- 1Key Laboratory of Applied Psychology, Chongqing Normal University, Chongqing, China
- 2Research Center for Brain and Cognitive Science, Chongqing Normal University, Chongqing, China
Previous studies have reported that individuals with autistic traits, like those with Autism Spectrum Disorder (ASD), may have impaired empathic responses when observing static stimuli of others' pain. However, it remains unclear whether individuals with autistic traits exhibit impaired empathy for pain in response to dynamic stimuli. The present study addressed this question by recruiting 529 individuals whose autistic traits were assessed using the autism-spectrum quotient (AQ) questionnaire. Thirty participants who scored within the top 10% and bottom 10% on the AQ were selected into High-AQ and Low-AQ groups, respectively. This study employed painful whole-body action pictures and videos as static and dynamic stimuli. Both groups were instructed to judge whether the models in the stimuli were experiencing pain, and their reaction times, accuracy and event-related potential (ERP) data were recorded. Results showed that the P2 amplitudes were larger in the High-AQ group than in the Low-AQ group when viewing painful static stimuli, while no difference between the two groups was found when viewing painful dynamic stimuli. These results suggest that autistic traits influenced the emotional processing of others' pain in response to static stimuli.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder (1). The condition is persistent and is characterized by repetitive and restrictive patterns in behaviors, activities, or interests in social interactions (2). It has been suggested that ASD exists along a continuum of autistic-like symptoms, such as social-cognitive impairments (3). Autistic traits (symptoms associated with ASD) are distributed in the general population (4). The Autism Spectrum Quotient (AQ) questionnaire is commonly used to assess autistic traits, and higher scores are associated with higher levels of autistic traits (5, 6). Like those with ASD (7), individuals who have autistic traits also show characteristics of reduced sensitivity to social information (8). Due to the similarities between individuals with ASD and those with autistic traits, it may be possible to better understand the social abnormalities observed in the ASD population by studying individuals with autistic traits (4).
Empathy is the ability to understand the feelings and emotions of others (9). Lack of empathy may lead not only to misunderstandings, and apparent insensitivity to others' feelings, but also to fundamental difficulties in social life (10). Empathy consists of two parts: (1) cognitive empathy, which refers to the ability to understand another person's point of view; and (2) emotional empathy, which is the observer's emotional response to another person's mental state (11). The mind-blindness theory of ASD indicates that people with ASD have difficulty understanding the feelings, thoughts, and beliefs of others due to impaired empathy, resulting in atypical social interaction patterns (3). Lack of empathy and difficulty understanding the emotions of others are important criteria for the diagnosis of ASD (12, 13). Some researchers believe that individuals with ASD have cognitive empathy deficits (14), and it has been suggested that impaired implicit endoreceptive reasoning may contribute to those deficits (15). However, some studies have suggested that individuals with ASD exhibit impaired emotional empathy, not cognitive empathy (16, 17). As the inability to feel and show empathy can effectively predict autism and autistic traits (18), research on empathy in individuals with autistic traits can help us better understand autism.
Some studies that have used static stimuli of human expressions have indicated that both individuals with ASD (19, 20), and those with autistic traits (21) may have difficulty experiencing empathy, and hypothesized that this deficit may be the core mechanism underlying their social impairments (22). However, other studies have not found evidence to support this theory in real-life environments (23, 24). When we express emotions with full-body movements and postures, real-life emotions tend to be vivid. It is believed that dynamic displays induce activation of the mirror neuron system, a neuronal network related to empathy, which means that dynamic displays enhance an individual's empathy (25).
Empathy for pain is the ability to perceive and judge the pain of others (26), which includes cognitive and emotional components of understanding others' pain (27). Most previous studies have used static stimuli of others' pain, such as pictures of injured human limbs or faces, to explore empathy for pain in individuals with autistic traits (4, 26, 28–30), because static stimuli of other's pain easily inducing a response of empathy for pain and with high reproducibility (31). Some studies have used dynamic stimuli of others' pain, such as cutting one's hand with a knife while chopping vegetables or getting it caught in a door while closing it to explore empathy for pain (32–34). Usually, both static and dynamic painful stimuli elicit N1, N2, P2, and P3, and late positive potential (LPP) components in ERP studies of empathy for pain. N1, N2, and P2 represent the early emotional component, while P3 and LPP represent the late cognitive component of empathy for pain (35–39). The early involuntary onset of pain empathy for emotion sharing and emotional contagion is reflected in early components, while late pain empathic processing, including pain recognition and judgment of self - others is reflected in late components (40).
Some studies have found that individuals with autistic traits exhibited difficulty recognizing pictures of others' limb pain (30), and produced larger P3 amplitudes when judging pictures of injured human faces (28, 29) compared to controls. This suggests that individuals with autistic traits may devote more cognitive and mental resources to processing others' painful static stimuli, requiring more complex cognitive assessment and control (28, 29). For individuals with ASD or autistic traits, the difficulty in empathy for pain may not be limited to static stimuli of parts of the human body, but may extend to processing information from whole-body postures and actions (41). However, to our knowledge, no study has used dynamic stimuli of whole-body pain to explore empathy for pain in individuals with autistic traits.
As in real-life, pain is dynamic and realistic, we investigated the influence of whole-body painful, dynamic and static stimuli on individuals with autistic traits' empathy for pain. We assumed that if their difficulty in empathy for pain is related to both static and dynamic information, the deficit may relate to their overall empathic ability. However, if the impairment exists only when recognizing painful static, but not dynamic stimuli, the deficit in recognizing others' pain may be related to the decoding of painful static stimuli. It will provide new perspectives regarding empathy in ASD in the future.
Methods
Ethics
The study was approved by the Chongqing Normal University Research Ethics Committee, and all procedures were performed in accordance with ethical guidelines and regulations. Written informed consent was provided by all participants prior to participation in the experiment in accordance with the Declaration of Helsinki.
Participants
A total of 529 adults from the Chongqing Normal University were recruited to complete a paper and pencil version of the Mandarin Version of the AQ questionnaire (5) to identify those with high and low autistic traits. The AQ questionnaire consists of five subscales: social skills, attention shifting, attention to detail, communication, and imagination, each of which is comprised of 10 questions. The questions are answered using a 4-point scale, ranging from “agree completely”, “agree slightly”, “disagree slightly”, to “disagree completely”. Higher scores reflect more autistic traits.
