- 1Department of Rehabilitation Medicine, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China
- 2School of Psychology, Central China Normal University, Wuhan, Hubei, China
- 3The Seventh Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 4Brain Cognition and Brain Disease Institute (BCBDI), Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
- 5Department of Rehabilitation Medicine, Yue Bei People’s Hospital, Shaoguan, Guangdong, China
- 6Department of Rehabilitation Medicine, Tianyang District People’s Hospital, Baise, Guangxi, China
- 7School of Educational Science, Huazhong University of Science and Technology, Wuhan, Hubei, China
Background: Previous studies have shown that cognitive impairment is common after stroke. Transcranial direct current stimulation (tDCS) is a promising tool for rehabilitating cognitive impairment. This study aimed to investigate the effects of tDCS on the rehabilitation of cognitive impairment in patients with stroke.
Methods: Twenty-two mild–moderate post-stroke patients with cognitive impairments were treated with 14 tDCS sessions. A total of 14 healthy individuals were included in the control group. Cognitive function was assessed using the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). Cortical activation was assessed using functional near-infrared spectroscopy (fNIRS) during the verbal fluency task (VFT).
Results: The cognitive function of patients with stroke, as assessed by the MMSE and MoCA scores, was lower than that of healthy individuals but improved after tDCS. The cortical activation of patients with stroke was lower than that of healthy individuals in the left superior temporal cortex (lSTC), right superior temporal cortex (rSTC), right dorsolateral prefrontal cortex (rDLPFC), right ventrolateral prefrontal cortex (rVLPFC), and left ventrolateral prefrontal cortex (lVLPFC) cortical regions. Cortical activation increased in the lSTC cortex after tDCS. The functional connectivity (FC) between the cerebral hemispheres of patients with stroke was lower than that of healthy individuals but increased after tDCS.
Conclusion: The cognitive and brain functions of patients with mild-to-moderate stroke were damaged but recovered to a degree after tDCS. Increased cortical activation and increased FC between the bilateral cerebral hemispheres measured by fNIRS are promising biomarkers to assess the effectiveness of tDCS in stroke.
Introduction
Post-stroke cognitive impairment is a common complication after a stroke and an important risk predictor of declining quality of life (1, 2). For example, up to 55% of patients with stroke have episodic memory deficits, 40% have executive function deficits, and 23% have language deficits (3). The high prevalence of cognitive dysfunction after stroke makes cognitive rehabilitation interventions crucial.
After a stroke, people have been found to have abnormal cortical activation in the brain, which is associated with persistent functional impairment. Several studies have found that the extent of cortical activation in patients with stroke is related to the degree of functional damage (4, 5). In addition, the balance between the cerebral hemispheres of patients with stroke is destroyed; the decreased excitability of one hemisphere cortex may lead to the enhancement of the opposite hemisphere’s cortex (6). The competition model between cerebral hemispheres explains this phenomenon, showing that homologous regions in healthy brains inhibit each other. When a stroke occurs, this interhemispheric inhibition (IHI) is destroyed, leading to disinhibition of the contralateral cortex and excessive activation, further reducing brain function (7–9). Thus, according to the competition model between cerebral hemispheres, the functional connectivity (FC) between the bilateral cerebral hemispheres is weakened or increased when a stroke occurs. Caliandro analyzed the small-world properties of the resting-state FC of patients with acute stroke using electroencephalography (EEG) and found that the increase or decrease of the small-world properties in patients with stroke depends on the frequency band analyzed (10). Lu and Arun used task-state FC based on functional near-infrared spectroscopy (fNIRS). They found that compared with the resting state, the FC of the stroke group increased during the task state (11). Compared with the healthy group, the FC of the stroke group during the task state was increased (11, 12). Hence, there is no consensus regarding this issue. In addition, it is critical to regulating stroke based on the levels of cortical activation due to abnormal brain cortical activation. Abnormal FC is very common in stroke patients with cognitive impairment.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique (13) that has been widely used to improve cognitive function in patients with stroke (2, 14). Previous studies indicated three different electrode placement schemes for tDCS. First, the anode electrode was placed above the affected cerebral hemisphere to increase the excitability of neurons; the cathode was set as the reference electrode. Second, the cathode was placed above the healthy hemisphere to reduce the excitability of neurons; the anode was set as the reference electrode. Third, the anode was placed on the affected side and the cathode on the healthy side to balance the excitability of neurons in both hemispheres (15–17). In this study, we placed the anodes and cathodes on F3 and F4 electrodes (10–20 EEG electrodes) with a 2 mA intensity.
Some research has shown that using tDCS with anodes and cathodes on F3 and F4 electrodes can improve cognitive function in patients with stroke. Baker found that anodal tDCS with anodes and cathodes on F3 and F4 electrodes enhanced naming accuracy in patients with stroke (18). Park found that using anode tDCS and cognitive rehabilitation training simultaneously positively impacted mild-to-moderate cognitive dysfunction in patients with stroke (19). Shaker used tDCS/sham tDCS to investigate the effect of tDCS on cognitive functions in patients with stroke. They found that cognitive functions were higher after tDCS than sham tDCS in terms of attention and concentration, figural memory, logical reasoning, and reaction behavior (2).
Using tDCS with the anodes and cathodes on F3 and F4 electrodes can improve the cognitive ability of individuals with cognitive impairment after stroke (20) and change their cortical excitability (21). In animal studies (22, 23), direct currents have been shown to change brain excitability. Based on this discovery in animal models, Nitsche and Paulus conducted a study on tDCS in humans (23). They found that anodic stimulation increased cortical excitability, whereas cathodic stimulation decreased it. Due to IHI after stroke, tDCS can increase ipsilateral and reduce contralateral excitability (24). For example, Feltman and Sarkis found that placing anodes and cathodes on F3 and F4 electrodes with 2 mA intensity could activate the dorsolateral prefrontal cortex (DLPFC) and enhance cognitive function (25–27). Some studies have reported that tDCS positively affects the treatment of patients with stroke (24, 28). This study aimed to investigate whether the cortical activation of patients with stroke after anodic tDCS would change. We hypothesized that anodic tDCS would increase cortical excitability in the left cerebral hemisphere.
Using tDCS with anodes and cathodes on F3 and F4 electrodes also changed FC in patients with stroke. Some studies using fMRI technology have found that tDCS can widely regulate interhemispheric connectivity in patients with stroke (29), increasing connectivity between the hemispheres (30, 31). This study aimed to investigate whether the FC of patients with stroke after anodic tDCS would be changed. We hypothesized that anodic tDCS would increase the FC between the bilateral cerebral hemispheres.
Various techniques can be used to observe the cortical activation in the brain. Several characteristics of fNIRS, a non-invasive optical technique, including portability, non-invasive, cost-effectiveness, and tolerance, make it a favorable tool for clinical nursing and neuroscience research (32). fNIRS can indirectly detect the oxy-hemoglobin (oxy-Hb) and the deoxy-hemoglobin (deoxy-Hb) concentration change in particular brain regions of the participants while performing tasks. fNIRS observes the activation of the cerebral cortex by determining the intensity of the scattered light in the cerebral cortex (33). The cortical activity observed based on fNIRS can also be used to further study brain functional connectivity (34, 35). Regarding the identification of oxy-Hb vs. deoxy-Hb indicators, oxy-Hb has a stronger signal amplitude than deoxy-Hb, thus, the former is often used for fNIRS research. Several neuroimaging studies have consistently shown that mild cognitive impairment and dementia involve reduced cerebral blood flow compared to normal cognition controls. This is largely localized to the medial temporal lobe, posterior cingulate, prefrontal cortex (PFC), and inferior parietal cortex (36–38). It is generally believed that oxy-Hb changes are important indicators of activity intensity and cognitive function in the brain. fNIRS has also been used to study cortical activation in patients with stroke. For example, Kim used fNIRS to study the oxy-Hb changes in patients with stroke in robotic mirror therapy to observe the efficacy of the therapy (39). Mihara also used fNIRS to study cortical activation differences between patients with stroke and healthy peers during ataxia gait (40). Therefore, this study used fNIRS to study the oxy-Hb changes in cortical activation and FC after tDCS in patients with stroke.
The verbal fluency task (VFT) has been used to measure executive functions (41). There are indications that the sensitivity of fNIRS is sufficient to detect small metabolic changes during the execution of cognitive tasks, including the VFT of letters or categories (42). Moreover, the VFT is the most widely used task with impaired understanding activation (43). When participants performed the VFT, the prefrontal cortex was extensively activated, especially the DLPFC (2, 44). A series of studies have found that lesions after post-stroke may be widespread in the frontal and temporal regions (45, 46). Therefore, this study used the VFT to measure executive function in stroke patients with cognitive impairment.
