- 1Peking University HuiLongGuan Clinical Medical School, Beijing HuiLongGuan Hospital, Beijing, China
- 2Department of Physiology, Faculty of Medicine, Institute of Biomedicine and Translational Medicine, University of Tartu, Tartu, Estonia
Innate immune components involved in host defense have been implicated in schizophrenia (SCZ). However, studies exploring their clinical utility in SCZ diagnosis are limited. The main purpose of this study was to evaluate whether circulating endotoxin, high mobility group box 1 protein (HMGB1) and complement component 4 (C4) could act as peripheral biomarkers to distinguish first-episode schizophrenia (FES, n = 42) patients from healthy controls (HCs, n = 35) in associations with psychopathological symptoms and cognitive dysfunctions. Also, their changes after 8-week antipsychotic treatment were investigated. The Positive and Negative Syndrome Scale (PANSS), Psychotic Symptom Rating Scale (PSYRATS), and MATRICS Consensus Cognitive Battery (MCCB) were administered. Receiver operating characteristic (ROC) curves were conducted to evaluate the diagnostic effectiveness of the three biological indicators. Compared to HCs, levels of endotoxin, HMGB1, and C4 were remarkably increased in FES patients after controlling for age, gender, body mass index (BMI) and education years, and the combination of the three biomarkers demonstrated desirable diagnostic performance (AUC = 0.933). Moreover, the endotoxin level was positively correlated with the severity of auditory hallucinations. After 8 weeks of treatment, HMGB1 was decreased significantly in patients but still higher than that in HCs, whereas endotoxin and C4 did not change statistically. The baseline levels of endotoxin, HMGB1, and C4, as well as their changes were not associated with changes in any PANSS subscale score and total score. Our preliminary results suggest that a composite peripheral biomarker of endotoxin, HMGB1, and C4 may have accessory diagnostic value to distinguish SCZ patients from HCs. Additionally, endotoxin might be implicated in the pathogenesis of auditory hallucinations.
Introduction
Although the exact etiology of schizophrenia (SCZ) is still largely unknown, accumulating evidence has demonstrated that immune dysfunctions, especially innate immunity as the first line of host defense, are intimately implicated in the pathophysiology of SCZ. The complex interactions between the immune system and brain may contribute to a diverse range of changes in cognition, mood and behavior in psychiatric disorders and has received considerable attention in recent years (1, 2). Accumulated evidence has demonstrated that SCZ is accompanied by an ongoing chronic low-grade inflammation as indicated by simultaneous increase of both pro-inflammatory (IL-1, IL-6, and TNF-α) and anti-inflammatory cytokines (IL-10), as well as complement activation (1).
Pattern recognition receptors (PRRs) and their associated ligands, as well as the complement system are essential components of the innate immunity. PRRs, such as toll-like receptors (TLRs) and receptors for advanced glycation end products (RAGE), not only detect the components of exogenous pathogens (i.e., pathogen-associated molecular patterns or PAMPs), such as Gram-negative bacterial endotoxin (i.e., lipopolysaccharide, LPS), but also are capable of recognizing endogenous molecules released by dying cells or damaged tissues (i.e., damage-associated molecular patterns or DAMPs) (3). High mobility group box 1 protein (HMGB1), a DNA binding protein, is released from damaged or necrotic cells to the extracellular matrix, then acts as a pro-inflammatory cytokine trigging immune-inflammatory responses primarily via binding RAGE and several TLR family members (i.e., TLR2, TLR4, and TLR9) (3–5) and is required in endotoxin tolerance (6). By activating TLRs and RAGE signaling pathways, these molecules initiate the activation of immune and inflammatory responses (3), which need to be tightly regulated so that pathogens can be cleared while avoiding inflammatory overactivation. Notably, as PAMPs/DAMPs and complement components are essential for innate immune signaling, the importance of not only their individual functions but also their interplay in inflammatory processes have been realized recently (7). For instance, complement components are key regulators of PRR-mediated clearance of PAMPs and DAMPs in host organic innate immune responses (7, 8).
Endotoxin has been implied in SCZ due to its harmful effect on the brain and behavior, as demonstrated in neurodevelopmental conditions such as maternal immune activation (MIA) (9–11). On the other hand, a population-based cohort study with linkage of Danish national registers also indicated that infection was more likely to increase the risk of SCZ in susceptible individuals, whereas bacterial infection was associated with the highest risk (12). HMGB1 can disrupt the blood brain barrier (BBB), activate microglia and induce neurotoxic effects as a late mediator of inflammation (13, 14), and it has been implicated in a variety of neurological diseases such as ischemic stroke, epilepsy, multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease, etc. (13, 15, 16). Nevertheless, there have been few studies examining endotoxin or HMGB1 in SCZ patients (17–21), especially in first-episode individuals.
Additionally, a growing body of evidence shows that the complement pathway appears to be more active in SCZ (22), especially complement component 4 (C4) due to its complex genetic variation leading to increased neuronal complement deposition and synapse elimination, and the development of psychotic symptoms (23, 24). C4 overexpression by MIA in late gestation in animal models has been proven to induce hypoconnectivity in the prefrontal cortex (PFC) circuitry and SCZ-like deficits (25, 26). However, serological studies have yielded mixed results regarding dysregulation of C4 in patients with SCZ including first-episode schizophrenia (FES) or psychosis (FEP) (27–30). Anyhow, given the importance of C4 in SCZ, studies focusing on early phenotype of SCZ and minimizing confounding factors would provide unique research value.
Our previous study showed that FES patients had more dampened instead of enhanced monocytic TLR4 response to LPS as compared to the HCs (31). We speculated that it might be associated with endotoxin- or HMGB1-induced tolerance, in other words, the FES patients might have higher levels of TLR4 ligands in their peripheral blood. Additionally, endotoxin, HMGB1, and C4 may holistically affect SCZ patients, yet studies exploring them collectively to understand their joint potential clinical utility are extremely limited. Therefore, the primary purpose of this study was to evaluate whether these molecules could act as peripheral biomarkers individually or jointly to distinguish FES patients from HCs, alongside their relationships with psychopathological symptoms and cognitive functions. Moreover, we endeavored to look whether their levels would change after 8-week antipsychotic treatment, and their correlations with treatment efficacy.
Materials and methods
Subjects
Forty-two FES patients within 2 weeks of cumulative psychotropic drugs exposure and 35 healthy volunteers, who all were Han Chinese people, were recruited from the Beijing HuiLongGuan Hospital and local community, respectively. The disease duration of all the patients was less than 2 years (16.1 ± 5.4 months), i.e., they were in an early stage of the illness. These samples were previously utilized and have been described in detail in our previous immune-psychiatry study (31), except one healthy subject in which had not plasma left. After 8 weeks of treatment, only 20 patients left blood samples and completed assessment of the Positive and Negative Syndrome Scale (PANSS). All blood samples were obtained in the morning after overnight fasting. This research adhered to the Helsinki Declaration and was approved by the Medical Ethical Committee of Beijing HuiLongGuan Hospital.
