- 1Psychiatric Hospital of Ziyang, Ziyang, China
- 2Zigong Affiliated Hospital of Southwest Medical University, Zigong Psychiatric Research Center, Zigong, China
Aim: We investigated the relationship between the sarcopenia-indicating Ishii test scores and pneumonia risk in stable schizophrenia patients.
Methods: This prospective investigation involves schizophrenic inpatients from two mental health centers in western China. Patient baseline information was gathered over 1 month from September 1 to 30 in 2020. All pneumonia-related patient information, including diagnosis and treatment, was acquired over 1 year between October 2020 and October 2021. Patients with schizophrenia were screened for sarcopenia utilizing a threshold value established by Ishii et al. Using regression analysis, the link between Ishii test scores and pneumonia risk in schizophrenia patients was investigated.
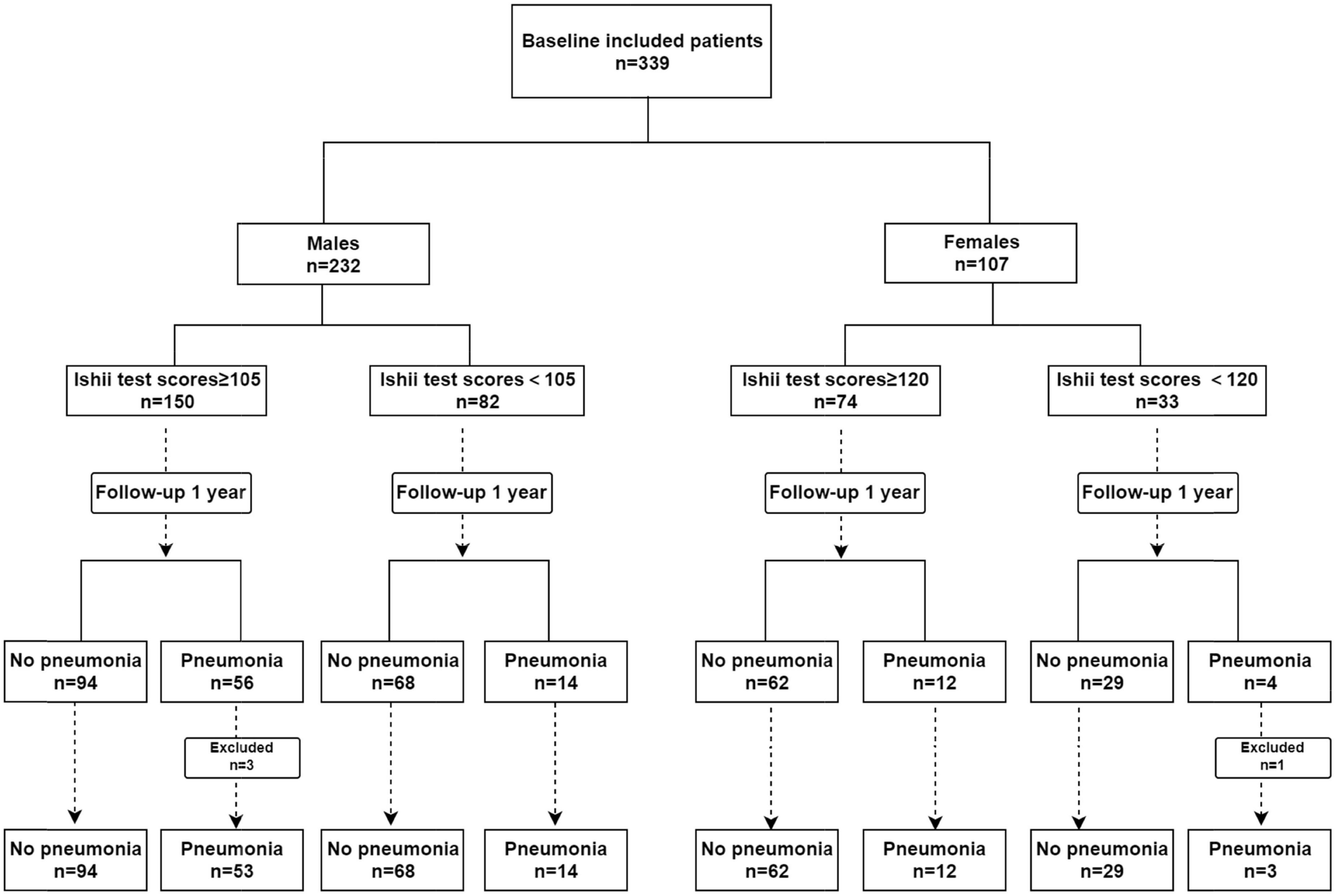
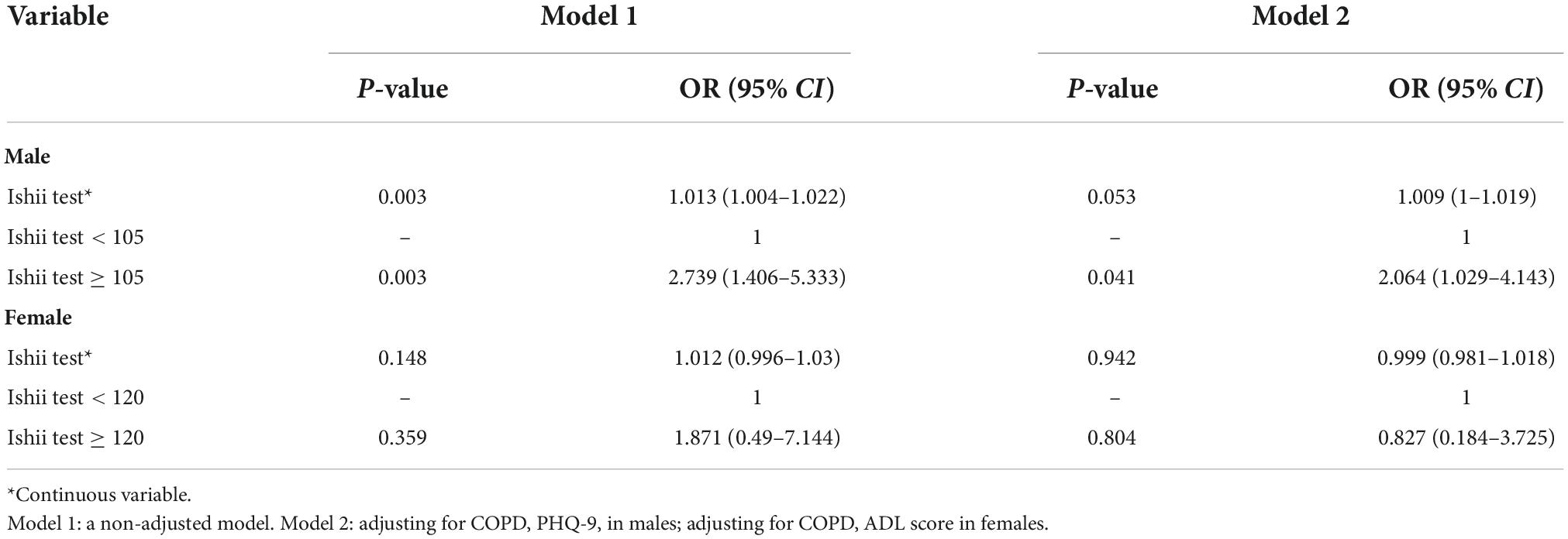
Result: This study recruited 232 males and 107 females with schizophrenia over the age of 50 and older. During a 1-year follow-up period, four patients (3 males and 1 female) acquired pneumonia within 1 week of relapse in schizophrenia; therefore, these patients were excluded from the study. Finally, data were collected for 335 patients. The pneumonia incidences were 29.3% in males and 14.2% in females. Our analysis confirmed that compared to the male schizophrenia patients with Ishii test scores < 105 (non-sarcopenia), those with Ishii test scores ≥ 105 (sarcopenia) exhibited an elevated pneumonia risk (OR = 2.739, 95%CI: 1.406–5.333). Following confounders adjustment, Ishii test scores ≥ 105 remained a risk factor for pneumonia (OR = 2.064, 95%CI: 1.029–4.143). Among females with schizophrenia, the Ishii test scores were not associated with pneumonia risk.
Conclusion: In conclusion, our results demonstrated that the Ishii test scores ≥ 105 were strongly associated with pneumonia risk in stable schizophrenic male patients.
