
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry , 26 October 2022
Sec. Schizophrenia
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.1025414
 Olga Stȩpień-Wyrobiec1,2*
Olga Stȩpień-Wyrobiec1,2* Marta Nowak3
Marta Nowak3 Grzegorz Wyrobiec3
Grzegorz Wyrobiec3 Emilia Morawiec4,5,6
Emilia Morawiec4,5,6 Magdalena Wierzbik-Strońska7
Magdalena Wierzbik-Strońska7 Rafał Staszkiewicz4,8
Rafał Staszkiewicz4,8 Beniamin Oskar Grabarek4,6,9
Beniamin Oskar Grabarek4,6,9Schizophrenia is a chronic, highly individualized disease with many symptoms that can occur with varying severity in different patients. Schizophrenia affects 1% of the population, but occurs in almost 20% of patients after 40 years of age. It should be noted that the next peak in the incidence of schizophrenia occurs at the age of 60 years, affects mostly females, and is closely associated with a high risk of developing memory disorders. Therefore, postadolescent schizophrenia includes two distinct groups of patients: those whose symptoms onset at the age of 45 or 60. The purposes of this literature review were as follows: (1) synthetically characterize the clinical manifestations of schizophrenia; (2) discuss difficulties in the diagnosis of schizophrenia, especially in patients over 40 years of age; (3) discuss the clinical utility of different classes of marker in diagnostic and differentiating schizophrenia from neurodegenerative diseases in elderly people; (4) discuss therapeutic options for schizophrenia, pharmacotherapy, and psychotherapy, emphasizing the role of caregivers of people with psychosis in therapy, in preadolescence and postadolescence schizophrenia. We have tried to primarily discuss the findings of original articles from the last 10 years with an indication of their clinical implications with the issues discussed in the various subsections. Moreover, despite many years of research, no specific, precise algorithm has been developed that can be used in clinical practice during the diagnosis of schizophrenia. For this reason, the diagnosis of schizophrenia is primarily based on an interview with the patient and his family, as well as on the experience of a psychiatrist. It also seems that schizophrenia treatment should be carried out holistically, including pharmacotherapy, psychotherapy, and the support of caregivers of patients who have this psychosis, which increases the achievement of therapeutic success. Finally, we must be aware of the difficulties in diagnosing schizophrenia in the elderly and the need to modify pharmacological treatment. Currently, no guidelines have been developed for the differentiation of negative symptoms in elderly patients with schizophrenia from amotivation/avolition/apathy symptoms in elderly patients with neurodegenerative disorders.
Officially, the term “schizophrenia” was introduced as a new name for the disease entity “dementia praecox,” previously defined. The concept of “dementia praecox” was inspired by the works of Morel, who believed that psychiatric disorders result from the malfunctioning of three mental components: emotions, thinking, and behavior. In addition, he emphasized the genetic and environmental factors underlying these disorders, such as alcohol or drug addiction of parents of the sick patients (1–3). The separation of affective disorders from schizophrenia and thus, the diagnosis, was facilitated by introducing specific antipsychotic drugs into the market. In the 1950s, the first neuroleptic chlorpromazine was created, which marked the beginning of the era of psychotropic drugs. In the last two decades of the 20th century, there has been enormous progress in the neurobiology of schizophrenia due to the development of neuropathological, neuroimaging, pharmacological, biochemical, and genetic studies (4–7).
The incidence of schizophrenia is 1% of the population, but in almost 20% of patients, it appears after the age of 40 years (8, 9). According to data on the Polish population, the mean age at diagnosis is 27 years and the mean age of patients with schizophrenia is approximately 38 years (6). It is worth paying attention that the next peak in the incidence of schizophrenia falls at the age of 60 years, which concerns mainly females and is closely related to the high risk of developing memory disorders. Therefore, postadolescent schizophrenia encompasses two different groups of patients: those who develop symptoms around the age of 45 or 60 (10). Schizophrenia is a chronic, highly individualized disease with many symptoms that may occur with varying intensity in different patients. However, the proper diagnosis is made too late, especially in the elderly, and therefore, appropriate treatment is initiated too late (11).
The etiology of this disease is not entirely clear, and its underlying cause is multifactorial. In addition to genetic determinants, the role of disturbances related to dopaminergic, serotonergic, and glutaminergic transmission has been indicated, which is particularly emphasized as the underlying cause of cognitive disorders (8, 10, 12). Abnormalities in glutamatergic transmission and dysfunction of M1 and M4 muscarinic receptors significantly reduce learning opportunities (13). In the pathogenesis of schizophrenia, several factors are distinguished, such as dysregulation of the hypothalamic-pituitary-adrenal axis, accumulation of insoluble proteins, abnormal integration of white matter detected by fractional anisotropy, and a number of environmental factors, such as drug abuse, for example, amphetamines, methamphetamine, cocaine, and some behavioral factors such as physical and sexual violence, lack of family support, and loneliness, which are associated with an increased risk of suicide (8, 9, 13). Several studies have emphasized the role of interconnection between the thalamic nuclei and cortical areas (e.g., prefrontal and subcortical structures such as the hippocampus, striatum, and amygdala) in the pathogenesis of both positive and negative symptoms and cognitive disorders in schizophrenia. Furthermore, it is emphasized that the same mechanisms are at the root of negative symptoms and cognitive dysfunction, such as dysfunction of glutaminergic transmission, neuroinflammation, and oxidative stress (14, 15). Primary negative symptoms are an integral part of the schizophrenic process and are idiopathic. They are characterized by stability and chronicity, significant resistance to available therapeutic methods, and relatively low incidence, estimated at 15–25% of patients with schizophrenia (16). In clinical practice, it is difficult to distinguish and differentiate them from secondary negative symptoms. The Scale for Assessment of Negative Symptoms (SANS), developed by Nancy Andreasen, is most commonly used for this purpose (16). The scale describes five areas: shallowing of affect, alogia, avolition, apathy, attention deficit disorder, anhedonia, and unsocialization (16). This scale does not initially differentiate between the two types of negative symptoms; therefore, in research papers, the presence of a symptom in all five domains is considered the criterion for diagnosing primary negative symptoms and scoring in the range of 2–5 is considered its presence. Meanwhile, secondary negative symptoms do not arise directly from the disease process but appear due to the co-occurrence of various additional symptoms and factors associated with schizophrenia. Currently, the most important of these are considered positive (psychotic) symptoms, depression, anxiety (psychotic and non-psychotic), side effects of pharmacotherapy, addiction, and social deprivation (16). According to many authors, negative symptoms represent “what the disease took from a person and what is missing.” Symptoms progress slowly, and over time patients distance themselves from the environment and, at the same time, are open to the world of internal experiences, delusions, and hallucinations that are experienced very intensely. There is social withdrawal (autism), limited family and professional relationships, and poor form statements and content (17, 18).
