- 1Department of Neuropsychiatry, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama, Japan
- 2Research Center for Idling Brain Science, University of Toyama, Toyama, Japan
- 3Arisawabashi Hospital, Toyama, Japan
- 4Department of Radiology, University of Toyama Graduate School of Medicine and Pharmaceutical Sciences, Toyama, Japan
Introduction: Patients with schizophrenia have a higher number of insular gyri; however, it currently remains unclear whether the brain characteristics of patients with schizotypal personality disorder (SPD), a mild form of schizophrenia, are similar. It is also unknown whether insular gross anatomical features are associated with the illness stages and clinical subtypes of schizophrenia.
Materials and methods: This magnetic resonance imaging study examined gross anatomical variations in the insular cortex of 133 patients with schizophrenia, 47 with SPD, and 88 healthy controls. The relationships between the insular gross anatomy and schizophrenia subgroups (71 first-episode and 58 chronic groups, 38 deficit and 37 non-deficit subtype groups) were also investigated.
Results: The number of insular gyri was higher in the schizophrenia and SPD patients than in the controls, where the patients were characterized by well-developed accessory, middle short, and posterior long insular gyri. The insular gross anatomy did not significantly differ between the first-episode and chronic schizophrenia subgroups; however, the relationship between the developed accessory gyrus and more severe positive symptoms was specific to the first-episode group. The prevalence of a right middle short gyrus was higher in the deficit schizophrenia group than in the non-deficit group.
Discussion: These findings suggest that schizophrenia and SPD patients may share an altered insular gross morphology as a vulnerability factor associated with early neurodevelopmental anomalies, which may also contribute to positive symptomatology in the early illness stages and clinical subtypes of schizophrenia.
Introduction
The insular cortex is involved in a range of cognitive functions as a “limbic integration cortex” (1) and is characterized by large inter-individual variations in the gross gyral organization (2, 3). The anterior subdivision (short insular cortex) is typically composed of an accessory and three principal short gyri (anterior, middle, and posterior), while the accessory gyrus (AG) and middle short gyrus (MSG) are frequently underdeveloped or absent (up to 50–70%) in general population (4–6). The posterior subdivision (long insular cortex) consists of the anterior and posterior long insular gyri, where the posterior long gyrus (PLG) is missing in between 10 and 20% of human brains (4–6). The significance of the effects of these anatomical variations on the function of the insular cortex has not yet been established; however, we recently reported a higher number of insular gyri with a well-developed AG, MSG, and PLG in patients with first-episode schizophrenia than in controls (7). Since gross brain folding patterns do not markedly change after birth (8), this finding in schizophrenia potentially reflects anomalous neurodevelopment during the mid to late fetal period, during which insular cortical folds are formed (9, 10). Nevertheless, there is currently no evidence to show similar features of the insular gross anatomy in patients with schizophrenia spectrum disorders, who may share early neurodevelopmental pathologies associated with vulnerability to psychosis (11).
Schizotypal personality disorder (SPD) (12), or schizotypal disorder (13), is a milder form within the schizophrenia spectrum and is characterized by attenuated forms of schizophrenic features without overt psychosis. SPD patients are considered to share biological similarities with patients with full-blown schizophrenia, potentially reflecting a common vulnerability (11, 14). Schizophrenia spectrum disorders partly share brain abnormalities, such as diverse cortical hyper-gyrification (15, 16), which may reflect deviations in early neurodevelopment (17, 18). On the other hand, gray matter reductions in the insular cortex appear to be specific to schizophrenia among schizophrenia spectrum disorders (19–23). To the best of our knowledge, magnetic resonance imaging (MRI) studies have not yet specifically examined variations in the insular gross anatomy in SPD patients.
We previously demonstrated that the gross anatomical features of the insular cortex correlated with positive symptomatology in first-episode schizophrenia (7); however, their potential contribution to clinical characteristics at later illness stages and clinical subtypes was not examined. Patients with the deficit subtype of schizophrenia, who are found in approximately 15% of first-episode and 25–30% of more chronic patients, have a trait-like feature of primary and persistent negative symptoms even during remission periods (24, 25). Unlike the DSM/ICD subtypes of schizophrenia (12, 13) based on symptom profiles (e.g., paranoid, disorganized, and undifferentiated), the deficit/non-deficit categorization is highly stable over time and the patients with deficit subtype have rather homogeneous poor outcome as demonstrated in longitudinal clinical follow-up (25). Previous MRI studies on deficit schizophrenia revealed that the patients with this specific subtype may exhibit prominent abnormalities in neurodevelopment (26, 27), as suggested by gross brain changes, including an altered surface morphology in the fronto-temporal regions (28, 29). To obtain a more detailed understanding of the role of insular gross anatomical features in the pathophysiology of schizophrenia, further studies in different illness stages and in specific clinical subtypes, particularly those with a prominent neurodevelopmental pathology (i.e., deficit schizophrenia), are warranted.
We herein used MRI to examine the insular gross anatomy of SPD patients and patients with schizophrenia of different illness stages (first-episode and chronic) and subtypes (deficit and non-deficit). Due to shared neurodevelopmental pathologies in the schizophrenia spectrum and prominent neurodevelopmental abnormalities in deficit schizophrenia as described above, we expected SPD patients to have an elevated number of insular gyri, similar to schizophrenia, and this change to be prominent in the deficit schizophrenia subgroup. We also investigated whether the insular gross anatomy affects clinical characteristics even in the chronic stages of schizophrenia.
