- 1Department of Gynecology and Obstetrics, Shanghai Jiao Tong University of Medicine Affiliated Sixth People’s Hospital, Shanghai, China
- 2Department of Gynecology and Obstetrics, Shanghai Eighth People’s Hospital, Affiliated to Jiangsu University, Shanghai, China
Objective: To delineate the association between sleep characteristics and renal function in peri-post menopause free of Chronic kidney disease (CKD) as well as cardiometabolic and hormone indicators.
Methods: Cross-sectional data from a total of 823 Han-Chinese women aged 40–67 years who visited the Menopause Clinic in the Shanghai Sixth People’s Hospital from November 2011 to November 2020 were analyzed through the Pittsburgh Sleep Quality Index (PSQI) and serum cystatin C (Cys-C). Logistic regression models were used to assess the association between cumulative/each sleep parameter and renal function after adjusting for cardiometabolic variables.
Results: After confounding factors, we identified that poor perceived sleep quality, shorter sleep duration (<6 h), low sleep efficiency (<75%), delayed sleep latency and worse sleep disturbance elevated more than doubled the odds ratio for declining renal function (≥0.91 mg/dL, the highest Cys-C) in postmenopause in a graded fashion. Meanwhile, multiple logistic regression analysis revealed that sleep disorder (PSQI ≥ 8), late postmenopause, highest quartile independently increased the odds ratio for declining renal function (OR 2.007, 95% CI: 1.408–2.861, OR = 3.287, 95%CI: 3.425–8.889, OR = 2.345, 95% CI: 1.310–4.199, respectively), while participants with menopausal hormone replacement (MHT) lower the odds of declining renal function (OR = 0.486, 95% CI: 0.324–0.728).
Conclusion: The findings proposed that maintaining good sleep quality should be attached great importance to postmenopausal women, which provides clinical evidence for the feasible early detection and effective prevention such as MHT of renal disease progression in postmenopausal women.
Introduction
Chronic kidney disease (CKD) is determined as ongoing deterioration of renal function manifested by decreased glomerular filtration rate (GFR), leading to an increasing risk of hospitalization and mortality (1), and thus results in substantial health economic burden globally (2). As an endocrine organ, the kidney serves as the main target for hormone action (3). Many studies have revealed that menopause was supported to be associated with a higher risk of developing CKD due to diminished ovarian hormones (3–5). Therefore, identification of potential patients in menopause is paramount for the initiation of effective therapies to slow or delay disease progression.
Although estimated GFR (eGFR), based on the measurement of serum creatinine, is the most commonly used method to evaluate renal function, the change of creatinine is not significant in the early stages of renal impairment and influenced by muscle mass and body weight (6), which could result in less sensitivity of eGFR measurements (7). While Serum cystatin C (Cys-C), constantly secreted from all nucleated cells, has been purported as a more sensitive and specific biomarker than serum creatinine (8, 9). Therefore, Cys-C may be highly applicable as an early marker of preclinical renal disease. In addition, it serves as a much better-diagnostic tool for kidney function independent of age, sex, inflammation, liver disease, diet, and individual constitution and muscle mass (10, 11).
Menopause is a critical physiological stage of women’s life with various complaints. Besides vasomotor symptoms, sleep disorder is another marker of menopause (12). Women who experience poor sleep are more vulnerable to diseases, which is of great concern for women’s life quality and long-term health. In addition, a review proposed that OSAHS (obstructive sleep apnea-hypopnea syndrome) may contribute to CKD development either indirectly through its influences on diabetes, obesity and hypertension, or directly through the sympathetic nervous system and renin-angiotensin-aldosterone system (13). However, literature on the relationship between sleep disorder and clinically latent renal disease in menopause is scant.
As the burden of CKD in women after menopause is increasing, there is an emerging need for menopausal women-based research designed to disentangle the interactions between sleep disorder and renal function. In this study, we aim to investigate the association between sleep characteristics evaluated by the Pittsburgh Sleep Quality Index (PSQI) and serum Cys-C in terms of menopause, to identify the potential predicting value of sleep disorder for preclinical kidney disease among Chinese women without CKD in different menopausal status.
Materials and methods
Study design and participants
This cross-sectional study enrolled participants who visited the Menopause Clinic in the Shanghai Sixth People’s Hospital from November 2011 to November 2020. Han-Chinese woman aged 40–67 years were recruited. The study protocol was approved by the Ethics Committee of Shanghai Sixth People’s Hospital, and the study was performed in accordance with the approved guidelines. All the participants provided written informed consents after full explanation of the study. All study protocols were performed in accordance with the principles of the Declaration of Helsinki. Participants were excluded as follows: (1) suspected renal insufficiency, with an eGFR less than 60 mL/min/1.73 m2; (2) history of chronic nephritis, nephrotic syndrome, nephrectomy, polycystic kidney disease, organ or bone marrow transplant, immunosuppressive drugs for kidney disease in the past 6 months; (3) night work shifts and irregular sleep schedule; (4) menopausal hormone replacement (MHT) past users (women who reported past not current use for over previous 6 months); (5) current smoking (at least once per week for the previous 6 months); (6) progressive malignancy currently undergoing radiotherapy or chemotherapy; (7) with missing data. Ultimately, 823 participants were recruited in this study.
General questionnaire
A general questionnaire (14–16) was used by well-trained investigators through face-to-face interview to collect sociodemographic information, including age, last menstrual period, education, marital status, employment status, income per month, years since menopause, MHT use (including estradiol + dydrogesterone, estradiol only, tibolone), history of chronic disease (hypertension, diabetes mellitus, CKD, metabolic syndrome, dyslipidemia, obesity, as well as medication use), lifestyle (i.e., smoking, alcohol consumption). Women who reported current use ≥ 6 months were classified as current MHT users, while women who had never taken were classified as never users. On the basis of the Stages of Reproductive Aging Workshop (STRAW + 10) (17), participants were categorized into peri-menopausal group (consecutive irregularities over 7 days of menstrual cycle), early postmenopausal group (absence of menstrual periods for 1–5 years) and late postmenopausal group (absence of menstrual periods for 5 years or more) (14).
Anthropometric and lab parameters
Height (cm) and weight (kg) were recorded and used to computed Body mass index BMI (kg/m2). Blood pressure was measured with the average of the 3 readings after 5-min sitting 0.19 Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mm Hg, diastolic blood pressure (DBP) ≥ 90 mm Hg or use of antihypertensive medications (16).
