
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 03 February 2023
Sec. Anxiety and Stress Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.1035469
This article is part of the Research Topic Obsessive-Compulsive Related Disorders (OCRD) Across the Lifespan View all 10 articles
 Michele Di Ponzio1*
Michele Di Ponzio1* Nikos Makris2,3,4,5
Nikos Makris2,3,4,5 Carlotta Tenerini1
Carlotta Tenerini1 Eleonora Grassi1
Eleonora Grassi1 Samuele Ragone1
Samuele Ragone1 Stefano Pallanti1,6*
Stefano Pallanti1,6*Introduction: Repetitive Transcranial Magnetic Stimulation (rTMS) is not only a therapeutic option but also an investigational tool to explore circuits and subjective dimensions in pathological conditions. Obsessive-Compulsive Related Disorders (OCRDs) shared similarities with Substance Use Disorder (SUD), suggesting the involvement of the reward system. This study aimed to verify the efficacy of targeting the reward system with rTMS in OCRDs.
Methods: Patients with trichotillomania, hoarding disorder and skin picking disorder were treated with rTMS over the left DorsoLateral PreFrontal Cortex (DLPFC) at 15 Hz, targeting the reward system via the connection with the nucleus accumbens and the ventral tegmental area. All patients were administered with psychometric scales assessing depression symptoms and severity of OCRDs symptoms at the baseline, at the end of the treatment and a 1-month follow-up.
Results: Analysis of the results showed a reduction in symptom severity at the end of the treatment in all three groups (p < 0.0001) as well as a reduction in depression symptoms (p < 0.01). Improvements at 1-month follow-up were maintained only in younger patients. Indeed, when changes in scores at the follow-up were analyzed separately for younger (<30 years) and older patients (>60 years), the elderly showed again an increase in symptoms severity, suggesting that the stability of TMS effects over time reduces with age, possibly as an effect of age-related reduction in brain plasticity.
Discussion: This study adopted with promising results a protocol (15 Hz over the left DLPFC) targeting the reward system, typically employed in addictions. These results can be in line with the view of OCRDs as behavioral addictions, suggesting the implication of common circuits, such as the reward system, in the mechanisms at the basis of these disorders.
The Diagnostic and Statistical Manual-Fifth Edition [DSM-5; (1)] introduced the new diagnostic category of Obsessive-Compulsive and Related Disorders (OCRDs). It comprises trichotillomania (TTM; hair-pulling disorder), excoriation disorder (skin picking; SPD), obsessive-compulsive disorder (OCD), body dysmorphic disorder (BDD), and hoarding disorder (HD). Obsessions (repeated, upsetting, intrusive thoughts, visions, or desires) and compulsions (ritualized acts performed to relieve discomfort from obsessions) are the key symptoms of OCD (1). HD refers to the difficult in discarding, also worthless, possessions (1). Recurring hair pulling, which causes hair loss, is a defining feature of TTM (1). SP entails regular skin picking, which causes lesions (1).
All these disorders share compulsive behaviors as a cardinal feature, which are also typical of addictions (2). Based on this and other analogies, OCD has been proposed to be considered a behavioral addiction (3). Furthermore, an addiction model of TTM (4) and of SPD (5) has been proposed mainly based on similar clinical manifestations, including compulsivity, diminished inhibitory control, urge or craving state before the engagement in the hair pulling and the hedonic quality of performing hair pulling or skin picking. Furthermore, all compulsive behaviors indicate impaired reward processing, lack of inhibitory control, and cognitive inflexibility (2). Patients with OCD as well as with SPD and TTM (6) showed impaired motor and cognitive inhibitory mechanisms, suggesting impairment of frontostriatal circuitries which regulate inhibitory control (7). At the same time, reward processing dysfunction, which is one of the main feature of addictions (8), has been implicated in the etiology and sustention of SPD and TTM (9), suggesting that the intense craving and pleasure experienced during the behavior could be the result of abnormal brain reward processing (10).
Repetitive Transcranial Magnetic Stimulation (rTMS) has emerged as a valid therapeutic option for the treatment of OCD. Furthermore, its application might work as an investigational tool exploring circuits and subjective dimensions involved in the impulsive-compulsive phenomena. Mainly, four brain areas have been the different targets of rTMS in OCD, as emerged from a literature review (11): the DorsoLateral PreFrontal Cortex (DLPFC), the Supplementary Motor Area (SMA), the OrbitoFrontal Cortex (OFC) and the Anterior Cingulate Cortex (ACC). Positive outcomes have been reported for all the aforementioned targets. This evidence highlights the heterogeneity of OCD. In the specific case of OCRDs, only two studies have reported the effect of rTMS. One study was a case series report (12), in which patients with TTM were treated with low-frequency rTMS over the pre-SMA. Then, a prospective study failed to report the effects of rTMS over the pre-SMA in SPD (13). Concerning hoarding, only one case study reported the efficacy of prefrontal direct current stimulation (14). At our knowledge, no study investigated the effect of TMS in hoarding. Furthermore, no study specifically targeted DLPFC in OCRD, although encouraging results have been shown in OCD both with TMS (11) and direct current stimulation (15, 16). However, controversial results emerged concerning the optimal frequency of stimulation. Different studies have chosen to treat OCD patients with rTMS over the left DLPFC at 10 hz or 20 Hz (17–20). Then, rTMS over left DLPFC at 15 Hz has been previously shown to be effective in addiction to reducing craving and compulsive behaviors (21, 22), due to its involvement in reward circuitries (23, 24). No FDA-approved treatment for OCRD exists.
In light of the addiction hypothesis of OCRD and given the negative results of pre-SMA stimulations, we have proposed rTMS over the left DLPFC at 15 Hz for the treatment of patients with OCRD in our center (Istituto di Neuroscienze, Florence, Italy). In our center, we use rTMS for different disorders and all the data are collected in our databases. Herein, data are reported and analyzed retrospectively, to examine the clinical profile of patients with TTM, SPD, and HD treated with rTMS at 15 Hz over the left DLPFC before, after treatment and at 1-month follow-up, with the aim also to propose the possibility that OCRDs are linked with addictions. Moreover, the potential effect of age was analyzed.