Materials and methods
Participants
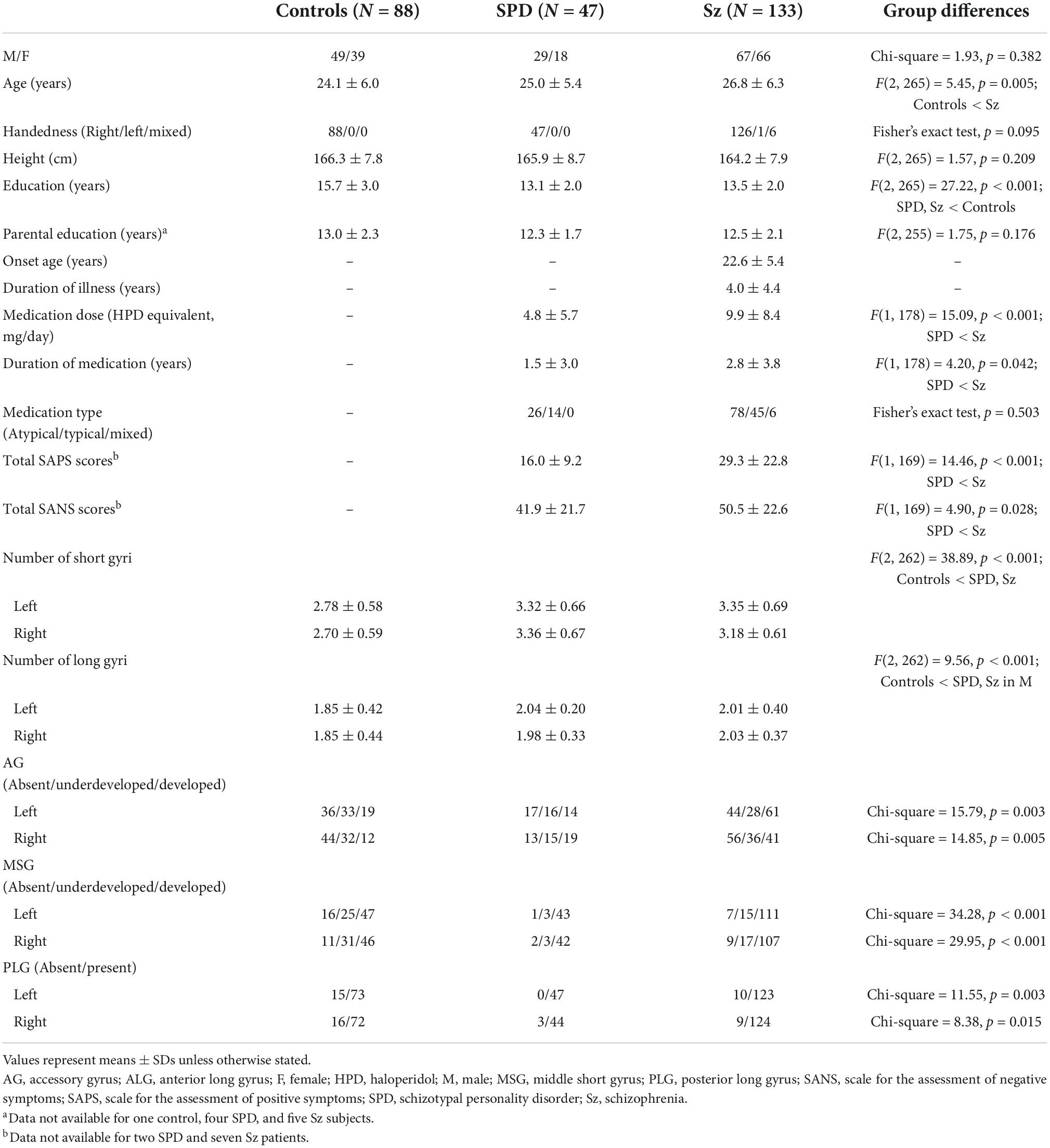
Participants in the present study comprised 133 patients with schizophrenia, 47 with SPD, and 88 healthy controls (Table 1). Their physical condition was good at the time of MRI and they had no previous history of serious illnesses requiring medical treatment (e.g., hypertension, seizure, head injury, diabetes, and thyroid diseases), oral steroid use, or substance use disorders. Among 268 participants, the insular gross anatomy of 66 first-episode schizophrenia patients and 66 healthy controls was reported elsewhere (7). This study aimed to examine the insular anatomy in our SPD sample as well as in an expanded schizophrenia sample with different illness duration (i.e., first-episode vs. chronic patients) and clinical characteristics (i.e., deficit vs. non-deficit subtypes) to explore the role of vulnerability to psychosis, illness stages, and subtypes. The recruitment strategies and inclusion criteria of participants were fully described in previous studies (16, 23, 30, 31).
Briefly, we enrolled schizophrenia and SPD patients at the Department of Neuropsychiatry, Toyama University Hospital and they were diagnosed by experienced psychiatrists based on a structured interview using the Comprehensive Assessment of Symptoms and History (32) and the Scale for the Assessment of Negative Symptoms and the Scale for the Assessment of Positive Symptoms (SANS/SAPS) (33). The schizophrenia group was also assessed using the Brief Psychiatric Rating Scale (BPRS) (34) for the purpose of clinical subgrouping.
Schizophrenia patients fulfilling the ICD-10 research criteria (13) were operationally categorized into first-episode [illness duration ≤ 1 year (N = 54) or under psychiatric hospitalization for the first time (N = 17)] and chronic [illness duration ≥ 3 years (N = 58)] subgroups (35, 36). As previously described in detail (28, 29, 37), we divided the patients into deficit and non-deficit schizophrenia subgroups based on scores for Proxy for the Deficit Syndrome (PDS) (38), which were obtained as follows using BPRS scores: blunted affect – (anxiety + guilt feelings + depressive mood + hostility items). To reduce false classification, patients with the top and bottom 25% of PDS scores among the whole schizophrenia sample were categorized into the deficit and non-deficit subgroups, respectively (39).
All schizotypal patients met the DSM Axis II diagnosis of SPD, with 13 having a history of transient quasi-psychotic episodes fulfilling the DSM Axis I diagnosis of brief psychotic disorder (12). They also fulfilled the ICD-10 research criteria of schizotypal disorder (13). None of these patients had developed schizophrenia during clinical follow-ups for at least 2 years. Table 1 shows the status of medication and clinical data on schizophrenia and SPD patients.
Following screening by a questionnaire on personal and family medical histories (40), healthy controls were enrolled from the community, hospital staff, and university students. None had a personal or family history of psychiatric illness among first-degree relatives. The Committee of Medical Ethics of the University of Toyama approved this study (ID: I2013006). In accordance with the Declaration of Helsinki, written informed consent was obtained from all participants after a full description of the study protocol. If a participant was younger than 20 years old, written consent was also obtained from a parent/guardian.
MR image acquisition and processing
One-millimeter-thick T1-weighted images were obtained in the sagittal plane with the three-dimensional gradient-echo sequence FLASH (fast low-angle shots) using a 1.5T Magnetom Vision MR scanner (Siemens Medical System, Inc., Erlangen, Germany) under the following imaging conditions: TR = 24 ms, TE = 5 ms, flip angle = 40°, field of view = 256 mm, matrix = 256 × 256 pixels, and voxel size = 1 × 1 × 1 mm.
Using Dr. View software (Infocom, Tokyo, Japan), MR images were reconstructed into 1-mm-thick coronal images perpendicular to the inter-commissural line after three-dimensional tilt correction.
Assessment of anatomical variations in the insula
As described in detail elsewhere (7), one rater who was blinded to the identities of subjects assessed the insular gross anatomy primarily using the sagittal view (Figure 1). Briefly, the AG and MSG were classified as absent, underdeveloped (i.e., identifiable, but does not extend to the convex surface of the insula), or developed based on the criteria reported by Wysiadecki et al. (6). The PLG was classified as present or absent because it is developed in most hemispheres (> 85%) and hypoplasia is rarely observed (4, 6). The anterior short gyrus (ASG), posterior short gyrus (PSG), and anterior long gyrus (ALG) were well-developed in all participants in this study. Regarding the number of insular gyri, only well-developed gyri were counted.