An a priori power analysis using G*Power 3 (42) was conducted using a conservative average of moderate effect sizes from previous sharing empathy for pain studies (Cohen's d = 0.79–0.44) (43). The analysis yielded a sample size of n = 14 per group to detect a medium effect size of d = 0.44 at a standard error probability of α = 0.05 and power of 1–β = 0.95. Following this, two subsets of 30 participants, those exhibiting the top 10% and bottom 10% of AQ scores (26, 44) from the total of 529 adults were randomly selected and divided into High-AQ (n = 30, female = 15) and Low-AQ (n = 30, female = 15) groups. We invited a total of 63 participants, although three refused. All participants were paid a small sum of money for participating in the experiment. Detailed demographic characteristics of the High-AQ and Low-AQ groups are summarized in Table 1. Criteria for inclusion were: normal or corrected-to-normal vision and no history of neurological or psychiatric disorders.
Stimuli
The stimuli used in the experiment were static and dynamic stimuli whole-body actions showing actors in painful or non-painful situations. Twenty adult actors (10 female), aged between 18 and 24 years (mean ± SD = 22.30 ± 2.12 years) were recruited to record the stimuli. To eliminate distractions, they were asked to dress in a uniform (white T-shirt and black shorts), remove any accessories (e.g., earrings) and to not wear make-up. Before recording, actors received remuneration and voluntarily signed informed consent forms for the use of portrait rights.
Dynamic stimuli
Actors were given instructions and guided to express either painful or neutral actions in 1 s durations. Prior to recording, they were encouraged to imagine personal situations to evoke painful and non-painful feelings. During the recording of painful dynamic stimuli, each actor was asked to exhibit painful feelings evoked by imagining pain in different body parts. Similarly, the actors were asked to exhibit neutral feelings in corresponding body parts when recording non-painful dynamic stimuli. Finally, 160 dynamic stimuli were obtained (80 painful and 80 non-painful video clips, recorded by 8 males and 8 females).
Actors were filmed in an evenly lit green-screen studio with an ambient temperature of ~26°C, using a Sony FDR-AXP55 (Sony Group Corporation), at a distance of ~3 m from the actors. The camera height was 1.2 m, and each video clip was 2,160 × 1,280 pixels, using a 60 fps progressive scan.
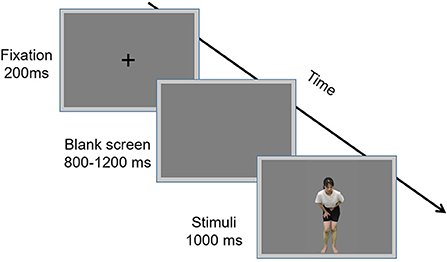
The video footage was edited using Adobe Premiere Adobe Premiere Pro2020 (Adobe Systems Incorporated). The green background was changed to gray and the actor was isolated from all other contextual information. Each video was edited to 1 s duration, Mp4 format, 768 × 432 pixels, and 60 fps. The luminance, contrast, and color were matched between the painful and non-painful dynamic stimuli. Examples of the dynamic stimuli are displayed in Figure 1, Left panel.
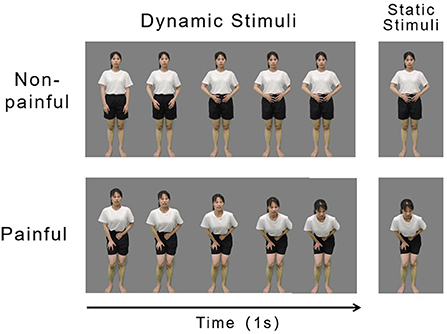
Figure 1. Examples of non-painful (Top) and painful (Bottom) stimuli. Examples of dynamic (Left) and static (Right) stimuli.
Static stimuli
The static stimuli used in the experiment were 160 screenshots that were derived from the 160 dynamic stimuli. A frame of an image that best represented the painful and non-painful feelings of an actor in each dynamic stimulus was cut out and used as static stimuli (examples of which are illustrated in the right panel of Figure 1). Each picture was 15.24 × 27.9 cm (width × height) and 72 pixels per inch.
Estimation of the stimuli
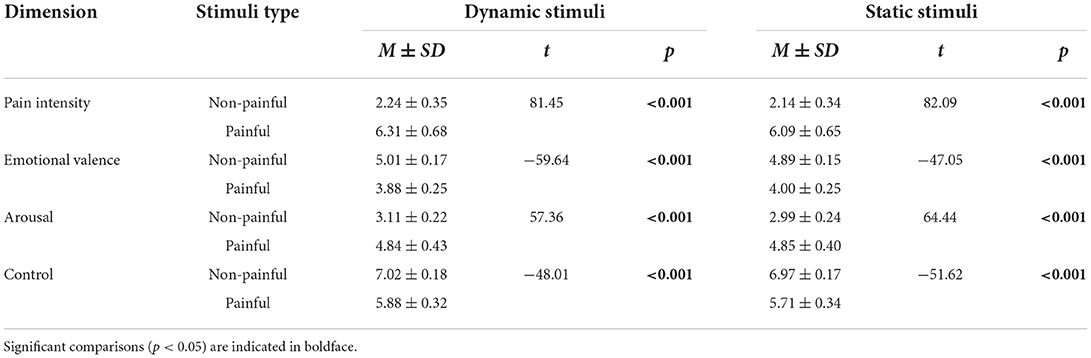
To assess how well the static and dynamic stimuli accurately reflected painful and non-painful feelings, seventy adults (35 females and 35 males), aged between 18 and 25 (mean ± SD = 21.96 ± 1.94) years from the Chongqing Normal University were recruited and were asked to evaluate the stimuli. They were not involved in the main experiment. In the experiment we used a 9-point Likert scales to assess: pain intensity (1 = no sensation, 4 = pain threshold, 9 = unbearable pain), emotional valence (1 = very unhappy, 9 = very happy), arousal (1 = extremely peaceful, 9 = extremely exciting), and control (1 = beyond control, 9 = under control). These results are summarized in Table 2.
Procedure
Study participants were seated in a quiet room with an ambient temperature about 26°C. Stimuli were presented on a 24-inch computer screen, using the E-Prime (3.0) program (Psychology Software Tools, Inc, Pittsburgh, PA, USA). All participants were seated about 80 cm from the screen, subtending a visual angle of 5.6° × 10° at viewing.