Materials and methods
Participants
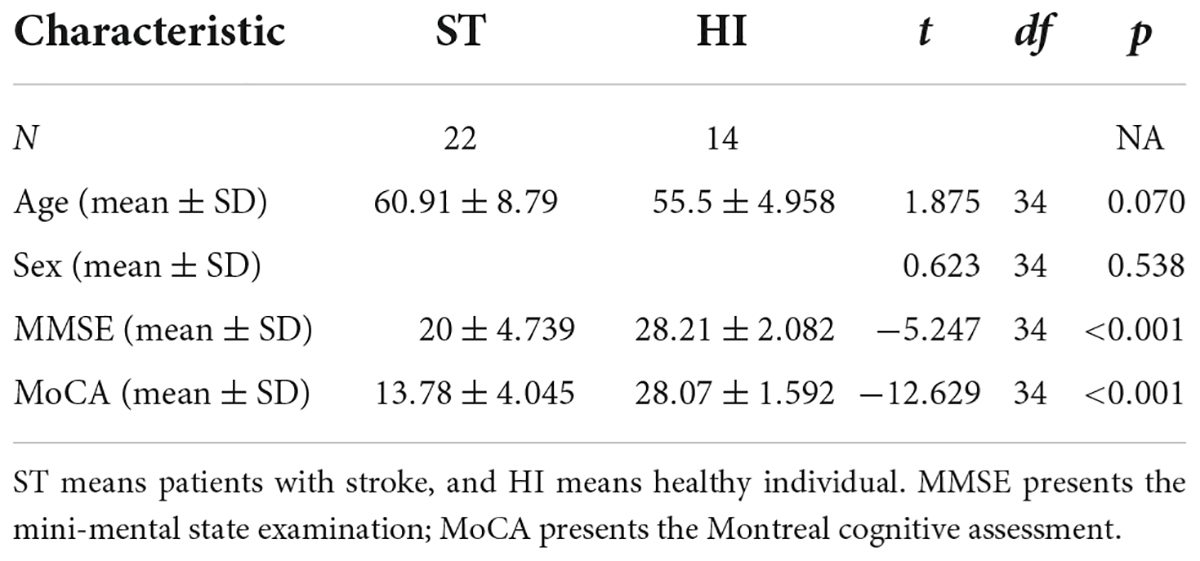
There were 22 patients with mild–moderate stroke (15 men and 7 women) and 14 healthy individuals (10 men and 4 women). Twenty-two mild–moderate stroke patients with post-stroke cognitive impairment met the criteria of stroke diagnosis and treatment guidelines. The onset time was within 2 weeks to 6 months. Education level was higher than 6 years. The cognitive impairment occurred after the stroke. Their National Institutes of Health Stroke Scale (NIHSS) score on admission was less than 20 (47). Those with severe visual impairment, hearing impairment, and other factors affecting cognitive examination such as sensory aphasia were excluded. All patients were right-handed. The demographic data of the participant are shown in Table 1.
Informed consent was signed by the patient himself or by his immediate family. The study was approved by the Ethics Committee of Yuebei People’s Hospital Affiliated with Shantou University Medical College (Ethical approval number: SUMC-IRB-2020) on 29 April 2020 and registered in the Chinese Clinical Trials Registry (registration No. ChiCTR2000032804).
Research process
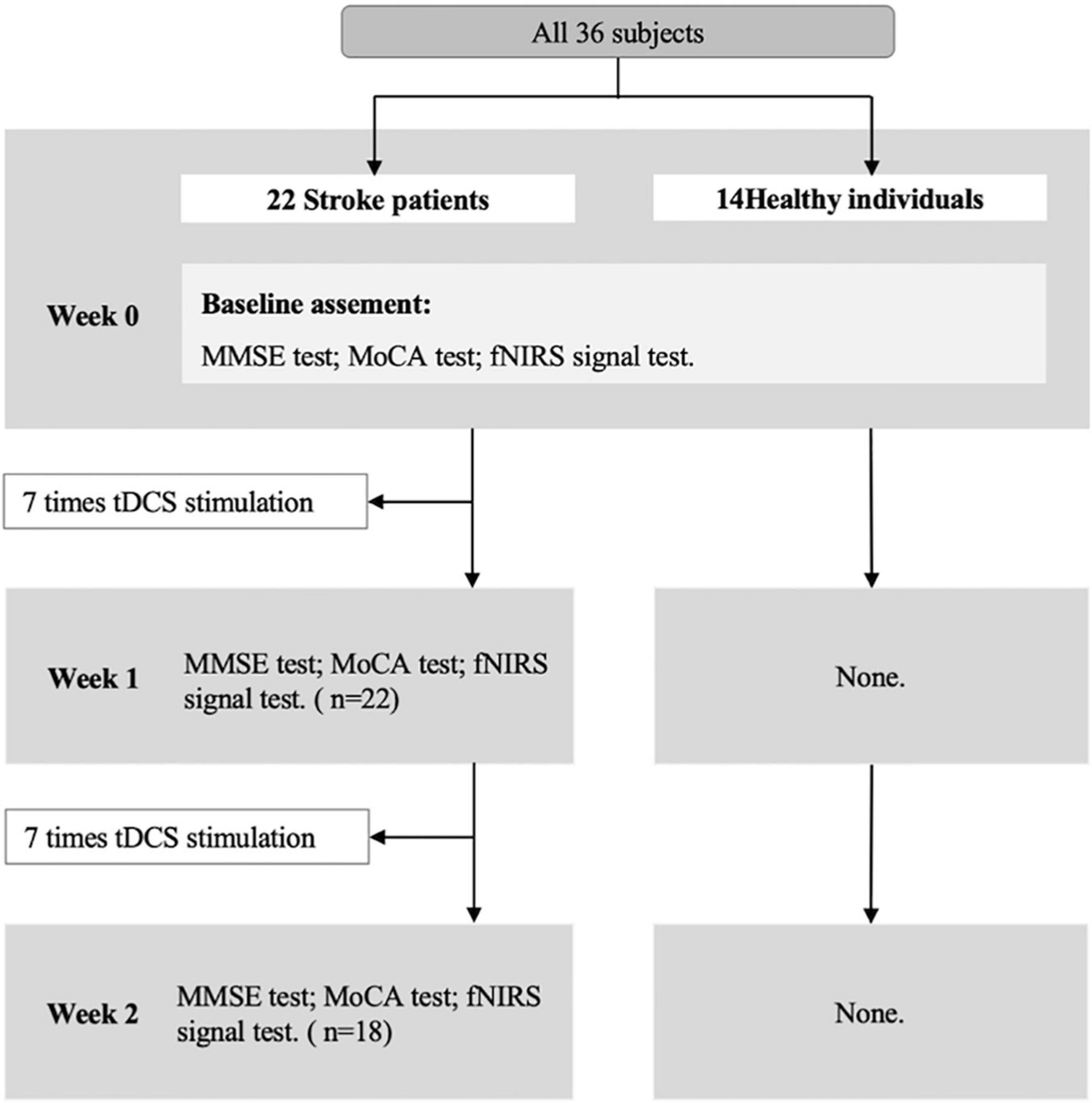
The Montreal Cognitive Assessment (MoCA) and the Mini-Mental State Examination (MMSE) were used to detect cognitive states before and after tDCS. The fNIRS with VFT was performed before and after tDCS. The tDCS intervention was taken only in the stroke group. The research process is shown in Figure 1.
Transcranial direct current stimulation
Treatment procedure: Patients with stroke receive tDCS treatment 7 times a week for 2 weeks (once a day). The duration of each tDCS application is 30 min. In the tDCS setting, the anode was attached over the F3 (10–20 EEG electrodes) (48) with 2 mA intensity. The return electrode was positioned over the F4 (10–20 EEG electrodes). After each tDCS application, the patients completed a brief adverse effect questionnaire (49). Before the tDCS, after the 7 times tDCS, and after the 14 times tDCS, the participants were tested for cognitive and cerebral hemodynamic signals with fNIRS, respectively.
Cognitive function measurement
All subjects were treated with comprehensive clinical evaluation. The MMSE is the most often used short screening tool for providing a clear impression of overall cognitive decline and checking the recovery of cognitive function after stroke (50, 51). Its validity has been demonstrated in patients with stroke (52). The MoCA can be used to assess people’s cognitive ability, including aspects of memory, executive function, attention, concentration, language, abstract reasoning, and orientation, with a maximum score of 30 (53, 54). Its validity has been demonstrated in patients with stroke (54).
Functional near-infrared spectroscopy measurement
Verbal fluency task process
The VFT flow is shown in Figure 2. The task time was about 170 s. The first 40 s and the last 70 s of the experiment are used to collect the resting-state signal of the participants. During these two stages, the participants are required to count from one to five repeatedly. During the second stage, the participants performed VFT of three consecutive word-generating tasks, each of which lasted 20 s.