In this retrospective study, clinical data of patients with a diagnosis of Obsessive-Compulsive Related Disorders (SPD, TTM, and HD) according to DSM-5 criteria were extracted from databases containing information on patients of the psychiatric clinic at the Istituto di Neuroscience, Florence (Italy). Patients’ age ranged from 16 to 76 years old. All patients had a history of cognitive-behavioral therapy, but no one was under psychotherapy while treated with TMS. Moreover, all patients were resistant to treatment, based on the operational definition by Pallanti and Quercioli (25). It is important to mention that the database used for the analysis contained only the data of patients who accepted treatment among all the ones to which was proposed during the normal clinical practice: 41 accepted out of 60 to which was proposed (information obtained from the clinic’s internal system). The reason for the ones who did not accept to start the protocol, despite the indication for treatment with rTMS, were the choice for other types of medications or their inability (for personal reasons) to follow the entire cycle of TMS. rTMS was added to ongoing pharmacological treatments. All patients were treated stably for 2 months with Selective Serotonin Reuptake Inhibitors (SSRIs) at a fluoxetine equivalent dosage of 30 mg. Demographical data are reported in Table 1 (see also Supplementary Table 1). After the complete description of the study to participants, written informed consent was obtained from each one for the inclusion of their data in this study.
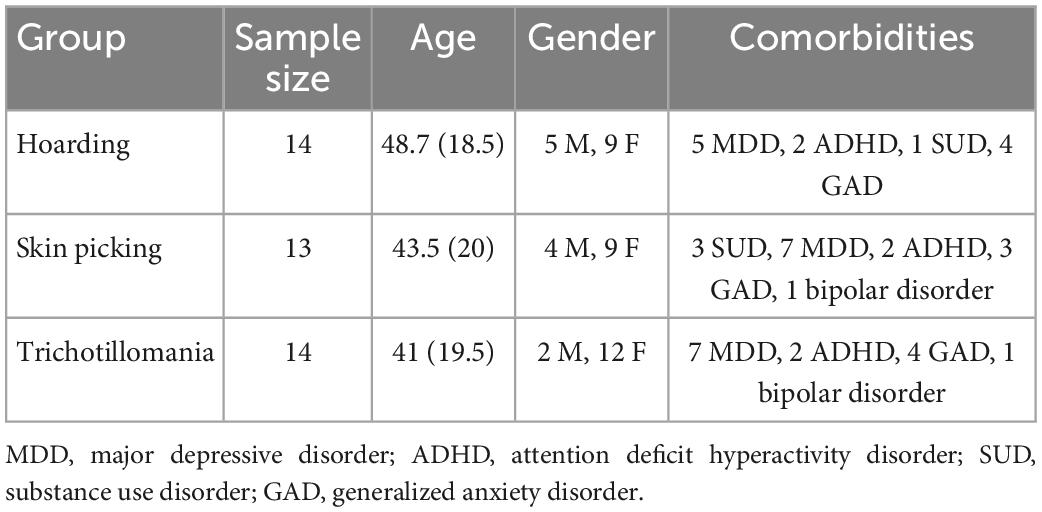
Table 1. Descriptive statistics for each group, reporting sample size, age mean and standard deviation, and male/female ratios.
Repetitive Transcranial Magnetic Stimulation was administered with the Magstim Rapid Stimulator (Magstim Company Ltd., Whitland, UK) using a 70-mm, 8-shaped coil. Stimulation parameters were 15 Hz, 2,400 pulses/day at 100% of resting motor threshold (MT), once a day, 6 days/week for 4 weeks (24 sessions total). Stimulation was applied on the left DLPFC, identified for each subject through neuronavigation. Resting MT was defined as the minimum magnetic flux needed to elicit a response in a resting target muscle (abductor pollicis brevis) in 5/10 trials using single-pulse TMS administered to the contralateral primary motor cortex.
Baseline assessments were performed before the first rTMS session and repeated at the end of the treatment. Follow-up assessments were performed 1 month after the end of the treatment. The assessment has been performed by a panel of trained raters but blind to the treatment administered.
The Massachusetts General Hospital Hair pulling Scale (MGH) (26) assesses the frequency, intensity, and distress of trichotillomania behavior. It consists of seven items with a maximum score of 28. A score between 0–7 refers to subclinical symptomatology, between 8–14 to mild symptomatology, between 15–21 to moderate symptomatology, and between 22–28 to severe symptomatology. Since the questionnaire was not available in the Italian, two independent native Italian speakers fluent in English translated the original scale into Italian. This translated version was then translated back into English by two separate native English speakers who were also fluent in Italian. No significant differences were found between the original and the newly translated version. The Cronbach’s alpha of the Italian version of the scale administered here was 0.89, indicating excellent internal consistency.
The Hoarding Rating Scale-Interview [HRS-I; (27)] is a 5-item semi-structured interview that assesses clutter, difficulty discarding, acquiring, distress, and impairment. Each item is rated on a 9-point scale from 0 to 8, and the item scores are summed to create a total score (range = 0–40). A score higher than 14 is associated with significant impairment in daily life due to difficulty discarding. The Italian version, validated by Faraci et al. (28) was used.
The Yale-Brown Obsessive Compulsive Scale Modified for Neurotic Excoriation (NE-YBOCS) is valid and reliable scale used to evaluate the severity of SPD. Responses to the 10 items were coded on a 4-point scale and summed to produce a composite score ranging from 0 to 40, with higher scores reflecting greater illness severity. Since the questionnaire was not available in the Italian, two independent native Italian speakers fluent in English translated the original scale into Italian. This translated version was then translated back into English by two separate native English speakers who were also fluent in Italian. No significant differences were found between the original and the newly translated version. The Cronbach’s alpha of the Italian version of the scale administered here was 0.92, indicating excellent internal consistency.
The Italian version of the Symptoms of Depression Questionnaire [SDQ; (29)] was used in this study. It is a 44-item, Likert-type, self-report scale developed for measuring symptom severity across several subtypes of depression. SDQ encloses five subscales, investigating the following dimensions: lassitude, mood, cognitive/social functioning; anxiety, agitation, anger and irritability; the desire to be dead; disruptions in sleep quality; changes in appetite and weight.
The baseline demographic and clinical characteristics of the sample were tabulated with descriptive statistics. Parametric (t-test) and non-parametric (Wilcoxon) tests were used according to variables’ distribution (tested with the Shapiro-Wilk test) to analyze changes in scores over time and to compare scores at the baseline between those who accepted to be treated with TMS and those who refused. A regression analysis (Pearson’s correlation) was used to test the effect of age and to verify whether the change in symptoms severity (score of each symptomatologic scale) was dependent to the change in SDQ scores between the pre- and post-treatment. For all statistical analyses, the alpha level of significance was set at 0.05. All the statistical analyses were performed using the statistical programming language R (version 4.0.5) (30).
The study included 41 patients, which were dived into three groups based on the diagnosis. The SPD group consisted of 13 patients (9 females; mean age: 43.5; SD: 20). The TTM group consisted of 14 patients (12 females; mean age: 41; SD: 19.5). The HD group consisted of 14 patients (9 females: mean age: 48.7; SD: 18.5) (see Table 1). Scores statistics are reported in Table 2. For detailed score report, please see Supplementary Tables 2–4.