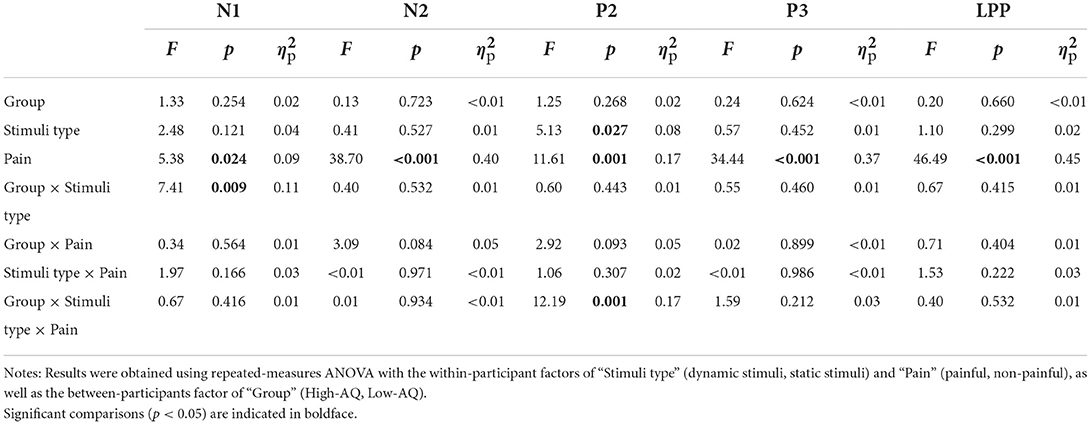
Participants were then instructed to assess whether the actor in each stimulus was experiencing pain. At the commencement of each trial, participants were presented with a 800–1,200 ms blank gray screen upon which a 200 ms fixation cross was presented, which was followed by the presentation of a 1,000 ms stimulus. Each stimulus was randomly presented once only. Participants were instructed to respond as accurately and quickly as possible by pressing a key (“1” or “2”) to indicate whether the actor depicted pain. Key-pressing was counterbalanced across participants to control for potential order effects. The inter-trial interval was 1,000 ms. The experiment comprised four blocks with 80 trials per block: two blocks included dynamic stimuli and two blocks included static stimuli. The four blocks were counterbalanced across all participants to control for possible order effects, and there was a 2–5 min break between blocks. The experimental procedure is illustrated in Figure 2.
After the experiment, participants were instructed to rate the attributes of pain intensity (1 = no sensation, 4 = pain threshold, 9 = unbearable pain), and emotional valence (1 = very unhappy, 9 = very happy), for each stimulus, using 9-point Likert scales.
EEG recording and data analyses
Electroencephalography (EEG) data were recorded from 64 scalp sites using tin electrodes mounted on an actiCHamp system (Brain Vision LLC, Morrisville, NC, US). The electrode on the frontal mastoid was used as a recording reference, and the one on the medial frontal aspect was used as a ground electrode. All electrode impedances remained below 5 kΩ.
EEG data were pre-processed and analyzed via MATLAB R2016a (MathWorks, Natick, MA, USA), and the EEGLAB toolbox (45). Continuous EEG signals were band-passed, filtered (0.1–40 Hz), and segmented using a 1,200 ms time window. Time windows of 200 ms before and 1,000 ms after the onset of stimuli were extracted from the continuous EEG. EEG epochs were baseline-corrected by a 200 ms time interval prior to stimuli onset. Epochs with amplitude values exceeding ± 80 μV at any electrode were excluded from the analyses. EEG epochs were also visually inspected, and trials containing significant noise from gross movements were removed–removed epochs accounted for 2.73 ± 4.85% of the total number of epochs. Electro-oculographic artifacts were corrected with an independent component analysis algorithm (46).
After confirming scalp topographies in both the single-participant and group-level event-related potential (ERP) waveforms, as well as on the basis of previous studies (47, 48), dominant ERP components and the electrodes included in the statistical analyses were identified as follows: N1, N2, and P2 (F1, Fz, F2, FC1, FCz, and FC2); P3 and late positive peak (LPP) (P3, Pz, P4, PO3, POz, and PO4). Amplitudes of N1, N2, P2, and P3 components were calculated as mean amplitudes with a latency interval of peak ± 20 ms at electrodes displaying maximal responses. The LPP was extracted within a time window of 400–600 ms.
Statistical analyses
Data analyses were performed using SPSS 15.0 software (IBM Corp., Armonk, NY, USA). Behavioral data [reaction times (RTs), accuracies (ACC)], subjective ratings (pain intensity and emotional valence) and ERP data (amplitudes of dominant ERP components) were analyzed using a three-way repeated-measure ANOVA. The within-participants' factors included: “Stimuli type” (dynamic stimuli, static stimuli) and “Pain” (painful, non-painful), with the between-participants' factor being “Group” (High-AQ, Low-AQ). The degrees of freedom for the F-ratios were corrected according to the Greenhouse-Geisser method. If the interactions between the three factors were significant, simple effects between groups analyses were performed for each condition. In total, nine 3-way ANOVAs were calculated.
Finally, we investigated the relationship between neural responses and autistic traits by assessing the correlation between participants' behavioral data and ERP amplitudes (N1, P2, N2, P3, and LPP), using Pearson's Correlation. To account for multiple comparisons, the false discovery rate (FDR) procedure (49) was used to correct p value.
Results
Behavioral results
Reaction time
RTs were modulated by the main effects of “Stimuli type” (F(1, 58) = 11.87, p = 0.001, = 0.17) and “Pain” (F(1, 58) = 29.92, p < 0.001, = 0.34). RTs were longer for dynamic stimuli (1,156.12 ± 55.43 ms) than for static stimuli (1,016.18 ± 44.81 ms), while RTs were longer for non-painful stimuli (1,185 ± 55.74 ms) than for painful stimuli (986.45 ± 42.59 ms).
Accuracies
ACCs were modulated by the main effect “Pain” (F(1, 58) = 11.37, p = 0.001, = 0.16) with the ACCs for painful stimuli (91.40 ± 1.60 %) being higher than those for non-painful stimuli (88.00 ± 1.80 %).