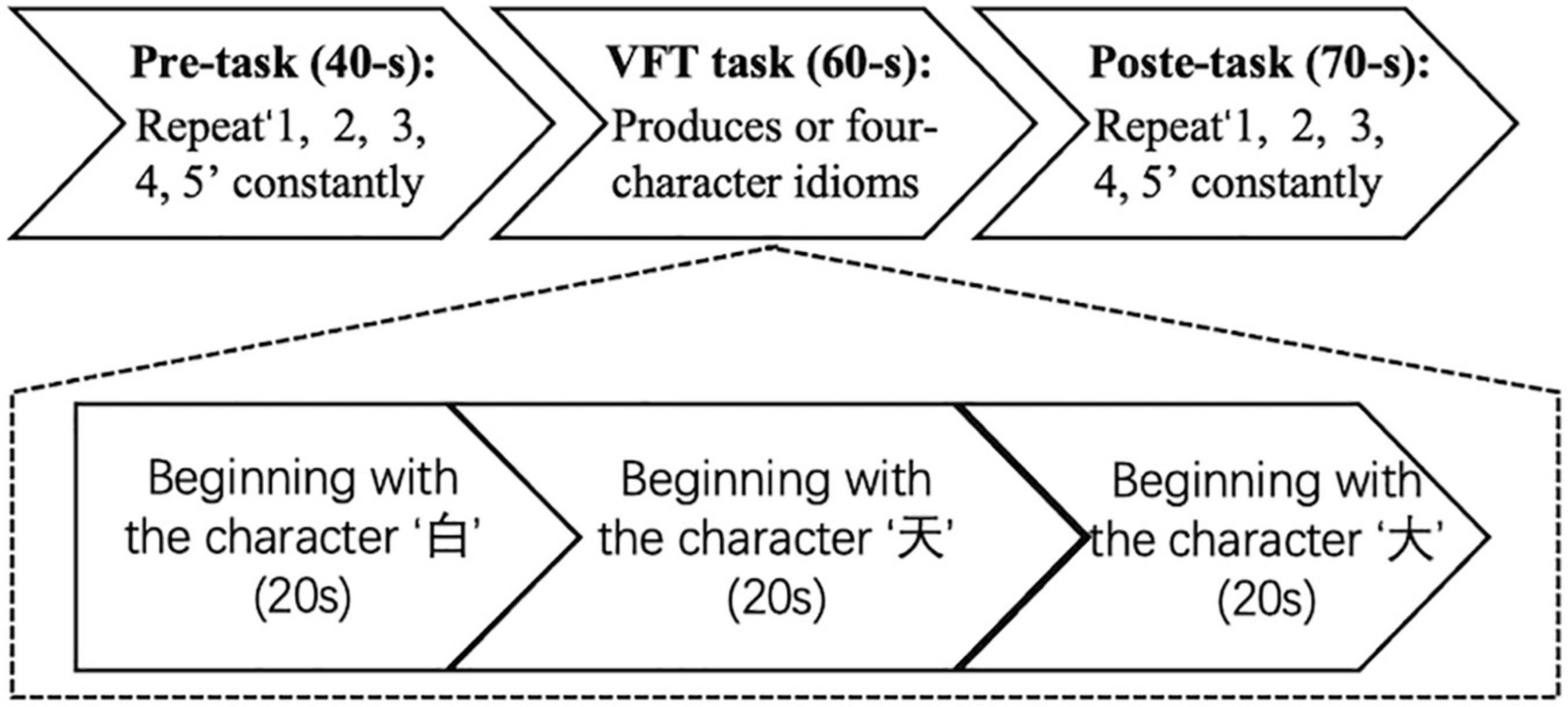
Figure 2. Experimental flowchart. The experiment has three procedures: 40-s pre-task, 60-s verbal fluency task (VFT) task, and 70-s post-task.
Functional near-infrared spectroscopy signal acquisition
During the VFT experiment, we used a 52-channel fNIRS device (ETG-4000 Optical Topography System) to estimate changes in regional cortical Hb concentration during the cognitive activation task, as described previously (55). The probes (17 emitters and 16 detectors, alternating) were fixed using 3 × 11 thermoplastic shells with an inter-optode distance of 3.0 cm. Each adjoining pair of an emitter and detector was referred to as a “channel,” resulting in 52 channels in total (Figure 3A). The lowermost probes were positioned along the Fp1–Fp2 line according to the International 10–20 EEG system (Figure 3B). The probes can measure Hb values bilaterally from the prefrontal and temporal surface regions at a depth of 20–30 mm from the scalp. This depth range corresponds roughly to the surface of the cerebral cortex. NIRS measures relative changes in oxy- and deoxy-Hb concentrations (in mM) using two wavelengths (695 and 830 nm) of near-infrared light based on the modified Beer–Lambert law (56). The sampling frequency is 10 Hz. The experiment was carried out in a quiet room with an appropriate temperature. After the experiment, the subjects were asked to sit quietly in the most comfortable position and minimize eye and other body movements.
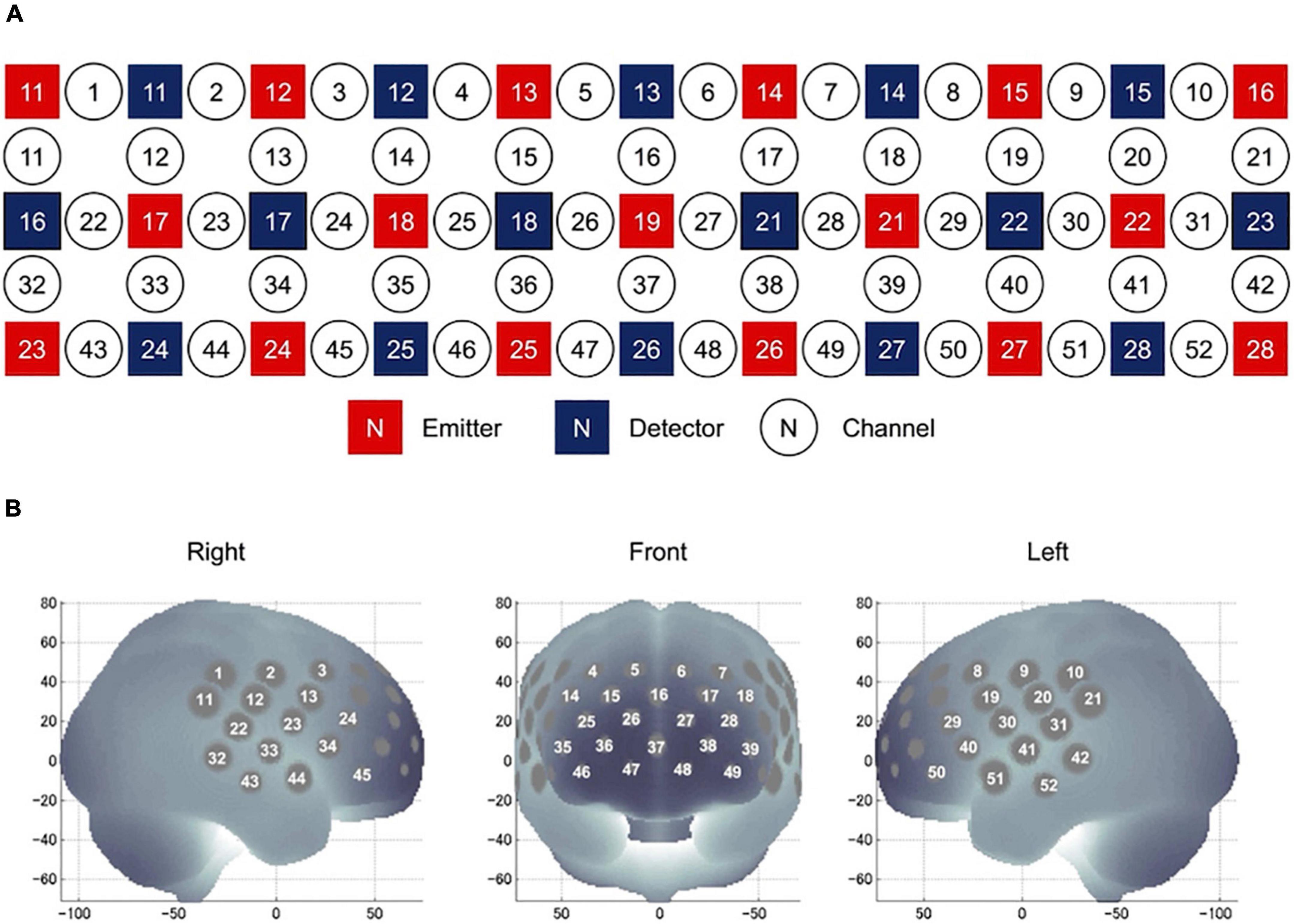
Figure 3. Location of NIRS channels. (A) Arrangement of the 17 emitters and 16 detectors and definition of the channels. (B) The anatomical site corresponding to each channel.
Nine ROIs were set at the following regions (57): right superior frontal cortex (rSFC) (detected by the 1st, 2nd, 11th, and 12th channels), right superior temporal cortex (rSTC) (detected by the 22nd, 23rd, 32nd, 33rd, 43rd, and 44th channels), right dorsolateral prefrontal cortex (rDLPFC) (detected by the 3rd, 4th, 13th, 14th, 15th, 24th, and 25th channels), right ventrolateral prefrontal cortex (rVLPFC) (detected by the 34th, 35th, 45th, and 46th channels), medial prefrontal cortex (mPFC) (detected by the 5th, 6th, 16th, 26th, 27th, 36th, 37th, 38th, 47th, and 48th channels), left dorsolateral prefrontal cortex (lDLPFC) (detected by the 7th, 8th, 17th, 18th, 19th, 28th, and 29th channels), left ventrolateral prefrontal cortex (lVLPFC) (detected by the 39th, 40th, 49th, and 50th channels), left superior frontal cortex (lSFC) (detected by the 9th, 10th, 20th, and 21st channels), and left superior temporal cortex (lSTC) (detected by the 30th, 31st, 41st, 42nd, 51st, and 52nd channels).
Data processing of functional near-infrared spectroscopy data
The fNIRS data were analyzed using the Homer2 package in MATLAB and customized MATLAB-based script (58). First, the raw light intensity file has been converted to homer2 file format (.nirs). Then, the raw fNIRS data were first converted to optical density (function: hmrIntensity2OD), and using the manufacturer’s recommendations, channels with a variation coefficient greater than 15% are considered bad channels and deleted from the analysis. A wavelet transform was used to correct motion artifact (function: hmrMotionCorrectWavelet) using the default interquartile range (0.1), as this is optimal for motion correction. Any remaining motion artifact was then removed through the motion artifact detection tool (function: hmrMotionArtifact, tMotion = 0.5, tMask = 1.0, STDEVthresh = 20, AMPthresh = 5.0). The signal was then bandpass-filtered (function: hmrBandpassFilt, hpf = 0.000, lpf = 0.10) to remove baseline drift and physiological noise. Finally, the concentration changes of oxy-Hb were then computed according to the modified Beer–Lambert law. Additionally, we took the final 5 s of the pre-task rest period as the baseline. The oxy-Hb values were then saved as text files for each subject. Finally, the oxy-Hb time series for each subject was z-scored by channel. Note that post-analysis in this study was solely based on oxy-Hb, since the oxy-Hb signal is known to be more robust and sensitive than deoxy-Hb to task-associated changes (59–61).