Introduction
Due to schizophrenic patients being hospitalized for long periods, taking antipsychotics, and having a weakened immune system, they have higher risks of other body diseases, including pneumonia (1, 2). A retrospective study showed that the incidence of hospital-acquired pneumonia in patients with schizophrenia aged ≥ 50 years was 7.8% (1). In a Taiwanese study, the incidence of pneumonia in patients with schizophrenia was 1.77 times higher than in patients without schizophrenia (2). Patients with schizophrenia after pneumonia are more likely than those without schizophrenia to have adverse outcomes, such as admission to the intensive care unit, acute respiratory failure, need for mechanical ventilation, and death (2, 3).
Schizophrenic patients usually receive relatively closed treatment in psychiatric hospitals for a long time, and the space is small not to get enough exercise. At the same time, patients take antipsychotic drugs, which have a specific sedative effect; they are often sedentary. Moreover, there is also a lack of professional rehabilitation experts to guide the exercise muscle program. Lack of exercise and being sedentary are risk factors for sarcopenia (4).
Sarcopenia is a progressive and systemic muscle disease (5) that is associated with poor cardiac outcomes (6), respiratory disease (including pneumonia) (7, 8), decreased quality of life (9), and even an increased risk of death (10). Sarcopenia and schizophrenia share some common mechanisms; interleukin (IL)-6 has been implicated in the pathogenesis of sarcopenia (11–13). On the other hand, it has also been implicated in the etiology of schizophrenia due to its ability to cross the blood-brain barrier (14). In addition, human S100B, a small multifunctional protein, is markedly overexpressed in sarcopenia and schizophrenia patients (15). Hence, schizophrenia patients may be more prone to sarcopenia because of specific common mechanisms.