However, it should be noted that the currently used methods for treating schizophrenia have three main limitations. First, their effectiveness, defined as the patient’s ability to lead an independent life, affects only approximately 50% of people with schizophrenia (19). Second, the therapeutic effect they exert is primarily directed at alleviating positive symptoms, improving only to a mild degree negative and cognitive symptoms (20). Third, their use may be associated with severe side effects leading to sexual dysfunction or agranulocytosis (clozapine). The partial effectiveness of pharmacotherapy for schizophrenia results from the pathomechanism of the disease, which is not yet fully understood (21).
Considering the complex etiopathogenesis of schizophrenia, including the difficulty of differential diagnosis of this psychosis with neurodegenerative diseases, especially in the elderly, as well as taking into account the dynamic development of medicine, among others, including non-imaging, the search for new biochemical and molecular markers, as well as the development of personalized, predictive, preventive, and participatory (4P) medicine, the collection and presentation of current knowledge and trends in the diagnosis and therapy of schizophrenia is cognitively interesting and essential for clinical practice.
The purposes of this literature review were as follows: (1) synthetically characterize the clinical manifestations of schizophrenia; (2) discuss difficulties in the diagnosis of schizophrenia, especially in patients over 40 years of age; (3) discuss the clinical utility of different classes of marker in diagnostic and differentiating schizophrenia from neurodegenerative diseases in elderly people; (4) discuss therapeutic options for schizophrenia, pharmacotherapy, and psychotherapy, emphasizing the role of caregivers of people with psychosis in therapy, in preadolescence and postadolescence schizophrenia.
The work we presented was classified as a “narrative literature review.” When looking for articles included in this study and critically reviewing them, we focused on the following keywords: “schizophrenia,” “marker,” “long-acting injectable medications,” “antipsychotic drugs,” “psychotherapy,” “caregiver,” and “elderly.” Therefore, given the nature of our review, there was no explicit and rigorous methodology for selecting articles, as in the case of systematic reviews. The search strategies were based on the experience of the authors of this study. In this article, we conducted an evaluation to indicate the importance of the topic we discussed and the extent to which it has been studied. We attempted to objectively present the current knowledge of the topic under discussion and base it on previously published studies. We have tried to primarily discuss the findings of original articles from the last 10 years with an indication of their clinical implications with the issues discussed in the various subsections. We searched PubMed and Google Scholar to find appropriate references. It should be noted that even in the case of a systematic review, where the selection paradigm of reports used in the target article is specified, this is not a fully objective method. Furthermore, narrative literature reviews in the case of the issue discussed in this study are justified, as it is still unclear what other more detailed questions can be posed and which can be valuably solved through a more precise systematic review (22).
It has been emphasized that people with schizophrenia are also more likely to suffer from several somatic diseases: myocardial infarction, arterial hypertension, chronic obstructive pulmonary disease, chronic bronchitis, emphysema, and lipid disorders, mainly hypertriglyceridemia, diabetes and lung cancer (23). There is a 30% higher 1 year mortality rate after a heart attack in people with schizophrenia compared to those without schizophrenia, mainly due to treatment discontinuation (24, 25). It is also estimated that the risk of attempting suicide in these patients is more than 12 times higher, and the overall mortality of people with schizophrenia is higher in the general population than in people with bipolar disorder, anxiety, and depression (26, 27). It is assumed that, on average, this disease is associated with an approximately 20 years shorter survival time (24).
Undoubtedly, we live in times when societies worldwide are aging gradually. Therefore, we are dealing with disease entities that previously did not occur or appeared sporadically. Polypathology and polypharmacy, closely related to old age, are important factors that hinder diagnosis and treatment, and modify the course of many diseases. The complexity of clinical situations observed in the elderly is the cause of many diagnostic and treatment difficulties observed in the geriatric population. Every disease entity may have a different, usually more complicated, and non-specific course in old age compared to the younger population. Diseases, including neurological and psychiatric disorders, deserve special attention because this group of disturbances frequently occurs, particularly in older people. Dementia, anxiety, depressive disorders, and multi-factor sleep disorders that appear de novo in the discussed group of patients are most common in the geriatric population. Some disease entities, such as schizophrenia, most often appear in earlier stages of life, so in geriatric age, the time of treatment reaches several, and often several dozen years (25).
The onset of clinical symptoms of schizophrenia in elderly patients is different from that observed in younger patients. In this age group, the disease mainly affects females and has a better prognosis than the early onset form (28). The presence of genetic determinants of the disease is indicated; negative symptoms of a chronic nature and cognitive impairment dominate, while positive symptoms are less frequent and less severe (28).