After an overnight fast for at least 10 h, venous blood samples were collected for all study participants for biochemical measurements analysis. Serum Cys-C, creatine, total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and creatinine were measured using an automated AU-5800 analyzer (Beckman Coulter, Brea, CA, USA). Fasting plasma glucose (FPG) was measured with the glucose oxidase method using an automated AU-5800 analyzer (Beckman Coulter, Brea, CA, USA). Female sex hormone levels were evaluated by chemiluminescence (Cobas E601; Roche, Basel, Switzerland). The sensitivity for follicle-stimulating hormone (FSH) detection was 0.100 mIU/mL, and the range of measurement was 0.100–200.0 mIU/mL; for estradiol (E2), the sensitivity and range of measurement was 5 pg/mL and 5–3,000 pg/mL, respectively. Intra- and inter-assay coefficients of variation were always <5% for FSH and E2.
Definitions of study outcomes
Dyslipidemia was defined as previous diagnosis or meeting any of the following criteria: (1) TC ≥ 6.22 mmol/L; (2) TG ≥ 2.26 mmol/L; (3) LDL ≥ 4.14 mmol/L; (4) HDL<1.05 mmol/L (18). Hypertension and diabetes were diagnosed based on self-reported previous diagnosis, or by criteria ≥ 140/90 mmHg and FPG ≥ 7 mmol/L (18, 19). BMI ≥ 28 kg/m2 was regarded as obesity. Definition of metabolic syndrome (MetS) was defined as the presence of two or more of the following components (20): (1) obesity (waist-hip-ratio > 0.85 and/or BMI ≥ 28 kg/m2); (2) TG ≥ 1.7 mmol/L; (3) HDL-c < 1.05 mmol/L; (4) blood pressure ≥ 140/90 mmHg or current use of antihypertensive medications; (5) FBG ≥ 7.0 mmol/L. CKD was defined as eGFR < 60 (mL/min/1.73 m2), which was calculated by recently revised CKD Epidemiology Collaboration (CKD-EPI) equation for the Chinese population (21).
Assessment of sleep quality
The validated Chinese version Pittsburgh Sleep Quality Index (PSQI) (22) was used to evaluate sleep quality over the past month for the participants. In brief, 18 items, including in the PSQI, were used to weigh scores based on the following 7 subscales: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleeping medications, and daytime dysfunction. Each parameter ranges from 0 to 3 scale. Subjective sleep quality was categorized into 0 to 3 scores, corresponding to very good, good, poor, very poor, sleep duration into >7 h, 6–7 h, 5–6 h, <5 h, sleep efficiency into>85%, 75–85%, 65–75%, <65%, sleep use of medication into none, <1 time/week, 1–2 times/week, ≥ 3 times/week. The total PSQI ranged from 0 to 21, with a higher score indicating worse sleep quality. A PSQI score of 8 or higher was indicative of sleep disorder (14, 23), which has been recommended in Chinese clinical practice and research.
Statistical analysis
All the variables were tested for normal distribution by Kolmogorov-Smirnov test. Levene’s test of homogeneity of variance were further performed. They were depicted as means ± standard deviation (SD) or number (%). One-way ANOVA (normal distributions), the Kruskal Wallis H-test (skewed continuous variables) and χ2 test (categorical variables) were carried out to compare the differences among the four groups on the quartiles of serum Cys-C levels (Q1:<0.70 mg/dL, Q2:0.71–0.72 mg/dL, Q3:0.73–0.90 mg/dL, Q4: ≥ 0.91 mg/dL). We defined Cys-C with 0.91 mg/dL (Q4, the highest quartile group) as declining renal function. The multivariate logistic regression analyses were performed to examine independent determinants for Q4. In addition, the association between each sleep parameter and Q4 was computed by logistic regression analysis. A two-sided p < 0.05 was considered to be a significant difference. Logistic regression model was assessed by the Hosmer-Lemeshow test. All statistical analyses were performed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Characteristics of the study participants based on cystatin-C quartiles
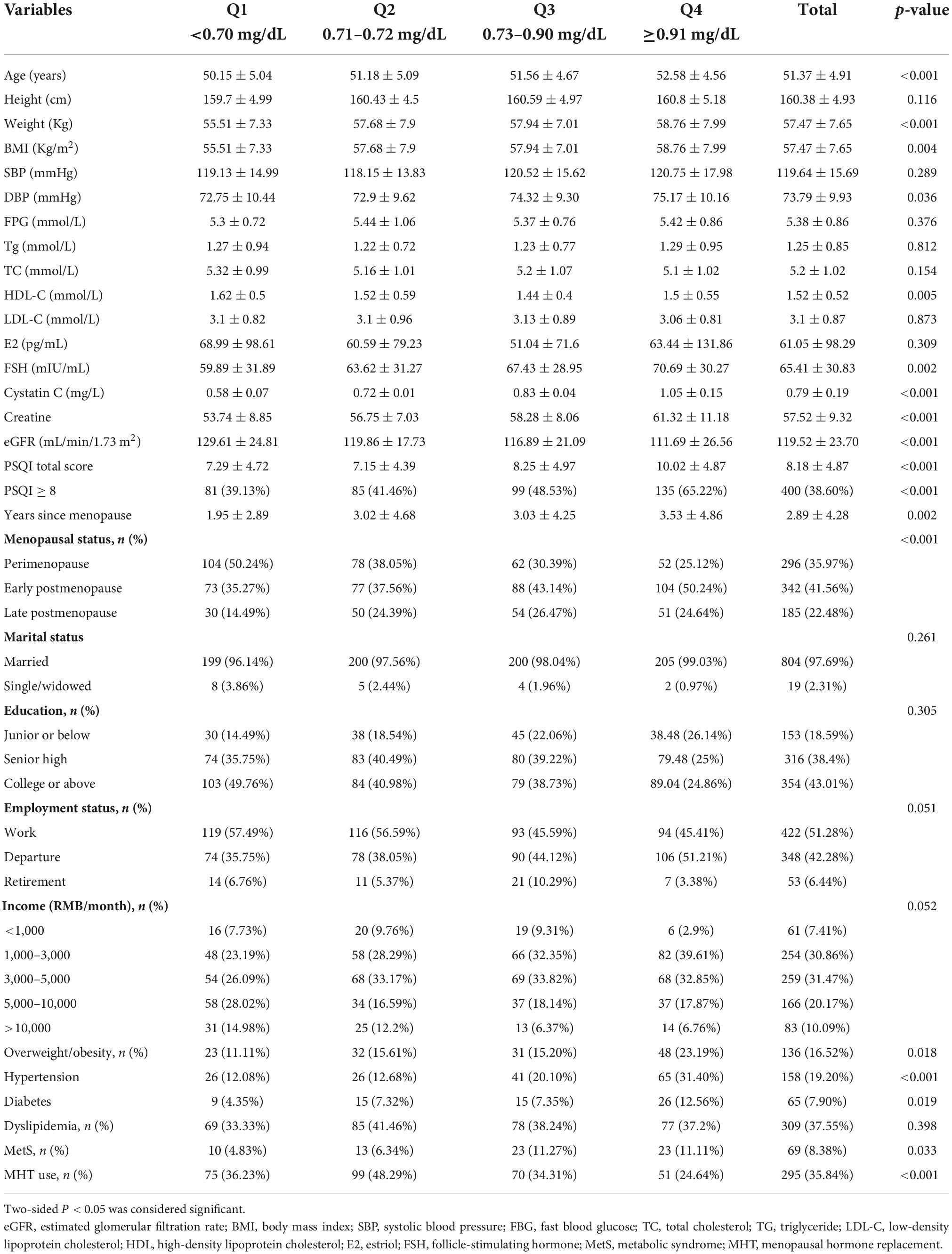
In a total of 823 eligible subjects, the baseline characteristics among quartile groups divided by the serum Cys-C: (Q1: <0.70 mg/dL, Q2: 0.71–0.72 mg/dL, Q3: 0.73–0.90 mg/dL, Q4: ≥ 0.91 mg/dL) were presented in Table 1. The prevalence of sleep disorder (PSQI ≥ 8) was 38.6% in our study.