The European Sarcopenia Working Group recommends employing the Ishii test scores for sarcopenia diagnosis (5). The Ishii test scores assess patient age, grip strength, and calf circumference (CC) to identify older patients with sarcopenia. It has a relatively high sensitivity and AUC value (16), with good operability and objectivity, and it is very suitable for institutions without specialized sarcopenia equipment. The Ishii test scores are associated with an increased risk of many clinical adverse outcomes. In a study by Morandi et al. the Ishii test scores were provided to older adults in hospitalized rehabilitation. They revealed that the Ishii test scores-identified sarcopenia patients exhibited an enhanced risk of worse functional status and lower walking ability at discharge (17). Wu et al. also reported that the Ishii test scores-screened sarcopenia strongly correlates with an enhanced risk of cognitive impairment (18). Moreover, the Ishii test scores were successfully employed for the future prediction of undesirable events in patients with heart failure and chronic kidney disease (19, 20). Li et al. demonstrated that the Ishii test scores could predict undesirable outcomes in older patients in clinical practice, including falls, fractures, readmissions, and death (21). Tang et al. also revealed that the Ishii test scores-screened sarcopenia could predict the 3-year mortality of hospitalized older adults (22).
However, no researchers employed the Ishii test scores to estimate the pneumonia risk of schizophrenia patients. In our preliminary study, the Ishii test scores were successfully applied to screen for sarcopenia in schizophrenia patients (4). Therefore, we wanted to examine the association between Ishii test scores and the risk of pneumonia in patients with schizophrenia.
Materials and methods
Study design and patient profiles
This study recruited stable schizophrenia inpatients from two mental health centers in Western China to establish a sarcopenia cohort. Baseline patient information was gathered from 1st to 30th September 2020. Additionally, we collected clinical data from pneumonia patients diagnosed and treated at the aforementioned mental health centers between October 2020 and October 2021. Our baseline inclusion and exclusion criteria were based on a previously published article (4). During the follow-up period, cases were eliminated from analysis if pneumonia developed within 1 week of a change in patient status.
This investigation strictly followed the Declaration of Helsinki and was legitimated by the Institutional Review Board (IRB) of mental institutions in western China (IRB number: 20191001; zjsjsbyy-kyxm-2019-2). Before starting the study, informed consent was obtained from each patient or their legal guardians.
The Ishii test scores
The Ishii test scores were calculated based on age, grip strength, and CC assessments. A digital dynamometer (Camry EH101, El Monte, CA, USA) was employed for patient grip strength measurement. The measurement of CC refers to the measurement methods described in the published articles of our research group (23). All measures were taken by trained personnel from the medical centers where the study was carried out. For the specific calculation method of the Ishii test scores: the male score is calculated as follows: 0.62 × (age – 64) – 3.09 × (grip strength – 50) –4.64 × (CC – 42). The female score is calculated as follows: 0.8 × (age – 64) – 5.09 × (grip strength – 34) – 3.28 × (CC – 42). The diagnostic threshold value of the Ishii test scores followed the recommendations of Ishii et al., and it was as follows: ≥ 105 and ≥ 120 points were the diagnostic cutoffs for male and female sarcopenia, respectively (24).
Outcome indicator
Pneumonia diagnoses are made by clinicians and treated with medication. Antibiotics are the drugs that must be used. Only patients who suffered from pneumonia for the first time during follow-up were selected for this study. All participants received a chest radiograph or/and computed tomography (CT) scan. Pneumonia was described as an acute lung parenchymal inflammation, combined with acute infiltration on a chest radiograph or CT, along with ≥ 2 of the following symptoms: increased body temperature (≥ 38°C), hypothermia (< 36°C), chills, sweating, novel cough, alteration of discharge color, chest discomfort, or difficulty breathing (25).