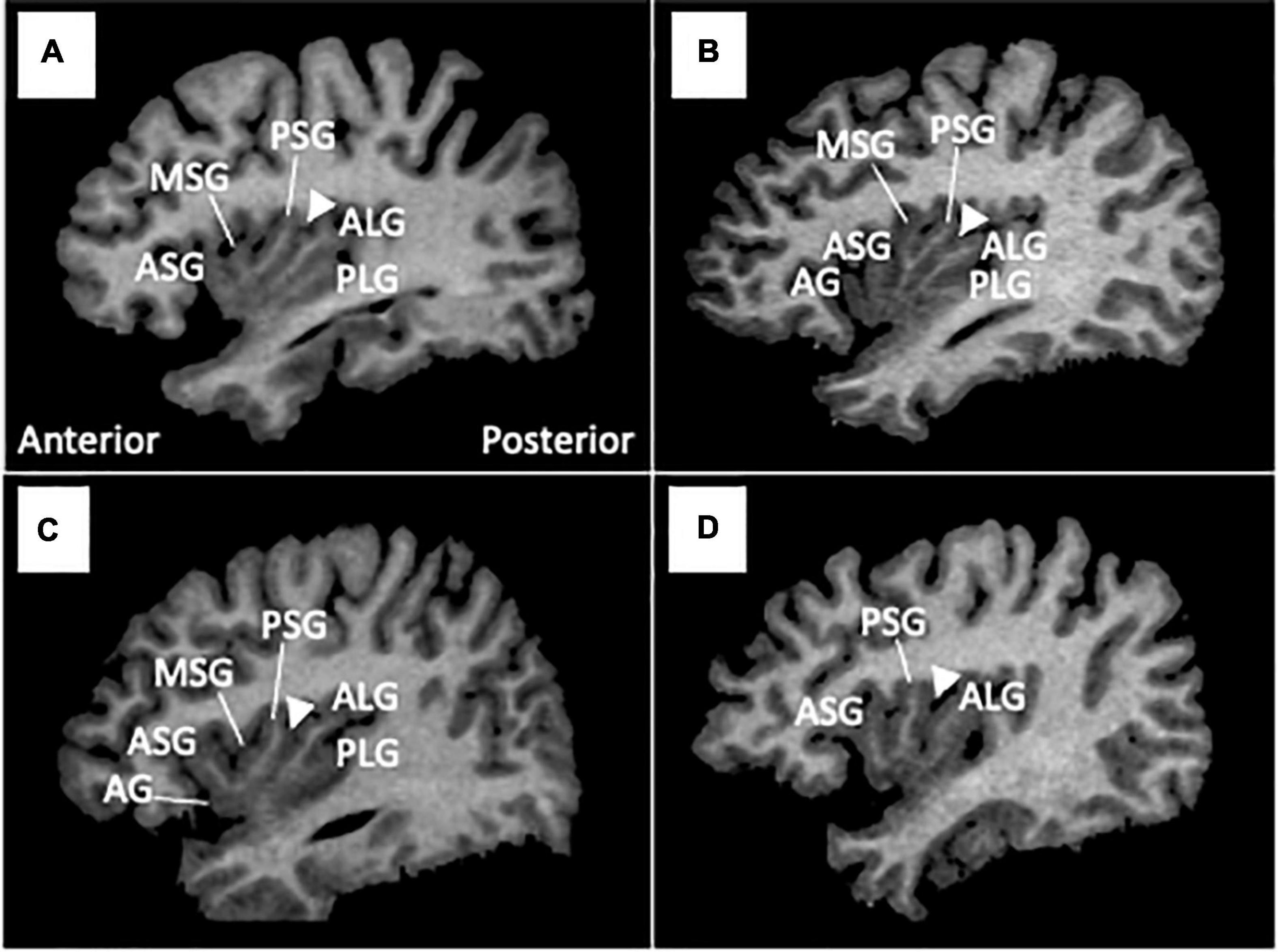
Figure 1. Insular gross anatomical variations in sample MR images in sagittal views. Coronal and axial views were simultaneously referred to in assessments of gyral development. Arrowheads indicate the location of the central insular sulcus, which subdivides the short (anterior) and long (posterior) insular cortices. The ASG, PSG, and ALG were well-developed in all participants in this study, while the AG and MSG were absent [subject (D)], underdeveloped [subject (C)], or well-developed [subject (B)]. The PLG was present in most subjects (A–C), but was not observed in subject (D). AG, accessory gyrus; ALG, anterior long gyrus; ASG, anterior short gyrus; MSG, middle short gyrus, PLS, posterior long gyrus; PSG, posterior short gyrus.
The classification reliabilities of insular gyri were examined in a subset of 10 brains that were randomly selected (20 hemispheres); intra- (TT) and inter-rater (TT and DS) reliabilities were > 0.89 for the number (intraclass correlation coefficients) and development classification (Cronbach’s α).
Statistical analysis
Group differences in demographic and clinical data were assessed by the χ2-test or an analysis of variance (ANOVA).
The developmental patterns of the AG, MSG, and PLG were exploratory compared between 3 groups (controls, SPD, and schizophrenia) using the χ2-test or Fisher’s exact test, where Benjamini-Hochberg procedure was used to decrease the false discovery rate. Lower-order comparisons (e.g., between two groups), which were not corrected for multiple comparisons due to prior hypothesis that both patient groups would similarly have well-developed insular gyri, were performed when significant group differences were found. The number of short (AG, ASG, MSG, and PSG) and long (ALG and PLG) gyri was log-transformed because of a skewed distribution (tested by Kolmogorov–Smirnov tests) and then compared between groups by ANOVA, with diagnosis and sex as between-subject factors and hemisphere as a within-subject variable.
Spearman’s correlation analysis with the Bonferroni correction was used to investigate relationships between the number of short insular gyri and clinical variables (age at disease onset in schizophrenia, the dose of medication and total SANS/SAPS scores in both patient groups). We did not use the durations of illness and medication here, because these variables were unlikely to be related to the insular gross anatomy, a stable brain characteristic. The first-episode and chronic schizophrenia groups were separately treated to explore the role of illness stages. Long gyri were not used in correlation analyses because there were two in most hemispheres (87.2%). The potential effects of insular gyral development on these clinical variables were examined by ANOVA, with the development pattern (developed vs. underdeveloped or absent) as a between-subject factor. Clinical variables were log-transformed, except for the total SAPS score in the schizotypal group and the SANS score, due to their non-normal distributions (Kolmogorov–Smirnov tests). Post hoc Scheffé’s tests were employed. The significance level was defined as p < 0.05.
Results
Demographic and clinical characteristics
No significant differences were observed in sex, handedness, or parental education between the groups; however, schizophrenia patients were older than healthy controls (Table 1). The education level was higher in healthy controls than in patient groups. The schizophrenia group was more symptomatic and received more medication than the SPD group (Table 1).
No significant differences were observed in age, handedness, personal or parental education, age at disease onset, illness duration, or medication (dose, type, and duration) between the deficit and non-deficit schizophrenia subgroups (27–29); however, a difference was noted in the sex ratio (Table 2). The deficit subgroup was characterized by a prominent blunted affect with less severe positive symptoms (Table 2).