Pain intensity
Pain intensity ratings were modulated by the main effect of “Pain” (F(1, 58) = 1,028.93, p < 0.001, = 0.95). The pain intensity for painful stimuli (6.35 ± 0.12) was higher than that of the non-painful stimuli (2.05 ± 0.08).
Emotional valence
Emotional valence ratings were modulated by the main effects of “Stimuli type” (F(1, 58) = 6.73, p = 0.012, = 0.10), and “Pain” (F(1, 58) = 58.66, p < 0.001, = 0.50). The emotional valence was more negative for static stimuli (4.28 ± 0.11) than for dynamic stimuli (4.37 ± 0.10), while the emotional valence was more negative for painful stimuli (3.60 ± 0.15) than for non-painful stimuli (5.06 ± 0.14).
ERP results
ERP waveforms and scalp topographies are shown in Figure 3, and bar charts for ERP amplitudes are shown in Figure 4. Results of the statistical analyses of the ERP amplitudes are summarized in Table 3.
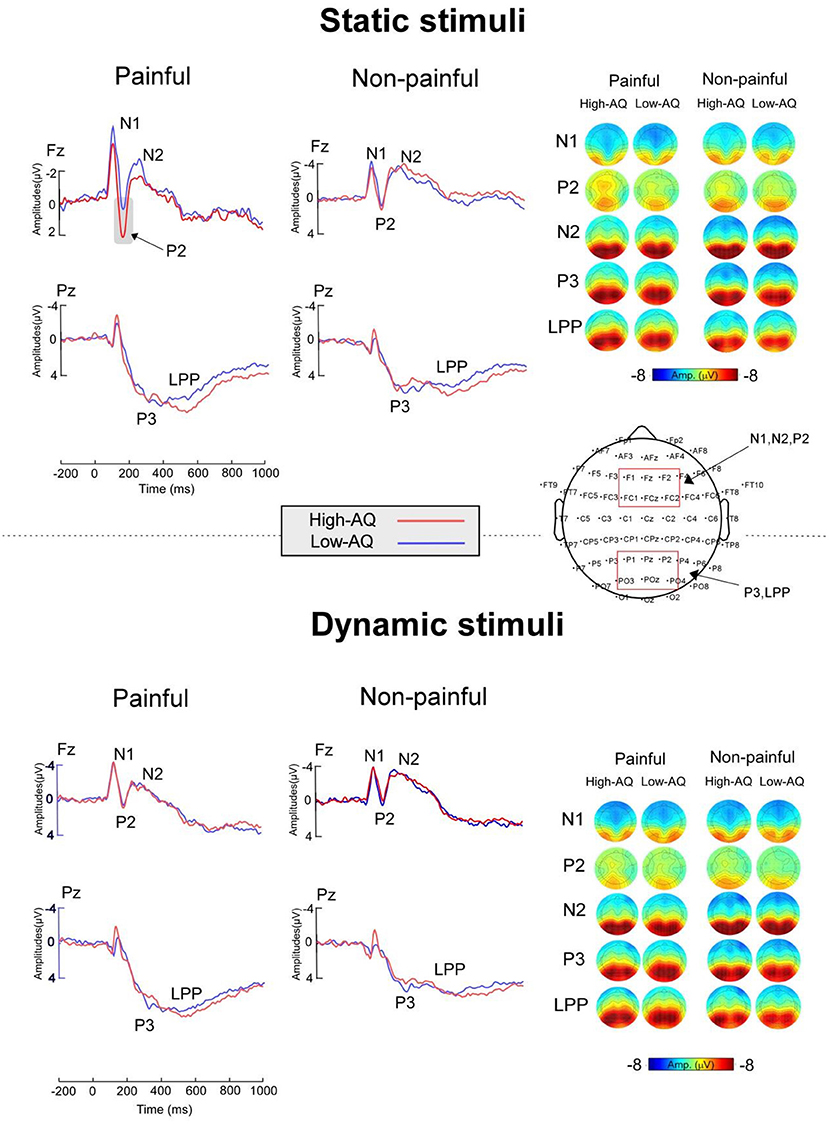
Figure 3. ERP waveforms (Right) and scalp topography distributions (Left) exhibited by the High-AQ (blue lines) and Low-AQ (red lines) groups in response to static stimuli (Top) and dynamic stimuli (Bottom). Electrodes used to estimate the ERP amplitudes are marked using the red squares on their respective topographic distributions.
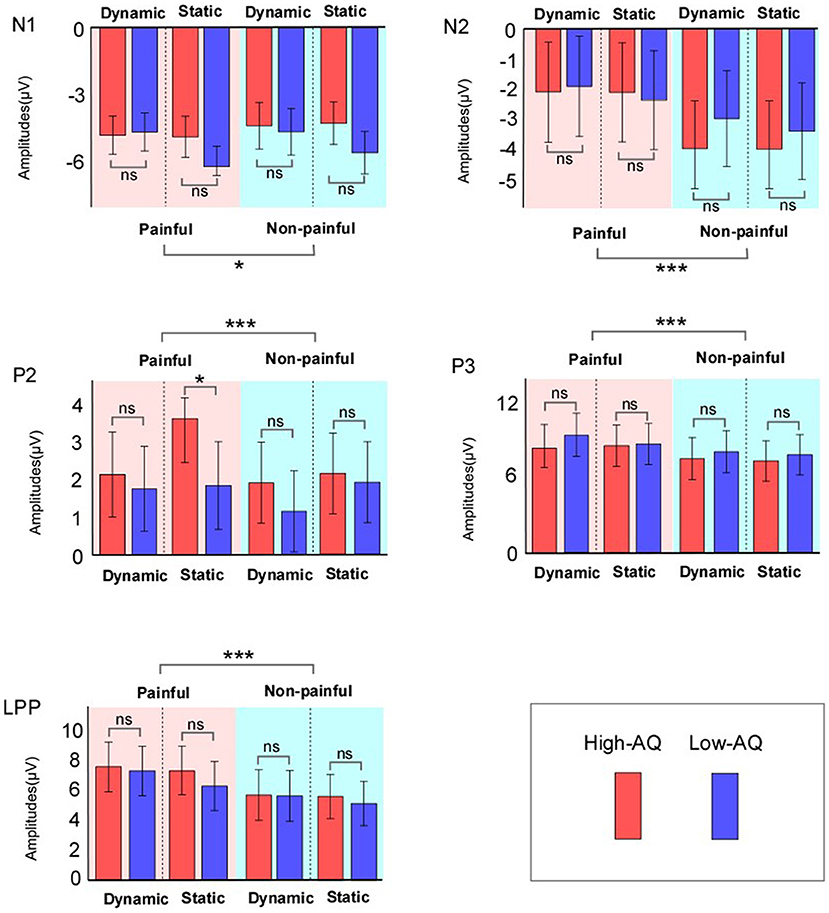
Figure 4. ERP amplitudes of High-AQ (blue bar) and Low-AQ (red bar) groups in response to static and dynamic stimuli. Data in the charts are expressed as Mean ± 2SEM. ns: p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001.