Covariates
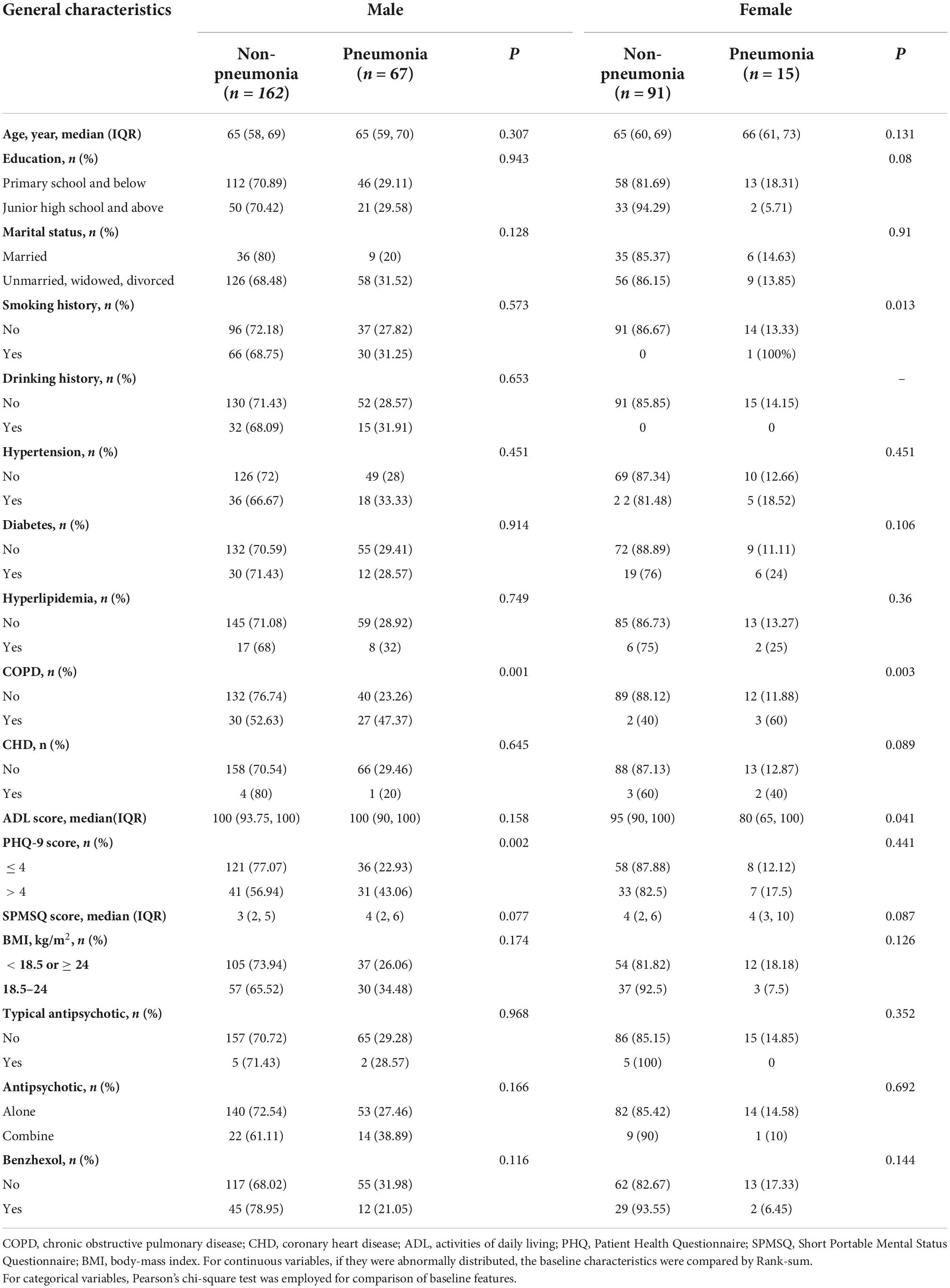
Using the hospital electronic system, we identified schizophrenic patients who underwent hospitalization for over 6 months. Next, we collected patient covariate information via personal interviews with patients who fit our inclusion criteria. The collected information was as follows: age, sex, weight, height, and smoking and drinking history. Alcohol intake was described as consuming ≥ 150 g ethanol each week (26). Smoking was described as an active smoking history of > 100 cigarettes, and a smoking history included the past 1 year (26). Also assessed were reports of chronic diseases, such as diabetes, hypertension, coronary heart disease (CHD), chronic obstructive pulmonary disease (COPD), and hyperlipidemia, which were treated as additional covariates in this study. Furthermore, Activities of Daily Living (ADL) score (27), depression (PHQ-9 score, Patient Health Questionnaire-9 score) (28), cognitive ability (SPMSQ, The Short Portable Mental Status Questionnaire) (29), antipsychotic (typical antipsychotic and drug kinds), and benzhexol were also assessed as covariates in our analysis. We constructed two models: Model 1: non-adjusted and Model 2: adjusted for covariates like COPD, PHQ-9 in males, COPD, and ADL score in females. This study included a single factor < 0.05 in model 2 for correction. Although there were differences in the distribution of smoking history among females, the frequency of occurrence was too insignificant to be included for correction.
Statistical analyses
All data analyses employed the SPSS 25.0 software. Two-sided p-values < 0.05 were set as the significance threshold. If continuous variables were normally distributed, they were expressed as mean ± SD; otherwise, they were expressed as median (quartile). The baseline characteristics were compared by Student’s t-test or Rank-sum, respectively. Data were reported as numbers (percentages) for the statistical description of categorical variables, and Pearson’s chi-square test was employed to compare baseline features. Binomial logistic regression analysis determined the relationship between the Ishii test scores and pneumonia risk in schizophrenia patients.
Results
In total, 339 schizophrenia patients aged 50 years and older were enrolled in this study. There were 232 males and 107 females among them. During the 1-year follow-up period, four patients (three males and one female) developed pneumonia within 1 week of schizophrenia relapse; hence, they were eliminated from the analysis. Ultimately, we collected data from 335 patients (Figure 1). The median age of the patients we included was 65, the minimum age was 52, and the maximum age was 81. The incidence of pneumonia was 29.3% among males and 14.2% among females. Among the schizophrenic males, the differences in COPD and the PHQ-9 scores between pneumonia and non-pneumonia cohorts were statistically significant. Among the schizophrenic females, the smoking history, COPD, and ADL scores were statistically significant among pneumonia and non-pneumonia patients (Table 1).