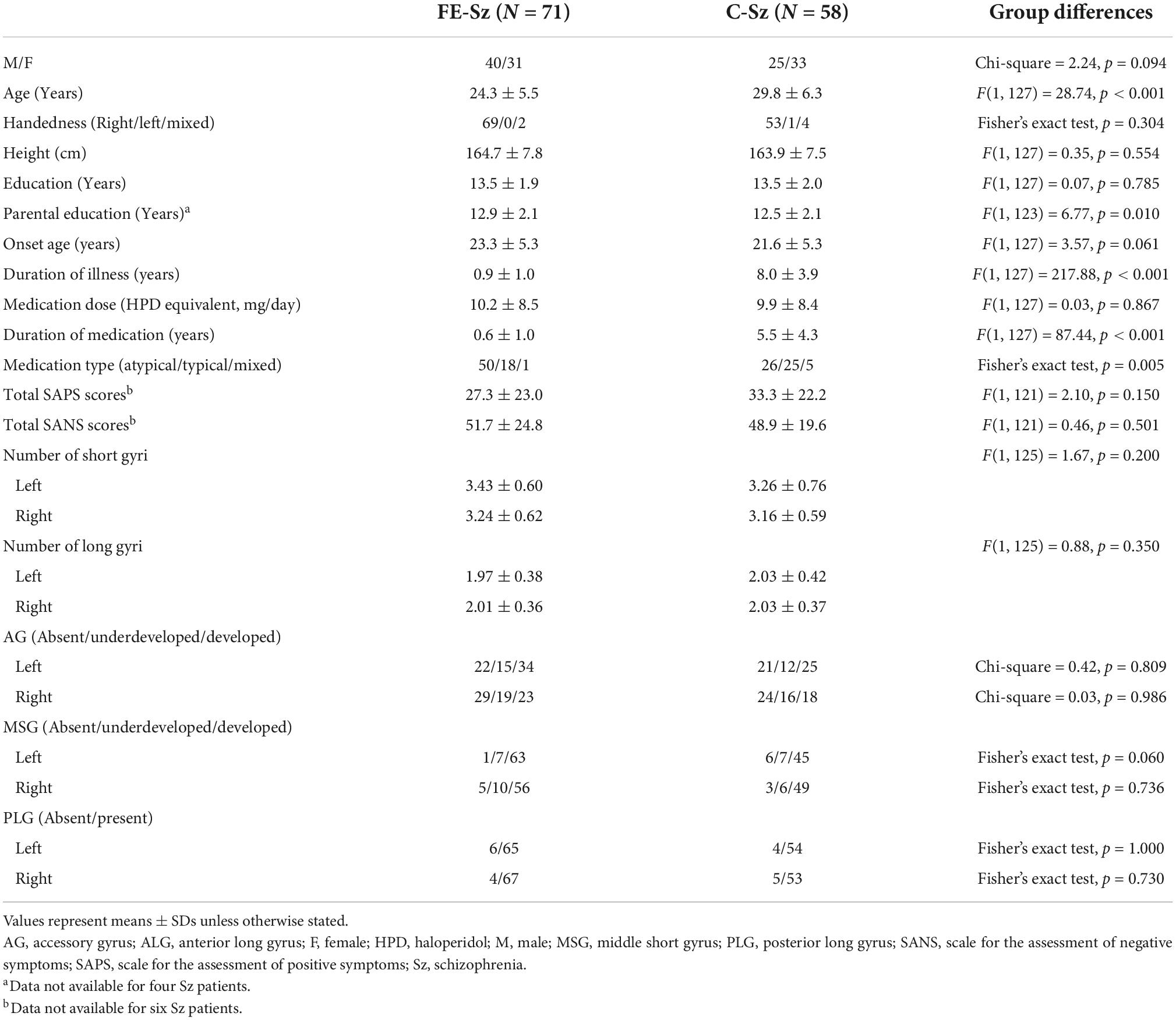
Table 2. Sample characteristics and gross insular morphology of first-episode (FE) and chronic (C) schizophrenia.
Gross variations in insular gyri
The degree of gyral development for the AG, MSG, and PLG significantly differed between healthy controls and patient groups (i.e., schizophrenia and SPD groups), but not between patient groups (Table 1 and Figure 2). The AG (left, χ2 = 13.51, p < 0.001; right, χ2 = 8.58, p = 0.003), MSG (left, χ2 = 19.99, p < 0.001; right, χ2 = 19.74, p < 0.001), and PLG (left, χ2 = 4.79, p = 0.029; right, χ2 = 6.88, p = 0.009) were significantly more well-developed bilaterally in schizophrenia patients than in healthy controls. The right AG (χ2 = 12.43, p < 0.001), bilateral MSG (left, Fisher’s exact test, p < 0.001; right, χ2 = 18.57, p < 0.001), and left PLG (Fisher’s exact test, p = 0.001) were significantly more well-developed in the SPD group than in healthy controls. Among schizophrenia patients, the right MSG was more developed in males than in females (χ2 = 4.97, p = 0.026), while no other significant effects were noted involving sex and hemisphere for the degree of insular gyral development.
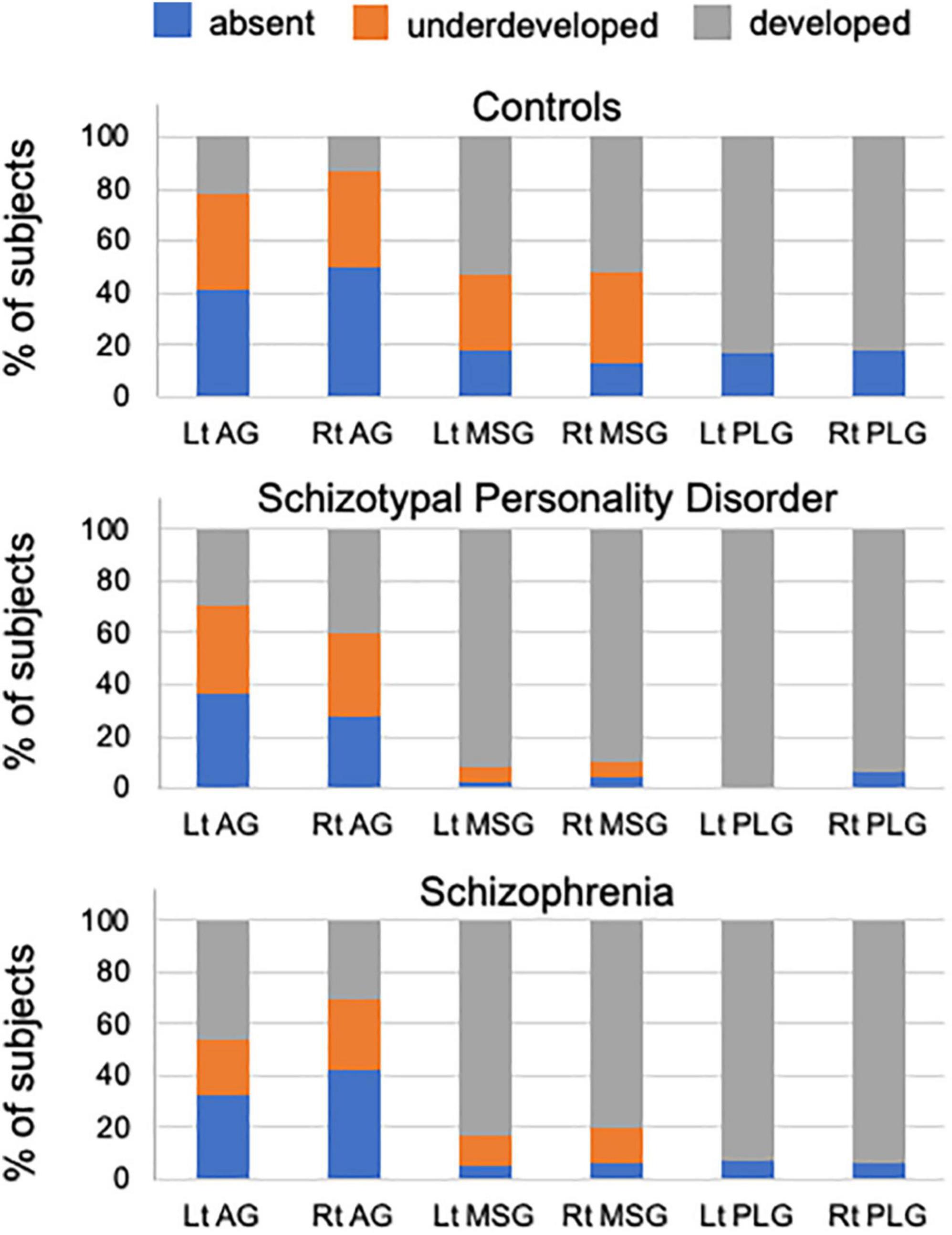
Figure 2. Percentage of insular gyral development in healthy controls, schizotypal patients, and schizophrenia patients. AG, accessory gyrus; MSG, middle short gyrus; PLG, posterior long gyrus.
