- 1Department of Psychiatry, School of Mental Health, Jining Medical University, Jining, China
- 2Department of Psychiatry, Shandong Daizhuang Hospital, Jining, Shandong Province, China
- 3Department of Cardiology, Jining No.1 Hospital, Jining, Shandong Province, China
Introduction: Schizophrenia is regarded as one of the most severe, disabling, and costly mental illnesses. Hence, early effective prevention and treatment are critical to the prognosis of patients. Amisulpride, a first-line atypical antipsychotic medication that acts as a blocker of the D2 and D3 dopamine receptors, is used in varying doses for the treatment of both positive and negative symptoms of schizophrenia. Reversible amisulpride-induced elevation of the myocardial enzyme spectrum with bradycardia is a rare condition.
Case presentation: We report a 26-year-old patient diagnosed with first-episode schizophrenia. This patient was treated with amisulpride (400 mg/d), but no clinical benefits were obtained. Meanwhile, amisulpride caused elevation of the myocardial enzyme spectrum with asymptomatic bradycardia. After stopping the medication, these parameters normalized.
Conclusion: We described a rare side reaction of amisulpride. Psychiatrists should take this side effect seriously in the clinical setting. The mechanism of this adverse reaction warrants further investigation and debate. When this side effect occurs during treatment, reducing the dosage of amisulpride and subsequently discontinuing medication, along with monitoring the electrocardiogram and serum myocardial enzymes, may be the most appropriate treatment protocol.
Introduction
Schizophrenia is identified as one of the most severe, incapacitating, and expensive mental illnesses, affecting up to 1% of the population (1). Schizophrenia is characterized by a cluster of positive or negative symptoms and deterioration in social, work, or interpersonal relationships. As a globally critical health concern, it still needs more research funding to investigate its causes, prevention, and treatment. Amisulpride, an atypical antipsychotic, selectively blocks the D2/D3 receptor, making it a first-line of medication for schizophrenia (2, 3). It has been proven that amisulpride has a significant effect on the positive symptoms of schizophrenia and a modest efficacy in treating negative symptoms (4–6). For the safety of the medication, it is essential to monitor the adverse effects of amisulpride, such as hyperprolactinemia, extrapyramidal side effects, and QT prolongation (6, 7). In this case, we report a rare side reaction during treatment, the reversible amisulpride-induced elevation of CK-MB and bradycardia.
Case presentation
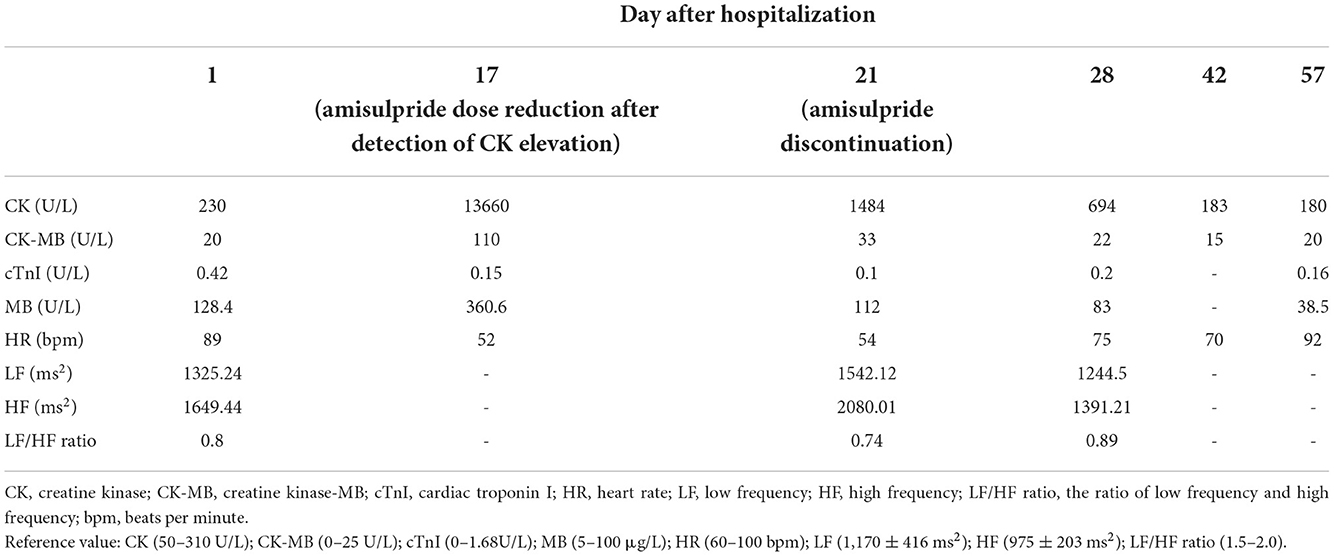
A 26-year-old male diagnosed with first-episode schizophrenia had predominant negative symptoms for nearly 6 years without anti-psychotic medication because of stigma and family neglect of his symptoms until his first visit to our hospital in February 2022. The patient, from a rural family of middle socio-economic status, had been unemployed since he graduated from high school. Due to lack of insight, the patient did not seek any medical care by himself. When the patient's family realized that the patient had psychiatric symptoms, they brought the patient to our hospital for treatment. The main clinical manifestations of this patient are affective blunting, alogia, avolition, attentional impairment, and delusion of reference. The major