Measurement of plasma endotoxin, high mobility group box 1 protein, and C4 concentration
Plasma levels of endotoxin, HMGB1, and C4 were determined at baseline and at 8 weeks of follow-up. Four (4) ml blood samples were collected in EDTAK2 tubes at the time of our previous research (31), which were stored at −80°C until analyses. Concentrations of the three substances were determined using commercially available enzyme-linked immunosorbent assay kits from Andy gene, Beijing, China: endotoxin (Human0939, sensitivity: 1.0 ng/L), HMGB1 (Human1775, sensitivity: 1.0 ng/L), and C4 (Human1584, sensitivity: 5.0 ng/L). All assays were conducted in blinded manner, and each sample was analyzed in duplicate.
Assessment of psychopathological symptoms and cognitive functions
PANSS and Matrics Consensus Cognitive Battery (MCCB) were used to evaluate the psychopathological symptoms and cognition, respectively, as previously described (31). Furthermore, the Psychotic Symptom Rating Scale (PSYRATS) was administered to some FES patients at baseline (n = 30). PSYRATS, a clinician-administered and semi-structured interview, were developed by Haddock et al. to evaluate the dimensionality of psychotic experiences, which consists of auditory hallucinations rating scale (AHRS) including 11 items and delusions rating scale (DRS) including 6 items scoring from 0 to 4 points, with higher scores indicating greater severity of symptoms (32).
Statistical methods
Data were analyzed using the IBM SPSS Statistics for Windows version 21.0. The Shapiro-Wilkinson test was used to examine data distribution. Group comparisons were performed using independent t-test or Mann-Whitney U-test for continuous variables and Chi-square test for categorical data. Analysis of covariance with age, gender, body mass index (BMI) and education years as covariates were applied to compare the levels of endotoxin, HMGB1, and C4 between FES patients and HCs, and then partial η2 was further calculated separately to determine the effect size of the group differences. A paired sample t-test was used to evaluate paired data.
Pearson and Spearman correlation analyses were applied as appropriate to investigate correlations of endotoxin, HMGB1, and C4 levels with clinical data at baseline. To explore the relationships between the three biological measures and clinical efficacy, we conducted partial correlation analyses controlling for baseline values.
The Receiver Operating Characteristic (ROC) curve analysis was performed to evaluate the diagnostic effectiveness of each baseline biological measure for differentiating FES patients from HCs. Then a combined model including endotoxin, HMGB1, and C4 jointly was constructed with multivariate logistic regression analysis, and its performance was compared with each substance alone by calculating areas under the ROC curves (AUC) with DeLong’s test. These analyses were performed using the pROC package in R 3.6.3. Plots were generated with ggplot2. Benjamini-Hochberg FDR (BH-FDR) was used for multiple testing correction. Two-tailed p < 0.05 was considered as statistically significant.
Results
Comparisons of demographic characteristics and plasma endotoxin, high mobility group box 1 protein, and C4 levels between first-episode schizophrenia and healthy controls
The demographic and clinical data of all participants at baseline are listed in Table 1. There were no statistically significant differences in age, gender, smoking status, BMI, and inflammatory marker high-sensitivity C-reactive protein (hs-CRP). However, the educational level was lower in FES patients than HC group (p < 0.001). As expected, FES patients showed impaired performance across all MCCB domains relative to normal controls (all p < 0.01; Table 1).
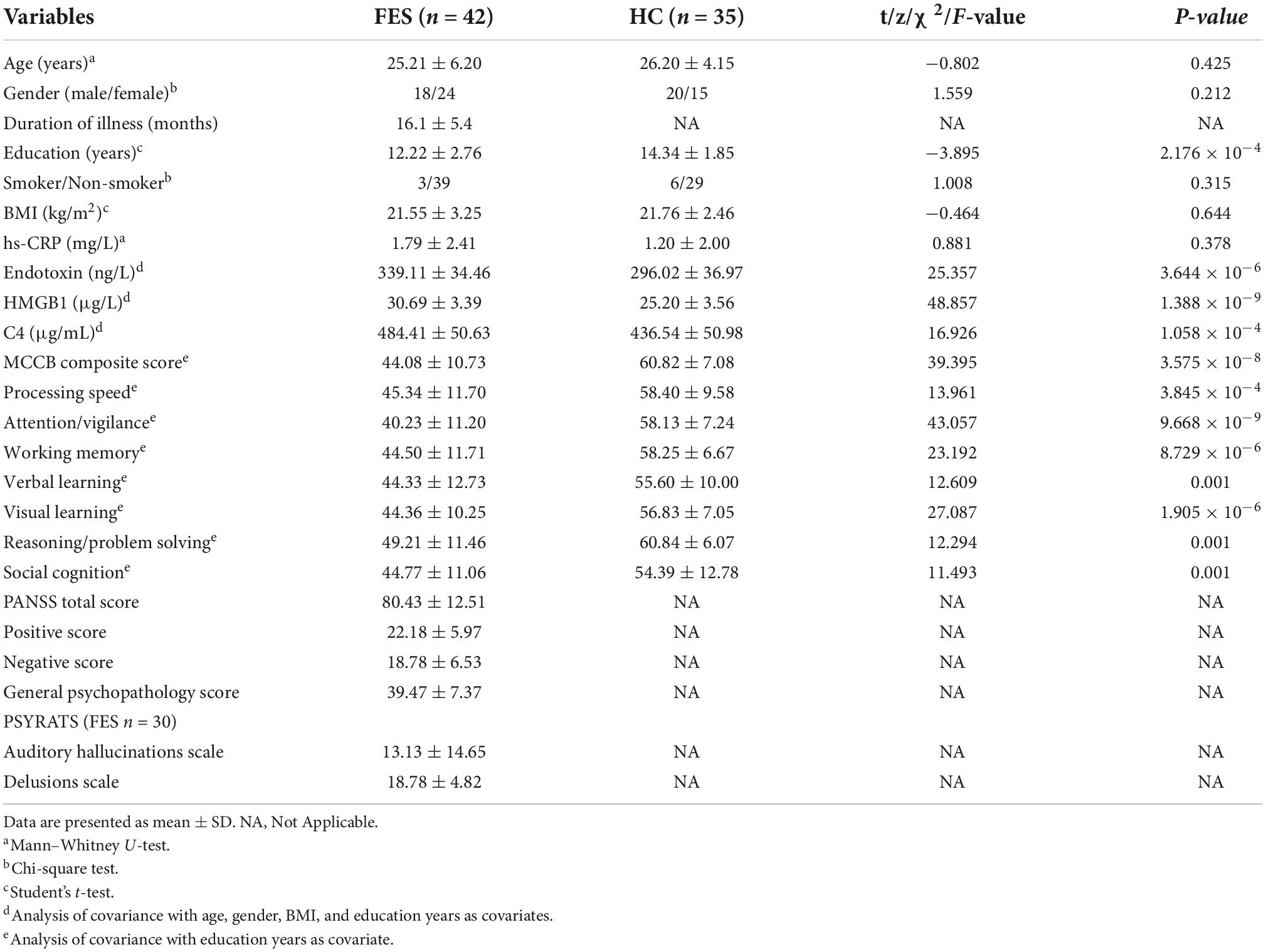
Table 1. The demographic and clinical characteristics of first-episode schizophrenia patients and healthy controls at baseline.