The number of short gyri was higher in the schizophrenia and SPD groups (Scheffé’s test, p < 0.001) than in healthy controls (Table 1). A significant group-by-sex interaction [F(2, 262) = 6.36, p = 0.002] was observed for long gyri, with male schizophrenia (Scheffé’s test, p = 0.005) and SPD (Scheffé’s test, p = 0.001) patients having a higher number than healthy male controls. These results remained the same even when age and medication (dose/duration) were used as covariates.
No significant differences were noted in the number or development patterns of insular gyri between the first-episode and chronic subgroups (Table 2). Because the results of a largely overlapping (N = 66/71) first-episode schizophrenia cohort have been reported elsewhere (7), we also demonstrate the results excluding the first-episode schizophrenia patients as Supplementary Table 1; the results remained essentially the same as the original results using whole schizophrenia sample (N = 133).
No significant differences were observed in the number of insular gyri between the deficit and non-deficit schizophrenia subgroups, whereas the prevalence of a well-developed right MSG was higher in the deficit subgroup than in the non-deficit subgroup (χ2 = 4.79, p = 0.029) (Table 3).
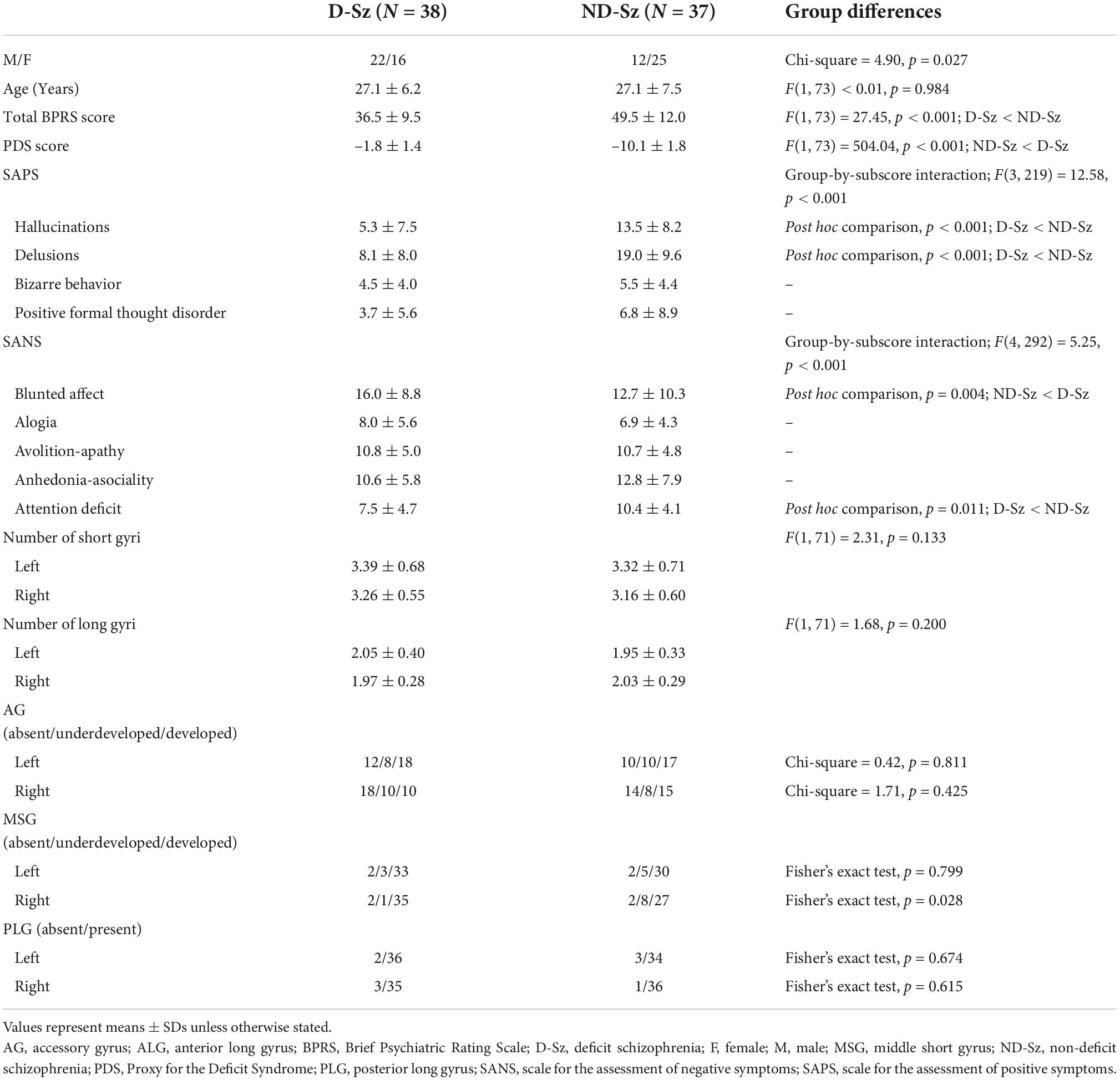
Table 3. Sample characteristics and gross insular morphology of deficit and non-deficit subtypes of schizophrenia.
Relationships between the insular anatomy and clinical variables
A higher number of left short gyri was associated with a younger onset age (rho = –0.366, p = 0.002) and higher medication dose (rho = 0.288, p = 0.015) in first-episode schizophrenia patients, but not in chronic schizophrenia patients. The number of right short gyri in SPD patients was also related to a higher SANS score (rho = 0.214, p = 0.013). Among these results, the relationship with onset age in first-episode schizophrenia patients remained after the Bonferroni correction for multiple comparisons [22 comparisons, p < 0.0023 (0.05/22)].
Schizophrenia patients with a left developed AG had a younger onset age than those without in the first-episode subgroup [F(1, 69) = 9.47, p = 0.003], but not in the chronic subgroup. Similarly, schizophrenia patients with a right developed AG had a higher SAPS score than those without only for the first-episode subgroup [F(1, 67) = 4.43, p = 0.039]. A developed right AG was also related to a higher SANS score in the SPD group [F(1, 43) = 4.11, p = 0.049].
Discussion
To the best of our knowledge, this is the first MRI study to demonstrate that patients with established schizophrenia (both first-episode and chronic stages) and SPD had a higher number of short and long insular gyri than healthy controls, potentially representing a common neurodevelopmental pathology within the schizophrenia spectrum. We also showed that a well-developed AG in schizophrenia was associated with an earlier onset and severe positive symptoms in first-episode, but not chronic patients. Furthermore, a well-developed MSG in schizophrenia was related to a subgroup with primary and persistent negative symptoms (i.e., deficit schizophrenia). Therefore, the gross anatomy of the insular cortex appears to contribute to vulnerability to psychosis, clinical features in early illness stages, and the clinical subtype of schizophrenia.