Late-onset schizophrenia (LOS), affecting people over 45 years of age, has a confirmed genetic predisposition, while schizophrenia onset after the age of 60 years belongs to the group of diseases known as very late-onset schizophrenia-like psychosis (VLOSLP), which is closely related to a high risk of developing neurodegenerative diseases, mainly dementia, Alzheimer’s disease, and Lewy bodies (29). Currently, very few studies have been carried out to support the hypothesis that VLOSLP is closely related to the risk of dementia; rather, it is emphasized that this group of diseases may be a harbinger of the development of dementia (30, 31). There is a high risk of misdiagnosis of schizophrenia or dementia because, in schizophrenia, memory disturbances are often predominant in the initial stage; conversely, early symptoms of dementia syndrome may suggest the presence of developing negative symptoms (29–31). In patients with VLOSLP, deficits in reasoning, attention, processing speed and remembering new information, working memory, and language function are more common, while short-term memory and visual-spatial function disorders are less severe (32). There is an average decrease in Mini–Mental State Examination (MMSE) score of one point per year over the 6 years duration of the disease, with most studies conducted in institutionalized patients, that is, those at a priori higher risk of cognitive impairment. Tang et al. (33) pointed out that the degree of severity of cognitive disorders, especially time perception, is positively correlated with the patient’s age, disease duration, and systolic blood pressure values.
Currently, no guidelines have been developed for the differentiation of negative symptoms in elderly patients with schizophrenia from amotivation/avolition/apathy symptoms in elderly patients with neurodegenerative disorders.
Meanwhile, in Table 1, we tried to present the main differences between LOS and dementia. Meanwhile, in the Table 2 we present key findings from studies about difficulties on schizophrenia with onset at different ages.
Considering the large variation in the intensity of symptoms in individual patients, the coexistence of schizophrenia with other diseases and difficulties in the differential diagnosis of schizophrenia from other neurodegenerative diseases, research is currently being conducted to find specific markers based on which it is possible to diagnose schizophrenia quickly and without error. This is essential because, in people aged >45 years, schizophrenia coexists with other diseases, as well as some of the symptoms, such as dementia, memory impairment, and psychosis. Therefore, for this reason, predictive markers are important (29–31). Below we present current developments in finding the “ideal marker” for schizophrenia, including LOS-like psychosis and VLOSLP.
The first group of markers is related to the development of non-invasive brain imaging techniques using magnetic resonance imaging (MRI).
The Consensus Report of the APA Work Group on Neuroimaging Markers of Psychiatric Disorders from 2012 indicates that so far no biomarkers of brain imaging have been developed that would have diagnostic value in psychiatry (34). The applied neuroimaging marker systems are primarily related to the diagnosis of neurocognitive disorders such as dementia. Currently, more research is needed on potential neuroimaging biomarkers in schizophrenia due to inconsistencies, small sample sizes, and the lack of multimodal studies (35).
These observations are consistent with those of Olabi et al. (36), who stated that the structural changes in the brain in the course of schizophrenia tend to progress and include white and gray matter.
Cropley et al. (37) indicated that structural changes in the brains of patients with schizophrenia depend on their age. In young people, there is a rapid loss of gray matter. Then, in middle age, this process slows significantly, and in old age the loss of white matter dominates.
In addition, Kambeitz et al. (38) showed that the sensitivity of MRI significantly depends on age, imaging modality, disease stage, while specificity depends on positive to negative symptom ratio, and antipsychotic drugs.
The most serious of these is that in patients most commonly diagnosed with schizophrenia, gray matter loss, thinning of the cerebral cortex, changes in white matter volume, and microarchitecture are, to some extent, age-related physiological phenomena (39, 40). Chung et al. (41) showed in the group of patients aged 12–17 years that the more severe neuroanatomic changes from the patient’s record age, the greater the risk of developing schizophrenia and the worse the prognosis. In turn, de Wit (42) noted that decreases in surface area of the prefrontal, cingulate, and parahippocampal are associated with less effective treatment of schizophrenia, but only among young people.
These observations indicate that the age of the patient should be considered when interpreting the neuroimaging results. Although there are specific brain neuroanatomical patterns characteristic of schizophrenia, they have certain limitations.
Table 3 summarizes the most important findings from the studies on the potential use of neuroimaging markers in schizophrenia.
Biochemical and molecular markers are the second group of markers that could be useful in differentiating schizophrenia from other conditions. This class of markers provides a chance to make a more objective and reliable diagnosis, especially when the diagnosis is uncertain based on clinical history (43).
S100 protein, nerve growth factor, and brain-derived nerve factor (BDNF) are also potentially important biomarkers of schizophrenia. Serum BDNF levels in untreated patients with schizophrenia are decreased and associated with cognitive impairment (44, 45). However, it should be noted that BDNF is not suggested as a specific marker for schizophrenia. Research suggests its role in the pathogenesis and treatment of other neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease, and depressive disorders (44, 45).
A blood multiset consisting of 51 markers (VeriPsych) was developed in the study by Schwarz et al. (46); however, in this case, the specificity was unsatisfactory.
Moreover, given that schizophrenia is a disease with high inheritance potential, the detection of gene expression and polymorphisms, e.g., disrupted-in-schizophrenia 1 (DISC1), is not insignificant in the context of biomarkers. However, polymorphic variants of this gene are not specific to schizophrenia. The DISC1 polymorphism has been noted, among other things, in patients with schizoaffective disorder, bipolar disorder, major depression, autism, and Asperger’s syndrome (47, 48). It has also been suggested that in schizophrenia, there is a reduction in the concentration of essential polysaturated fatty acids (EPUFAs), vitamin E, and creatinine (49), an increased concentration of asparagine, glutamine, methionine, ornithine, and taurine, and reduced levels of aspartate, glutamate, and alpha-aminoadipic acid (alpha-AAA) (50).
To date, no specific, biochemical or molecular marker has been developed that can be used in clinical practice to diagnose schizophrenia, including elderly patients.
In Table 4, we summarize studies on the possibility of using biochemical and molecular markers in schizophrenia.
