip (n = 61, 1.0%); target person was ill (n = 75, 1.3%); and target person refused participation (n = 786, 13.3%).
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.26068/mhhrpm/20221017-000.
The studies involving human participants were reviewed and approved by Ethical Review Committee of the University of Leipzig (298/21-ek). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conceived and designed the data acquisition: EB. Analyzed the data: AW and MZ. Contributed reagents, materials, and analysis tools: EB, MM, and MZ. Wrote the manuscript: AW. Interpretation of the data: AW, MN, MM, EB, and MZ. All authors contributed to the manuscript revision, read, and approved the submitted version.
Data acquisition for the SF-36 has been financially supported by the publisher Hogrefe.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1062426/full#supplementary-material
1. Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie. (2003) 26:140–4. doi: 10.1159/000069834
2. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. (2006) 10:63–76. doi: 10.1016/j.smrv.2005.05.004
3. Matura LA, Malone S, Jaime-Lara R, Riegel B. A systematic review of biological mechanisms of fatigue in chronic illness. Biol Res Nurs. (2018) 20:410–21. doi: 10.1177/1099800418764326
4. Menting J, Tack CJ, Bleijenberg G, Donders R, Droogleever Fortuyn HA, et al. Is fatigue a disease-specific or generic symptom in chronic medical conditions? Health Psychol. (2018) 37:530–43. doi: 10.1037/hea0000598
5. Ferentinos P, Kontaxakis V, Havaki-Kontaxaki B, Dikeos D, Papadimitriou G. The fatigue questionnaire: standardization in patients with major depression. Psychiatry Res. (2010) 177:114–9. doi: 10.1016/j.psychres.2009.01.029
6. Ferentinos PP, Kontaxakis VP, Havaki-Kontaxaki BJ, Paplos KG, Soldatos CR. The measurement of fatigue in depression. Psychopathology. (2007) 40:133–4. doi: 10.1159/000098494
7. Joli J, Buck P, Zipfel S, Stengel A. Post-COVID-19 fatigue: a systematic review. Front Psychiatry. (2022) 13:947973. doi: 10.3389/fpsyt.2022.947973
8. Aoun Sebaiti M, Hainselin M, Gounden Y, Sirbu CA, Sekulic S, Lorusso L, et al. Systematic review and meta-analysis of cognitive impairment in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Sci Rep. (2022) 12:2157. doi: 10.1038/s41598-021-04764-w
9. Maksoud R, Du Preez S, Eaton-Fitch N, Thapaliya K, Barnden L, Cabanas H, et al. A systematic review of neurological impairments in myalgic encephalomyelitis/ chronic fatigue syndrome using neuroimaging techniques. PLoS ONE. (2020) 15:e0232475. doi: 10.1371/journal.pone.0232475
10. Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. (1993) 37:147–53. doi: 10.1016/0022-3999(93)90081-P
11. Kieffer JM, Starreveld DE, Boekhout A, Bleiker EM. A questionable factor structure of the multidimensional fatigue inventory in the general Dutch population. J Clin Epidemiol. (2021) 137:266–76. doi: 10.1016/j.jclinepi.2021.05.005
12. Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. (2009) 37:107–28. doi: 10.1016/j.jpainsymman.2007.08.019
13. Friedrich M, Hinz A, Kuhnt S, Schulte T, Rose M, Fischer F. Measuring fatigue in cancer patients: a common metric for six fatigue instruments. Qual Life Res. (2019) 28:1615–26. doi: 10.1007/s11136-019-02147-3
14. Hjollund NH, Andersen JH, Bech P. Assessment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health Qual Life Outcomes. (2007) 5:12. doi: 10.1186/1477-7525-5-12
15. Smets EMA, Garssen B, Bonke B, de Haes JCJM. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. (1995) 39:315–25. doi: 10.1016/0022-3999(94)00125-O
16. Binz C, Osmanovic A, Thomas NH, Stolte B, Freigang M, Cordts I, et al. Validity and reliability of the German multidimensional fatigue inventory in spinal muscular atrophy. Ann Clin Transl Neurol. (2022) 9:351–62. doi: 10.1002/acn3.51520
17. Lin J-MS, Brimmer DJ, Maloney EM, Nyarko E, BeLue R, Reeves WC. Further validation of the multidimensional fatigue inventory in a US adult population sample. Popul Health Metr. (2009) 7:18. doi: 10.1186/1478-7954-7-18
18. Paslakis G, Fischer-Jacobs J, Pape L, Schiffer M, Gertges R, Tegtbur U, et al. Assessment of use and preferences regarding internet-based health care delivery: cross-sectional questionnaire study. J Med Internet Res. (2019) 21:e12416. doi: 10.2196/12416
19. Song S-W, Kang S-G, Kim K-S, Kim M-J, Kim K-M, Cho D-Y, et al. Reliability and validity of the Korean version of the multidimensional fatigue inventory (MFI-20): a multicenter, cross-sectional study. Pain Res Manag. (2018) 2018:3152142. doi: 10.1155/2018/3152142
20. Morfeld M, Kirchberger I, Bullinger M. SF-36 - Fragebogen zum Gesundheitszustand [2., ergänzte und überarbeitete Auflage [German version of the short form 36 health survey (SF-36)]]. Göttingen: Hogrefe (2011).