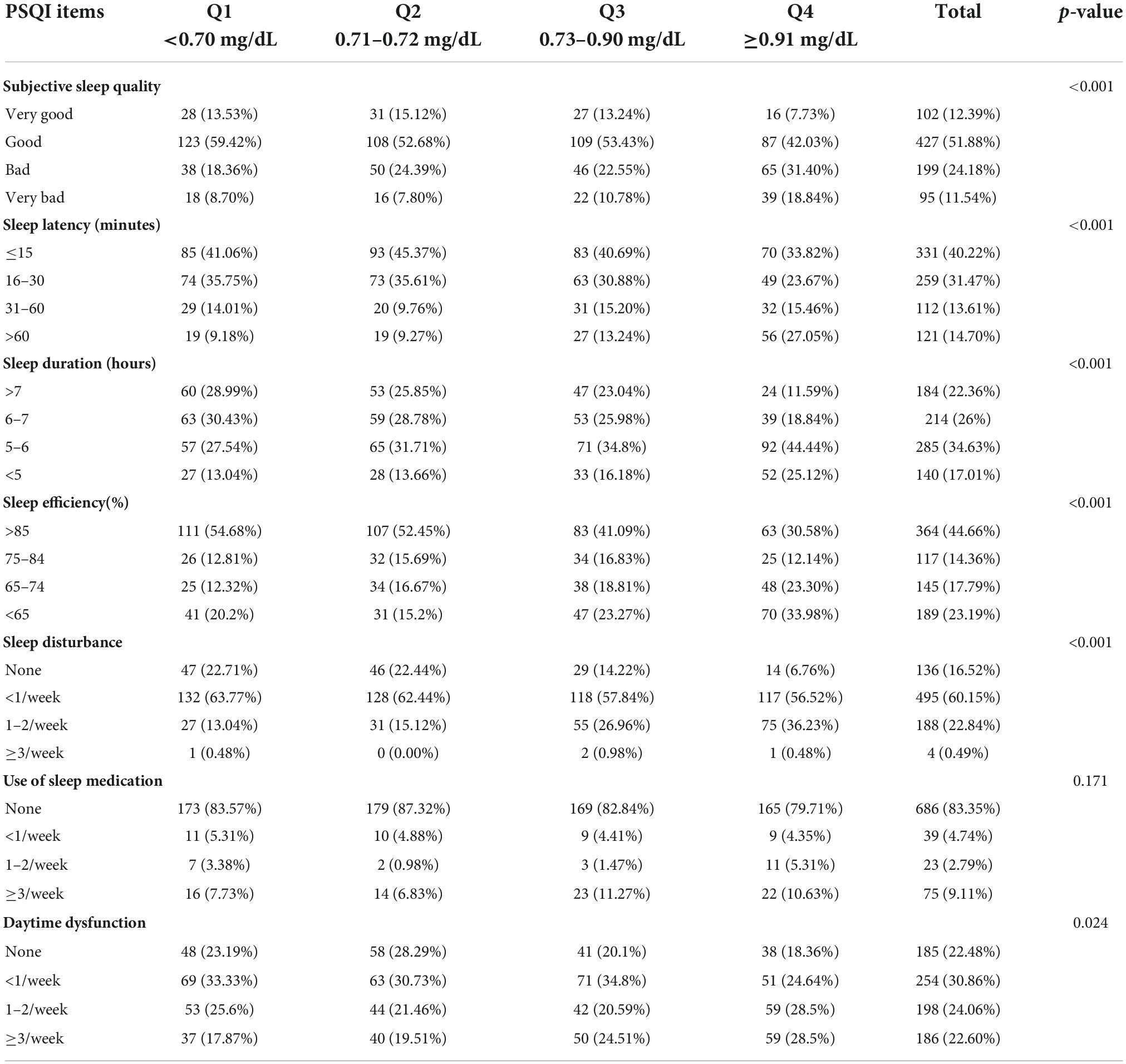
Interestingly, the prevalence of sleep disorder (PSQI ≥ 8) increased in a dose-dependent manner according to the serum of Cys-C: 39.13% (Q1) vs.41.46% (Q2) vs.48.53% (Q3) vs.65.22% (Q4) (p< 0.001). We also observed the growing value of FSH, years since menopause, age, BMI, DBP, creatine, while decreasing level of eGFR from Q1 to Q4 quartiles (p < 0.001). Moreover, there showed a decreasing incidence of MHT use, while an ascending incidence of early, late postmenopause (compared with perimenopause), as well as hypertension, diabetes mellitus with the increasing of Cys-C quartiles (p < 0.05). On the other hand, lipid profiles (including TC, TG, LDL-C, HDL-C), FBG, E2, SBP did not show significant trends across Cys-C quartiles. Furthermore, Table 2 showed score for sub-scales of PSQI based on Cys-C quartiles. There were significant differences in six sub-scales of PSQI (subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance and daytime dysfunction) (All p < 0.05).
Independent determinant factors for declining renal function
To investigate the independent determinant factors for declining renal function, we conducted a multivariate logistic stepwise regression analysis. As shown in Figure 1, after adjusting for confounding covariates, including age, BMI, menopausal status, years since menopause, FBG, lipid profiles (TC, TG, LDL-C, HDL-C), SBP, hypertension, diabetes, obesity, MetS, we identified the independent risk factors for declining renal function as PSQI ≥ 8 [odds ratio (OR) 2.007, 95% confidential interval (CI): 1.408–2.861], hypertension (OR 2.659, 95% CI: 1.624–4.353), diabetes (OR 2.008, 95% CI: 1.008–3.706), early postmenopause (OR 1.624, 95% CI: 1.050–2.511), BMI (OR 1.089, 95% CI: 1.018–1.165), age (OR 1.047, 95% CI: 1.002–1.094), while MHT served as a protective role in renal function (OR = 0.486, 95% CI: 0.324–0.728). In addition, the regression analysis revealed that the ORs of declining renal function were twofold higher (OR = 2.345, 95% CI: 1.310–4.199, p < 0.001) in the highest FSH quartile compared to the lowest.