Analysis showed that concentrations of endotoxin (partial η2 = 0.269), HMGB1 (partial η2 = 0.415), and C4 (partial η2 = 0.197) were all significantly higher in FES patients as compared to the HCs after controlling for age, gender, BMI, and education years (all p < 0.001 and FDR corrected p < 0.001; Table 1), with the largest effect size for HMGB1.
Diagnostic performance evaluation of baseline plasma endotoxin, high mobility group box 1 protein, and C4
For a better utilization of the three peripheral biomarkers in the clinical diagnosis of SCZ, we evaluated their respective and combined diagnostic performance between the FES patients and the HCs. ROC analysis showed that the individual AUCs of endotoxin, HMGB1, and C4 ranged from 0.741 to 0.862 (95% CI ranged from 0.631 to 0.941), and the combination model of the three indicators significantly improved diagnostic power with an AUC of 0.933 (95% CI: 0.878–0.987; Figure 1 and Table 2). These results suggest that a composite biomarker had a better discrimination ability than any singular indicator (three comparisons, all FDR corrected p < 0.05; Table 2), with both sensitivity and specificity at 85.7% above the predicted probability cut-off value of 0.495 (Table 2; endotoxin: β = 0.033, p < 0.01; HMGB1: β = 0.411, p < 0.001; C4: β = 0.018, p < 0.05).
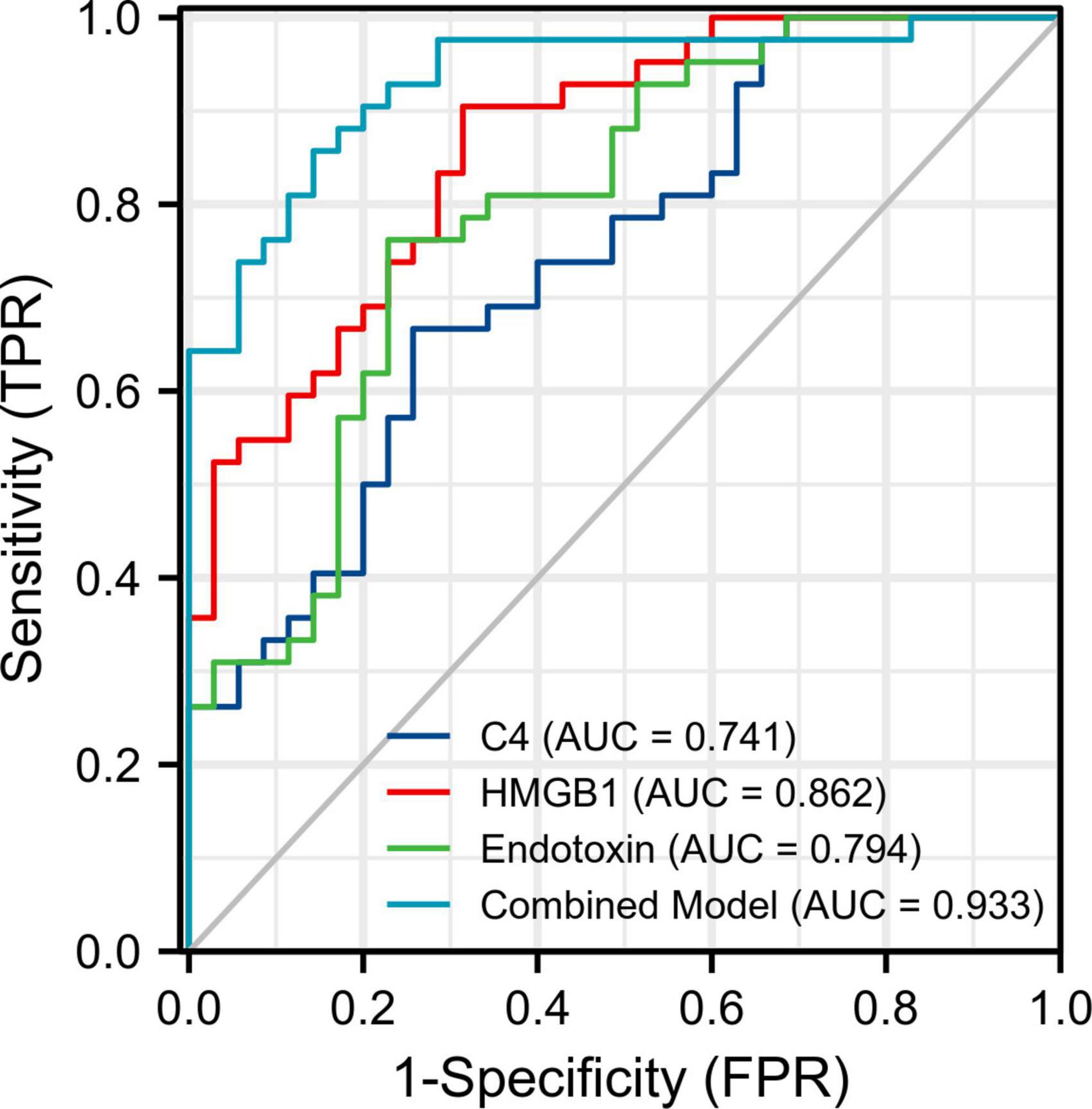
Figure 1. Receiver operating characteristic (ROC) curve analysis for discriminating first-episode schizophrenia patients from healthy controls. The areas under the ROC curves (AUC) of endotoxin, HMGB1, and C4 were calculated individually and in combination. FPR, false positive rate; TPR, true positive rate.
