
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 15 November 2022
Sec. Schizophrenia
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.1022472
Background: Many studies have explored the link between the gut microbiota and schizophrenia. To date, there have been no bibliometric analyses to summarize the association between the gut microbiota and schizophrenia. We aimed to conduct a bibliometric study of this association to determine the current status and areas for advancement in this field.
Materials and methods: Publications related to the gut microbiota and schizophrenia were retrieved from the Web of Science Core Collection (WoSCC). The WoSCC literature analysis wire and VOSviewer 1.6.16 were used to conduct the analysis.
Results: In total, 162 publications were included in our study. The publications generally showed an upward trend from 2014. A total of 873 authors from 355 organizations and 40 countries/regions contributed to this field. The leading authors were Timothy Dinan, John F Cryan, and Emily Severance. The leading institutions were Johns Hopkins University, the University College Cork, and the University of Toronto. The most productive countries were the United States (US), China, and Canada. In total, 95 journals contributed to this field. Among them, the top three productive journals were Schizophrenia Research, Progress in Neuro Psychopharmacology Biological Psychiatry, and Frontiers in Psychiatry. The important keywords in the clusters were gut microbiome, bipolar disorder, schizophrenia, antipsychotics, weight gain, metabolic syndrome, gut-brain axis, autism, depression, inflammation, and brain.
Conclusion: The main research hotspots involving the connection between schizophrenia and the gut microbiota were the characteristics of the microbiota composition in schizophrenia patients, the gut-brain axis, and microbial-based interventions for schizophrenia. The studies about the association between gut microbiota and schizophrenia are limited, and more studies are needed to provide new insights into the gut microbiota in the pathogenesis and treatment of schizophrenia.
Schizophrenia is a serious psychiatric illness that affects approximately one in a hundred people worldwide (1–5). The rate of early mortality is 2- to 3-fold higher in patients with schizophrenia than that in the general population (6–10). The current interventions for schizophrenia are mainly antipsychotic medications, including olanzapine, risperidone, aripiprazole, and clozapine; modified electroconvulsive therapy (MECT); and repetitive transcranial magnetic stimulation (rTMS). Drugs for schizophrenia may have some side effects, including weight gain and metabolic disturbances (11–14).
In recent years, the link between schizophrenia and the gut microbiota has received increasing attention. Recent studies have shown that the gut microbiota can modulate brain function through the gut-brain axis (15–19). The gut microbiota may provide a possible mechanism for the development of schizophrenia. The alterations and dysbiosis in the function and composition of the gut microbiome are found to be associated with schizophrenia though the modulation of glutamatergic neurotransmission metabolism and tryptophan–kynurenine metabolism (18, 20–23). The mice received the gut microbiome from patients with schizophrenia displayed altered lipid metabolism and amino acid, and decreased brain glutamate and disruptions in the glutamate–glutamine–GABA cycle, which were implicated in the pathophysiology of schizophrenia (20–22). In mice received the gut microbiome from patients with schizophrenia, the serotonin pathway of tryptophan catabolism was markedly reduced, while the Kyn–Kyna pathway of tryptophan catabolism was increased, which is related to schizophrenia by the modulation of tryptophan–kynurenine metabolism (23). Moreover, microbial-based therapies may be effective for antipsychotic-induced weight gain and metabolic disturbances. Emerging preclinical and clinical studies have demonstrated potential associations between schizophrenia and the gut microbiota. Bibliometric analysis has been widely used to determine the current status and explore developmental trends by the quantitative analysis of patterns in the scientific literature. By this method, researchers can understand the range of research topics and predict future directions in specific field. To date, there have been no bibliometric analyses to summarize the link between the gut microbiota and schizophrenia. We aimed to conduct a bibliometric study of the association between gut microbiota and schizophrenia to determine the current status and areas for advancement in this field.
In our study, publications about schizophrenia and the gut microbiota were downloaded from the WoSCC. The following search terms were used: TS = (microbiome* OR flora microbiot* OR bacteria OR microflora) AND TS = (intestin* OR gut OR gastro-intestin* OR gastrointestin*) AND TS = (schizophrenia OR “schizoaffective disorder” OR “schizophreniform disorder” OR “first episode psychosis” OR “schizophrenia spectrum disorder”). The search results were confined by the publication date from the inception of the study to 30 May 2022; types of publications, including articles and reviews; and English language. In our study, we conducted a literature search and screening according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24).