Based on the MATLAB-based Nirs_Kit software (62), we used the GLM model (63, 64), using mission oxygenated hemoglobin (oxy-Hb) β value minus rest oxygenated hemoglobin β value, representing the cortical activation. Then, we used an independent sample t-test to compare the cortical activation between patients with stroke and healthy control groups and used the ANOVA test to compare the cortical activation among before tDCS, after 7 times tDCS, and after 14 times tDCS in the stroke group.
Data analysis of functional connectivity
As stroke can disrupt FC and cause brain-wide network changes (65), it is important to investigate brain-wide network dynamics during post-stroke recovery. This study uses a technology similar to the existing research to study FC (66, 67). The Nirs_Kit toolbox was used to conduct statistical comparisons (62). The primary threshold (test statistic) for electrode pairs was set to a conservative value of t = 3.1 (equivalent to p = 0.001) to ensure that only highly robust and reliable connectivity differences would be compared at the cluster level (68). A value of p < 0.05 (two-tailed) was used as the secondary significance threshold for family-wise corrected cluster analysis (5,000 permutations) (68). Subsequent visualization of brain networks was performed using the BrainNet viewer toolbox (69).
Results
Mini-mental state examination and Montreal cognitive assessment
First, based on previous studies and clinical observations, we assume that the cognitive function of the stroke group is lower than that of the healthy control group. We used the independent sample t-test to compare the MMSE and MoCA of the stroke group and the healthy control group. The results show that both MMSE score (m = 20 ± 4.739) and MoCA score (m = 13.78 ± 4.045) in the stroke group were lower than that in the healthy control group (m = 28.21 ± 2.082), (m = 28.07 ± 1.592), [t(34) = –5.247, p < 0.001], [t(34) = –12.629, p < 0.001]. This shows that compared with the healthy control group, the cognitive function of the stroke group has decreased.
Second, we assume that after the tDCS, the cognitive function of patient with stroke will increase. Therefore, we conducted an ANOVA test on MMSE and MoCA scores of three tests in the stroke group. The results showed that there was a significant difference in MoCA score among before tDCS, after 7 times tDCS, and after 14 times tDCS [F(1,58) = 11.860, p < 0.001]; the results of multiple comparisons show that the MoCA score after 7 times tDCS (m = 18.10 ± 5.612) is significantly higher than that before tDCS (m = 14.38 ± 4.631) [p = 0.025]; the results of multiple comparisons show that the MoCA score after 14 times tDCS (m = 20.17 ± 5.426) is significantly higher than that before tDCS (m = 14.38 ± 4.631) [p < 0.001], as shown in Figure 4. There were marginally significant differences in MMSE score among before tDCS, after 7 times tDCS, and after 14 times tDCS [F(1,58) = 3.782, p = 0.057]; the results of multiple comparisons show that the MMSE score after 14 times tDCS (m = 24.44 ± 4.105) was marginally significantly higher than that before tDCS (m = 21.33 ± 5.453) [p = 0.057]. This shows that after 7 times tDCS and 14 times tDCS, the cognitive function measured by MoCA of patients with stroke has been significantly improved.
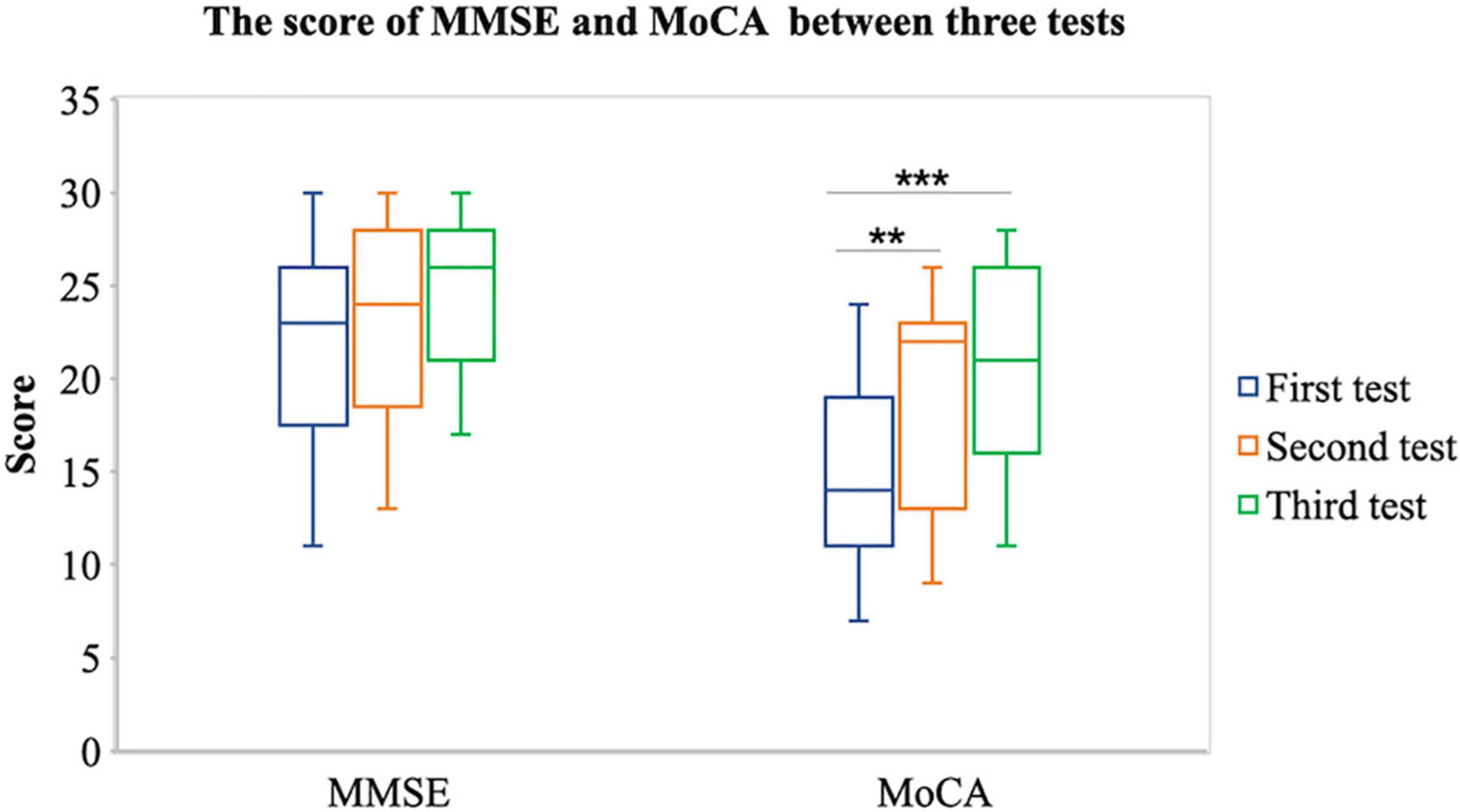
Figure 4. Comparison of MMSE and MoCA between the first, second, and third tests. **P < 0.05 and ***P < 0.001.
Cortical activation
First, based on the phenomenon that the cognitive function of the stroke group is lower than that of the healthy control group, we assume that the cortical activation of the stroke group is lower than that of the healthy control group. Therefore, we compared the cortical activation of the stroke group and the healthy control group in nine brain cortexes, including rSFC, rSTC, rDLPFC, rVLPFC, mPFC, lDLPFC, lVLPFC, lSFC, and lSTC. The independent sample t-test results show that the β value of the stroke group was lower than that of the healthy control group in the rSTC [m(stroke) = 0.510 ± 0.970, m(control) = 1.705 ± 1.381, t(34) = –2.966, p = 0.006], rDLPFC [m(stroke) = 0.035 ± 0.522, m(control) = 0.592 ± 0.599, t(34) = –2.852, p = 0.008], rVLPFC [m(stroke) = 0.163 ± 0.711, m(control) = 0.906 ± 0.894, t(34) = –2.683, p = 0.011], lVLPFC [m(stroke) = 0.400 ± 0.391, m(control) = 0.951 ± 0.674, t(34) = –2.677, p = 0.016], and lSTC [m(stroke) = 0.360 ± 0.635, m(control) = 1.641 ± 1.754, t(34) = –2.533, p = 0.023], as shown in Figure 5A. This shows that compared with the healthy control group, the brain cortical activation of the stroke group during the VFT has decreased in a wide range of brain regions.