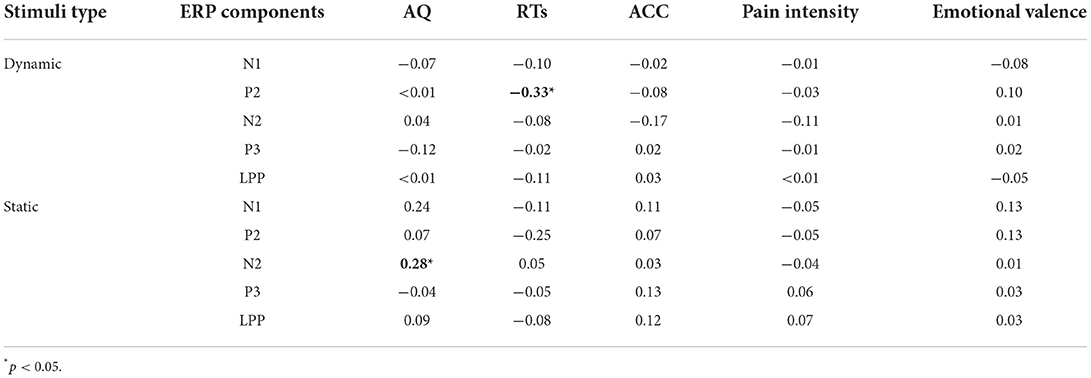
N1 amplitudes were modulated by “Pain” (F(1, 58) = 5.38, p = 0.024, = 0.09), and painful stimuli (−5.03 ± 0.27 μV) elicited larger N1 amplitudes than non-painful stimuli (−4.63 ± 0.33 μV). N1 amplitudes were also modulated by the interaction between “Group” and “Stimuli type” (F(1, 58) = 7.41, p = 0.009, = 0.11), with static stimuli (−5.64 ± 0.42 μV) eliciting larger N1 amplitudes than dynamic stimuli (−4.69 ± 0.45 μV) in the Low-AQ group. No other main effects or interactions were found (p > 0.05).
N2 amplitudes were modulated by “Pain” (F(1, 58) = 38.70, p < 0.001, = 0.40), with non-painful stimuli (−3.56 ± 0.53 μV) eliciting larger N2 amplitudes than painful stimuli (−2.09 ± 0.55 μV).
P2 amplitudes were modulated by the main effects of “Stimuli type” (F(1, 58) = 5.13, p = 0.027, = 0.08) and “Pain” (F(1, 58) = 11.61, p = 0.001, = 0.17). Static stimuli (2.40 ± 0.38 μV) elicited larger P2 amplitudes than dynamic stimuli (1.77 ± 0.38 μV), and painful stimuli (2.37 ± 0.37 μV) elicited larger P2 amplitudes than non-painful stimuli (1.79 ± 0.36 μV). P2 amplitudes were also modulated by the interaction between “Group”, “Stimuli type”, and “Pain” (F(1, 58) = 12.19, p = 0.001, = 0.17). Simple effect analyses indicated that P2 amplitudes were larger in the High-AQ group (3.65 ± 0.59 μV) than in the Low-AQ group (1.87 ± 0.59 μV, p = 0.035) in response to painful static stimuli. However, no group difference was found in response to other stimuli (i.e., non-painful static stimuli, painful and non-painful dynamic stimuli) (p > 0.05).
P3 and LPP amplitudes were modulated by “Pain” (P3: F(1, 58) = 34.44, p < 0.001, = 0.37; LPP: F(1, 58) = 46.49, p < 0.001, = 0.45), with painful stimuli (P3: 8.65 ± 0.56 μV; LPP: 7.21 ± 0.55 μV) eliciting larger amplitudes than non-painful stimuli (P3: 7.58 ± 0.55 μV; LPP: 5.59 ± 0.54 μV).
Correlation between ERP data and behavioral data
Results of the correlation analysis indicated that participant's AQ scores were positively correlated with the P2 amplitudes of painful static stimuli (r = 0.28, p = 0.033). A significant correlation between P2 amplitudes and RTs for painful dynamic stimuli (r = −0.38, p = 0.011) was found. No other significant correlations were found between ERP data and behavioral data (p > 0.05). The correlation results for ERP amplitudes and behavioral data (painful dynamic/static stimuli) and AQ scores are presented in Table 4.
Discussion
In order to explore the potential influence of autistic traits on empathy for pain when observing dynamic and static stimuli, we investigated the behavioral and neural responses to others' pain in individuals with autistic traits utilizing videos and pictures of painful whole-body actions as experimental materials. Results showed that P2 amplitudes were larger in the High-AQ group than in the Low-AQ group when viewing painful static stimuli only and no difference between the two groups was found in response to dynamic stimuli. These results suggest that the empathic difficulties experienced by individuals with autistic traits may be influenced by the type of stimuli. In addition, the altered decoding process when interpreting painful static stimuli may be an important cause of difficulties individuals with autistic traits have when identifying others' pain in experimental settings.
Consistent with previous studies (35–39), the present study found that both behavioral (RTs, ACCs) and ERP (N1, N2, P2, P3, and LPP amplitudes) results were significantly modulated by the main effect of “Pain” as participants responded more quickly and more accurately, and showed larger ERP amplitudes to painful than to non-painful stimuli. In previous studies of empathy for pain, a subset of fixed ERP components have been shown to be good indicators. Examples include the N1, P2, and N2 components, which arise above the frontoparietal lobe and reflect early pain perception and emotion sharing, and the P3 and LPP components, which arise above the parieto-occipital lobe and reflect later cognitive appraisal (50). Like previous studies which used painful pictures or videos of human limbs (38, 51, 52), faces (53), and robot hands (52), the present study utilized dynamic and static stimuli of human whole-body actions to demonstrate feelings of pain and elicit similar empathic responses to others' pain.