The h-index was used to evaluate the citations of the researcher’s publications. The WoSCC literature analysis wire and VOSviewer 1.6.16 were used to conduct the analysis. The WoSCC literature analysis wire was used to analyze the publication years, categories, document types, the distribution of authors, institutions, countries/regions, and h-index. VOSviewer1.6.16 software was used to conduct assessments of the coauthorship of authors, keywords, countries/regions, and institutions. The total link strength (TLS) was used to evaluate the cooperation relationship. In the keyword co-occurrence analysis, we merged the synonyms of “induced weight-gain,” weight-gain and “weight gain” to the term “weight gain”; “probiotic supplementation” and “probiotics” into “probiotics”; “autism spectrum disorders,” “autism spectrum disorder,” and “autism” into the term “autism”; and “microbiome,” “microbiota,” “intestinal microbiota,” “gut microbiome,” “gut microbiota,” and “fecal microbiota” into the term “gut microbiota.”
A total of 162 publications containing 78 reviews and 84 articles were included, as shown in Figure 1. The publications generally showed an upward trend from 2014. The top subject categories were psychiatry with 70 publications and neurosciences with 51 publications (Figure 2).
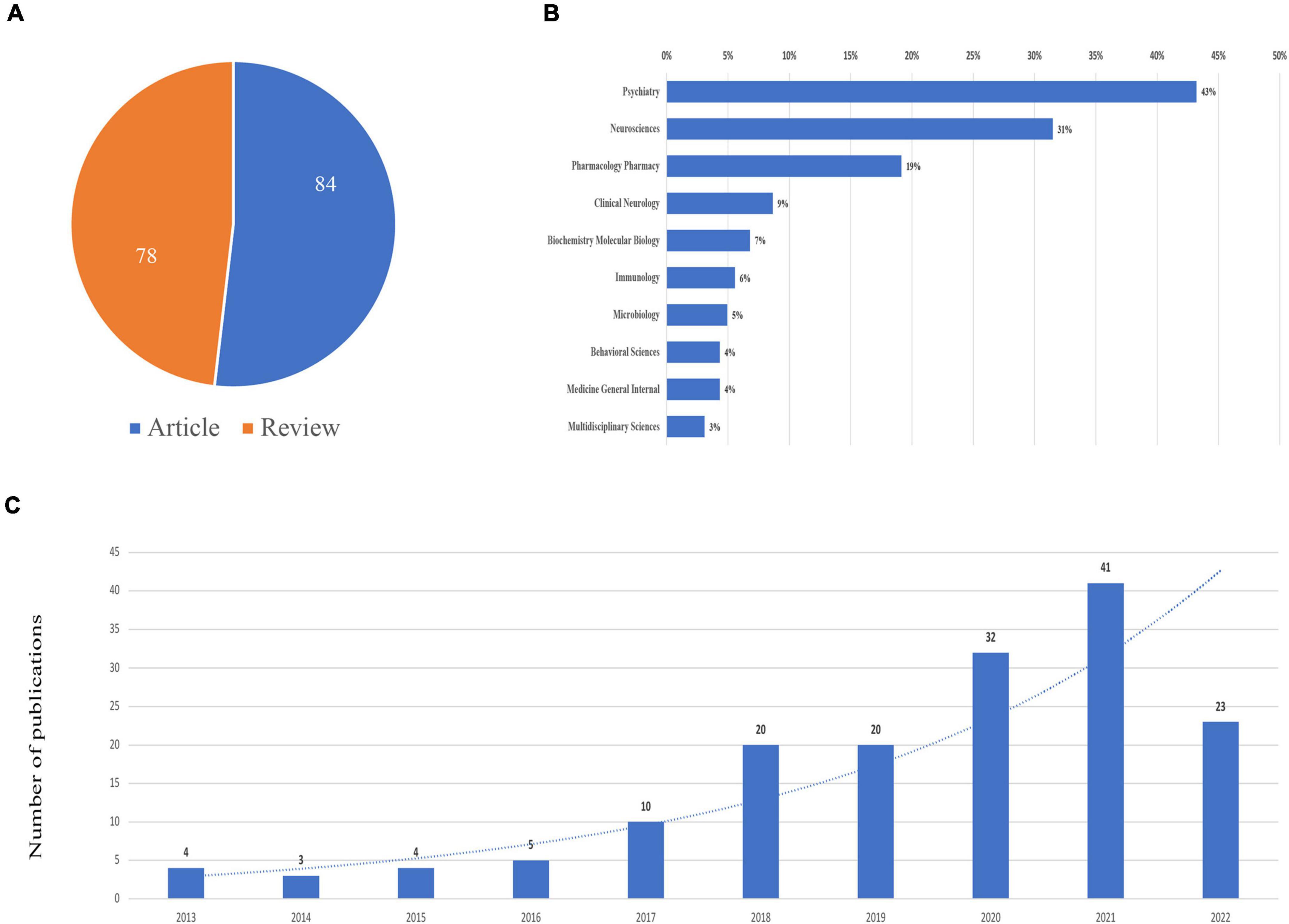
Figure 2. Publications on schizophrenia and gut microbiota from inception to 2022. (A) Literature types distribution. (B) Subject categories distribution. (C) Annual publications quantitative distribution.