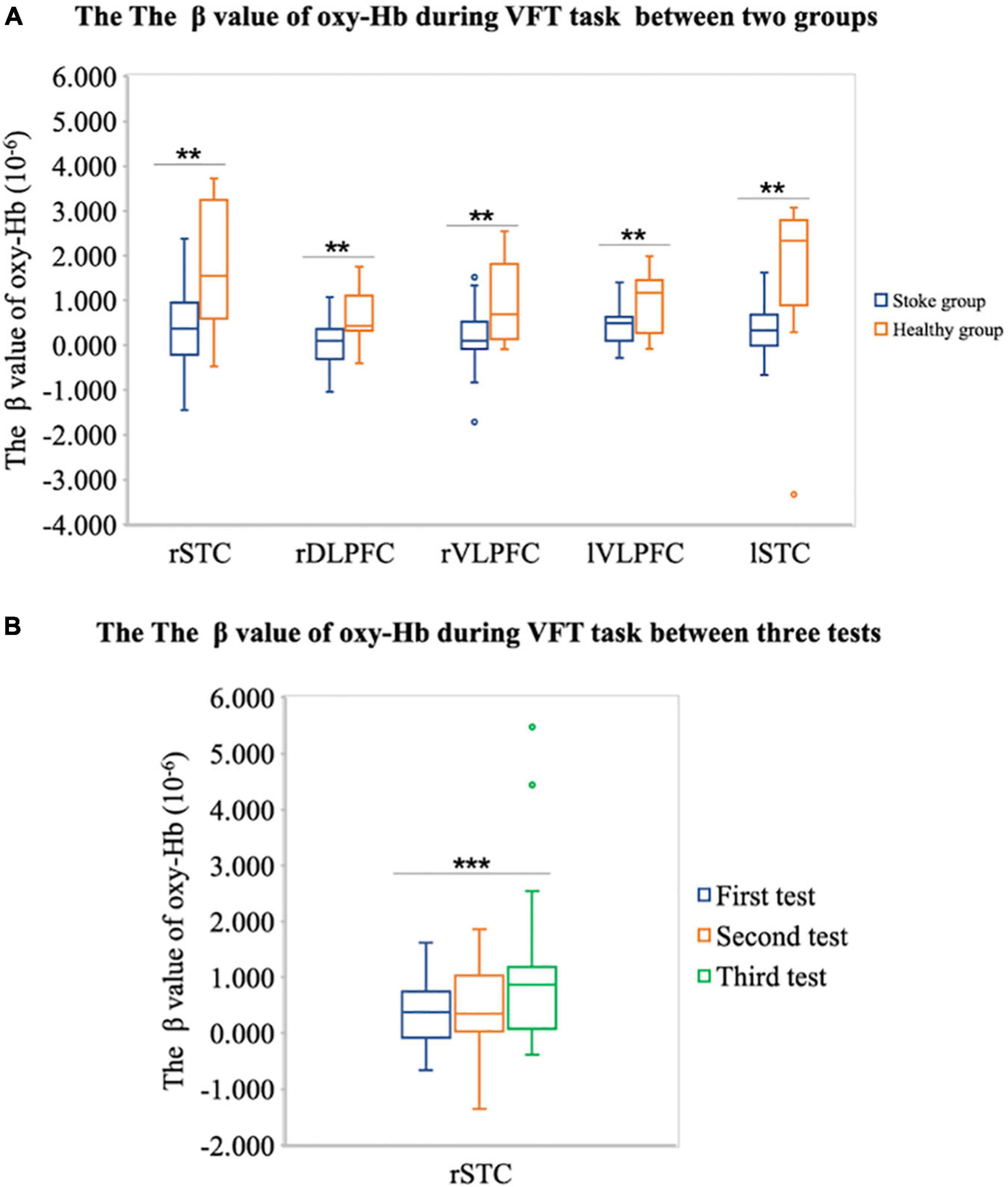
Figure 5. (A) Difference of the β value of oxy-Hb during VFT between the stroke group and the healthy individual group on rSTC, rDLPFC, rVLPFC, and lVLPFC areas. (B) The difference of the β value of oxy-Hb on the right superior temporal cortex (rSTC) during VFT between three tests in patient with stroke. **P < 0.05 and ***P < 0.001.
Second, we assume that after the tDCS, the cortical activation in lSTC of patient with stroke will increase. Therefore, we conducted an ANOVA test on cortical activation in lSTC of three tests in the stroke group. The results showed that there were significant differences in the lSTC in the stroke group among before tDCS, after seven times tDCS, and after 14 times tDCS [F(1,58) = 4.488, p = 0.038], as shown in Figure 6; the results of multiple comparisons showed that the β value on the lSTC during VFT after 14 times tDCS (m = 0.360 ± 0.643) was significantly higher than that before tDCS (m = 1.122 ± 1.576) [p = 0.038], as shown in Figures 5B, 6. This shows that after 14 times tDCS, the cortical activation in the lSTC in the stroke group has been significantly improved.
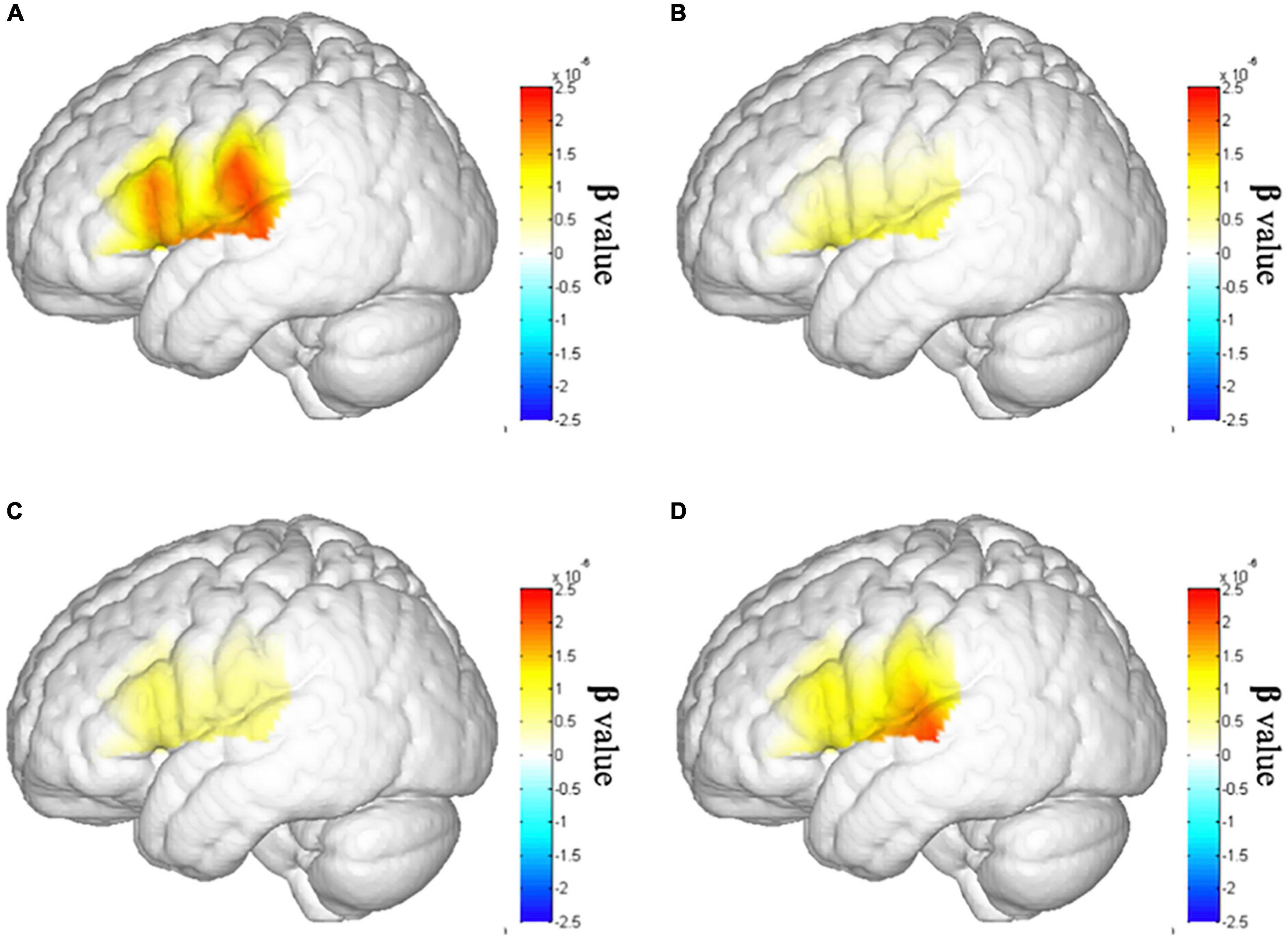
Figure 6. Brain activation map of the left superior temporal cortex (lSTC). (A) Brain activation map of lSTC in the healthy individual group. (B) Brain activation map of lSTC in the stroke group before tDCS. (C) Brain activation map of lSTC in the stroke group after seven times tDCS. (D) Brain activation map of lSTC in the stroke group after 14 times tDCS.
Third, we further explored whether the cortical activation of the patient with stroke has recovered to normal level after 14 times tDCS. we assume that there is no difference between the cortical activation of patients with stroke after 14 times tDCS and that of the healthy control group. The independent sample t-test results show that there is no difference between the cortical activation on all cortexes in patients with stroke after 14 times tDCS and that in the healthy control group. This shows that after 14 times tDCS, the cortical activation of patients with stroke has recovered to the same level as that of healthy peers.
Functional connectivity
First, as the balance between the cerebral hemispheres of patients with stroke is destroyed (6), we propose the assumption that the FC between bilateral cerebral hemispheres in the stroke group is lower than that in the healthy control group. We used the correlation between bilateral cerebral hemispheric channels’ oxy-Hb during VFT to represent FC. The result shows that the FC between rSTC and lSTC in the stroke group is significantly lower than that in the healthy control group. The FC between rDLPFC and lSTC in the stroke group is significantly lower than that in the healthy control group, as shown in Figure 7A. This shows that compared with the healthy control group, the FC between bilateral cerebral hemispheres of the stroke group during the VFT has decreased, especially the FC between the lSTC and the rSTC and the FC between the lSTC and the rDLPFC.