In previous studies, individuals with high scores on the AQ questionnaire were considered to have autistic traits (26, 28, 29, 54, 55) and it was used to select participants in the present study. The P2 amplitudes in the High-AQ group were larger than those in the Low-AQ group when they viewed painful static stimuli, while the P2 amplitudes of the two groups did not differ in response to painful dynamic stimuli. In addition, P2 amplitudes in response to painful static stimuli were positively correlated with participants' AQ scores, with higher AQ scores corresponding to larger P2 amplitudes in response to painful static stimuli. As P2 represents the emotional component of empathy for pain (56, 57) our findings suggest that emotional processing in response to painful static stimuli may be influenced by autistic traits, and individuals with autistic traits require more mental resources for emotional processing when viewing painful static stimuli or vice versa.
However, no differences were found between the groups in response to painful dynamic stimuli in the present study, which may be because the dynamic stimuli provided more real-world information than the static stimuli. Previous studies have also found individuals with ASD did not exhibit empathic deficits in real-world environments (58), but exhibited empathic difficulties in response to static stimuli in an experimental environment (59). This evidence suggests that the empathy deficit seen in people with ASD and autistic traits may be related to the decoding of emotional painful static stimuli. Previous research has shown that an important feature of mirror neurons is the link between vision and movement. When an individual sees an action performed by another person, the neurons representing that action are activated in the individual's premotor cortex. Thus, individuals are better able to understand the actions performed by others (60). Dynamic simulation of pain provides more detail than static simulation, such as a change in one's facial? expression when feeling pain, and rubbing the painful area when feeling pain. Empathy impairment in individuals with ASD may be related to the level of mirror neural system involvement, and to the type of stimuli used (61). For example, a previous study has found that activation of the mirror neural system was associated with emotional empathy (62), and lower levels of mirror neural system involvement in individuals with ASD may result in greater difficulty decoding painful static stimuli when processing such stimuli. The observation that individuals with ASD do not exhibit empathic deficits when processing painful dynamic stimuli may account for the better decoding of dynamic pain stimuli that was found in individuals with autistic traits in the present study.
Finally, while our study was methodologically sound and conducted in a rigorous manner, there are several limitations that should be acknowledged. First since we focused on the difference in temporal resolution between dynamic and static stimuli in this study, we chose EEG rather than fMRI. The ERP technique used in this study did not provide evidence regarding the area of brain activation, which may be further explored in future studies using fMRI. Second, the participants in the present study were individuals with autistic traits, and future investigations may benefit by including individuals with ASD. Finally, it has also been suggested that individuals with ASD have difficulty empathizing because of alexithymia, rather than an impairment in empathy. Alexithymia has a relatively high prevalence rate in the ASD population, and is characterized by individuals' difficulty identifying and describing their emotional states (12, 13). However, this issue was not analyzed in our experiments, and we plan to investigate it in future studies.
Conclusions
This study investigated the influence of autistic traits on empathy for pain when viewing dynamic and static stimuli. Our findings of larger P2 amplitudes by the High-AQ group compared to the Low-AQ group in response to painful static, but not dynamic stimuli, suggests that individuals with autistic traits may have altered emotional processing in response to others' painful static stimuli.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Chongqing Normal University Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: conceptualization, methodology, software, data curation, and writing-original draft preparation. ZW and MS: editor of the revised manuscript. MH: writing-original draft preparation. DY: methodology, software, and writing-original draft preparation. LL: software and data curation. JM: conceptualization, methodology, funding acquisition, and writing-reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ministry of Education in China, Humanity and Social Science Youth Foundation Project (Grant number 19YJC190016) and Fundamental Research Funds for the National Natural Science Foundation of China (31400882).
Acknowledgments
We are grateful to Lingxiao Li, Xiaocui Liu, and Jin Jiang for their support. We thank all actors and participants who took part in this study. This work was supported by Program for Chongqing Scholars and Innovative Research Team in University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ASD, Autism spectrum disorder; AQ, Autism Spectrum Quotient; ERP, Event-related Potentials; DSM-5, Diagnostic and Statistical Manual of Mental Disorders (5th ed.); IAPS, International Affective Picture System; CAPS, Chinese Affective Picture System; ISI, inter-stimulus interval; ANOVA, Analysis of variance; EEG, Electroencephalography; ACCs, Accuracies; RTs, Reaction times.
References
1. Xu XJ, Cai XE, Meng FC, Song TJ, Wang XX, Wei YZ, et al. Comparison of the metabolic profiles in the plasma and urine samples between autistic and typically developing boys: a preliminary study. Front Psychiatry. (2021) 12:657105. doi: 10.3389/fpsyt.2021.657105
2. McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J Child Psychol. Psychiatry. (2004) 45:1235–45. doi: 10.1111/j.1469-7610.2004.00318.x
3. Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge: MIT press (1997).