In total, 873 authors contributed to this field. Timothy Dinan was the leading author who had 13 publications with 1,933 citations and an h-index of 12, followed by John F Cryan who had 12 publications with 1,929 citations and an h-index of 12, Emily Severance who had 11 publications with 580 citations and an h-index of 9, Robert Yolken who had 11 publications with 580 citations and an h-index of 9, and Michael Maes who had 7 publications with 160 citations and an h-index of 7. Figure 3A shows the coauthorship map of the authors. The Severance Emily and Yolken Robert had the highest TLS, indicating that they participated in the most collaborations with other authors.
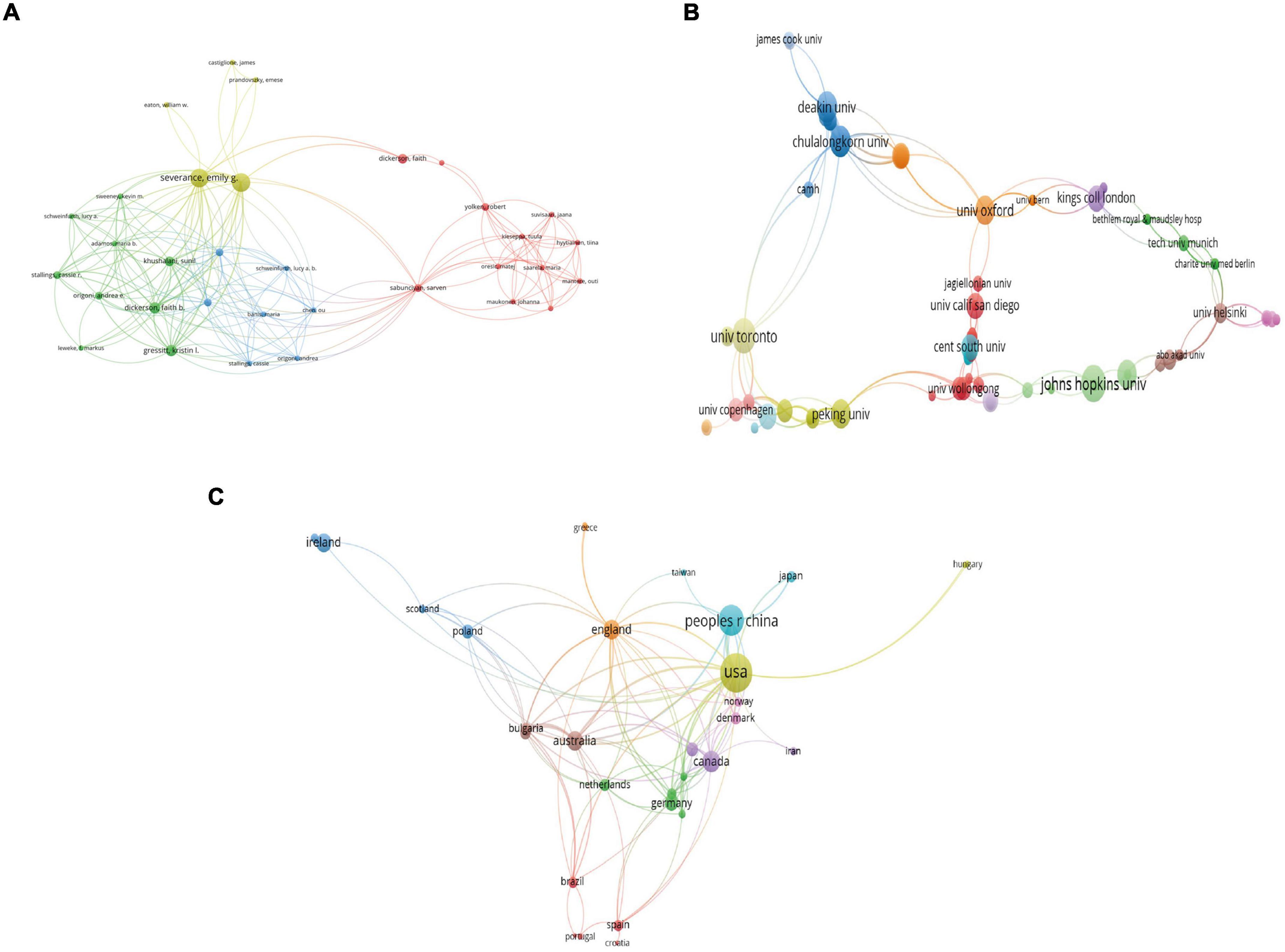
Figure 3. Visualization knowledge maps of the co-authorship. (A) Authors. (B) Organizations. (C) Countries/regions.