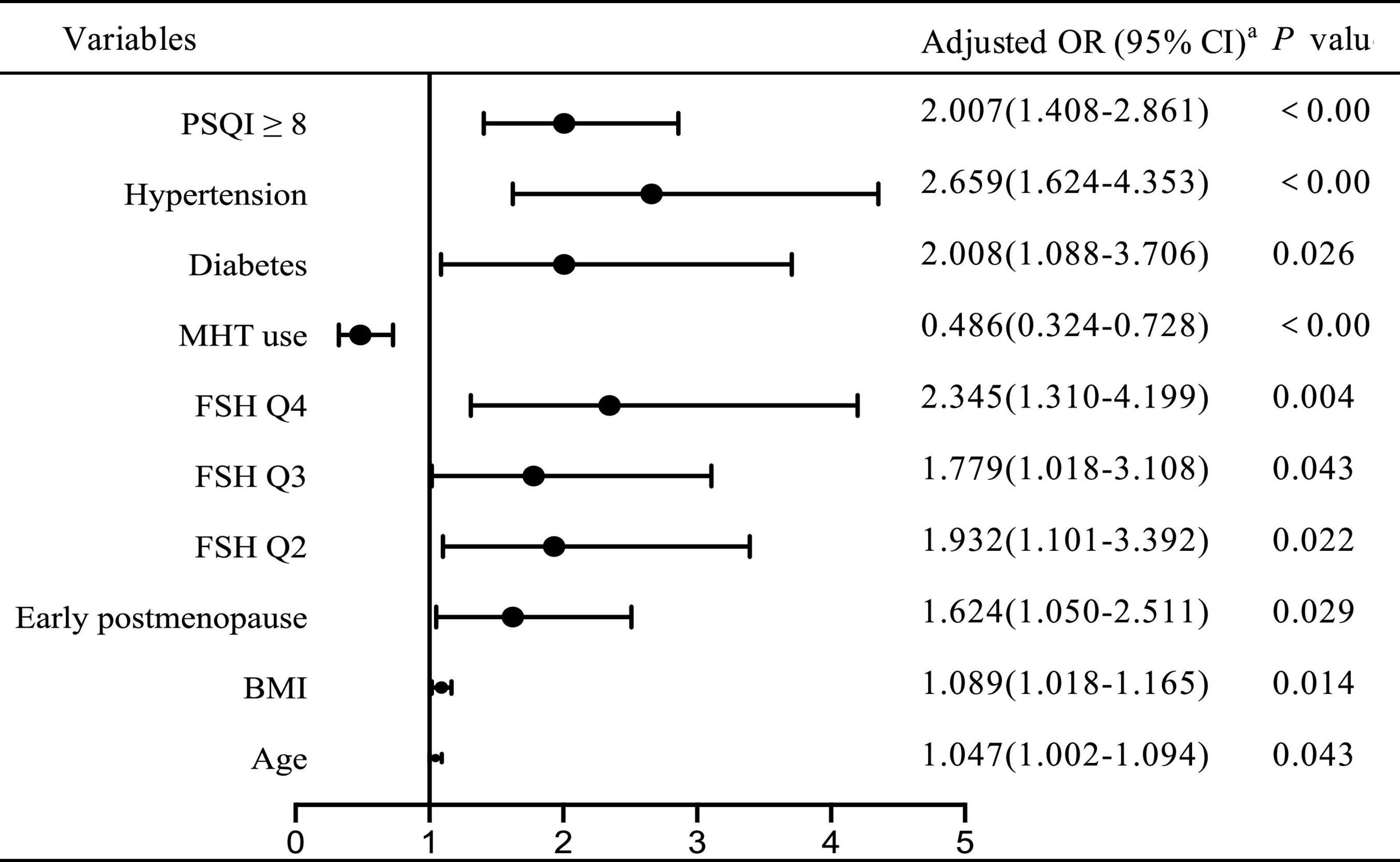
Figure 1. Adjusted odds ratio for declining renal function by logistic regression aadjusted for age, BMI, menopausal status, years since menopause, SBP, FBG, lipid profiles, hypertension, diabetes, obesity, MetS. OR, odds ratio; CI, confidential interval.
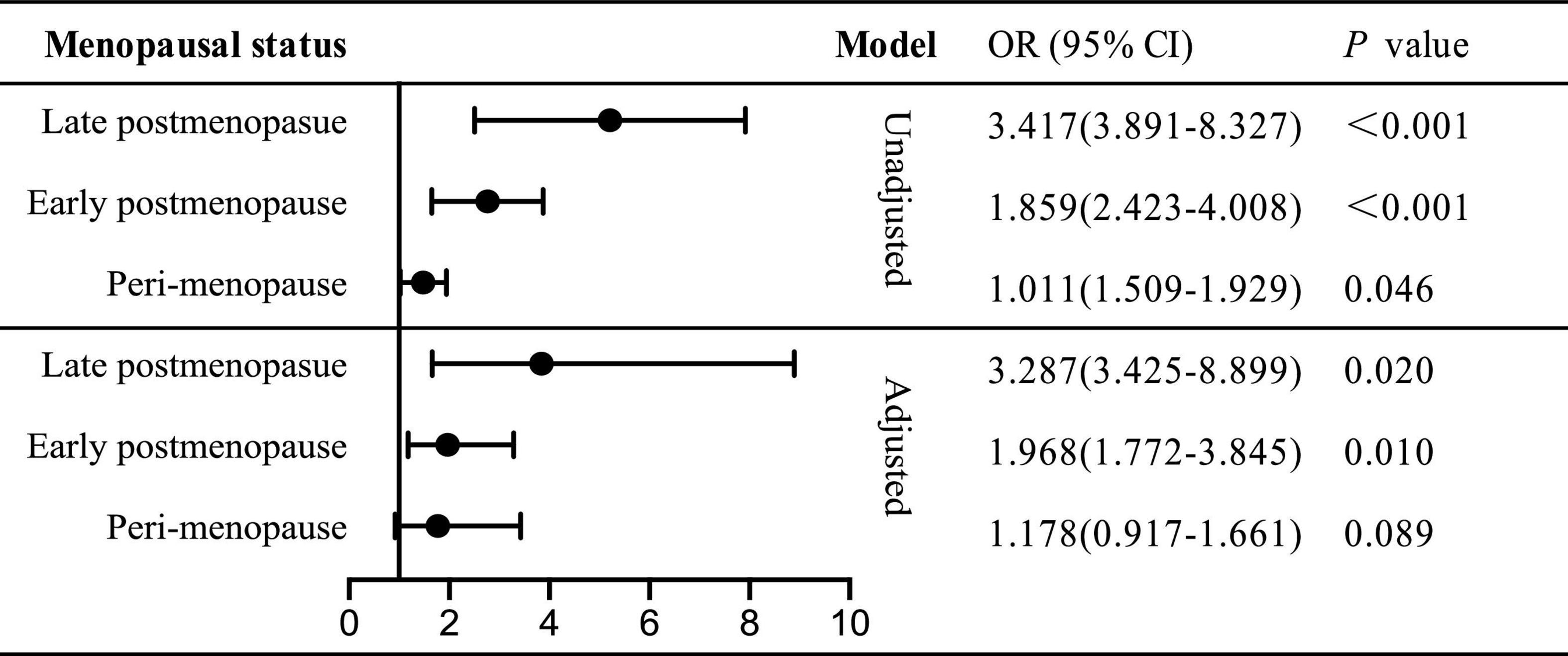
Odds ratio of sleep disorder for declining renal function stratified by menopause status
We further investigated the role of menopause in sleep disorder-renal function relation in unadjusted or multi-covariates adjusted model (Figure 2). Then the total subjects were divided into three groups: peri-, early post- and late post-menopause. However, in peri-menopause, the risk indicator of PSQI ≥ 8 for declining renal function vanished (OR = 1.178, 95% CI: 0.917–1.661, p = 0.089), while the ORs of declining renal function were twofold (OR = 1.968, 95% CI: 1.772–3.845) and threefold (OR = 3.287, 95% CI: 3.425–8.889) higher in early and late postmenopausal status, respectively. Thus, we put forward that in