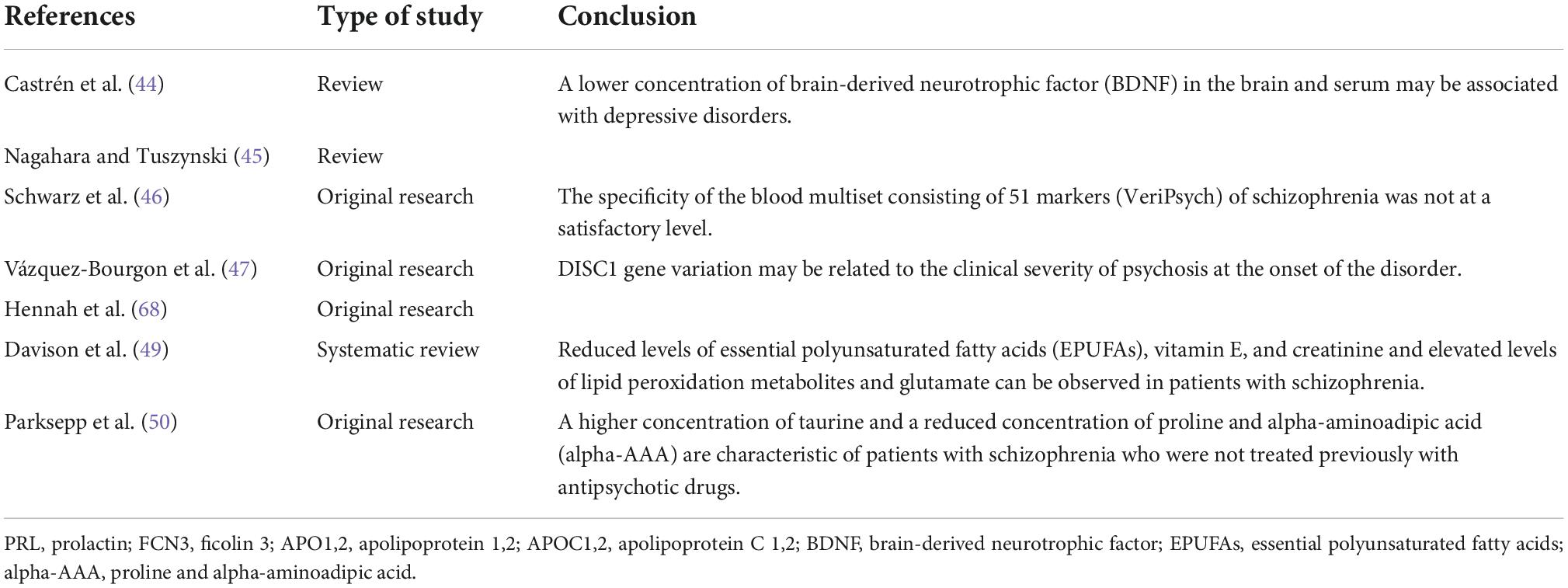
Table 4. Current knowledge about the possibility of using biochemical and molecular markers in schizophrenia.
Machine learning is a promising approach to better differentiate schizophrenia from other mental disorders or neurodegenerative diseases, which is a multidimensional statistical approach to solving this problem. The combination of neuroimaging and non-imaging data significantly contributes to the predictive potential of biomarkers. In this regard, the multi-center PSYSCAN project aims to collect neuroimaging, clinical, cognitive, and genetic data and thus identify a marker useful for predicting outcomes and developing new software for data analysis in mental disorders, with particular emphasis on schizophrenia (accessed August 10, 2022)1 (51). The FSA score was calculated using machine learning from images obtained during functional magnetic resonance imaging (fMRI). FSA is characterized by satisfactory accuracy (80%), sensitivity (79.3%), and specificity (81.5%) in differentiating patients with schizophrenia from the control group (52). In addition, this indicator appears to be useful for predicting the response to treatment and monitoring its effectiveness (53). Table 5 shows the usefulness of machine learning in schizophrenia.
In general, elderly patients require lower doses of antipsychotic drugs compared to younger age groups, the principle of “start low, go slow” applies, with the starting dose of the drug being 25–50% of the dose used in young patients (54). This pharmacokinetic hypothesis may not necessarily apply to all drugs, especially to all antipsychotics; for example, during treatment with clozapine, age does not affect serum drug concentration, while smoking and male sex positively correlate with increased excretion (54). Similar observations were made for olanzapine, risperidone, and ziprasidone, in which the patient’s age did not influence the blood levels of these drugs (55). Because the active metabolite of risperidone, paliperidone, is excreted only in the urine, dose modification is required in patients with renal failure (54). Patients who do not follow medical recommendations are more often admitted to emergency psychiatric care and experience an increased number and duration of hospitalizations, which entails an increase in the costs of therapy and care for this group of patients (17). According to research, a break in the therapy of 1–10 days causes an almost 2-fold increase in the risk of disease relapse and rehospitalization (56). The risk of non-compliance in elderly patients with schizophrenia is mainly due to cognitive impairment in the first 7–10 days after hospitalization, 15–25% of patients discontinued treatment, and another crisis was observed 6 months after the disease had stabilized (56).
Hypersensitivity to antipsychotics is particularly frequent in elderly patients, which is based on an impaired blood-brain barrier and dysfunction of the P-glycoprotein, which contributes to the increased direct penetration of the drug into the central nervous system (57). Second, with age, the concentration of endogenous dopamine decreases, which leads to a decrease in the activity of the dopaminergic system as well as the synthesis of dopamine enzymes and precursors (57). Moreover, the activity of monoamine oxidase B, which is involved in the catabolism of dopamine, increases, and the concentration of the dopamine transporter responsible for its reuptake decreases (57). In the elderly population, where negative symptoms dominate, clozapine and cariprazine, a partial agonist of D3 and D2 receptors with a special affinity for D3 receptors and a partial agonist of 5-HT1A serotonin receptors, play a special role. Cariprazine is recommended as first-line monotherapy in situations where secondary negative symptoms predominate (57). In the case of intolerance, low doses of amisulpride (50–300 mg/day) are recommended, especially when negative symptoms are accompanied by depressive syndrome (57). Amisulpride has an affinity for D2 and D3 receptors and has a positive effect on symptoms such as avolition or attention deficits (57). Asenapine binds to receptors for dopamine, serotonin, histamine, and noradrenaline, showing greater efficiency in reducing negative symptoms than risperidone or haloperidol (52). A number of drugs, such as selegiline, duloxetine, citalopram, fluvoxamine, and mirtazapine, also have a beneficial effect in reducing negative symptoms. Selective serotonin reuptake inhibitors (SSRIs) reduce IL-6 and CRP levels (58). Minocycline is a second-generation tetracycline that works by reducing proinflammatory cytokines, and its effectiveness is emphasized, especially in the early and stable stages of the disease (58).