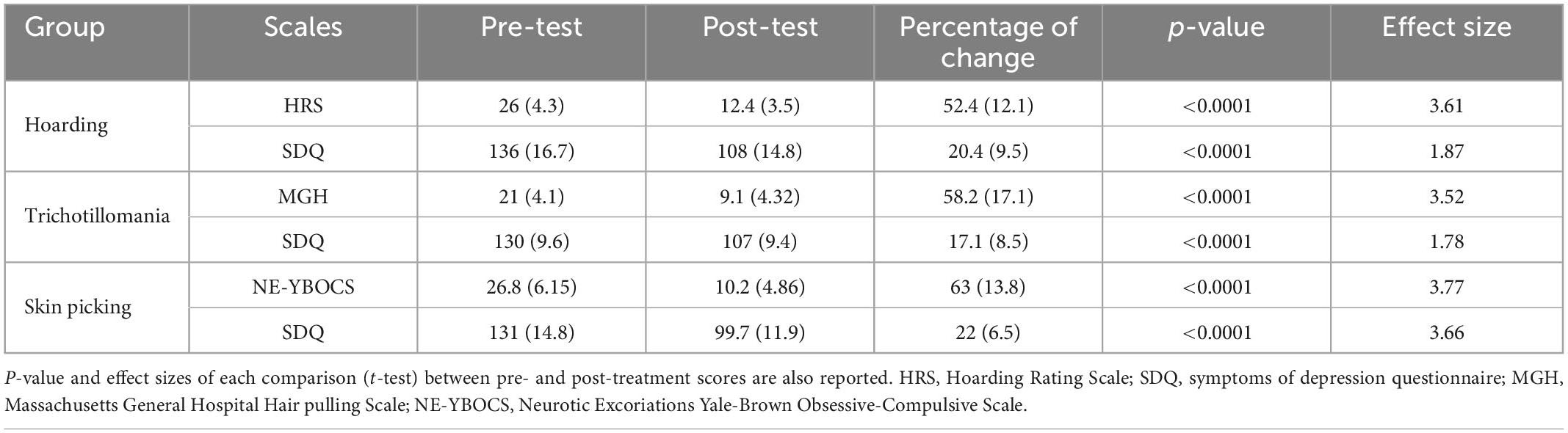
Table 2. Mean scores (and standard deviations) of the psychometrics scale are reported for each group at the pre- and post-treatment timepoints, as well as the percentage of score reduction after treatment.
Baseline scale measures were compared between the 41 patients who accepted to be treated and the ones who refused TMS treatment as well as age distribution, in order to verify whether there were differences between these two groups. No statistically significant differences were found.
As HRS scores in the HD group were normally distributed (verified through the Shapiro–Wilk test), a multiple paired t-test was used to determine whether there were differences in scores between pre-and post-treatment and between post-treatment and follow-up. All patients improved at the end of the treatment (Table 2), with a mean percentage of improvement of 52%. HRS scores before and after treatment were statistically different (p < 0.0001), while there was no statistically significant difference between post-treatment and follow-up scores.
As MGH scores in the TTM group were normally distributed (verified through the Shapiro–Wilk test), a multiple paired t-test was used to determine whether there were differences in scores between pre- and post-treatment and between post-treatment and follow-up. All patients improved at the end of the treatment (Table 2), with a mean percentage of improvement of 58%. MGH scores before and after treatment were statistically different (p < 0.0001), while there was no statistically significant difference between post-treatment and follow-up scores.
As NE-YBOCS scores in the SPD group were normally distributed (verified through the Shapiro–Wilk test), a multiple paired t-test was used to determine whether there were differences in scores between pre- and post-treatment and between post-treatment and follow-up. All patients improved at the end of the treatment (Table 3), with a mean percentage of improvement of 62%. NE-YBOCS scores before and after treatment were statistically different (p < 0.0001), while there was no statistically significant difference between post-treatment and follow-up scores.
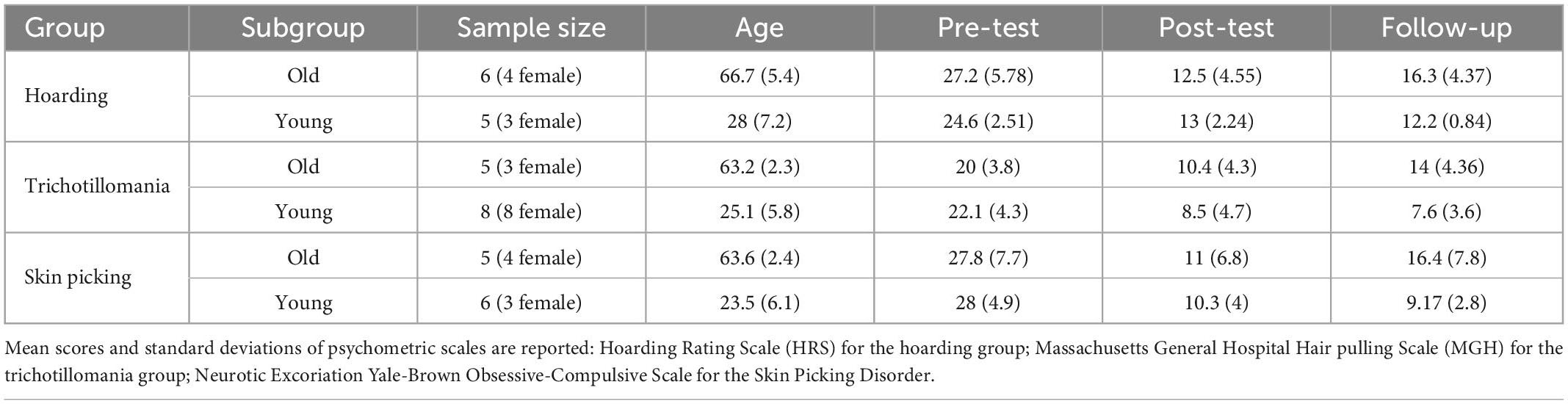
Table 3. Demographical data (sample size, age mean and standard deviation) are here reported for each group (Hoarding, Trichotillomania, and Skin Picking) in the subgroups: older (>60 years of age) vs. younger adults (<35 years of age).
As SDQ scores, for all groups, were normally distributed (verified through the Shapiro–Wilk test), a multiple paired t-test was used to determine whether there were differences in scores between pre-and post-treatment and between post-treatment and follow-up for each group. In the hoarding group, the mean reduction corresponded to 20%; in the TTM group, 17% and in the SPD group, 23%. SDQ scores before and after treatment were statistically different (p < 0.0001), while there was no statistically significant difference between post-treatment and follow-up scores for each group.