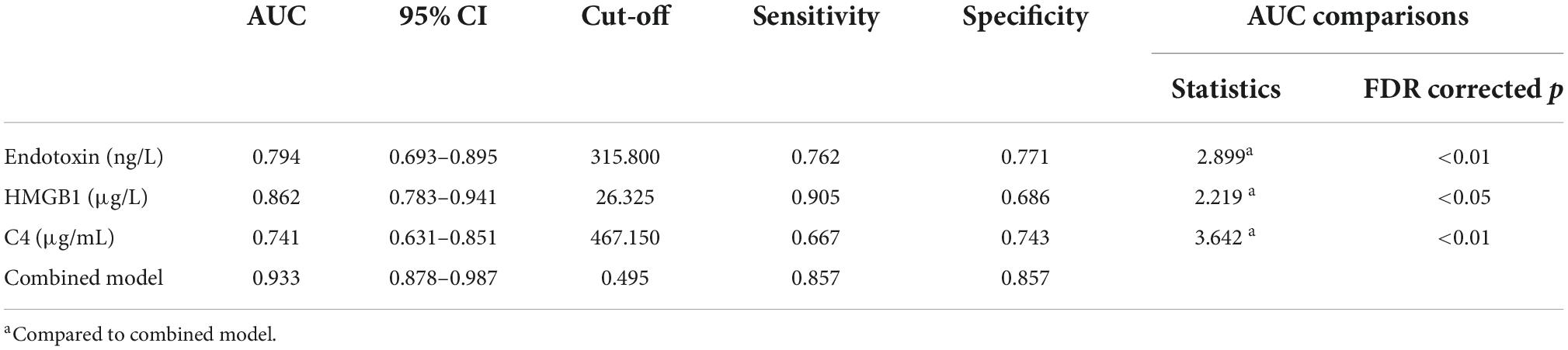
Table 2. ROC curve analysis for differentiating first-episode schizophrenia patients from healthy controls.
Associations of baseline plasma endotoxin, high mobility group box 1 protein, and C4 with clinical assessments
No correlations of the baseline plasma endotoxin, HMGB1, and C4 were observed with age, gender, disease duration, BMI, smoking status, family history of psychiatric disorder, PANSS subscale scores, and total scores, as well as all cognitive dimensions evaluated by MCCB in FES group (all p > 0.05). Since our previous study had found that LPS stimulation resulted in a lower TLR4 expression on monocytes in FES patients as compared to HCs (31), and the blood samples in the present study were also from the same subjects, we further explored the associations of endotoxin, HMGB1, and C4 with monocytic TLR4 levels before and after LPS challenge. However, no significant relationships were observed (all p > 0.05). Additionally, the plasma endotoxin, HMGB1, and C4 were independent from each other in patient group (all p > 0.05). Results were the same in HC group.
Regarding the PSYRATS evaluation in the SCZ patients, we found a positive association between endotoxin concentration and baseline score of AHRS (Spearman rho = 0.504, p < 0.01; Figure 2). Beyond that, we observed no significant relationships neither between HMGB1, C4, and AHRS scores, nor their correlations with DRS scores (all p > 0.05).
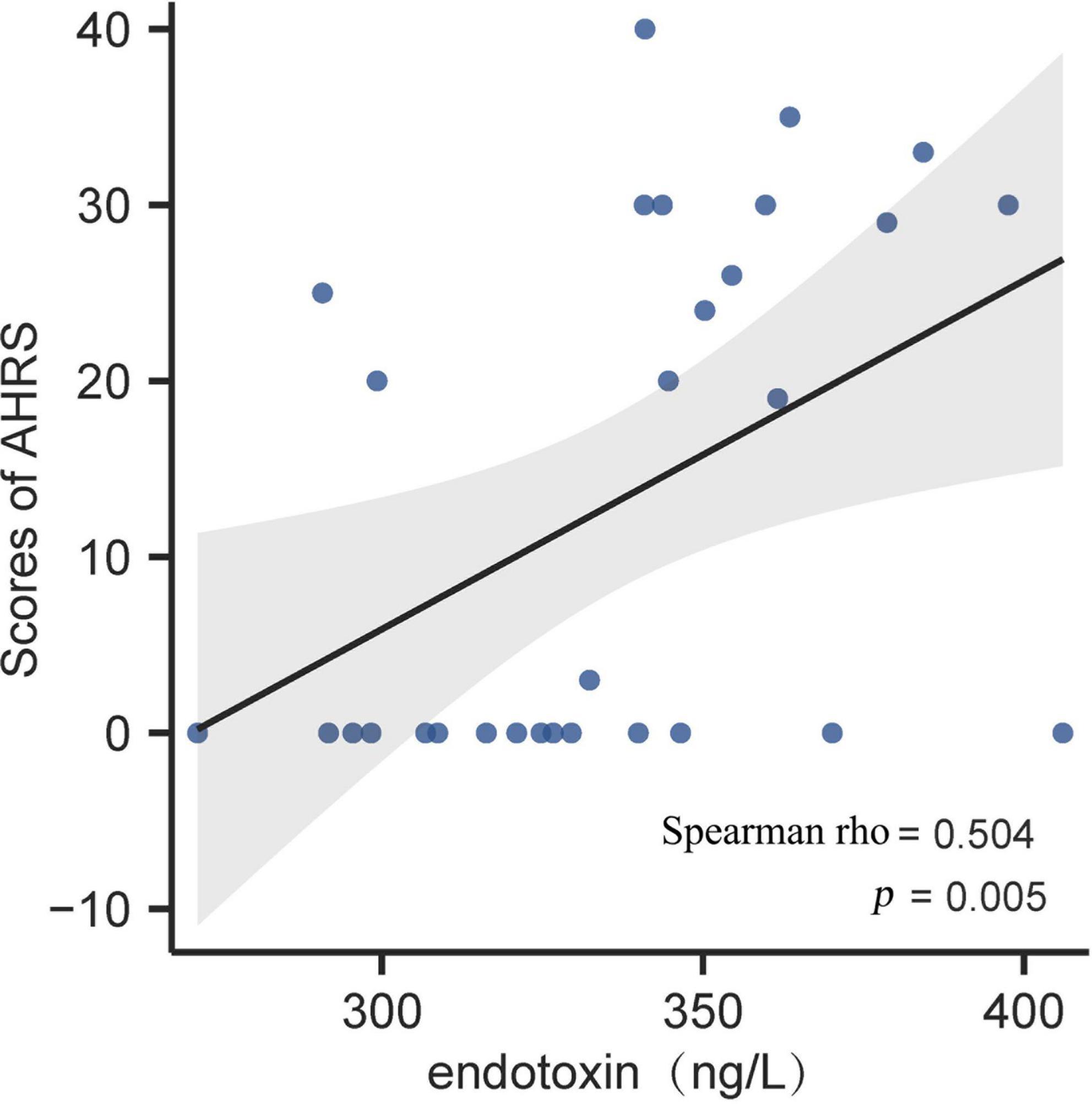
Figure 2. Correlation analysis between plasma endotoxin and AHRS score in first-episode schizophrenia (FES) patients at baseline. Scatter plots indicated that there was a significantly positive association of endotoxin concentration with auditory hallucinations in FES patients. The gray band represents 95% confidence interval. AHRS, auditory hallucinations rating scale.
Changes in plasma endotoxin, high mobility group box 1 protein, and C4 after 8-week treatment as well as their associations with treatment efficacy
Eight-week follow up data were available for 20 patients with SCZ. There were significant improvements in PANSS positive scores, general psychopathology scores and total scores (all p < 0.001; Table 3) over the 8 weeks of treatment. As expected, negative symptoms did not improve in the short term (p > 0.05).