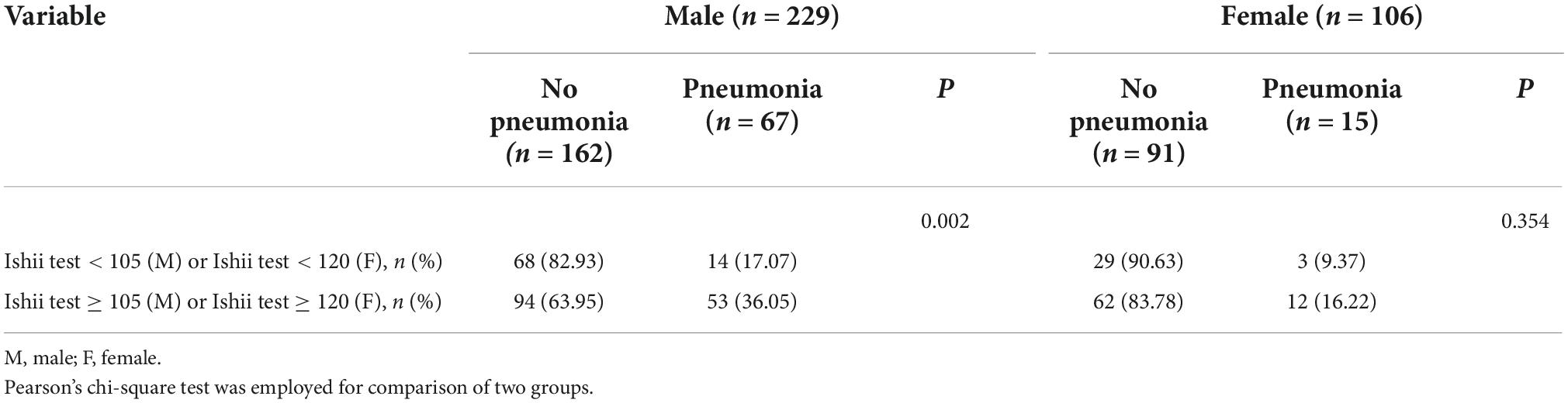
Among the schizophrenic males, patients with pneumonia exhibited a high Ishii test score relative to those without pneumonia (P = 0.003, Figure 2). There was no statistical significance among schizophrenic females (P = 0.148, Figure 2). The schizophrenic males, who were screened for sarcopenia via the Ishii test scores, experienced a higher incidence of pneumonia than those without sarcopenia (sarcopenia vs. non-sarcopenia, 36.05% vs. 17.07%, P = 0.002). Interestingly, the schizophrenic females showed no such statistical significance (Table 2).
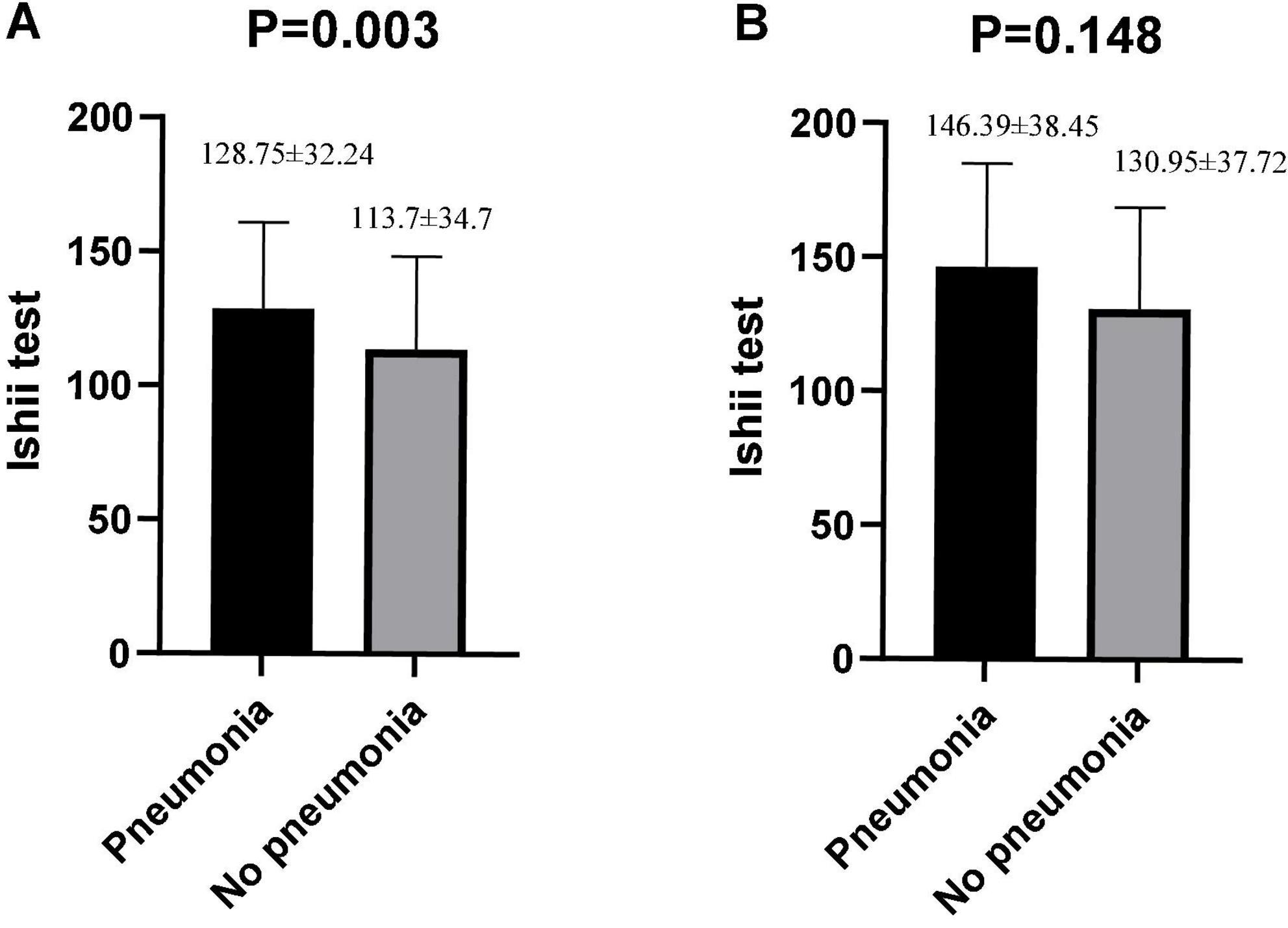
Figure 2. Differences in Ishii test scores between pneumonia and non-pneumonia in males and females. (A) Males. (B) Females. The Student’s t-test was employed for comparison of two groups.
We observed that compared to the male schizophrenic patients with Ishii test scores < 105 (non-sarcopenia), those with Ishii test scores ≥ 105 (sarcopenia) experienced a higher risk of pneumonia (OR = 2.739, 95%CI: 1.406–5.333). We adjusted the model for specific confounders, and an Ishii test score ≥ 105 remained a risk factor for pneumonia (OR = 2.064, 95%CI: 1.029–4.143; Table 3). Among females with schizophrenia, the Ishii test scores were not associated with pneumonia risk (Table 3).
Discussion
In our study, Ishii test scores were linked to pneumonia in males with schizophrenia, but not in females. This is the first study to use the Ishii test scores to predict pneumonia in schizophrenic patients. In this study, physicians used a simple, accurate sarcopenia screening tool to determine the risk of pneumonia among stable male schizophrenia patients. The advantages of using the Ishii test scores results in clinical practice include their accessibility, accuracy, low cost, and non-invasive aspect. This is a useful platform for psychiatric hospitals that lack specialized equipment.
In our study, the Ishii test scores ≥ 105 were considered sarcopenia and were a risk factor for pneumonia in male patients with schizophrenia. The possible mechanisms are as follows: first, we included people 50 years of age and older who had schizophrenia and had decreased immunity (1). If they with sarcopenia, it will aggravate the decline of immune function (30–32). There is a common molecular mechanism (IL-6) between pneumonia and sarcopenia. IL-6 is secreted during infection or