postmenopausal not perimenopause women, sleep disorder served as an independent determinant for declining renal function, while the odds ratio was higher in late postmenopause than in early one.
Odds ratio of each sleep parameter of Pittsburgh Sleep Quality Index for declining renal function
Next, we investigated the association between each sleep parameter and declining renal function. As shown in Figure 3, after multivariable adjustment of confounding factors, compared with >7 h (score 0), 5–6 h [score 2 OR 95% CI: 3.073 (1.824–5.178)] and <5 h [score 3 OR 95% CI: 3.295 (1.837–5.91), p<0.001] were associated with threefold higher odds ratio for declining renal function. Compared with sleep efficiency 85% (score 0), 65–75% [score 2 OR 95% CI: 2.145 (1.351–3.403)] and<65% [score 3 OR 95% CI: 2.338 (1.522–3.591), p<0.001] were associated with twofold higher odds for declining renal function.
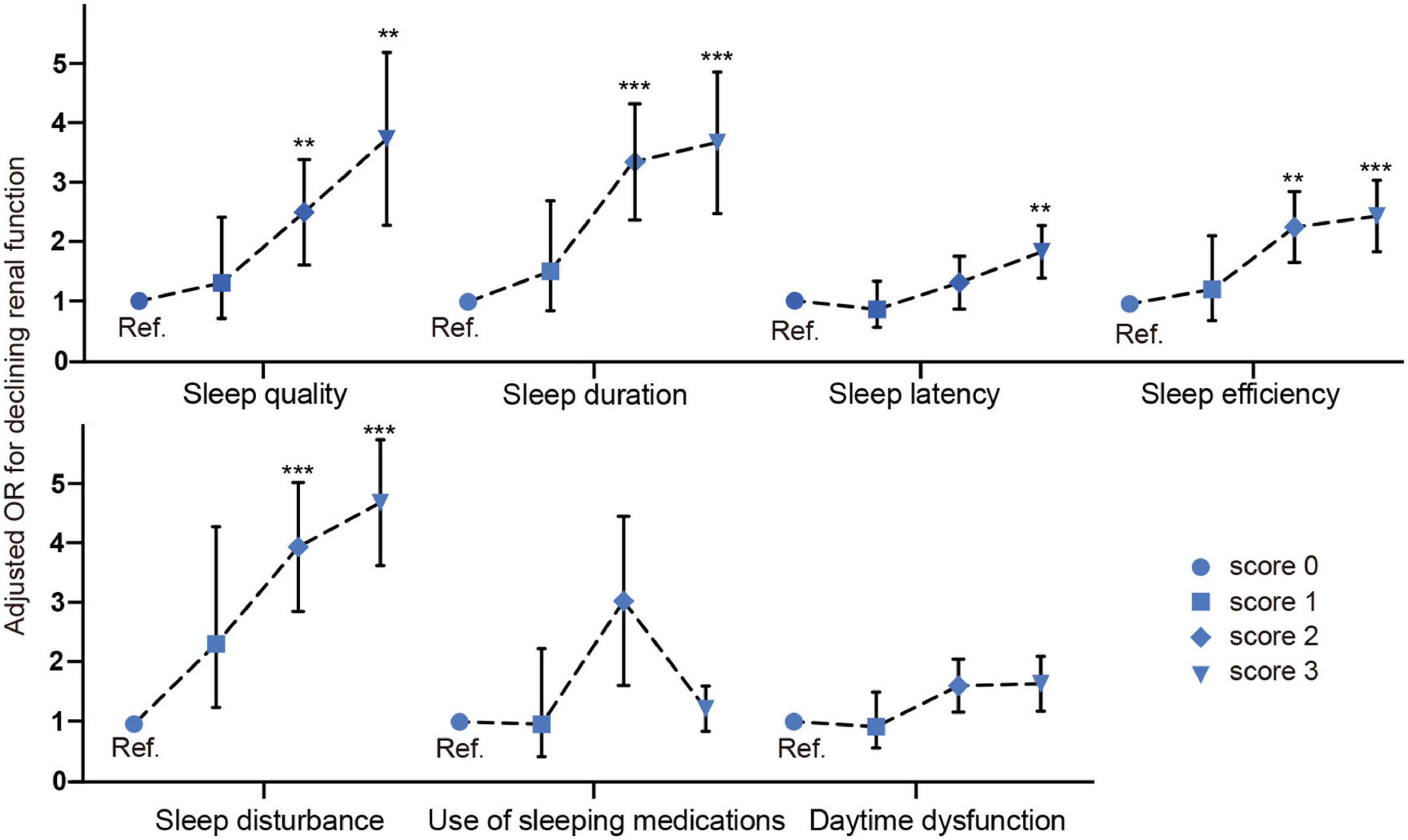
Figure 3. Odds ratio of each sleep parameter of PSQI for declining renal function. **p < 0.05, ***p < 0.001.