All publications were from 355 organizations and 40 countries/regions. The top three most productive institutions were Johns Hopkins University with 14 publications and 931 citations, University College Cork with 14 publications and 1,942 citations, and the University of Toronto with 10 publications and 341 citations. Figure 3B shows the coauthorship map of institutions. Regarding countries/regions, the United States (US) had the most publications with 59 documents, followed by China with 37 documents, Canada with 18 documents, Australia with 15 documents, and England with 15 documents. Figure 3C shows the coauthorship map of the countries/regions. Table 1 shows the top 10 high-yield institutions, authors, and countries/regions.
In total, 95 journals contributed to this field. Among them, the top three productive journals were Schizophrenia Research with 14 documents and 809 citations, Progress in Neuro Psychopharmacology Biological Psychiatry with 9 documents and 115 citations, and Frontiers in Psychiatry with 6 documents and 51 citations. Figure 4A and Table 2 show the coauthorship map of journals and the top 10 high-yield journals.
Table 3 shows the top twenty most-cited publications (23, 25–43). The first highest-cited article with 918 citations was published in Physiological Reviews and authored by Cryan et al. in 2019. This review demonstrates that the gut microbiota is associated with many diseases, including schizophrenia and Parkinson’s disease. Animal models have been paramount in linking the activation of microglia induced by the microbiome to the regulation of fundamental neural processes, and translational human studies are ongoing and will greatly enhance this field. The second most-cited article, with 307 citations, was published in Translational Psychiatry and authored by Hoban et al. in 2016. They found that the microbiome was necessary for dynamic and appropriate regulation of myelin-related genes and may be a therapeutic target for schizophrenia and autism. The third most-cited article, with 223 citations, was published in Science advances and authored by Zheng et al in 2019. They found that patients with schizophrenia exhibited marked disturbances in gut microbial composition and a decreased microbiome α-diversity index. The abundances of organisms assessed in microbial panel, including Aerococcaceae, Rikenellaceae, Pasteurellaceae, Brucellaceae, and Bifidobacteriaceae, decreased in patients with schizophrenia. Their results demonstrated that the microbiome may alter neurologic function and neurochemistry, which is related to the pathology of schizophrenia. The fourth most-cited article, with 155 citations, was published in Schizophrenia research and authored by Schwarz et al. in 2018. They found that Lactobacillus abundance was elevated in patients with first-episode psychosis (FEP) and was related to the severity of schizophrenia. Their results showed that microbiota alterations benefited remission and treatment response in schizophrenia patients. Figure 4B show the coauthorship map of documents.
Figure 5 shows the co-occurrence map of keywords, which indicates four research directions. The cluster represented in green includes the important keywords of gut microbiome and bipolar disorder. The cluster represented in blue includes the important keywords of schizophrenia, antipsychotics, weight gain, and metabolic syndrome. The cluster represented in red includes the important keywords of gut-brain axis, autism, and depression. The cluster represented in yellow includes the important keywords of inflammation and brain.