tissue damage (33). As a pro-inflammatory molecule, IL-6 enhances T cell recruitment and expansion and stimulates B cells to produce antibodies (33). Wussler et al. also demonstrated that IL-6 levels in emergency department patients with pneumonia were significantly higher than in non-pneumonic patients (34). On the other hand, IL-6 is a component of age-related chronic low-grade inflammation. In addition, IL-6 attenuates muscle anabolism, energy homeostasis, and even directly mediates muscle catabolism, promotes muscle atrophy, and leads to sarcopenia disease (11–13). Second, schizophrenia patients take long-term antipsychotic drugs, which have a certain sedative effect, the patient’s cough reflex is relatively weak; if there were sarcopenia patients, their respiratory muscle strength will be weakened and cannot effectively regulate cough to clear the airway and more prone to pneumonia (7). Third, sarcopenia also can impair swallowing functions, which leads to aspiration pneumonia (7).
In our study, however, sarcopenia only predicted pneumonia risk in schizophrenic males but not females. There may be differences in the development of sarcopenia between males and females due to differences in sex hormones (35). Absolute and relative reductions in muscle mass were greater in aged males (35). Second, the loss of muscle strength in males was greater and faster, and the decline in physical function was more pronounced (35).
The current study has certain limitations. First, we did not collect information on the pneumonia severity, nor did we gather sufficient mortality data to investigate further the association between the Ishii test scores and pneumonia severity or death. However, follow-up studies are continuing, potentially addressing this issue in the future. Second, the cohort size of this study was relatively small and included only a few schizophrenic patients from western China. Therefore, the results cannot be generalized to other ethnicities and countries; hence, a larger patient population is strongly needed for further validation. Third, there are other suggested cutoffs for the Ishii test’s scores (36). However, in this study, other cutoff values were not explored. Finally, we only included schizophrenic patients aged 50 years and older but did not explore the relationship between the Ishii test scores and pneumonia risk in young adults with schizophrenia.