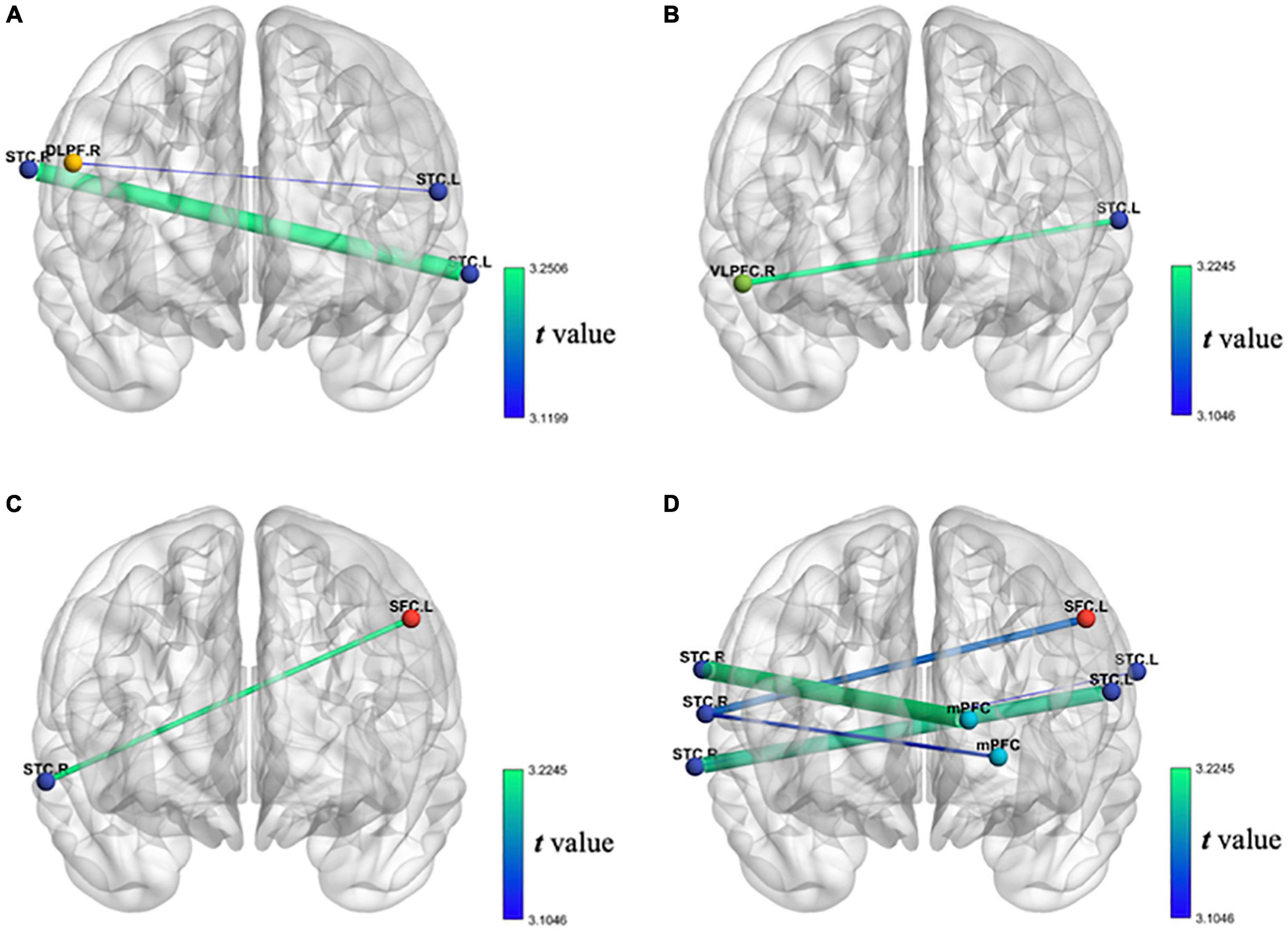
Figure 7. (A) Decrease of FC of oxy-Hb during VFT in patients with stroke compared with the healthy individual group; (B) the decrease of FC of oxy-Hb during VFT of the first test compared with the second test in patients with stroke; (C) the increase of FC of oxy-Hb during VFT of the first test compared with the third test in patients with stroke; (D) the increase of FC of oxy-Hb during VFT of the second test compared with the third test in patients with stroke.
Second, we assume that after the tDCS, the FC between bilateral cerebral hemispheres of patient with stroke will increase. Similarly, we used the correlation between bilateral cerebral hemispheric channels’ oxy-Hb during VFT to represent FC. The unexpected results showed that the FC between rVLPFC and lSTC after seven times tDCS is significantly lower than that before tDCS in the stroke group, as shown in Figure 7B, but the expected results showed that the FC between lSFC and rSTC after 14 times tDCS is significantly higher than that before tDCS in the stroke group, as shown in Figure 7C. Similarly, the expected results showed that the FC between lSFC and rSTC after 14 times tDCS is significantly higher than that after seven times tDCS in the stroke group. The FC between the mPFC and the rSTC after 14 times tDCS is significantly higher than that after seven times tDCS in the stroke group. The FC between the lSTC and rSTC after 14 times tDCS is significantly higher than that after seven times tDCS in the stroke group, as shown in Figure 7D. This shows that after 14 times tDCS, the FC between bilateral cerebral hemispheres of the stroke group during the VFT has increased. In addition, during the whole 14 times of tDCS, the stimulation from the 7th to the 14th time was the most effective.
Discussion
Post-stroke cognitive impairment is a common complication after stroke (1, 2). After a stroke, people have been found to have decreased brain cortical activation (4, 5) and FC (10–12), which is associated with persistent functional impairment. Therefore, regulating neural activity in stroke patients with cognitive impairment is critical. tDCS is a promising tool for increasing cortical activity (20) and FC (29–31). fNIRS is a non-invasive optical technique that can indirectly observe the activation of the cerebral cortex (33). Therefore, we investigated the effect of tDCS with anodes and cathodes on F3 and F4 electrodes on the rehabilitation of cognitive impairment in patients with stroke using fNIRS technology. This study’s results showed that the cognitive function of patients with stroke was lower than that of healthy individuals but improved after tDCS. The cortical activation of patients with stroke was lower than that of healthy individuals in the lSTC, rSTC, rDLPFC, rVLPFC, and lVLPFC cortical regions; cortical activation increased in the lSTC cortex after tDCS. The FC between the cerebral hemispheres of patients with stroke was lower than that of healthy individuals and increased after 14 tDCS sessions. This shows that the cognitive and brain function of patients with mild-to-moderate stroke were damaged but could recover after tDCS.
Cognitive function in older adult patients with stroke is impaired and can be improved after tDCS. This research shows that the cognitive function identified by the MMSE and the MoCA scores in patients with stroke was lower than in healthy peers but improved after tDCS. Post-stroke cognitive impairment is a known complication after stroke and a critical risk predictor of a decline in quality of life (1–3). Some researchers applied tDCS to stimulate the brain cortex. They found that cognitive functions, such as naming accuracy (18), the scores of attention and concentration, figural memory, logical reasoning, reaction behavior (2), working memory (20, 70), and executive function (27), improved in patients with stroke. Therefore, it can be inferred that the tDCS intervention is an effective treatment to improve cognitive function for stroke patients with cognitive impairment.
Cortical brain activation in older adult patients with stroke is decreased and can be re-activated after tDCS. This research shows that cortical activation in patients with stroke was lower than in healthy individuals in the cortices of the lSTC, rSFC, rDLPFC, rVLPFC, and lVLPFC. This increased to the same level as normal peers in the lSTC after tDCS. Consistent with the results of this study, previous studies have also found that the decreased extent of cortical activation in patients with stroke is related to the degree of functional damage (4, 5). Post-stroke, the lesions may be widespread in the frontal and temporal regions (45, 46). Fortunately, a series of studies have found that tDCS can alter the excitability of the cortex (21, 24–28, 71). Anodic stimulation increases cortical excitability, whereas cathodic stimulation decreases it (71). In addition, regarding the IHI state after stroke, tDCS can increase ipsilateral excitability and reduce contralateral excitability due to the destruction of IHI after stroke (24). This study placed the anodes and cathodes on F3 and F4 electrodes (10–20 EEG electrodes) with 2 mA intensity and found that the cortical activation of patients with stroke increased in the lSTC cortex after tDCS. Therefore, tDCS positively affects the recovery of cerebral cortical function in patients with stroke (24, 28).
The brain FC between the bilateral cerebral hemispheres of older adult patients with stroke is decreased but can be re-connected after tDCS. This research showed that the FC between the bilateral cerebral hemispheres in patients with stroke was lower than in healthy individuals and increased after 14 tDCS sessions. Consistent with the results of this study, previous studies found that the damage to neural systems caused brain-wide network changes. The balance between the cerebral hemispheres of patients with stroke is destroyed (6) when a stroke occurs, leading to the disinhibition of the contralateral cortex and excessive activation, further reducing brain function (7–9). The promising findings are that tDCS can widely increase this interhemispheric connectivity in patients with stroke (29–31).
This research makes an important contribution to the diagnosis and effective treatment of brain function in patients with cognitive impairment after stroke. Theoretically, this research confirms that patients with stroke have brain function damage (4, 5, 45, 46). Second, this study expanded the research range of brain function damage to the connectivity between the bilateral cerebral hemispheres. Based on the theoretical model of competition inhibition between the bilateral cerebral hemispheres (6–9), this study found that in patients with stroke, the FC between the bilateral cerebral hemispheres is abnormally reduced. More importantly, based on previous studies, this study confirmed that tDCS is an effective intervention for post-stroke cognitive impairment. In placing the anodes and cathodes on F3 and F4 electrodes during tDCS, we can effectively improve cognitive impairment after stroke, re-activate the cerebral cortex, and re-connect the FC of the bilateral cerebral hemispheres. In this study, we found that increased cortical activation and FC between bilateral cerebral hemispheres measured by fNIRS are promising biomarkers to assess the effectiveness of tDCS in patients with stroke. These two neural indicators can be used clinically to measure cerebral function recovery in stroke patients with cognitive impairment.
Limitations
This study had some limitations. There was no control group with healthy older adult peers in the tDCS intervention study, and the use of drugs in treating patients with stroke has not been controlled. Future researchers can verify these results using more cognitive task paradigms. Moreover, they can explore the mechanism of action of tDCS and drug use in treating patients with stroke.