To the best of our knowledge, this is the first bibliometric study to explore the link between schizophrenia and the gut microbiota. In total, 162 publications were included in our study. The publications generally showed an upward trend from 2014. The most top subject categories were psychiatry with 70 publications. A total of 873 authors from 355 organizations and 40 countries/regions contributed to this field. The leading authors were Timothy Dinan who had 13 publications with 1,933 citations and an h-index of 12, John F Cryan who had 12 publications with 1,929 citations and an h-index of 12, and Emily Severance who had 11 publications with 580 citations and an h-index of 9. The Severance Emily and Yolken Robert participated in the most collaborations with other authors. The leading institutions were Johns Hopkins University with 14 publications, the University College Cork with 14 publications, and the University of Toronto with 10 publications. The most productive countries were the US with 59 documents, China with 37 documents, and Canada with 18 documents. In total, 95 journals contributed to this field. Among them, the top three productive journals were Schizophrenia Research with 14 documents, Progress in Neuro Psychopharmacology Biological Psychiatry with 9 documents, and Frontiers in Psychiatry with 6 documents. There are four research directions in the clusters. The cluster represented in green includes the important keywords of gut microbiome and bipolar disorder. The cluster represented in blue includes the important keywords of schizophrenia, antipsychotics, weight gain, and metabolic syndrome. The cluster represented in red includes the important keywords of gut-brain axis, autism, and depression. The cluster represented in yellow includes the important keywords of inflammation and brain.
In the field of schizophrenia and gut microbiota, most of the articles were published in the US, and the percentage of articles from the US was 38% (155/388). Among the top ten institutions, six were located in the US. Among the top ten productive authors, five were from the US. The prominent country in this field was the US. The number of studies from other countries/regions should be improved.
Based on the important keywords and the top 20 most-cited references, the research hotspots and areas for advancement in research on the link between schizophrenia and the gut microbiota were found to be as follows: (1) The characteristics of the microbiota composition in schizophrenia. In the top 20 most-cited references, 5 publications explored the characteristics of the microbiota composition in schizophrenia patients. A systematic review (44) demonstrated that there were significant differences in beta diversity but not alpha diversity between patients with and without schizophrenia. Zheng et al. showed that Veillonella abundance was significantly higher while Ruminococcus and Roseburia abundances were significantly lower in patients with schizophrenia. Germ-free mice that received fecal microbiota transplantation (FMT) from the microbiota of schizophrenia patients had higher glutamine and GABA levels and lower glutamate levels in the hippocampus. There was considerable discord between these results due to factors including small sample sizes, potential confounders, and the measurement methods. More large-scale prospective studies should be conducted to identify whether specific microbiome compositions are associated with an increased risk of developing schizophrenia. (2) The role of the gut-brain axis. In the top 20 most-cited references, 7 publications explored the gut-brain axis and its role in the association between schizophrenia and the gut microbiota, and the important keywords gut-brain axis, inflammation, and brain were in the cluster represented in blue. The gut microbiota was found to be associated with schizophrenia via processes involved in the gut-brain axis, including immune-regulating pathways, neurotransmitter synthesis, the production of bioactive microbial metabolites, and tryptophan metabolism (34, 45–48). Among the involved molecular features, immune mediators are the most important intermediaries between schizophrenia and the gut microbiota (49–53). Gastrointestinal symptoms were found to be chronic comorbidities observed in schizophrenia patients. The gut microbiota may play an important role in the development of the neuroimmune system, neuronal remodeling, synaptic pruning, and myelination. The pathophysiology of schizophrenia is associated with immune system alterations. The gut microbiota is also involved in the activation and maturation of microglia, which may play an important role in the development of schizophrenia (54–56). (3) Microbial-based interventions for schizophrenia. In the top 20 most-cited references, 7 publications explored the potential role of targeting the gut microbiota as an intervention for schizophrenia, and the important keywords antipsychotics, weight gain and metabolic syndrome were in the cluster represented in blue. Prebiotics and probiotics are potential treatments to improve cognition, neural activity, anxiety, and gastrointestinal symptoms for patients with schizophrenia (41, 57–60). In addition, prebiotics and probiotics could alleviate the metabolic side effects induced by antipsychotics (61–67). More studies should be performed to explore microbiome-mediated treatment for ameliorating cognitive dysfunction and antipsychotic-associated weight gain.
There are some limitations to our study. First, all publications were from the WoSCC database because it was the best citation-based database and was the most widely used in bibliometric and visual analysis. Second, only publications in English were included in our study. Additionally, the literature produced in 2022 was not fully assessed because of the study cut-off time.
The studies about the association between gut microbiota and schizophrenia are limited although the gut microbiota may play an important role in the pathogenesis and treatment of schizophrenia. More studies are needed in the future. Most of existing studies are of cross-sectional design, so it is challenging to establish the causality. Further researches should focus on the role of gut microbiota in the pathogenesis of schizophrenia and the effective treatment for schizophrenia using data analysis approaches that deal more effectively to avoid confounding factors.