differential diagnosis was major depression. The diagnosis of major depressive disorder was excluded because this patient with markedly impaired social functioning had no obvious depressed mood or anhedonia, and his clinical course was persistent over 6 years. Physical examination, electrocardiogram, and routine blood tests were performed on admission with no abnormal findings. After admission, routine examinations were applied to rule out organic disease and improve clinical medication safety. The cranial computed tomography and electroencephalogram did not reveal any abnormalities. Furthermore, laboratory examination showed normal renal, liver, and thyroid functions. Serum electrolytes, blood glucose and lipid levels, and serum myocardial enzymes were also within normal limits. The patient was treated with amisulpride 400 mg/d. After 2 weeks of treatment, routine serum laboratory tests showed that high creatine kinase of MB (CK-MB) (104 U/L), serum creatine kinase (CK) (13660 U/L), lactate dehydrogenase (LDH) (387 U/L), and myoglobin (Mb) (360.6 ug/L) with normal levels of cardiac troponin I (cTnI). Furthermore, the patient had significantly high levels of aspartate aminotransferase (AST) (146 U/L) and alanine aminotransferase (ALT) (71 U/L). And when the ECG revealed bradycardia (52 beats per minute), the Holter monitor showed high-frequency heart rate variability (HF-HRV) (2081.01 ms2) and low-frequency heart rate variability (LF-HRV) (1542.12 ms2) increased significantly compared to the baseline HF-HRV (1649.44 ms2) and LF-HRV (1325.24 ms2), while the LF/HF ratio (0.74) decreased than the baseline (0.80) (Table 1). There were no reports of discomfort, such as chest pain or myalgia, and echocardiography revealed no abnormalities. Moreover, the patient had no medical history or family history of cardiovascular disease. Because of the poor therapeutic efficacy, bradycardia, and CK-MB elevation, the medicine was reduced (200 mg/d) on the 17th day after admission and discontinued on the 21st day after admission. After that, the level of the myocardial enzyme spectrum decreased and eventually recovered to the normal range. Furthermore, due to low overall efficacy and the adverse effect of amisulpride, the treatment scheme was modified to include aripiprazole medication. After the aripiprazole medication, this patient's serum myocardial enzymes and heart rate were within the normal range (Table 1). The negative symptoms of this patient improved significantly and remained stable.
Discussion
It is well-known that amisulpride has several cardiac side effects. A preclinical study showed that amisulpride induced cardiac lesions in rabbits, such as necrosis and deformation of the nuclei (8). Qu et al. (9) indicated that amisulpride could affect cardiac functions, and the changed levels of LDH and CK were related to the plasma concentration of amisulpride. According to a recent meta-analysis, the common cardiac side effect of amisulpride is QTc prolongation (7). Moreover, He et al. (10) illustrated that amisulpride performed strong cardiotoxicities, such as cardiomyopathy, QTc prolongation/Torsade de Pointes and cardiac arrhythmia, among ten antipsychotics (clozapine, olanzapine, etc.) based on the analysis of side effect records on the Adverse Event Reporting System database. And there were several case reports that demonstrated amisulpride could induce bradycardia (11, 12) and hypertension (13) as well as elevation of CK (14, 15).