Table 6 summarizes the most important findings related to pharmacotherapy for schizophrenia, including postadolescent schizophrenia.
People diagnosed with schizophrenia are perceived as demanding or even difficult patients because psychotherapists experience negative emotions and sometimes do not know how to deal with such a patient. When working with people with psychosis, it is necessary to have not only a specific approach to the patient but also the therapist should have the appropriate characteristics (55, 59). Support for such individuals can be provided through supportive counseling, family interventions, cognitive-behavioral approaches, and psychodynamic psychotherapy. Psychoanalysis is a method that aims to reveal past emotional experiences of the patients and its role in influencing their current mental life. This is intended to discover the conflicts and mechanisms that precede their pathological mental state and provide guidance for psychotherapeutic management. The method of psychoanalysis is based on the use of free association, dream recall and interpretation, and interpretation of the phenomena of transference and resistance. Psychoanalysis should be conducted regularly, 3–5 times a week, for no less than 30 min, with a trained psychoanalyst (60). Furthermore, the psychoanalysis should be scheduled for at least 1 year (60). Psychoanalytic psychotherapists place a relatively high value on conceptualizing the properties of mental life (60).
Meanwhile, in psychodynamic psychotherapy, patient’s individual sessions with a psychotherapist are based on various insight-oriented, supportive or directive strategies applied flexibly (60). The main goal of psychotherapy in the case of psychosis is to support the patient with less focus on exploratory work. It focuses on defining the emotions felt by the patient, most often using techniques of clarifying, normalizing, and containing patient experiences. Psychotherapy in patients with schizophrenia occurs mainly in mainstream psychodynamic or psychoanalytical therapy (61, 62). In the psychodynamic approach, the goals are to support and provide a corrective experience, resolve emotional conflicts of the patients, and avoid situations that could cause regression (63).
Cognitive-behavioral therapy is considered the most important skill to improve the standard of social life, the acquisition of cognitive ways to cope with difficult situations, solving current problems, and developing the ability to compensate for deficits resulting from neurocognitive failure (64).
Therapists agree that working with people with schizophrenia is different from working with patients diagnosed with depression, anxiety disorders, and personality disorders. This is related to the fact that the patient-psychotherapist relationship is more intense, as well as the psychological boundaries between them. Patients with schizophrenia often experience acceptance, being heard, understood, and “accepted as they are” only in contact with the therapist (65–67). The patients themselves perceive therapy as a place where they can come and talk about difficult events, and the therapist will not be scared of it but will allow the patient to reflect on the experienced emotions. Most psychoanalysts believe that emotional problems are of primary importance for patients with schizophrenia, although they are more difficult to study systematically and empirically (68).
The main limitations of research on psychotherapy for schizophrenia are as follows: clinical heterogeneity of schizophrenia, methodological rigidity (no possibility of changing clinical management), differences in the motivation and needs of patients, differences in the possibility of using individual psychotherapy (some patients have good results, others worsen, because psychotherapy is inadequate for them; in the statistical analysis, the results cancel each other out); and finally, most patients require an integrated approach, and the study of a single treatment method does not give any insight into the possibilities of comprehensive treatment. Perhaps, researchers’ attention should be shifted from the methods to the needs of patients, and to determine what happens in effective and ineffective psychotherapy (69, 70). Therefore, controlled research should be postponed until more is known about the factors that contribute to the formation and maintenance of this therapeutic relationship (69, 70). Table 7 summarizes the most important findings related to psychotherapy for schizophrenia. Based on the available literature, there is no differentiation of psychotherapy methods in patients with schizophrenia according to age.
This study had several limitations. First, this study was not a systematic review; thus, it may have missed some relevant studies that would have been identified by systematic search. Second, in this study, although we attempted to discuss the findings presented in the original articles, we also included several review publications when, to the best of our knowledge, they contributed to the discussion of the issues raised. Third, the non-rigorous approach to the period in which the articles included in our literature review were published. Fourth, even with original works, the minimum group size necessary to include an article in our study was not considered. In addition, due to the type of the study (review article), the qualifications of the articles included in our study are probably characterized by a certain bias, as they were based on the experience of the authors of the articles.
However, it should be kept in mind, as Shrier et al. (71) noted, that even if a systematic review (with) meta-analysis is characterized by greater objectivity than other review papers, bias during interpretation does arise. Evidence-based practitioners should be aware that conclusions and recommendations based on a systematic review with a meta-analysis should be read cautiously, even if the methodology is rigorous (71).
Although the term schizophrenia was defined in 1893, research is still being carried out on the optimization of the pharmacotherapy used in the treatment of this psychosis based on individual and population variability following the principle of “personalization of medicine.” In addition, the search for “ideal” biomarkers of schizophrenia, based on specific structural and dynamic changes in the brains of people with schizophrenia biochemical and molecular markers, is constantly being sought. However, to date, nothing can replace the experience of a psychiatrist and a thorough interview with the patients and their families. This review also draws attention to the fact that therapy should be holistic, not only including pharmacological treatment but also psychotherapy, and considers the key role of caregivers for people with schizophrenia. It is worth mentioning that the most important aspect is to diagnose schizophrenia as early as possible and thus start appropriate treatment as soon as possible. Unfortunately, we often deal with situations where schizophrenia is diagnosed several years after the onset of symptoms when the severity of symptoms and the decline in the patient’s functioning in society are already significant (40). There are several possible reasons for this finding. First, as indicated in this study, the “ideal” marker of schizophrenia has yet to be identified. Second, physicians’ experience in recognizing symptoms and the low public awareness of symptoms are factors of great importance. Third, schizophrenia, especially in the elderly, is relatively rare and its symptoms, especially the negative ones, may be confused with amotivation/avolition/apathy symptoms accompanying neurodegenerative disorders, which are common in old age.