The other significant sleep parameters were sleep quality [score 2 OR 95% CI: 2.179 (1.139–4.169), score 3 OR 95% CI: 3.165 (1.543–6.491), p < 0.05], sleep latency [score 3 OR 95% CI: 1.719 (1.117–2.647), p < 0.05], sleep disturbance [score 2 OR 95% CI: 4.047 (2.084–7.858), score 3 OR 95% CI: 4.233 (3.215–6.798), p < 0.05], sleep efficiency [score 2 OR 95% CI: 2.145 (1.351–3.403), score 3 OR 95% CI: 2.338 (1.522–3.591), p < 0.001]. In a summary, our fully adjusted model revealed that subjective poor sleep quality, shorter sleep duration (<6 h), low sleep efficiency (<75%) and longer sleep latency and higher sleep disturbance were independently associated with declining renal function in a graded fashion.
Sleep disorder and menopausal hormone replacement on the prevalence of declining renal function
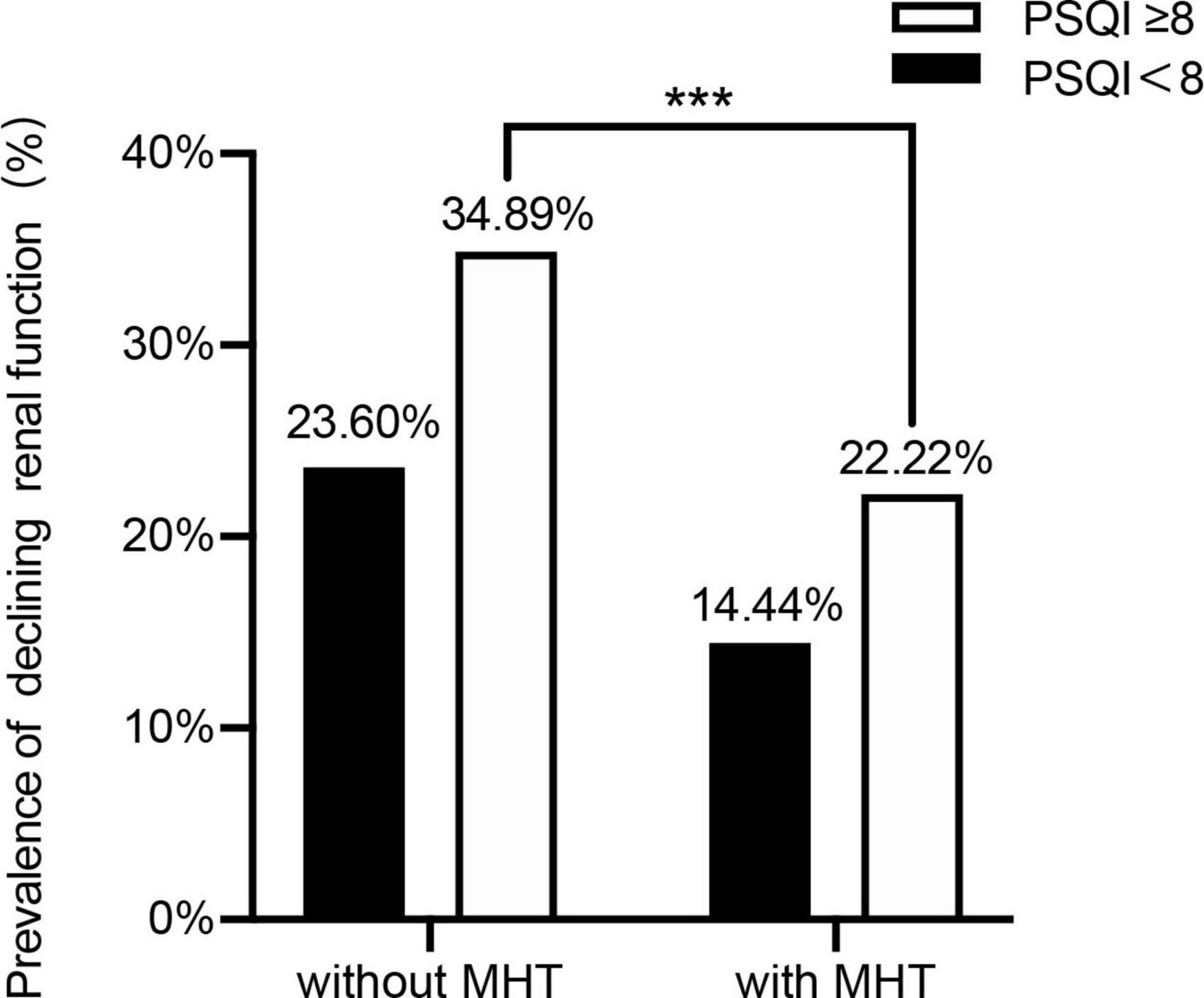
To investigate the role of MHT in sleep disorder-renal function relation, we then distinguished the participants by using MHT and sleep disorder to analyze the prevalence of declining kidney function (Cys-C ≥ 0.91 mg/dL, Q4) with different combinations of two-score based PSQI and MHT.
Compared with MHT non-users, the prevalence of sleep disorder for declining kidney function was significantly lower than in MHT user group (34.89 vs. 22.22%) (p < 0.05). On the whole, we found that the prevalence of declining renal function in sleep disorder was higher than without sleep disorder group, while MHT may lower the prevalence (Figure 4).
Discussion
To our knowledge, this is the first study to document the relationship between sleep characteristics (total PSQI score as well as each sleep parameter) and latent renal function (estimated by serum Cys-C) in peri-post menopausal women without CKD in addition to cardiometabolic and hormone indicators. In our study, we found that sleep disorder (PSQI ≥ 8) served as a determinant risk factor for declining renal function (Cys-C ≥ 0.91 mg/dL, Q4) independent of cardiovascular variables, while the risk was higher in late postmenopause than in early postmenopause. In addition, we observed that poor subjective sleep quality, shorter sleep duration (<6 h), low sleep efficiency (<75%) and delayed sleep latency and higher sleep disturbance showed increasing odds ratio for declining renal function in a dose-manner fashion.
Previous studies have supported that short sleep duration (<5 or 6 h), poor sleep quality and obstructive sleep apnea (OSA) are associated with a higher prevalence of CKD progression estimated by eGFR in patients with diabetic kidney disease, type 2 diabetes (16, 24–26). In addition, a 16.8 years of follow-up study demonstrated that both short and long sleep durations were associated with a higher risk of end-stage renal disease in Chinese population (27). However, little attention was paid to the menopausal women. The strength of our study was that we focused on the interaction of sleep characteristics and clinically latent renal disease in terms of menopause. Accordingly, we suggested that sleep disorder (both total PSQI score and sleep parameter) adversely impacted renal function independent of cardiovascular risk factors. Additionally, we indicated that longer years since menopause served as an incremental role of sleep disorder for declining renal function.