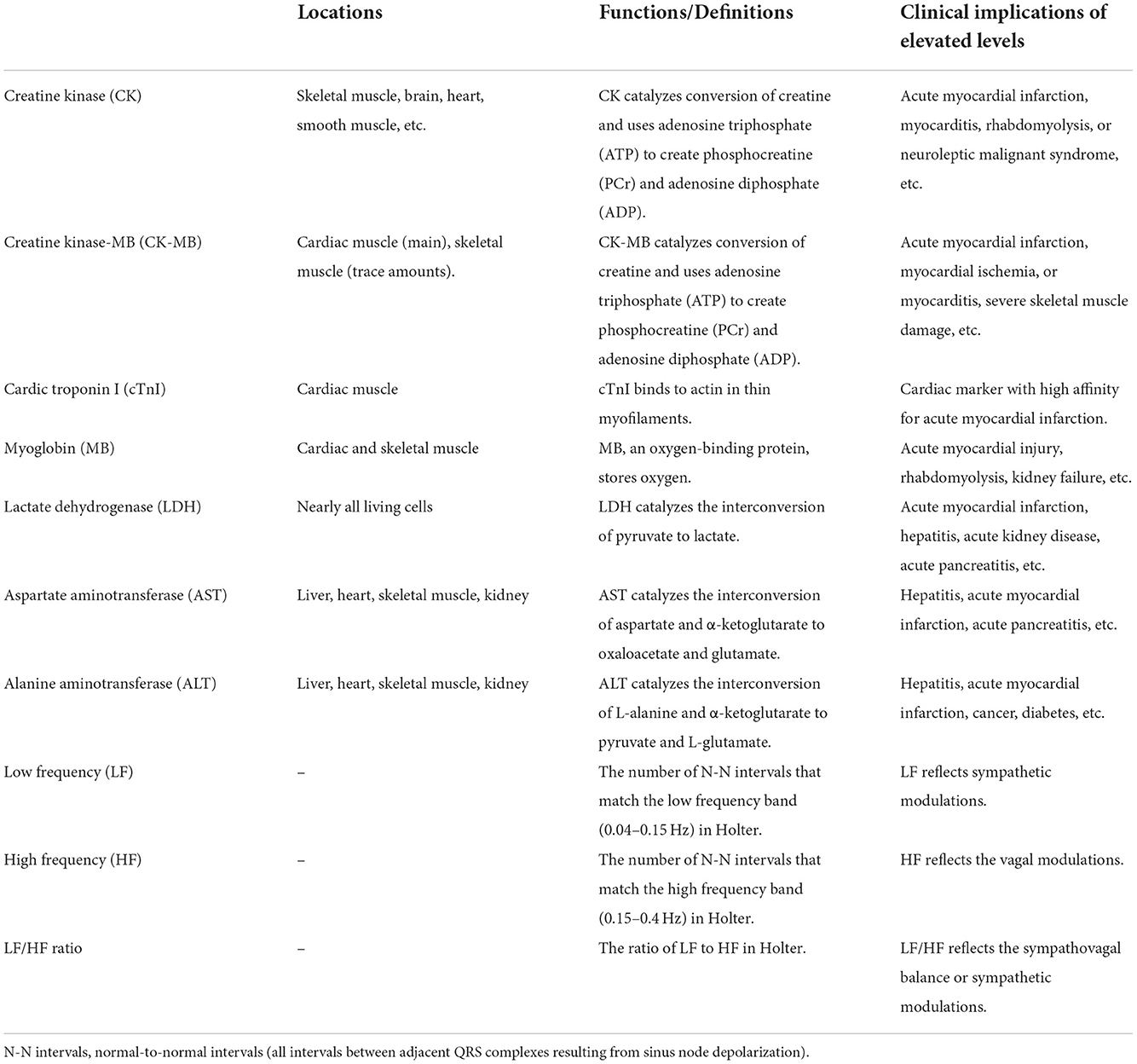
We experienced a rare case of a patient who had a reversible elevation of CK-MB accompanied by bradycardia. Naranjo Scale, a method designed by Naranjo et al. for estimating the probability of adverse drug reactions, was applied to assess the relationship between amisulpride and this rare side reaction (16). As the Naranjo Scale score was 6, this side effect was probably related to amisulpride. This case has several explanations, but none fully explain this phenomenon. First, CK-MB, an isozyme of CK, mainly exists in cardiac muscle. And there are trace amounts of CK-MB in skeletal muscle (Table 2). CK-MB and cTnI as cardiac markers assist in diagnoses of myocardial infarction (17). Considering the normal level of cTnI, the evidence of myocardial injury is insufficient (18). However, because of the CK-MB elevation, the functional abnormality of the cardiocyte remains conceivable. Secondly, the neuroleptic malignant syndrome is excluded due to the absence of related symptoms including fever and rigid muscles (19). Thirdly, based on the high level of CK (13,660 U/L), a diagnosis of asymptomatic rhabdomyolysis should be considered, though without myoglobinuria, electrolyte abnormalities, or acute kidney injury. The exact pathophysiology of the antipsychotic-induced CK-MB and CK elevation is not known. It may be connected with the low-effect central action on dopamine receptors in the striatum or direct toxic effects on the sarcolemma (20, 21). The increased HF-HRV and decreased LF/HF ratio suggested that the activation of the brain's muscarinic cholinergic system by amisulpride may have a role in bradycardia (Table 2) (22). The patient rejected additional testing, electromyography, and musculoskeletal biopsy. Therefore, the diagnosis and differential diagnosis is uncertain. Because the amisulpride drug concentration test is not available in our hospital, it is difficult to determine if there is a drug overdose without the patient's plasma level of amisulpride. There appears to be no literature reporting the amisulpride-induced elevation of CK-MB with asymptomatic bradycardia. To our knowledge, there is no report of this adverse reaction on pharmacovigilance databases such as WHO's VigiAccess (23) and Side Effect Resouce 4.1 (SIDER 4.1) (24).
The mechanism of this adverse reaction deserves more investigation and discussion. This rare side effect should be considered in amisulpride intervention. When this side effect occurs during treatment, reducing the dosage of amisulpride and subsequently discontinuing medication, along with monitoring the electrocardiogram and serum myocardial enzymes, may be the most appropriate treatment protocol.