Conclusion
This investigation demonstrated that the Ishii test scores ≥ 105 were strongly related to an enhanced pneumonia risk in stable male schizophrenic patients but not females. Therefore, we recommend the application of the Ishii test scores as a reliable screening marker for pneumonia risk among male stable schizophrenia patients.
Data availability statement
The original contributions presented in this study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Zigong Medical Foundation, the Psychiatric Hospital of Ziyang. The patients/participants provided their written informed consent to participate in this study.
Author contributions
QY, SH, MC, TZ, QL, and XC: study concept and design. QY, TZ, MC, and QL: acquisition of data. XC and SH: analysis and interpretation of data. SH and QY: drafting of the manuscript. XC: critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Psychiatric Hospital of Ziyang Support Project (Project Nos. Zysjsbyy-kyxm-2021-8 and Zysjsbyy-kyxm-2022-6) and the 2021 Key Science and Technology Plan of Zigong City (Project No. 2021YXY12). The funders had no role in the study design, methodology, data collecting, analysis, or writing of this manuscript.
Acknowledgments
We thank all personnel for their contribution to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CC, calf circumference; IRB, Institutional Review Board; CT, computed tomography; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; ADL, Activities of Daily Living; PHQ-9, Patient Health Questionnaire-9; SPMSQ, The Short Portable Mental Status Questionnaire; IL, interleukin.
References
1. Yang M, Li Q, Wang C, Li L, Xu M, Yan F, et al. Influencing factors of hospital-acquired pneumonia infection in the middle-aged and elderly patients with schizophrenia. Front Psychiatry. (2021) 12:746791. doi: 10.3389/fpsyt.2021.746791
2. Chou FH-C, Tsai K-Y, Chou Y-M. The incidence and all-cause mortality of pneumonia in patients with schizophrenia: a nine-year follow-up study. J Psychiatr Res. (2013) 47:460–6. doi: 10.1016/j.jpsychires.2012.12.007
3. Chen Y-H, Lin H-C, Lin H-C. Poor clinical outcomes among pneumonia patients with schizophrenia. Schizophr Bull. (2011) 37:1088–94. doi: 10.1093/schbul/sbq019
4. Chen M, Lei X, Zhu T, Li Q, Chen X. Evaluation of the accuracy of six simple screening tools for sarcopenia in schizophrenic patients. J Nutr Health Aging. (2022) 26:571–5. doi: 10.1007/s12603-022-1799-3
5. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
6. Curcio F, Testa G, Liguori I, Papillo M, Flocco V, Panicara V, et al. Sarcopenia and Heart failure. Nutrients. (2020) 12:211. doi: 10.3390/nu12010211
7. Okazaki T, Ebihara S, Mori T, Izumi S, Ebihara T. Association between sarcopenia and pneumonia in older people. Geriatr Gerontol Int. (2020) 20:7–13. doi: 10.1111/ggi.13839
8. Altuna-Venegas S, Aliaga-Vega R, Maguiña JL, Parodi JF, Runzer-Colmenares FM. Risk of community-acquired pneumonia in older adults with sarcopenia of a hospital from Callao, Peru 2010-2015. Arch Gerontol Geriatr. (2019) 82:100–5. doi: 10.1016/j.archger.2019.01.008
9. Beaudart C, Biver E, Reginster J-Y, Rizzoli R, Rolland Y, Bautmans I, et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for sarcopenia. J Cachexia Sarcopenia Muscle. (2017) 8:238–44. doi: 10.1002/jcsm.12149
10. De Buyser SL, Petrovic M, Taes YE, Toye KR, Kaufman JM, Lapauw B, et al. Validation of the FNIH sarcopenia criteria and SOF frailty index as predictors of long-term mortality in ambulatory older men. Age Ageing. (2016) 45:602–8. doi: 10.1093/ageing/afw071
11. Alemán H, Esparza J, Ramirez FA, Astiazaran H, Payette H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing. (2011) 40:469–75. doi: 10.1093/ageing/afr040
12. Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. (2005) 98:911–7. doi: 10.1152/japplphysiol.01026.2004
13. Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. (2017) 8:1045. doi: 10.3389/fphys.2017.01045
14. Lacina L, Brábek J, Král V, Kodet O, Smetana K. Interleukin-6: a molecule with complex biological impact in cancer. Histol Histopathol. (2019) 34:125–36. doi: 10.14670/HH-18-033
15. Zaręba-Kozioł M, Burdukiewicz M, Wysłouch-Cieszyńska A. Intracellular protein s-nitrosylation-a cells response to extracellular S100B and rage receptor. Biomolecules. (2022) 12:613. doi: 10.3390/biom12050613
16. Locquet M, Beaudart C, Reginster J-Y, Petermans J, Bruyère O. Comparison of the performance of five screening methods for sarcopenia. Clin Epidemiol. (2018) 10:71–82. doi: 10.2147/CLEP.S148638