Conclusion
In conclusion, post-stroke cognitive impairment is a common complication of stroke. After a stroke, people have been found to have abnormal cortical activation in the brain, which is associated with persistent cognitive impairment. tDCS is a promising tool for assessing changes in cortical activation. fNIRS is a non-invasive optical technique that can indirectly observe cortical activation. This study investigated the effect of tDCS on the rehabilitation of cognitive impairment in patients with stroke using fNIRS technology. This study’s results showed that the cognitive ability of patients with stroke, measured on the MMSE and MoCA, was lower than that of healthy individuals but was improved after tDCS. The cortical activation of patients with stroke was lower than that of healthy individuals on the lSTC, rSTC, rDLPFC, rVLPFC, and lVLPFC cortical regions; this increased in the lSTC cortex after tDCS. The FC between the cerebral hemispheres of patients with stroke was lower than that of healthy individuals and increased after 14 tDCS. This shows that the cognitive and brain function of mild-to-moderate patients with stroke were damaged but could be recovered after tDCS. Increased cortical activation and increased FC between the bilateral cerebral hemispheres measured by fNIRS are promising biomarkers to assess the effectiveness of tDCS in stroke.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Yue Bei People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PW, YZ, CY, KH, and HL participated in this study and proposed an experimental design. PW, CY, TZ, and KH participated in the experimental process of this study. TZ and CY participated in the data analysis of this study. TZ, MX, and CY participated in the writing of this study. PW, YZ, CY, and TZ participated in the modification of this study. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the Regional Joint Key Program of the National Natural Science Foundation of China (No. U21A20479).
Acknowledgments
The authors would like to thank all the participants for their active participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nys GMS, Zandvoort MJEV, Worp HBVD, Haan EHFD, Kort PLMD, Jansen BPW, et al. Early cognitive impairment predicts long-term depressive symptoms and quality of life after stroke. J Neurol Sci. (2006) 247:149–56. doi: 10.1016/j.jns.2006.04.005
2. Shaker HA, Sawan SAE, Fahmy EM, Ismail RS, Elrahman SAEA. Effect of transcranial direct current stimulation on cognitive function in stroke patients. Egypt J Neurol Psychiatr Neurosurg. (2018) 54:32. doi: 10.1186/s41983-018-0037-8
3. Horvath JC, Forte JD, Carter O. Quantitative review finds no evidence of cognitive effects in healthy populations from single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimul. (2015) 8:535–50. doi: 10.1016/j.brs.2015.01.400
4. Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. (2003) 126:1430–48. doi: 10.1093/brain/awg145
5. Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. (2003) 126:2476–96. doi: 10.1093/brain/awg245
6. Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. (2004) 55:400–9. doi: 10.1002/ana.10848
7. Grefkes C, Ward NS. Cortical reorganization after stroke: how much and how functional? Neuroscientist. (2014) 20:56–70. doi: 10.1177/1073858413491147
8. Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. (2009) 23:641–56. doi: 10.1177/1545968309336661
9. Cheng MY, Aswendt M, Steinberg GK. Optogenetic approaches to target specific neural circuits in post-stroke recovery. Neurotherapeutics. (2016) 13:325–40. doi: 10.1007/s13311-015-0411-5
10. Caliandro P, Vecchio F, Miraglia F, Reale G, Della Marca G, La Torre G, et al. Small-World characteristics of cortical connectivity changes in acute stroke. Neurorehabil Neural Repair. (2017) 31:81–94. doi: 10.1177/1545968316662525
11. Lu K, Xu G, Li W, Huo C, Liu Q, Lv Z, et al. Frequency-specific functional connectivity related to the rehabilitation task of stroke patients. Med Phys. (2019) 46:1545–60. doi: 10.1002/mp.13398
12. Arun KM, Smitha KA, Sylaja PN, Kesavadas C. Identifying resting-state functional connectivity changes in the motor cortex using fNIRS During Recovery from Stroke. Brain Topogr. (2020) 33:710–9. doi: 10.1007/s10548-020-00785-2
13. Schlaug G, Renga V. Transcranial direct current stimulation: a noninvasive tool to facilitate stroke recovery. Expert Rev Med Devices. (2008) 5:759–68. doi: 10.1586/17434440.5.6.759
14. Monti A, Ferrucci R, Fumagalli M, Mameli F, Cogiamanian F, Ardolino G, et al. Transcranial Direct Current Stimulation (tDCS) and language. J Neurol Neurosurg Psychiatry. (2013) 84:832–42. doi: 10.1136/jnnp-2012-302825
15. Wang C, Chen Y, Song P, Yu H, Du J, Zhang Y, et al. Varied response of EEG rhythm to different tDCS protocols and lesion hemispheres in stroke subjects with upper limb dysfunction. Neural Plast. (2022) 2022:7790730. doi: 10.1155/2022/7790730
16. Begemann MJ, Brand BA, Uri-Blake B, Aleman A, Sommer IE. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-Analysis. Psychol Med. (2020) 50:1–22. doi: 10.1017/S0033291720003670
17. Berryhill ME, Martin D. Cognitive effects of transcranial direct current stimulation in healthy and clinical populations: an overview. J ECT. (2018) 34:e25–35. doi: 10.1097/YCT.0000000000000534
18. Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. (2010) 41:1229–36. doi: 10.1161/STROKEAHA.109.576785
19. Park SH, Koh EJ, Choi HY, Ko MH. A double-blind, sham-controlled, pilot study to assess the effects of the concomitant use of transcranial direct current stimulation with the computer assisted cognitive rehabilitation to the prefrontal cortex on cognitive functions in patients with stroke. J Korean Neurosurg Soc. (2013) 54:484–8. doi: 10.3340/jkns.2013.54.6.484
20. Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. (2011) 4:84–9. doi: 10.1016/j.brs.2010.06.004
21. Chan MMY, Han YMY. Effects of Transcranial Direct Current Stimulation (tDCS) in the normalization of brain activation in patients with neuropsychiatric disorders: a systematic review of neurophysiological and neuroimaging studies. Neural Plast. (2020) 2020:8854412. doi: 10.1155/2020/8854412
22. Bindman LJ, Lippold OCJ, Redfearn JWT. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced by polarizing currents. Nature. (1962) 196:584–5. doi: 10.1038/196584a0
23. Bindman LJ, Lippold O, Redfearn J. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. (1964) 172:369–82. doi: 10.1113/jphysiol.1964.sp007425
24. Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial Direct Current Stimulation (tDCS). Clin Neurophysiol. (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.087
25. Baschi R, Sava S, Salvia VL, Pasqua VD, Schoenen J, Magis D. EHMTI-0317. Transcranial direct current stimulation in chronic migraine: a pilot trial combining cathodal visual and anodal dlpfc stimulation. J Headache Pain. (2014) 15(Suppl. 1):G4. doi: 10.1186/1129-2377-15-S1-G4
26. Feltman KA, Hayes AM, Bernhardt KA, Emmanuel N, Kelley AM. Viability of tDCS in military environments for performance enhancement: a systematic review. Mil Med. (2020) 185:e53–60. doi: 10.1093/milmed/usz189
27. Sarkis RA, Kaur N, Camprodon JA. Transcranial Direct Current Stimulation (tDCS): modulation of executive function in health and disease. Curr Behav Neurosci Rep. (2014) 1:74–85. doi: 10.1007/s40473-014-0009-y
28. Elsner B, Kugler J, Mehrholz J. Transcranial direct current stimulation (tDCS) for upper limb rehabilitation after stroke: future directions. J Neuroeng Rehabil. (2018) 15:106. doi: 10.1186/s12984-018-0459-7
29. Lee J, Park E, Lee A, Chang WH, Kim D-S, Shin Y-I, et al. Modulating brain connectivity by simultaneous dual-mode stimulation over bilateral primary motor cortices in subacute stroke patients. Neur Plastic. (2018) 2018:1458061. doi: 10.1155/2018/1458061
30. Lefebvre S, Dricot L, Laloux P, Desfontaines P, Evrard F, Peeters A, et al. Increased functional connectivity one week after motor learning and tDCS in stroke patients. Neuroscience. (2017) 340:424–35. doi: 10.1016/j.neuroscience.2016.10.066
31. Hordacre B, Moezzi B, Ridding MC. Neuroplasticity and network connectivity of the motor cortex following stroke: a transcranial direct current stimulation study. Hum Brain Mapp. (2018) 39:3326–39. doi: 10.1002/hbm.24079
32. Chen WL, Wagner J, Heugel N, Sugar J, Lee YW, Conant L, et al. Functional near-infrared spectroscopy and its clinical application in the field of neuroscience: advances and future directions. Front Neurosci. (2020) 14:724. doi: 10.3389/fnins.2020.00724
33. Irani F, Platek SM, Bunce S, Ruocco AC, Chute D. Functional near infrared spectroscopy (fNIRS): an emerging neuroimaging technology with important applications for the study of brain disorders. Clin Neuropsychol. (2007) 21:9–37. doi: 10.1080/13854040600910018