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the current study in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Z-RH: conceptualization, data curation, and writing—original draft preparation. Z-ZY: investigation and formal analysis. X-BW: data curation and visualization. H-SC: writing—review and editing. C-XL: supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Graduate Education Reform Research Project of Shandong Province (SDYJG19212) and the Key Research and Development Project in Jining (2021YXNS054).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. (2022) 399:473–86. doi: 10.1016/S0140-6736(21)01730-X
2. Barnes TR, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British association for psychopharmacology. J Psychopharmacol. (2020) 34:3–78. doi: 10.1177/0269881119889296
3. Chinese medical association. Guidelines for the Prevention and Treatment of Schizophrenia. Peking University Medical Press (2007). 179 p. https://book.douban.com/subject/2346901/ [accessed November 7, 2022]
4. Bressan RA, Erlandsson K, Jones HM, Mulligan R, Flanagan RJ, Ell PJ, et al. Is regionally selective D2/D3 dopamine occupancy sufficient for atypical antipsychotic effect? An in vivo quantitative [123I] epidepride SPET study of amisulpride-treated patients. AJP. (2003) 160:1413–20. doi: 10.1176/appi.ajp.160.8.1413
5. Leucht S, Crippa A, Siafis S, Patel MX, Orsini N, Davis JM. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. AJP. (2020) 177:342–53. doi: 10.1176/appi.ajp.2019.19010034
6. Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. (2013) 382:951–62. doi: 10.1016/S0140-6736(13)60733-3
7. Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. (2019) 394:939–51. doi: 10.1016/S0140-6736(19)31135-3
8. Belhani D, Frassati D, Mégard R, Tsibiribi P, Bui-Xuan B, Tabib A, et al. Cardiac lesions induced by neuroleptic drugs in the rabbit. Exp Toxicol Pathol. (2006) 57:207–12. doi: 10.1016/j.etp.2005.09.003
9. Qu K, Zhou Q, Tian L, Shen Y, Zhou Z. Amisulpride steady-state plasma concentration and adverse reactions in patients with schizophrenia: a study based on therapeutic drug monitoring data. Int Clin Psychopharmacol. (2022) 37:255–62. doi: 10.1097/YIC.0000000000000420
10. He L, Yu Y, Wei Y, Huang J, Shen Y, Li H. Characteristics and spectrum of cardiotoxicity induced by various antipsychotics: a real-world study from 2015 to 2020 based on FAERS. Front Pharmacol. (2022) 12:815151. doi: 10.3389/fphar.2021.815151
11. Huang LC, Huang LY, Tseng SY, Hou YM, Hsiao CC. Amisulpride and symptomatic bradycardia: a case report. General Hospital Psychiatry. (2015) 37:497. doi: 10.1016/j.genhosppsych.2013.12.005
12. Prikryl R, Ustohal L, Prikrylova-Kucerova H. Amisulpride therapeutic dose-induced asymptomatic bradycardia. Prog Neuro-Psychopharmacol Biol Psychiatry. (2011) 35:290. doi: 10.1016/j.pnpbp.2010.10.006