In addition, our multiple logistic regression analysis identified that a higher level of FSH was independently associated with declining renal function. In particular, participants in the fourth FSH were more likely to have renal dysfunction, which was in agreement with the results of the previous study that higher FSH was an independent risk factor declined eGFR and CKD in postmenopausal women (5). Of interest, we also found that MHT was an independent protective indicator associated with declining renal function. Our finding was consistent with the previous study that MHT can delay CKD progression and improved eGFR (28). Menopause is known to be a vital physiological stage of women’s lives characterized by increased FSH concentrations as a result of ovarian failure. Thus, our above results confirmed that the higher FSH level caused by menopause contributed to the worsening of renal function in women, whereas MHT may reversely attenuate the kidney injury. While the possible mechanism was that FSH promoted renal fibrosis in aging women via bonding to FSH receptor, which was expressed in kidney tissue (29, 30).
Other classical independent factors such as hypertension, diabetes, older age, BMI, postmenopause for declining renal function were compatible with previous studies (5, 29–31).
Although the underlying mechanisms by which sleep disorder induced declining renal function in menopause are not fully understood, the admissible mechanisms can be boiled into direct and indirect ways. Sleep disorder can lead to inflammation, oxidative stress, endothelial dysfunction, increased sympathetic tone, activation of the renin-angiotensin system, circadian timing dysfunction and subsequent systemic and intraglomerular pressure, which hereby adversely affects kidney function (24–26, 31, 32). Another possible explanation is the impact of sleep disorder on hypertension (33, 34), diabetes (35, 36), obesity (37), and metabolic syndrome (38), which were known to accelerate deterioration of kidney function.
Several limitations deserve mention in this research. First, our study is a cross-sectional analysis, the inherent drawback of an observational survey may weaken the causal relationship. Secondly, sleep quality ascertained by questionnaire would produce memory bias. Therefore, further longitudinal study is needed to confirm these relationships. Our team is now working on the following-up investigation.
Conclusion
Taken together, our study demonstrated that both cumulative (PSQI total score) and separate sleep dimension (subjective sleep quality, shorter sleep duration, low sleep efficiency delayed sleep latency and higher sleep disturbance) were independently associated with declining renal function (the highest Cys-C, Q4) in postmenopausal women. Thus preclinical prevention such as MHT should be taken for postmenopausal women with sleep problems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study protocol was approved by the Ethics Committee of Shanghai Sixth People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MT conceived and designed the study. YZ analyzed the data. JT wrote the manuscript. CL and JH took part in the investigation and data collection. YT revised the manuscript. All authors read and approved the final manuscript and contributed to the article and approved the submitted version.
Funding
This research was supported by grants from the Science and Technology Commission of Shanghai Municipality (Grant nos. 20Y11907800 and 21YF1434600).
Acknowledgments
We would like to thank the study participants and the research associates in the department of the medical center who made it possible to complete this research project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CKD, chronic kidney disease; GFR, glomerular filtration rate; eGFR, estimated GFR; Cys-C, cystatin C; OSHS, obstructive sleep apnea-hypopnea syndrome; PSQI, Pittsburgh Sleep Quality Index; MHT, menopausal hormone replacement; STRAW, Stages of Reproductive Aging Workshop; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FSH, follicle-stimulating hormone; FBG, Fasting plasma glucose; MetS, metabolic syndrome; OSA, obstructive sleep apnea; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
References
1. Oh TR, Choi HS, Kim CS, Bae EH, Oh YK, Kim YS, et al. Association between health related quality of life and progression of chronic kidney disease. Sci Rep. (2019) 9:19595. doi: 10.1038/s41598-019-56102-w
2. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. (2013) 382:260–72. doi: 10.1016/S0140-6736(13)60687-X
3. Qian D, Wang ZF, Cheng YC, Luo R, Ge SW, Xu G. Early menopause may associate with a higher risk of CKD and all-cause mortality in postmenopausal women: an analysis of NHANES, 1999-2014. Front Med. (2022) 9:823835. doi: 10.3389/fmed.2022.823835
4. Kummer S, von Gersdorff G, Kemper MJ, Oh J. The influence of gender and sexual hormones on incidence and outcome of chronic kidney disease. Pediatr Nephrol. (2012) 27:1213–9. doi: 10.1007/s00467-011-1963-1
5. Li Q, Zheng D, Lin H, Zhong F, Liu J, Wu Y, et al. High circulating follicle-stimulating hormone level is a potential risk factor for renal dysfunction in post-menopausal women. Front Endocrinol. (2021) 12:627903. doi: 10.3389/fendo.2021.627903
6. Hwang JA, Song Y, Shin J, Cho E, Ahn SY, Ko GJ, et al. Changes in mortality according to creatinine/cystatin C ratio in chronic kidney disease and non-chronic kidney disease patients. Front Med. (2022) 9:810901. doi: 10.3389/fmed.2022.810901
7. Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. (2004) 141:929–37. doi: 10.7326/0003-4819-141-12-200412210-00009
8. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. (2002) 40:221–6. doi: 10.1053/ajkd.2002.34487
9. Kato K, Takata Y, Usui Y, Shiina K, Asano K, Hashimura Y, et al. Severe obstructive sleep apnea increases cystatin C in clinically latent renal dysfunction. Respir Med. (2011) 105:643–9. doi: 10.1016/j.rmed.2010.11.024
10. Li HW, Wong BP, Ip WK, Yeung WS, Ho PC, Ng EH. Comparative evaluation of three new commercial immunoassays for anti-Müllerian hormone measurement. Hum Reprod. (2016) 31:2796–802. doi: 10.1093/humrep/dew248
11. Guo DX, Zhu ZB, Zhong CK, Bu XQ, Chen LH, Xu T, et al. Serum cystatin C levels are negatively correlated with post-stroke cognitive dysfunction. Neural Regen Res. (2020) 15:922–8. doi: 10.4103/1673-5374.268928
12. Jiang K, Jin Y, Huang L, Feng S, Hou X, Du B, et al. Black cohosh improves objective sleep in postmenopausal women with sleep disturbance. Climacteric. (2015) 18:559–67. doi: 10.3109/13697137.2015.1042450
13. Petrov ME, Kim Y, Lauderdale DS, Lewis CE, Reis JP, Carnethon MR, et al. Objective sleep, a novel risk factor for alterations in kidney function: the CARDIA study. Sleep Med. (2014) 15:1140–6. doi: 10.1016/j.sleep.2014.05.021
14. Zhou Y, Yang R, Li C, Tao M. Sleep disorder, an independent risk associated with arterial stiffness in menopause. Sci Rep. (2017) 7:1904. doi: 10.1038/s41598-017-01489-7
15. Zhou Y, Liu F, Li C, Zheng Y, Hu J, Zhou Y, et al. Association of snoring and body composition in (peri-post) menopausal women. BMC Womens Health. (2020) 20:175. doi: 10.1186/s12905-020-01025-2
16. Zhou Y, Zheng Y, Li C, Hu J, Zhou Y, Geng L, et al. Association of body composition with menopausal symptoms in (peri-)menopausal women. Climacteric. (2018) 21:179–83. doi: 10.1080/13697137.2018.1428295
17. Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. (2012) 97:1159–68. doi: 10.1210/jc.2011-3362
18. Aschner P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res Clin Pract. (2017) 132:169–70. doi: 10.1016/j.diabres.2017.09.002
19. Chaturvedi S. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC 7): is it really practical? Natl Med J India. (2004) 17:227.