However, studies on the search for “ideal biomarkers” for prognostic, diagnostic, predictive, and optimizing treatment of schizophrenia should not be stopped because this is supported by progress in medicine and related sciences. In Figure 1, we have included the most important challenges identified in this study regarding the diagnosis and treatment of schizophrenia and the following steps.
OS-W and MN: conceptualization. MW-S: formal analysis. OS-W, MN, and GW: writing – original draft preparation. EM and RS: writing – review and editing. OS-W and BG: supervision. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Berrios GE, Luque R, Villagrán JM. Schizophrenia: a conceptual history. Int J Psychol Psychological Ther. (2003) 3:111–40.
2. Katschnig H. Psychiatry’s contribution to the public stereotype of schizophrenia: historical considerations. J Eval Clin Pract. (2018) 24:1093–100. doi: 10.1111/jep.13011
4. Polskiego RA. Schizofrenia od antyku po lata współczesne. (2018). Available online at: https://www.aptekarzpolski.pl/wiedza/schizofrenia-od-antyku-po-lata-wspolczesne-%EF%BB%BF/. (accessed August 9, 2022).
5. Seeman MV. History of the dopamine hypothesis of antipsychotic action. World J Psychiatry. (2021) 11:355–64. doi: 10.5498/wjp.v11.i7.355
6. Cunningham Owens D, Johnstone EC. The development of antipsychotic drugs. Brain Neurosci Adv. (2018) 2:2398212818817498. doi: 10.1177/2398212818817498
7. Riederer P, Laux G, Nagatsu T, Le W, Riederer C. Neuropsychopharmacotherapy. Cham: Springer (2020). doi: 10.1007/978-3-319-56015-1_387-1
8. Fatima A, Barkat U, Mahmood KT, Zaka M. Schizophrenia in elderly patients. J Pharm Sci. (2011) 3:952–60.
9. Berger T, Schnitzer J, Kettenmann H. Developmental changes in the membrane current pattern, K+ buffer capacity, and morphology of glial cells in the corpus callosum slice. J Neurosci. (1991) 11:3008–24. doi: 10.1523/JNEUROSCI.11-10-03008.1991
10. Convert H, Védie C, Paulin P. [Late-onset schizophrenia or chronic delusion]. Encéphale. (2006) 32:957–61. doi: 10.1016/s0013-7006(06)76273-x
11. Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatr. (2014) 13:153–60. doi: 10.1002/wps.20128
12. Mueser KT, McGurk SR. Schizophrenia. Lancet. (2004) 363:2063–72. doi: 10.1016/S0140-6736(04)16458-1
13. Ross CA, Margolis RL, Reading SAJ, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. (2006) 52:139–53. doi: 10.1016/j.neuron.2006.09.015
14. Jiang Y, Patton MH, Zakharenko SS. A case for thalamic mechanisms of schizophrenia: perspective from modeling 22q11.2 deletion syndrome. Front Neural Circuits. (2021) 2021:769969. doi: 10.3389/fncir.2021.769969
15. Nucifora LG, MacDonald ML, Lee BJ, Peters ME, Norris AL, Orsburn BC, et al. Increased protein insolubility in brains from a subset of patients with schizophrenia. Am J Psychiatry. (2019) 176:730–43. doi: 10.1176/appi.ajp.2019.18070864
16. Galderisi S, Kaiser S, Bitter I, Nordentoft M, Mucci A, Sabé M, et al. EPA guidance on treatment of negative symptoms in schizophrenia. Eur Psychiatry. (2021) 64:e21. doi: 10.1192/j.eurpsy.2021.13
17. Rumian K, Pollok-Waksmańska W, Łukasik RJ. Wiedza pacjentów ze schizofrenią dotycząca rozpoznania objawów zwiastujących nawrót choroby. Psychiatria. (2020) 17:186–95. doi: 10.5603/PSYCH.2020.0032
18. Rybakowski J. Etiopathogenesis of schizophrenia – the state of the art for 2021. Psychiatr Pol. (2021) 55:261–74. doi: 10.12740/PP/132953
19. Stroup TS, Lieberman A, Swartz M, McEvoy JP. Comparative effectiveness of antipsychotic drugs in schizophrenia. Dial Clin Neurosci. (2000) 2:373–9. doi: 10.31887/DCNS.2000.2.4/tstroup
20. Carbon M, Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. (2014) 19:38–52. doi: 10.1017/S1092852914000601
21. De Berardis D, Rapini G, Olivieri L, Di Nicola D, Tomasetti C, Valchera A, et al. Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine. Ther Adv Drug Saf. (2018) 9:237–56. doi: 10.1177/2042098618756261
22. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18:143. doi: 10.1186/s12874-018-0611-x
23. Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatr. (2017) 16:163–80. doi: 10.1002/wps.20420
24. Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. (2017) 4:295–301. doi: 10.1016/S2215-0366(17)30078-0
25. Ermakov D, Fomina E, Kartashova O. Specific features of rational pharmacotherapy in elderly patients. Eur J Hosp Pharm. (2021) 2021:2980. doi: 10.1136/ejhpharm-2021-002980
26. Kershenbaum A, Cardinal RN, Chen S, Underwood BR, Seyedsalehi A, Lewis J, et al. Investigation of risk of dementia diagnosis and death in patients in older people’s secondary care mental health services. Int J Geriatr Psychiatry. (2021) 36:573–82. doi: 10.1002/gps.5455
27. Almeida OP, Ford AH, Hankey GJ, Yeap BB, Flicker L. Risk of dementia associated with psychotic disorders in later life: the health in men study (HIMS). Psychol Med. (2019) 49:232–42. doi: 10.1017/S003329171800065X