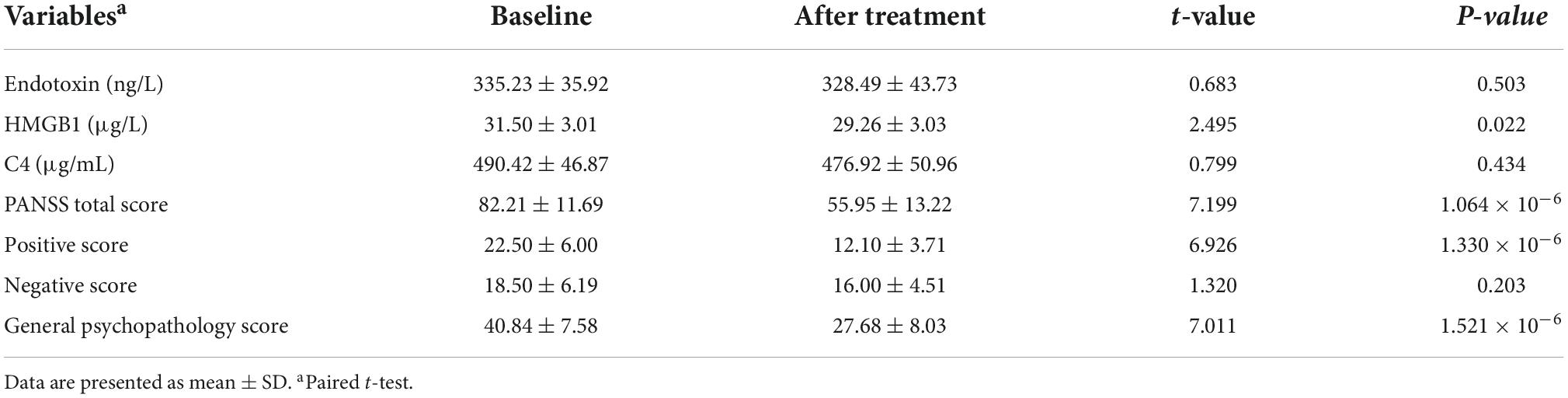
Table 3. Changes in plasma endotoxin, HMGB1, C4, and psychopathological symptoms of 20 patients after 8-week treatment.
There was a significant decrease in HMGB1 level after 8 weeks of treatment (p < 0.05; Table 3), but which was still remarkably higher than that in HCs (29.26 ± 3.03 vs. 25.20 ± 3.56, t = 4.283, p = 7.792 × 10–5). The levels of endotoxin and C4 did not change significantly (both p > 0.05; Table 3).
The levels of endotoxin, HMGB1, and C4 before and after treatment, as well as their changes were not associated with any PANSS subscale score, total score at 8 weeks and changes in PANSS scores by partial correlation controlling for baseline psychopathological symptoms (all p > 0.05).
Discussion
Innate immune dysfunctions have been implied in the pathophysiology of SCZ. In this naturalistic longitudinal study, we selected endotoxin, HMGB1, and C4 as typical representatives of PAMPs, DAMPs, and complement system, respectively, and FES patients with minimal exposure to neuroleptic medications as subjects. There were three important findings. Firstly, circulating levels of endotoxin, HMGB1, and C4 were remarkably increased in FES patients as compared to HCs, and the combination of the three biomarkers showed desirable diagnostic performance in individuals during early stage of SCZ, with an impressive AUC of 0.933. Secondly, the baseline plasma endotoxin levels were found to be positively correlated with the severity of auditory hallucinations, indicating involvement of endotoxin in the pathophysiology of psychosis in FES. Thirdly, the level of plasma HMGB1, but not endotoxin or C4, was decreased significantly after 8 weeks of therapy, albeit still higher than that in HCs.
Immune-inflammatory response triggered by a variety of PAMPs and DAMPs, through PRRs, as well as complement pathway play critical roles in innate immune defense both individually and jointly (7). Normally, small amounts of endotoxin, released from dead Gram-negative bacteria primarily in the gut, are absorbed into the circulation through the intestinal mucosa. Noteworthily, many studies have demonstrated that SCZ is accompanied by increased intestinal permeability and impaired mucosal barrier function (leaky gut), giving rise to bacterial and endotoxin translocation to peripheral circulation (20, 33–35), which may explain the elevated endotoxin levels in FES patients observed in the current study. Endotoxin can destroy the BBB through a variety of mechanisms, and recruit immune mediators into the brain parenchyma, leading to neuroinflammation and disturbed neurotransmitter balance, with downstream consequences of sickness behavior (36, 37).
A recent study by Wang et al. revealed that serum levels of LPS and LPS-responded soluble CD14 were significantly higher in SCZ patients with aggression than in HCs and patients without any aggression, and they were not only positively correlated to proinflammation cytokines but also the severity of aggression (20). Moreover, peripheral anti-LPS IgA antibodies levels were also found to be associated with IL-6 elevation and recent suicide attempts in psychosis (18, 19). To our knowledge, ours is the first study to directly investigate plasma endotoxin level in FES, and we found a positive correlation between it and auditory hallucinations, which are a main positive symptom of SCZ. However, the underlying neurobiological mechanisms remain unclear. One possible explanation is that endotoxin-induced low-grade neuroinflammation could interfere with neurotransmitters, such as dopamine (38), and neurosteroids (39) in the cortical circuits. In an animal study, the corticocortical loop from the auditory association cortex to the orbitofrontal cortex, an underlying neural circuit involved in auditory hallucinations, was dramatically sensitive to dopamine and neurosteroids (40). In addition, it is also important to consider that auditory hallucination itself could evoke chronic psychological stress, which is a well-described gastrointestinal barrier disrupting factor (41), resulting in more endotoxin translocation to bloodstream.
Our result showed that HMGB1 concentration had the largest effect size for difference between the FES patients and HCs, as indicated by partial η2, among the three molecules we measured. A few studies have described the involvement of HMGB1 in psychosis, and our results are highly consistent with existing findings in different stages of SCZ, including patients with FES (21), patients in acute exacerbation phase and chronic patients (17, 42, 43), which all display that SCZ is characterized by increased levels of HMGB1 as compared with HCs. HMGB1 is released by two distinct pathways: passive release by damaged or necrotic cells, and active secretion from activated innate immune cells (such as monocytes, macrophages, and microglia) (15). We speculated that both mechanisms might contribute to the elevated peripheral HMGB1 levels in FES patients. HMGB1 can cause BBB breakdown and aggravate neuroinflammation and oxidative stress in neurological diseases (13–16). Additionally, it is worth mentioning that growing evidence (44–47), including our previous study (31), demonstrates blunted cytokine response to immune stimulations in vitro, implicating the state of immune tolerance or senescence in SCZ. Indeed, endotoxin and extracellular HMGB1 play a critical role in immune tolerance (6, 48, 49), which is not conducive to pathogen clearance and ultimately result in persistent low-grade systemic inflammation and in turn cause further detrimental injury to the intestinal mucosal barrier and tissue cells, thereby forming a vicious cycle.