20. Meigs JB, D’Agostino RB Sr., Wilson PW, Adrienne Cupples L, Nathan DM, Singer DE. Risk variable clustering in the insulin resistance syndrome: the Framingham offspring study. Diabetes. (1997) 46:1594–600. doi: 10.2337/diacare.46.10.1594
21. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. (2012) 60:850–86. doi: 10.1053/j.ajkd.2012.07.005
22. Tsai PS, Wang SY, Wang MY, Su CT, Yang TT, Huang CJ, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res. (2005) 14:1943–52. doi: 10.1007/s11136-005-4346-x
23. Chen H, He Y, Zeng X, Chen Q, Zhou N, Yang H, et al. Sleep quality is an independent predictor of blood glucose and gestational diabetes mellitus: a longitudinal study of 4550 Chinese women. Nat Sci Sleep. (2022) 14:609–20. doi: 10.2147/NSS.S353742
24. Neborak JM, Mokhlesi B. Short sleep, sleep apnoea-associated hypoxaemic burden and kidney function: more questions than answers. Thorax. (2021) 76:638–9. doi: 10.1136/thoraxjnl-2020-216618
25. Turek NF, Ricardo AC, Lash JP. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis. (2012) 60:823–33. doi: 10.1053/j.ajkd.2012.04.027
26. Ricardo AC, Knutson K, Chen J, Appel LJ, Bazzano L, Carmona-Powell E, et al. The association of sleep duration and quality with CKD progression. J Am Soc Nephrol. (2017) 28:3708–15. doi: 10.1681/ASN.2016121288
27. Geng TT, Jafar TH, Yuan JM, Koh WP. Sleep duration and risk of end-stage renal disease: the Singapore Chinese health study. Sleep Med. (2019) 54:22–7. doi: 10.1016/j.sleep.2018.10.007
28. Fung MM, Poddar S, Bettencourt R, Jassal SK, Barrett-Connor E. A cross-sectional and 10-year prospective study of postmenopausal estrogen therapy and blood pressure, renal function, and albuminuria: the Rancho Bernardo study. Menopause. (2011) 18:629–37. doi: 10.1097/gme.0b013e3181fca9c4
29. Zhang K, Kuang L, Xia F, Chen Y, Zhang W, Zhai H, et al. Follicle-stimulating hormone promotes renal tubulointerstitial fibrosis in aging women via the AKT/GSK-3β/β-catenin pathway. Aging Cell. (2019) 18:e12997. doi: 10.1111/acel.12997
30. Chen H, Cui Y, Yu S. Expression and localisation of FSHR, GHR and LHR in different tissues and reproductive organs of female yaks. Folia Morphol. (2018) 77:301–9. doi: 10.5603/FM.a2016.0095
31. Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. (2011) 80:806–21. doi: 10.1038/ki.2011.198
32. Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. (2004) 65:1568–76. doi: 10.1111/j.1523-1755.2004.00552.x
33. Prejbisz A, Florczak E, Pręgowska-Chwała B, Klisiewicz A, Kuśmierczyk-Droszcz B, Zieliński T, et al. Relationship between obstructive sleep apnea and markers of cardiovascular alterations in never-treated hypertensive patients. Hypertens Res. (2014) 37:573–9. doi: 10.1038/hr.2014.43
34. Tsioufis C, Thomopoulos C, Dimitriadis K, Amfilochiou A, Tsiachris D, Selima M, et al. Association of obstructive sleep apnea with urinary albumin excretion in essential hypertension: a cross-sectional study. Am J Kidney Dis. (2008) 52:285–93. doi: 10.1053/j.ajkd.2008.05.001
35. Nishimura A, Kasai T, Kikuno S, Nagasawa K, Okubo M, Narui K, et al. Effect of sleep-disordered breathing on albuminuria in 273 patients with Type 2 Diabetes. J Clin Sleep Med. (2018) 14:401–7. doi: 10.5664/jcsm.6986
36. Zamarrón E, Jaureguizar A, García-Sánchez A, Díaz-Cambriles T, Alonso-Fernández A, Lores V, et al. Obstructive sleep apnea is associated with impaired renal function in patients with diabetic kidney disease. Sci Rep. (2021) 11:5675. doi: 10.1038/s41598-021-85023-w
37. Perticone M, Maio R, Scarpino PE, Mancuso L, Volpentesta M, Caroleo B, et al. Continuous positive airway pressure improves renal function in obese patients with obstructive sleep apnea syndrome. Front Med. (2021) 8:642086. doi: 10.3389/fmed.2021.642086
Keywords: sleep characteristics, cystatin C, renal function, menopause, menopausal hormone therapy (HT)
Citation: Tong J, Li C, Hu J, Teng Y, Zhou Y and Tao M (2022) Association of sleep characteristics with renal function in menopausal women without recognized chronic kidney disease. Front. Psychiatry 13:1024245. doi: 10.3389/fpsyt.2022.1024245
Received: 21 August 2022; Accepted: 21 October 2022;
Published: 09 November 2022.
Edited by:
Haitham Jahrami, Arabian Gulf University, BahrainReviewed by:
Enas Abdelaziz, Al Jouf University, Saudi ArabiaYongbing Liu, Yangzhou University, China
Copyright © 2022 Tong, Li, Hu, Teng, Zhou and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Zhou, ZWl2aXJhMTlAMTYzLmNvbQ==; Minfang Tao, dGFvbWZAc2p0dS5lZHUuY24=
†These authors have contributed equally to this work
 Jianqian Tong1,2†
Jianqian Tong1,2† Yincheng Teng
Yincheng Teng Yang Zhou
Yang Zhou Minfang Tao
Minfang Tao