Linear regression was performed to verify a potential age effect in all three groups, due to the wide range of ages in this sample. Linear regression was performed before between the score difference between pre- and post-treatment and age, then between the score difference between the post-treatment and the follow-up. Regarding the HD group and TTM group, no age effect was found in the score difference between pre- and post-treatment. While the correlation between age and the difference in scores between post-treatment and follow-up scores was significant with a p-value of < 0.01. Regarding the SPD group, no age effect was found in the score difference between pre- and post-treatment. While the correlation between age and the difference in scores between post-treatment and follow-up scores was significant with a p-value of < 0.001.
To further investigate the effect of age, given the results obtained with the correlation, participants in each group were divided into two subgroups based on their age. The young adult group included patients younger than 35 years of age and the older adults group included patients older than 60 years of age (see Table 3).
The comparison (see Figure 1) between the HRS scores between post-treatment and follow-up was significant only in the old group (p < 0.01). The comparison (see Figure 2) between the MGH scores between post-treatment and follow-up was significant only in the old group (p < 0.01). The comparison (see Figure 3) between the NE-YBOCS scores between post-treatment and follow-up was significant only in the old group (p < 0.05).
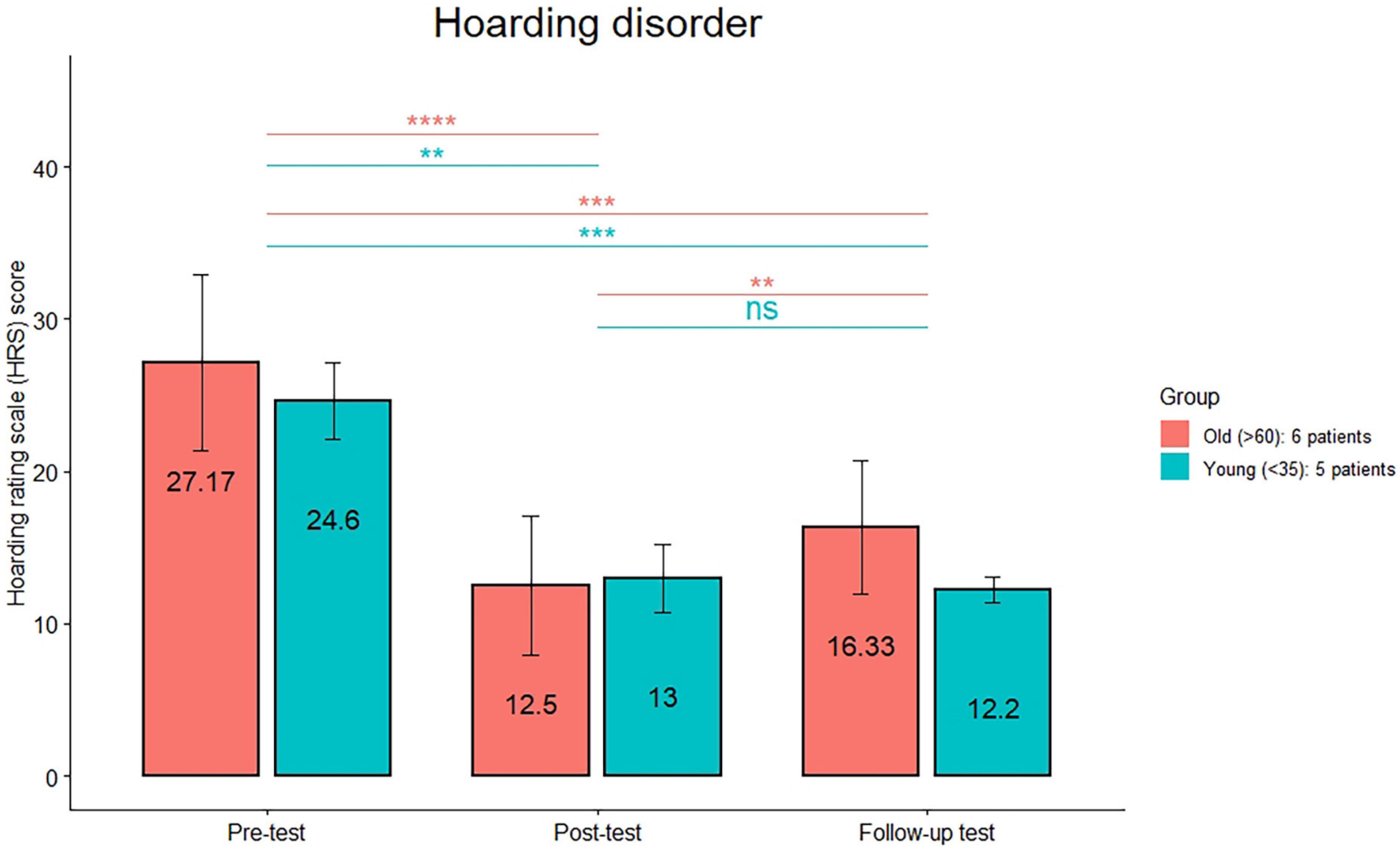
Figure 1. Hoarding Rating Scale mean scores are reported for the young and old subgroups in the hoarding group at the three timepoints (pre-treatment, post-treatment, and 1 month follow-up). ****p < 0.0001; ***p < 0.001; **p < 0.01; n.s.: not significant.