4. Zhang W, Zhuo S, Li X, Peng W. Autistic traits and empathy for others' pain among the general population: test of the mediating effects of first-aand pain sensitivity. J Autism Dev Disord. (2022) 1–15. doi: 10.1007/s10803-022-05471-9
5. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. (2001) 31:5–17. doi: 10.1023/A:1005653411471
6. Becker C, Caterer E, Chouinard PA, Laycock R. Alterations in rapid social evaluations in individuals with high autism traits. J Autism Dev Disord. (2021) 51:3575–85. doi: 10.1007/s10803-020-04795-8
7. Sevgi M, Diaconescu AO, Henco L, Tittgemeyer M, Schilbach L. Social bayes: using bayesian modeling to study autistic trait-related differences in social cognition. Biol Psychiatry. (2020) 87:185–93. doi: 10.1016/j.biopsych.2019.09.032
8. Poljac E, Poljac E, Wagemans J. Reduced accuracy and sensitivity in the perception of emotional facial expressions in individuals with high autism spectrum traits. Autism. (2013) 17:668–80. doi: 10.1177/1362361312455703
9. Peng W, Lou W, Huang X, Ye Q, Tong RK, Cui F. Suffer together, bond together: Brain-to-brain synchronization and mutual affective empathy when sharing painful experiences. Neuroimage. (2021) 238:118249. doi: 10.1016/j.neuroimage.2021.118249
10. Rum Y, Perry A. Empathic accuracy in clinical populations. Front Psychiatry. (2020) 11:457. doi: 10.3389/fpsyt.2020.00457
11. Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP, Kelly C. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Soc Cogn Affect Neurosci. (2012) 7:727–37. doi: 10.1093/scan/nsr051
12. Bird G, Cook R. Mixed emotions: the contribution of alexithymia to the emotional symptoms of autism. Transl Psychiatry. (2013) 3:e285. doi: 10.1038/tp.2013.61
13. Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. (2010) 133:1515–25. doi: 10.1093/brain/awq060
14. Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, et al. Dissociation of cognitive and emotional empathy in adults with asperger syndrome using the multifaceted empathy test (MET). J Autism Dev Disord. (2008) 38:464–73. doi: 10.1007/s10803-007-0486-x
15. Gu X, Eilam-Stock T, Zhou T, Anagnostou E, Kolevzon A, Soorya L, et al. Autonomic and brain responses associated with empathy deficits in autism spectrum disorder. Hum Brain Map. (2015) 36:3323–38. doi: 10.1002/hbm.22840
16. Mazza M, Pino MC, Mariano M, Tempesta D, Ferrara M, De Berardis D, et al. Affective and cognitive empathy in adolescents with autism spectrum disorder. Front Hum Neurosci. (2014) 8:791. doi: 10.3389/fnhum.2014.00791
17. Scambler DJ, Hepburn S, Rutherford MD, Wehner EA, Rogers SJ. Emotional responsivity in children with autism, children with other developmental disabilities, and children with typical development. J Autism Dev Disord. (2007) 37:553–63. doi: 10.1007/s10803-006-0186-y
18. Shalev I, Warrier V, Greenberg DM, Smith P, Allison C, Baron-Cohen S, et al. Reexamining empathy in autism: Empathic disequilibrium as a novel predictor of autism diagnosis and autistic traits. Autism Res. (2022) 15:1917–28. doi: 10.1002/aur.2794
19. Bookheimer SY, Wang AT, Scott A, Sigman M, Dapretto M. Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J Int Neuropsychol Soc. (2008) 14:922–32. doi: 10.1017/S135561770808140X
20. Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Res Cognit Brain Res. (2005) 24:190–8. doi: 10.1016/j.cogbrainres.2005.01.014
21. Robertson AE, Simmons DR. The relationship between sensory sensitivity and autistic traits in the general population. J Autism Dev Disord. (2013) 43:775–84. doi: 10.1007/s10803-012-1608-7
22. Zhao X, Li X, Song Y, Shi W. Autistic traits and prosocial behaviour in the general population: test of the mediating effects of trait empathy and state empathic concern. J Autism Dev Disord. (2019) 49:3925–38. doi: 10.1007/s10803-018-3745-0
23. Evers K, Steyaert J, Noens I, Wagemans J. Reduced recognition of dynamic facial emotional expressions and emotion-specific response bias in children with an autism spectrum disorder. J Autism Dev Disord. (2015) 45:1774–84. doi: 10.1007/s10803-014-2337-x
24. Jones CR, Pickles A, Falcaro M, Marsden AJ, Happé F, Scott SK, et al. A multimodal approach to emotion recognition ability in autism spectrum disorders. J Child Psychol Psychiatry. (2011) 52:275–85. doi: 10.1111/j.1469-7610.2010.02328.x
25. Rymarczyk K, Zurawski Ł, Jankowiak-Siuda K, Szatkowska I. Do dynamic compared to static facial expressions of happiness and anger reveal enhanced facial mimicry? PLoS ONE. (2016) 11:e0158534. doi: 10.1371/journal.pone.0158534
26. Meng J, Shen L, Li Z, Peng W. Top-down effects on empathy for pain in adults with autistic traits. Sci Rep. (2019) 9:8022. doi: 10.1038/s41598-019-44400-2
27. Ece Ozdemir O, Seyda C. Empathy for Pain. In W. Viduranga Yashasvi, B. Ines, and B. Jelena (Eds.), Pain Management. Rijeka: IntechOpen (2021). p. 5. doi: 10.5772/intechopen.95276c
28. Li X, Li Z, Xiang B, Meng J. Empathy for pain in Individuals with autistic traits influenced by attention cues: evidence from an ERP study. Acta Psychologica Sinica. (2020) 52:294. doi: 10.3724/SP.J.1041.2020.00294
29. Li X, Liu Y, Ye Q, Lu X, Peng W. The linkage between first-hand pain sensitivity and empathy for others' pain: attention matters. Hum Brain Mapp. (2020) 41:4815–28. doi: 10.1002/hbm.25160
30. Meng J, Li Z, Shen L. Responses to others' pain in adults with autistic traits: The influence of gender and stimuli modality. PLoS ONE. (2017) 12:e0174109. doi: 10.1371/journal.pone.0174109
31. Ren Q, Lu X, Zhao Q, Zhang H, Hu L. Can self-pain sensitivity quantify empathy for others' pain? Psychophysiology. (2020) 57:e13637. doi: 10.1111/psyp.13637
32. Chen C, Yang CY, Cheng Y. Sensorimotor resonance is an outcome but not a platform to anticipating harm to others. Soc Neurosci. (2012) 7:578–90. doi: 10.1080/17470919.2012.686924
33. Fan YT, Chen C, Chen SC, Decety J, Cheng Y. Empathic arousal and social understanding in individuals with autism: evidence from fMRI and ERP measurements. Soc Cogn Affect Neurosci. (2014) 9:1203–13. doi: 10.1093/scan/nst101