17. Morandi A, Onder G, Fodri L, Sanniti A, Schnelle J, Simmons S, et al. The association between the probability of sarcopenia and functional outcomes in older patients undergoing in-hospital rehabilitation. J Am Med Dir Assoc. (2015) 16:951–6. doi: 10.1016/j.jamda.2015.05.010
18. Wu B, Lyu YB, Cao ZJ, Wei Y, Shi WY, Gao X, et al. Associations of sarcopenia, handgrip strength and calf circumference with cognitive impairment among Chinese older adults. Biomed Environ Sci. (2021) 34:859–70. doi: 10.3967/bes2021.119
19. Onoue Y, Izumiya Y, Hanatani S, Tanaka T, Yamamura S, Kimura Y, et al. A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int J Cardiol. (2016) 215:301–6. doi: 10.1016/j.ijcard.2016.04.128
20. Hanatani S, Izumiya Y, Onoue Y, Tanaka T, Yamamoto M, Ishida T, et al. Non-invasive testing for sarcopenia predicts future cardiovascular events in patients with chronic kidney disease. Int J Cardiol. (2018) 268:216–21. doi: 10.1016/j.ijcard.2018.03.064
21. Li M, Kong Y, Chen H, Chu A, Song G, Cui Y. Accuracy and prognostic ability of the SARC-F questionnaire and Ishii’s score in the screening of sarcopenia in geriatric inpatients. Braz J Med Biol Res. (2019) 52:e8204. doi: 10.1590/1414-431X20198204
22. Tang T, Wu L, Yang L, Jiang J, Hao Q, Dong B, et al. A sarcopenia screening test predicts mortality in hospitalized older adults. Sci Rep. (2018) 8:2923. doi: 10.1038/s41598-018-21237-9
23. Ren S, Huang S, Chen M, Zhu T, Li Q, Chen X. Association between the mid-upper arm circumference (MUAC) and calf circumference (CC) screening indicators of sarcopenia with the risk of pneumonia in stable patients diagnosed with schizophrenia. Front Psychiatry. (2022) 13:931933. doi: 10.3389/fpsyt.2022.931933
24. Ishii S, Tanaka T, Shibasaki K, Ouchi Y, Kikutani T, Higashiguchi T, et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. (2014) 14(Suppl 1.):93–101. doi: 10.1111/ggi.12197
25. Phua J, See KC, Chan YH, Widjaja LS, Aung NW, Ngerng WJ, et al. Validation and clinical implications of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Thorax. (2009) 64:598–603. doi: 10.1136/thx.2009.113795
26. He X-X, Yuan S-Y, Li W-B, Yang H, Ji W, Wang ZQ, et al. Improvement of Asia-Pacific colorectal screening score and evaluation of its use combined with fecal immunochemical test. BMC Gastroenterol. (2019) 19:226. doi: 10.1186/s12876-019-1146-2
27. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of adl: a standardized measure of biological and psychosocial function. JAMA. (1963) 185:914–9. doi: 10.1001/jama.1963.03060120024016
28. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
29. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. (1975) 23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x
30. Wang P-Y, Xu L-D, Chen X-K, Xu L, Yu YK, Zhang RX, et al. Sarcopenia and short-term outcomes after esophagectomy: a meta-analysis. Ann Surg Oncol. (2020) 27:3041–51. doi: 10.1245/s10434-020-08236-9
31. Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. (2019) 49:381–8. doi: 10.1016/j.ebiom.2019.10.034
32. Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr. (1995) 62:30–9. doi: 10.1093/ajcn/62.1.30
33. Schaper F, Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. (2015) 26:475–87. doi: 10.1016/j.cytogfr.2015.07.004
34. Wussler D, Kozhuharov N, Tavares Oliveira M, Bossa A, Sabti Z, Nowak A, et al. Clinical utility of procalcitonin in the diagnosis of pneumonia. Clin Chem. (2019) 65:1532–42. doi: 10.1373/clinchem.2019.306787
35. Anderson LJ, Liu H, Garcia JM. Sex differences in muscle wasting. Adv Exp Med Biol. (2017) 1043:153–97. doi: 10.1007/978-3-319-70178-3_9
Keywords: Ishii test, sarcopenia, schizophrenia, pneumonia, risk
Citation: Yang Q, Huang S, Chen M, Zhu T, Li Q and Chen X (2022) Association of Ishii test scores with pneumonia in stable schizophrenic subjects. Front. Psychiatry 13:1034905. doi: 10.3389/fpsyt.2022.1034905
Received: 02 September 2022; Accepted: 27 September 2022;
Published: 13 October 2022.
Edited by:
Silvia Giovannini, Catholic University of the Sacred Heart, ItalyReviewed by:
Gabriela Cabett Cipolli, State University of Campinas, BrazilClaudia Loreti, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2022 Yang, Huang, Chen, Zhu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Chen, Mzc5NTMxNzIyQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qin Yang1†
Qin Yang1† Sha Huang
Sha Huang Xiaoyan Chen
Xiaoyan Chen