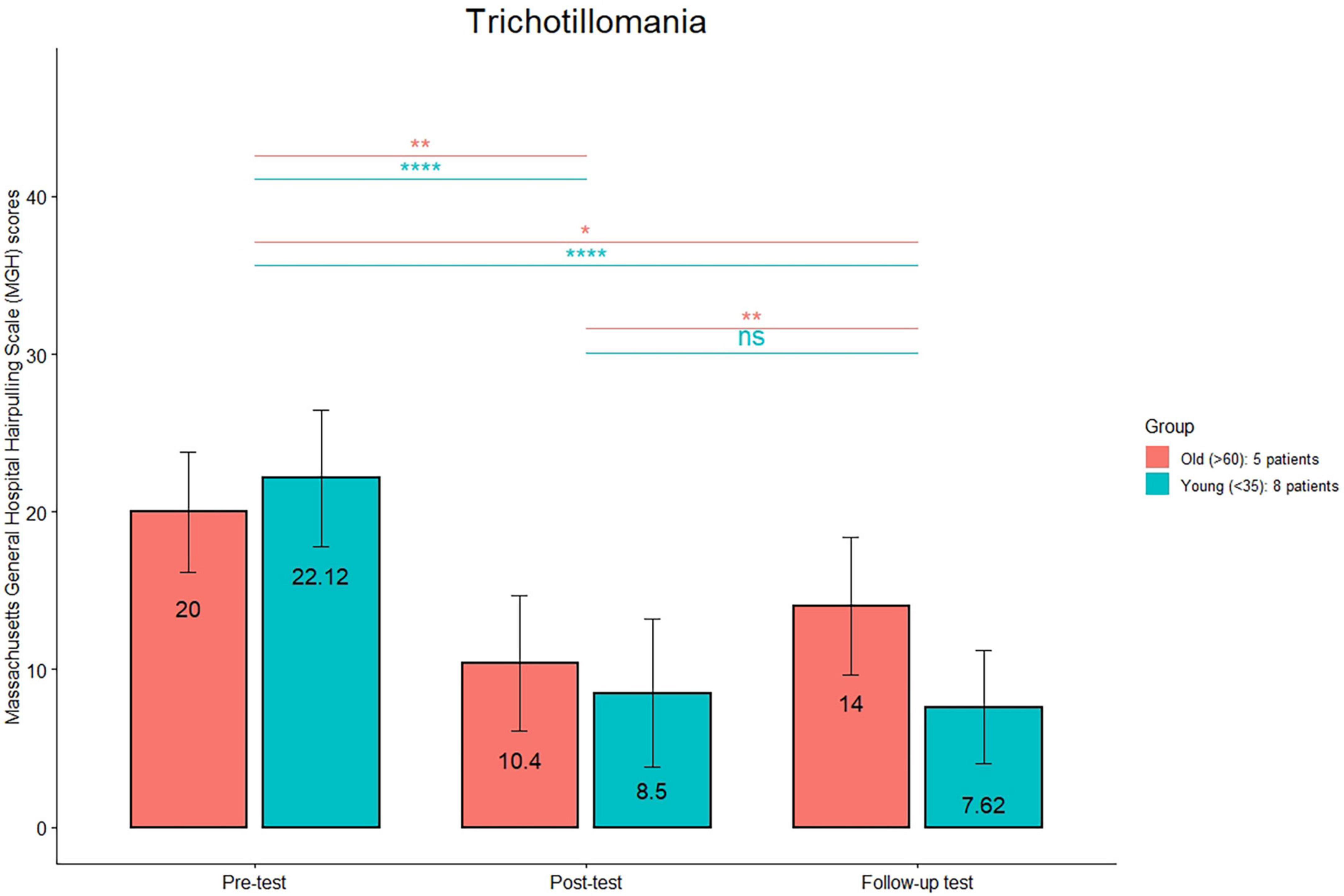
Figure 2. Massachusetts General Hospital Hair pulling Scale (MGH) mean scores are reported for the young and old subgroups in the trichotillomania group at the three timepoints (pre-treatment, post-treatment, and 1 month follow-up). ****p < 0.0001; **p < 0.01; *p < 0.05; n.s.: not significant.
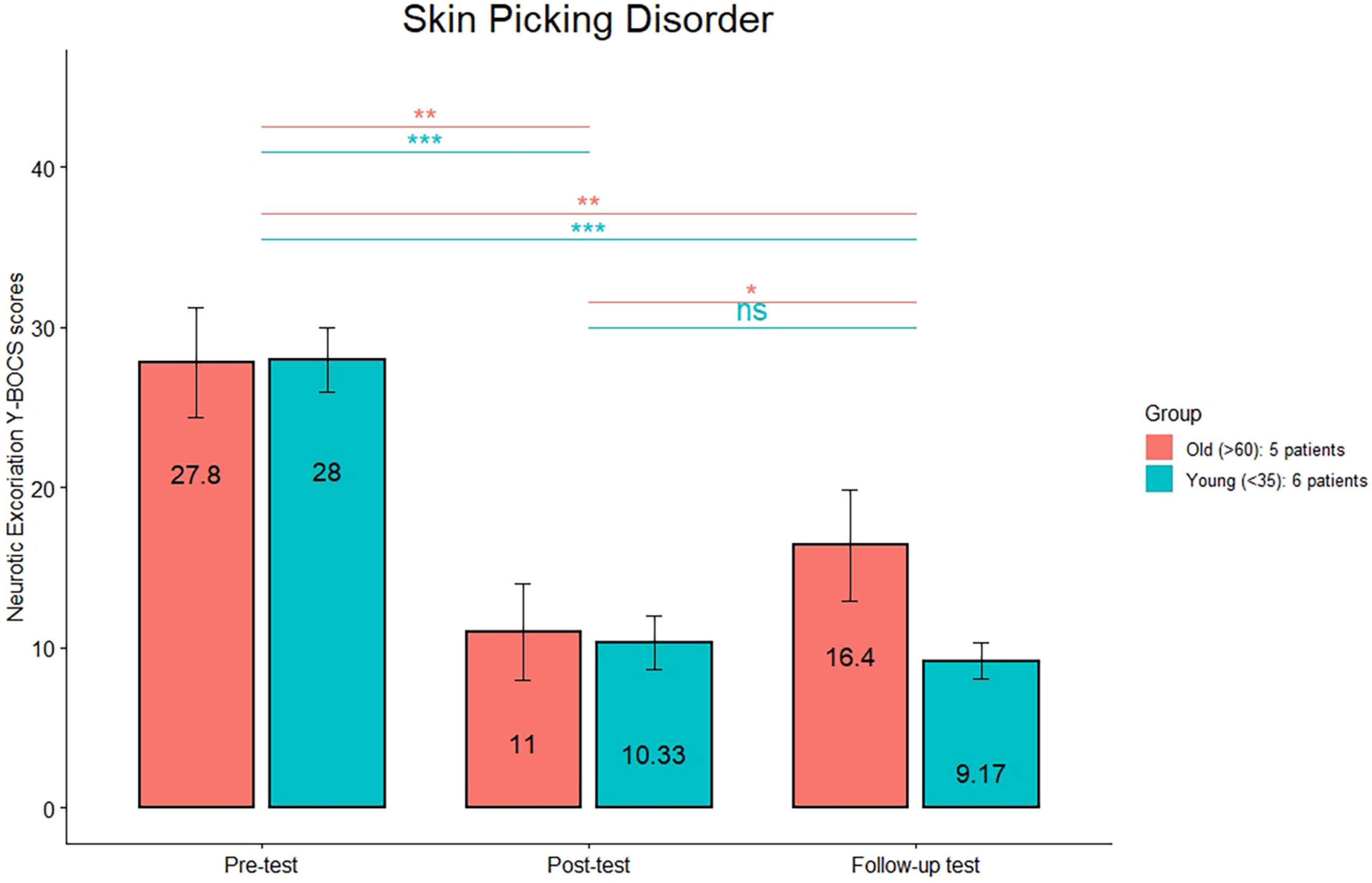
Figure 3. Neurotic Excoriations Yale-Brown Obsessive-Compulsive Scale mean scores are reported for the young and old subgroups in the trichotillomania group at the three timepoints (pre-treatment, post-treatment, and 1 month follow-up). ***p < 0.0001; **p < 0.01; *p < 0.05; n.s.: not significant.