Our result also showed a significant increase in C4 plasma levels in FES patients relative to HCs, which is consistent with a recent study conducted in patients with FEP (27). Genetic and animal studies have provided convincing evidence for the relationship between C4 and risk of developing SCZ via perturbation of synaptic remodeling (23, 25). So far, serological studies on complement levels in SCZ have remained inconsistent according to a previous systematic review and meta-analysis (50). Nevertheless, more recent individual studies still reported significant increases of C4 in SCZ (51–53). The causes for these discrepancies may relate to ethnicity, disease stage, heterogeneity of SCZ, and the impact of antipsychotics.
Additionally, our ROC analysis further revealed that endotoxin, HMGB1, and C4 concentrations in peripheral blood singly had moderate diagnostic effectiveness with AUC values ranging from 0.741 (C4) to 0.862 (HMGB1), while a combination of the three parameters could improve the diagnostic efficacy to 0.933. As is well established, an AUC value > 0.9 may imply a highly accurate diagnostic performance in clinical practice, suggesting that the three innate immune-related biomarkers selected in our study may be a promising combination for distinguishing FES from HCs.
In the present study, 20 patients were followed up prospectively for their clinical outcome at 8 weeks. We found the concentration of plasma HMGB1 in FES patients was significantly decreased after antipsychotic medication but still higher than HCs, whereas endotoxin and C4 were not affected by the treatment. Coupled with aforementioned cross-sectional results on the peripheral levels of the three biomarkers in SCZ patients, our finding indicates that they may be trait markers in SCZ. To date, only two studies have investigated their changes after treatment in SCZ patients, involving HMGB1 and C4, respectively (21, 53). Interestingly, these two studies were also about Chinese subjects. Consistent with our findings, Zhu et al. also showed a decrease of HMGB1 after risperidone treatment for 6 months, yet higher than HCs (21). However, Su et al. revealed that plasma C4 level could be suppressed by 4-week treatment with antipsychotics, becoming comparable to those of HCs (53). We speculate that the different disease duration and the length of follow-up might be, in part, responsible for this discordance. On the other hand, our results on the absence of correlations of endotoxin, HMGB1, and C4 levels with clinical efficacy as assessed by PANSS were generally similar to those observed in these two studies. Nevertheless, a prospective study enrolling 25 patients with FEP showed that higher baseline C4 level predicted worse clinical outcome at 1-year follow-up (54). In this study, clinical outcome was evaluated by WHO Personal and Psychiatric History Schedule, the focus of which lies on assessing disease relapse, whether or not accompanied with personality changes (54). It is worth noting that all the follow-up sample sizes in our and the other three studies were small, hence necessitating further research to draw a solid conclusion.
Several limitations should be mentioned in our study. Firstly, due to the small sample size, we did not consolidate our findings with another validation dataset. Therefore, caution should be taken in interpreting our results. Secondly, the present study could not establish the causal associations between increased endotoxin, HMGB1, C4, and SCZ. Moreover, the FES patients in our study had short-term psychotropic drugs exposure at baseline, hence, we cannot completely rule out the possible influences of this confounder on the plasma levels of the three biomarkers. Thirdly, we only examined total C4 levels rather than two isoforms of C4 including C4a and C4b, which are two functionally distinct isotypes (55). It is noted that evidence points to a more specific association of C4a in the peripheral blood and brain with SCZ risk (22, 23, 56, 57). Fourthly, as mentioned previously, we cannot draw definitive conclusions regarding whether endotoxin, HMGB1, and C4 could be affected by antipsychotic medication or remission status of disease, and thus these need to be further confirmed in longitudinal studies with larger sample size, longer follow-up periods and more comprehensive assessment of clinical outcomes. Finally, due to the naturalistic follow-up design, we did not stipulate the type and dosage of antipsychotics, notwithstanding different drugs might have different impacts on the three biomarkers, which should be clarified in the future.
Conclusion
Despite these limitations, this study is the first to holistically explore endotoxin, HMGB1, and C4 in FES individuals with minimal exposure to antipsychotics, revealing that a combination of the three biomarkers in the peripheral blood can be used to distinguish FES patients from HCs, indicating its clinical usefulness. In addition, we found that endotoxin showed a significant positive correlation with auditory hallucinations, suggesting that endotoxin might be implicated in the pathogenesis of this important psychotic symptom, and might also be a potentially interesting target for future therapy. Future study should determine whether the immune variables measured here are specific to SCZ, in other words, whether they have the ability of differential diagnosis among various mental disorders.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethical Committee of Beijing HuiLongGuan Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LT and YT initiated and directed the study. SC, MG, WC, and MX were responsible of recruitment of study subjects and clinical assessments. LT, YT, and SC performed statistical analysis and interpreted results. SC and LT wrote the first draft of the manuscript in consultation with YT. HF invited in evolving the ideas, analyzing data, and editing the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82001415, SC and 82171507, YT) and the Estonian Research Council-European Union Regional Developmental Fund Mobilitas Pluss Program No. MOBTT77 (LT).