28. Huq R, Mithani F, Cullum S, Chacko R. An atypical progression of schizophrenia in an elderly woman. Proc (Bayl Univ Med Cent). (2020) 34:321–2. doi: 10.1080/08998280.2020.1834906
29. Stafford J, Dykxhoorn J, Sommerlad A, Dalman C, Kirkbride JB, Howard R. Association between risk of dementia and very late-onset schizophrenia-like psychosis: a Swedish population-based cohort study. Psychol Med. (2021) 2021:1–9. doi: 10.1017/S0033291721002099
30. Bora E. Neurodevelopmental origin of cognitive impairment in schizophrenia. Psychol Med. (2015) 45:1–9. doi: 10.1017/S0033291714001263
31. Stafford J, Howard R, Dalman C, Kirkbride JB. The incidence of nonaffective, nonorganic psychotic disorders in older people: a population-based cohort study of 3 million people in Sweden. Schizophr Bull. (2019) 45:1152–60. doi: 10.1093/schbul/sby147
32. Yang VX, Sin Fai Lam CC, Kane JPM. Cognitive impairment and development of dementia in very late-onset schizophrenia-like psychosis: a systematic review. Ir J Psychol Med. (2021) 2021:1–13. doi: 10.1017/ipm.2021.48
33. Tang W, Fan KL, Zhao SZ, Zhang YY, Li Y, Shao SM, et al. Correlations between age, biomedical variables, and cognition in patients with schizophrenia. Schizophr Res Cogn. (2020) 22:100182. doi: 10.1016/j.scog.2020.100182
34. Vogelstein JT, Bridgeford EW, Pedigo BD, Chung J, Levin K, Mensh B, et al. Connectal coding: discovering the structures linking cognitive phenotypes to individual histories. Curr Opin Neurobiol. (2019) 55:199–212. doi: 10.1016/j.conb.2019.04.005
35. Aydin O, Unal Aydin P, Arslan A. Development of neuroimaging-based biomarkers in psychiatry. Adv Exp Med Biol. (2019) 1192:159–95. doi: 10.1007/978-981-32-9721-0_9
36. Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. (2011) 70:88–96. doi: 10.1016/j.biopsych.2011.01.032
37. Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, et al. Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. (2017) 174:286–95. doi: 10.1176/appi.ajp.2016.16050610
38. Kambeitz J, Kambeitz-Ilankovic L, Leucht S, Wood S, Davatzikos C, Malchow B, et al. Detecting neuroimaging biomarkers for schizophrenia: a meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology. (2015) 40:1742–51. doi: 10.1038/npp.2015.22
39. Ahn SY, Sohn SH, Lee SY, Park HL, Park YW, Kim H, et al. The effect of lipopolysaccharide-induced obesity and its chronic inflammation on influenza virus-related pathology. Environ Toxicol Pharmacol. (2015) 40:924–30. doi: 10.1016/j.etap.2015.09.020
40. Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. (2020) 8:570. doi: 10.1016/S2213-8587(20)30183-2
41. Chung Y, Allswede D, Addington J, Bearden CE, Cadenhead K, Cornblatt B, et al. Cortical abnormalities in youth at clinical high-risk for psychosis: findings from the NAPLS2 cohort. NeuroImage Clin. (2019) 23:101862. doi: 10.1016/j.nicl.2019.101862
42. de Wit S, Ziermans TB, Nieuwenhuis M, Schothorst PF, van Engeland H, Kahn RS, et al. Individual prediction of long-term outcome in adolescents at ultra-high risk for psychosis: applying machine learning techniques to brain imaging data. Hum Brain Mapp. (2017) 38:704–14. doi: 10.1002/hbm.23410
43. Galińska-Skok B, Waszkiewicz N. Markers of schizophrenia—a critical narrative update. J Clin Med. (2022) 11:3964. doi: 10.3390/jcm11143964
44. Castrén E, Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis. (2017) 97:119–26. doi: 10.1016/j.nbd.2016.07.010
45. Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. (2011) 10:209–19. doi: 10.1038/nrd3366
46. Schwarz E, Izmailov R, Spain M, Barnes A, Mapes JP, Guest PC, et al. Validation of a blood-based laboratory test to aid in the confirmation of a diagnosis of schizophrenia. Biomark Insights. (2010) 5:39–47. doi: 10.4137/bmi.s4877
47. Vázquez-Bourgon J, Mata I, Roiz-Santiáñez R, Ayesa-Arriola R, Suárez Pinilla P, Tordesillas-Gutiérrez D, et al. A disrupted-in-schizophrenia 1 gene variant is associated with clinical symptomatology in patients with first-episode psychosis. Psychiatry Investig. (2014) 11:186–91. doi: 10.4306/pi.2014.11.2.186
48. Hennah W, Thomson P, McQuillin A, Bass N, Loukola A, Anjorin A, et al. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol Psychiatry. (2009) 14:865–73. doi: 10.1038/mp.2008.22
49. Davison J, O’Gorman A, Brennan L, Cotter DR. A systematic review of metabolite biomarkers of schizophrenia. Schizophr Res. (2018) 195:32–50. doi: 10.1016/j.schres.2017.09.021
50. Parksepp M, Leppik L, Koch K, Uppin K, Kangro R, Haring L, et al. Metabolomics approach revealed robust changes in amino acid and biogenic amine signatures in patients with schizophrenia in the early course of the disease. Sci Rep. (2020) 10:13983. doi: 10.1038/s41598-020-71014-w
51. Tognin S, van Hell HH, Merritt K, Winter-van Rossum I, Bossong MG, Kempton MJ, et al. Toward precision medicine in psychosis: benefits and challenges of multimodal multicenter studies-PSYSCAN: translating neuroimaging findings from research into clinical practice. Schizophr Bull. (2020) 46:432–41. doi: 10.1093/schbul/sbz067