In order to verify whether the improvement in symptoms severity was due to the improvement in comorbid depression, a linear regression was used to assess whether there was a relationship between the change between the pre and post treatment in SDQ scores and the change in HRS, NE-YBOCS, and MGH scores. No significant results were obtained for any measure. Importantly, no side effects were reported by the patients.
This is the first study to report the effects of high-frequency (15 Hz) rTMS over the left DLPFC in OCRD. The main finding of this retrospective study was the positive response of OCRD patients to treatment with a reduction of symptoms severity of more than 35%, which is the conventional threshold to discriminate between respondents and not respondents to treatment in OCD (25). Moreover, an improvement in depression symptoms was also observed. Therefore, given the lack of approved treatments for OCRD and the promising results here reported, this study suggests that this protocol can be a possible treatment for OCRD, that could open a new therapeutic pathway as already occurred in Substance Use Disorder.
There is no consensus on the optimal target and protocol of TMS in OCD. Low-frequency TMS of the supplementary motor cortex has been shown to alleviate OCD symptoms in many but not all studies (11). Studies investigating high-frequency (10 Hz) stimulation over the DLPFC also showed controversial results (11), as well as studies adopting 20 hz frequency stimulations (19, 20). Recently, Khurshid (31) hypothesized that high-frequency rTMS of pre-SMA can reduce OCD symptoms. Here, instead, we tested the efficacy of high-frequency (15 hz) stimulation over left DLPFC. High-frequency 15 HZ rTMS over DLPFC is a treatment for addictions, such as cocaine (21, 22), due to the modulation of activity in subcortical reward circuitry involving the dopaminergic midbrain ventral tegmental area and nucleus accumbens (23, 32). One study provided strong evidence that stimulation of left DLPFC influences the ACC (33), which has a specific role in reward decision-making (34). ACC shows alterations in OCD and also in skin picking (35). Therefore, the positive outcomes here reported in OCRD suggested an implication of reward circuits. It can be hypothesized that, given the positive outcomes of a protocol usually employed for addictions, our results are consistent with the emerging view of OCD as a behavioral addiction (36), a conclusion that could be spread to the entire spectrum. As a matter of fact, people with OCRDs have an high comorbidity rates of addiction (37) and are more likely than controls to have first-degree relatives with Substance Use Disorder (38). Furthermore, double-blind placebo-controlled trials have shown that pharmacological treatments targeting the reward processing by modulating glutamate and dopamine are effective in OCRDs (39). Moreover, the involvement of reward circuitry in OCRDs has been supported by a fMRI study which found alterations in reward circuitry (9).
The hypothesis is that there is a continuum that goes from impulsivity through compulsivity to addiction and the transition to addiction involved a shift from hyperactivation of the ventral striatum to the dorsal striatum (40) and also a progressive loss of top-down, executive control resulting from a loss of PFC and cingulate cortex function (36).
Concerning the controversial results of HF l-DLPFC in OCD (11), it is reasonable to believe that since OCD is an heterogeneous disorder, the individuals who benefited the most from that treatment were the one with features more similar to OCRD. In this sense, they could be clustered into a “reward deficiency group,” adopting the terminology by Lochner et al. (41). Again, this could result in a different neurocircuitry involvement, with a preferential involvement of the complex DLPFC/ACC in “reward deficiency group” and a pre-SMA involvement in the “impulsive” group. Furthermore, it could be that these two groups experience differently their symptoms, with a different level of awareness. The same explanation could apply to the differences in outcomes between the TTM and SPD groups and HD group. The last one showed a percentage of improvement inferior to the ones obtained in the other two groups. Reasonably, HD could be characterized by features, such as the attentional component (42), that may not match perfectly the ones of the “reward deficiency group.”
These results are in line with a multidimensional perspective of OCD (43), which lies in the middle between a lumping and a splitting view. According to the lumping view, OCD is a unitary disorder; while, the splitting perspective claims that different subtypes of OCD exist which all represent different disorders, with different causes and different treatments. But, according to an intermediate view, OCD is a spectrum of overlapping disorders, which have their specificities but share also some similarities. Accordingly, they can share the same neural substrates, such as DLPFC alterations. Although this speculation is beyond the actual implications of this study, the fact that the previous study (13) failed to replicate for SPD the same results that have been obtained for OCD and the fact that instead the study here presented replicated them for DLPFC can mean that the common link could be an alteration of DLPFC. Indeed, considering that compulsive behaviors are a cardinal feature of the OCD spectrum, recently, Fremont et al. (44) have found that reductions in the left DLPFC were associated with the development of compulsive behaviors not accompanied by obsessions. Coherently, in TTM and SPD compulsions are not necessarily triggered by obsessional thoughts, as they are not in the DSM–5 diagnostic criteria (1).
Regarding the other results of this study, no difference was found between scores at the end of the treatment and 1-month follow-up, suggesting that the rTMS effect can last beyond the end of the treatment. Interestingly, when patients were divided into two groups based on their age, differences emerged concerning the maintenance of beneficial effects of rTMS at the follow-up. Indeed, results showed that in older adults symptoms severity at the follow-up worsened again, while in young adults the results were stable over time. Reasonably, this result can be a consequence of a reduction of plasticity in older brains. This result is coherent with other findings (45). For example, in a study with an adult age ranging from 19 to 81 years, Freitas et al. (46) found the duration and magnitude of corticospinal excitability modulation by rTMS were inversely and significantly correlated with age. Furthermore, a recent study by D’Urso et al. (47) found an inverse correlation between age and clinical response to TMS treatment in resistant-depression. These data provide direct experimental evidence that, in humans, long-term plasticity becomes increasingly less efficient with advancing age.
The present study has some limitations, including its retrospective nature, the lack of a control group, addressing the potential placebo effect (although blind raters were involved to minimize the confounding effects) and the low sample size. Furthermore, although the inclusion of a follow-up assessment and the stability of effects in younger participants, it is a relative short term follow-up, considering that Aydin et al. (12) found a re-worsening of symptoms in TTM patients after TMS at a 3-month follow-up. In this sense, we believe that, based on unpublished data in our possession, a monthly follow-up booster session could be helpful in the stability of the effects over time. Furthermore, right DLPFC has also been implicated in reward functioning. We cannot conclude about the potential effect of targeting right DLPFC at high-frequency in OCRDs. Future research should overcome these limitations and should prospectively analyze the effects of rTMS in OCRD over the DLPFC and should also investigate the neuroimaging correlates, in order to corroborate the hypotheses here formulated. Being a naturalistic study, it was not possible to control for comorbidities, such as ADHD, which appeared to be frequent in our sample, as reported in the methods section. ADHD is characterized by frontal dysfunctions, but, as Cardullo et al. (48) reported, ADHD comorbidity with psychiatric disorders did not interfere with rTMS application.