The main research hotspots regarding the connection between schizophrenia and the gut microbiota were the characteristics of the microbiota composition in schizophrenia patients, the gut-brain axis, and microbial-based interventions for schizophrenia. The studies about the association between gut microbiota and schizophrenia are limited, and more studies are needed to provide new insights into the gut microbiota in the pathogenesis and treatment of schizophrenia.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
CY, XW, HL, and XL were responsible for the data collection, investigation, figures and tables construction and writing the original draft. SW and JH contributed to the discussion and final review and editing. All authors reviewed and edited the final manuscript.
This research was supported by the fellowship of China Postdoctoral Science Foundation (Grant No. 2021M692802) and the Key Research and Development Program of Zhejiang Province (Grant No. 2020C03018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bighelli I, Rodolico A, García-Mieres H, Pitschel-Walz G, Hansen WP, Schneider-Thoma J, et al. Psychosocial and psychological interventions for relapse prevention in schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry. (2021) 8:969–80. doi: 10.1016/S2215-0366(21)00243-1
2. Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. (2018) 5:727–38. doi: 10.1016/S2215-0366(18)30269-4
3. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. (2016) 388:86–97. doi: 10.1016/S0140-6736(15)01121-6
4. Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. (2020) 7:64–77. doi: 10.1016/S2215-0366(19)30416-X
5. Schneider-Thoma J, Chalkou K, Dörries C, Bighelli I, Ceraso A, Huhn M, et al. Comparative efficacy and tolerability of 32 oral and long-acting injectable antipsychotics for the maintenance treatment of adults with schizophrenia: a systematic review and network meta-analysis. Lancet. (2022) 399:824–36. doi: 10.1016/S0140-6736(21)01997-8
6. Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. (2015) 2:452–64. doi: 10.1016/S2215-0366(15)00115-7
7. Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. (2019) 394:939–51. doi: 10.1016/S0140-6736(19)31135-3
8. Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. (2022) 399:473–86. doi: 10.1016/S0140-6736(21)01730-X
9. Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. (2015) 2:258–70. doi: 10.1016/S2215-0366(14)00122-9
10. Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. (2013) 382:951–62. doi: 10.1016/S0140-6736(13)60733-3
11. Cernea S, Dima L, Correll CU, Manu P. Pharmacological management of glucose dysregulation in patients treated with second-generation antipsychotics. Drugs. (2020) 80:1763–81. doi: 10.1007/s40265-020-01393-x
12. Chang SC, Goh KK, Lu ML. Metabolic disturbances associated with antipsychotic drug treatment in patients with schizophrenia: state-of-the-art and future perspectives. World J Psychiatry. (2021) 11:696–710. doi: 10.5498/wjp.v11.i10.696
13. Cooper SJ, Reynolds GP, Barnes T, England E, Haddad PM, Heald A, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. (2016) 30:717–48. doi: 10.1177/0269881116645254
14. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. (2010) 35:1520–30. doi: 10.1038/npp.2010.21
15. Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG. The role of the gut microbiome in the development of schizophrenia. Schizophr Res. (2021) 234:4–23. doi: 10.1016/j.schres.2020.02.010
16. Minichino A, Brondino N, Solmi M, Del Giovane C, Fusar-Poli P, Burnet P, et al. The gut-microbiome as a target for the treatment of schizophrenia: a systematic review and meta-analysis of randomised controlled trials of add-on strategies. Schizophr Res. (2021) 234:1–13. doi: 10.1016/j.schres.2020.02.012
17. Morkl S, Wagner-Skacel J, Lahousen T, Lackner S, Holasek SJ, Bengesser SA, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. (2018) [Online ahead of print]. doi: 10.1159/000492834
18. Szeligowski T, Yun AL, Lennox BR, Burnet PWJ. The gut microbiome and schizophrenia: the current state of the field and clinical applications. Front Psychiatry. (2020) 11:156. doi: 10.3389/fpsyt.2020.00156
19. Xu Y, Shao M, Fang X, Tang W, Zhou C, Hu X, et al. Antipsychotic-induced gastrointestinal hypomotility and the alteration in gut microbiota in patients with schizophrenia. Brain Behav Immun. (2022) 99:119–29. doi: 10.1016/j.bbi.2021.09.014
20. McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. (2020) 19:15–33. doi: 10.1002/wps.20693
21. Stan AD, Ghose S, Zhao C, Hulsey K, Mihalakos P, Yanagi M, et al. Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Mol Psychiatry. (2015) 20:433–9. doi: 10.1038/mp.2014.54
22. Tsamakis K, Galinaki S, Alevyzakis E, Hortis I, Tsiptsios D, Kollintza E, et al. Gut microbiome: a brief review on its role in schizophrenia and first episode of psychosis. Microorganisms. (2022) 10:1121. doi: 10.3390/microorganisms10061121
23. Zhu F, Guo RJ, Wang W, Ju Y, Wang Q, Ma Q, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. (2020) 25:2905–18. doi: 10.1038/s41380-019-0475-4
24. Page MJMJ, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet Part B Neuropsychiatr Genet. (2017) 174:651–60. doi: 10.1002/ajmg.b.32567
26. Codagnone MG, Spichak S, O’Mahony SM, O’Leary OF, Clarke G, Stanton C, et al. Programming bugs: microbiota and the developmental origins of brain health and disease. Biol Psychiatry. (2019) 85:150–63. doi: 10.1016/j.biopsych.2018.06.014
27. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immunity. (2017) 62:46–52. doi: 10.1016/j.bbi.2016.12.010