The present results showing an increased number of insular gyri in SPD, similar to schizophrenia, is considered to reflect common insults in the process of fetal insular gyration that predominantly occur between 17 and 35 weeks of gestation (9, 10). Previous MRI studies on shared abnormalities in early neurodevelopmental markers, such as the small adhesio interthalamica (41), an altered surface morphology in the orbitofrontal region (42, 43), and diverse cortical hyper-gyrification (16), support common neurodevelopmental pathologies among schizophrenia spectrum disorders (11, 14). Since aberrant neurodevelopmental processes associated with gyral formation in uteri may lead to neural dysconnectivity (17, 18), our results showing gross insular changes and their contribution to negative symptoms in SPD patients are partly consistent with the diffusion tensor imaging findings of schizotypal subjects with altered connectivity involving the insular cortex, which is associated with clinical symptoms and cognitive impairments (44, 45). The insular gray matter volume, which exhibits a progressive decline in the early stages of schizophrenia (20, 21), is preserved in SPD (19, 23); therefore, the results obtained in the present study appear to support the insular morphology in schizophrenia spectrum disorders having multiple pathological processes. Gross anatomical features may represent a vulnerability to psychosis that is attributable to prenatal neurodevelopment, while the gray matter volume more reflects dynamic brain pathologies related to the onset of overt psychosis. Interestingly, clinical high-risk individuals for psychosis (20, 46, 47), but not genetic high-risk subjects (48–50), likely exhibit gray matter reduction of the insular cortex especially for those who later develop psychosis. However, as far as we know, no MRI studies to date have specifically examined the insular gross anatomy in these high-risk groups. Thus, future studies will be warranted to examine whether the insular morphology is associated with vulnerability or genetic liability to psychosis and later psychosis onset.
The present study replicated our previous findings (7) in an expanded schizophrenia sample in which patients had an altered gyral organization with well-developed insular gyri (AG, MSG, and PLG), and also revealed no significant differences in the gross anatomical features of the insular cortex between the first-episode and chronically medicated subgroups. Previous gyrification studies in schizophrenia have demonstrated both hyper- and hypo-gyrification depending on the illness stages and brain regions (18); the patients likely have hyper-gyrification of diverse cortical regions in early stages (15, 51) but exhibit a progressive decline in brain gyrification predominantly in the fronto-temporal regions during the course of the illness (52). On the other hand, the present results appear to support insular gross anatomical features representing a stable neurodevelopmental marker regardless of illness stages. However, their contribution to clinical characteristics differed with the illness stage, with a developed AG being associated with an early illness onset, which implies prominent early developmental abnormalities (53), and severe positive symptoms specifically in the first-episode subgroup. We also demonstrated that a higher number of left short gyri was associated with a higher medication dose specifically in first-episode schizophrenia patients, supporting that gross anatomical features of the insular cortex may contribute to severe symptomatology that requires higher dose of medication at early illness stages. Interestingly, previous studies on first-episode schizophrenia also supported hyper-gyrification (15) and dysfunctional connectivity (54) in the anterior insular subdivision being associated with the severity of positive symptoms, implicating the contribution of early developmental processes associated with gyral formation in the anterior insula to the later production of psychotic symptoms. On the other hand, the relationships between the insular gross anatomy, a stable brain feature, and clinical features in chronic patients need to be interpreted with caution because the latter may be affected by a number of factors (e.g., medication and the chronicity of illness). However, it is possible that the insular gross morphology also contributes to the clinical course (e.g., treatment response) and stable clinical characteristics associated with specific subtypes.
Indeed, the present results suggest that the insular gross anatomy is associated with a persistent trait-like clinical feature of schizophrenia. No significant differences were observed in the number of insular gyri between the deficit and non-deficit subtypes of schizophrenia; however, the deficit subgroup was characterized by a more well-developed right MSG than the non-deficit subgroup. While etiological factors related to deficit schizophrenia have yet to be identified, the relationships between deficit schizophrenia and premorbid maladjustment (25, 55), general cognitive impairments (56), and neurological anomalies (27) appear to support pervasive abnormalities in neurodevelopment in this specific subtype (26, 27). The present results are consistent with previous MRI findings showing enhanced interregional cortical coupling, which may reflect reduced network differentiation during early neurodevelopment (39), and alterations in the gross brain morphology (e.g., gyrification patterns) (28, 30) specifically in deficit schizophrenia. Although the exact role of the MSG in the human brain remains unclear, the present result showing its relationship with persistent negative symptoms in schizophrenia supports functional neuroimaging evidence showing the crucial involvement of the regional functional organization within the insular cortex of the short insula (incl. the MSG) in social-emotional networks, particularly on the right hemisphere (1, 3, 57).
There are several potential limitations in this study that need to be addressed. Although the insular cortex has a number of functions in a range of cognitive domains (57) that are impaired in schizophrenia (e.g., emotional, auditory processing, and language-related functions) (58), the present study did not systematically assess cognition in participants. Therefore, it remains unclear whether the insular gross anatomy in schizophrenia spectrum disorders is associated with cognitive impairments. Furthermore, since insular gross anatomical diversity itself is widely observed in healthy subjects, its relationship with brain function warrants further study, for example, using functional/connectivity neuroimaging. Another limitation in the present study is that the deficit and non-deficit schizophrenia subgroups were not matched for sex, potentially reflecting the general tendency that male sex is associated with deficit schizophrenia (59). Since male schizophrenia patients had a higher prevalence of a developed right MSG than female patients, our results on the schizophrenia subtype need to be replicated in a larger and/or more sex-balanced cohort. Moreover, schizophrenia patients were older than healthy controls. However, the present results did not change even when we statistically controlled for the age difference. In addition, because altered brain gyrification is also observed in other neuropsychiatric disorders, such as bipolar disorder [reviewed by Sasabayashi et al. (18)], the disease specificity of our gross insular findings in schizophrenia spectrum disorders needs to be examined in further studies.