Recognition that neural networks are interconnected and communicate at different levels can facilitate a better understanding of the neurobiological concepts related to psychiatric disorders and also of treatment with rTMS. In the future, targets for rTMS should be no more anatomical but should look at the functional connections of the target. In this sense, the target should be chosen depending on its connectivity (49). Our study points in this direction. Indeed, its positive outcomes acquire sense only by looking at the connections and at the neural networks in which the left DLPFC is involved.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from each participant for the inclusion of their potentially identifiable data in this study.
SP and MD: conceptualization. MD: formal analysis. SP, EG, and MD: investigation. EG, CT, and SR: data curation. SP, MD, EG, CT, SR, and NM: writing—original draft preparation. SP and NM: supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1035469/full#supplementary-material
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Virginia: American Psychiatric Association (2013).
2. Figee M, Pattij T, Willuhn I, Luigjes J, van den Brink W, Goudriaan A, et al. Compulsivity in obsessive–compulsive disorder and addictions. Eur Neuropsychopharmacol. (2016) 26:856–68. doi: 10.1016/j.euroneuro.2015.12.003
3. Holden C. ’Behavioral’ addictions: do they exist? Science. (2001) 294:980–2. doi: 10.1126/science.294.5544.980
4. Grant JE, Odlaug BL, Potenza MN. Addicted to hair pulling? How an alternate model of trichotillomania may improve treatment outcome. Harvard Rev Psychiatry. (2007) 15:80–5. doi: 10.1080/10673220701298407
6. Grant JE, Odlaug BL, Chamberlain SR. A cognitive comparison of pathological skin picking and trichotillomania. J Psychiatr Res. (2011) 45:1634–8. doi: 10.1016/j.jpsychires.2011.07.012
7. Penades R, Catalan R, Rubia K, Andres S, Salamero M, Gasto C. Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry. (2007) 22:404–10. doi: 10.1016/j.eurpsy.2006.05.001
8. Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. (2008) 59:29–53.
9. Grant JE, Peris TS, Ricketts EJ, Bethlehem RA, Chamberlain SR, O’Neill J, et al. Reward processing in trichotillomania and skin picking disorder. Brain Imaging Behav. (2022) 16:547–56. doi: 10.1007/s11682-021-00533-5
10. Roos A, Grant JE, Fouche JP, Stein DJ, Lochner C. A comparison of brain volume and cortical thickness in excoriation (skin picking) disorder and trichotillomania (hair pulling disorder) in women. Behav Brain Res. (2015) 279:255–8. doi: 10.1016/j.bbr.2014.11.029
11. Lusicic A, Schruers KR, Pallanti S, Castle DJ. Transcranial magnetic stimulation in the treatment of obsessive–compulsive disorder: current perspectives. Neuropsychiatr Dis Treat. (2018) 14:1721. doi: 10.2147/NDT.S121140
12. Aydin EP, Kenar JG, Tutan A, Akil Özer Ö, Oğuz Karamustafalıoğlu K. Repetitive transcranial magnetic stimulation for treatment of trichotillomania: case series. Clin Psychopharmacol Neurosci. (2020) 18:631–5. doi: 10.9758/cpn.2020.18.4.631
13. Aydin EP, Kenar JG, Altunay IK, Kaymak D, Özer ÖA, Karamustafalioglu KO. Repetitive transcranial magnetic stimulation in the treatment of skin picking disorder: an exploratory trial. J ECT. (2020) 36:60–5. doi: 10.1097/YCT.0000000000000616
14. Handrack M, Voderholzer U, Schwartz C, Hasan A, Padberg F, Palm U. Prefrontal direct current stimulation in hoarding disorder: a case report. Brain Stimul. (2018) 11:634–5. doi: 10.1016/j.brs.2018.02.001
15. D’Urso G, Mantovani A, Patti S, Toscano E, de Bartolomeis A. Transcranial direct current stimulation in obsessive-compulsive disorder, posttraumatic stress disorder, and anxiety disorders. J ECT. (2018) 34:172–81.
16. Brunelin J, Mondino M, Bation R, Palm U, Saoud M, Poulet E. Transcranial direct current stimulation for obsessive-compulsive disorder: a systematic review. Brain Sci. (2018) 8:37. doi: 10.3390/brainsci8020037
17. De Wit SJ, Van Der Werf YD, Mataix-Cols D, Trujillo JP, Van Oppen P, Veltman DJ, et al. Emotion regulation before and after transcranial magnetic stimulation in obsessive compulsive disorder. Psychol Med. (2015) 45:3059–73. doi: 10.1017/S0033291715001026
18. Sachdev PS, Loo CK, Mitchell PB, McFarquhar TF, Malhi GS. Repetitive transcranial magnetic stimulation for the treatment of obsessive compulsive disorder: a double-blind controlled investigation. Psychol Med. (2007) 37:1645–9. doi: 10.1017/S0033291707001092
19. Greenberg BD, George MS, Martin JD, Benjamin J, Schlaepfer TE, Altemus M, et al. Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary study. Am J Psychiatry. (1997) 154:867–9.
20. Badawy AA, El Sawy H, El Hay MA. Efficacy of repetitive transcranial magnetic stimulation in the management of obsessive compulsive disorder. Egypt J Neurol Psychiatry Neurosurg. (2010) 47:393–7.
21. Lolli F, Salimova M, Scarpino M, Lanzo G, Cossu C, Bastianelli M, et al. A randomised, double-blind, sham-controlled study of left prefrontal cortex 15 Hz repetitive transcranial magnetic stimulation in cocaine consumption and craving. PLoS One. (2021) 16:e0259860. doi: 10.1371/journal.pone.0259860
22. Madeo G, Terraneo A, Cardullo S, Gómez Pérez LJ, Cellini N, Sarlo M, et al. Long-term outcome of repetitive transcranial magnetic stimulation in a large cohort of patients with cocaine-use disorder: an observational study. Front Psychiatry. (2020) 11:158. doi: 10.3389/fpsyt.2020.00158
23. Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. (2011) 31:10340–6. doi: 10.1523/JNEUROSCI.0895-11.2011
24. Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. (2010) 35:4–26.