13. Su CH, Chen CS, Huang MF. Asymptomatic bradycardia and hypotension associated with amisulpride: a case report. Asia-Pacific Psychiatry. (2016) 8:175–175. doi: 10.1111/appy.12226
14. Ursini F, Succurro E, Grembiale A, Arturi F. Acute rhabdomyolysis during treatment with amisulpride and metformin. Eur J Clin Pharmacol. (2010) 66:321–2. doi: 10.1007/s00228-009-0773-x
15. Laoutidis ZG, Konstantinidis A, Grohmann R, Luckhaus C, Mobascher J, Cordes J. Reversible amisulpride-induced elevation of creatine kinase (CK): a case series from the German AMSP pharmacovigilance project. Pharmacopsychiatry. (2015) 48:178–81. doi: 10.1055/s-0035-1549997
16. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. (1981) 30:239–45. doi: 10.1038/clpt.1981.154
17. Lee TH. Serum enzyme assays in the diagnosis of acute myocardial infarction recommendations based on a quantitative analysis. Ann Intern Med. (1986) 105:221. doi: 10.7326/0003-4819-105-2-221
18. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
19. Schneider M, Regente J, Greiner T, Lensky S, Bleich S, Toto S, et al. Neuroleptic malignant syndrome: evaluation of drug safety data from the AMSP program during 1993-2015. Eur Arch Psychiatry Clin Neurosci. (2020) 270:23–33. doi: 10.1007/s00406-018-0959-2
20. Laoutidis ZG, Kioulos KT. Antipsychotic-induced elevation of creatine kinase: a systematic review of the literature and recommendations for the clinical practice. Psychopharmacology. (2014) 231:4255–70. doi: 10.1007/s00213-014-3764-2
21. Berman BD. Neuroleptic malignant syndrome. The Neurohospitalist. (2011) 1:41–7. doi: 10.1177/1941875210386491
22. Electrophysiology TF of the ES of C the NA. Heart Rate Variabil Circ. (1996) 93:1043–65. doi: 10.1161/01.CIR.93.5.1043
23. VigiAccess. Available online at: https://vigiaccess.org/ (accessed November 5, 2022).
Keywords: schizophrenia, serum myocardial enzyme spectrum, amisulpride, bradycardia, case report
Citation: Hu Z-R, Yang Z-Z, Wang X-B, Chu H-S and Liu C-X (2022) Case report: Amisulpride therapy induced reversible elevation of creatine kinase-MB and bradycardia in schizophrenia. Front. Psychiatry 13:1037738. doi: 10.3389/fpsyt.2022.1037738
Received: 06 September 2022; Accepted: 24 November 2022;
Published: 14 December 2022.
Edited by:
Markus M. Kosel, Hôpitaux Universitaires de Genève (HUG), SwitzerlandReviewed by:
Valentin Matei, Carol Davila University of Medicine and Pharmacy, RomaniaMichał Seweryn Karbownik, Medical University of Lodz, Poland
Copyright © 2022 Hu, Yang, Wang, Chu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan-Xin Liu, bGl1Y2h1YW54aW5AbWFpbC5qbm1jLmVkdS5jbg==
 Ze-Rui Hu
Ze-Rui Hu Zhen-Zhen Yang2
Zhen-Zhen Yang2