In summary, the present MRI study on gross anatomical features in the insular cortex support schizophrenia and SPD patients having similar brain characteristics possibly on the basis of common vulnerability associated with early neurodevelopmental anomalies. In schizophrenia, the insular gross anatomy appears to be associated with symptom severity, particularly in the early illness stages, as well as persistent traits associated with the deficit syndrome. However, the functional significance of this gross anatomical variation needs to be investigated in more detail in patients with neuropsychiatric disorders and healthy controls.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Committee of Medical Ethics of the University of Toyama. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
MS and TT conceived the idea and methodology of the study. TT conducted the statistical analyses and wrote the manuscript. DS, YT, and HK recruited subjects and were involved in clinical and diagnostic assessments. TT and DS analyzed the MRI data. KN provided technical support for MRI scanning and data processing. AF and YY managed the MRI and clinical data. MS and YT contributed to the writing and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by JSPS KAKENHI (Grant Nos. JP18K07550 to TT, JP18K15509 to DS, and JP20H03598 to MS) and Health and Labour Sciences Research Grants for Comprehensive Research on Persons with Disabilities from the Japan Agency for Medical Research and Development (AMED) (Grant Nos. JP19dk0307029 to MS and JP22dk0307103h0002 to TT).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1050712/full#supplementary-material
References
1. Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. (1996) 22:229–44. doi: 10.1016/S0165-0173(96)00011-2
2. Türe U, Yasargil DCH, Al-Mefty O, Yasargil MG. Topographic anatomy of the insular region. J Neurosurg. (1999) 90:720–33. doi: 10.3171/jns.1999.90.4.0720
3. Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. (2017) 34:300–6. doi: 10.1097/WNP.0000000000000377
4. Cunha-Cabral D, Silva SM, Alves H, Vaz RP, Pereira PA, Andrade JP. Neurosurgical anatomy of the insular cortex. Clin Neurol Neurosurg. (2019) 186:105530. doi: 10.1016/j.clineuro.2019.105530
5. Rosen A, Chen DQ, Hayes DJ, Davis KD, Hodaie M. Neuroimaging strategy for the three-dimensional in vivo anatomical visualization and characterization of insular gyri. Stereotact Funct Neurosurg. (2015) 93:255–64. doi: 10.1159/000380826
6. Wysiadecki G, Małkiewicz A, Rożniecki J, Polguj M, Haładaj R, żytkowski A, et al. Anatomical variations of the insular gyri: a morphological study and proposal of unified classification. Clin Anat. (2018) 31:347–56. doi: 10.1002/ca.23060
7. Takahashi T, Sasabayashi D, Takayanagi Y, Furuichi A, Kobayashi H, Yuasa Y, et al. Gross Anatomical Variations in the Insular Cortex in First-episode Schizophrenia (in submission).
8. Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. (1995) 5:56–63. doi: 10.1093/cercor/5.1.56
9. Afif A, Bouvier R, Buenerd A, Trouillas J, Mertens P. Development of the human fetal insular cortex: study of the gyration from 13 to 28 gestational weeks. Brain Struct Funct. (2007) 212:335–46. doi: 10.1007/s00429-007-0161-1
10. Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. (1977) 1:86–93. doi: 10.1002/ana.410010109
11. Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. (2004) 161:398–413. doi: 10.1176/appi.ajp.161.3.398
12. American Psychiatric Association [APA]. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Association Press (1994).
13. World Health Organization [WHO]. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization (1993).
14. Siever LJ, Kalus OF, Keefe RSE. The boundaries of schizophrenia. Psychiatr Clin N Am. (1993) 16:217–44.
15. Sasabayashi D, Takayanagi Y, Nishiyama S, Takahashi T, Furuichi A, Kido M, et al. Increased frontal gyrification negatively correlates with executive function in patients with first-episode schizophrenia. Cereb Cortex. (2017) 27:2686–94. doi: 10.1093/cercor/bhw101
16. Sasabayashi D, Takayanagi Y, Takahashi T, Nemoto K, Furuichi A, Kido M, et al. Increased brain gyrification in the schizophrenia spectrum. Psychiatry Clin Neurosci. (2020) 74:70–6.
17. Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. (2013) 36:275–84.
18. Sasabayashi D, Takahashi T, Takayanagi Y, Suzuki M. Anomalous brain gyrification patterns in major psychiatric disorders: a systematic review and transdiagnostic integration. Transl Psychiatry. (2021) 11:176. doi: 10.1038/s41398-021-01297-8
19. Takahashi T, Suzuki M, Zhou SY, Hagino H, Tanino R, Kawasaki Y, et al. Volumetric MRI study of the short and long insular cortices in schizophrenia spectrum disorders. Psychiatry Res. (2005) 138:209–20. doi: 10.1016/j.pscychresns.2005.02.004
20. Takahashi T, Wood SJ, Yung AR, Phillips LJ, Soulsby B, McGorry PD, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. (2009) 111:94–102. doi: 10.1016/j.schres.2009.03.024
21. Takahashi T, Wood SJ, Soulsby B, McGorry PD, Tanino R, Suzuki M, et al. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr Res. (2009) 108:49–56. doi: 10.1016/j.schres.2008.12.029
22. Takahashi T, Wood SJ, Soulsby B, Tanino R, Wong MT, McGorry PD. Diagnostic specificity of the insular cortex abnormalities in first-episode psychotic disorders. Prog Neuropsychopharmacol Biol Psychiatry. (2009) 33:651–7. doi: 10.1016/j.pnpbp.2009.03.005
23. Takahashi T, Kido M, Sasabayashi D, Nakamura M, Furuichi A, Takayanagi Y, et al. Gray matter changes in the insular cortex during the course of the schizophrenia spectrum. Front Psychiatry. (2020) 11:659. doi: 10.3389/fpsyt.2020.00659
24. Carpenter WT Jr., Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. (1988) 145:578–83. doi: 10.1176/ajp.145.5.578
25. Kirkpatrick B, Galderisi S. Deficit schizophrenia: an update. World Psychiatry. (2008) 7:143–7. doi: 10.1002/j.2051-5545.2008.tb00181.x
26. Galderisi S, Maj M, Mucci A, Cassano GB, Invernizzi G, Rossi A, et al. Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. Am J Psychiatry. (2002) 159:983–90. doi: 10.1176/appi.ajp.159.6.983
27. Peralta V, Moreno-Izco L, Sanchez-Torres A, García de Jalón E, Campos MS, Cuesta MJ. Characterization of the deficit syndrome in drug-naive schizophrenia patients: the role of spontaneous movement disorders and neurological soft signs. Schizophr Bull. (2014) 40:214–24. doi: 10.1093/schbul/sbs152
28. Takahashi T, Takayanagi Y, Nishikawa Y, Nakamura M, Komori Y, Furuichi A, et al. Brain neurodevelopmental markers related to the deficit subtype of schizophrenia. Psychiatry Res Neuroimaging. (2017) 266:10–8. doi: 10.1016/j.pscychresns.2017.05.007
29. Takahashi T, Sasabayashi D, Takayanagi Y, Furuichi A, Kobayashi H, Noguchi K, et al. Different Heschl’s gyrus Duplication patterns in deficit and non-deficit subtypes of schizophrenia. Front Psychiatry. (2022) 13:867461. doi: 10.3389/fpsyt.2022.867461
30. Suzuki M, Zhou SY, Takahashi T, Hagino H, Kawasaki Y, Niu L, et al. Differential contributions of prefrontal and temporolimbic pathology to mechanisms of psychosis. Brain. (2005) 128:2109–22. doi: 10.1093/brain/awh554
31. Takayanagi Y, Sasabayashi D, Takahashi T, Furuichi A, Kido M, Nishikawa Y, et al. Reduced cortical thickness in schizophrenia and schizotypal disorder. Schizophr Bull. (2020) 46:387–94. doi: 10.1093/schbul/sbz051
32. Andreasen NC, Flaum M, Arndt S. The comprehensive assessment of symptoms and history (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. (1992) 49:615–23. doi: 10.1001/archpsyc.1992.01820080023004
33. Andreasen NC. Scale for the Assessment of Negative Symptoms/Scale for the Assessment of Positive Symptoms. Iowa City: The University of Iowa (1984).