34. Li X, Lou W, Zhang W, Tong RK, Hu L, Peng W. Ongoing first-hand pain facilitates somatosensory resonance but inhibits affective sharing in empathy for pain. Neuroimage. (2022) 263:119599. doi: 10.1016/j.neuroimage.2022.119599
35. Chen J, Chang B, Li W, Shi Y, Shen H, Wang R, et al. Dispositional self-construal modulates the empathy for others' pain: an ERP study. Front Psychol. (2020) 11:508141. doi: 10.3389/fpsyg.2020.508141
36. Cui F, Ma N, Luo YJ. Moral judgment modulates neural responses to the perception of other's pain: an ERP study. Sci Rep. (2016) 6:20851. doi: 10.1038/srep20851
37. Cui F, Zhu X, Luo Y. Social contexts modulate neural responses in the processing of others' pain: an event-related potential study. Cognit Affect Behav Neurosci. (2017) 17:850–7. doi: 10.3758/s13415-017-0517-9
38. Fan Y, Han S. Temporal dynamic of neural mechanisms involved in empathy for pain: An event-related brain potential study. Neuropsychologia. (2008) 46:160–73. doi: 10.1016/j.neuropsychologia.2007.07.023
39. Song J, Wei Y, Ke H. The effect of emotional information from eyes on empathy for pain: A subliminal ERP study. PLoS ONE. (2019) 14:e0226211. doi: 10.1371/journal.pone.0226211
40. Peng W, Meng J, Lou Y, Li X, Lei Y, Yan D. Reduced empathic pain processing in patients with somatoform pain disorder: evidence from behavioral and neurophysiological measures. Int J Psychophysiol Official J Int Organiz Psychophysiol. (2019) 139:40–7. doi: 10.1016/j.ijpsycho.2019.03.004
41. Mazzoni N, Ricciardelli P, Actis-Grosso R, Venuti P. Difficulties in recognising dynamic but not static emotional body movements in autism spectrum disorder. J Autism Dev Disord. (2022) 52:1092–105. doi: 10.1007/s10803-021-05015-7
42. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
43. Rütgen M, Seidel EM, Riečanský I, Lamm C. Reduction of empathy for pain by placebo analgesia suggests functional equivalence of empathy and first-hand emotion experience. J Neurosci Official J Soc Neurosci. (2015) 35:8938–47. doi: 10.1523/JNEUROSCI.3936-14.2015
44. Meng J, Li Z, Shen L. Altered neuronal habituation to hearing others' pain in adults with autistic traits. Sci Rep. (2020) 10:15019. doi: 10.1038/s41598-020-72217-x
45. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. (2004) 134:9–21. doi: 10.1016/j.jneumeth.2003.10.009
46. Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Analysis and visualization of single-trial event-related potentials. Hum Brain Mapp. (2001) 14:166–85. doi: 10.1002/hbm.1050
47. Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. Cogn Neurosci. (2008) 20:977–88. doi: 10.1162/jocn.2008.20066
48. Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol. (2010) 35:129–55. doi: 10.1080/87565640903526504
49. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
50. Li X, Zhang Y, Xiang B, Meng J. Differences between empathy for face and body pain: Cognitive and neural responses. Brain Sci Adv. (2019) 5:256–64. doi: 10.26599/BSA.2019.9050022
51. Ibáñez A, Hurtado E, Lobos A, Escobar J, Trujillo N, Baez S, et al. Subliminal presentation of other faces (but not own face) primes behavioral and evoked cortical processing of empathy for pain. Brain Res. (2011) 1398:72–85. doi: 10.1016/j.brainres.2011.05.014
52. Suzuki Y, Galli L, Ikeda A, Itakura S, Kitazaki M. Measuring empathy for human and robot hand pain using electroencephalography. Sci Rep. (2015) 5:15924. doi: 10.1038/srep15924
53. Meng J, Li X, Peng W, Li Z, Shen L. The interaction between pain and attractiveness perception in others. Sci Rep. (2020) 10:5528. doi: 10.1038/s41598-020-62478-x
54. Peled-Avron L, Shamay-Tsoory SG. Don't touch me! autistic traits modulate early and late ERP components during visual perception of social touch. Autism Res. (2017) 10:1141–54. doi: 10.1002/aur.1762
55. Yang D, Tao H, Ge H, Li Z, Hu Y, Meng J. Altered processing of social emotions in individuals with autistic traits. Front Psychol. (2022) 13:746192. doi: 10.3389/fpsyg.2022.746192
56. Decety J, Yang CY, Cheng Y. Physicians down-regulate their pain empathy response: an event-related brain potential study. Neuroimage. (2010) 50:1676–82. doi: 10.1016/j.neuroimage.2010.01.025
57. Sheng F, Han S. Manipulations of cognitive strategies and intergroup relationships reduce the racial bias in empathic neural responses. Neuroimage. (2012) 61:786–97. doi: 10.1016/j.neuroimage.2012.04.028
58. Ponnet K, Buysse A, Roeyers H, De Corte K. Empathic accuracy in adults with a pervasive developmental disorder during an unstructured conversation with a typically developing stranger. J Autism Dev Disord. (2005) 35:585–600. doi: 10.1007/s10803-005-0003-z
59. Lassalle A, Zürcher NR, Porro CA, Benuzzi F, Hippolyte L, Lemonnier E, et al. Influence of anxiety and alexithymia on brain activations associated with the perception of others' pain in autism. Social Neurosci. (2019) 14:359–77. doi: 10.1080/17470919.2018.1468358
60. Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. (2004) 27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230
61. Lassalle A, Zürcher NR, Hippolyte L, Billstedt E, Porro CA, Benuzzi F, et al. Effect of visual stimuli of pain on empathy brain network in people with and without autism spectrum disorder. Eur J Neurosci. (2018) 48:2333–42. doi: 10.1111/ejn.14138
Keywords: autism spectrum disorder, autism, empathy, empathy for pain, event related potential
Citation: Li Y, Wei Z, Shao M, Hong M, Yang D, Luo L and Meng J (2022) Empathy for pain in individuals with autistic traits during observation of static and dynamic stimuli. Front. Psychiatry 13:1022087. doi: 10.3389/fpsyt.2022.1022087
Received: 18 August 2022; Accepted: 31 October 2022;
Published: 16 November 2022.
Edited by:
Markus Rütgen, Karolinska Institutet (KI), SwedenReviewed by:
Helena Hartmann, Essen University Hospital, GermanyWeiwei Peng, Shenzhen University, China
Copyright © 2022 Li, Wei, Shao, Hong, Yang, Luo and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Meng, cXVmdW1qQHFxLmNvbQ==
†These authors have contributed equally to this work
 Yanting Li
Yanting Li Zilong Wei1,2†
Zilong Wei1,2† Jing Meng
Jing Meng