28. Hornig M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr Opin Rheumatol. (2013) 25:488–95.
29. Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. (2015) 17:27. doi: 10.1007/s11920-015-0574-0
30. Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG. Cross talk: the microbiota and neurodevelopmental disorders. Front Neurosci. (2017) 11:490. doi: 10.3389/fnins.2017.00490
31. Nguyen TT, Kosciolek T, Eyler LT, Knight R, Jeste DV. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J Psychiatr Res. (2018) 99:50–61. doi: 10.1016/j.jpsychires.2018.01.013
32. Dinan TG, Borre YE, Cryan JF. Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry. (2014) 19:1252–7. doi: 10.1038/mp.2014.93
33. Zhu F, Ju YM, Wang W, Wang Q, Guo R, Ma Q, et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun. (2020) 11:1612. doi: 10.1038/s41467-020-15457-9
34. Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M. The microbiota-gut-brain axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.17116/jnevro202212210157
35. Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, et al. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One. (2014) 9:e115225. doi: 10.1371/journal.pone.0115225
36. Dinan TG, Cryan JF. Brain-gut-microbiota axis and mental health. Psychosom Med. (2017) 79:920–6. doi: 10.1097/PSY.0000000000000519
37. Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. (2019) 204:23–9. doi: 10.1016/j.schres.2018.09.014
38. Schwarz E, Maukonen J, Hyytiainen T, Kieseppä T, Orešič M, Sabunciyan S, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. (2018) 192:398–403. doi: 10.1016/j.schres.2017.04.017
39. Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. (2013) 148:130–7. doi: 10.1016/j.schres.2013.05.018
40. Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res. (2016) 176:23–35. doi: 10.1016/j.schres.2014.06.027
41. Shen Y, Xu JT, Li ZY, Huang Y, Yuan Y, Wang J, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophr Res. (2018) 197:470–7. doi: 10.1016/j.schres.2018.01.002
42. Zheng P, Zeng BH, Liu ML, Chen J, Pan J, Han Y, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. (2019) 5:eaau8317. doi: 10.1126/sciadv.aau8317
43. Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. (2016) 6:e774. doi: 10.1038/tp.2016.42
44. Vindegaard N, Speyer H, Nordentoft M, Rasmussen S, Benros ME. Gut microbial changes of patients with psychotic and affective disorders: a systematic review. Schizophr Res. (2021) 234:1–10. doi: 10.1016/j.schres.2019.12.014
45. Liu JCW, Gorbovskaya I, Hahn MK, Muller DJ. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. (2021) 13:1152. doi: 10.3390/nu13041152
46. Nikolova VL, Hall MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. (2021) 78:1343–54. doi: 10.1001/jamapsychiatry.2021.2573
47. Spichak S, Bastiaanssen TFS, Berding K, Vlckova K, Clarke G, Dinan TG, et al. Mining microbes for mental health: determining the role of microbial metabolic pathways in human brain health and disease. Neurosci Biobehav Rev. (2021) 125:698–761. doi: 10.1016/j.neubiorev.2021.02.044
48. Zeng C, Yang P, Cao T, Gu Y, Li N, Zhang B, et al. Gut microbiota: an intermediary between metabolic syndrome and cognitive deficits in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 106:110097. doi: 10.1016/j.pnpbp.2020.110097
49. Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. (2012) 488:178. doi: 10.1038/nature11319
50. Cuomo A, Maina G, Rosso G, Crescenzi BB, Bolognesi S, Di Muro A, et al. The microbiome: a new target for research and treatment of schizophrenia and its resistant presentations? A systematic literature search and review. Front Pharmacol. (2018) 9:1040. doi: 10.3389/fphar.2018.01040
51. Skonieczna-Zydecka K, Loniewski I, Misera A, Stachowska E, Maciejewska D, Marlicz W, et al. Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology. (2019) 236:1491–512. doi: 10.1007/s00213-018-5102-6
52. Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. (2015) 21:166–72. doi: 10.1038/nm.3766
53. Solas M, Milagro FI, Ramirez MJ, Martinez JA. Inflammation and gut-brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Curr Opin Pharmacol. (2017) 37:87–92. doi: 10.1016/j.coph.2017.10.005
54. Boehme M, van de Wouw M, Bastiaanssen TFS, Olavarría-Ramírez L, Lyons K, Fouhy F, et al. Mid-life microbiota crises: middle age is associated with pervasive neuroimmune alterations that are reversed by targeting the gut microbiome. Mol Psychiatry. (2020) 25:2567–83. doi: 10.1038/s41380-019-0425-1
55. Cryan JF, Dinan TG. GUT microbiota: microbiota and neuroimmune signalling-Metchnikoff to microglia. Nat Rev Gastroenterol Hepatol. (2015) 12:495–6. doi: 10.1038/nrgastro.2015.127
56. Erny D, de Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18:965–977. doi: 10.1038/nn.4030
57. Castanon N, Luheshi G, Laye S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front Neurosci. (2015) 9:229. doi: 10.3389/fnins.2015.00229
58. Ma X, Asif H, Dai LL, Zheng W, Wang D, Ren H, et al. Alteration of the gut microbiome in first-episode drug-naive and chronic medicated schizophrenia correlate with regional brain volumes. J Psychiatr Res. (2020) 123:136–44. doi: 10.1016/j.jpsychires.2020.02.005
59. Pelka-Wysiecka J, Kaczmarczyk M, Baba-Kubis A, Liśkiewicz P, Wroński M, Skonieczna-Żydecka K, et al. Analysis of gut microbiota and their metabolic potential in patients with schizophrenia treated with olanzapine: results from a six-week observational prospective cohort study. J Clin Med. (2019) 8:1605. doi: 10.3390/jcm8101605
60. Xu R, Wu B, Liang J, He F, Gu W, Li K, et al. Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav Immunity. (2020) 85:120–7. doi: 10.1016/j.bbi.2019.06.039
61. Clarke G, Sandhu KV, Griffin BT, Dinan TG, Cryan JF, Hyland NP. Gut reactions: breaking down xenobiotic-microbiome interactions. Pharmacol Rev. (2019) 71:198–224. doi: 10.1124/pr.118.015768
62. Cussotto S, Clarke G, Dinan TG, Cryan JF. Psychotropics and the microbiome: a chamber of secrets. Psychopharmacology. (2019) 236:1411–32. doi: 10.1007/s00213-019-5185-8
63. Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, et al. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology. (2019) 236:1671–85.
64. Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. (2012) 221:155–69. doi: 10.1007/s00213-011-2555-2
65. Dinan TG, Cryan JF. Schizophrenia and the microbiome: time to focus on the impact of antipsychotic treatment on the gut microbiota. World J Biol Psychiatry. (2018) 19:568–70. doi: 10.1080/15622975.2018.1540793
66. Gorbovskaya I, Kanji S, Liu JCW, MacKenzie NE, Agarwal SM, Marshe VS, et al. Investigation of the gut microbiome in patients with schizophrenia and clozapine-induced weight gain: protocol and clinical characteristics of first patient cohorts. Neuropsychobiology. (2020) 79:5–12. doi: 10.1159/000494696
Keywords: gut microbiota, bibliometric analysis, gut-brain axis, schizophrenia, antipsychotics
Citation: Yang C, Lin X, Wang X, Liu H, Huang J and Wang S (2022) The schizophrenia and gut microbiota: A bibliometric and visual analysis. Front. Psychiatry 13:1022472. doi: 10.3389/fpsyt.2022.1022472
Received: 18 August 2022; Accepted: 24 October 2022;
Published: 15 November 2022.
Edited by:
Peng Zhong, University of Nebraska Medical Center, United StatesReviewed by:
Xinyu Fang, Nanjing Brain Hospital Affiliated to Nanjing Medical University, ChinaCopyright © 2022 Yang, Lin, Wang, Liu, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinyu Huang, emRzeWhqeTA5MDJAemp1LmVkdS5jbg==; Shuai Wang, ZHJ3YW5nc2h1YWlAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.