25. Pallanti S, Quercioli L. Treatment-refractory obsessive-compulsive disorder: methodological issues, operational definitions and therapeutic lines. Progress Neuro Psychopharmacol Biol Psychiatry. (2006) 30:400–12. doi: 10.1016/j.pnpbp.2005.11.028
26. Keuthen NJ, O’Sullivan RL, Ricciardi JN, Shera D, Savage CR, Borgmann AS, et al. The massachusetts general hospital (MGH) hairpulling scale: 1. development and factor analyses. Psychother Psychosom. (1995) 64:141–5. doi: 10.1159/000289003
27. Tolin DF, Frost RO, Steketee G. A brief interview for assessing compulsive hoarding: the Hoarding Rating Scale-Interview. Psychiatry Res. (2010) 178:147–52. doi: 10.1016/j.psychres.2009.05.001
28. Faraci P, Perdighe C, Del Monte C, Saliani AM. Hoarding rating scale-interview: reliability and construct validity in a nonclinical sample. Int J Psychol Psychol Ther. (2019) 19:345–52.
29. Salerno L, Burian I, Pallanti S. A new generation rating scale for depression: reliability and validity of the Italian version of Symptoms of Depression Questionnaire (SDQ), an RDoC-oriented depression comprehensive assessment. J Psychopathol. (2017) 23:160–71.
30. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2021).
31. Khurshid KA. High frequency repetitive transcranial magnetic stimulation of supplementary motor cortex for obsessive compulsive disorder. Med Hypothes. (2020) 137:109529.
32. Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. (2009) 4:e6725. doi: 10.1371/journal.pone.0006725
33. Tik M, Hoffmann A, Sladky R, Tomova L, Hummer A, de Lara LN, et al. Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. (2017) 162:289–96. doi: 10.1016/j.neuroimage.2017.09.022
34. Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. (2002) 99:523–8. doi: 10.1073/pnas.012470999
35. Grant JE, Odlaug BL, Hampshire A, Schreiber L, Chamberlain SR. White matter abnormalities in skin picking disorder: a diffusion tensor imaging study. Neuropsychopharmacology. (2013) 38:763–9. doi: 10.1038/npp.2012.241
36. Lüscher C, Robbins T, Everitt B. The transition to compulsion in addiction. Nat Rev Neurosci. (2020) 21:247–63. doi: 10.1038/s41583-020-0289-z
37. Snorrason I, Belleau EL, Woods DW. How related are hair pulling disorder (trichotillomania) and skin picking disorder? A review of evidence for comorbidity, similarities and shared etiology. Clin Psychol Rev. (2012) 32:618–29. doi: 10.1016/j.cpr.2012.05.008
38. Schlosser S, Black DW, Repertinger S, Freet D. Compulsive buying: demography, phenomenology, and comorbidity in 46 subjects. Gene Hosp Psychiatry. (1994) 16:205–12. doi: 10.1016/0163-8343(94)90103-1
39. Deepmala SJ, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. (2015) 55:294–321. doi: 10.1016/j.neubiorev.2015.04.015
40. Grant JE, Brewer JA, Potenza MN. The neurobiology of substance and behavioral addictions. CNS Spectrums. (2006) 11:924–30. doi: 10.1017/s109285290001511x
41. Lochner C, Hemmings SM, Kinnear CJ, Niehaus DJ, Nel DG, Corfield VA, et al. Cluster analysis of obsessive-compulsive spectrum disorders in patients with obsessive-compulsive disorder: clinical and genetic correlates. Comp Psychiatry. (2005) 46:14–9. doi: 10.1016/j.comppsych.2004.07.020
42. Grassi G, Micheli L, Mannelli LDC, Compagno E, Righi L, Ghelardini C, et al. Atomoxetine for hoarding disorder: a pre-clinical and clinical investigation. J Psychiatr Res. (2016) 83:240–8. doi: 10.1016/j.jpsychires.2016.09.012
43. Mataix-Cols D. Deconstructing obsessive–compulsive disorder: a multidimensional perspective. Curr Opin Psychiatry. (2006) 19:84–9. doi: 10.1097/01.yco.0000194809.98967.49
44. Fremont R, Dworkin J, Manoochehri M, Krueger F, Huey E, Grafman J. Damage to the dorsolateral prefrontal cortex is associated with repetitive compulsive behaviors in patients with penetrating brain injury. BMJ Neurol Open. (2022) 4:e000229. doi: 10.1136/bmjno-2021-000229
45. Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, et al. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. (2011) 24:302–15. doi: 10.1007/s10548-011-0196-8
46. Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, et al. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. (2011) 3:5. doi: 10.3389/fnagi.2011.00005
47. D’Urso G, Dini M, Bonato M, Gallucci S, Parazzini M, Maiorana N, et al. Simultaneous bilateral frontal and bilateral cerebellar transcranial direct current stimulation in treatment-resistant depression—clinical effects and electrical field modelling of a novel electrodes montage. Biomedicines. (2022) 10:1681. doi: 10.3390/biomedicines10071681
48. Cardullo S, Gómez Pérez LJ, Cuppone D, Sarlo M, Cellini N, Terraneo A, et al. A retrospective comparative study in patients with cocaine use disorder comorbid with attention deficit hyperactivity disorder undergoing an rTMS protocol treatment. Front Psychiatry. (2021) 12:659527. doi: 10.3389/fpsyt.2021.659527
Keywords: rTMS (repetitive transcranial magnetic stimulation), Obsessive-Compulsive Related Disorders, OCD (obsessive-compulsive disorder), reward system, brain plasticity, brain stimulation, behavioral addictions
Citation: Di Ponzio M, Makris N, Tenerini C, Grassi E, Ragone S and Pallanti S (2023) rTMS investigation of resistant Obsessive-Compulsive Related Disorders: Efficacy of targeting the reward system. Front. Psychiatry 13:1035469. doi: 10.3389/fpsyt.2022.1035469
Received: 02 September 2022; Accepted: 28 December 2022;
Published: 03 February 2023.
Edited by:
Katarzyna Prochwicz, Jagiellonian University, PolandReviewed by:
Ghazal Jahanbakhsh, Golestan Mental Health Clinic, IranCopyright © 2023 Di Ponzio, Makris, Tenerini, Grassi, Ragone and Pallanti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele Di Ponzio,  ZGlwb256aW9taWNoZWxlQGdtYWlsLmNvbQ==; Stefano Pallanti,
ZGlwb256aW9taWNoZWxlQGdtYWlsLmNvbQ==; Stefano Pallanti,  c3RlZmFub3BhbGxhbnRpQHlhaG9vLml0
c3RlZmFub3BhbGxhbnRpQHlhaG9vLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.