21. Rodrigue JR, Fleishman A, Schold JD, Morrissey P, Whiting J, Vella J, et al. Patterns and predictors of fatigue following living donor nephrectomy: findings from the KDOC study. Am J Transplant. (2020) 20:181–9. doi: 10.1111/ajt.15519
22. Gecaite-Stonciene J, Bunevicius A, Burkauskas J, Brozaitiene J, Neverauskas J, Mickuviene N, et al. Validation of the multidimensional fatigue inventory with coronary artery disease patients. Int J Environ Res Public Health. (2020) 17:8003. doi: 10.3390/ijerph17218003
24. Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. (2012) 48:1–36. doi: 10.18637/jss.v048.i02
25. Hayes AF, Coutts JJ. Use omega rather than cronbach's alpha for estimating reliability. But. Comm Methods Meas. (2020) 14:1–24. doi: 10.1080/19312458.2020.1718629
26. Nájera Catalán HE. Reliability, population classification and weighting in multidimensional poverty measurement: a monte carlo study. Soc Indic Res. (2019) 142:887–910. doi: 10.1007/s11205-018-1950-z
27. Revelle W, Zinbarg RE. Coefficients alpha, beta, omega, and the glb: comments on sijtsma. Psychometrika. (2009) 74:145–54. doi: 10.1007/s11336-008-9102-z
28. Hemmerich WA. StatistikGuru: Multivariate Normalverteilung online prüfen. (2018). Available online at: https://statistikguru.de/rechner/multivariate-normalverteilung.html (accessed June 8, 2022).
29. Baussard L, Carayol M, Porro B, Baguet F, Cousson-Gelie F. Fatigue in cancer patients: development and validation of a short form of the multidimensional fatigue inventory (MFI-10). Eur J Oncol Nurs. (2018) 36:62–7. doi: 10.1016/j.ejon.2018.07.005
30. Hinz A, Benzing C, Brähler E, Zenger M, Herzberg PY, Finck C, et al. Psychometric properties of the multidimensional fatigue inventory MFI-20, derived from seven samples. J Pain Symptom Manage. (2020) 59:717–23. doi: 10.1016/j.jpainsymman.2019.12.005
31. Wintermann G-B, Rosendahl J, Weidner K, Strauß B, Hinz A, Petrowski K. Fatigue in chronically critically ill patients following intensive care - reliability and validity of the multidimensional fatigue inventory (MFI-20). Health Qual Life Outcomes. (2018) 16:1–10. doi: 10.1186/s12955-018-0862-6
32. Hewlett S, Dures E, Almeida C. Measures of fatigue: bristol rheumatoid arthritis fatigue multi-dimensional questionnaire (BRAF MDQ), bristol rheumatoid arthritis fatigue numerical rating scales (BRAF NRS) for severity, effect, and coping, chalder fatigue questionnaire (CFQ), checklist individual strength (CIS20R and CIS8R), fatigue severity scale (FSS), functional assessment chronic illness therapy (fatigue) (FACIT-F), multi-dimensional assessment of fatigue (MAF), multi-dimensional fatigue inventory (MFI), pediatric quality of life (PedsQL) multi-dimensional fatigue scale, profile of fatigue (ProF), short form 36 vitality subscale (SF-36 VT), and visual analog scales (VAS). Arthritis Care Res. (2011) 63:S263–86. doi: 10.1002/acr.20579
33. Smets EMA, Garssen B, Cull A, de Haes JCJM. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br J Cancer. (1996) 73:241–5. doi: 10.1038/bjc.1996.42
Keywords: fatigue, multidimensional fatigue inventory, MFI-20, validation, German norm values
Citation: Westenberger A, Nöhre M, Brähler E, Morfeld M and de Zwaan M (2022) Psychometric properties, factor structure, and German population norms of the multidimensional fatigue inventory (MFI-20). Front. Psychiatry 13:1062426. doi: 10.3389/fpsyt.2022.1062426
Received: 05 October 2022; Accepted: 22 November 2022;
Published: 20 December 2022.
Edited by:
Katrin Giel, Tübingen University Hospital, GermanyReviewed by:
Panagiotis Ferentinos, National and Kapodistrian University of Athens, GreeceCopyright © 2022 Westenberger, Nöhre, Brähler, Morfeld and de Zwaan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adrian Westenberger, d2VzdGVuYmVyZ2VyLmFkcmlhbkBtaC1oYW5ub3Zlci5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.