34. Rhoades HM, Overall JE. The semistructured BPRS interview and rating guide. Psychopharmacol Bull. (1988) 24:101–4.
35. Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. (2000) 57:692–9. doi: 10.1001/archpsyc.57.7.692
36. Schooler N, Rabinowitz J, Davidson M, Emsley R, Harvey PD, Kopala L, et al. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry. (2005) 162:947–53. doi: 10.1176/appi.ajp.162.5.947
37. Takayanagi Y, Sasabayashi D, Takahashi T, Komori Y, Furuichi A, Kido M, et al. Altered brain gyrification in deficit and non-deficit schizophrenia. Psychol Med. (2019) 49:573–80. doi: 10.1017/S0033291718001228
38. Kirkpatrick B, Buchanan RW, Breier A, Carpenter WT Jr. Case identification and stability of the deficit syndrome of schizophrenia. Psychiatry Res. (1993) 47:47–56. doi: 10.1016/0165-1781(93)90054-k
39. Wheeler AL, Wessa M, Szeszko PR, Foussias G, Chakravarty MM, Lerch JP, et al. Further neuroimaging evidence for the deficit subtype of schizophrenia: a cortical connectomics analysis. JAMA Psychiatry. (2015) 72:446–55. doi: 10.1001/jamapsychiatry.2014.3020
40. Takahashi T, Suzuki M, Tsunoda M, Kawamura Y, Takahashi N, Maeno N, et al. The association of genotypic combination of the DRD3 and BDNF polymorphisms on the adhesio interthalamica and medial temporal lobe structures. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:1236–42. doi: 10.1016/j.pnpbp.2008.03.014
41. Takahashi T, Suzuki M, Zhou SY, Nakamura K, Tanino R, Kawasaki Y, et al. Prevalence and length of the adhesio interthalamica in schizophrenia spectrum disorders. Psychiatry Res. (2008) 164:90–4. doi: 10.1016/j.pscychresns.2008.03.001
42. Nishikawa Y, Takahashi T, Takayanagi Y, Furuichi A, Kido M, Nakamura M. Orbitofrontal sulcogyral pattern and olfactory sulcus depth in the schizophrenia spectrum. Eur Arch Psychiatry Clin Neurosci. (2016) 266:15–23. doi: 10.1007/s00406-015-0587-z
43. Takahashi T, Nakamura M, Nishikawa Y, Takayanagi Y, Furuichi A, Kido M, et al. Decreased number of orbital sulci in schizophrenia spectrum disorders. Psychiatry Res Neuroimaging. (2016) 250:29–32. doi: 10.1016/j.pscychresns.2016.03.005
44. Gurrera RJ, Nakamura M, Kubicki M, Dickey CC, Niznikiewicz MA, Voglmaier MM, et al. The uncinate fasciculus and extraversion in schizotypal personality disorder: a diffusion tensor imaging study. Schizophr Res. (2007) 90:360–2. doi: 10.1016/j.schres.2006.10.003
45. Nakamura M, McCarley RW, Kubicki M, Dickey CC, Niznikiewicz MA, Voglmaier MM, et al. Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol Psychiatry. (2005) 58:468–78. doi: 10.1016/j.biopsych.2005.04.016
46. Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. (2012) 38:1297–307. doi: 10.1093/schbul/sbr134
47. Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, et al. Neuroimaging predictors of transition to psychosis–a systematic review and meta-analysis. Neurosci Biobehav Rev. (2010) 34:1207–22. doi: 10.1016/j.neubiorev.2010.01.016
48. Fusar-Poli P, Smieskova R, Serafini G, Politi P, Borgwardt S. Neuroanatomical markers of genetic liability to psychosis and first episode psychosis: a voxelwise meta-analytical comparison. World J Biol Psychiatry. (2014) 15:219–28. doi: 10.3109/15622975.2011.630408
49. Lin B, Li XB, Ruan S, Wu YX, Zhang CY, Wang CY, et al. Convergent and divergent gray matter volume abnormalities in unaffected first-degree relatives and ultra-high risk individuals of schizophrenia. Schizophrenia. (2022) 8:55. doi: 10.1038/s41537-022-00261-9
50. Smieskova R, Marmy J, Schmidt A, Bendfeldt K, Riecher-Rössler A, Walter M. Do subjects at clinical high risk for psychosis differ from those with a genetic high risk?–A systematic review of structural and functional brain abnormalities. Curr Med Chem. (2013) 20:467–81. doi: 10.2174/0929867311320030018
51. Sasabayashi D, Takayanagi Y, Takahashi T, Koike S, Yamasue H, Katagiri N, et al. Increased occipital gyrification and development of psychotic disorders in individuals with an at-risk mental state: a multicenter study. Biol Psychiatry. (2017) 82:737–45. doi: 10.1016/j.biopsych.2017.05.018
52. Pham TV, Sasabayashi D, Takahashi T, Takayanagi Y, Kubota M, Furuichi A, et al. Longitudinal changes in brain gyrification in schizophrenia spectrum disorders. Front Aging Neurosci. (2021) 13:752575. doi: 10.3389/fnagi.2021.752575
53. Chen BY, Tsai IN, Lin JJ, Lu MK, Tan HP, Jang FL, et al. Risk model assessment in early-onset and adult-onset schizophrenia using neurological soft signs. J Clin Med. (2019) 8:1443. doi: 10.3390/jcm8091443
54. Schmidt A, Palaniyappan L, Smieskova R, Simon A, Riecher-Rössler A, Lang UE, et al. Dysfunctional insular connectivity during reward prediction in patients with first-episode psychosis. J Psychiatry Neurosci. (2016) 41:367–76. doi: 10.1503/jpn.150234
55. Bucci P, Mucci A, Piegari G, Nobile M, Pini S, Rossi A, et al. Characterization of premorbid functioning during childhood in patients with deficit vs. non-deficit schizophrenia and in their healthy sibling. Schizophr Res. (2016) 174:172–6. doi: 10.1016/j.schres.2016.01.032
56. Mucci A, Merlotti E, Üçok A, Aleman A, Galderisi S. Primary and persistent negative symptoms: concepts, assessments and neurobiological bases. Schizophr Res. (2007) 186:19–28. doi: 10.1016/j.schres.2016.05.014
57. Nieuwenhuys R. The insular cortex: a review. Prog Brain Res. (2012) 195:123–63. doi: 10.1016/B978-0-444-53860-4.00007-6
58. Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. (2022) 399:473–86. doi: 10.1016/S0140-6736(21)01730-X
Keywords: magnetic resonance imaging, schizotypal, deficit schizophrenia, insula, gyrification, early neurodevelopment
Citation: Takahashi T, Sasabayashi D, Takayanagi Y, Furuichi A, Kobayashi H, Yuasa Y, Noguchi K and Suzuki M (2022) Gross anatomical features of the insular cortex in schizophrenia and schizotypal personality disorder: Potential relationships with vulnerability, illness stages, and clinical subtypes. Front. Psychiatry 13:1050712. doi: 10.3389/fpsyt.2022.1050712
Received: 22 September 2022; Accepted: 07 November 2022;
Published: 18 November 2022.
Edited by:
Yasuhiro Kawasaki, Kanazawa Medical University, JapanCopyright © 2022 Takahashi, Sasabayashi, Takayanagi, Furuichi, Kobayashi, Yuasa, Noguchi and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tsutomu Takahashi, dHN1dG9tdUBtZWQudS10b3lhbWEuYWMuanA=
 Tsutomu Takahashi
Tsutomu Takahashi Daiki Sasabayashi
Daiki Sasabayashi Yoichiro Takayanagi1,3
Yoichiro Takayanagi1,3 Michio Suzuki
Michio Suzuki