Acknowledgments
We thank all individuals who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, et al. The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol Neurobiol. (2020) 57:778–97. doi: 10.1007/s12035-019-01737-z
2. van Mierlo HC, Schot A, Boks MPM, de Witte LD. The association between schizophrenia and the immune system: review of the evidence from unbiased ‘Omic-studies’. Schizophr Res. (2020) 217:114–23. doi: 10.1016/j.schres.2019.05.028
3. Ibrahim ZA, Armour CL, Phipps S, Sukkar MB. RAGE and TLRs: relatives, friends or neighbours? Mol Immunol. (2013) 56:739–44. doi: 10.1016/j.molimm.2013.07.008
4. Bertheloot D, Latz E. HMGB1, IL-1alpha, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. (2017) 14:43–64. doi: 10.1038/cmi.2016.34
5. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. (2005) 5:331–42. doi: 10.1038/nri1594
6. Li S, Luo C, Yin C, Peng C, Han R, Zhou J, et al. Endogenous HMGB1 is required in endotoxin tolerance. J Surg Res. (2013) 185:319–28. doi: 10.1016/j.jss.2013.05.062
7. Gaboriaud C, Lorvellec M, Rossi V, Dumestre-Perard C, Thielens NM. Complement system and alarmin HMGB1 crosstalk: for better or worse. Front Immunol. (2022) 13:869720. doi: 10.3389/fimmu.2022.869720
8. Karasu E, Nilsson B, Kohl J, Lambris JD, Huber-Lang M. Targeting complement pathways in polytrauma- and sepsis-induced multiple-organ dysfunction. Front Immunol. (2019) 10:543. doi: 10.3389/fimmu.2019.00543
9. Bergdolt L, Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog Neurobiol. (2019) 175:1–19. doi: 10.1016/j.pneurobio.2018.12.002
10. Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. (2010) 24:881–97. doi: 10.1016/j.bbi.2010.03.005
11. Talukdar PM, Abdul F, Maes M, Binu VS, Venkatasubramanian G, Kutty BM, et al. Maternal immune activation causes schizophrenia-like behaviors in the offspring through activation of immune-inflammatory, oxidative and apoptotic pathways, and lowered antioxidant defenses and neuroprotection. Mol Neurobiol. (2020) 57:4345–61. doi: 10.1007/s12035-020-02028-8
12. Nielsen PR, Benros ME, Mortensen PB. Hospital contacts with infection and risk of schizophrenia: a population-based cohort study with linkage of Danish national registers. Schizophr Bull. (2014) 40:1526–32. doi: 10.1093/schbul/sbt200
13. Festoff BW, Sajja RK, van Dreden P, Cucullo L. HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease. J Neuroinflammation. (2016) 13:194. doi: 10.1186/s12974-016-0670-z
14. Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. (2011) 31:1081–92. doi: 10.1523/JNEUROSCI.3732-10.2011
15. Rana T, Behl T, Mehta V, Uddin MS, Bungau S. Molecular insights into the therapeutic promise of targeting HMGB1 in depression. Pharmacol Rep. (2021) 73:31–42. doi: 10.1007/s43440-020-00163-6
16. Taverna S, Tonacci A, Ferraro M, Cammarata G, Cuttitta G, Bucchieri S, et al. High mobility group box 1: biological functions and relevance in oxidative stress related chronic diseases. Cells. (2022) 11:849. doi: 10.3390/cells11050849
17. Al-Dujaili AH, Mousa RF, Al-Hakeim HK, Maes M. High mobility group protein 1 and dickkopf-related protein 1 in schizophrenia and treatment-resistant schizophrenia: associations with interleukin-6, symptom domains, and neurocognitive impairments. Schizophr Bull. (2021) 47:530–41. doi: 10.1093/schbul/sbaa136
18. Delaney S, Fallon B, Alaedini A, Yolken R, Indart A, Feng T, et al. Inflammatory biomarkers in psychosis and clinical high risk populations. Schizophr Res. (2019) 206:440–3. doi: 10.1016/j.schres.2018.10.017
19. Dickerson F, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, et al. The association between immune markers and recent suicide attempts in patients with serious mental illness: a pilot study. Psychiatry Res. (2017) 255:8–12. doi: 10.1016/j.psychres.2017.05.005
20. Wang C, Zhang T, He L, Fu JY, Deng HX, Xue XL, et al. Bacterial translocation associates with aggression in schizophrenia inpatients. Front Syst Neurosci. (2021) 15:704069. doi: 10.3389/fnsys.2021.704069
21. Zhu Q, Li X, Hie G, Yuan X, Lu L, Song X. [Analysis of the changes of serum high mobility group protein B1 and cytokines in first-episode schizophrenia patients]. Zhonghua Yi Xue Za Zhi. (2015) 95:3818–22.
22. Woo JJ, Pouget JG, Zai CC, Kennedy JL. The complement system in schizophrenia: where are we now and what’s next? Mol Psychiatry. (2020) 25:114–30. doi: 10.1038/s41380-019-0479-0
23. Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. (2016) 530:177–83. doi: 10.1038/nature16549
24. Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. (2019) 22:374–85. doi: 10.1038/s41593-018-0334-7
25. Comer AL, Jinadasa T, Sriram B, Phadke RA, Kretsge LN, Nguyen TPH, et al. Increased expression of schizophrenia-associated gene C4 leads to hypoconnectivity of prefrontal cortex and reduced social interaction. PLoS Biol. (2020) 18:e3000604. doi: 10.1371/journal.pbio.3000604
26. Duchatel RJ, Meehan CL, Harms LR, Michie PT, Bigland MJ, Smith DW, et al. Increased complement component 4 (C4) gene expression in the cingulate cortex of rats exposed to late gestation immune activation. Schizophr Res. (2018) 199:442–4. doi: 10.1016/j.schres.2018.03.035
27. Chen Y, Zhao Z, Lin F, Wang L, Lin Z, Yue W. Associations between genotype and peripheral complement proteins in first-episode psychosis: evidences from C3 and C4. Front Genet. (2021) 12:647246. doi: 10.3389/fgene.2021.647246
28. Kopczynska M, Zelek W, Touchard S, Gaughran F, Di Forti M, Mondelli V, et al. Complement system biomarkers in first episode psychosis. Schizophr Res. (2019) 204:16–22. doi: 10.1016/j.schres.2017.12.012
29. Laskaris L, Zalesky A, Weickert CS, Di Biase MA, Chana G, Baune BT, et al. Investigation of peripheral complement factors across stages of psychosis. Schizophr Res. (2019) 204:30–7. doi: 10.1016/j.schres.2018.11.035
30. Xia XW, Li LJ, Chen ZC, Qiu Y, Zhao JS, Wu JY, et al. [Correlation of the peripheral serum complement protein levels and cognitive function in first-episode drug-naive patients with schizophrenia]. Zhonghua Yi Xue Za Zhi. (2020) 100:3081–5. doi: 10.3760/cma.j.cn112137-20200425-01316
31. Chen S, Tian L, Chen N, Xiu MH, Wang ZR, Wang YC, et al. More dampened monocytic Toll-like receptor 4 response to lipopolysaccharide and its association with cognitive function in Chinese Han first-episode patients with schizophrenia. Schizophr Res. (2019) 206:300–6. doi: 10.1016/j.schres.2018.11.001
32. Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med. (1999) 29:879–89. doi: 10.1017/s0033291799008661