34. Sun W, Wu X, Zhang T, Lin F, Sun H, Li J. Narrowband resting-state fNIRS functional connectivity in Autism spectrum disorder. Front Hum Neurosci. (2021) 15:643410. doi: 10.3389/fnhum.2021.643410
35. Tang TB, Chong JS, Kiguchi M, Funane T, Lu CK. Detection of Emotional Sensitivity Using fNIRS based dynamic functional connectivity. IEEE Trans Neural Syst Rehabil Eng. (2021) 29:894–904. doi: 10.1109/TNSRE.2021.3078460
36. Coutinho AMN, Porto FHG, Duran FLS, Prando S, Ono CR, Feitosa EAAF, et al. Brain metabolism and cerebrospinal fluid biomarkers profile of non-amnestic mild cognitive impairment in comparison to amnestic mild cognitive impairment and normal older subjects. Alzheimers Res Ther. (2015) 7:58. doi: 10.1186/s13195-015-0143-0
37. Li HJ, Hou XH, Liu HH, Yue CL, He Y, Zuo XN. Toward systems neuroscience in mild cognitive impairment and alzheimer’s disease: a meta-analysis of 75 fMRI Studies. Hum Brain Mapp. (2015) 36:1217–32. doi: 10.1002/hbm.22689
38. Mky A, Ascb C. Functional near-infrared spectroscopy reveals decreased resting oxygenation levels and task-related oxygenation changes in mild cognitive impairment and dementia: a systematic review. J Psychiatr Res. (2020) 124:58–76. doi: 10.1016/j.jpsychires.2020.02.017
39. Kim DH, Lee KD, Bulea TC, Park HS. Increasing motor cortex activation during grasping via novel robotic mirror hand therapy: a pilot fNIRS study. J Neuroeng Rehabil. (2022) 19:8. doi: 10.1186/s12984-022-00988-7
40. Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? Neuroimage. (2007) 37:1338–45. doi: 10.1016/j.neuroimage.2007.06.014
41. Kelley R. Primary mental abilities.by L. L. Thurstone. J Am Stat Assoc. (1938) 33:753–5. doi: 10.2307/2279078
42. Chen WL, Wagner J, Heugel N, Sugar J, Whelan HT. Functional near-infrared spectroscopy and its clinical application in the field of neuroscience: advances and future directions. Front Neurosci. (2020) 14:724. doi: 10.3389/fnins.2020.00724
43. Hong KS, Yaqub MA. Application of functional near-infrared spectroscopy in the healthcare industry. J Innov Optic Health Sci. (2019) 12:1930012. doi: 10.1142/S179354581930012X
44. Ehlis AC, Haeussinger F, Gastel A, Fallgatter AJ, Plewnia C. Task-dependent and polarity-specific effects of prefrontal transcranial direct current stimulation on cortical activation during word fluency. Neuroimage. (2016) 140:134–40. doi: 10.1016/j.neuroimage.2015.12.047
45. Wilson SM, Schneck SM. Neuroplasticity in post-stroke aphasia: a systematic review and meta-analysis of functional imaging studies of reorganization of language processing. Neurobiol Lang. (2020) 2:1–757. doi: 10.1162/nol_a_00025
46. Yang ZH, Zhao XQ, Wang CX, Chen HY, Zhang YM. Neuroanatomic correlation of the post-stroke aphasias studied with imaging. Neurol Res. (2008) 30:356–60. doi: 10.1179/174313208X300332
47. Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. (2003) 34:1717–22. doi: 10.1161/01.STR.0000078657.22835.B9
48. Darkow R, Martin A, Wurtz A, Floel A, Meinzer M. Transcranial direct current stimulation effects on neural processing in post-stroke aphasia. Hum Brain Mapp. (2017) 38:1518–31. doi: 10.1002/hbm.23469
49. Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. (2011) 14:1133–45. doi: 10.1017/S1461145710001690
50. Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. (2007) 78:1298–303. doi: 10.1136/jnnp.2006.109074
51. Momeni M, Meyer S, Docquier M-A, Lemaire G, Kahn D, Khalifa C, et al. Predicting postoperative delirium and postoperative cognitive decline with combined intraoperative electroencephalogram monitoring and cerebral near-infrared spectroscopy in patients undergoing cardiac interventions. J Clin Monit Comput. (2019) 33:999–1009. doi: 10.1007/s10877-019-00253-8
52. Lees R, Selvarajah J, Fenton C, Pendlebury ST, Langhorne P, Stott DJ, et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke. (2014) 45:3008–18. doi: 10.1161/STROKEAHA.114.005842
53. Katz MJ, Wang C, Nester CO, Derby CA, Zimmerman ME, Lipton RB, et al. T-MoCA: a valid phone screen for cognitive impairment in diverse community samples. Alzheimers Dement. (2021) 13:e12144. doi: 10.1002/dad2.12144
54. Sharma R, Mallick D, Llinas RH, Marsh EB. Early post-stroke cognition: in-hospital predictors and the association with functional outcome. Front Neurol. (2020) 11:613607. doi: 10.3389/fneur.2020.613607
55. Figeys M, Zeeman M, Kim ES. Effects of Transcranial Direct Current Stimulation (tDCS) on cognitive performance and cerebral oxygen hemodynamics: a systematic review. Front Hum Neurosci. (2021) 15:623315. doi: 10.3389/fnhum.2021.623315
56. Cope M, Delpy DT, Reynolds EO, Wray S, Wyatt J, Van D. Methods of Quantitating Cerebral near Infrared Spectroscopy Data. Berlin: Springer (1988). doi: 10.1007/978-1-4615-9510-6_21
57. Yang J, Ji X, Quan W, Liu Y, Wei B, Wu T. Classification of schizophrenia by functional connectivity strength using functional near infrared spectroscopy. Front Neuroinform. (2020) 14:40. doi: 10.3389/fninf.2020.00040
58. Aasted CM, Yücel MA, Steele SC, Peng K, Boas DA, Becerra L, et al. Parameters used for Homer2 processing stream. San Francisco, CA: Public library of science (2016).
59. Sato H, Yahata N, Funane T, Takizawa R, Katura T, Atsumori H, et al. A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. Neuroimage. (2013) 83:158–73. doi: 10.1016/j.neuroimage.2013.06.043
60. Duan L, Zhang YJ, Zhu CZ. Quantitative comparison of resting-state functional connectivity derived from fNIRS and fMRI: a simultaneous recording study. Neuroimage. (2012) 60:2008–18. doi: 10.1016/j.neuroimage.2012.02.014
61. Pinti P, Aichelburg C, Gilbert S, Hamilton A, Hirsch J, Burgess P, et al. A review on the use of wearable functional near-infrared spectroscopy in naturalistic environments. Jpn Psychol Res. (2018) 60:347–73. doi: 10.1111/jpr.12206
62. Hou X, Zhang Z, Zhao C, Duan L, Gong Y, Li Z, et al. NIRS-KIT: a MATLAB toolbox for both resting-state and task fNIRS data analysis. Neurophotonics. (2021) 8:010802. doi: 10.1117/1.NPh.8.1.010802
63. Tak S, Ye JC. Statistical analysis of fNIRS data: a comprehensive review. Neuroimage. (2014) 85:72–91. doi: 10.1016/j.neuroimage.2013.06.016
64. von Lühmann A, Ortega-Martinez A, Boas DA, Yücel MA. Using the general linear model to improve performance in fNIRS single trial analysis and classification: a perspective. Front Hum Neurosci. (2020) 14:30. doi: 10.3389/fnhum.2020.00030
65. Silasi G, Murphy TH. Stroke and the connectome: how connectivity guides therapeutic intervention. Neuron. (2014) 83:1354–68. doi: 10.1016/j.neuron.2014.08.052
66. Yao Z, Shi J, Zhang Z, Zheng W, Hu T, Li Y, et al. Altered dynamic functional connectivity in weakly-connected state in major depressive disorder. Clin Neurophysiol. (2019) 130:2096–104. doi: 10.1016/j.clinph.2019.08.009
67. Hill AT, Hadas I, Zomorrodi R, Voineskos D, Farzan F, Fitzgerald PB, et al. Modulation of functional network properties in major depressive disorder following electroconvulsive therapy (ECT): a resting-state EEG analysis. Sci Rep. (2020) 10:17057. doi: 10.1038/s41598-020-74103-y
68. Cao KX, Ma ML, Wang CZ, Iqbal J, Si JJ, Xue YX, et al. TMS-EEG: An emerging tool to study the neurophysiologic biomarkers of psychiatric disorders. Neuropharmacology. (2021) 197:108574. doi: 10.1016/j.neuropharm.2021.108574
69. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. (2013) 8:e68910. doi: 10.1371/journal.pone.0068910
70. Schwippel T, Papazova I, Strube W, Fallgatter AJ, Hasan A, Plewnia C. Beneficial effects of anodal transcranial direct current stimulation (tDCS) on spatial working memory in patients with schizophrenia. Eur Neuropsychopharmacol. (2018) 28:1339–50. doi: 10.1016/j.euroneuro.2018.09.009
Keywords: post-stroke patients, transcranial direct current stimulation (tDCS), functional near-infrared spectroscopy (fNIRS), cognitive impairment (CI), cortical activation, functional connectivity
Citation: Yang C, Zhang T, Huang K, Xiong M, Liu H, Wang P and Zhang Y (2022) Increased both cortical activation and functional connectivity after transcranial direct current stimulation in patients with post-stroke: A functional near-infrared spectroscopy study. Front. Psychiatry 13:1046849. doi: 10.3389/fpsyt.2022.1046849
Received: 17 September 2022; Accepted: 14 November 2022;
Published: 07 December 2022.
Edited by:
Zhong Zheng, Sichuan University, ChinaReviewed by:
Lingyun Zeng, Shenzhen KangNing Hospital, ChinaJie Yang, Second Xiangya Hospital, Central South University, China
Lian Duan, Shenzhen University, China
Copyright © 2022 Yang, Zhang, Huang, Xiong, Liu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pu Wang, aHhrZndwQDE2My5jb20=; Yan Zhang, emhhbmd5YW4xOTgxQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work
 Caihong Yang
Caihong Yang Tingyu Zhang
Tingyu Zhang Kaiqi Huang3
Kaiqi Huang3 Menghui Xiong
Menghui Xiong Pu Wang
Pu Wang Yan Zhang
Yan Zhang