52. Correll CU, Kane JM. Ranking antipsychotics for efficacy and safety in schizophrenia. JAMA Psychiatry. (2020) 77:225–6. doi: 10.1001/jamapsychiatry.2019.3377
53. Li A, Zalesky A, Yue W, Howes O, Yan H, Liu Y, et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat Med. (2020) 26:558–65. doi: 10.1038/s41591-020-0793-8
54. Krause M, Huhn M, Schneider-Thoma J, Rothe P, Smith RC, Leucht S. Antipsychotic drugs for elderly patients with schizophrenia: a systematic review and meta-analysis. Eur Neuropsychopharmacol. (2018) 28:1360–70. doi: 10.1016/j.euroneuro.2018.09.007
55. Lotterman AC. Psychotherapy techniques for patients diagnosed with schizophrenia. Am J Psychother. (2016) 70:63–78. doi: 10.1176/appi.psychotherapy.2016.70.1.63
56. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among california medicaid patients with schizophrenia. Psychiatr Serv. (2004) 55:886–91. doi: 10.1176/appi.ps.55.8.886
57. Wu Q, Wang X, Wang Y, Long YJ, Zhao JP, Wu RR. Developments in biological mechanisms and treatments for negative symptoms and cognitive dysfunction of schizophrenia. Neurosci Bull. (2021) 37:1609–24. doi: 10.1007/s12264-021-00740-6
58. Tavakoli Ardakani M, Mehrpooya M, Mehdizadeh M, Beiraghi N, Hajifathali A, Kazemi MH. Sertraline treatment decreased the serum levels of interleukin-6 and high-sensitivity C-reactive protein in hematopoietic stem cell transplantation patients with depression; a randomized double-blind, placebo-controlled clinical trial. Bone Marrow Transplant. (2020) 55:830–2. doi: 10.1038/s41409-019-0623-0
59. Pec O, Lysaker PH, Probstova V, Leonhardt BL, Hamm JA, Bob P. The psychotherapeutic treatment of schizophrenia: psychoanalytical explorations of the metacognitive movement. J Contemp Psychother. (2020) 50:205–12. doi: 10.1007/s10879-020-09452-w
60. Malmberg L, Fenton M, Rathbone J. Individual psychodynamic psychotherapy and psychoanalysis for schizophrenia and severe mental illness. Cochrane Database Syst Rev. (2001) 2012:CD001360. doi: 10.1002/14651858.CD001360
61. Lincoln TM, Pedersen A. An overview of the evidence for psychological interventions for psychosis: results from meta-analyses. Clin Psychol Eur. (2019) 1:1–23. doi: 10.32872/cpe.v1i1.31407
62. Bjornestad J, Veseth M, Davidson L, Joa I, Johannessen JO, Larsen TK, et al. Psychotherapy in psychosis: experiences of fully recovered service users. Front Psychol. (2018) 9:1675. doi: 10.3389/fpsyg.2018.01675
63. Ridenour JM, Hamm JA, Neal DW, Lysaker PH. From fragmentation to coherence: psychodynamic psychotherapy for psychosis through the lens of metacognition. Psychodyn Psychiatry. (2020) 48:455–76. doi: 10.1521/pdps.2020.48.4.455
64. Sawicka M, Żochowska A. Positive interventions in the therapy of schizophrenia patients. Curr Probl Psychiatry. (2018) 19:239–47. doi: 10.2478/cpp-2018-0018
65. Babl A, Berger T, Decurtins H, Gross I, Frey T, Caspar F, et al. A longitudinal analysis of reflective functioning and its association with psychotherapy outcome in patients with depressive and anxiety disorders. J Couns Psychol. (2022) 69:337–47. doi: 10.1037/cou0000587
66. Lingiardi V, Lonati C, Delucchi F, Fossati A, Vanzulli L, Maffei C. Defense mechanisms and personality disorders. J Nerv Ment Dis. (1999) 187:224–8. doi: 10.1097/00005053-199904000-00005
67. Cheli S, Lysaker PH, Dimaggio G. Metacognitively oriented psychotherapy for schizotypal personality disorder: a two-case series. Personal Ment Health. (2019) 13:155–67. doi: 10.1002/pmh.1447
68. Krupa A, Styła R. Specyfika psychoterapii psychodynamicznej skierowanej do osób z rozpoznaniem schizofrenii. Badanie jakościowe operate na wywiadach z terapeutami. Psychiatria. (2015) 12:119–27.
69. Fonagy P. The effectiveness of psychodynamic psychotherapies: an update. World Psychiatry. (2015) 14:137–50. doi: 10.1002/wps.20235
70. Lysaker PH, Lancaster RS, Lysaker JT. Narrative transformation as an outcome in the psychotherapy of schizophrenia. Psychol Psychother. (2003) 76:285–99. doi: 10.1348/147608303322362505
Keywords: geriatric population, schizophrenia, cognitive impairment, antipsychotic drugs, aging
Citation: Stȩpień-Wyrobiec O, Nowak M, Wyrobiec G, Morawiec E, Wierzbik-Strońska M, Staszkiewicz R and Grabarek BO (2022) Crossroad between current knowledge and new perspective of diagnostic and therapy of late-onset schizophrenia and very late-onset schizophrenia-like psychosis: An update. Front. Psychiatry 13:1025414. doi: 10.3389/fpsyt.2022.1025414
Received: 22 August 2022; Accepted: 11 October 2022;
Published: 26 October 2022.
Edited by:
Luca De Peri, Cantonal Sociopsychiatric Organization, SwitzerlandReviewed by:
Octavian Vasiliu, Dr. Carol Davila University Emergency Military Central Hospital, RomaniaCopyright © 2022 Stȩpień-Wyrobiec, Nowak, Wyrobiec, Morawiec, Wierzbik-Strońska, Staszkiewicz and Grabarek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olga Stȩpień-Wyrobiec, d3lyb2JpZWNvQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.