33. Fan Y, Gao Y, Ma Q, Yang Z, Zhao B, He X, et al. Multi-omics analysis reveals aberrant gut-metabolome-immune network in schizophrenia. Front Immunol. (2022) 13:812293. doi: 10.3389/fimmu.2022.812293
34. Rudzki L, Szulc A. “Immune gate” of psychopathology-the role of gut derived immune activation in major psychiatric disorders. Front Psychiatry. (2018) 9:205. doi: 10.3389/fpsyt.2018.00205
35. Szeligowski T, Yun AL, Lennox BR, Burnet PWJ. The gut microbiome and schizophrenia: the current state of the field and clinical applications. Front Psychiatry. (2020) 11:156. doi: 10.3389/fpsyt.2020.00156
36. Peng X, Luo Z, He S, Zhang L, Li Y. Blood-brain barrier disruption by lipopolysaccharide and sepsis-associated encephalopathy. Front Cell Infect Microbiol. (2021) 11:768108. doi: 10.3389/fcimb.2021.768108
37. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. (2019) 99:21–78. doi: 10.1152/physrev.00050.2017
38. Wang X, Northcutt AL, Cochran TA, Zhang X, Fabisiak TJ, Haas ME, et al. Methamphetamine Activates toll-like receptor 4 to induce central immune signaling within the ventral tegmental area and contributes to extracellular dopamine increase in the nucleus accumbens shell. ACS Chem Neurosci. (2019) 10:3622–34. doi: 10.1021/acschemneuro.9b00225
39. Izumi Y, Cashikar AG, Krishnan K, Paul SM, Covey DF, Mennerick SJ, et al. A proinflammatory stimulus disrupts hippocampal plasticity and learning via microglial activation and 25-hydroxycholesterol. J Neurosci. (2021) 41:10054–64. doi: 10.1523/JNEUROSCI.1502-21.2021
40. Tripathi A, Sato SS, Medini P. Cortico-cortical connectivity behind acoustic information transfer to mouse orbitofrontal cortex is sensitive to neuromodulation and displays local sensory gating: relevance in disorders with auditory hallucinations? J Psychiatry Neurosci. (2021) 46:E371–87. doi: 10.1503/jpn.200131
41. Ilchmann-Diounou H, Menard S. Psychological stress, intestinal barrier dysfunctions, and autoimmune disorders: an overview. Front Immunol. (2020) 11:1823. doi: 10.3389/fimmu.2020.01823
42. Kozlowska E, Brzezinska-Blaszczyk E, Agier J, Wysokinski A, Zelechowska P. Alarmins (IL-33, sST2, HMGB1, and S100B) as potential biomarkers for schizophrenia. J Psychiatr Res. (2021) 138:380–7. doi: 10.1016/j.jpsychires.2021.04.019
43. Yilmaz N, Yelboga Z, Yilmaz Y, Demirpence O. High mobility group box-1 levels in schizophrenia: potential biomarker of remission phase. J Med Biochem. (2021) 40:295–301. doi: 10.5937/jomb0-28108
44. Gao Y, Fan Y, Yang Z, Ma Q, Zhao B, He X, et al. Systems biological assessment of altered cytokine responses to bacteria and fungi reveals impaired immune functionality in schizophrenia. Mol Psychiatry. (2022) 27:1205–16. doi: 10.1038/s41380-021-01362-0
45. Keri S, Szabo C, Kelemen O. Uniting the neurodevelopmental and immunological hypotheses: neuregulin 1 receptor ErbB and Toll-like receptor activation in first-episode schizophrenia. Sci Rep. (2017) 7:4147. doi: 10.1038/s41598-017-03736-3
46. Muller N, Wagner JK, Krause D, Weidinger E, Wildenauer A, Obermeier M, et al. Impaired monocyte activation in schizophrenia. Psychiatry Res. (2012) 198:341–6. doi: 10.1016/j.psychres.2011.12.049
47. Tarantino N, Leboyer M, Bouleau A, Hamdani N, Richard JR, Boukouaci W, et al. Natural killer cells in first-episode psychosis: an innate immune signature? Mol Psychiatry. (2021) 26:5297–306. doi: 10.1038/s41380-020-01008-7
48. Lopez-Collazo E, del Fresno C. Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit Care. (2013) 17:242. doi: 10.1186/cc13110
49. Watanabe H, Son M. The immune tolerance role of the HMGB1-RAGE axis. Cells. (2021) 10:564. doi: 10.3390/cells10030564
50. Mongan D, Sabherwal S, Susai SR, Focking M, Cannon M, Cotter DR. Peripheral complement proteins in schizophrenia: a systematic review and meta-analysis of serological studies. Schizophr Res. (2020) 222:58–72. doi: 10.1016/j.schres.2020.05.036
51. Ji E, Boerrigter D, Cai HQ, Lloyd D, Bruggemann J, O’Donnell M, et al. Peripheral complement is increased in schizophrenia and inversely related to cortical thickness. Brain Behav Immun. (2022) 101:423–34. doi: 10.1016/j.bbi.2021.11.014
52. Mohd Asyraf AJ, Nour El Huda AR, Hanisah MN, Norsidah KZ, Norlelawati AT. Relationship of selective complement markers with schizophrenia. J Neuroimmunol. (2022) 363:577793. doi: 10.1016/j.jneuroim.2021.577793
53. Su J, Feng X, Chen K, Fang Z, Zhang H. Plasma complement component 4 alterations in patients with schizophrenia before and after antipsychotic treatment. Asian J Psychiatr. (2022) 73:103110. doi: 10.1016/j.ajp.2022.103110
54. Mondelli V, Di Forti M, Morgan BP, Murray RM, Pariante CM, Dazzan P. Baseline high levels of complement component 4 predict worse clinical outcome at 1-year follow-up in first-episode psychosis. Brain Behav Immun. (2020) 88:913–5. doi: 10.1016/j.bbi.2020.01.014
55. Chung EK, Yang Y, Rennebohm RM, Lokki ML, Higgins GC, Jones KN, et al. Genetic sophistication of human complement components C4A and C4B and RP-C4-CYP21-TNX (RCCX) modules in the major histocompatibility complex. Am J Hum Genet. (2002) 71:823–37. doi: 10.1086/342777
56. Hatzimanolis A, Foteli S, Stefanatou P, Ntigrintaki AA, Ralli I, Kollias K, et al. Deregulation of complement components C4A and CSMD1 peripheral expression in first-episode psychosis and links to cognitive ability. Eur Arch Psychiatry Clin Neurosci. (2022) 272:1219–28. doi: 10.1007/s00406-022-01409-5
Keywords: first-episode schizophrenia (FES), endotoxin, high mobility group box 1 protein (HMGB1), complement component 4, biomarker
Citation: Chen S, Gou M, Chen W, Xiu M, Fan H, Tan Y and Tian L (2022) Alterations in innate immune defense distinguish first-episode schizophrenia patients from healthy controls. Front. Psychiatry 13:1024299. doi: 10.3389/fpsyt.2022.1024299
Received: 24 August 2022; Accepted: 26 September 2022;
Published: 13 October 2022.
Edited by:
Anilkumar Pillai, University of Texas Health Science Center at Houston, United StatesReviewed by:
Xinyu Fang, Nanjing Brain Hospital Affiliated to Nanjing Medical University, ChinaHussein Kadhem Al-Hakeim, University of Kufa, Iraq
Copyright © 2022 Chen, Gou, Chen, Xiu, Fan, Tan and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlong Tan, eWx0YW4yMUAxMjYuY29t; Li Tian, bGkudGlhbkB1dC5lZQ==
 Song Chen
Song Chen Mengzhuang Gou1
Mengzhuang Gou1 Meihong Xiu
Meihong Xiu Hongzhen Fan
Hongzhen Fan Yunlong Tan
Yunlong Tan Li Tian
Li Tian